Key Findings.
-
▪
A 63-year-old man without obstructive sleep apnea who reported dizziness with running was found to have frequent nocturnal episodes of asystole, predominantly during rapid eye movement (REM) sleep, lasting up to 12.1 seconds. This presentation is consistent with a rarely described syndrome called “REM-related asystole.”
-
▪
Members of the Heart Rhythm Society were surveyed as to how they would manage this clinical scenario, and many reported having encountered similar cases with various treatment approaches.
-
▪
We review 22 prior cases of REM-related asystole described in the literature. Most of these patients had no significant cardiovascular past medical history and had normal daytime cardiovascular testing. However, nearly all were implanted with pacemakers.
-
▪
Little is known about the mechanism of REM-related asystole, its prevalence, or its long-term outcomes. Although the most recent ACC/AHA/HRS guidelines recommend against pacing in patients for the indication of sleep-related sinus pauses, more research is needed to elucidate the prognosis of this condition and inform future guidelines.
Introduction
In the general population, sleep is associated with bradyarrhythmias owing to increased vagal tone.1, 2, 3, 4 Up to 5.7% of asymptomatic, healthy patients have been reported to have nocturnal sinus pauses >2 seconds on cardiac monitoring, with the most prolonged pause of 2.8 seconds in these studies.1,2,5,6 Such asymptomatic pauses are typically considered physiologic, not requiring intervention. In contrast, between 10% and 15.6% of patients with known obstructive sleep apnea (OSA) have been reported to have sinus pauses >2 seconds during sleep studies, with the most extended pause of 13 seconds.7, 8, 9 For this reason, the 2018 ACC/AHA/HRS guidelines give a Class I recommendation to screen for symptoms of OSA in patients with nocturnal bradycardia, with subsequent confirmatory testing as directed by clinical suspicion.10 If OSA is confirmed, a Class I recommendation is given for treatment directed specifically at OSA, since continuous positive airway pressure has been shown to effectively suppress these bradyarrhythmias during sleep.9,11 These same guidelines, however, give a Class III recommendation against pacing in patients with sleep-related sinus pauses unless other indications for pacing are present. We present a case of a 63-year-old man without OSA who was found to have frequent episodes of asystole, predominantly during rapid eye movement (REM) sleep, lasting up to 12.1 seconds, and we review what is currently known about this condition.
Case report
A 63-year-old man with a past medical history only significant for treated Lyme disease and no significant family history presented to his primary care provider for several months of brief dizziness episodes that would occur while running, often after episodes of belching. His primary care provider recommended that he wear a Zio XT monitor for 14 days to evaluate for potential cardiac causes of dizziness.
The patient’s monitor report showed a predominant underlying sinus rhythm with an overall average heart rate of 50 beats per minute (bpm). However, 971 sinus pauses >3 seconds were observed, with the majority occurring between 10 PM and 6 AM. Of these, the 2 longest pauses were 12.1 and 8.8 seconds, both occurring between 1 and 2 AM. The monitor also detected 4 runs of supraventricular tachycardia (SVT), with a longest of 4 beats at 138 bpm. In addition, there were 11 patient-initiated triggers during symptomatic events, which were associated mainly with normal sinus rhythm, sinus tachycardia, and sinus slowing with junctional rhythm (76–79 bpm). There were no patient triggers associated with sinus pauses.
Given these findings, he was urged by a covering physician to present to his local emergency room for further evaluation and monitoring. His electrocardiogram (ECG), echocardiogram, and other laboratory work were largely unremarkable, although he was noted to have a 3.5-second sinus pause during sleep while inpatient. Given a lack of symptomatic bradycardia, he was discharged and told to follow up as an outpatient.
He ultimately established care with the cardiac electrophysiology clinic at Johns Hopkins Medicine. Owing to suspicion of OSA as a potential cause of asystole, he was referred for an in-lab polysomnography, which included assessment of sleep staging, oximetry, respiratory airflow and effort, movement, and a single-lead ECG. During this sleep study, the ECG demonstrated 40 episodes of asystole lasting 2–6 seconds, mostly occurring during REM sleep. Notably, there was no significant degree of sleep apnea. He was awoken from sleep during one episode to ensure safety, and he reported no symptoms. After continued events, he was advised to present to the emergency room, which he declined. For daytime cardiac evaluation, an exercise stress test was ordered to evaluate for chronotropic incompetence. He exercised for 11 minutes, and his heart rate appropriately increased from 49 to 139 bpm. However, he did have abrupt sinus slowing and a junctional beat during the initial portion of this test. A subsequently performed tilt table test was negative. He later underwent testing for genetic variants associated with arrhythmias and cardiomyopathy, which identified him only as a carrier for FKRP, a gene associated with autosomal recessive muscular dystrophy.
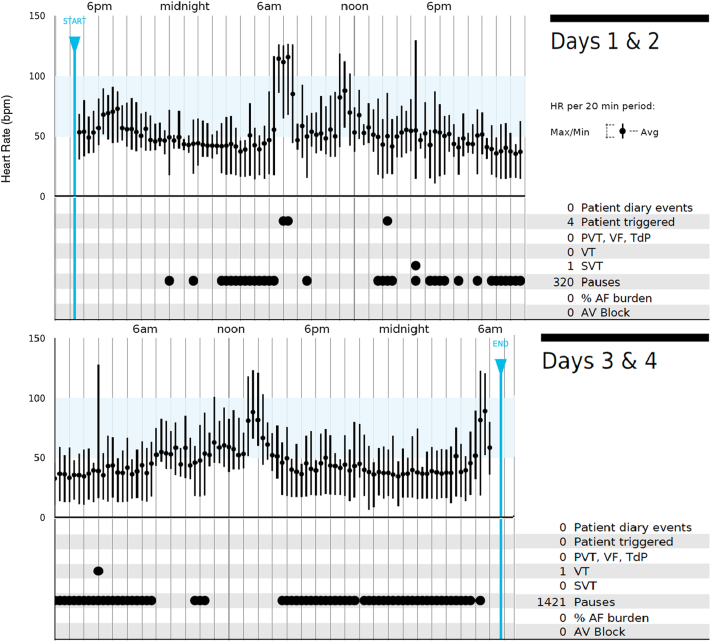
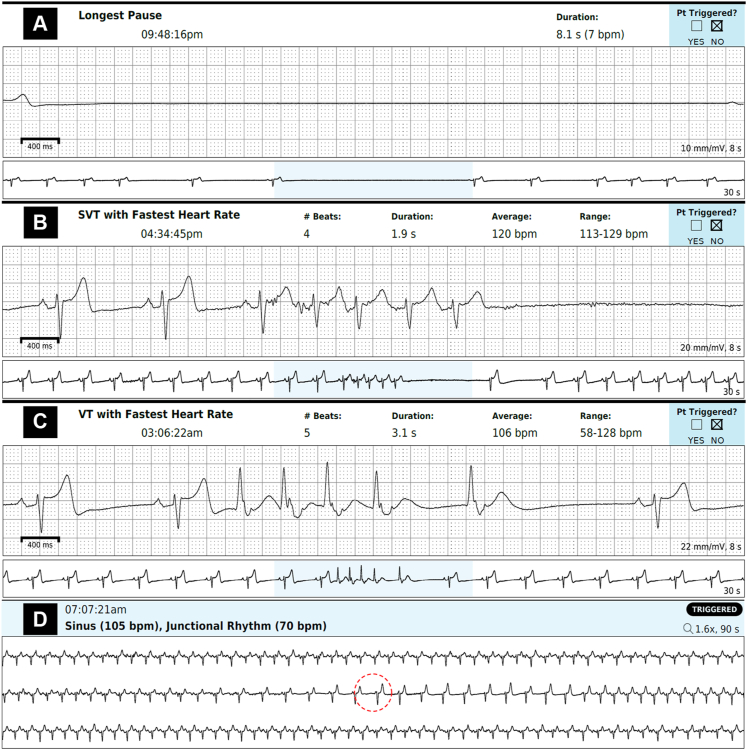
To be certain that this was not a self-terminating condition, he underwent a repeat 48-hour Zio XT monitoring weeks later, which again showed an underlying sinus rhythm with an average heart rate of 50 bpm. Additionally, 1741 sinus pauses occurred, predominantly at night, with longest pauses of 8.1 and 8.0 seconds occurring around 10 PM (Figure 1, Figure 2A). He also had a 4-beat run of SVT (maximum rate 129 bpm, Figure 2B) and a 5-beat run of nonsustained ventricular tachycardia (maximum rate 128 bpm, Figure 2C). Again, patient triggers were associated with normal sinus rhythm, sinus tachycardia, and sinus slowing with junctional rhythm (70 bpm, Figure 2D); none of the 4 patient triggers were associated with sinus pauses.
Figure 1.
Event log adapted from patient’s Zio XT report.
Figure 2.
A–D: Key tracings adapted from patient’s Zio XT report. The red dashed circle in panel D indicates the timing of a patient trigger during a symptomatic event. SVT = supraventricular tachycardia; VT = ventricular tachycardia.
We reviewed the literature (discussed in the following section) and found little data on the prognosis of REM-related sinus pauses. Given guidelines against pacemaker implantation for sleep-related sinus pauses, our group initially agreed that it would be best to refrain from permanent pacemaker (PPM) implantation. However, to survey broader expert opinion, we posted a brief description of our patient’s case in the Heart Rhythm Society (HRS) Member Open Forum. We asked whether other HRS members would implant a PPM in this scenario. Within a week of posting, we received responses from 27 different cardiac electrophysiologists from around the world. Of these responses, we categorized 2 responses as “yes,” 8 as “maybe,” and 17 as “no” (Table 1). The most frequently cited rationale behind “no,” consistent with our internal discussions, was that his pauses appeared to be asymptomatic. Interestingly, these respondents mentioned encounters with 15 patients with findings similar to ours. Of those 15 patients, 8 were followed without treatment, 5 received a PPM, and 2 underwent cardioneural ablation.
Table 1.
Heart Rhythm Society member open forum responses
| Resp. | Rec. PPM? | Comments |
|---|---|---|
| 1 | Maybe | Had a similar pt for 10 years without issue. Highlighted need for more data |
| 2 | No | Not without symptoms |
| 3 | Maybe | Highlighted need for topic to be mentioned in guidelines |
| 4 | No | Consider CNA |
| 5 | No | No PPM or CNA without symptoms |
| 6 | No | Had 2 similar pts who developed pauses and symptoms while awake, chose CNA with good results |
| 7 | No | Not without symptoms |
| 8 | No | No PPM regardless of pause length |
| 9 | Maybe | Had a similar pt with 12-second pauses and shaking, would frequently fall out of bed. PPM resolved symptoms. Highlighted need for more data/sleep EEGs. |
| 10 | No | Consider CNA if daytime symptoms |
| 11 | No | CNA if severe daytime bradycardia, or PPM if severe nocturnal pauses with pause-dependent wide QRS ventricular escape beats or NSVT |
| 12 | No | Had 3 similar pts (2 complete HB, 1 sinus pauses) whose arrhythmia burden improved with SSRI (chosen for pro-adrenergic and pro-serotoninergic actions) |
| 13 | Maybe | Had a similar pt in whom PPM resolved diurnal “brain fog.” Recommended broad definition of “symptomatic.” Wondered about thrombotic risk with long pauses |
| 14 | No | Commented that vagotonia from running is not responsible since REM vagal tone is less predominant. Suggested removing culprit medications |
| 15 | Maybe | Had 2 similar pts without issue. Highlighted need for more guidance in literature |
| 16 | No | Recommended de-training in athletic pts but recognized difficulty of adherence |
| 17 | No | Not without daytime symptoms, recommended extended monitoring to detect association with daytime symptoms |
| 18 | Yes | Had a similar pt whom he implanted after discussing with Dr Guilleminault (author of 2011 review)13 owing to theoretical risk of sudden cardiac death |
| 19 | No | Had a pt with pause of 13.8 seconds, no issue with 7 years of follow-up |
| 20 | No | Not without symptoms |
| 21 | No | Would not implant without symptoms but wondered about subclinical brain damage during prolonged asystole |
| 22 | No | Not without presyncope or syncope |
| 23 | Maybe | Had 2 similar pts found on cardiac monitor after cryptogenic stroke. Wondered if pauses caused stroke and if related cerebral hypoperfusion is damaging in elderly pts. Highlighted need for outcomes research |
| 24 | Maybe | Had a similar pt in whom an HCM4 mutation was discovered and subsequently underwent PPM implantation. Recommended genetic testing. |
| 25 | Yes | Had a similar pt who received PPM, AAI mode. Later developed more exertional fatigue on runs; AAIR mode resolved symptoms |
| 26 | Maybe | Highlighted difficulties with lack of prognostic data. Mentioned that a colleague implants PPM for pauses >8 seconds |
| 27 | No | Not without daytime symptoms |
CNA = cardioneural ablation; EEG = electroencephalogram; HB = heart block; NSVT = nonsustained ventricular tachycardia; PPM = permanent pacemaker; Pt(s) – patient(s); Rec. = recommended; REM = rapid eye movement sleep; Resp = respondent; SSRI = selective serotonin release inhibitor.
Shortly after this workup and discussion, however, our patient had a syncopal episode while running and sustained an ankle injury. Unfortunately, he did not have a monitor in place during this incident, so we do not know his heart rhythm at the time of syncope. However, with the development of new exercise-related syncope in the setting of profound sinus pauses (predominantly but not exclusively nocturnal), symptomatic episodes of sinus slowing with junctional rhythm, and runs of SVT and nonsustained ventricular tachycardia, we ultimately decided with the patient to pursue pacemaker implantation.
Discussion
Our patient’s history is consistent with a condition, first described in 1984, referred to as “REM-related bradyarrhythmia syndrome.”12,13 This term encompasses both REM-related asystole (as seen in our patient) and REM-related complete heart block. Although sinus bradycardia during non-REM sleep can be attributed to a physiologic increase in parasympathetic tone, a REM-specific bradyarrhythmia warrants a different explanation. Unlike non-REM sleep, REM sleep is typically associated with decreased vagal tone and concomitant surges in sympathetic activity during phasic events, resulting in increased heart rates.13 Thus, REM-related bradyarrhythmias may result from a dysfunction in autonomic modulation, with a nonphysiologic predominance of vagal tone compared to sympathetic activation during this phase of sleep. It is unclear whether this imbalance primarily results from abnormally heightened vagal tone during REM sleep or from a withdrawal of sympathetic input during phasic events. Additionally, it is unknown whether this abnormal modulation results from altered central influences on the autonomic nervous system or from an abnormal baroreceptor reflex.13
REM sleep–related asystole has rarely been reported in the literature, and this condition’s prevalence and natural course remain unknown. We found 22 similar cases of REM-related asystole in the literature, of which 18 were described in a 2011 review13 and 4 have been described in subsequent case reports.14, 15, 16, 17 The overall characteristics of these patients in addition to our patient are summarized in Table 2, and the individual case characteristics are presented in Table 3. The vast majority of these patients were male (87%), and the average age at diagnosis was 30.5 years. Our case describes the eldest patient (63 years old) known to be diagnosed with this condition. Of these 23 patients, only 1 had any significant cardiovascular past medical history (hypertension), and none had illicit drug use.
Table 2.
Summary of rapid eye movement sleep–related sinus arrest cases (N = 23 patients)
| Age, years | 30.5 (± 12.0) |
| Male | 20 (87.0%) |
| No prior cardiovascular history | 22 (95.7%) |
| Longest pause on cardiac monitor, seconds | 10.4 (± 2.6) |
| Longest pause during REM on PSG, seconds | 7.6 (± 3.1) |
| Pacemaker implantation | 19 (82.6%) |
For continuous variables, numbers are represented as % (± SD); for categorical variables, numbers are represented as n (%).
PSG = polysomnography; REM = rapid eye movement sleep.
Table 3.
Individual cases of rapid eye movement sleep–related sinus arrest
| Year, ref. | Age, sex | PMH | Symptoms | Normal diurnal cardiac eval. | Abnormal diurnal cardiac eval. | Cardiac monitor, longest pause (s) | PSG, longest pause (s) | Device? |
|---|---|---|---|---|---|---|---|---|
| 198412 | 30, F | None | Syncope after awakening, CP, palpitations | ECG, EST, TTE, Cath, EPS, ANS | None | 9 | 5 | PPM |
| 198412 | 27, M | None | CP, dizziness | ECG, EST, Cath, ANS | None | 8 | 5 | PPM |
| 198412 | 35, M | None | Syncope after awakening, CP | ECG, EST, Cath, ANS | None | 7 | 6 | PPM |
| 198412 | NR, M | None | CP | ECG, EST, EPS, ANS | None | 8 | 6 | None |
| 199919 | 27, M | None | Syncope w/ nocturnal BMI, CP, dizziness | EST, TTE, EPS, Tilt, ANS | None | 7.2 | 5 | None |
| 200421 | 23, M | None | None | ECG, EST, TTE, Tilt, EPS | None | 11.8 | 5.5 | PPM |
| 200722 | 54, M | BMI 37 | CP, presyncope w/exercise | EST, TTE, ANS | None | 7.0 | 6.7 | PPM |
| 200722 | 41, M | None | CP | TTE, Cath, Tilt | None | 8.2 | 11 | PPM |
| 201113 | 29, M | None | Presyncope w/ exercise | ECG, Cath, EST | None | 12 | 10 | PPM |
| 201113 | 23, M | None | Presyncope w/ early AM urination | ECG, Cath, EST | None | 15 | 7 | PPM |
| 201113 | 20, F | None | Syncope w/menstruation | ECG, Cath | None | 11 | 8 | PPM |
| 201113 | 22, M | None | Unknown | ECG, Cath, EPS | None | 13 | 7 | PPM |
| 201113 | 24, M | None | Presyncope w/exercise | ECG, Cath, EPS | None | 12 | 10 | PPM |
| 201113 | 21, M | None | Presyncope w/intercourse | ECG, Cath, EPS | None | 14 | 6 | PPM |
| 201113 | 19, M | None | Malaise w/exercise | ECG, Cath, EPS | None | 12 | 5 | PPM |
| 201113 | 18, F | None | Malaise w/exercise | ECG, Cath, EPS | None | 12 | 10 | PPM |
| 201113 | 23, M | None | Presyncope after awakening | ECG, Cath, EPS | None | 9 | 9 | PPM |
| 201113 | 24, M | None | Presyncope w/exercise | ECG, Cath, EPS | None | 12 | 8 | PPM |
| 201214 | 43, M | None | None | None | Brady. on ECG | 13.8 | 5.1 | None |
| 201515 | 36, M | None | Syncope after awakening | ECG, TTE | EPS with pauses up to 4.1 s | 7.8 | 8.6 | PPM |
| 201816 | 49, M | BMI 34, HTN, pAF | Dyspnea, snoring | EST | TTE w/LVH, diastolic dys. | 5.5 | 5.5 | PPM |
| 202217 | 21, M | None | Presyncope after awakening | ECG, TTE, EST, EPS | None | 10.9 | 19.5 | PPM |
| Current | 63, M | Lyme (treated) | Syncope, presyncope w/exercise | ECG, TTE, EST | None | 12.1 | 6 | PPM |
ANS = autonomic nervous system study (eg, Valsalva ratio, heart rate variability); BMI = body mass index; Cath = cardiac catheterization (right- vs left-sided not specified); Brady. = bradycardia; CP = chest pain; dys = dysfunction; ECG = electrocardiogram; EPS = electrophysiology study; EST = exercise stress test; Eval. = evaluation; LVH = left ventricular hypertrophy; F = female; M = male; NR = not reported; pAF = paroxysmal atrial fibrillation; PMH = past medical history; PSG = polysomnography; Ref. = reference; Tilt = tilt table test; TTE = transthoracic echocardiogram.
The patients underwent initial evaluation owing to a range of symptoms, including daytime chest pain, daytime dizziness, and occasional presyncopal or (in 4 cases) syncopal events near the time of awakening. Daytime cardiac evaluations were almost universally normal, including ECGs, transthoracic echocardiograms, diagnostic electrophysiology studies, cardiac catheterizations, and tilt table tests.16 In each case, the patient was diagnosed with REM-related asystole via a sleep study obtained in response to nocturnal pauses detected on prolonged cardiac monitoring. The longest pauses in these patients during sleep studies ranged from 5 to 19.5 seconds. REM-related asystole was not associated with sleep apnea in these patients.
To investigate a possible overlap between REM-related asystole and sinus node dysfunction (SND), genetic testing was suggested by a member of the HRS Online Forum who had identified a similar patient with SND harboring a pathogenic variant in the cardiac pacemaker channel HCN4. Although the 2018 ACC/AHA/HRS guidelines state that genetic testing is not routinely indicated for SND,10 the newer 2022 European guidelines note that genetic testing may be considered as part of the diagnostic evaluation for patients with familial or isolated but otherwise unexplained SND, or with SND and concomitant atrial fibrillation, cardiac conduction disease, structural heart disease, or extracardiac syndromal features, especially in the setting of a positive family history.18 In line with the notion that most sinus node disease is acquired and that there is low sensitivity for detecting a causal genetic variant, no pathogenic mutation was identified in our patient.
Given the relatively scant data on REM-related asystole, there has been no consensus on how to treat these patients. Multiple drugs have been trialed, with variable success. Atropine (used in 2 patients) and protriptyline (1 patient) decreased episodes of REM-related sinus arrest,12 but amitriptyline (1 patient) was ineffective.19 These drugs were discontinued in each case owing to adverse effects. Of the 22 patients previously described, 18 ultimately underwent PPM implantation. Long-term follow-up was reported in only 7 of these patients, but all remained asymptomatic after implantation.
Although PPM implantation was performed in 18 of these prior cases, 2018 ACC/AHA/HRS pacing guidelines recommend against pacing for the indication of sleep-related sinus pauses. These guidelines recommend a sleep apnea evaluation, but REM-related asystole as a condition is not mentioned. In contrast, the 2021 European Society of Cardiology (ESC) pacing guidelines recommend that these patients be evaluated for both sleep apnea and REM-related bradycardia.20 These guidelines state that most patients with REM-related asystole have received PPMs but acknowledge that there is little evidence for this practice. More generally, the 2021 ESC guidelines give a Class IIb recommendation for pacing in patients who have experienced syncope and asymptomatic sinus pauses >6 seconds, which would apply to our patient. Notably, however, only 5 of the patients in the REM-related asystole literature presented with syncope. Those described in prior cases and in the HRS online forum generally displayed nonspecific symptoms, and REM-related asystole was discovered incidentally. Interestingly, a common anecdote from the literature and HRS forum was that vague presenting symptoms often improved after PPM implantation. One athlete (not included in the literature review owing to a lack of sleep study) with incidentally discovered nocturnal pauses up to 15 seconds even reported increased energy and significantly improved running times after PPM implantation, despite being reportedly asymptomatic before implantation.4
As a result of underdiagnosis and underreporting, we do not have data on important prognostic questions regarding this condition, such as whether it may lead to increased stroke risk or unexplained cases of sudden cardiac death during sleep. As a result, it is clear from our literature review and HRS online forum survey that the management decisions regarding these patients have been quite variable. As wearable cardiac monitors become increasingly widespread, we will encounter this finding more frequently, and we will need to ensure that we are treating patients appropriately. Thus, more research is necessary to describe the prognosis of this condition, determine the best management, and inform evidence-based guidelines.
Acknowledgments
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Dr Calkins is supported by the Hugh Calkins, Marvin H. Weiner, and Jacqueline J Bernstein Cardiac Arrhythmia Center.
Disclosures
J.R.S., A.S.B., J.C.J., R.D.B., and C.E. report no disclosures. J.C. receives honoraria from Biosense Webster and Abbott. C.J.L. serves on the medical advisory board for Medtronic and Philips; has received research support from Boston Scientific; has received honoraria from Cook Medical, Abbott, and Philips; and has been compensated for travel for training by Abbott. H.C. is a consultant and/or has received honoraria from Medtronic, Boston Scientific, and Abbott.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
Verbal consent was obtained from the patient for use of his de-identified health information.
Ethics Statement
The research reported in this paper adhered to CARE case report guidelines.
References
- 1.Brodsky M., Wu D., Denes P., Kanakis C., Rosen K.M. Arrhythmias documented by 24 hour continuous electrocardiographic monitoring in 50 male medical students without apparent heart disease. Am J Cardiol. 1977;39:390–395. doi: 10.1016/s0002-9149(77)80094-5. [DOI] [PubMed] [Google Scholar]
- 2.Bjerregaard P. Mean 24 hour heart rate, minimal heart rate and pauses in healthy subjects 40-79 years of age. Eur Heart J. 1983;4:44–51. doi: 10.1093/oxfordjournals.eurheartj.a061370. [DOI] [PubMed] [Google Scholar]
- 3.Viitasalo M.T., Kala R., Eisalo A. Ambulatory electrocardiographic recording in endurance athletes. Br Heart J. 1982;47:213–220. doi: 10.1136/hrt.47.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Northcote R.J., Canning G.P., Ballantyne D. Electrocardiographic findings in male veteran endurance athletes. Br Heart J. 1989;61:155–160. doi: 10.1136/hrt.61.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sobotka P.A., Mayer J.H., Bauernfeind R.A., Kanakis C., Jr., Rosen K.M. Arrhythmias documented by 24-hour continuous ambulatory electrocardiographic monitoring in young women without apparent heart disease. Am Heart J. 1981;101:753–759. doi: 10.1016/0002-8703(81)90611-6. [DOI] [PubMed] [Google Scholar]
- 6.Clarke J.M., Hamer J., Shelton J.R., Taylor S., Venning G.R. The rhythm of the normal human heart. Lancet. 1976;1:508–512. doi: 10.1016/s0140-6736(76)90801-1. [DOI] [PubMed] [Google Scholar]
- 7.Guilleminault C., Connolly S.J., Winkle R.A. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am J Cardiol. 1983;52:490–494. doi: 10.1016/0002-9149(83)90013-9. [DOI] [PubMed] [Google Scholar]
- 8.Miller W.P. Cardiac arrhythmias and conduction disturbances in the sleep apnea syndrome. Prevalence and significance. Am J Med. 1982;73:317–321. doi: 10.1016/0002-9343(82)90716-1. [DOI] [PubMed] [Google Scholar]
- 9.Harbison J., O'Reilly P., McNicholas W.T. Cardiac rhythm disturbances in the obstructive sleep apnea syndrome: effects of nasal continuous positive airway pressure therapy. Chest. 2000;118:591–595. doi: 10.1378/chest.118.3.591. [DOI] [PubMed] [Google Scholar]
- 10.Kusumoto F.M., Schoenfeld M.H., Barrett C., et al. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;140:e382–e482. doi: 10.1161/CIR.0000000000000628. [DOI] [PubMed] [Google Scholar]
- 11.Becker H., Brandenburg U., Peter J.H., Von Wichert P. Reversal of sinus arrest and atrioventricular conduction block in patients with sleep apnea during nasal continuous positive airway pressure. Am J Respir Crit Care Med. 1995;151:215–218. doi: 10.1164/ajrccm.151.1.7812557. [DOI] [PubMed] [Google Scholar]
- 12.Guilleminault C., Pool P., Motta J., Gillis A.M. Sinus arrest during REM sleep in young adults. N Engl J Med. 1984;311:1006–1010. doi: 10.1056/NEJM198410183111602. [DOI] [PubMed] [Google Scholar]
- 13.Holty J.E., Guilleminault C. REM-related bradyarrhythmia syndrome. Sleep Med Rev. 2011;15:143–151. doi: 10.1016/j.smrv.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Serafini A., Dolso P., Gigli G.L., et al. Rem sleep brady-arrhythmias: an indication to pacemaker implantation? Sleep Med. 2012;13:759–762. doi: 10.1016/j.sleep.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Duba A.S., Jasty S., Mahajan A., et al. Rare case of rapidly worsening REM sleep induced bradycardia. Case Rep Cardiol. 2015;2015 doi: 10.1155/2015/546712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jakutis G., Juknevicius V., Barysiene J., et al. A rare case of REM sleep-related bradyarrhythmia syndrome with concomitant severe hypertension: a case report and a review of literature. Acta Med Litu. 2018;25:1–6. doi: 10.6001/actamedica.v25i1.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yasar S., Gokoglan Y., Gormel S., Asil S., Kabul H.K. A rare case of REM sleep-related bradyarrhythmia syndrome: 19.5-s asystole during REM sleep. J Interv Card Electrophysiol. 2022;63:227–228. doi: 10.1007/s10840-021-01075-y. [DOI] [PubMed] [Google Scholar]
- 18.Wilde A.A.M., Semsarian C., Marquez M.F., et al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) Expert Consensus Statement on the state of genetic testing for cardiac diseases. Europace. Apr 4 2022 doi: 10.1093/europace/euac030. [DOI] [PubMed] [Google Scholar]
- 19.Coccagna G., Capucci A., Pierpaoli S. A case of sinus arrest and vagal overactivity during REM sleep. Clin Auton Res. 1999;9:135–138. doi: 10.1007/BF02281626. [DOI] [PubMed] [Google Scholar]
- 20.Glikson M., Nielsen J.C., Kronborg M.B., et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2021;42:3427–3520. doi: 10.1093/eurheartj/ehab364. [DOI] [PubMed] [Google Scholar]
- 21.Sanna N., Della Marca G., Bianco M., et al. Prolonged asystolia in a young athlete: a case of sinus arrest during REM sleep. Int J Sports Med. 2004;25:457–460. doi: 10.1055/s-2004-820940. [DOI] [PubMed] [Google Scholar]
- 22.Janssens W., Willems R., Pevernagie D., Buyse B. REM sleep-related brady-arrhythmia syndrome. Sleep Breath. 2007;11:195–199. doi: 10.1007/s11325-007-0105-2. [DOI] [PubMed] [Google Scholar]