Abstract
Background
Depression is a psychiatric disorder characterized by low mood and loss of interest in daily activities. Allopurinol, a xanthine oxidase blocker, is widely administered for the treatment of hyperuricemia. Recently, its effects on serotonin and depressive like behaviors have been reported. On the other hand, the level of brain-derived neurotrophic factor (BDNF), a protective and regenerative neurotrophic, has been increased by many antidepressants. The purpose of this study was to evaluate the antidepressant effects of allopurinol and changes in serum level of BDNF compared to those of fluoxetine.
Methods
Thirty-five male Wistar albino rats divided into five groups (control, 10 mg/kg fluoxetine, 25, 50 and 100 mg/kg allopurinol; n = 7 per group), that received all treatments intraperitoneally, every day. Forced swimming tests (FST), tail suspension test (TST) and open field test (OFT) were performed after 21 days of drug administration. Finally, the serum BDNF levels were measured using the sandwich ELISA method.
Results
All doses of allopurinol and fluoxetine reduced the duration of immobility time in FST and TST. No significant changes were observed in the number of lines crossed in OFT between either allopurinol or fluoxetine groups and control group. Serum level of BDNF were significantly higher in fluoxetine and allopurinol 50 and 100 mg/kg groups.
Conclusions
Long-term administration of allopurinol 50 and 100 mg/kg have shown antidepressant effects in behavioral tests along with an increase in the amount of serum BDNF concentration. The OFT results suggested that allopurinol did not have any significant effects on motor activity. The increased serum level of the BDNF in the allopurinol group was correlated with FST and TST results. However, it is still not clear whether the antidepressant effects of allopurinol are due to a direct effects on serotonin and/or BDNF or an indirect effect related to its xanthin oxidase inhibition.
Keywords: Neurotrophic factor, Serum, Depression, Rat, Allopurinol, BDNF, Fluoxetine
Introduction
Depression is a widespread chronic psychiatric condition and disabling illness which can affect the physical, emotional, and social development of adults (Johnson et al., 2018, Stevanovic et al., 2011). It is characterized by persistent sadness which can lead to increased suicidal ideation in depressed individuals. The point prevalence of the disease has been reported 8–12 % in different countries (Al-Windi, 2005).
It is a complex condition with several contributing factors at different, genetic (Flint and Kendler, 2014, Shadrina et al., 2018), psychological, biological and social levels (Chirita et al., 2015, Ikechukwu Ugwua et al., 2022, Ben-Azu et al., 2020, Oladapo et al., 2021, Akinluyi et al., 2020, Nutt, 2002). Neuroimmune, neurotrophic and neurochemical homeostasis have important role in psychosocial stress-induced psychopathologies. It has been shown that neuroactive compounds, like quercetin, protects against psychosocial stress-induced psychiatric disturbances particularly via neurochemical mechanisms (Ikechukwu Ugwua et al., 2022). Targeting neuroinflammation and oxidative stress for the prevention of psychosocial stress-induced psychiatric disorders are potential strategies. In another study the effect of morin on social-defeat stress (SDS)-induced behavioral, neurochemical, neuroimmune and neuro-oxidative changes showed promising results (Ben-Azu et al., 2020, Akinluyi et al., 2020). Also, the effect of naringin on social-defeat stress (SDS)-induced behavioral, GABAergic, cholinergic, oxidative, nitrergic, and neuroinflammatory changes has been well established in psychiatric disturbances (Oladapo et al., 2021).
The currently available antidepressants needs long time to start any therapeutic effect. In addition, they have many unfavorable adverse effect profile. They may cause side effects such as gastric distress, sedation, palpitations, urinary retention, postural hypotension, sexual dysfunction, delirium and manic states, etc. Furthermore, the hepatic metabolism of some antidepressants is mainly dependent on the activity of hepatic cytochrome enzymes sometimes producing clinically significant drug interactions. Moreover, developing of tolerance or physical dependence to their effects have been reported (Al-Windi, 2005, Flint and Kendler, 2014, Cipriani et al., 2018).
Allopurinol which is an effective medication in the treatment of hyperuricemia and gout, is known to function as a xanthine oxidase enzyme inhibitor. Interestingly, high level of xanthine oxidase have been detected in depressed patients (Herken et al., 2007) and its role in depression is previously demonstrated by clinical and preclinical investigations (Gibney et al., 2014, Reus et al., 2015). It is reported that allopurinol can increase levels of tryptophan (TRP) which is positively correlated with the concentration of serotonin (Gurbuz Ozgur et al., 2015).
The brain-derived neurotrophic factor (BDNF) is one of the neurotrophies that is important in several mental disorders. Its expression and regulation could affect depressive-like behaviors in animal models and in depressed patients (Dwivedi, 2009, Yang et al., 2020). Many studies have shown the relation between serotonin level and serum level of BDNF (Nutt, 2002, Yang et al., 2020).
Therefore, the present work aimed to determine the antidepressant-like effects of allopurinol in chronic administration in FST, TST and OFT and to evaluate the changes in BDNF serum levels in the male albino rats.
Materials and methods
Animals and drug administration and design of study
Animals were prepared from Pasteur Institute of Tehran, Iran. They were kept with 12 h of light and 12 h of darkness, a constant temperature of 23 ± 1 °C, and humidity levels of 54 ± 1 %. Thirty-five male Wistar albino rats (8 weeks old) were divided into five groups: (control (D.W), 10 mg/kg fluoxetine (PAK-DAROU Pharmaceutical Co, Tehran Iran), 25, 50 and 100 mg/kg allopurinol (Hakim Pharmaceutical Co., Tehran, Iran); n = 7 per group). The allopurinol doses have been chosen based on previous work (Gurbuz Ozgur et al., 2015). They received all treatments intraperitoneally (i.p) every day. Forced swimming test (FST) tail suspension test (TST) and open field test (OFT) were performed once before and after 21 days of drug administration. Finally, the serum BDNF levels were measured using the sandwich ELISA method.
Animals’ maintenance and all the steps of the experiment were performed based on the ethical principles mentioned in the Helsinki Declaration related to the care and work of laboratory animals and were approved by the Ethics Committee in Animal Research of Tehran Islamic Azad University of Medical Sciences by ethic code of IR.IAU.PS.REC.1399.006.
Forced swimming test
One of the most valid and common tests for induction and evaluation of rodent depressive-like effects is the forced swimming test (FST). Briefly, the rats were placed individually into a cylindrical container made of acrylic glass with a diameter of 16 which was filled to a height of 30 cm with water of 23–24 °C for 6 min in exam day. After the first 2 min, a video tracking system recorded the animal immobility (in seconds) for the last 4 min. Conventionally, the cessation of movements of the rat's limbs and its floating is considered as immobility and its duration is considered as immobility time (Brandes et al., 1992).
Tail suspension test
Tail suspension test (TST) is also performed to assess the depressive features of rats and the extent of frustration and lack of struggle for survival. As described by Steru et al. each rat was suspended for 6 min by the tail (1–2 cm from the end of the tail) using adhesive tape. Likewise FST, after the first 2 min of familiarization with the situation, a video tracking system recorded the animal immobility (based on seconds) during 4 min (Steru et al., 1985). By definition, an animal was considered immobile when it ceased any movement in the body.
Open field test
The open field consists of a black plastic square board with a side size of 50 * 50 cm divided into 25 equal squares by white lines that is surrounded by 50 cm high black walls. Rats were placed in the arena and allowed to explore it for 6 min and the number of lines crossed is for last 4 min. The movements were recorded by a video tracking system and stored on a computer.
Sandwich ELISA for BDNF serum level evaluation
Finally, the animals were euthanized by Ketamine hydrochloride and Xylazin (100 mg/kg and 10 mg/kg, i.p., respectively), and blood samples were collected from their hearts then centrifuged for 10 min at 2000 rpm. We used a commercially available sandwich ELISA kit (EK-3127–97) as described by the manufacturer. Briefly, 50 μl of sequentially diluted Rat BDNF Standard was poured into each well of rabbit anti-human BDNF pre-coated 96-well plates. Then 40 μl of each serum sample was transferred to the wells and then 10 μl of biotin-labeled antibodies were added to each. In the next step, 50 μl of streptavidin-HPR was added to all wells and incubated for 60 min at 37 ℃. At this stage, plates were washed 5 times with buffer. Then 50 μl of chromogen A and 50 μl of chromogen B solutions were added to all wells, respectively. The microplate was then incubated for 10 min at 37 ℃ to determine the amount of BDNF in the serum sample, which is sandwiched between two antibodies. At the end of the incubation period, the stop solution was added to the plates. Finally, the microplate was placed in the ELISA reader (Vira-teb-tajiz, Iran) for 15 min at a wavelength of 450 nm.
Statistical analyses
Statistical analyses were performed in SPSS software version 25. In all cases, P value less than 0.05 considered significant. In data analysis, first, the normality of the data was examined using a single sample Kolmogorov-Smirnov test, with confirmation of the normality, appropriate parametric methods such as Student's t-test and analysis of variance were used, and in the absence of normal distribution, Kruskal-Wallis and Mann-Whitney were applied.
Results
Evaluation of depressive-like effects by FST
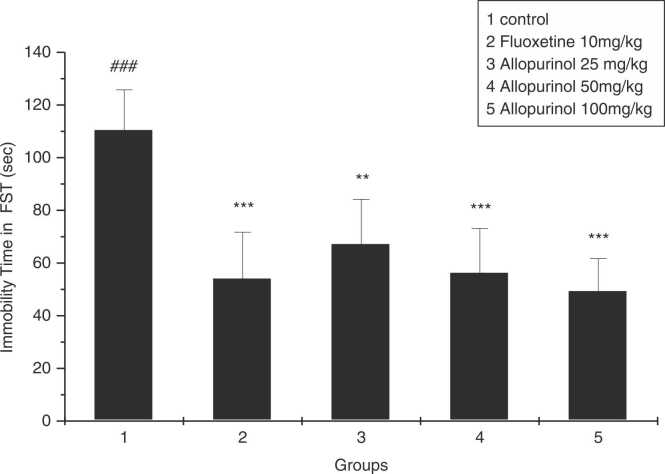
As shown in the Fig. 1, swimming times of rats received either fluoxetine 10 mg/kg) or allopurinol at all doses (25, 50, and 100 mg / kg) were significantly increased (dose-dependently in case of allopurinol) compared to the control group (P = 0.0001, P = 0.002, P = 0.0001, P = 0.0001, respectively).
Fig. 1.
The effect of allopurinol 25, 50, and 100 mg/kg, and fluoxetine 10 mg/kg after 3 weeks in immobility time in FST. * * p < 0.01 and * ** p < 0.001 compared to control group; ### p < 0.001 compared to fluoxetine group (n = 7).
Evaluation of motor function activities by OFT
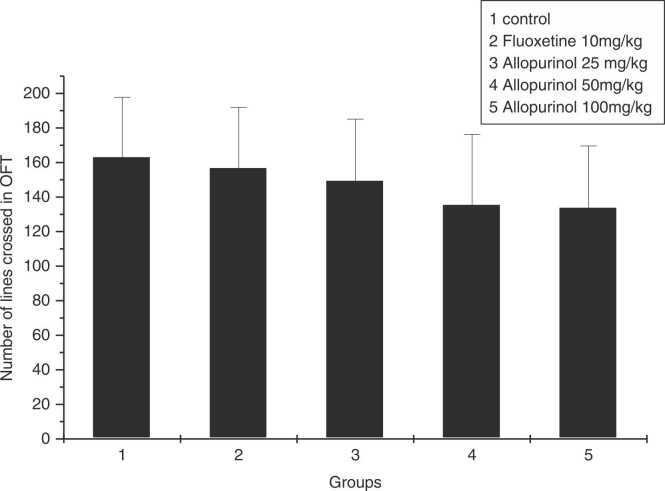
OFT was performed to assess the mobility of rats and exploration the field. Analysis revealed that there was no significant difference in the mean number of lines crosses in the OFT between diverse groups (p = 0.592). This indicates that neither fluoxetine nor allopurinol at doses of 25, 50, and 100 mg/kg had stimulatory effect on mobility of rats (Fig. 2).
Fig. 2.
The effect of allopurinol 25, 50, and 100 mg/kg, and fluoxetine 10 mg/kg after 3 weeks, in number of lines crossed in OFT None of the groups showed statistically different performance in OFT (n = 7 per group).
Evaluation of depressive-like behaviors in TST
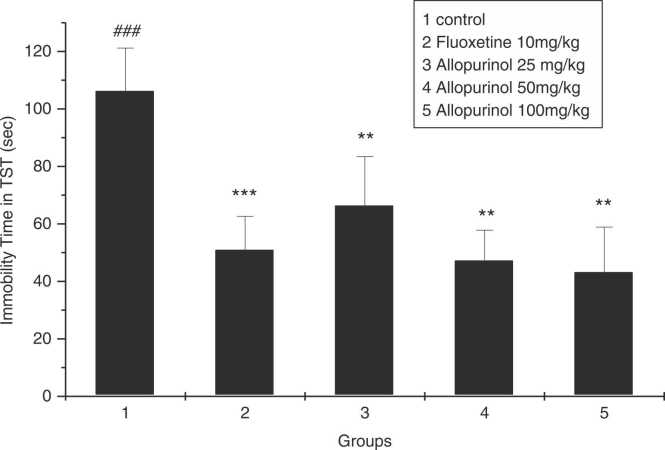
As shown in Fig. 3, all groups receiving allopurinol 25, 50, 100 mg/kg and fluoxetine 10 mg\kg had significantly reduced immobility time compared to the control group (P = 0.009, P = 0.004, P = 0.004, P = 0.0001 respectively), but these three groups did not have a significant difference in reducing the immobility time of rats compared to the group receiving fluoxetine (P = 0.177, P = 0.589, P = 0.537 respectively).
Fig. 3.
The effect of allopurinol 25, 50, and 100 mg/kg, and fluoxetine 10 mg/kg after 3 weeks in immobility time in TST. * * p < 0.01 and * ** p < 0.001 compared to control group; ### p < 0.001 compared to fluoxetine group (n = 7).
Changes in serum levels of BDNF using Sandwich ELISA
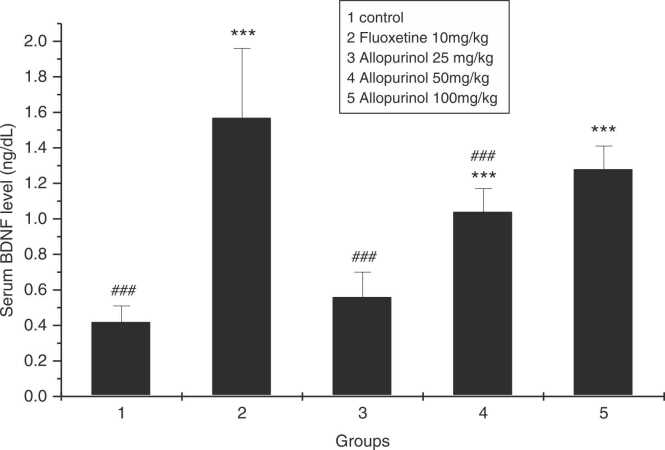
As it is reported in Fig. 4, BDNF concentration in the serum sample of rats which received allopurinol 25 mg/kg was not significantly different from the control group (P₌0.812), while BDNF serum levels in allopurinol 50 and 100 mg/kg was significantly different from the control group (P₌0.000 and P₌0.000, respectively). The serum level of BDNF in the group receiving fluoxetine was highest. Allopurinol 100 mg/kg showed a comparable effect on the BDNF serum level as well as the fluoxetine.
Fig. 4.
The effect of allopurinol 25, 50, and 100 mg/kg after 3 weeks, compared to fluoxetine and control groups in serum levels of BDNF. BDNF, Brain-derived neurotrophic factor; * ** p < 0.001 compared to control group; ## p < 0.01 compared to fluoxetine group; ### p < 0.001 compared to fluoxetine group (n = 7).
Discussion
In recent years, increasing attention has been paid to the antidepressant effects of atypical drugs. Allopurinol is a xanthine oxidase enzyme inhibitor that apart from its usefulness in gout, helps to improve various diseases, including hypoxia-ischemic encephalopathy in newborns (Annink et al., 2017), ischemia-reperfusion in various organs (Prickaerts et al., 2014), and had been shown to have pro-epileptic effects (Lakatos et al., 2018). Xanthine oxidase and xanthine has been reported to induce mood swings and depressive symptoms that can be due to oxidative stress in the brain (Salim et al., 2011).
The FST is the most commonly used model of depressive like behaviour in rodents. This test is very sensitive and relatively specific to all major classes of anti-depressant agents. It is widely used in rodents to predict anti-depressant potential of drugs. In the test, the immobility time of the animal in swimming is ascertained and a drug having anti-depressant like effect, decreases the immobility time. This immobility, referred to as behavioral despair in animals, is claimed to reproduce a condition similar to human depression (Gurbuz Ozgur et al., 2015).
In this study, the effects of allopurinol 25, 50, 100 mg/kg on depressive-like features has been examined and this is the first time that changes in serum BDNF also have been measured after 3 weeks in male Wistar rats and compared to fluoxetine and control groups. In all 3 groups that received allopurinol, a significant decrease in immobility time was observed dose dependently, in both FST and TST, which indicates an improvement in depressive symptoms similar to the group that received fluoxetine. Our results are in line with some published articles that mentioned the effect of allopurinol in CNS (Prickaerts et al., 2014, Kessing et al., 2019, Tovchiga and Shtrygol, 2012). Changes in serum BDNF level at higher doses of allopurinol (50 and 100 mg/kg) were also correlated with behavioral experiments. Allopurinol had no effect on movement profile of animals in OFT that means the drug lack any anxiogenic or excitatory activity. Based on the findings, 25 mg/kg of Allopurinol did not significantly increase serum BDNF compared to control group, even though the performance of these rats in FST and TST was significantly improved compared to controls. Therefore, non-BDNF related anti-depression mechanisms mediated by Allopurinol might be involved.
BDNF expression in brain is an important sign of depression since its reduction was associated with stress and depressive symptoms. Increased BDNF indicates improvement in mood and could be effective in depression treatment (Duman et al., 1997, Faraguna et al., 2008, Huber et al., 2007, Molteni et al., 2006). Yamgata et al. in 1999 proposed that BDNF has protective effects in TrkB-expressing PC12h cells against H2O2 free radicals and oxidative stress. One of the important sources of free radical production is the xanthine oxidase enzyme and BDNF is a factor that had been shown to do its effect by interrupting this enzyme’s activity (Yamagata et al., 1999). Therefore, it could be suggested that the increased serum level of BDNF (as an indicator for measuring the anti-depressants effect) is the result of inhibiting xanthine oxidase activity and free radicals’ production by allopurinol. In this regard, the results of our study revealed that higher doses of allopurinol, which increased the inhibition of xanthine oxidase, could be associated with increased serum levels of BDNF and improved depressive-like behaviors.
Another recent study also reported that tryptophan 2,3 dioxygenase activity, which metabolizes tryptophan, was reduced by xanthine oxidase inhibitors (including allopurinol), increased tryptophan and elevated serotonin in the brain and exerted antidepressant effects (Gurbuz Ozgur et al., 2015). In fact, our results also showed antidepressant effects in both behavioral and serum BDNF. In another study Karve et al. examined the antidepressant effects of allopurinol and Febuxostat using a forced swimming test and showed their strong effect on depressive-like behaviors (Kessing et al., 2019).
However, at pharmacological point of view, it is still not clear whether the antidepressant effects of allopurinol are due to a direct effects on the BDNF or an indirect effect related to its xanthin oxidase inhibition.
Conclusion
Allopurinol showed depressive-like features in rats and increased serum BDNF levels dose-dependently at high concentrations. This is the first time that effects of allopurinol on the serum level of BDNF is reported specially in chronic administration. The results compared with three behavioral well-known animal model tests (FST, TST and OFT) and the correlation with them at high doses is reported. Besides, allopurinol had no effects on movement profile of animals with no stimulatory action. However, the exact mechanism of allopurinol in rising BDNF is not clear. Regarding lower concentration of allopurinol, other mechanisms might be involved rather than BDNF rising.
CRediT authorship contribution statement
M H Jahromy: conceptualization, funding acquisition, Data curation, experiment supervising. B Baghchesara: conceptualization, funding acquisition, Data curation, performing the experiment. S Javanshir: writing original draft, review and editing, Data analysis. R Sarallah: writing – original draft, review and editing, Data analysis.
Conflicts of Interest
The authors of this study had no conflict of interest to declare.
References
- Akinluyi Elizabeth, Aderibigbe A., Adeoluwa O., Ben-Azu B. Morin hydrate attenuates chronic stress-induced memory impairment and degeneration of hippocampal subfields in mice: the role of oxidative, nitrergic and neuroinflammatory pathways. Metab. Brain Dis. 2020;35:1145–1156. doi: 10.1007/s11011-020-00595-2. [DOI] [PubMed] [Google Scholar]
- Al-Windi A. Depression in general practice. Nord J. Psychiatry. 2005;59(4):272–277. doi: 10.1080/08039480500227733. [DOI] [PubMed] [Google Scholar]
- Annink K.V., Franz A.R., Derks J.B., Rudiger M., Bel F.V., Benders M. Allopurinol: old drug, new indication in neonates? Curr. Pharm. Des. 2017;23(38):5935–5942. doi: 10.2174/1381612823666170918123307. [DOI] [PubMed] [Google Scholar]
- Ben-Azu B., Emokpae O., Mayowa Ajayi A., et al. Repeated psychosocial stress causes glutamic acid decarboxylase isoform-67, oxidative-Nox-2 changes and neuroinflammation in mice: prevention by treatment with a neuroactive flavonoid, morin. Brain Res. 2020;1744(1) doi: 10.1016/j.brainres.2020.146917. [DOI] [PubMed] [Google Scholar]
- Brandes L.J., Arron R.J., Bogdanovic R.P., Tong J., Zaborniak C.L., Hogg G.R., et al. Stimulation of malignant growth in rodents by antidepressant drugs at clinically relevant doses. Cancer Res. 1992;52(13):3796–3800. [PubMed] [Google Scholar]
- Chirita A.L., Gheorman V., Bondari D., Rogoveanu I. Current understanding of the neurobiology of major depressive disorder. Rom. J. Morphol. Embryol. 2015;56(2 Suppl):651–658. [PubMed] [Google Scholar]
- Cipriani A., Furukawa T.A., Salanti G., Chaimani A., Atkinson L.Z., Ogawa Y., et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Focus (Am. Psychiatr. Publ.) 2018;16(4):420–429. doi: 10.1176/appi.focus.16407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman R.S., Heninger G.R., Nestler E.J. A molecular and cellular theory of depression. Arch. Gen. Psychiatry. 1997;54(7):597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y. Brain-derived neurotrophic factor: role in depression and suicide. Neuropsychiatr. Dis. Treat. 2009;5:433–449. doi: 10.2147/ndt.s5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraguna U., Vyazovskiy V.V., Nelson A.B., Tononi G., Cirelli C. A causal role for brain-derived neurotrophic factor in the homeostatic regulation of sleep. J. Neurosci. 2008;28(15):4088–4095. doi: 10.1523/JNEUROSCI.5510-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint J., Kendler K.S. The genetics of major depression. Neuron. 2014;81(3):484–503. doi: 10.1016/j.neuron.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibney S.M., Fagan E.M., Waldron A.M., O'Byrne J., Connor T.J., Harkin A. Inhibition of stress-induced hepatic tryptophan 2,3-dioxygenase exhibits antidepressant activity in an animal model of depressive behaviour. Int. J. Neuropsychopharmacol. 2014;17(6):917–928. doi: 10.1017/S1461145713001673. [DOI] [PubMed] [Google Scholar]
- Gurbuz Ozgur B., Aksu H., Birincioglu M., Dost T. Antidepressant-like effects of the xanthine oxidase enzyme inhibitor allopurinol in rats. A comparison with fluoxetine. Pharm. Biochem. Behav. 2015;138:91–95. doi: 10.1016/j.pbb.2015.09.016. [DOI] [PubMed] [Google Scholar]
- Herken H., Gurel A., Selek S., Armutcu F., Ozen M.E., Bulut M., et al. Adenosine deaminase, nitric oxide, superoxide dismutase, and xanthine oxidase in patients with major depression: impact of antidepressant treatment. Arch. Med. Res. 2007;38(2):247–252. doi: 10.1016/j.arcmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Huber R., Tononi G., Cirelli C. Exploratory behavior, cortical BDNF expression, and sleep homeostasis. Sleep. 2007;30(2):129–139. doi: 10.1093/sleep/30.2.129. [DOI] [PubMed] [Google Scholar]
- Ikechukwu Ugwua P., Ben-Azuc B., Ugonne Ugwub S., et al. Preventive putative mechanisms involved in the psychopathologies of mice passively coping with psychosocial defeat stress by quercetin. Brain Res. Bull. 2022;183(1):127–141. doi: 10.1016/j.brainresbull.2022.03.004. [DOI] [PubMed] [Google Scholar]
- Johnson D., Dupuis G., Piche J., Clayborne Z., Colman I. Adult mental health outcomes of adolescent depression: a systematic review. Depress Anxiety. 2018;35(8):700–716. doi: 10.1002/da.22777. [DOI] [PubMed] [Google Scholar]
- Kessing L.V., Rytgaard H.C., Gerds T.A., Berk M., Ekstrom C.T., Andersen P.K. New drug candidates for depression - a nationwide population-based study. Acta Psychiatr. Scand. 2019;139(1):68–77. doi: 10.1111/acps.12957. [DOI] [PubMed] [Google Scholar]
- Lakatos R.K., Dobolyi A., Kovacs Z. Uric acid and allopurinol aggravate absence epileptic activity in Wistar Albino Glaxo Rijswijk rats. Brain Res. 2018;1686:1–9. doi: 10.1016/j.brainres.2018.02.012. [DOI] [PubMed] [Google Scholar]
- Molteni R., Calabrese F., Bedogni F., Tongiorgi E., Fumagalli F., Racagni G., et al. Chronic treatment with fluoxetine up-regulates cellular BDNF mRNA expression in rat dopaminergic regions. Int. J. Neuropsychopharmacol. 2006;9(3):307–317. doi: 10.1017/S1461145705005766. [DOI] [PubMed] [Google Scholar]
- Nutt D.J. The neuropharmacology of serotonin and noradrenaline in depression. Int. Clin. Psychopharmacol. 2002;17(1):S1–S12. doi: 10.1097/00004850-200206001-00002. [DOI] [PubMed] [Google Scholar]
- Oladapo O., Ben-Azu B., Mayowa Ajayi A. Naringin confers protection against psychosocial defeat stress-induced neurobehavioral deficits in mice: involvement of glutamic acid decarboxylase isoform-67, oxido-nitrergic stress, and neuroinflammatory mechanisms. J. Mol. Neurosci. 2021;71:431–445. doi: 10.1007/s12031-020-01664-y. [DOI] [PubMed] [Google Scholar]
- Prickaerts J., Gieling E.T., Bruder A.K., van der Staay F.J., Vanmierlo T. Long-term effects of prenatal allopurinol treatment on brain plasticity markers in low and normal birth weight piglets. Int. J. Dev. Neurosci. 2014;33:29–32. doi: 10.1016/j.ijdevneu.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Reus G.Z., Jansen K., Titus S., Carvalho A.F., Gabbay V., Quevedo J. Kynurenine pathway dysfunction in the pathophysiology and treatment of depression: Evidences from animal and human studies. J. Psychiatr. Res. 2015;68:316–328. doi: 10.1016/j.jpsychires.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim S., Asghar M., Taneja M., Hovatta I., Chugh G., Vollert C., et al. Potential contribution of oxidative stress and inflammation to anxiety and hypertension. Brain Res. 2011;1404:63–71. doi: 10.1016/j.brainres.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadrina M., Bondarenko E.A., Slominsky P.A. Genetics factors in major depression disease. Front Psychiatry. 2018;9:334. doi: 10.3389/fpsyt.2018.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steru L., Chermat R., Thierry B., Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacol. (Berl.) 1985;85(3):367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Stevanovic D., Jancic J., Lakic A. The impact of depression and anxiety disorder symptoms on the health-related quality of life of children and adolescents with epilepsy. Epilepsia. 2011;52(8):e75–e78. doi: 10.1111/j.1528-1167.2011.03133.x. [DOI] [PubMed] [Google Scholar]
- Tovchiga O., Shtrygol S. The influence of oxonate-induced hyperuricemia and allopurinol on behavioral reactions of random-bred mice. J. Basic Clin. Physiol. Pharm. 2012;23(4):147–151. doi: 10.1515/jbcpp-2012-0027. [DOI] [PubMed] [Google Scholar]
- Yamagata T., Satoh T., Ishikawa Y., Nakatani A., Yamada M., Ikeuchi T., et al. Brain-derived neurotropic factor prevents superoxide anion-induced death of PC12h cells stably expressing TrkB receptor via modulation of reactive oxygen species. Neurosci. Res. 1999;35(1):9–17. doi: 10.1016/s0168-0102(99)00062-0. [DOI] [PubMed] [Google Scholar]
- Yang T., Nie Z., Shu H., Kuang Y., Chen X., Cheng J., et al. The role of BDNF on neural plasticity in depression. Front Cell Neurosci. 2020;14:82. doi: 10.3389/fncel.2020.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]