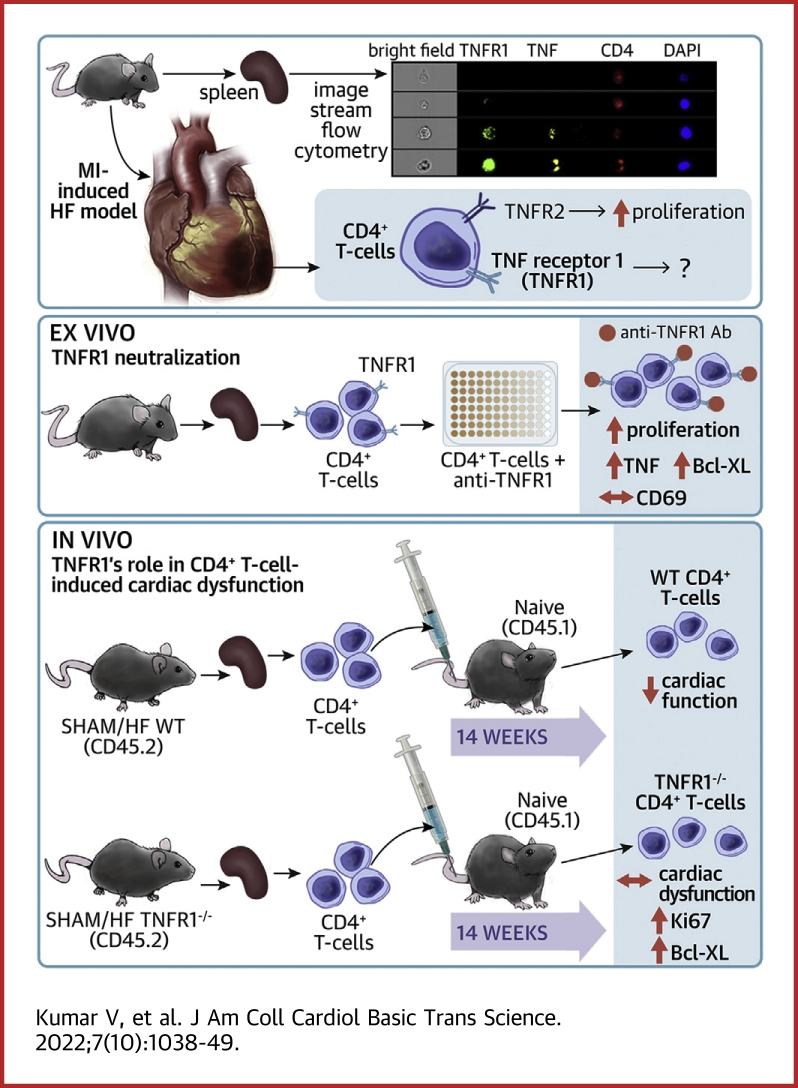
Visual Abstract
Key Words: heart failure, left ventricular remodeling, myocardial infarction, T lymphocytes, tumor necrosis factor receptors
Abbreviations and Acronyms: AT, adoptive transfer; HF, heart failure; IL, interleukin; LV, left ventricular; MFI, mean fluorescence intensity; MI, myocardial infarction; mLN, mediastinal lymph node; PBS, phosphate-buffered saline; Tcm, memory T cell; TCR, T-cell receptor; TNF, tumor necrosis factor; TNFR1, tumor necrosis factor receptor 1; WT, wild type
Highlights
-
•
CD4+ T lymphocytes exhibit a temporal phenotypic shift associated with increased TNF-α expression during ischemic heart failure. Importantly, TNF-α expression is highest in TNFR1+ versus TNFR1− T cells during HF.
-
•
Neutralization of TNFR1 promotes prosurvival signaling and proliferation of TCR-stimulated CD4+ T cells, suggesting an important role of the TNF-α–TNFR1 axis in regulating prosurvival and proliferative pathways in CD4+ T cells.
-
•
The loss of TNFR1 amplifies the life span and proliferation of adoptively transferred HF-activated CD4+ T cells without altering their trafficking or pathogenicity, indicating that TNFR1 does not regulate pathogenicity of CD4+ T cells during HF. Stimulation of TNF-α–TNFR1 signaling could be an attractive immunomodulatory strategy to promote activation-induced cell death of CD4+ T cells during HF.
Summary
CD4+ T cells turn pathological during heart failure (HF). We show that the expression of tumor necrosis factor (TNF)-α and tumor necrosis factor receptor (TNFR1) increases in HF-activated CD4+ T cells. However, the role of the TNF-α/TNFR1 axis in T-cell activation/proliferation is unknown. We show that TNFR1 neutralization during T-cell activation (ex vivo) or the loss of TNFR1 in adoptively transferred HF-activated CD4+ T cells (in vivo) augments their prosurvival and proliferative signaling. Importantly, TNFR1 neutralization does not affect CD69 expression or the pathological activity of HF-activated TNFR1−/− CD4+ T cells. These results show that during HF TNFR1 plays an important role in quelling prosurvival and proliferative signals in CD4+ T cells without altering their pathological activity.
Inadequate healing and sustained inflammation post–myocardial infarction (MI) mediate left ventricular (LV) remodeling and progressive cardiac dysfunction during heart failure (HF).1,2 In patients with ischemic or nonischemic HF, LV remodeling and progressive cardiac dysfunction are strongly associated with increased proinflammatory biomarkers such as tumor necrosis factor (TNF)-α,3 interleukin (IL)-1β,4 IL-6,3 and monocyte chemoattractant protein-1.5 However, immunomodulation strategies aimed at neutralizing cytokines such as TNF-α6 and IL-67 failed to show clinical benefit and, paradoxically, enhanced mortality and morbidity in HF patients.6 These findings underscore the intricacies of immune responses and a need to understand inflammatory mechanisms in a cell- and time-dependent manner.
TNF-α is a pleiotropic cytokine that exists in soluble and transmembrane forms. It exerts its effects via tumor necrosis factor receptor 1 (TNFR1) and 2 (TNFR2), which are expressed on several cell types, including cardiomyocytes and immune cells.8,9 Although TNFR1 is expressed on almost all tissues including the lymphoid system, TNFR2 expression is confined to certain tissues, such as the myocardium, and certain immune cell populations. TNFR1 can be activated by both soluble and membranous forms of TNF-α, but TNFR2 requires activation by membrane-bound TNF-α.8 A prior study by Hamid et al9 showed that although both the receptors mediate oxidative stress and diastolic dysfunction post-MI, global TNFR1−/− mice exhibit improved LV remodeling and contractile function, and global TNFR2−/− mice show exaggerated tissue remodeling during chronic HF.9 Similarly, Duerrschmid et al10 showed that in response to angiotensin II, TNFR1−/− mice develop less cardiac hypertrophy, remodeling, and hypertension compared with wild-type (WT) and TNFR2−/− mice. Considering that immune cells also express these receptors, it is unknown if these responses were, in part, also mediated by altered immune responses.
We11 and others12 have shown that ischemic and nonischemic HF is associated with T-cell activation, proliferation, and infiltration into rodent hearts. Moreover, cardiac T cells exhibit a restricted T-cell receptor (TCR) repertoire,13 and adoptive transfer (AT) of HF-activated CD4+ T cells can induce cardiac dysfunction and LV remodeling in naive mice,11 suggestive of autoimmune-like behavior. T cells express high levels of TNFR2, which is critical for TCR-mediated T-cell activation and proliferation.14 In contrast, TNFR1 expression on T cells is more restricted and is known to be expressed during pathogenic conditions such as rheumatoid arthritis.15 Nonetheless, it is unknown if TNFR1 is also expressed in HF-activated T cells and, more importantly, if TNFR1 is involved in regulating the activation or proliferation of T cells during HF.
In these studies, we show that T cells activated during ischemic HF (8 weeks post-MI) are phenotypically different than protective T cells activated at 3 days post-MI. Despite increased CD4+ TNFR1+ T cells at both the time points, we show that the dynamics of TNF-α expression in T cells are significantly different between the 2 time points. We further show that TNFR1 inhibition enhances prosurvival signaling and promotes the proliferation of CD4+ T cells without affecting their activation. These findings were confirmed in vivo by adoptively transferring HF-activated CD4+ T cells isolated from WT or TNFR1−/− mice to naive mice.
Ethics Statement
Animal studies were approved by the Institutional Animal Care and Use Committee at the Ohio State University (IACUC# 2018A00000078) and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Department of Health and Human Services Publication No. 85-23, revised 1996). Mice were kept in temperature- and humidity-controlled vivarium and had access to food and water ad libitum. A total of 165 mice were used.
Mouse model, surgical protocol, and sample collection
Male 10- to 12-week old C57BL/6 mice (WT) (000664, The Jackson Laboratory) or TNFR1−/− (002818, The Jackson Laboratory) mice underwent permanent left anterior descending coronary artery ligation (n = 98) to induce ischemic HF. Sham animals (n = 45) underwent a similar surgical protocol with thoracotomy and passing of the needle through the cardiac wall without ligating the artery to mimic immune activation originating from just the surgical intervention required to access the left anterior descending coronary artery. At 8 weeks, tissues were harvested to isolate cardiac or splenic CD4+ T cells as previously described.16
AT studies
Splenic CD4+ T cells from WT or TNFR1−/− mice were isolated using the MojoSort CD4+ T-cell isolation kit (Catalog #480033, BioLegend) and the RoboSep cell separation system (Stemcell Technologies) following the manufacturers’ protocols. Live cells were counted using trypan blue staining, and 8 to 9 × 106 live cells were injected via the tail vein into naive CD45.1-recipient mice. Peripheral blood (∼100 μL) was collected from the facial vein at different time intervals. At 14 weeks post-transfer, all the mice were sacrificed to harvest the heart, spleen, and lymph nodes (mediastinal and inguinal) to measure donor and recipient cells by flow cytometry.
Echocardiography
Cardiac function in recipient mice was measured at baseline (day 0) and at 14 weeks after AT using echocardiography. Parasternal B-mode imaging was performed under 1% to 1.5% isoflurane using VisualSonics Vevo 3100 equipped with the MX550D scan head and adjustable heated rail system.
Immune cell isolation, fixation, and flow cytometric staining
Immune cells from the blood, spleen, heart, and lymph nodes were isolated and fixed using our previously published protocols.16 The antibodies are listed in Supplemental Table 1.
T-cell proliferation assays
Flat-bottom 96-well plates were coated with 50 μL 4.5 μg/mL anti-Hamster IgG (Catalog #H1643, MilliporeSigma) for 1 hour, washed using sterile phosphate-buffered saline (PBS), and subsequently coated with 50 μL 2 μg/mL antimouse CD3 antibody (Catalog #102116, BioLegend) at room temperature. All wells were washed using sterile PBS, and 1 × 105 Tag-it Violet (Catalog #425101, BioLegend) labeled CD4+ T cells (in 100 μL RPMI-1640 (Gibco) media supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin) were added to each well followed by 100 μL complete RPMI-1640 media containing 4 μg/mL anti-mouse CD28 antibody (Catalog #102116, BioLegend). Varying concentrations of neutralizing anti-TNFR1 antibody (Catalog #16-1202-85, Invitrogen) were added to measure changes in cell proliferation and activation. At 72 hours postincubation (37 °C and 5% v/v CO2), cells were harvested and labeled with a live/dead dye (Catalog #77184, BioLegend) for 10 minutes. Excess dye was washed using cold PBS, and cells were stained with antimouse CD4-PECy7, CD69-PerCP, and TNF-α-PE using protocols described earlier.16 Data were captured either by the LSRFortessa (Becton Dickinson) or NL3000 Northern Lights (Cytek) flow cytometer and were analyzed using FlowJo v10.1 (BD Biosciences).
Statistical analysis
All data are presented as mean ± SD. The Shapiro-Wilk test was used to assess normality, and log transformation was used for data that did not show normal distribution. For the statistical analysis of 2 groups, either the paired or unpaired Student’s t-test was used for within- and between-group comparisons. For more than 2 groups, either 1- or 2-way analysis of variance with the Bonferroni, Tukey, or 2-stage linear step-up procedure of Benjamini, Krieger and Yekutieli [to control the false discovery rate (FDR)] was used for multiple pairwise comparisons. All statistical tests used were 2-sided, and P < 0.05 was considered significant with ∗P < 0.05, ∗∗P < 0.01, and ∗∗P < 0.001 representing the level of significance with respect to the indicated groups. Specific statistical methods are detailed in the individual figure legends. GraphPad Prism version 9.2.0 was used to plot and analyze all data.
Results
CD4+ T cells exhibit temporal phenotypic differences post-MI
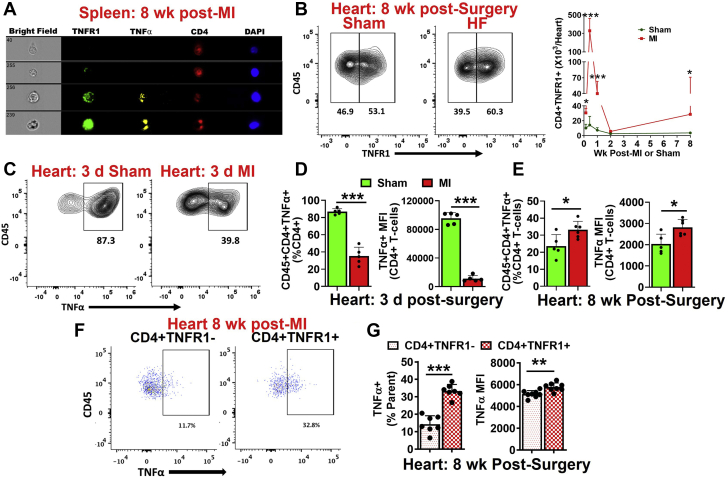
CD4+ T cells mediate wound healing and scar formation post-MI.17 However, we have previously shown that CD4+ T cells turn pathological and exacerbate LV remodeling and cardiac dysfunction during ischemic HF,11 suggesting temporal changes in T-cell phenotype and function. CD4+ T cells are known to express TNFR2, which promotes T-cell activation and proliferation, whereas TNFR1 expression on CD4+ T cells is limited and is known to be expressed during disease conditions.15 To check if HF-activated CD4+ T cells also express TNFR1, we conducted image-stream analysis of HF-activated splenic CD4+ T cells. Indeed, we found that a subset of CD4+ T cells expressed TNFR1 during HF (Figure 1A). Thus, we measured TNFR1 expression on CD4+ T cells infiltrated into the hearts at different time intervals post-MI. As shown in Figure 1B, TNFR1+ CD4+ T cells exhibited biphasic kinetics with ∼23-fold higher cell counts at 3 days post-MI, which decreased almost to baseline levels by 2 weeks compared with sham controls. These levels were increased again and were almost 7- to 8-fold higher than the sham by 8 weeks post-MI. We also observed significantly increased frequencies of TNFR1 expressing CD4+ T cells at both the time points (Supplemental Figures 1A and 1B). Because TNF-α levels have been shown to directly correlate with mortality and morbidity in HF patients,3 we measured their expression in cardiac CD4+ T cells at 3 days and 8 weeks post-MI. At 3 days post-MI, TNF-α+ (frequency and mean fluorescence intensity [MFI]) CD4+ T cells were significantly decreased (Figures 1C and 1D). In contrast, at 8 weeks, post-MI CD4+ T cells exhibited a significant increase in TNF-α compared with sham controls (Figure 1E). We further compared TNF-α expression in TNFR1+ CD4+ and TNFR1− CD4+ T cells. Interestingly, TNF-α expression (frequency and MFI) was significantly higher in CD4+ TNFR1+ T cells compared with CD4+ TNFR1− T cells in the hearts (Figures 1F and 1G) as well as the spleens (Supplemental Figure 1C) of HF mice.
Figure 1.
T Cells Activated During HF Are Proinflammatory
(A) Representative image-stream pictures showing TNF-α and TNFR1 in splenic CD4+ T cells. (B) Representative flow contour plots showing cardiac TNFR1+ CD4+ T cells in HF and sham mice (left) and their group quantitation at 1 day, 3 days, 1 week, 2 weeks, and 8 weeks postsurgery (right). (C) Representative flow contour plots showing TNF-α in cardiac CD4+ T cells at 3 days post-MI or sham surgery. Group quantitation for the frequency of cardiac TNF-α+ CD4+ T cells (left) and TNF-α mean fluorescence intensity in CD4+ T cells (right) at 3 days (D) and 8 weeks (E) post-MI or sham surgery. (F) Representative flow cytometry scatterplots showing the expression of TNF-α in CD4+ TNFR1− and CD4+ TNFR1+ T cells in the failing hearts and (G) its group quantitation (frequency and MFI). N = 5-10 in each group in B. Data were normalized using logarithmic transformation in B and analyzed using 2-way analysis of variance with the Bonferroni post hoc test. To avoid overcrowding of data, especially at early time points, individual data points are not shown. Data in DandE were analyzed using the unpaired Student’s t-test, whereas the paired Student’s t-test was used to analyze data in G. HF = heart failure; MFI = mean fluorescence intensity; MI = myocardial infarction; TNF = tumor necrosis factor; TNFR1 = tumor necrosis factor receptor 1.
TNFR1 inhibition promotes proliferative and prosurvival signaling in CD4+ T cells
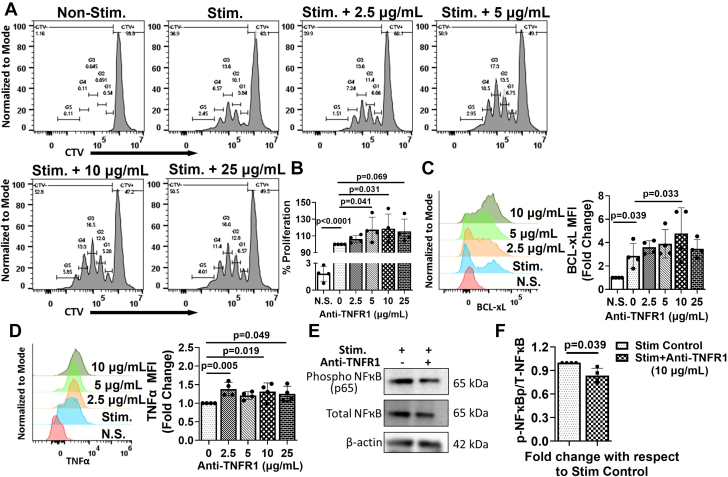
To determine the role of TNFR1 on T cells, we conducted TCR-mediated T-cell proliferation assays in the absence and presence of a neutralizing anti-TNFR1 antibody. As shown in Figures 2A and 2B, we observed significantly increased T-cell proliferation in the presence of 5 to 10 μg/mL anti-TNFR1 antibody. This effect was observed at each generation, with G5 (cells that underwent 5 or more divisions) exhibiting the most increase with 10 μg/mL anti-TNFR1 antibody (Supplemental Figure 2A). We also observed significant increases in Bcl-xL (Figure 2C), an antiapoptotic protein, and TNF-α expression (Figure 2D) with TNFR1 neutralization. Interestingly, we did not observe any significant changes in CD69, an activation marker for CD4+ T cells (Supplemental Figure 2B). Western blot analysis showed that upon treatment with 10 μg/mL anti-TNFR1 antibody, the proportion of phospho-NFκB to total NFkB (Figures 2E and 2F) was significantly decreased in TCR-activated T cells indicative of decreased NFκB-mediated proapoptotic pathways. These results suggest a potential role of TNFR1 in regulating the proliferation and survival of TCR-activated T cells.
Figure 2.
TNFR1 Neutralization Promotes Pro-Survival Signaling
(A) Representative flow histograms showing the proliferation of CD4+ T cells either nonstimulated or stimulated with anti-CD3/CD28 antibodies in the absence and presence of different concentrations of neutralizing anti-TNFR1 antibody and (B) their group quantitation. (C) Representative flow histograms for Bcl-xL expression in proliferated T cells treated with different concentrations of neutralizing anti-TNFR1 antibody (left), and fold change (right) with respect to nonstimulated (N.S.) control. (D) Representative flow histograms for TNF-α expression in proliferated T cells treated with different concentrations of anti-TNFR1 antibody (left) and fold change (right) with respect to stimulated control. (E) Representative Western blots and (F) densitometric ratio of phospho- to total-NFκB (p65) (normalized to β-actin) in stimulated T cells in the absence and presence of 10 μg/mL anti-TNFR1 antibody. Experiments were repeated 4 to 5 times with 3 to 4 replicates in each, and the average of each experiment is shown as individual points. Data were analyzed using 1-way analysis of variance, and P values were adjusted using the false discovery rate in B, C, andD. Data in F were analyzed using the unpaired Student’s t-test. Stimulated T cells were compared either with the nonstimulated (N.S.) T cells or with stimulated T cells treated with different concentrations of anti-TNFR1 antibody. Abbreviations as in Figure 1.
TNFR1−/− T cells exhibit improved survival in the circulation and lymphoid tissues
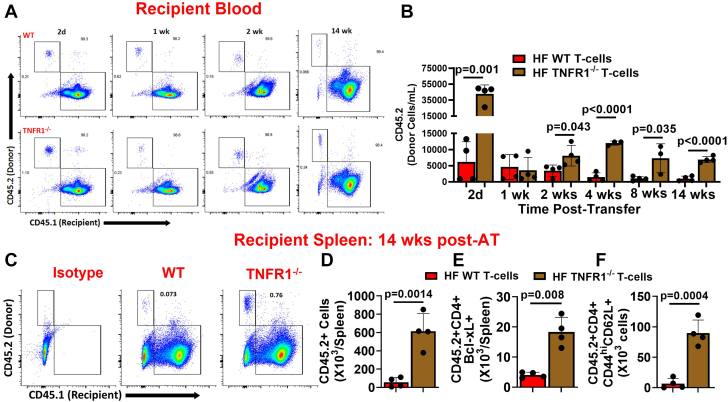
Previously, we have shown that during HF central memory and activated CD69+ T cells increase in the spleen, and the AT of HF-activated splenic CD4+ T cells induce cardiac dysfunction in naive mice.11 We used this finding to further characterize the functional role of TNFR1 on CD4+ T cells in an in vivo environment. We isolated splenic CD4+ T cells either from CD45.2+ WT or TNFR1−/− mice (also CD45.2+) at 8 weeks post-MI and adoptively transferred them to CD45.1 naive mice. Prior studies by others9 have shown that global TNFR1−/− mice exhibit improved LV remodeling. This was a significant confounding factor in our experimental design because observed differences in the biological activities of adoptively transferred T cells (if any) in the recipient mice could also be attributed to the difference in the degree of cardiac remodeling in the donor mice. To avoid this confounding factor, we tested cardiac function of all the donor mice before harvesting T cells, and only those mice that had <30% ejection fraction were selected (Supplemental Figure 3). We evaluated donor CD45.2 cells in different tissues of the recipient mice as a measure of difference in survival/proliferation, whereas cardiac function was used as an outcome of differences in the functional activity of HF-activated WT and TNFR1−/− T cells. As shown in Figures 3A and 3B, we observed significantly higher numbers of donor TNFR1−/− T cells in the blood of recipient mice at 2 days postinjection compared with WT CD4+ T cells. Interestingly, this difference was lost by 7 days and reappeared again by 14 days, and higher levels of TNFR1−/− T cells (compared with WT) were thereafter sustained for up to 14 weeks. At 14 weeks post-AT, we harvested splenocytes from recipient mice and measured donor-derived CD4+ T cells. The loss of TNFR1 resulted in 10- to 11-fold higher levels of donor CD4+ T cells compared with TNFR1-intact WT CD4+ T cells in the naive mice (Figures 3C and 3D). We also found ∼4-fold increased levels of Bcl-xL+ CD4+ T cells among donor CD45.2+ TNFR1−/− T cells (Figure 3E). In a subset of mice, we also measured donor T cells in the mediastinal lymph nodes (mLNs), which drain into the heart, and inguinal lymph nodes, which do not drain into the hearts, considering that HF-activated T cells might preferentially be localizing into mLNs. However, both LNs showed the presence of HF-activated donor CD4+ T cells with significantly higher levels of TNFR1−/− T cells compared with WT T cells (Supplemental Figure 4A). In the spleens of recipient mice, we also measured donor-derived CD44high CD62L+ central memory T cells (Tcms) and CD44high CD69+ activated T cells (Figure 3F, Supplemental Figure 4B) and found a higher level of both of these cells in the recipient mice that were injected with HF-activated TNFR1−/− T cells compared with WT T cells. Interestingly, Tcms derived from donor TNFR1−/− or WT CD4+ T cells were ∼3- to 4-fold higher than the CD69+-activated T cells in the spleens of recipient mice, suggesting an increased accumulation of donor-derived Tcms compared with activated T cells irrespective of the phenotypes (Figure 3F, Supplemental Figure 4B). Overall, these results indicate improved survival of CD4+ T cells in the circulation and the lymphoid tissues upon TNFR1 loss and support our ex vivo results.
Figure 3.
TNFR1−/− Improves Survival of HF-Activated T Cells
(A) Representative flow scatterplots for donor CD45.2+ CD4+ T cells isolated either from WT or TNFR1−/− mice in the blood of CD45.1+ mice at 2 days and 1, 2, and 14 weeks and (B) their group quantitation at 2 days and 1, 2, 4, 8, and 14 weeks post-AT. (C) Representative flow scatterplots and (D) group quantitation for donor CD45.2+ CD4+ T cells in the spleens of CD45.1 recipient mice at 14 weeks. Group quantitation for (E) donor-derived CD45.2+ CD4+ Bcl-xL+ and (F) CD45.2+ CD4+ CD44high CD62L+ cells at 14 weeks post-AT. The study was repeated 2 times (n = 3-5 in each study), and data from a representative experiment are shown. Data in B were analyzed using repeated measures 2-way analysis of variance, and P values were adjusted using the false discovery rate. Data in D, E, andF were analyzed using the unpaired Student’s t-test. AT = adoptive transfer; WT = wild type; other abbreviations as in Figure 1.
TNFR1 expression is not critical for pathological effects of HF-activated T cells
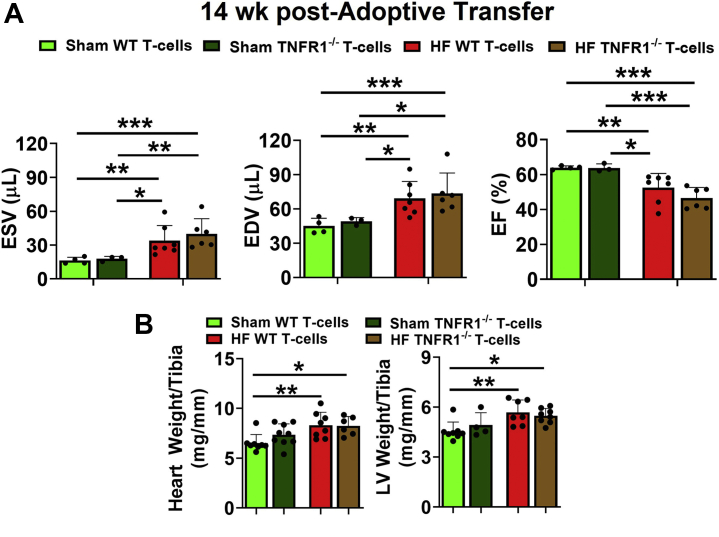
To determine if TNFR1 is important for the pathological effects of HF-activated CD4+ T cells, we measured cardiac function before (baseline) and after 14 weeks of AT while maintaining heart rate >500 bpm (Supplemental Figure S5A). At baseline, the cardiac function of all recipient mice was normal and comparable between all the groups (Supplemental Figure 5B). As shown in Figure 4A and consistent with our previous findings,11 AT of HF-activated WT CD4+ T cells (vs sham CD4+ T cells) to naive mice induced significant dysfunction characterized by increased end-systolic and end-diastolic volumes and decreased ejection fraction at 14 weeks. However, the degree of cardiac dysfunction induced by HF-activated TNFR1−/− T cells was similar to T cells from WT HF mice, and both groups had comparable increases in the end-systolic volume and the end-diastolic volume and a decrease in ejection fraction. Consistently, we also observed significant increases in the tibia-normalized heart and LV weights of naive mice injected either with HF-activated WT or TNFR1−/− T cells compared with T cells from sham mice (Figure 4B). Importantly, no significant differences were observed in tissue weights (Figure 4B) of the naive mice injected with HF-activated T cells of either phenotype (WT or TNFR1−/−).
Figure 4.
TNFR1 Does Not Regulate Pathogenicity of HF-Activated T Cells
(A) Group quantitation for end-systolic and end-diastolic volumes (ESV and EDV), and ejection fraction (EF) of CD45.1+ recipient mice at 14 weeks post-AT of sham or HF-activated splenic CD45.2+ CD4+ T cells isolated either from WT or TNFR1−/− mice. (B) Tibia normalized heart and LV weights of recipient mice at 14 weeks post-AT. The study was repeated 2 times (n = 2-5 in each study), and cumulative data from both the experiments are shown. Data in AandB were analyzed using 2-way analysis of variance with the Tukey post hoc test. LV = left ventricular; other abbreviations as in Figures 1 and 3.
TNFR1 expression is not critical for CD4+ T-cell trafficking into the hearts but regulates their proliferative potential
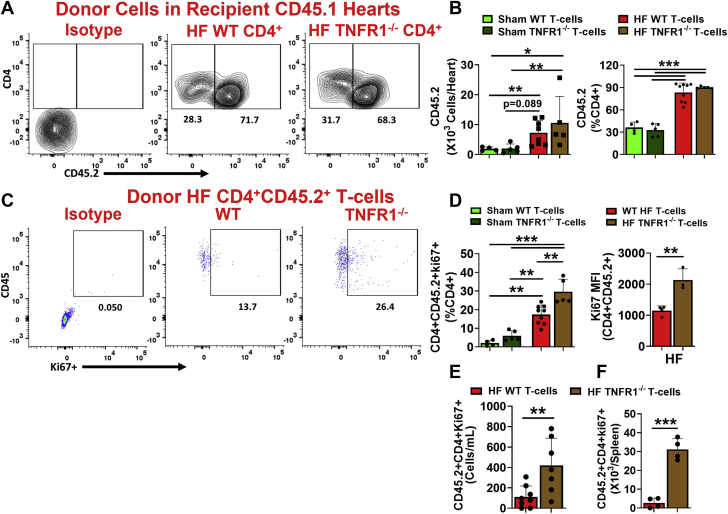
At 14 weeks post-AT, we measured donor CD45.2+ cells in the hearts of recipient mice. As shown in Figures 5A and 5B, both WT and TNFR1−/− HF-activated donor CD4+ T cells were significantly higher than the sham WT or TNFR1−/− T cells in the hearts of the naive recipient mice. Although only 30% to 36% of cardiac CD4+ T cells were donor-derived CD45.2+ sham cells (WT and TNFR1−/−), 83% to 90% of CD4+ T cells were CD45.2+ donor T cells in the hearts of the mice injected with HF-activated WT or TNFR1−/− T cells. This suggested a preferential accumulation of HFactivated CD4+ T cells into the recipient hearts compared with sham T cells. We also observed increased frequencies of donor-derived CD69+, interferon-γ+ (T helper cell 1), and IL-17+ (T helper cell 17) T cells in the hearts of naive mice injected with WT or TNFR1−/− HF-activated T cells compared with mice injected with sham WT or TNFR1−/− T cells (Supplemental Figure S6). However, neither the total cell count nor the frequency of HF-activated donor TNFR1−/− T cells (10.5 × 103 ± 8.9 × 103 and 90.1% ± 1.4%, respectively) was different than the HF-activated WT T cells (7.2 × 103 ± 4.1 × 103 and 83.4% ± 12.6%, respectively) in the hearts of naive mice (Figures 5A and 5B). Overall, these results indicate that TNFR1 expression is not required for migration, localization, activation, or proinflammatory polarization of T cells into the peripheral tissues.
Figure 5.
TNFR1−/− Promotes Proliferation of HF-Activated T Cells
(A) Representative flow contour plots for HF-activated WT or TNFR1−/− donor CD45.2+ CD4+ T cells in the hearts of CD45.1+ recipient mice and (B) their group quantitation for the total number of cells (left) and their frequency (right) with respect to total cardiac CD4+ T cells at 14 weeks. (C) Representative flow scatterplots showing ki67+ cells in CD45.2+ CD4+ T cells infiltrated into the hearts of CD45.1 recipient mice and (D) their group quantitation (frequency [left] and ki67 MFI [right]) at 14 weeks post-AT. Donor-derived CD45.2+ CD4+ Ki67+ T cells in the (E) blood and (F) spleen of recipient CD45.1 mice at 14 weeks post-AT. Studies were repeated 2 times (n = 2-5 in each), and cumulative data are shown. Ki67 MFI and splenic cell counts are representative from 1 experiment. Data were analyzed using 2-way analysis of variance with P values adjusted using the false discovery rate in BandD (left) and the unpaired Student’s t-test in D (right), E, andF.
Because our ex vivo studies showed that TNFR1 neutralization promotes CD4+ T-cell proliferation, we also measured donor-derived ki67+ CD4+ T cells in the hearts, spleens, and blood of recipient mice. As shown in Figure 5C, only 2% to 5% of donor CD4+ T cells derived from sham mice (WT or TNFR1−/−) expressed Ki67 in the hearts of recipient mice. In contrast, HF-activated donor CD45.2+ ki67+ CD4+ T cells were 5- to 6-fold higher in the hearts of recipient mice (Figures 5C and 5D). Importantly, HF-activated donor TNFR1−/− T cells showed significantly increased levels of Ki67+ T cells (and MFI) compared with HF-activated WT donor T cells (Figures 5C and 5D). Increased levels of ki67+ CD4+ T cells derived from donor TNFR1−/− T cells compared with WT were also observed in the blood (Figure 5E), spleen (Figure 5F), and mLNs (Supplemental Figure 7A). Although donor-derived CD4+ T cells were also present in the inguinal lymph nodes (Supplemental Figure 4A), interestingly, none of those were found to have ki67 expression (Supplemental Figure 7B), suggesting that the increased proliferative potential of HF-activated CD4+ T cells was restricted specifically to the heart-draining mLNs.
Discussion
Circulating levels of TNF-α and soluble forms of its receptors (sTNFR1 and sTNFR2) directly correlate with increased mortality and morbidity in HF patients.3 However, trials aimed at antagonizing TNF-α failed in HF patients,6 suggesting significant beneficial effects of TNF-α.18 Studies in rodents further showed that TNFR1 and TNFR2 activate disparate signaling pathways with improved healing in TNFR1−/− mice and exacerbation of LV remodeling in TNFR2−/− mice, implying receptor-specific effects of TNF-α.9 However, none of the previous studies explored the role of TNF-α–TNFR1 signaling in regulating immune function, especially the protective (during MI) versus the pathological (during HF) role of T cells.
CD4+ T cells express high levels of TNFR214 to promote the transcription of prosurvival genes through noncanonical NFkB signaling19 and aid T-cell proliferation.14 In contrast, TNFR1 contains a death domain and activates apoptosis through canonical NFkB activation and the TNFR1-associated death domain.19 Thus, balanced TNFR1 versus TNFR2 signaling regulates proapoptotic versus prosurvival signaling, respectively, in cells and could dictate tolerogenic versus immunogenic responses in T cells.8 Although the role of TNFR2 in CD4+ T cells is well established,14 the expression and function of TNFR1 in CD4+ T cells during ischemic HF has not been explored previously. For the first time, our studies show that despite increased levels of TNFR1-expressing CD4+ T cells at 3 days and 8 weeks post-MI, CD4+ T cells exhibited significant phenotypic differences with respect to TNF-α expression at both the time points. In contrast to decreased TNF-α expression at 3 days post-MI, CD4+ T cells activated during HF exhibited amplified TNF-α expression explaining their proinflammatory and pathological role in LV remodeling.11 Considering that TNF-α–TNFR signaling mediates T-cell anergy20 and activation-induced cell death,21 increased levels of TNF-α in TNFR1+ T cells (versus TNFR1− T cells) would further suggest the activation of apoptotic signaling to contain overt T-cell activation. However, this is an extremely simplistic view of a very complex and highly regulated TNF-α mechanism to balance activation14 versus anergy20 because almost all T cells also express TNFR2 on their surface and TNFR2 levels are significantly higher than TNFR1.14 Thus, both TNFR1+ and TNFR1− T cells during HF also expressed TNFR2, which can promote the activation and proliferation of autoreactive T cells in the presence of high TNF-α levels.14 Increased activity of the TNF-α–TNFR2 axis has been sown in several autoimmune disorders, such as multiple sclerosis, systemic lupus erythematosus, Crohn disease, and ankylosing spondylitis, all characterized by the increased survival of autoreactive T cells.22,23 Thus, it is possible that TNFR2 and not TNFR1 is the major regulator of T-cell activation, proliferation, and survival during HF as well. This would suggest that T-cell pathogenicity as observed during chronic HF probably is not caused by increased TNFR1, and it is likely that the expression of TNFR2 is more critical in this regard.
Dendritic cells and monocytes/macrophages also express significant levels of TNFR1 and TNFR2 and by binding sTNF-α to their TNFR1 can cross-present it to TNFR2 expressed on T cells.24,25 This can shift the balance toward TNFR2 over TNFR1, a condition more relevant to an in vivo environment, such as that seen in HF. These influences were absent in our ex vivo assays because they were conducted without other antigen-presenting cells. Although this strategy eliminated effects originating from other immune cells and helped us understand the role of TNFR1 on T-cell biology, this also limited the conclusions that can be drawn to explain the exact role of increased levels of TNFR1+ T cells in failing hearts. However, our AT studies partly alleviate these concerns, and our findings of the increased survival and proliferation of HF-activated TNFR1−/− T cells compared with WT in the blood and lymphoid tissues partly support the role of TNFR1 in regulating proapoptotic signals in T cells. This is also consistent with our data showing increased ki67 expression in donor TNFR1−/− T cells compared with WT in the hearts, blood, and spleen of recipient mice.
During ischemic injury, such as in MI and HF, a controlled expansion and contraction of the immune system is essential to promote wound healing and scar formation followed by immune resolution. This regulation is vital during the activation of antigen-specific T cells to avoid autoimmune responses against self-antigens during sterile injury. In this regard, the dualistic role of cytokines, such as IL-2, is critical to mediate clonal expansion followed by eventual cell death.26 We show a similar dualistic role for TNF-α, which promotes T-cell survival and proliferation via TNFR214 and activates apoptosis through TNFR1 (responses determined by the cues from cell-cell and cell-matrix interactions). Importantly, this dualistic regulation is a common program throughout the TNFR superfamily; other TNFR family members such as CD95, CD27, CD30, CD40, and LT-β can also initiate death and activation programs.27
TNFR1 loss only modestly (although significantly) increased T-cell proliferation but resulted in drastic increases in Bcl-xL and ki67 without affecting CD69 expression or the degree of cardiac dysfunction induced by HF-activated T cells. This suggests that TNFR1 does not mediate activation or the pathological effects of T cells during HF and only suppresses prosurvival and proliferative signals. The limited effects on T-cell proliferation could also be caused by the fact that TNFR2 expression on T cells is higher than TNFR1, and TNFR2-dependent proliferative signaling is predominant over TNFR1-mediated apoptosis28 during early phases of T-cell activation (as in our ex vivo studies). Studies have shown that during the initial stages of T-cell activation, TNFR2 mediates the proliferation of CD4+ T cells and renders them resistant to TNF-mediated apoptosis. However, with time, this resistance wanes and T cells become sensitive to TNF-α–mediated apoptosis,28 suggesting the involvement of TNFR1-mediated proapoptotic signaling in late activation-induced cell death.26 Therefore, it is possible that anti-TNFR1 treatment during late stages of activation could result in drastic changes in cell proliferation; this needs to be considered in future studies.
Study limitations
In this study we used TNFR1 neutralization (or TNFR1-/-) to show its role in CD4+ T-cell activation and proliferation, and did not use gain of function approaches due to unavailability of agonistic TNFR1 antibodies. TNFR1 is also expressed on other immune cells such as monocytes, macrophages, dendritic cells and CD8+ cytotoxic T-cells, and TNFR1 signaling from these cells can cross-present TNF-α to TNFR1/TNFR2 on CD4+ T-cells to alter overall cellular response.
Conclusions
Our studies show that TNFR1 exerts multifaceted and temporal effects on T cells. Considering the global expression of TNFR1 and the potential of T cells to upregulate its expression to mediate inter- and intracellular cross-talk, specifically during pathological states, further adds to this complexity. Therefore, it is necessary to revisit the role of TNF-α–TNFR signaling in a cell- and time-dependent manner to dissect its protective versus pathological effects. An improved understanding of these effects could also provide new mechanisms explaining the failure of the ATTACH (Anti-TNF Therapy Against Congestive Heart Failure). Thus, immunomodulation of TNF-α–TNFR signaling during HF may need a multipronged approach in which proapoptotic pathways need to be activated while simultaneously inhibiting proliferative stimuli in T cells.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Failure of the ATTACH trial suggested a complex role of proinflammatory cytokines that cannot be gleaned by measuring their circulating concentrations. Subsequent rodent studies underscored these complexities by showing the differential effects of TNF-α mediated by its 2 receptors. Our studies complement those findings and show that the TNF-α–TNFR axis also regulates immune responses by regulating T-cell survival and proliferation. Our studies dissect the role of the TNF-α–TNFR axis in mediating T-cell activation, survival, and proliferation during ischemic HF.
TRANSLATIONAL OUTLOOK: T-cell activation mediates wound healing post-MI. However, persistently elevated T cells promote progressive cardiac dysfunction and LV remodeling during HF. Although TNFR2 is known to promote T-cell proliferation, we show that the TNF-α–TNFR1 axis regulates proapoptotic signals in T cells. To our knowledge, this is the first study to show improved survival and proliferation of HF-activated TNFR1−/− T cells compared with WT in the blood and lymphoid tissues without affecting their pathogenicity or activation potential. We show that immunomodulation of TNF-α during HF requires a multipronged approach in which simultaneous activation of TNFR1 and inhibition of TNFR2 may be warranted to activate proapoptotic signals while inhibiting proliferation, respectively, of pathological T cells.
Funding Support and Author Disclosures
This work was supported by the National Institutes of Health grants to Dr Bansal (R00 HL132123 and R01 HL153164). Funding sources did not influence any part of this work. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Pål Aukrust, MD, served as the Guest Associate Editor for this paper. Michael Bristow, MD, PhD, served as the Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental table and figures, please see the online version of this paper.
Appendix
References
- 1.Burchfield J.S., Xie M., Hill J.A. Pathological ventricular remodeling: mechanisms: part 1 of 2. Circulation. 2013;128:388–400. doi: 10.1161/CIRCULATIONAHA.113.001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nehra S., Gumina R.J., Bansal S.S. Immune cell dilemma in ischemic cardiomyopathy: to heal or not to heal. Curr Opin Physiol. 2021;19:39–46. doi: 10.1016/j.cophys.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deswal A., Petersen N.J., Feldman A.M., Young J.B., White B.G., Mann D.L. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST) Circulation. 2001;103:2055–2059. doi: 10.1161/01.cir.103.16.2055. [DOI] [PubMed] [Google Scholar]
- 4.Ridker P.M., Everett B.M., Thuren T., et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 5.Stumpf C., Lehner C., Raaz D., et al. Platelets contribute to enhanced MCP-1 levels in patients with chronic heart failure. Heart. 2008;94:65–69. doi: 10.1136/hrt.2007.115006. [DOI] [PubMed] [Google Scholar]
- 6.Chung E.S., Packer M., Lo K.H., Fasanmade A.A., Willerson J.T. Anti TNFTACHFI. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107:3133–3140. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
- 7.Giles J.T., Sattar N., Gabriel S., et al. Cardiovascular safety of tocilizumab versus etanercept in rheumatoid arthritis: a randomized controlled trial. Arthritis Rheumatol. 2020;72:31–40. doi: 10.1002/art.41095. [DOI] [PubMed] [Google Scholar]
- 8.Faustman D., Davis M. TNF receptor 2 pathway: drug target for autoimmune diseases. Nat Rev Drug Discov. 2010;9:482–493. doi: 10.1038/nrd3030. [DOI] [PubMed] [Google Scholar]
- 9.Hamid T., Gu Y., Ortines R.V., et al. Divergent tumor necrosis factor receptor-related remodeling responses in heart failure: role of nuclear factor-kappaB and inflammatory activation. Circulation. 2009;119:1386–1397. doi: 10.1161/CIRCULATIONAHA.108.802918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duerrschmid C., Crawford J.R., Reineke E., et al. TNF receptor 1 signaling is critically involved in mediating angiotensin-II-induced cardiac fibrosis. J Mol Cell Cardiol. 2013;57:59–67. doi: 10.1016/j.yjmcc.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bansal S.S., Ismahil M.A., Goel M., et al. Activated t lymphocytes are essential drivers of pathological remodeling in ischemic heart failure. Circ Heart Fail. 2017;10 doi: 10.1161/CIRCHEARTFAILURE.116.003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nevers T., Salvador A.M., Grodecki-Pena A., et al. Left ventricular T-cell recruitment contributes to the pathogenesis of heart failure. Circ Heart Fail. 2015;8:776–787. doi: 10.1161/CIRCHEARTFAILURE.115.002225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang T.T., Zhu Y.C., Dong N.G., et al. Pathologic T-cell response in ischaemic failing hearts elucidated by T-cell receptor sequencing and phenotypic characterization. Eur Heart J. 2019;40:3924–3933. doi: 10.1093/eurheartj/ehz516. [DOI] [PubMed] [Google Scholar]
- 14.Chen X., Nie Y., Xiao H., et al. TNFR2 expression by CD4 effector T cells is required to induce full-fledged experimental colitis. Sci Rep. 2016;6 doi: 10.1038/srep32834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossol M., Schubert K., Meusch U., et al. Tumor necrosis factor receptor type I expression of CD4+ T cells in rheumatoid arthritis enables them to follow tumor necrosis factor gradients into the rheumatoid synovium. Arthritis Rheum. 2013;65:1468–1476. doi: 10.1002/art.37927. [DOI] [PubMed] [Google Scholar]
- 16.Covarrubias R., Ismahil M.A., Rokosh G., et al. Optimized protocols for isolation, fixation, and flow cytometric characterization of leukocytes in ischemic hearts. Am J Physiol Heart Circ Physiol. 2019;317:H658–H666. doi: 10.1152/ajpheart.00137.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hofmann U., Beyersdorf N., Weirather J., et al. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation. 2012;125:1652–1663. doi: 10.1161/CIRCULATIONAHA.111.044164. [DOI] [PubMed] [Google Scholar]
- 18.Papathanasiou S., Rickelt S., Soriano M.E., et al. Tumor necrosis factor-alpha confers cardioprotection through ectopic expression of keratins K8 and K18. Nat Med. 2015;21:1076–1084. doi: 10.1038/nm.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gough P., Myles I.A. Tumor necrosis factor receptors: pleiotropic signaling complexes and their differential effects. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.585880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ledo C., Gonzalez C.D., Poncini C.V., Mollerach M., Gomez M.I. TNFR1 signaling contributes to t cell anergy during staphylococcus aureus sepsis. Front Cell Infect Microbiol. 2018;8:259. doi: 10.3389/fcimb.2018.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otano I., Alvarez M., Minute L., et al. Human CD8 T cells are susceptible to TNF-mediated activation-induced cell death. Theranostics. 2020;10:4481–4489. doi: 10.7150/thno.41646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chatzikyriakidou A., Georgiou I., Voulgari P.V., Drosos A.A. The role of tumor necrosis factor (TNF)-alpha and TNF receptor polymorphisms in susceptibility to ankylosing spondylitis. Clin Exp Rheumatol. 2009;27:645–648. [PubMed] [Google Scholar]
- 23.Kodama S., Davis M., Faustman D.L. The therapeutic potential of tumor necrosis factor for autoimmune disease: a mechanistically based hypothesis. Cell Mol Life Sci. 2005;62:1850–1862. doi: 10.1007/s00018-005-5022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maney N.J., Reynolds G., Krippner-Heidenreich A., Hilkens C.M.U. Dendritic cell maturation and survival are differentially regulated by TNFR1 and TNFR2. J Immunol. 2014;193:4914–4923. doi: 10.4049/jimmunol.1302929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossol M., Meusch U., Pierer M., et al. Interaction between transmembrane TNF and TNFR1/2 mediates the activation of monocytes by contact with T cells. J Immunol. 2007;179:4239–4248. doi: 10.4049/jimmunol.179.6.4239. [DOI] [PubMed] [Google Scholar]
- 26.Lenardo M.J. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 27.Ward-Kavanagh L.K., Lin W.W., Sedy J.R., Ware C.F. The TNF receptor superfamily in co-stimulating and co-inhibitory responses. Immunity. 2016;44:1005–1019. doi: 10.1016/j.immuni.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pimentel-Muinos F.X., Seed B. Regulated commitment of TNF receptor signaling: a molecular switch for death or activation. Immunity. 1999;11:783–793. doi: 10.1016/s1074-7613(00)80152-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.