Abstract
Objective
We sought to investigate whether long-term clinical outcomes differ following percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) in patients with three-vessel disease (3VD) and lesions in the proximal left anterior descending artery (P-LAD).
Methods
This post-hoc analysis of the Synergy between PCI with Taxus and Cardiac Surgery (SYNTAX) Extended Survival study included patients with 3VD who were classified according to the presence or absence of lesions located in the P-LAD. Ten-year all-cause death and 5-year major adverse cardiac or cerebrovascular events (MACCE) were assessed.
Results
Among 1088 patients with 3VD, 559 (51.4%) had involvement of P-LAD and their 10-year mortality was numerically higher following PCI versus CABG (28.9% vs 21.9%; HR: 1.39, 95% CI 0.99 to 1.95). Although patients without P-LAD lesions had significantly higher 10-year mortality following PCI compared with CABG, there was no evidence of a treatment-by-subgroup interaction (28.8% vs 20.2%; HR: 1.47, 95% CI 1.03 to 2.09, pinteraction=0.837). The incidence of MACCE at 5 years was significantly higher with PCI than CABG, irrespective of involvement of P-LAD (with P-LAD: HR: 1.86, 95% CI 1.36 to 2.55; without P-LAD: HR: 1.54, 95% CI 1.11 to 2.12; pinteraction=0.408). Individualised assessment using the SYNTAX Score II 2020 established that a quarter of patients with P-LAD lesions had significantly higher mortality with PCI than CABG, whereas in the remaining three-quarters CABG had similar mortality.
Conclusions
Among patients with 3VD, the presence or absence of a P-LAD lesion was not associated with any treatment effect on long-term outcomes following PCI or CABG.
Trial registration number
SYNTAXES: NCT03417050; SYNTAX: NCT00114972.
Keywords: Coronary Artery Bypass, Percutaneous Coronary Intervention, Coronary Artery Disease
WHAT IS ALREADY KNOWN ON THIS TOPIC
The optimal mode of revascularisation for lesions in the proximal left anterior descending artery (P-LAD) remains unclear, especially when associated with three-vessel disease (3VD).
WHAT THIS STUDY ADDS
In this post-hoc analysis of the Synergy between PCI with Taxus and Cardiac Surgery (SYNTAX) Extended Survival study, the 5-year major adverse cardiac or cerebrovascular event was significantly higher with percutaneous coronary intervention (PCI) compared with coronary artery bypass grafting (CABG), irrespective of the presence of a P-LAD lesion.
In addition, the presence of a P-LAD lesion did not have any impact on the treatment benefit of CABG over PCI in terms of 10-year mortality.
The SYNTAX Score II 2020 identified individuals with P-LAD lesions who benefited more from CABG than from PCI.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
In patients with 3VD, there is no need for a lesion in P-LAD to be taken into account more than it currently is in the anatomical SYNTAX score.
Further studies are warranted to elucidate the clinical importance of a P-LAD lesion in contemporary practice.
Introduction
Coronary artery disease (CAD) involving the proximal left anterior descending artery (P-LAD) is considered a high-risk lesion for adverse outcomes in light of the large area of the myocardium subtended by this vessel.1–4 Consequently, revascularisation of these patients with concomitant one-vessel or two-vessel CAD has a class I recommendation for percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) in the current European Society of Cardiology (ESC) guidelines.5 Recent studies suggest a similar risk of mortality with PCI or CABG in patients with isolated P-LAD lesions6–8; however, there is an absence of data regarding the optimal mode of revascularisation to improve very long-term prognosis in patients with a P-LAD lesion and multivessel disease (MVD).9 10 Cavalcante et al 10 reported a numerically lower rate of mortality with CABG (7.0%) compared with PCI (10.1%) at 5-year follow-up (p=0.06). Given the potential superior long-term patency of a left internal mammary artery (LIMA) graft on the left anterior descending artery (LAD) compared with stenting, it is of genuine interest whether the presence of a P-LAD lesion can amplify the beneficial effects of CABG over PCI during long-term follow-up beyond 5 years.
The Synergy between PCI with Taxus and Cardiac Surgery (SYNTAX) trial compared PCI with CABG in patients with left main CAD (LMCAD) and/or three-vessel disease (3VD).11 The aim of this subgroup analysis of the SYNTAX Extended Survival (SYNTAXES) study was to investigate the impact of a P-LAD lesion on long-term clinical outcomes among patients with 3VD and to compare PCI with CABG in patients with 3VD with or without a P-LAD lesion.
Methods
Study design and patient population
The present study is a post-hoc subgroup analysis of the SYNTAXES study (NCT03417050),12 which was an investigator-driven, extended, 10-year follow-up of the randomised SYNTAX trial (NCT00114972) beyond its original follow-up of 5 years.11 13 14 In brief, the SYNTAX trial was a multicentre, randomised controlled trial performed in 85 hospitals across 18 North American and European countries which adopted an ‘all-comers’ design involving consecutive enrolment of all eligible patients with 3VD or LMCAD, except for those presenting with a myocardial infarction (MI). A total of 1800 patients with de novo 3VD and/or LMCAD who were deemed eligible for both PCI and CABG based on clinical judgement and consensus of a heart team were enrolled and randomised in a 1:1 fashion to receive PCI (n=903), with the uniform use of Taxus Express paclitaxel drug-eluting stents (Boston Scientific Corporation, Marlborough, Massachusetts, USA), or CABG (n=897). If patients were deemed ineligible for either treatment, they were entered into the nested CABG (PCI-ineligible patients) or PCI (CABG-ineligible patients) registry.
All patients provided written informed consent prior to participation in the SYNTAX trial.
P-LAD subgroup
In this study, patients with LMCAD were excluded to focus on the prognostic impact of P-LAD lesions independent of LMCAD. Patients were classified in the P-LAD group if they had at least one lesion of ≥50% diameter stenosis in the P-LAD, which was anatomically defined as the segment between the branching point of the left main stem and the first major septal branch, and represents segment 6 of the American Heart Association classification and the anatomical SYNTAX score.15 16
Study endpoint
The primary endpoint of this study was all-cause death at 10 years. Vital status was confirmed by electronic healthcare record review and using national death registries. Patients with missing vital status were included in the analysis and censored at the time of ‘lost to follow-up’ or at 5 years when the recruiting centres did not participate in the SYNTAXES study for 10-year extended follow-up (a total of five patients in two centres).
We also assessed major adverse cardiac or cerebrovascular events (MACCE; defined as the composite of all-cause death, MI, stroke and any repeat revascularisation) at 5 years and its components, which were adjudicated by an independent clinical events committee.
Statistical analysis
All analyses were performed on the intention-to-treat population. Continuous variables are expressed as median and IQR and compared using the Kruskal-Wallis H test. Categorical variables are presented as counts and percentages and compared using the χ2 test or Fisher’s exact test as appropriate. The Kaplan-Meier method was used as the primary analysis to estimate the cumulative rates of events over time, and the log-rank test was performed to examine the differences between groups, with CIs for 95% ratios of the probability of events at 5 or 10 years.
As a supplementary analysis, the hazards of clinical endpoints were compared between the P-LAD and the non-P-LAD group using unadjusted and adjusted Cox proportional hazards models to calculate the HR and 95% CI. The covariables in the adjusted models were baseline variables of age, sex, body mass index, medically treated diabetes, hypertension, dyslipidaemia, current smokers, previous MI, previous cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease, chronic kidney disease, prescription of statin at discharge, any bifurcations and anatomical SYNTAX score, which had been selected based on prior knowledge of the association of these variables with all-cause mortality and MACCE.14 17 The HRs between PCI and CABG were also assessed in the unadjusted Cox proportional hazards models stratified by P-LAD and non-P-LAD groups, with evaluation of the treatment-by-subgroup interaction.
In addition, the predicted 10-year all-cause mortality for PCI and CABG was also calculated using the SYNTAX Score II 2020, with assessment of calibration plots in each P-LAD subgroup18 and comparisons of the treatment benefit.18
Statistical significance was defined as a two-sided p≤0.05. All analyses were performed using SPSS Statistics V.26 (IBM Corp., Armonk, 281 N.Y., USA) and R V.3.5.1 software (R Foundation for Statistical Computing, Vienna, Austria).
Patient and public involvement
No patients were involved in the design, conduct, reporting or dissemination plans of the present study.
Results
Baseline characteristics
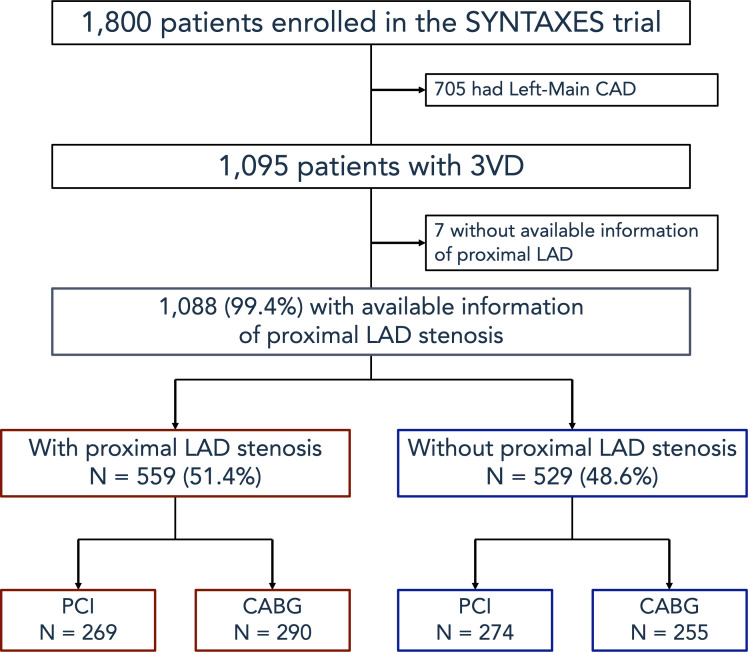
Out of the 1800 patients enrolled in the SYNTAX trial, 705 with LMCAD were excluded from the current analysis. Among the remaining 1095 patients with 3VD, 1088 (99.4%) were included in the present study, with 7 patients excluded due to missing information on P-LAD lesion. Out of the 1088 patients, 559 (51.4%) had at least one P-LAD lesion and were classified in the P-LAD group (figure 1).
Figure 1.
Flow chart of the present study. 3VD, three-vessel disease; CABG, coronary artery bypass graft; CAD, coronary artery disease; LAD, left anterior descending artery; PCI, percutaneous coronary intervention; SYNTAX, Synergy between PCI with Taxus and Cardiac Surgery; SYNTAXES, SYNTAX Extended Survival.
The baseline characteristics of the patients according to presence or absence of a P-LAD lesion are summarised in table 1. Compared with those without a P-LAD lesion, the P-LAD group had a lower prevalence of previous MI, higher anatomical SYNTAX score and more frequent bifurcation lesions. There were no significant between-group differences in other characteristics, including age, proportion of women and average predicted rate of mortality by the SYNTAX Score II 2020 (table 1).
Table 1.
Baseline characteristics of patients with or without proximal LAD stenosis
| Proximal LAD n=559 |
Non-proximal LAD n=529 |
P value | |
| Mid-LAD lesion | 73.7 (412/559) | 93.4 (494/529) | <0.001 |
| Apical LAD lesion | 14.8 (83/559) | 20.4 (108/529) | 0.017 |
| Age (years) | 66 (58–72) | 65 (58–72) | 0.663 |
| Sex | 1.000 | ||
| Male | 80.1 (448/559) | 80.2 (424/529) | |
| Female | 19.9 (111/559) | 19.8 (105/529) | |
| Body mass index (kg/m2) | 28 (25–30) | 27 (25–31) | 0.769 |
| Diabetes | 27.7 (155/559) | 26.5 (140/529) | 0.682 |
| On insulin | 11.3 (63/559) | 10.8 (57/529) | 0.847 |
| Metabolic syndrome | 48.9 (217/444) | 49.6 (210/423) | 0.839 |
| Hypertension | 66.0 (369/559) | 69.4 (367/529) | 0.244 |
| Dyslipidaemia | 77.4 (429/554) | 78.2 (408/522) | 0.826 |
| Current smoking | 20.2 (113/559) | 19.3 (102/528) | 0.761 |
| Previous MI | 33.6 (186/554) | 40.2 (210/522) | 0.027 |
| Previous cerebrovascular disease | 14.0 (78/556) | 13.5 (71/527) | 0.860 |
| Previous stroke | 4.5 (25/557) | 4.8 (25/526) | 0.885 |
| Previous transient ischemic attack | 4.1 (23/556) | 5.9 (31/526) | 0.209 |
| Previous carotid artery disease | 8.6 (48/559) | 6.6 (35/529) | 0.253 |
| Peripheral vascular disease | 8.9 (50/559) | 9.5 (50/529) | 0.834 |
| COPD | 7.9 (44/559) | 9.3 (49/529) | 0.448 |
| Chronic kidney disease | 19.7 (99/502) | 18.6 (90/483) | 0.686 |
| Creatinine clearance (mL/min) | 80 (63–102) | 82 (65–104) | 0.703 |
| LVEF (%) | 60 (50–67) | 60 (50–65) | 0.909 |
| Congestive heart failure | 5.2 (29/554) | 4.8 (25/522) | 0.781 |
| Clinical presentation | |||
| Silent ischaemia | 13.1 (73/559) | 14.6 (77/529) | 0.483 |
| Stable angina | 61.2 (342/559) | 56.0 (296/529) | 0.085 |
| Unstable angina | 25.8 (144/559) | 29.5 (156/529) | 0.175 |
| EuroSCORE | 4 (2–5) | 3 (2–5) | 0.771 |
| Parsonnet score | 6 (3–12) | 6 (3–12) | 0.825 |
| Disease type | 0.016 | ||
| 2VD (no LMCAD) | 1.9 (10/529) | 4.5 (25/559) | |
| 3VD (no LMCAD) | 98.1 (519/529) | 95.5 (534/559) | |
| Number of lesions | 5 (4–6) | 5 (4–6) | 0.797 |
| SYNTAX score | 30 (24–37) | 24 (18–30) | <0.001 |
| SYNTAX score tercile | |||
| Low | 20.8 (116/559) | 44.6 (236/529) | <0.001 |
| Intermediate | 39.9 (223/559) | 36.3 (192/529) | 0.236 |
| High | 39.4 (220/559) | 19.1 (101/529) | <0.001 |
| Predicted 10-year mortality rates by SYNTAX Score II 2020 (%) | 20.9 (12.1–34.2) | 20.2 (11.3–33.6) | 0.259 |
| Any total occlusion | 28.8 (161/559) | 28.4 (150/529) | 0.893 |
| Any bifurcation | 77.8 (435/559) | 71.3 (377/529) | 0.015 |
| Number of stents | 5 (4–6) | 5 (4–6) | 0.448 |
| Total stent length per patient | 92 (68–120) | 92 (64–124) | 0.723 |
| Off-pump CABG | 14.3 (40/279) | 14.1 (34/241) | 1.000 |
| Number of total conduits | 3 (2–3) | 3 (2–3) | 0.934 |
| Number of arterial conduits | 1 (1–2) | 1 (1–2) | 0.235 |
| Number of venous conduits | 1 (1–2) | 1 (1–2) | 0.375 |
| Complete revascularisation | 55.9 (307/549) | 53.0 (274/517) | 0.356 |
| Medication at discharge | |||
| Any antiplatelet therapy | |||
| Aspirin | 91.3 (501/549) | 91.1 (471/517) | 1.000 |
| Thienopyridine | 56.8 (312/549) | 61.5 (318/517) | 0.135 |
| Statin | 78.9 (433/549) | 81.2 (420/517) | 0.358 |
| Beta blocker | 80.7 (443/549) | 79.3 (410/517) | 0.592 |
| ACEI | 50.1 (275/549) | 53.8 (278/517) | 0.244 |
| ARB | 9.3 (51/549) | 8.7 (45/517) | 0.749 |
Data are presented as median (IQR) or percentage (n).
P values highlighted in bold suggest statistical significance (p<0.05).
ACEI, ACE inhibitors; ARB, angiotensin II receptor blocker; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; LAD, left anterior descending artery; LMCAD, left main coronary artery disease; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; SYNTAX, Synergy between PCI with Taxus and Cardiac Surgery; 2VD, two-vessel disease; 3VD, three-vessel disease.
The baseline characteristics stratified according to the randomised revascularisation strategy in the P-LAD and the non-P-LAD group are presented in online supplemental table 1 and are generally well balanced.
heartjnl-2022-320934supp001.pdf (695.9KB, pdf)
Clinical outcomes between the P-LAD and the non-P-LAD group
The presence or absence of a lesion in P-LAD did not impact significantly on the unadjusted or adjusted risk of any clinical endpoints including MACCE at 5 years (unadjusted HR: 0.95, 95% CI 0.77 to 1.19, p=0.674; adjusted HR: 0.89, 95% CI 0.70 to 1.14, p=0.351) and its components and all-cause death at 10 years (unadjusted HR: 1.02, 95% CI 0.80 to 1.30, p=0.863; adjusted HR: 0.95, 95% CI 0.72 to 1.26, p=0.718; table 2).
Table 2.
Unadjusted and adjusted hazard risks for long-term clinical outcomes between patients with and without proximal LAD lesion
| Endpoints | n | Unadjusted | Adjusted | ||
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| All-cause death at 10 years | 263/1088 | 1.02 (0.80 to 1.30) | 0.863 | 0.95 (0.72 to 1.26) | 0.718 |
| All-cause death at maximum follow-up | 330/1088 | 1.03 (0.83 to 1.28) | 0.786 | 0.98 (0.77 to 1.26) | 0.886 |
| MACCE at 5 years | 323/1088 | 0.95 (0.77 to 1.19) | 0.674 | 0.89 (0.70 to 1.14) | 0.351 |
| All-cause death at 5 years | 124/1088 | 0.96 (0.68 to 1.37) | 0.840 | 0.87 (0.58 to 1.31) | 0.512 |
| Cardiac death at 5 years | 68/1088 | 0.88 (0.55 to 1.42) | 0.601 | 0.89 (0.51 to 1.56) | 0.682 |
| MI at 5 years | 72/1088 | 0.88 (0.55 to 1.39) | 0.583 | 0.83 (0.50 to 1.38) | 0.471 |
| Stroke at 5 years | 32/1088 | 1.22 (0.60 to 2.44) | 0.584 | 1.15 (0.54 to 2.46) | 0.710 |
| Revascularisation at 5 years | 190/1088 | 0.88 (0.66 to 1.17) | 0.386 | 0.82 (0.60 to 1.12) | 0.207 |
The covariables in the adjusted models included age, sex, body mass index, medically treated diabetes, hypertension, dyslipidaemia, current smokers, previous myocardial infarction, previous cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease, chronic kidney disease, prescription of statin at discharge, any bifurcations and anatomical SYNTAX score.
‘n’ refers to the number of patients having events/total number of patients in the model.
LAD, left anterior descending artery; MACCE, major adverse cardiac or cerebrovascular events; MI, myocardial infarction; SYNTAX, Synergy between PCI with Taxus and Cardiac Surgery.
Clinical outcomes of PCI versus CABG in the P-LAD and the non-P-LAD group
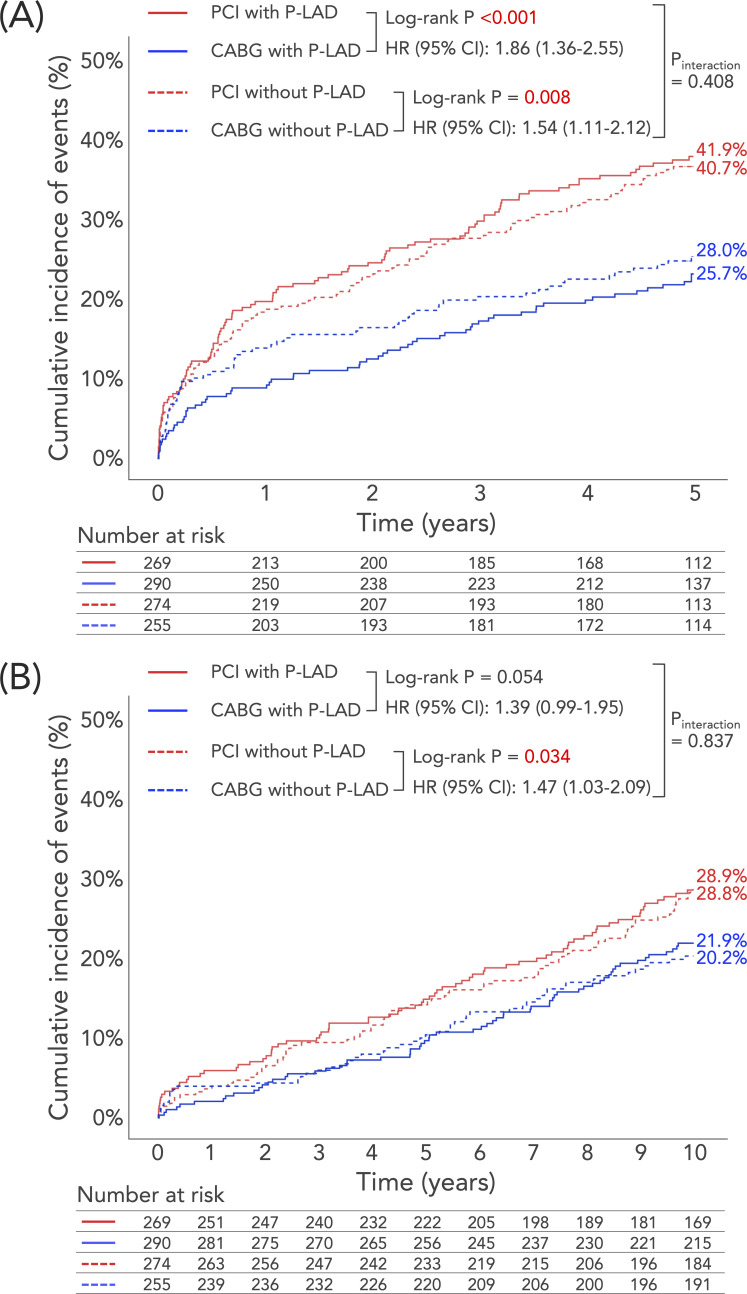
At 5 years, the rate of MACCE was significantly higher with PCI than CABG in both the P-LAD group (41.9% vs 25.7%; unadjusted HR: 1.86, 95% CI 1.36 to 2.55, p<0.001) and the non-P-LAD group (40.7% vs 28.0%; unadjusted HR: 1.54, 95% CI 1.11 to 2.12, p=0.008, p value for interaction=0.408; figure 2A and table 3). As a component of MACCE, the rate of MI was significantly higher with PCI than CABG in the P-LAD group, which was not observed in the non-P-LAD group, with a statistically significant treatment-by-subgroup interaction (p value for interaction=0.038). No other treatment-by-subgroup interactions were seen for any of the other components of MACCE.
Figure 2.
Cumulative incidence of (A) MACCE up to 5 years and (B) all-cause death up to 10 years after PCI or CABG in patients with or without P-LAD stenosis. (A) The incidence of MACCE at 5 years was significantly higher in PCI than in CABG irrespective of the involvement of a P-LAD lesion. (B) In the P-LAD group, all-cause death at 10 years did not differ significantly between PCI and CABG, whereas in the non-P-LAD group PCI was associated with a significantly higher risk of all-cause death at 10 years compared with CABG, although there was no significant treatment-by-subgroup interaction. CABG, coronary artery bypass grafting; MACCE, major adverse cardiac or cerebrovascular events; PCI, percutaneous coronary intervention; P-LAD, proximal left anterior descending artery.
Table 3.
HR of PCI versus CABG on clinical outcomes in the proximal LAD and the non-proximal LAD group
| Endpoints | Proximal LAD | Non-proximal LAD | P value for interaction | ||||
| n | HR (95% CI) | P value | n | HR (95% CI) | P value | ||
| All-cause death at 10 years | 136/559 | 1.39 (0.99 to 1.95) | 0.055 | 127/529 | 1.47 (1.03 to 2.09) | 0.035 | 0.837 |
| All-cause death at maximum follow-up | 170/559 | 1.36 (1.01 to 1.84) | 0.044 | 160/529 | 1.35 (0.98 to 1.84) | 0.063 | 0.955 |
| MACCE at 5 years | 164/559 | 1.86 (1.36 to 2.55) | <0.001 | 159/529 | 1.54 (1.11 to 2.12) | 0.009 | 0.408 |
| All-cause death at 5 years | 63/559 | 1.74 (1.04 to 2.89) | 0.033 | 61/529 | 1.57 (0.93 to 2.64) | 0.093 | 0.780 |
| Cardiac death at 5 years | 33/559 | 3.96 (1.72 to 9.13) | 0.001 | 35/529 | 1.50 (0.76 to 2.98) | 0.246 | 0.078 |
| MI at 5 years | 35/559 | 6.52 (2.53 to 16.81) | <0.001 | 37/529 | 1.90 (0.95 to 3.78) | 0.068 | 0.038 |
| Stroke at 5 years | 18/559 | 0.53 (0.20 to 1.40) | 0.199 | 14/529 | 1.59 (0.53 to 4.76) | 0.403 | 0.136 |
| Revascularisation at 5 years | 93/559 | 2.46 (1.59 to 3.79) | <0.001 | 97/529 | 1.99 (1.30 to 3.06) | 0.002 | 0.501 |
HRs are calculated with the ratios of the PCI arm compared with the CABG arm.
‘n’ refers to the number of patients having events/total number of patients in the model.
P values highlighted in bold suggest statistical significance (p<0.05).
CABG, coronary artery bypass grafting; LAD, left anterior descending artery; MACCE, major adverse cardiac or cerebrovascular event; MI, myocardial infarction; PCI, percutaneous coronary intervention.
In the P-LAD group, the primary endpoint of all-cause death at 10 years was numerically higher with PCI than with CABG (28.9% vs 21.9%; HR: 1.39, 95% CI 0.99 to 1.95, p=0.054), whereas in the non-P-LAD group PCI was associated with a significantly higher incidence of all-cause death at 10 years compared with CABG (28.8% vs 20.2%; HR: 1.47, 95% CI 1.03 to 2.09, p=0.034); however, there was no evidence of a treatment-by-subgroup interaction (p value for interaction=0.837; figure 2B and table 3).
Assessment of the SYNTAX Score II 2020 and treatment benefit in patients with or without P-LAD lesion
For exploratory purposes, we also investigated the clinical outcomes among patients with 3VD stratified according to the presence of P-LAD lesions and the SYNTAX Score II 2020. Online supplemental figure 1 shows the calibration plots of the SYNTAX Score II 2020 for 10-year mortality in the P-LAD and non-P-LAD groups, and confirms the score’s acceptable discrimination and calibration, irrespective of the presence or absence of a P-LAD lesion.
Online supplemental figure 2 shows the absolute risk differences between CABG and PCI in each quarter for patients with and without a P-LAD lesion, respectively. In the P-LAD group, the (red) curve for absolute risk difference in mortality (treatment benefit of CABG over PCI) was helpfully calibrated, whereas in the non-P-LAD group the (blue) curve lacked precision.
When stratifying patients according to the treatment benefit predicted by the SYNTAX Score II 2020, PCI was predicted to be superior or equivalent to CABG in the first three quartiles, whereas CABG was superior to PCI in terms of lowering 10-year mortality in only the fourth quartile, irrespective of the presence or absence of a P-LAD lesion (online supplemental figures 3–5).
Discussion
P-LAD lesion and long-term clinical events in patients with 3VD and/or LMCAD
Compared with isolated lesions in other epicardial vessels, a focal lesion in P-LAD is considered high risk for adverse events and derives a mortality benefit from revascularisation. The anatomical SYNTAX score gives a P-LAD lesion the second highest score following a lesion in the LMCAD. In fact, in the present study, the P-LAD group had a significantly higher mean anatomical SYNTAX score compared with the non-P-LAD group (table 1). However, in the context of an average treatment effect, the risk of MACCE at 5 years or all-cause death at 10 years did not differ significantly between the presence or absence of a P-LAD lesion. Hence, in the context of 3VD, the presence or absence of a P-LAD lesion does not significantly increase adverse events, including MI and restenosis,9 as was also predicted by the SYNTAX Score II 2020 (table 1).
Impact of P-LAD lesion on the treatment effects of CABG over PCI
LIMA is the preferred conduit for grafting the LAD during a CABG operation, given its proven very long-term patency. Therefore, an isolated P-LAD lesion would be an ideal target for surgery,19 20 as supported by its class I recommendation in the ESC guidelines.5 However, in the current study, the survival benefit of CABG over PCI was only observed in patients without involvement of the P-LAD, with no statistically significant benefit observed in patients with a P-LAD lesion, even despite their significantly higher SYNTAX score compared with the non-P-LAD group.
In the PCI arm the risk of mortality or MACCE did not differ significantly irrespective of the involvement of a P-LAD, which is in line with recent reports,21–23 and presumably due to the fact that lesion characteristics such as large reference vessel diameter, minimal tortuosity and higher blood flow are all favourable for PCI. Only for MI there was a statistically significant treatment-by-subgroup interaction between revascularisation strategies and a P-LAD lesion (p=0.038). This finding might indicate that the beneficial effect of CABG in preventing MI is greater when the myocardial area subtended by the bypass graft is larger (ie, the lesion is located more proximally than in mid or distal). Nevertheless, no long-term survival benefit was observed with CABG over PCI in the P-LAD compared with the non-P-LAD group. Therefore, this finding may be a play of chance due to the limited number of MIs (35 in the P-LAD group and 37 in the non-P-LAD group) occurring during follow-up. Although further investigations will be needed to clarify whether CABG has a true beneficial effect over PCI for a P-LAD lesion, our findings suggest that when selecting a revascularisation strategy in cases of 3VD, a P-LAD lesion does not need any special consideration other than that already accounted for in the anatomical SYNTAX score.10 23
Of note, the prescription rates of postprocedural medical therapies were significantly lower following CABG compared with PCI in both the P-LAD and non-P-LAD groups (table 1), which may partially negate the potential benefit of CABG.24
Recently, there is growing interest in hybrid coronary revascularisation procedures combining LIMA graft to the LAD with PCI for lesions in other epicardial vessels.25 Given the limited patency of saphenous vein grafts,26 this hybrid revascularisation strategy may be an important treatment option especially in patients with MVD, including a P-LAD lesion. Although this is beyond the scope of the current study, further studies are warranted to address this.
Average treatment effect versus personalised treatment benefit in patients with or without P-LAD
A dichotomic selection by a practitioner of privileged therapy between two treatment options mostly relies on the outcome of an average treatment effect, traditionally provided by an absolute difference of risk or benefit derived from Kaplan-Meier estimates in randomised trials. However, it is always appealing to identify a single major prognostic factor which more specifically predicts MACCE or mortality. Classically, a multivariable analysis will identify a variety of risk factors and their potential positive or negative interaction with one or the other alternative treatment.
From the patient’s perspective, however, it remains of paramount importance to have a personalised assessment of risk and benefit by combining multiple independent determinants of outcome while integrating their mutual interactions.16 The goal is to identify in an heterogeneous population who is going to benefit from the novel treatment, who is going to have an equivocal outcome and who is going to be harmed by this new therapy.
The methodological foundation of this approach lies in the accuracy of the predicted outcomes from each modality of treatment, allowing personalised and individual predictions of the treatment benefit of one treatment versus the other.16 18 Given the fact that the SYNTAX Score II 2020 had similar impact on predicting mortality in both the P-LAD and non-P-LAD groups, a P-LAD lesion may not need to be taken into account when using the SYNTAX score II 2020 in patients with 3VD.
Limitations
Our study has several limitations. First, the current subanalysis of the SYNTAXES trial was not prespecified and the patients were not randomised or stratified according to the presence or absence of a P-LAD lesion. Therefore, all the results were non-confirmatory and should be regarded as hypothesis-generating only. Second, patients with extremely complex CAD who were deemed not to be able to undergo PCI were excluded in the randomised cohort and were entered in the nested CABG registry.27 Hence, our results might not be applicable to those with very complex P-LAD lesions. Third, the SYNTAX trial was conducted between 2005 and 2007 with an unrestricted use of first-generation paclitaxel drug-eluting stents for treatment with PCI. The technological improvements of PCI devices as well as medical treatment strategies may limit the generalisability of our findings to current practice. It is, however, unavoidable that the findings from long-term follow-up data are based on outdated technology while the evidence for contemporary technology can be derived only from short-term follow-up studies. Fourth, the SYNTAX Score II 2020 was developed from the SYNTAX(ES) study population, which is the same as the current study and the original landmark SYNTAX trial. Hence, the current findings derived from the SYNTAX Score II 2020 may be overestimated and the predictive ability of the score when applied to real-world populations may be inferior to what was observed in the current study. However, the SYNTAX Score II 2020 has been externally validated in four randomised trials (the Evaluation of XIENCE versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization (EXCEL), the Premier of Randomized Comparison of Bypass Surgery versus Angioplasty Using Sirolimus-Eluting Stent in Patients with Left Main Coronary Artery Disease (PRECOMBAT), the Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus-Eluting Stent Implantation in the Treatment of Patients with Multivessel Coronary Artery Disease (BEST), the Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM)) and in a large, contemporary registry and showed a predictive performance as good as in the original SYNTAX cohort.18 28 29 Finally, the extended follow-up of the SYNTAXES trial up to 10 years was only for survival status, with data for other clinical endpoints with independent adjudication limited to 5 years.
Conclusions
Among patients with 3VD, the presence of a P-LAD lesion was not associated with a higher incidence of MACCE at 5 years or all-cause death at 10 years and there was also no evidence that the presence of a P-LAD impacted on the treatment effects of PCI and CABG. The SYNTAX Score II 2020 identified individuals who benefited from differential long-term survival after CABG or PCI in patients with 3VD and a P-LAD lesion.
Acknowledgments
Dr. Serruys had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Collaborators: SYNTAX Extended Survival Principal Investigators: dr. Stuart J. Head, MD PhD, Erasmus MC, Rotterdam, The Netherlands dr. Piroze M. Davierwala, MD, German Heart Center, Leipzig, Germany Prof. dr. Friedrich-Wilhelm Mohr, MD PhD, German Heart Center, Leipzig, Germany Prof. dr. Patrick W.J.C. Serruys, MD PhD, Imperial College London, United Kingdom dr. Michael J. Mack, MD, Baylor Scott & White Health, Plano, TX, United States dr. David R. Holmes Jr, MD, Mayo Clinic, Rochester, MN, United States dr. Marie-Claude Morice, MD, ICPS Ramsay-Générale de Santé, Massy, France Prof. dr. A. Pieter Kappetein, MD PhD, Erasmus MC, Rotterdam, The Netherlands SYNTAX Extended Survival Chief Investigator/Study director: Daniel J.F.M. Thuijs, MD, Erasmus MC, Rotterdam, The Netherlands SYNTAX Extended Survival Participating sites with Local Chief Investigators: 1. Aalst, Onze Lieve Vrouw Ziekenhuis (OLVZ), Belgium: Filip Casselman, Bernard de Bruyne, MD 2. Aarhus, Aarhus Universitets hospital, Denmark: Evald Høj Christiansen, MD PhD 3. Alicante, Hospital General de Alicante, Spain: Juan M. Ruiz-Nodar, MD PhD 4. Antwerp, ZNA Middelheim, Belgium: Paul Vermeersch, MD 5. Bad Oeynhausen, Universitätsklinikum der Ruhr-Universität Bochum, Germany: Werner Schultz, MD 6. Barcelona, Hospital Clínic de Barcelona, Spain: Manel Sabaté, MD PhD 7. Bergamo, Ospedale Papa Giovanni di Bergamo, Italy; Giulio Guagliumi, MD 8. Berlin, Charité – Universitätsmedizin Berlin, Germany: Herko Grubitzsch, MD PhD, Karl Stangl, MD 9. Bordeaux, Clinique ST Augustin, France: Olivier Darremont, MD 10. Breda, Amphia Ziekenhuis, The Netherlands: M. Bentala, MD, Peter den Heijer, MD PhD 11. Budapest, Cardiovascular Center of the Semmelweis University, Hungary: Istvan Preda, MD PhD 12. Dallas, Baylor University Medical Centre, TX, USA: Robert Stoler, MD 13. Dallas, Medical City Dallas Hospital, TX, USA: Michael J. Mack, MD 14. Debrecen, University of Debrecen, Hungary: Tamás Szerafin, MD PhD 15. Denver, UCH Denver Colorado, USA: John K. Buckner, MD, and Myles S. Guber, MD 16. Eindhoven, Catharina Ziekenhuis, The Netherlands: Niels Verberkmoes, MD, Ferdi Akca, MD PhD 17. Evanston, Northshore University HealthSystem, IL, USA: Ted Feldman, MD 18. Freiburg, Heart Center, University of Freiburg, Germany: Friedhelm Beyersdorf, MD PhD 19. Gent, Universitair Ziekenhuis Gent, Belgium: Benny Drieghe, MD 20. Glasgow, Golden Jubilee National Hospital, Clydebank, UK: Keith Oldroyd, MD, Geoff Berg, MD 21. Gothenborg, Sahlgrenska University Hospital, Sweden; Anders Jeppsson, MD PhD 22. Grand Blanc, Genesys Regional Medical Center, Grand Blanc, MI, USA: Kimberly Barber, MD PhD 23. Grand Rapids, Spectrum Health Hospitals Cook Research Department, MI, USA; Kevin Wolschleger, MD, John Heiser, MD 24. Groningen, Universitair Medisch Centrum Groningen, The Netherlands: Pim van der Harst, MD PhD, Massimo A. Mariani, MD PhD 25. Hamburg, University Heart Center Hamburg, Germany: Hermann Reichenspurner, MD PhD 26. Helsinki, University of Helsinki Meilahti Hospital, Finland: Christoffer Stark, MD, Mika Laine, MD PhD 27. Honolulu, Kaiser Permanente, HI, USA: Paul C. Ho, MD and John C. Chen, MD 28. Hyannis, Cape Cod Hospital, MA, USA: Richard Zelman, MD 29. Iowa, University of Iowa Hospitals & Clinics, IA, USA: Phillip A. Horwitz, MD 30. Katowice, Centrum Badawczo-Rozwojowe, American Heart of Poland, Poland: Agata Krauze PhD, Andrzej Bochenek, MD PhD 31. Kiel, Universitätsklinikum Campus Kiel, Germany: Christina Grothusen, MD 32. Krakow, John Paul II Hospital, Poland: Dariusz Dudek, MD PhD 33. Langhorne, St. Mary Medical Center, PA, USA: George Heyrich, MD 34. Leipzig, Heart Center, University of Leipzig, Germany: Piroze Davierwala, MD, Thilo Noack, MD 35. Liege, University Hospital of Liege, Belgium: Victor LeGrand, MD PhD, Philippe Kolh, MD PhD 36. Lisbon, Hospital de Santa Marta, Portugal: Pedro Coelho, MD 37. Lubeck, University Medical Center Schleswig-Holstein, Campus Lübeck, Germany: Stephan Ensminger, MD, Boris Nasseri, MD 38. Lund, Skånes Universitetssjukvård, Sweden: Richard Ingemansson, MD PhD, Goran Olivecrona, MD PhD 39. Madrid, Hospital Clinico San Carlos, Spain: Javier Escaned, MD PhD, Reddy Guera, MD 40. Massa, Fondazione CNR/Regione Toscana per la Ricerca Medica e di Sanità Pubblica-Ospedale del Cuore G.Pasquinucci, Italy: Sergio Berti, MD 41. Massy, Cardiovascular Institute Paris-Sud (ICPS), Hopital privé Jacques Cartier, Ramsay, Générale de Santé Massy, France : Marie-Claude Morice, MD 42. Milan, San Raffaele Hospital, Milan, Italy: Alaide Chieffo, MD 43. Minneapolis, Minneapolis Heart Institute Foundation, MN, USA: Nicholas Burke, MD, Michael Mooney, MD 44. Mirano, Ospedale di Mirano, Italy: Alvise Spolaor, MD 45. Munich, Klinikum der Universität München, Campus Großhadern, Germany; Christian Hagl, MD, Michael Näbauer, MD 46. Nieuwegein, Sint-Antonius Ziekenhuis, The Netherlands: Maarten Jan Suttorp, MD PhD 47. Norfolk, Sentara Cardiovascular Research Institute, Norfolk, VA, USA: Ronald A. Stine, MD 48. Oklahoma, Oklahoma Cardiovascular Research Group, OK, USA: Thomas McGarry, MD PhD, Scott Lucas, MD 49. Olso, Oslo universitetssykehus HF, Norway: Knut Endresen, MD PhD 50. Orlando, Florida Hospital Cardiovascular Research, Florida, USA: Andrew Taussig, MD, Kevin Accola, MD 51. Pavia, IRCCS Policlinico San Matteo, Italy: Umberto Canosi, BS 52. Pecs, University Hospital of Pecs, Hungary: Ivan Horvath, MD PhD 53. Petoskey, Cardiac & Vascular Research Center of Northern Michigan, Michigan, USA; Louis Cannon, MD, John D. Talbott, MD, Chris W. Akins, MD 54. Portland, Maine Medical Center, ME, USA: Robert Kramer, MD 55. Prague, Interni Klinika VFN, Czech Republic: Michael Aschermann, MD 56. Raleigh, WakeMed Health & Hospitals, Raleigh, NC, USA; William Killinger, MD 57. Riga, Latvian Centre of Cardiology, Latvia: Inga Narbute, MD 58. Rochester, Mayo Clinic, MN, USA: David R. Holmes Jr., MD 59. Rome, Catholic University of the Sacred Heart, Italy: Francesco Burzotta, MD 60. Rotterdam, Erasmus University Medical Centre, The Netherlands: Ad J.J.C. Bogers, MD, Felix Zijlstra, MD, PhD 61. Rouen, Centre Hôpital Universitaire Rouen, Hôpital Charles Nicolle, France: Helene Eltchaninoff, MD 62. Rouen, Clinique Saint-Hilaire Rouen, France: Jacques Berland, MD 63. Rozanno, Instituto Clinico Humanitas, Italy: Giulio Stefanini, MD PhD 64. Salamanca, Hospital Clinico Salamanca, Spain: Ignacio Cruz Gonzalez, MD 65. Salzburg, Dept. of Cardiology, Paracelsus Medical University Salzburg, Austria: Uta Hoppe, MD 66. San Antonio, San Antonio Endovascular and Heart Inst., TX, USA: Radoslaw Stefan Kiesz, MD, Bartlomiej Gora, MD 67. Stockholm, Karolinska University Hospital, Sweden: Anders Ahlsson, MD PhD, Matthias Corbascio, MD PhD 68. Stonybrook, Stony Brook University, NY, USA: Thomas V. Bilfinger, MD 69. Toulouse, Centre Hôpital Universitaire Rangueil, France: Didier Carrie, MD 70. Toulouse, Groupe CardioVasculaire Interventionnel, Clinique Pasteur, France: Didier Tchétché, MD 71. Trier, Krankenhaus der Barmherzigen Bruder Trier, Germany: Karl-Eugen Hauptman, MD 72. Uppsala, University Hospital Uppsala, Sweden: Elisabeth Stahle, MD PhD, Stefan James, MD PhD 73. Vienna, Allgemeines Krankenhaus AKH, Austria: Sigrid Sandner, MD, Günther Laufer, MD, Irene Lang, MD 74. Warsaw, Institute of Cardiology, Poland: Adam Witkowski, MD PhD 75. Washington, Medstar Heart and Vascular Institute, DC, USA; Vinod Thourani, MD 76. Zwolle, Isala Zwolle, The Netherlands: Harry Suryapranata, MD PhD 77. London, Guys and St Thomas, UK; Simon Redwood, MD 78. London, Barts, UK: Charles Knight, MD 79. London, King’s College, UK: Philip MacCarthy, MD 80. Southampton, University Hospital Southampton NHS FT, UK: Nick Curzen, MD PhD 81. Brighton, Brighton and Sussex University Hospitals NHS Trust, UK: Adam de Belder, MD 82. Oxford, John Radcliffe Hospital, UK: Adrian Banning, MD 83. Leicester, University Hospitals of Leicester NHS Trust Glenfield Hospital, UK: Anthony Gershlick, MD 84. Rockford, St. Anthony's Medical Center, IL, USA: Robert Minor, MD (elected not to participate in SYNTAX Extended Survival, yet did contribute to the SYNTAX trial) 85. Sacramento, Mercy General, CA, USA: Michael Chang, MD (elected not to participate in SYNTAX Extended Survival, yet did contribute to the SYNTAX trial)
Contributors: MO gathered, analysed and interpreted the data, wrote the first draft of the article, and contributed to all revisions. PWS and YO designed the study, gathered and interpreted data, and contributed to all revisions. MJM, DH, M-CM, SH, APK, TN, PMD and FWM designed the study, gathered and interpreted the data, and contributed to critical revision of the manuscript. HH, CG, HK and RW gathered and cleaned the data and contributed to revision of the article. NO’L, JJW, SG and JJP interpreted the data and contributed to critical revision of the manuscript. PWS had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. As guarantor, PWS accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: The SYNTAX Extended Survival study, during the extension of follow-up to up to 10 years, was supported by the German Foundation of Heart Research (Frankfurt am Main, Germany). The SYNTAX trial, during follow-up of 0–5 years, was funded by Boston Scientific Corporation (Marlborough, Massachusetts, USA).
Disclaimer: The study funders had no role in the study design, data collection, data analyses and interpretation of the study data, nor were involved in the decision to publish the final manuscript. The principal investigators and authors had complete scientific freedom.
Competing interests: PWS reports personal fees from Biosensors, Micel Technologies, Sino Medical Sciences Technology, Philips/Volcano, Xeltis and HeartFlow, outside the submitted work. HH reports a grant for studying overseas from the Japanese Circulation Society and a grant from Fukuda Foundation for Medical Technology, outside the submitted work. JJP reports personal fees and non-financial support from Philips/Volcano, outside the submitted work. M-CM is CEO and shareholder of CERC, a CRO not involved in this trial, and is a minor shareholder of Electroducer. SH reports to work as employee of Medtronic, outside the submitted work. APK reports to work as employee of Medtronic, outside the submitted work. All other authors have no conflict of interest to declare.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
the SYNTAX Extended Survival Investigators:
Stuart J. Head, Piroze M. Davierwala, Friedrich-Wilhelm Mohr, Patrick W.J.C. Serruys, Michael J. Mack, David R. Holmes, Marie-Claude Morice, A. Pieter Kappetein, Daniel J.F.M. Thuijs, Bernard de Bruyne, Evald Høj Christiansen, Juan M. Ruiz-Nodar, Paul Vermeersch, Werner Schultz, Manel Sabaté, Giulio Guagliumi, Herko Grubitzsch, Karl Stangl, Olivier Darremont, M. Bentala, Peter den Heijer, Istvan Preda, Robert Stoler, Michael J. Mack, Tamás Szerafin, John K. Buckner, Myles S. Guber, Niels Verberkmoes, Ferdi Akca, Ted Feldman, Friedhelm Beyersdorf, Benny Drieghe, Keith Oldroyd, Geoff Berg, Anders Jeppsson, Kimberly Barber, Kevin Wolschleger, John Heiser, Pim van der Harst, Massimo A. Mariani, Hermann Reichenspurner, Christoffer Stark, Mika Laine, Paul C. Ho, John C. Chen, Richard Zelman, Phillip A. Horwitz, Agata Krauze, Andrzej Bochenek, Christina Grothusen, Dariusz Dudek, George Heyrich, Piroze Davierwala, Thilo Noack, Victor LeGrand, Pedro Coelho, Stephan Ensminger, Richard Ingemansson, Goran Olivecrona, Javier Escaned, Reddy Guera, Sergio Berti, Marie-Claude Morice, Alaide Chieffo, Nicholas Burke, Michael Mooney, Alvise Spolaor, Christian Hagl, Michael Näbauer, Maarten Jan Suttorp, Ronald A. Stine, Thomas McGarry, Scott Lucas, Knut Endresen, Andrew Taussig, Kevin Accola, Umberto Canosi, Ivan Horvath, Louis Cannon, John D. Talbott, Chris W. Akins, Robert Kramer, Michael Aschermann, William Killinger, Inga Narbute, David R. Holmes, Francesco Burzotta, Ad J.J.C. Bogers, Felix Zijlstra, Helene Eltchaninoff, Jacques Berland, Giulio Stefanini, Ignacio Cruz Gonzalez, Uta Hoppe, Radoslaw Stefan Kiesz, Anders Ahlsson, Matthias Corbascio, Thomas V. Bilfinger, Didier Carrie, Didier Tchétché, Karl-Eugen Hauptman, Elisabeth Stahle, Stefan James, Sigrid Sandner, Günther Laufer, Irene Lang, Adam Witkowski, Vinod Thourani, Harry Suryapranata, Simon Redwood, Charles Knight, Philip MacCarthy, Nick Curzen, Adam de Belder, Adrian Banning, Anthony Gershlick, Robert Minor, and Michael Chang
Data availability statement
Data are available upon reasonable request. Anonymised data that support the findings of this study are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants and the protocol was approved by the institutional review board of all 85 sites. The SYNTAX and SYNTAXES trials were approved by the ethics committees at each investigating centre. Follow-up was performed in accordance with local law and regulations of each participating institution and complied with the Declaration of Helsinki and Good Clinical Practice. Participants gave informed consent to participate in the study before taking part.
References
- 1. Leaman DM, Brower RW, Meester GT, et al. Coronary artery atherosclerosis: severity of the disease, severity of angina pectoris and compromised left ventricular function. Circulation 1981;63:285–99. 10.1161/01.CIR.63.2.285 [DOI] [PubMed] [Google Scholar]
- 2. Klein LW, Weintraub WS, Agarwal JB, et al. Prognostic significance of severe narrowing of the proximal portion of the left anterior descending coronary artery. Am J Cardiol 1986;58:42–6. 10.1016/0002-9149(86)90238-9 [DOI] [PubMed] [Google Scholar]
- 3. Yusuf S, Zucker D, Peduzzi P, et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the coronary artery bypass graft surgery Trialists collaboration. Lancet 1994;344:563–70. 10.1016/S0140-6736(94)91963-1 [DOI] [PubMed] [Google Scholar]
- 4. Sianos G, Morel M-A, Kappetein AP, et al. The SYNTAX score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention 2005;1:219–27. [PubMed] [Google Scholar]
- 5. Neumann F-J, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165. 10.1093/eurheartj/ehy394 [DOI] [PubMed] [Google Scholar]
- 6. Matsoukis IL, Karanasos A, Patsa C, et al. Long-Term clinical outcomes of coronary artery bypass graft surgery compared to those of percutaneous coronary intervention with second generation drug eluting stents in patients with stable angina and an isolated lesion in the proximal left anterior descending artery. Catheter Cardiovasc Interv 2021;98:447–57. 10.1002/ccd.29247 [DOI] [PubMed] [Google Scholar]
- 7. Hannan EL, Zhong Y, Cozzens K, et al. Revascularization for isolated proximal left anterior descending artery disease. Ann Thorac Surg 2021;112:555–62. 10.1016/j.athoracsur.2020.08.049 [DOI] [PubMed] [Google Scholar]
- 8. Li S, Zhang H, Xiao C, et al. Robotically assisted coronary artery bypass graft surgery versus drug-eluting stents for patients with stable isolated proximal left anterior descending disease. J Card Surg 2021;36:1864–71. 10.1111/jocs.15433 [DOI] [PubMed] [Google Scholar]
- 9. Garg S, Sarno G, Gutiérrez-Chico J-L, et al. Five-year outcomes of percutaneous coronary intervention compared to bypass surgery in patients with multivessel disease involving the proximal left anterior descending artery: an ARTS-II sub-study. EuroIntervention 2011;6:1060–7. 10.4244/EIJV6I9A185 [DOI] [PubMed] [Google Scholar]
- 10. Cavalcante R, Sotomi Y, Zeng Y, et al. Coronary bypass surgery versus stenting in multivessel disease involving the proximal left anterior descending coronary artery. Heart 2017;103:428–33. 10.1136/heartjnl-2016-309720 [DOI] [PubMed] [Google Scholar]
- 11. Serruys PW, Morice M-C, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009;360:961–72. 10.1056/NEJMoa0804626 [DOI] [PubMed] [Google Scholar]
- 12. Thuijs DJFM, Kappetein AP, Serruys PW, et al. Percutaneous coronary intervention versus coronary artery bypass grafting in patients with three-vessel or left main coronary artery disease: 10-year follow-up of the multicentre randomised controlled SYNTAX trial. Lancet 2019;394:1325–34. 10.1016/S0140-6736(19)31997-X [DOI] [PubMed] [Google Scholar]
- 13. Mohr FW, Morice M-C, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013;381:629–38. 10.1016/S0140-6736(13)60141-5 [DOI] [PubMed] [Google Scholar]
- 14. Ono M, Serruys PW, Hara H, et al. 10-Year follow-up after revascularization in elderly patients with complex coronary artery disease. J Am Coll Cardiol 2021;77:2761–73. 10.1016/j.jacc.2021.04.016 [DOI] [PubMed] [Google Scholar]
- 15. Austen WG, Edwards JE, Frye RL, et al. A reporting system on patients evaluated for coronary artery disease. Report of the AD hoc Committee for grading of coronary artery disease, Council on cardiovascular surgery, American heart association. Circulation 1975;51:5–40. 10.1161/01.CIR.51.4.5 [DOI] [PubMed] [Google Scholar]
- 16. Serruys PW, Chichareon P, Modolo R, et al. The SYNTAX score on its way out or … towards artificial intelligence: Part I. EuroIntervention 2020;16:44–59. 10.4244/EIJ-D-19-00543A [DOI] [PubMed] [Google Scholar]
- 17. Pocock SJ, McMurray JJV, Collier TJ. Statistical controversies in reporting of clinical trials: Part 2 of a 4-Part series on statistics for clinical trials. J Am Coll Cardiol 2015;66:2648–62. 10.1016/j.jacc.2015.10.023 [DOI] [PubMed] [Google Scholar]
- 18. Takahashi K, Serruys PW, Fuster V, et al. Redevelopment and validation of the SYNTAX score II to individualise decision making between percutaneous and surgical revascularisation in patients with complex coronary artery disease: secondary analysis of the multicentre randomised controlled SYNTAXES trial with external cohort validation. Lancet 2020;396:1399–412. 10.1016/S0140-6736(20)32114-0 [DOI] [PubMed] [Google Scholar]
- 19. Thiele H, Neumann-Schniedewind P, Jacobs S, et al. Randomized comparison of minimally invasive direct coronary artery bypass surgery versus sirolimus-eluting stenting in isolated proximal left anterior descending coronary artery stenosis. J Am Coll Cardiol 2009;53:2324–31. 10.1016/j.jacc.2009.03.032 [DOI] [PubMed] [Google Scholar]
- 20. Hannan EL, Zhong Y, Walford G, et al. Coronary artery bypass graft surgery versus drug-eluting stents for patients with isolated proximal left anterior descending disease. J Am Coll Cardiol 2014;64:2717–26. 10.1016/j.jacc.2014.09.074 [DOI] [PubMed] [Google Scholar]
- 21. Kjøller-Hansen L, Bligaard N, Kelbæk H, et al. Ten-year clinical outcome of patients treated with a drug-eluting stent in the proximal left anterior descending artery segment compared with patients stented in other non-left main coronary segments. EuroIntervention 2018;14:764–71. 10.4244/EIJ-D-18-00396 [DOI] [PubMed] [Google Scholar]
- 22. Takahashi K, Wang R, Kawashima H, et al. Efficacy and safety of one-month DAPT followed by 23-month ticagrelor monotherapy in patients undergoing proximal LAD stenting: insights from the global leaders trial. Int J Cardiol 2020;320:27–34. 10.1016/j.ijcard.2020.07.042 [DOI] [PubMed] [Google Scholar]
- 23. Codner P, Saada M, Sakhov O, et al. Proximal left anterior descending artery treatment using a Bioresorbable polymer coating sirolimus-eluting stent: real-world outcomes from the multicenter prospective e-Ultimaster registry. J Am Heart Assoc 2019;8:e013786. 10.1161/JAHA.119.013786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kawashima H, Serruys PW, Ono M, et al. Impact of Optimal Medical Therapy on 10-Year Mortality After Coronary Revascularization. J Am Coll Cardiol 2021;78:27–38. 10.1016/j.jacc.2021.04.087 [DOI] [PubMed] [Google Scholar]
- 25. Torregrossa G, Sá MP, Van den Eynde J, et al. Hybrid robotic off-pump versus conventional on-pump and off-pump coronary artery bypass graft surgery in women. J Card Surg 2022;37:895–905. 10.1111/jocs.16247 [DOI] [PubMed] [Google Scholar]
- 26. Davierwala PM, Gao C, Thuijs D. Single or multiple arterial bypass graft surgery vs. percutaneous coronary intervention in patients with three-vessel or left main coronary artery disease. Eur Heart J 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Head SJ, Holmes DR, Mack MJ, et al. Risk profile and 3-year outcomes from the SYNTAX percutaneous coronary intervention and coronary artery bypass grafting nested registries. JACC Cardiovasc Interv 2012;5:618–25. 10.1016/j.jcin.2012.02.013 [DOI] [PubMed] [Google Scholar]
- 28. Takahashi K, van Klaveren D, Steyerberg EW, et al. Concerns with the new SYNTAX score - Authors' reply. Lancet 2021;397:795–6. 10.1016/S0140-6736(21)00195-1 [DOI] [PubMed] [Google Scholar]
- 29. Hara H, Shiomi H, van Klaveren D, et al. External Validation of the SYNTAX Score II 2020. J Am Coll Cardiol 2021;78:1227–38. 10.1016/j.jacc.2021.07.027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2022-320934supp001.pdf (695.9KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Anonymised data that support the findings of this study are available from the corresponding author on reasonable request.