Highlights
-
•
Cruciferous vegetables as fermented products has been used since ancient times.
-
•
During fermentation of cruciferous vegetables complete fermentation of glucosinolates occur.
-
•
Fermentation decrease the content of complex polyphenols, while increase the content of polyphenols in free form.
-
•
Carotenoid content decrease during fermentation of cruciferous vegetables.
Keywords: Cruciferous vegetables, Fermentation, Carotenoids, Polyphenols, Glucosinolates
Abstract
Cruciferous vegetables are considered functional foods because of their content of health-related compounds. They are grown and consumed in various cultures around the world. Fermentation as a preservation method for cruciferous vegetables has been used since ancient times. This process results in fermented products that have a unique flavour and odour, high bioactivity, and a distinctly different phytochemical profile than raw vegetables. In this mini review, we summarize data on changes in phytochemical content during lactic-acid fermentation of various cruciferous vegetables. The main focus was on the changes in the group of glucosinolates, polyphenols and carotenoids.
Introduction
Cruciferous vegetables belong to the genus Brassica (family Brassicaceae), along with other oilseed (canola, mustard) crops (Baky, Shamma, Xiao, & Farag, 2022). The most commonly grown and utilized cruciferous vegetables include Brassica oleracea and Brassica rapa which are almost entirely edible (leaves, inflorescence, root, stem, and seed). In addition, Brassica juncea, Brassica nigra and Brassica carinata have been used as cruciferous vegetables for human consumption (Šamec & Salopek-Sondi, 2019). Their common name, Latin name and part used for human consumption as vegetables are listen in Table 1. (adopted from Šamec & Salopek-Sondi, 2019).
Table 1.
Cruciferous vegetables common name, Latin name and parts used for human consumption.
| Common name | Genus, species | Cultivar, croup | Part used for food |
|---|---|---|---|
| broccoli | B. oleracea | var. italica | inflorescence |
| broccolini, broccoflower | var. italica × alboglabra | inflorescence | |
| Brussels sprouts | var. gemmifera | buds | |
| cabbage (white, red, cone etc.) | var.capitata | leaves | |
| cauliflower, Romanesco broccoli | var. botrytis | inflorescence | |
| Chinese brocolli, Kai-lan | var. alboglabra | leaves | |
| collard greens | var. viridis | leaves | |
| kale, collard greens | var. acephala | leaves | |
| kohlrabi | var. gongylodes | stem | |
| Asian greens | B. rapa | ssp. narinosa (or rosularis) | leaves |
| Bok Choy, Pak Choy | ssp. chinensis | leaves | |
| Chinese cabbage, napa cabbage | ssp. pekinensis | leaves | |
| komatsuna, Japanese mustard spinach | ssp. pervidis | leaves | |
| mizuna | ssp. nipposinica | leaves | |
| rapini, broccoli rabe | ssp. parachinensis | Leaves, stem, flower buds | |
| tunrip | ssp. rapa | root | |
| mustard greens, Chinese leaf mustard | B. juncea | var. rugosa (or integrifolia) | leaves |
| Siberian kale | B. napus | var. pabularia | leaves |
Because of their good adaptability to different environmental conditions, different cultivars are now grown as vegetables on all continents and are part of the culinary and traditional medicine of different cultures. Cruciferous vegetables can be eaten raw, in salads, or cooked in various ways and served alone or in combination with vegetables and meats. New trends in gastronomy have also introduced the consumption of cruciferous vegetables as sprouts (Šamec, Pavlović, Radojčić Redovniković, & Salopek-Sondi, 2018). Since the earliest times, cruciferous vegetables have also been consumed after fermentation. Fermentation as a method of preservation for cruciferous vegetables was used in ancient times and is present in daily life as a process of food preservation. It is a metabolic process in which carbohydrates (among others) are converted into alcohol or organic acids with the help of enzymes in bacteria and/or yeasts (Nkhata, Ayua, Kamau & Shingiro, 2018). By applying different technologies from those that can be described as simple and domestic to those that are complex, advanced and industrial, different characteristics of fermented products can be obtained. To produce a product with the desired quality, it is necessary to monitor various process parameters such as temperature, oxygen and salt content, and microorganisms present. The quality of the raw material also plays an important role.
Fermentation is based on the production of lactic acid from sugar under microbial activity. The fermentation of white cabbage, which was first used in Central and Eastern Europe, is known worldwide. Products made from shredded fermented cabbage are known as sauerkraut, but the whole heads can also be fermented. In other parts of the world, different cruciferous vegetables are fermented, and in many cultures fermented cruciferous products are considered functional foods or superfood because they are a good source of vitamins, minerals, prebiotic fibers, polyphenols, active compounds derived from as glucosinolates, phytoestrogens and bioactive peptides (Septembre-Malaterre et al., 2018, Quirante-Moya et al., 2020, Ciska et al., 2021). These compounds may contribute to the antioxidant, chemo protective and immunomodulatory effects of fermented cruciferous vegetables (Šamec & Salopek-Sondi, 2019). Epidemiological studies associated the consumption of fermented cruciferous vegetables with a lower risk of breast cancer (Pathak et al., 2021). Recent studies even suggest a beneficial effect of fermented cabbage in mitigating COVID-19 severity (Bousquet et al., 2020). Despite the fact that all of these reports emphasize the benefits of fermentation, little is known about the background of these beneficial properties. Even less is known how metabolic change during fermentation induces mentioned health benefits (Kim, Choi, Park, & Kim, 2019).
All cruciferous vegetables have a similar phytochemical profile, with phytochemicals from/or derivate from glucosinolates, polyphenols, and carotenoids group associated with the health benefits of cruciferous vegetables (Šamec & Salopek-Sondi, 2019). However, the final product, after cruciferous vegetables fermentation, has completely different nutritional and phytochemical properties than the raw material (Swain, Anandharaj, Ray, & Parveen Rani, 2014). Therefore, we would like to summarize here the available data on the presence of phytochemicals in fermented cruciferous vegetables and the chemistry behind them.
Lactic acid fermentation
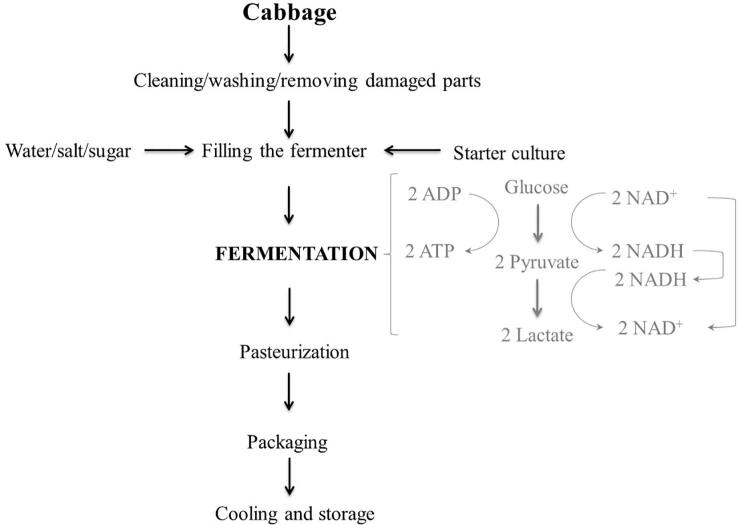
As mentioned earlier, lactic acid fermentation or commonly known as lactic-fermentation, is based on the production of lactic acid from sugar. Example of lactic-fermentation of cabbage are shown at Fig. 1. In the fermentation process, lactic acid production is important because it has a dual function: prevention and nutrient and sensory enrichment of the product. Acetic acid has a stronger antimicrobial effect than lactic acid, so it is believed that the formation of this acid is of great importance for proper process control. Fermentation is a complex process in which numerous physical, chemical and microbial changes affect the quality of the final product. The first step of the process is the collection of raw materials, followed by cleaning/washing, removal of stumps, washing, milling, packaging and fermentation. The fermentation process can be done spontaneously by indigenous microbial cultures on the vegetables or by adding starter cultures (Li & Gänzle, 2020). The mixture of autochthonous microorganisms responsible for fermentation processes on vegetables depends on the type of vegetable (chemical composition, biota, buffer capacity etc.), place of growth, temperature and harvesting conditions (Sajjad, Rasool, Bakr, Fazili, & Bhat, 2020). They include lactic acid bacteria (LAB) such as Enterococcus, Streptococcus, Leuconostoc, Lactobacillus, and Pediococcus and yeasts and molds Debaryomyces, Kluyveromyces, Saccharomyces, Geotrichium, Mucor, Penicillium, and Rhizopus species (Sharma et al., 2020, Torres et al., 2020, Wuyts et al., 2020). When the fermentation process occurs under optimal/favourable conditions, main role will have LAB belonging mostly to Leuconostoc (L. mesenteroides), bacillus (Lactiplantibacillus plantarum, Lactiplantibacillus pentosus, Levilactobacillus brevis, Limosilactobacillus fermentum, Lacticaseibacillus casei), Weissella, Enterococcus and Pediococcus (P. pentosaceus) genera. Lactiplantibacillus plantarum is one of the most commonly used fermentation species LAB (Brückner-Gühmann et al., 2019, Kimoto-Nira et al., 2019). On the other hand, despite the fact that fermentation is a natural process contamination with spoilage or pathogenic microorganisms can occur if the process is not controlled. To initiate fermentation process and to control the process more efficiently, starter cultures can be used (Di Cagno et al., 2013, Torres et al., 2020). Starter cultures are usually autochthonous cultures isolated and grown from raw materials (Petrova & Petro, 2020). This makes them highly adaptable to specific vegetables and allows for controlled fermentation to obtain the desired product. In addition, most of these starter cultures are potential probiotic cultures (Lactiplantibacillus plantarum, Lacticaseibacillus casei, Lactobacillus acidophilus, and Streptococcus lactis), which are an added benefit in food production (Torres, Fabersani, Marquez, & Gauffin-Cano, 2019) and for improvement of human well-being in general (cholesterol lowering, antioxidant activity, anticancer activity, etc.) (Viridiana, Lidia, Audry, & Humberto, 2018). Selectin the right LAB culture is one of the crucial process steps. The selected microorganisms have to compete against indigenous cultures and also overcome the growth of spoilage and pathogenic microorganisms. For this reason, it is not uncommon to also use allochthones cultures isolated from one source but applied to another product. Inoculation can be done with a pure culture or by so-called back-slopping, i.e. transferring a small amount of a previously successfully fermented material to a new batch.
Fig. 1.
Schematic process of cabbage lactic-fermentation.
In addition to the selection of LAB, some other parameters such as temperature, pH, salt concentration, oxygen, etc. have a significant impact on the fermentation process. The optimum salt concentration depends on the type of vegetable being fermented (Swain et al., 2021) and is added to the process to promote the growth of LAB over other bacteria present. For example, for the production of sauerkraut, a salt content (NaCl) between 2.25 % and 2.5 % is most favourable but this percentage is higher, about 6 %, when whole cabbage heads are fermented (Berger et al., 2020). As for temperature, a temperature around 18 °C is considered the best for cabbage fermentation. In the work of Mel, Karim, Jamal, Salleh, and Zakaria (2006), the authors concluded that pH has the greatest influence on lactic acid production. The pH is regulated by the addition of acids or buffers (acid + acid salts) during the fermentation process to inhibit the growth of molds, yeasts and other bacteria. In addition, some other ingredients such as sugars or spices (garlic, essential oils, mustard, red chili, etc.) may be added during the fermentation process to promote the growth of LAB or inhibit the growth of spoilage bacteria (Swain et al., 2014).
Changes in phytochemical content during cruciferous vegetables fermentation
As mentioned earlier, all cruciferous vegetables have a similar phytochemical profile. Their health benefits are associated with phytochemicals from the groups of glucosinolates, polyphenols, and carotenoids whose presence and relative abundance may be influenced by the species, cultivar and environmental conditions during cultivation (Šamec et al., 2018, Šamec et al., 2019). Preparation methods prior to consumption and storage also significantly affect phytochemical content. In this section we focused on fermentation as an important process which influence the presence and abundance of phytochemicals in fermented cruciferous vegetables.
Glucosinolates
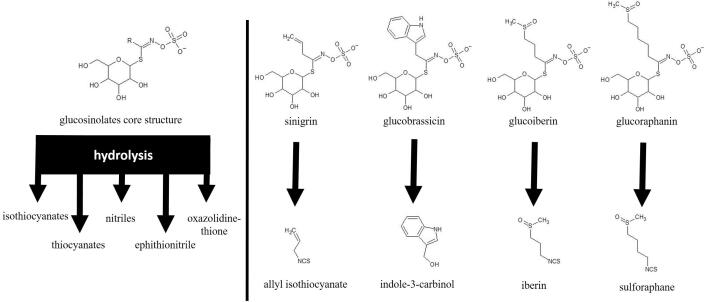
Glucosinolates are a group of specialized metabolites widely distributed in plants of the Brassicaceae family (Maina, Misinzo, Bakari, & Kim, 2020). They are characterized by a core structure consisting of a sulfated isothiocyanate group attached to thioglucose (Fig. 2). Today, about 200 different aliphatic, aromatic and indolic glucosinolates are known, but their presence and distribution in different cruciferous species/varieties is most likely genetically determined. Different cruciferous species have a characteristic glucosinolate profile that in most cases includes, more than ten different glucosinolates in each species/variety, although only 3 to 4 of them are predominant (Fahey, Stephenson, Wade, & Talalay, 2013). Glucosinolates have no biological activity; what is biologically active are their hydrolysis products formed during enzymatic hydrolysis by the endogenous plant enzyme myrosinase. The degradation products of glucosinolates that have bioactive properties include isothiocyanates, nitriles, thiocyanates, epithionitriles, and oxazolidinethiones (Sikorska-Zimny & Beneduce, 2021) (Fig. 2). In addition, these hydrolysis products can undergo further modifications.
Fig. 2.
Glucosinolates core structure, representative of glucosinolates and their hydrolysis products.
During fermentation of cruciferous vegetables, most glucosinolates are hydrolysed and undergo various additional transformations. In fermented products glucosinolates are absent or present in very small amounts in native form (Sarvan et al., 2013, Ciska et al., 2021). The degree of hydrolysis of glucosinolates during fermentation depends on their chemical and microbiological stability. For example, the fastes hydrolysis was observed for glucoiberverin, while 4- methoxyglucobrassicin is considered the most stable glucosinolates (Ciska et al., 2021). Fermented cruciferous vegetables are rich in degradation products of glucosinolates, and some representatives are shown in Fig. 2. Ciska et al. (2021) identified 12 breakdown products of aliphatic and aryl glucosinolates in fermented cabbage. According to them, sinigrin was the most abundant glucosinolate in shredded cabbage and it was hydrolysed to allyl isothiocyanate during fermentation, and part of this compound underwent cyclisation to epithiocyanate, 1-cyano-2,3-epithiopropane. They also reported that aliphatic glucosinolates, such as glucoiberine, glucoiberverin, 4-methylthiobutyl-GLS (glucoerucin) and glucoraphanin were hydrolysed to both isothiocyanates and cyanides. 3- Butenyl-GLS (gluconapin) and gluconasturtiin were hydrolysed only to isothiocyanates. 2-Hydroxy-3-butenyl-GLS (progoitrin) degraded with the formation of 5-vinyloxazolidine-2-thione, the cyclisation product of the unstable isothiocyanate.
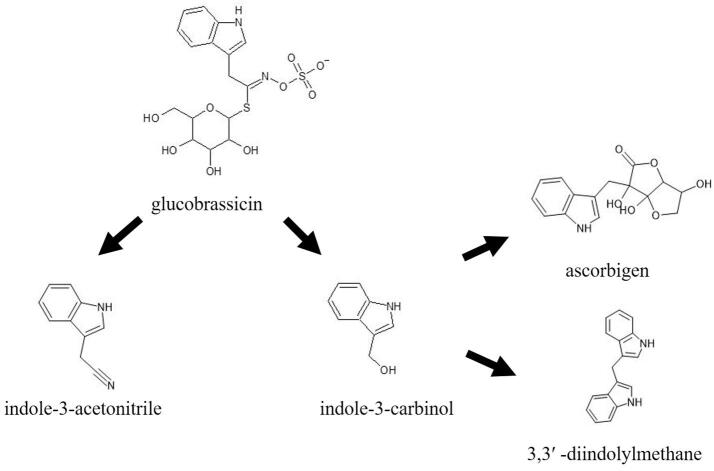
The vast majority of available literature dealing with glucosinolates in fermented cruciferous vegetables focuses on the degradation products of glucobrassicin (Fig. 3), probably due to the abundant evidence attributing beneficial properties to this molecule (Annunziata, Arnone, & Tenore, 2021). The enzymatic degradation of glucobrassicin by myrosinase leads to the formation of indole-3-carbinol and indole-3-acetonitrile (Chevolleau, Gasc, Rollin, Tulliez, 1997). In addition, these molecules can undergo further modifications. In the presence of ascorbic acid indole-3-carbinol can be converted to ascorbigen, depending on pH and temperature (Wagner & Rimbach, 2009). At low pH indole −3- carbinol is unstable and may also undergo dimerization and form 3,3′ -diindolylmethane (Annunziata et al., 2021). Both, ascorbigen and 3,3′ -diindolylmethane are considered molecules with high potential health benefits. Ascorbigen is considered one of the most potent bioactive compounds in cruciferous vegetables (Wagner & Rimbach, 2009) while 3,3′ -diindolylmethane has been reported to have cancer preventive activity. It is involved in all aspects of cancer cell–cycle regulation and survival including Akt-NFjB signaling, caspase activation, cyclin-dependent kinase activities, estrogen metabolism, estrogen receptor signaling, endoplasmic reticulum stress, and BRCA (BReast CAncer) gene expression (Weng, Tsai, Kulp, & Chen, 2008).
Fig. 3.
Glucobrassicin and their derivatives present in fermented cruciferous vegetables.
During fermentation, the content of glucobrassicin and its derivatives changes significantly. For example, according to Palani et al. (2016) raw shredded cabbage contains a small amount of ascorbigen and indole-3-carbinol, but during fermentation, the content of glucobrassicin decreases while the content of ascorbigen and indole-3-carbinol increases. They also reported that indole-3-acetonitrile content was highest in raw shredded cabbage and decreased during fermentation. Different trend was reported by Ciska et al. (2021) who found that ascorbigen was the major compound derived from glucobrassicin and that its content increased as glucobrassicin was degraded during fermentation. In their study other glucobrassicin derivatives, such as indole-3-carbinol, indole-3-acetonitrile and 3,3′ -diindolylmethane, were present in relatively small amounts but the content of 3,3′ -diindolylmethane increased during storage. According to Ciska and Pathak, 2004, Ciska et al., 2021, ascorbigen is the main degradation product of glucosinolates in fermented cabbage. Higher glucobrassicin content in fresh cabbage and lower amount of NaCl during the fermentation process are related to higher ascorbigen content in sauerkraut (Martinez-Villaluenga et al., 2009).
Although the hydrolysis products of glucosinolates are recognized as the major players contributing to the health benefits of cruciferous vegetables, further comprehensive metabolomic analyses are needed to further explain the chemical conversions of glucosinolates during fermentation.
Polyphenols
Polyphenols, a large, widely distributed group of specialized metabolites, have gain the attention of scientists for more than 30 years, mainly due to their health-related benefits (Aravind, Wichienchot, Tsao, Ramakrishnand, & Chakkaravarthia, 2021) but also because of their important role in plant-stress interactions (reviewed by Šamec, Karalija, Šola, Vujčić-Bok & Salopek-Sondi, 2021). The most widespread and diverse group of polyphenols in cruciferous vegetables are flavonoids (mainly flavonols), hydroxycinnamic acids, and in red varieties anthocyanins (reviewed by Cartea, Francisco, Soengas, & Velasco, 2011). In fresh cruciferous vegetables, polyphenols are rarely present in free form, but are often highly glycosylated and acrylated, forming complex molecules (Olsen, Aaby, & Borge, 2009).
During fermentation, several factors may change, leading to changes in polyphenol content, especially those in complex form. The reported data on changes in polyphenol content in various cruciferous vegetables during fermentation are summarized in Table 2. As can be seen from the Table 2, the results vary widely. Harbaum, Hubbermann, Zhu, and Schwarz (2008) studied the effects of fermentation on phenolic compounds in leaves of Pak Choi and Chinese leaf mustard. They found more flavonoid derivatives with lower molecular mass (di- and triglycosides) as well as flavonoids and hydroxycinnamic aglycones in fermented products than in fresh vegetables. During the fermentation process, the content of flavonoid derivatives and hydroxycinnamic acid derivatives decreases, but the total content of polyphenols increases due to the release of bound sugar moieties and the formation of free hydroxyl groups (Harbaum et al., 2008). Cai, Wang, McAuley, Augustin, and Terefe (2019) reported an increase in total polyphenol content of up to 83 % in fermented broccoli puree compared to raw broccoli. Fang, Hu, Liu, Chen, and Ye (2008) reported an increase in free phenolic acid content, but a decrease in total phenolic acids, total phenols, and antioxidant activities in fermented potherb mustard. Odongo et al. (2017) identified 37 different polyphenolic compounds and derivatives in fermented Ethiopian kale and found a 75 % decrease in phenolic compounds during fermentation. However, they noticed that the decrease depended on the type of compounds. Small hydroxycinnamic acid derivatives (e.g. caffeoylquinic acid) were more stable under fermentation conditions, while the more complex ones degraded more easily. Most flavonol glycosides were degraded by more than 50 %, but quercetin-3-O-sophoroside-7-O-d-glucoside, kaempferol-3-O-feruloylsophoroside-7-O-d-glucoside, and isorhamnetin-3-O-feruloyl-sophoroside-7-O-glucoside were found to be relatively stable (Odongo et al., 2017).
Table 2.
Studies related with changes of polyphenols during fermentation of cruciferous vegetables. Symbol ↑ represent increase, while ↓ represent decrease.
| Fermentated cruciferous | Used method | Identified polyphenols in fermentated product | Influence fermentation on polyphenols | References |
|---|---|---|---|---|
| potherb mustard | HPLC-DAD | gallic, protocatechuic, p-hydroxybenzoic, vanillic, caffeic, p-coumaric, ferulic and sinapic acid | ↑ free phenolic acids | Fang et al., 2008 |
| TPC | ↓ total phenolic acids, total phenolics, and antioxidant activities decreased. | |||
| Pak Choi Chinese leaf mustard |
HPLC-MS/MS | 40 different polyphenolic compounds | ↑ flavonoid derivatives with a lower molecular mass and flavonoids and hydroxycinnamic acids aglycones | Harbaum et al. (2008) |
| broccoli puree | TPC spectrophotometrically | – | ↑ TPC of ∼83 % compared to the raw broccoli | Cai et al., 2019 |
| curly kale leaves | LC-ESI-Q-TOF-MS | gentisic and salycilic acid | ↑ gentisic acid was detected only in fermentated leaves. | Michalak et al., 2020 |
| ↓ salycilic acid during fermentation. | ||||
| red cabbage shredded | HPLC-DAD- QTRAP | twenty different nonacylated and acylated anthocyanins with main structure of cyanidin-3-diglucoside-5-glucoside |
↓ Anthocyanins content by 24 % during fermentation | Wiczkowski, Szawara-Nowak, & Topolska, 2015 |
| Ethiopian kale | HPLC-DAD-ESI-MSn | 37 different polyphenolic compounds | ↓ phenolic compounds by 75 %. Small hydroxycinnamic acid derivatives (e.g. caffeoylquinic acid) were more stable under fermentation conditions, the more complex ones were degraded more easily. | Odongo et al., 2017 |
Carotenoids
Carotenoids are a group of about 850 known tetraterpene pigments distributed in photosynthetic organisms. They play an important role in human health by acting as precursors of vitamin A, photoprotectors, antioxidants, enhancers of immunity, and contributors to reproduction (Maoka, 2019). Cruciferous vegetables are considered a good source of carotenoids, with lutein and β-carotene being the predominant carotenoids (Lee, Zhang, Liang & Ong, 2020).
According to Szutowska et al. (2021), kale is one of the best sources of carotenoids among vegetables, with lutein and β-carotene being the most abundant. In fresh curly kale juice, lutein and β-carotene accounted for 40 % and 34.8 % of total carotenoids, respectively, while fermentation resulted in a 17–31 % decrease in carotenoid content (Szutowska et al., 2021). Similar results were reported by Odongo et al. (2017), who directly compared carotenoid content in raw and fermented Ethiopian kale. In raw samples, they identified lutein, zeaxanthin, and β-carotene. After fermentation, total carotenoid content decreased by 75 %, with the greatest loss in lutein at 98 %, while zeaxanthin concentration increased during fermentation. During lactic-acid fermentation, carotenoid content decreases because volatile carotenoid cleavage derivatives can form, contributing to the flavour and aroma of fermented products (reviewed by Mapelli-Brahm et al., 2020). However, a larger metabolomics study focusing on profiling carotenoids and their derivatives before and after lactic acid fermentation of cruciferous vegetables and explaining carotenoid chemistry and potential biodegradation is not currently available.
Conclusions and further directions
Fermented cruciferous vegetables are considered functional foods due to the presence of probiotics, prebiotics, and phytochemicals with beneficial effects on human health. In this review, we have summarised data on the presence of phytochemicals from the glucosinolates, polyphenols, and carotenoids groups in fermented cruciferous vegetables, and how fermentation affects their chemical structure.
Glucosinolates are completely hydrolyzed during lactic acid fermentation and only the hydrolysis products of glucosinolates are present in fermented products. The best studied to date are glucobrassicin and its hydrolysis products indole-3-carbinol, indole-3-acetoniril, ascorbigen, and 3,3′-diindolylmethane. Ascorbigen is reported to be the major product of glucosinolates in fermented cabbage. During fermentation, the content of complex polyphenol molecules decreases, but the total polyphenol content may increase due to the release of bound sugar units and the formation of free hydroxyl groups. Carotenoid content also decreases after fermentation, possibly due to the formation of carotenoid cleavage derivatives that may contribute to the flavour and aroma of fermented products.
Although the popularity of fermented cruciferous vegetables has increased in recent years, comprehensive metabolomic studies covering various groups of phytochemicals in fermented cruciferous vegetables are lacking. Metabolomic studies are becoming increasingly available and it is expected that these types of studies will be conducted in the future and contribute to a deeper understanding of the chemical changes of phytochemicals during fermentation of cruciferous vegetables.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
D.Š. is supported by the Grant for Scientific Research and Artistic Work from the University North under the grant no. UNIN-BIOTEH-21-1-1.
References
- Annunziata G., Arnone A., Tenore G.C. In: Nutraceuticals and Cancer Signaling. Jafari S.M., Nabavi S.M., Sanches Silva A., editors. Springer; Cham: 2021. Cruciferous Vegetables (Indole-3-Carbinol, Isothiocyanates) Against Cancer; pp. 129–144. [Google Scholar]
- Aravind S.M., Wichienchot S., Tsao R., Ramakrishnand S., Chakkaravarthia S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Research International. 2021;142 doi: 10.1016/j.foodres.2021.110189. [DOI] [PubMed] [Google Scholar]
- Baky M.H., Shamma S.N., Xiao J., Farag M.A. Comparative aroma and nutrients profiling in six edible versus nonedible cruciferous vegetables using MS based metabolomics. Food Chemistry. 2022;383 doi: 10.1016/j.foodchem.2022.132374. [DOI] [PubMed] [Google Scholar]
- Berger M.D., Vakula A., Horecki A.T., Rakić D., Pavlić B., Malbaša R.…Šumić Z. Cabbage (Brassica oleracea L. var. capitata) fermentation: Variation of bioactive compounds, sum of ranking differences and cluster analysis. LWT. 2020;133 doi: 10.1016/j.lwt.2020.110083. [DOI] [Google Scholar]
- Bousquet J., Anto J.M., Czarlewski W., Haahtela T., Fonseca S.C., Iaccarino G.…ARIA group Cabbage and fermented vegetables: From death rate heterogeneity in countries to candidates for mitigation strategies of severe COVID-19. Allergy. 2021;76(3):735–750. doi: 10.1111/all.14549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brückner-Gühmann M., Banovic M., Drusch S. Towards an increased plant protein intake: Rheological properties, sensory perception and consumer acceptability of lactic acid fermented, oat-based gels. Food Hydrocolloids. 2019;96:201–208. doi: 10.1016/j.foodhyd.2019.05.016. [DOI] [Google Scholar]
- Cai Y.X., Wang J.H., McAuley C., Augustin M.A., Terefe N.S. Fermentation for enhancing the bioconversion of glucoraphanin into sulforaphane and improve the functional attributes of broccoli puree. Journal of Functional Foods. 2019;61 doi: 10.1016/j.jff.2019.103461. [DOI] [Google Scholar]
- Cartea M.E., Francisco M., Soengas P., Velasco P. Phenolic compounds in Brassica vegetables. Molecules. 2011;16:251–280. doi: 10.3390/molecules16010251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevolleau S., Gasc N., Rollin P., Tulliez J. Enzymatic, Chemical, and Thermal Breakdown of 3H-Labeled Glucobrassicin, the Parent Indole Glucosinolate. Journal of Agriculture and Food Chemistry. 1997;45:4290–4296. [Google Scholar]
- Ciska E., Pathak D.R. Glucosinolate Derivatives in Stored Fermented Cabbage. Journal of Agriculture and Food Chemistry. 2004;52:7938–7943. doi: 10.1021/jf048986+. [DOI] [PubMed] [Google Scholar]
- Ciska E., Honke J., Drabińska N. Changes in glucosinolates and their breakdown products during the fermentation of cabbage and prolonged storage of sauerkraut: Focus on sauerkraut juice. Food Chemistry. 2021;365 doi: 10.1016/j.foodchem.2021.13049. [DOI] [PubMed] [Google Scholar]
- Cortés-Rodríguez V., Dorantes-Alvarez L., Peredo-Lovillo A., Hernández-Sánchez H. Reference Module in Food Science. 2018. Lactic Acid Bacteria Isolated From Vegetable Fermentations: Probiotic Characteristics. [DOI] [Google Scholar]
- Di Cagno R., Coda R., De Angelis M., Gobbetti M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiology. 2013;33(1):1–10. doi: 10.1016/j.fm.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Fahey J.W., Stephenson K.K., Wade K.L., Talalay P. Urease from Helicobacter pylori is inactivated by sulforaphane and other isothiocyanates. Biochemical and Biophysical Research Communications. 2013;435:1–7. doi: 10.1016/j.bbrc.2013.03.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z., Hu Y., Liu D., Chen J., Ye X. Changes of phenolic acids and antioxidant activities during potherb mustard (Brassica juncea, Coss.) pickling. Food Chemistry. 2008;108(3):811–817. doi: 10.1016/j.foodchem.2007.11.033. [DOI] [PubMed] [Google Scholar]
- Harbaum B., Hubbermann E.M., Zhu Z., Schwarz K. Impact of Fermentation on Phenolic Compounds in Leaves of Pak Choi (Brassica campestris L. ssp.chinensisvar.communis) and Chinese Leaf Mustard (Brassica juncea Coss) Journal of Agricultural and Food Chemistry. 2008;56(1):148–157. doi: 10.1021/jf072428o. [DOI] [PubMed] [Google Scholar]
- Kim J., Choi K.-B., Park J.H., Kim K.H. Metabolite profile changes and increased antioxidative and antiinflammatory activities of mixed vegetables after fermentation by Lactobacillus plantarum. PLoS One. 2019;14(5) doi: 10.1371/journal.pone.0217180. Article e0217180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimoto-Nira H., Moriya N., Nogata Y., Sekiyama Y., Toguchi Y. Fermentation of Shiikuwasha (Citrus depressa Hayata) pomace by lactic acid bacteria to generate new functional materials. International Journal of Food Science & Technology. 2019;54:688–695. doi: 10.1111/ijfs.13980. [DOI] [Google Scholar]
- Lee Hui Wen, Zhang Hui, Liang Xu, Ong Choon Nam. Simultaneous determination of carotenoids, tocopherols and phylloquinone in 12 Brassicaceae vegetables. LWT. 2020;130 doi: 10.1016/j.lwt.2020.109649. In this issue. [DOI] [Google Scholar]
- Li Q., Gänzle M.G. Host-adapted lactobacilli in food fermentations: Impact of metabolic traits of host adapted lactobacilli on food quality and human health. Current Opinion in Food Science. 2020;31:71–80. doi: 10.1016/j.cofs.2020.02.002. [DOI] [Google Scholar]
- Maina S., Misinzo G., Bakari G., Kim H.-Y. Human, Animal and Plant Health Benefits of Glucosinolates and Strategies for Enhanced Bioactivity: A Systematic Review. Molecules. 2020;25 doi: 10.3390/molecules25163682. article 3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maoka T. Carotenoids as natural functional pigments. Journal of Natural Medicine. 2020;74:1–16. doi: 10.1007/s11418-019-01364-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapelli-Brahm P., Barba F.J., Remize F., Garcia C., Fessard A., Khaneghah A.M.…Meléndez-Martínez A.J. The impact of fermentation processes on the production, retention and bioavailability of carotenoids: An overview. Trends in Food Science & Technology. 2020;99:389–401. doi: 10.1016/j.tifs.2020.03.013. [DOI] [Google Scholar]
- Martinez-Villaluenga C., Peñas E., Frias J., Ciska E., Honke J., Piskula M.K.…Vidal-Valverde C. Influence of Fermentation Conditions on Glucosinolates, Ascorbigen, and Ascorbic Acid Content in White Cabbage (Brassica oleracea var. capitata cv. Taler) Cultivated in Different Seasons. Journal of Food Science. 2009;74(1):C62–C67. doi: 10.1111/j.1750-3841.2008.01017.x. [DOI] [PubMed] [Google Scholar]
- Mel M., Karim M.I.A., Jamal P., Salleh M.R.M., Zakaria R.A. The Influence of Process Parameters on Lactic Acid Fermentation in Laboratory Scale Fermenter. Journal of Applied Sciences. 2006;6:2287–2291. doi: 10.3923/jas.2006.2287.2291. [DOI] [Google Scholar]
- Michalak M., Szwajgier D., Paduch R., Kukula-Koch W., Waśko A., Polak-Berecka M. Fermented curly kale as a new source of gentisic and salicylic acids with antitumor potential. Journal of Functional Foods. 2020;67 doi: 10.1016/j.jff.2020.103866. [DOI] [Google Scholar]
- Nkhata, S. G., Ayua, E., Kamau, E. H., & Shingiro, J.-B. (2018). Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Science & Nutrition, 6(8), 2446–2458. https://doi.org/10.1002/fsn3.846. [DOI] [PMC free article] [PubMed]
- Odongo G.A., Schlotz N., Herz C., Hanschen F.S., Baldermann S., Neugart S.…Lamy E. The role of plant processing for the cancer preventive potential of Ethiopian kale (Brassica carinata) Food & Nutrition Research. 2017;61(1):1271527. doi: 10.1080/16546628.2017.1271527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen H., Aaby K., Borge G.I. Characterization and quantification of flavonoids and hydroxycinnamic acids in curly kale (Brassica oleracea L. Convar. acephala Var. sabellica) by HPLC-DAD-ESI-MSn. Journal of Agriculture and Food Chemisry. 2009;57(7):2816–2825. doi: 10.1021/jf803693t. [DOI] [PubMed] [Google Scholar]
- Palani K., Harbaum-Piayda B., Meske D., Keppler J.K., Bockelmann W., Heller K.J., Schwarz K. Influence of fermentation on glucosinolates and glucobrassicin degradation products in sauerkraut. Food Chemistry. 2016;190:755–762. doi: 10.1016/j.foodchem.2015.06.012. [DOI] [PubMed] [Google Scholar]
- Pathak D.R., Stein A.D., He J.-P., Noel M.M., Hembroff L., Nelson D.A.…Willett W.C. Cabbage and Sauerkraut Consumption in Adolescence and Adulthood and Breast Cancer Risk among US-Resident Polish Migrant Women. International Journal of Environmental Research and Public Health. 2021;18 doi: 10.3390/ijerph182010795. Article 10795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova P., Petro K. Lactic acid fermentation of cereals and pseudocereals: Ancient nutritional biotechnologies with modern applications. Nutrients. 2020;12 doi: 10.3390/nu12041118. article 1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirante-Moya S., García-Ibanez P., Quirante-Moya F., Villano D., Moreno D.A. The Role of Brassica Bioactives on Human Health: Are We Studying It the Right Way? Molecules. 2020;25(7):1591. doi: 10.3390/molecules25071591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjad N., Rasool A., Bakr A., Fazili A.B.A., Bhat E. Fermentation of fruits and vegetables: A review. Plant Archives. 2020;20:1338–1342. [Google Scholar]
- Sarvan I., Valerio F., Lonigro S.L., de Candia S., Verkerk R., Dekker M., Lavermicocca P. Glucosinolate content of blanched cabbage (Brassica oleracea var. capitata) fermented by the probiotic strain Lactobacillus paracasei LMG-P22043. Food Research International. 2013;54(1):706–710. doi: 10.1016/j.foodres.2013.07.065. [DOI] [Google Scholar]
- Septembre-Malaterre A., Remize F., Poucheret P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Research International. 2018;104:86–99. doi: 10.1016/j.foodres.2017.09.031. [DOI] [PubMed] [Google Scholar]
- Sikorska-Zimny K., Beneduce L. The glucosinolates and their bioactive derivatives in Brassica: A review on classification, biosynthesis and content in plant tissues, fate during and after processing, effect on the human organism and interaction with the gut microbiota. Critical Reviews in Food Science and Nutrition. 2021;61(15):2544–2571. doi: 10.1080/10408398.2020.1780193. [DOI] [PubMed] [Google Scholar]
- Sharma R., Garg P., Kumar P., Bhatia S.K., Kulshrestha S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation. 2020;6:106. doi: 10.3390/fermentation6040106. [DOI] [Google Scholar]
- Swain M.R., Anandharaj M., Ray R.C., Parveen Rani R. Fermented Fruits and Vegetables of Asia: A Potential Source of Probiotics. Biotechnology Research International. 2014;1–19 doi: 10.1155/2014/250424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szutowska J., Gwiazdowska D., Rybicka I., Pawlak-Lemańska K., Biegańska-Marecik R., Gliszczyńska-Świgło A. Controlled fermentation of curly kale juice with the use of autochthonous starter cultures. Food Research International. 2021;149 doi: 10.1016/j.foodres.2021.110674. [DOI] [PubMed] [Google Scholar]
- Šamec D., Salopek-Sondi B. In: Nonvitamin and Nonmineral Nutritional Supplements. Nabavi S.M., Sanches Silva T., editors. Academic Press; Cambridge, MA, USA: 2019. Cruciferous (Brassicaceae) vegetables; pp. 195–202. [Google Scholar]
- Šamec D., Karalija E., Šola I., Vujčić Bok V., Salopek-Sondi B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants. 2021;10:118. doi: 10.3390/plants10010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šamec D., Pavlović I., Radojčić Redovniković I., Salopek-Sondi B. Comparative analysis of phytochemicals and activity of endogenous enzymes associated with their stability, bioavailability and food quality in five Brassicaceae sprouts. Food Chemistry. 2018;269:96–102. doi: 10.1016/j.foodchem.2018.06.133. [DOI] [PubMed] [Google Scholar]
- Šamec D., Urlić B., Salopek-Sondi B. Kale (Brassica oleracea var. acephala) as a superfood: Review of the scientific evidence behind the statement. Critical Reviews in Food Science and Nutrition. 2019;59(15):2411–2422. doi: 10.1080/10408398.2018.1454400. [DOI] [PubMed] [Google Scholar]
- Torres S.S., Veron H., Contreras L., Isla I.I. An overview of plant autochthonous microorganisms and fermented vegetable foods. Food Science and Human Wellness. 2020;9(2):112–123. [Google Scholar]
- Torres S., Fabersani E., Marquez A., Gauffin-Cano P. Adipose tissue inflammation and metabolic syndrome. The proactive role of probiotics. European Journal of Nutrition. 2019;58:27–43. doi: 10.1007/s00394-018-1790-2. [DOI] [PubMed] [Google Scholar]
- Wagner A.E., Rimbach G. Ascorbigen: Chemistry, occurrence, and biologic properties. Clinics in Dermatology. 2009;27:217–224. doi: 10.1016/j.clindermatol.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Wiczkowski W., Szawara-Nowak D., Topolska J. Changes in the content and composition of anthocyanins in red cabbage and its antioxidant capacity during fermentation, storage and stewing. Food Chemistry. 2015;167:115–123. doi: 10.1016/j.foodchem.2014.06.08. [DOI] [PubMed] [Google Scholar]
- Weng J.-R., Tsai C.-H., Kulp S.K., Chen C.-S. Indole-3-carbinol as a chemopreventive and anti-cancer agent. Cancer Letters. 2008;262:153–163. doi: 10.1016/j.canlet.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuyts S., Van Beeck W., Allonsius C.N., van den Broek M.F.L., Lebeer S. Applications of plant-based fermented foods and their microbes. Current Opinion in Biotechnology. 2020;61:45–52. doi: 10.1016/j.copbio.2019.09.023. [DOI] [PubMed] [Google Scholar]