Abstract
The nutritional and phytochemical content of foxtail millet (Cetaria italica) makes it a viable food grain. In this study, we looked at foxtail millet in Bangladesh and analyzed its nutritional value, functional and physical characteristics. In addition, methanol, ethanol, and acetone: water: acetic acid (70: 29.50: 0.50) extracts of foxtail millet flour (FMF) were analyzed for their antioxidant properties (total phenolic and flavonoid content, total antioxidant capacity, ferric reducing antioxidant power (FRAP) assay, and 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity). According to this study, foxtail millet has favorable physiological and functional properties. FMF had protein at 11.65 ± 0.45 g/100 g, fat at 3.48 ± 0.04 g/100 g, carbohydrates at 75.33 ± 0.53 g/100 g, and crude fiber at 2.21 ± 0.03 g/100 g. Calcium was found at 47 ± 0.48 mg/100 g, iron at 4.59 ± 0.14 mg/100 g, potassium at 393 ± 15.87, sodium at 27.4 ± 1.21, magnesium at 45.40 ± 2.22, manganese at 0.71 ± 0.02, copper at 0.58 ± 0.04 and zinc at 2.30 ± 0.18 mg/100 g. The total flavonoid content (TFC) of the methanolic extract (68.26 ± 1.51 mg quercetin equivalents (QE)/100 g) was significantly (p < 0.05) higher than the extract of acetone: water: acetic acid. Total antioxidant capacity (TAC) (169.40 ± 3.45 mg ascorbic acid equivalents (AAE)/100 g) and total phenolic content (TPC) (51.35 ± 1.35 mg gallic acid equivalents (GAE)/100 g) of the methanolic extracts were significantly (p < 0.05) higher than others. The ascending order of DPPH free radical scavenging activity of FMF extract is as follows: acetone: acetic acid: water < ethanol < methanol. In the ferric reducing antioxidant power (FRAP) test, the reducing power of FMF extracts increased with the rise in sample concentration. Foxtail millet has potential as a functional food that could influence rural residents' diets and health.
Keywords: Foxtail millet, Antioxidant activity, Nutritional properties, Functional properties
Foxtail millet; Antioxidant activity; Nutritional properties; Functional properties.
1. Introduction
Millets are a type of small seeded cereals that belongs to the poacea family. There are several varieties of millets. Pearl millet (Pennisetum glaucum), foxtail millet (Setaria italica), finger millet (Eleusine coracana), kodo millet (Paspalum scrobiculatum), proso millet (Panicum miliaceum), little millet (Panicum sumatrense), and barnyard millet (Echinochloa utilis) are the different varieties of millets (Saleh et al., 2013). The world's oldest crop is foxtail millet (Austin, 2006). This crop has been cultivated in China for 8000 years and presently grown in semi-arid and tropical regions of Africa, Asia, Australia, South America and especially in China, India, Mali, Nigeria, and Niger (Liang et al., 2018; Saleh et al., 2013). Foxtail millet has attracted research attention due to its short growing season, insect and disease resistance, high salinity stress tolerance, photosynthesis efficacy, nutritional and medicinal properties (Adekunle et al., 2013; Liu et al., 2011; Vetriventhan et al., 2012). Currently, millet is the second most-produced crop in the world, and foxtail millet is grown in 26 countries (Sharma & Niranjan, 2018). Foxtail millet (Setaria italic (L.) P. Beauv.) also called 'Kaon,' is an important crop in Bangladesh. In Bangladesh foxtail millets are mainly cultivated both Rabi and Kharif seasons in all types of soil as sole or mixed crop with chilli, aush rice, seasame, mustard etc. But the main area is mostly concentrated to flood prone riverbeds and marginal lands (Karim et al., 1993). Foxtail millet is extremely important to the food and agricultural industries in many third world countries because it can be grown in a wide variety of soils and agro-climatic conditions (Meherunnahar et al., 2018). Foxtail millet is a highly nutritious, gluten-free, and non-acid-forming food which is easy to digest. It is a low glycemic index food, so it may be an ideal food for celiac diseases and diabetes patients (Ramashia et al., 2019). Millets have phytochemicals, phenolic acids, flavonoids, essential amino acids, vitamin B, and minerals like iron, potassium, phosphorus, calcium, and zinc (Bandyopadhyay et al., 2017; Saleh et al., 2013; Singh et al., 2012). Foxtail millet contains crude fiber, which aids digestion and helps to promote bowel movement (Sharma & Niranjan, 2018). Due to its nutritional value, foxtail millet has become an important ingredient in biscuits, noodles, soups, drinks and cakes (Yang et al., 2013). Additionally, foxtail millet has several health advantages, such as cancer and cardiovascular disease prevention, reducing heart attack risk, helping in weight loss, and reducing the level of lipids in the blood (Gupta et al., 2012; A. Zhang et al., 2015). Geographical location can affect millets' chemical composition with some functional ingredients (Kitta et al., 2005; Wen et al., 2014). The type of extraction solvent has an impact on the yield of phenolic constituents from plant material. Researchers are currently attempting to generate data on the optimal extraction conditions for various plant matrices based on their polarity (polar, non-polar) and chemical structural properties (hydrophilic and hydrophobic) (Shaheen et al., 2012; Złotek et al., 2016). The distribution and amount of these bioactive compounds vary according to millet type and species (Akanbi et al., 2019; Banerjee et al., 2012; Shahidi and Chandrasekara, 2013). This study was conducted by the extraction of compounds from foxtail millets using three different solvents in the same experiment. Although foxtail millet has been well studied in different countries, limited study exists with exploring Bangladeshi foxtail millet. This study aims to assess the nutritional, functional, physical, and antioxidant characteristics of foxtail millet.
2. Materials and methods
2.1. Chemicals and reagents
Purchased from Sigma–Aldrich were the following chemicals: Folin–(FC) Ciocalteu's reagent, DPPH (1,1-diphenyl-2-picryl-hydrazyl), ascorbic acid, quercetin, and gallic acid (USA). The following chemicals were supplied by Merck (Germany): acetic acid, acetone, aluminium nitrate, ammonium molybdate, disodium hydrogen phosphate, ethanol, iron (III) chloride, methanol, potassium acetate, potassium ferricyanide, sodium carbonate, sodium nitrite, sulfuric acid, sodium dihydrogen phosphate, toluene, and trichloroacetic acid.
2.2. Preparation of foxtail millet flour (FMF)
Foxtail millet (variety: Bari Kaon-2) was collected from the Panchagarh district of Bangladesh. The coordinates of Panchagarh are 26° 20′ 7.3572″ North and 88° 33′ 6.1092″ East. First, the grain of foxtail millet is cleaned and rinsed. They were then dried in an oven at 600C for 8 h before being processed into flour using an electric grinder. Finally, sieves with a 60 micron (m) opening were used to pass the powder through the flour. In accordance with the flowchart in Figure 1, foxtail millet flour (FMF) was prepared. The FM flours were stored in sealed containers for further researches.
Figure 1.
Flow diagram for the preparation of foxtail millet flour (FMF).
2.3. Physical properties
By weighing the sample on a 4 digit electronic balance (ViBRA, Japan), and manually counting the number of grains, the weight per thousand grains was calculated (Balasubramanian and Viswanathan, 2010). The bulk density of foxtail millet grain was measured by the Appiah et al. (2011) method. The true density was measured using the toluene displacement method (Sunil et al., 2016). The following formula (equation-1) was used to determine the porosity (Balasubramanian and Viswanathan, 2010).
| (1) |
2.4. Functional properties
According to the method of Yousf et al. (2017) the water solubility index (WSI) and water absorption index (WAI) of FMF were measured. The methods of Siroha et al. (2016) were used to calculate the water absorption capacity (WAC) and oil absorption capacity (OAC) of FMF with minor modifications. Yasumatsu et al. (1972) methods were used to measure emulsion activity (EA) and emulsion stability (ES) of FMF. The Narayana and Narasinga Rao (1982) methods were used to measure the foaming capacity (FC) and foaming stability (FS) of FMF, with some minor modification. The method of Chandra et al. (2015) with some modification was used to calculate the swelling capacity (SC) of FMF.
2.5. Nutritonal analysis
2.5.1. Proximate
The proximate composition of FMF, including moisture, ash, protein, fat, and crude fiber was calculated using the AOAC (2005) standard analytical method. Drying the material at 1050C until a consistent mass was produced allowed us to determine the moisture content. After burning the sample for 8 h at 650 – 7000C, the ash was quantified by weighing the remaining residue. The Soxhlet technique was used to extract crude fat using petroleum ether and the standard AOAC (2005) method was used to assess crude fiber. The Micro-Kjeldahl method (N × 6.25) was used to calculate the crude protein. According to AOAC (2005) the quantity of carbohydrates in a food may be calculated by taking the total percentage of other ingredients, like moisture, ash, crude fiber, fat, and protein, and deducting that number from 100. Carbohydrate (%) = 100 – (% moisture + % fat + % crude fiber + % protein + % ash). Using the following formula, we were able to determine the total energy value of the samples: total energy (Kcal/100 g) = (% carbs + % protein) X 4 + (% fat × 9) (Farzana & Mohajan, 2015).
2.5.2. Mineral content
Mineral samples of the FMF were prepared by standard analytical methods according to AOAC (2005). A flame photometer (JENWAY, Model: PFP7, Germany) was used to measure sodium, calcium, and potassium, while an atomic absorption spectrophotometer (Thermo, Model: ICS-3000, USA) was used to measure iron, magnesium, manganese, copper, and zinc. Each mineral was diluted from standard stock solutions (1000 μg/mL) to prepare working solutions. In this process, approximately 2 g of FMF are placed in a crucible and burned in a muffle furnace at 650 – 7000C to form greyish ash. After cooling, the ash was dissolved in 3 mL of concentrated HCl and dried at a low temperature in a water bath. The remaining parts of the crucible were washed multiple times with de-ionized water to evaporate until the liquid became colorless. The solution was then filtered using ashless Whatman filter paper, and the total volume was made up to 100 mL with de-ionized water. To measure the concentrations of the different minerals, aliquots of this solution were used.
2.6. Antioxidant properties
2.6.1. Extraction procedure
For FMF (20 g) extraction, different solvents like methanol, ethanol, and acetone: water: acetic acid (70:29.5:0.5) were used. The mixture was shaken on a mechanical shaker for 48 h, then filtered and dried on a rotary evaporator (Stuart, UK) under reduced pressure (Khan et al., 2020; Shaheen et al., 2012; Złotek et al., 2016). The concentrated foxtail millet extract were stored at 40C. The antioxidant properties of methanolic (FMME), ethanolic (FMEE), and acetone: water: acetic acid (FMAE) extracts were tested by dissolving them in methanol at a 10 mg/ml concentration.
2.6.2. Determination of total phenolic content
A slightly modified folin-ciocalteu colorimetric method (Salar et al., 2012) was used to measure the total phenolic content (TPC). In brief, 1 mL of folin-ciocalteu reagent, 0.5 mL of extract (10 mg/mL), and 4.5 mL of distilled water were mixed together and incubated for 10 min at room temperature. After that, a 2.0 mL solution of 35% Na2CO3 was added, and the mixture was left to stand at room temperature for half an hour. Using a spectrophotometer (Specord 205, Analytik Jena, UK), absorbance at 765 nm was measured against a blank. Using a similar technique, a standard curve of gallic acid solutions at concentrations of 20, 40, 60, 80, and 100 mg/mL was prepared. Using the calibration curve, the total phenolic content of the sample extracts was calculated. The results were presented as mg of gallic acid equivalent per 100 g of sample (mg GAE/100 g).
2.6.3. Determination of total flavonoid content
The total flavonoid content (TFC) was measured using a slightly modified Eom et al. (2008) method. In brief, 0.1 mL of 1 M potassium acetate and 0.1 mL of 10% aluminum nitrate were mixed with an aliquot of 1 mL of the sample (10 mg/mL). The volume of the resulting solution was adjusted to 5 mL by adding 3.8 mL of methanol. After 30 min, absorbance at 430 nm was measured against a blank using a spectrophotometer. A standard curve of quercetin solutions (at concentrations of 20, 40, 60, 80, and 100 mg/mL) was prepared using a similar method. The calibration curve was used to calculate the total flavonoid content of the sample extracts. The results were represented as mg of quercetin equivalent per 100 g of sample (mg QE/100 g).
2.6.4. Determination of total antioxidant capacity
The method of Prieto et al. (1999), with a few changes, was used to measure the total antioxidant capacity (TAC). To sum up, 5 mL of the reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate) was added to 0.5 mL of the extract (10 mg/mL). The tubes were covered and incubated at 950C for 90 min. After cooling the sample, the absorbance was measured at 695 nm against a blank using a spectrophotometer. A standard curve for ascorbic acid solutions of 20, 40, 60, 80, and 100 mg/mL was prepared using a similar procedure. The calibration curve was used to calculate the total antioxidant capacity of the sample extracts. The antioxidant capacity was represented as mg of ascorbic acid equivalent per 100 g of sample (mg AAE/100 g).
2.6.5. DPPH radical scavenging activity
The FMF extract was tested for 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity as published by Pushparaj and Urooj (2014), with modifications. In a nutshell, 0.1 mM methanolic DPPH solution (4 mL) was combined with 1 mL of different doses of sample solution. The mixture was vortexed and left for 30 min at room temperature in the dark. The absorbance of the solutions was measured at 517 nm using a spectrophotometer and an appropriate blank. The activity was calculated using the following formula (eq. (2)):
| (2) |
where, A0 = Absorbance of control, and A1 = Absorbance of sample.
Instead of sample extract, 1.0 mL of ascorbic acid solutions (1–100 μg/mL) was used for standard testing. The IC50 (Half Inhibitory Concentration) value in the DPPH free radical scavenging method is the sample concentration that scavenged 50% of the DPPH free radical and was calculating using logarithmic regression.
2.6.6. Ferric reducing antioxidant power (FRAP) assay
We used the method developed by Wu et al. (2003) to determine the ferric reducing antioxidant power (FRAP) of FMF extract. In a nutshell, a 0.2 M phosphate buffer (pH 6.6) and 1% (w/v) potassium ferricyanide were added to a 2.5 mL extract. A total of 20 min were spent in a 500C incubator with the mixture. After cooling the solution, trichloroacetic acid (2.5 ml, 10% w/v) was added. For 10 min, the mixture was centrifuged at 3000 rpm. The supernatant was then combined with 2.5 ml of deionized water and 0.5 ml of a 0.1% (w/v) FeCl3 solution. After 10 min, the spectrophotometer reading for absorbance at 700 nm was taken. The high reducing power of the sample extracts was evidenced by the high absorbance of the reaction mixture.
2.7. Statistical analysis
Data were reported as mean ± SD (standard deviation) for three replications. To determine significant differences between the mean values, analysis of variance (one-way ANOVA) was performed using SPSS software (version 22.0).
3. Results and discussions
3.1. Physical properties of foxtail millet
The physical properties such as the thousand grain weight, thousand grain volume, true density, bulk density, and porosity of foxtail millet grain were observed in 1.46 ± 0.06 g, 1.70 ± 0.03 mL, 1.37 ± 0.01 g/cm3, 0.85 ± 0.01 g/cm3, and 38.00 ± 0.01% respectively (Table 1). According to Sunil et al. (2016) the thousand grain weight, true density, bulk density, and porosity of foxtail millet were observed 2.45 g, 1.26 g/cm3, 0.737 g/cm3, and 41.47 % respectively. Gouda et al. (2019) observed that thousand grain weight, true density, bulk density, and porosity of foxtail millet were 2.36 g, 1.196 g/cm3, 0.724 g/cm3, and 39.46 ± 2.20% respectively. The differences in the physical parameters of the study might be due to variations in the geographical location, environmental, and climate conditions (Wen et al., 2014).
Table 1.
Physical properties of foxtail millet grain.
| Parameters | Quantity |
|---|---|
| Thousand grain weight (g): | 1.46 ± 0.06 |
| Thousand grain volume (mL): | 1.70 ± 0.03 |
| Bulk density (g/cm3): | 0.85 ± 0.01 |
| True density (g/cm3): | 1.37 ± 0.01 |
| Porosity (%): | 38.00 ± 0.01 |
∗Each value represents the average of three determinations ± SD.
3.2. Functional properties of FMF
The functional properties of flour are important in the manufacture, flavor, taste, texture, stability, storage, and, transportation of food items. These characteristics depend on the chemical composition, variety, type, and particle size of flour. Functional properties are important physico-chemical properties that indicate the intricate interactions between the molecular conformation, composition, and structure of food components (Awuchi et al., 2019).
WSI and WAI are important characteristics for cereal flours used in beverage preparation. WAI indicates the portion of water absorbed by the flour, whereas WSI indicates water soluble ingredients in the flour. The WAI and WSI of FMF were observed at 7.16 ± 0.17 g/100 g, and 3.05 ± 0.18 g/100 g, respectively (Table 2). According to Devisetti et al. (2014) the WSI of FM is in the range of 2.55 – 3.92 g/100 g.
Table 2.
Functional properties of FMF.
| Parameters | Quantity |
|---|---|
| WAC (g/100 g): | 152.35 ± 2.81 |
| OAC (mL/100 g): | 79.38 ± 0.63 |
| WAI (g/100 g): | 7.16 ± 0.17 |
| WSI (g/100 g): | 3.05 ± 0.18 |
| EA (mL/100 mL): | 45.01 ± 0.24 |
| ES (mL/100 mL): | 37.27 ± 0.22 |
| FC (mL/100 mL): | 7.11 ± 0.11 |
| FS (mL/100 mL): | 46.90 ± 1.25 |
| SC (%): | 7.98 ± 0.31 |
∗Each value represents the average of three determinations±SD.
Here, WAI = Water absorption index, WSI = Water solubility index, WAC = Water absorption capacity, OAC = Oil absorption capacity, EA = Emulsion activity, ES = Emulsion stability, FC = Foaming capacity, FS = Foaming stability, SC = Swelling capacity.
The flour's water absorption capacity was 152.35 ± 2.81 g/100 g (Table 2). According to Devisetti et al. (2014) the WAC of foxtail millet ranged from 139.4 – 168.8 g/100 g. In food formulations, especially in the case of dough and finished products, water absorption capacity is an important functional property (Awuchi et al., 2019). Due to the high content of hydrophilic polysaccharides and proteins, especially polar amino acid residues in the flour, water absorption capacity may be higher. The quality of food products can be negatively impacted by very low or excessive water absorption.
The Oil absorption capacity is an important functional characteristic that improves the feeling in the mouth while maintaining the taste of food (Awuchi et al., 2019). The oil absorption capacity of the flour was 79.38 ± 0.63 mL/100 g (Table 2). Devisetti et al. (2014) reported that the OAC of foxtail millet was in the range of 69.6 – 91.1 mL/100 mL. Kamara et al. (2009) observed that the OAC of two FMF varieties was 78 and 50 mL/100 g. The water and oil absorption capacities of flour are influenced by surface polarity, amino acid composition, and protein conformation (Chandra, 2013). The flour's high water and oil absorption capacity can improve the flavor, moisture, and fat content of food.
Foaming properties are desirable in bakery food products to maintain their texture and structure during processing and storage. Protein is mainly responsible for foaming. The cereal protein in the dispersion produces a continuous cohesive layer surrounding the air bubbles in the foam. Non-polar residues in protein increase the stability of the foam film while polar residues reduce it. For flours used in the production of various baked goods like cookies, muffins, cakes, etc., good foam capacity and stability are desired characteristics (Awuchi et al., 2019). The FC and FS of FMF were 7.11 ± 0.10 and 46.90 ± 1.25 mL/100 mL, respectively (Table 2). Devisetti et al. (2014) reported that the FC and FS ranges for foxtail millets were 4.2 – 10.2 and 22.5 – 58.4 mL/100 mL, respectively. Meherunnahar et al. (2018) pointed out that a protein's solubility, concentration, and other factors affect the protein's ability to foam and its stability.
An emulsion is a fluid system in which one liquid (dispersed phase) is dispersed into another liquid (continuous phase), and the stability of emulsion is the ability of a food emulsion to resist any change in its properties over time (Yasumatsu et al., 1972). The EA and ES of FMF were 45.01 ± 0.24 mL/100 mL and 37.27 ± 0.22 mL/100 mL, respectively (Table 2). According to Meherunnahar et al. (2018), the EA and ES of foxtail millet were 40.01% and 41.41%, respectively. Devisetti et al. (2014) observed that the ES of foxtail millets is in the range of 32.4 – 53.4 mL/100 mL. Protein hydrophobicity has been related to their emulsifying characteristic. These characteristics are influenced by a variety of factors, such as concentration, pH, and solubility. The ability of proteins to enhance the composition and stability of emulsions is important for many food products applications, such as frozen desserts, coffee, whiteners, and cakes.
The SC of FMF was 7.98 ± 0.30% (Table 2). Ushakumari et al. (2004) found that the SC of FMF was 7.1 ± 0.01. Swelling capacity indicates the presence of non-covalent interactions between the starch molecules. The swelling capacities of flours are affected by the variety of species, processing method and particle size. The high swelling capacity of the floor is considered more suitable for the preparation of bakery products.
3.3. Proximate and mineral analysis of FMF
The proximate composition of FMF were moisture (5.94 ± 0.12 g/100 g), ash (1.39 ± 0.03 g/100 g), protein (11.65 ± 0.45 g/100 g), fat (3.48 ± 0.04 g/100 g), crude fiber (2.21 ± 0.03 g/100 g), carbohydrates (75.33 ± 0.53 g/100 g), and energy (379.23 ± 0.59 kcal/100 g) (Table 3). Liang et al. (2018) reported that the values of ash (1.39 g/100 g), protein (10.28 g/100 g), fat (3.40 g/100 g), dietary fiber (2.37 g/100 g) for FMF. According to Sharma and Niranjan (2018) the proximate composition were protein (12.3 g/100 g), fat (4.3 g/100 g), crude fiber (8 g/100 g), carbohydrates (60.90 g/100 g), and energy (351 kcal/100 g) for FMF. Whereas, according to Chen et al. (2013) the protein, carbohydrate, and crude fat contents of the various foxtail millet cultivars ranged from 9.5 – 18.9 g/100 g, 71.5 – 83.8 g/100 g, and 4.4 – 7.3 g/100 g, respectively. Mohamed et al. (2009) showed that the proximate composition were protein (11.41 ± 0.15 g/100 g), fat (2.91 ± 0.35 g/100 g), crude fiber (1.92 ± 0.02 g/100 g), and carbohydrates (73.00 ± 0.14 g/100 g) for FMF.
Table 3.
Proximate and mineral composition of FMF.
| Nutrient | ∗Mean Value | |
|---|---|---|
| Macronutrients | Moisture (g/100 g): | 5.94 ± 0.12 |
| Ash (g/100 g): | 1.39 ± 0.03 | |
| Protein (g/100 g): | 11.65 ± 0.45 | |
| Fat (g/100 g): | 3.48 ± 0.04 | |
| Crude fiber (g/100 g): | 2.21 ± 0.03 | |
| Carbohydrate (g/100 g): | 75.33 ± 0.53 | |
| Energy (Kcal/100 g): | 379.23 ± 0.59 | |
| Micronutrients | Sodium (mg/100 g): | 27.4 ± 1.21 |
| Potassium (mg/100 g): | 393 ± 15.87 | |
| Calcium m (g/100 g): | 47 ± 0.48 | |
| Iron (mg/100 g): | 4.59 ± 0.14 | |
| Copper (mg/100 g): | 0.58 ± 0.04 | |
| Magnesium (mg/100 g): | 45.40 ± 2.22 | |
| Manganese (mg/100 g): | 0.71 ± 0.02 | |
| Zinc (mg/100 g): | 2.30 ± 0.18 | |
Each value represents the average of three determinations ± SD.
The mineral content of FMF viz., sodium, calcium, potassium, iron, manganese, magnesium, zinc, and copper were 27.4 ± 1.21, 47 ± 0.48, 393 ± 15.87, 4.59 ± 0.14, 0.71 ± 0.02, 45.40 ± 2.22, 2.30 ± 0.18, and 0.58 ± 0.04 mg/100 g respectively (Table 3). According to Kamara et al. (2009) the value of sodium, calcium, potassium, iron, manganese, magnesium, zinc, and copper were 10.91, 21.17, 200.24, 2.03, 0.70, 98.3, 3.34, and 0.75 mg/100 g respectively. Kola et al. (2020) reported that the values of calcium, potassium, iron, manganese, zinc, and copper were 13.13 – 39.58, 219.43 – 349.47, 219.43 – 349.47, 3.69 – 7.51, 1.05 – 1.64, 4.54 – 5.71, and 0.60 – 1.09 mg/100 g among the 24 different varieties of foxtail millet. Vali Pasha et al. (2018) reported that the values of sodium, calcium, potassium, iron, manganese, magnesium, zinc, and copper were 54 – 62, 19 – 23, 543 – 923, 20.8 – 38.6, 2.4 – 3.9, 161 – 342, 7.2 – 8.4, and 1 – 2.1 mg/100 g among the different varieties of foxtail millet.
Minerals are important in many activities in the body, including calcium, which is essential for bones and teeth and helps prevent osteoporosis; sodium, which helps maintain osmotic equilibrium; potassium, which is essential for maintaining systemic blood pressure and electrolyte balance; magnesium is essential for neuron and muscular function; manganese is essential for bone development; iron is essential for oxygen storage and transport in the human body; zinc and copper are trace elements which are essential for many enzymes to function properly (Soetan et al., 2010).
3.4. Total phenolic content, flavonoid content and antioxidant capacity of FMF extracts
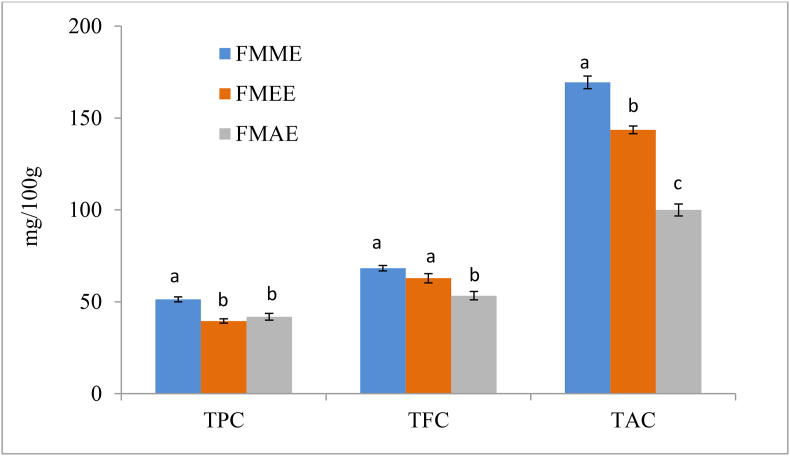
In this study, the TPC of the methanolic extract was significantly (p < 0.05) higher than others. It was observed that the methanolic extract had the highest TPC (51.35 ± 1.35 mg GAE/100 g) compared to the ethanol (39.55 ± 1.16 mg GAE/100 g) and acetone: water: acetic acid extract (41.81 ± 1.84 mg GAE/100 g) (Figure 2). According to L. Z. Zhang and Liu (2015) two varieties of foxtail millet the TPC were 78.79 ± 1.19 and 114.22 ± 4.63 mg GAE/100 g respectively. Devisetti et al. (2014) reported that two varieties of foxtail millet the TPC were 81, and 79 mg GAE/100 g respectively. The TFC of the methanolic extract was significantly (p < 0.05) higher than acetone: water: acetic acid extract and the ethanolic extract was significantly (p < 0.05) higher than acetone: water: acetic acid extract. The methanolic extract had the highest TFC (68.26 ± 1.51 mg QE/100 g) compared to the ethanol (62.82 ± 2.54 mg QE/100 g) and acetone: water: acetic acid extract (53.29 ± 2.28 mg QE/100 g) (Figure 2). Devisetti et al. (2014) reported that two varieties of FM the TFC were 75 ± 0.06 and 65 ± 0.04 mg catechin equivalents/100 g respectively. The TAC of the methanolic extract was significantly (p < 0.05) higher than others. Methanolic extract had the higher amount (169.40 ± 3.45 mg AAE/100 g) of TAC compared to ethanol (143.55 ± 2.1 mg AAE/100 g) and acetone: water: acetic acid extract (99.98 ± 3.29 mg AAE/100 g) (Figure 2). Kumari et al. (2017) reported that the TAC of extracts of FMF ranged from 0.7 – 2.3 μmol of trolox equiv/g sample. Environmental factors such as rainfall, soil type, and sun exposure have a major impact on plant polyphenol content (Manach et al., 2004). Due to the nature and polarity of the solvent used in the extraction process, methanol extract may contain high amounts of TPC, TFC, and TAC. Polyphenols are polar molecules that dissolve easily in polar solvents like methanol (Lin et al., 2016).
Figure 2.
Total phenolic content (TPC), total flavonoid content (TFC) and total antioxidant capacity (TAC) of FMF extracts. Values are expressed as mean ± SD (n = 3). Statistical analysis was carried out using one-way ANOVA. Different lowercase letters within the column indicate significant differences (p < 0.05)
3.5. DPPH scavenging activity of FMF extracts
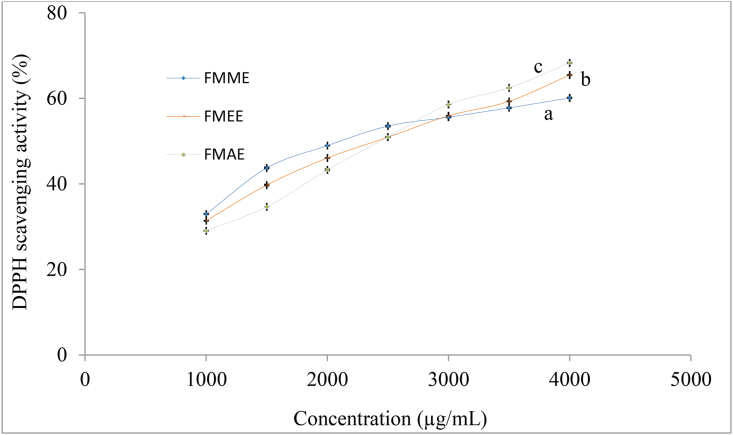
Significant different values (p < 0.05) were found that the free radical scavenging activity of various solvent extracts from FMF at different concentrations were measured by the DPPH. The IC50 value (μg/ml) is the concentration of antioxidants at which 50% inhibition of free radical activity is observed. The IC50 value of the methanolic extract was significantly higher (p < 0.05) than others. The IC50 value of methanol, ethanol, and acetone: water: acetic acid extracts were 2238.00 ± 19.46, 2293.66 ± 11.67, and 2311.67 ± 15.82 μg/mL respectively (Figure 3). Whereas, the IC50 value of standard ascorbic acid was 14.28 μg/mL Amadou et al. (2011) noted that the IC50 value of different solvents of foxtail millet bran extracts were 131–3118 μg/mL Jayawardana et al. (2018) reported that the IC50 value of different varieties of foxtail millet extracts were 3900 – 5230 μg/mL.
Figure 3.
DPPH scavenging activity (%) of FMF extracts. Values are expressed as mean ± SD (n = 3). Statistical analysis was carried out using one-way ANOVA. Different lowercase letters within the graph indicate significant differences (p < 0.05)
3.6. Ferric reducing antioxidant power (FRAP) assay of FMF extracts
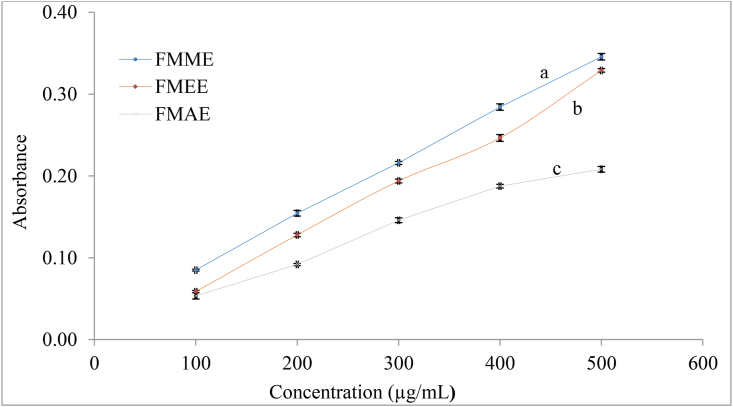
The ferric reducing antioxidant power (FRAP) of various extracts (10 mg/ml) of FMF is shown in Fig-4. In this method, the ferric-ferricyanide complex was reduced to the ferrous form due to the presence of antioxidants (Amarowicz et al., 2004). The methanolic, ethanolic, and acetone: water: acetic acid extracts in the FMF showed higher reducing power (A700 = 0.3456, 0.3289, and 0.2082) at a concentration of 500 μg/mL (Figure 4). The reducing power of the FMF extracts increased with increasing sample concentration. The FRAP potency of the FMF sample was compared with that of ascorbic acid. In this study, the FRAP activity of different extracts varied significantly (p < 0.05), and the order of FRAP activity was: methanol > ethanol > acetone: water: acetic acid extracts. Choi et al. (2007) reported that the reducing power of the methanolic extract of foxtail millet was 0.2 (absorbance) at a concentration of 4000 μg/mL. The reducing power of ethanolic extract was similar to the previous findings on foxtail millet at a concentration of 500 μg/mL (Kim et al., 2010).
Figure 4.
Ferric reducing antioxidant power (FRAP) of FMF extracts. Values are expressed as mean ± SD (n = 3). Statistical analysis was carried out using one-way ANOVA. Different lowercase letters within the graph indicate significant differences (p < 0.05)
4. Conclusions
Millet has both potential nutritional and health benefits. Foxtail millet is rich in protein, fiber, and minerals but low in fat content. It also contains phenolics and flavonoids as antioxidant constituents. Millets are generally not used in commercial foods and they remain underutilized cereals owing to the lack of product development technologies and consumer awareness of their health advantages. The present results highlight the utility and importance of foxtail millet as a food ingredient, with remarkable levels of nutrients and antioxidants. This study explored the potential use of foxtail millet grains in food product formulations, especially as a functional ingredient that can contribute to the development of health status and consumption behaviors.
Declarations
Author contribution statement
Md. Jaynal Abedin: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Abu Tareq Mohammad Abdullah: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Mohammed Abdus Satter: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Tasnim Farzana: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The study was conducted the Research and Development (R&D) work under Bangladesh Council of Scientific and Industrial Research (BCSIR), Ministry of Science and Technology, Bangladesh.
References
- Adekunle A.A., Ellis-Jones J., Ajibefun I., Nyikal R.A., Bangali S., Fatunbi O., Ange A. Forum for Agricultural Research in Africa (FARA) 2012. Agricultural innovation in sub-Saharan Africa: experiences from multiple-stakeholder approaches. Accra, Ghana. [Google Scholar]
- Akanbi T.O., Timilsena Y., Dhital S. In: Bioactive Factors and Processing Technology for Cereal Foods. Wang J., Sun B., Tsao R., editors. Springer; Singapore: 2019. Bioactives from millet: properties and effects of processing on bioavailability; pp. 171–183. [Google Scholar]
- Amadou I., Amza T., Shi Y.-H., Le G.-W. Chemical analysis and antioxidant properties of foxtail millet bran extracts. Songklanakarin J. Sci. Technol. 2011;33(5):509–515. [Google Scholar]
- Amarowicz R., Pegg R., Rahimi-Moghaddam P., Barl B., Weil J. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004;84(4):551–562. [Google Scholar]
- AOAC . eighteenth ed. Association of Official Analytical Chemists; Gaithersburg, MD.,USA: 2005. Official Methods of Analysis. [Google Scholar]
- Appiah F., Asibuo J., Kumah P. Physicochemical and functional properties of bean flours of three cowpea (Vigna unguiculata L. Walp) varieties in Ghana. Afr. J. Food Sci. 2011;5(2):100–104. [Google Scholar]
- Austin D.F. Fox-tail millets (Setaria: poaceae)—abandoned food in two hemispheres. Econ. Bot. 2006;60(2):143–158. [Google Scholar]
- Awuchi C.-G., Igwe V.S., Echeta C.K. The functional properties of foods and flours. Int. J. Adv. Acad. Res. 2019;5(11):139–160. [Google Scholar]
- Balasubramanian S., Viswanathan R. Influence of moisture content on physical properties of minor millets. J. Food Sci. Technol. 2010;47(3):279–284. doi: 10.1007/s13197-010-0043-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay T., Jaiswal V., Prasad M. In: The Foxtail Millet Genome. Compendium of Plant Genomes. Prasad M., editor. Springer; Cham: 2017. Nutrition potential of foxtail millet in comparison to other millets and major cereals; pp. 123–135. [Google Scholar]
- Banerjee S., Sanjay K., Chethan S., Malleshi N. Finger millet (Eleusine coracana) polyphenols: investigation of their antioxidant capacity and antimicrobial activity. Afr. J. Food Sci. 2012;6(13):362–374. [Google Scholar]
- Chandra S. Assessment of functional properties of different flours. Afr. J. Agric. Res. 2013;8(38):4849–4852. [Google Scholar]
- Chandra S., Singh S., Kumari D. Evaluation of functional properties of composite flours and sensorial attributes of composite flour biscuits. J. Food Sci. Technol. 2015;52(6):3681–3688. doi: 10.1007/s13197-014-1427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Ren X., Zhang Q., Diao X., Shen Q. Determination of protein, total carbohydrates and crude fat contents of foxtail millet using effective wavelengths in NIR spectroscopy. J. Cereal. Sci. 2013;58(2):241–247. [Google Scholar]
- Choi Y., Jeong H.-S., Lee J. Antioxidant activity of methanolic extracts from some grains consumed in Korea. Food Chem. 2007;103(1):130–138. [Google Scholar]
- Devisetti R., Yadahally S.N., Bhattacharya S. Nutrients and antinutrients in foxtail and proso millet milled fractions: evaluation of their flour functionality. LWT - Food Sci. Technol. 2014;59(2):889–895. [Google Scholar]
- Eom S.-H., Park H.-J., Jin C.-W., Kim D.-O., Seo D.-W., Jeong Y.-H., Cho D.-H. Changes in antioxidant activity with temperature and time in Chrysanthemum indicum L.(Gamguk) teas during elution processes in hot water. Food Sci. Biotechnol. 2008;17(2):408–412. [Google Scholar]
- Farzana T., Mohajan S. Effect of incorporation of soy flour to wheat flour on nutritional and sensory quality of biscuits fortified with mushroom. Food Sci. Nutr. 2015;3(5):363–369. doi: 10.1002/fsn3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouda G.P., Sharanagouda H., Nidoni U., Ramachandra C., Naik N., Ananada N., Ganjyal G. Studies on engineering properties of foxtail millet [Setaria italica (L.) Beauv.] J. Farm Sci. 2019;32(3):340–345. [Google Scholar]
- Gupta N., Srivastava A., Pandey V. Biodiversity and nutraceutical quality of some indian millets. Proc. Natl. Acad. Sci. India B Biol. Sci. 2012;82(2):265–273. [Google Scholar]
- Jayawardana N., Wimalasiri K., Samarasinghe G., Madhujith T. Bound and total phenolic contents and antioxidant potential of selected Sri Lankan millet varieties. Trop. Agric. 2018;29(3):316–321. [Google Scholar]
- Kamara M., Huiming Z., Kexue Z., Amadou I., Tarawalie F. Comparative study of chemical composition and physicochemical properties of two varieties of defatted foxtail millet flour grown in China. Am. J. Food Technol. 2009;4(6):255–267. [Google Scholar]
- Karim M., Arabinda S., Mohiuddin M., Rahman A., Salahuddin A. Study on the stages of development and agronomic parameters of foxtail millet (Setaria italica L. Beauv.) under Bangladesh conditions. J. Trop. Agric. 1993;37(1):28–31. [Google Scholar]
- Khan T., Ipshita A., Mazumdar R., Abdullah A., Islam G., Rahman M. Bioactive polyphenol profiling and in-vitro antioxidant activity of Tinospora cordifolia Miers ex Hook F and Thoms: a potential ingredient for functional food development. Bangladesh J. Sci. Ind. Res. 2020;55(1):23–34. [Google Scholar]
- Kim J., Hyun T.K., Kim M. Anti-oxidative activities of sorghum, foxtail millet and proso millet extracts. Afr. J. Biotechnol. 2010;9(18):2683–2690. [Google Scholar]
- Kitta K., Ebihara M., Iizuka T., Yoshikawa R., Isshiki K., Kawamoto S. Variations in lipid content and fatty acid composition of major non-glutinous rice cultivars in Japan. J. Food Compos. Anal. 2005;18(4):269–278. [Google Scholar]
- Kola G., Reddy P.C.O., Shaik S., Gunti M., Palakurthi R., Talwar H., Sekhar A.C. Variability in seed mineral composition of foxtail millet (Setaria italica L.) landraces and released cultivars. Curr. Trends Biotechnol. Pharm. 2020;14(3):239–255. [Google Scholar]
- Kumari D., Madhujith T., Chandrasekara A. Comparison of phenolic content and antioxidant activities of millet varieties grown in different locations in Sri Lanka. Food Sci. Nutr. 2017;5(3):474–485. doi: 10.1002/fsn3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang K., Liang S., Lu L., Zhu D., Cheng L. Geographical origin traceability of foxtail millet based on the combination of multi-element and chemical composition analysis. Int. J. Food Prop. 2018;21(1):1769–1777. [Google Scholar]
- Lin D., Xiao M., Zhao J., Li Z., Xing B., Li X., Kong M., Li L., Zhang Q., Liu Y., Chen H., Qin W., Wu H., Chen S. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules. 2016;21(10):1374. doi: 10.3390/molecules21101374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Bai G., Zhang D., Zhu C., Xia X., Cheng R., Shi Z. Genetic diversity and population structure of elite foxtail millet [Setaria italica (L.) P. Beauv.] germplasm in China. Crop Sci. 2011;51(4):1655–1663. [Google Scholar]
- Manach C., Scalbert A., Morand C., Rémésy C., Jiménez L. Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 2004;79(5):727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- Meherunnahar M., Chowdhury R., Hoque M., Satter M., Islam M. Comparison of nutritional and functional properties of BK2 foxtail millet with rice, wheat and maize flour. Progress. agric. 2018;29(2):186–194. [Google Scholar]
- Mohamed T.K., Zhu K., Issoufou A., Fatmata T., Zhou H. Functionality, in vitro digestibility and physicochemical properties of two varieties of defatted foxtail millet protein concentrates. Int. J. Mol. Sci. 2009;10(12):5224–5238. [Google Scholar]
- Narayana K., Narasinga Rao M. Functional properties of raw and heat processed winged bean (Psophocarpus tetragonolobus) flour. J. Food Sci. 1982;47(5):1534–1538. [Google Scholar]
- Prieto P., Pineda M., Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem. 1999;269(2):337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Pushparaj F.S., Urooj A. Antioxidant activity in two pearl millet (Pennisetum typhoideum) cultivars as influenced by processing. Antioxidants. 2014;3(1):55–66. doi: 10.3390/antiox3010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramashia S.E., Anyasi T.A., Gwata E.T., Meddows-Taylor S., Jideani A.I.O. Processing, nutritional composition and health benefits of finger millet in sub-saharan Africa. Food Sci. Technol. 2019;39(2):253–266. [Google Scholar]
- Salar R.K., Certik M., Brezova V. Modulation of phenolic content and antioxidant activity of maize by solid state fermentation with Thamnidium elegans CCF 1456. Biotechnol. Bioproc. Eng. 2012;17(1):109–116. [Google Scholar]
- Saleh A.S., Zhang Q., Chen J., Shen Q. Millet grains: nutritional quality, processing, and potential health benefits. Compr. Rev. Food Sci. Food Saf. 2013;12(3):281–295. [Google Scholar]
- Shaheen N., Goto M., Watanabe J., Takano-Ishikawa Y. Antioxidant capacity and total phenol content of commonly consumed indigenous foods of Asian tropical regions. J. Food Sci. Eng. 2012;2(4):187–195. [Google Scholar]
- Shahidi F., Chandrasekara A. Millet grain phenolics and their role in disease risk reduction and health promotion: a review. J. Funct.Foods. 2013;5(2):570–581. [Google Scholar]
- Sharma N., Niranjan K. Foxtail millet: properties, processing, health benefits, and uses. Food Rev. Int. 2018;34(4):329–363. [Google Scholar]
- Singh K., Mishra A., Mishra H. Fuzzy analysis of sensory attributes of bread prepared from millet-based composite flours. LWT - Food Sci. Technol. 2012;48(2):276–282. [Google Scholar]
- Siroha A.K., Sandhu K.S., Kaur M. Physicochemical, functional and antioxidant properties of flour from pearl millet varieties grown in India. J. Food Meas. Char. 2016;10(2):311–318. [Google Scholar]
- Soetan K., Olaiya C.O., Oyewole O.E. The importance of mineral elements for humans, domestic animals and plants-A review. Afr. J. Food Sci. 2010;4(5):200–222. [Google Scholar]
- Sunil C., Venkatachalapathy N., Shanmugasundaram S., Loganathan M. Engineering properties of foxtail millet (Setaria italic L): variety-HMT 1001. Int. j. sci. 2016;5(2):632–637. [Google Scholar]
- Ushakumari S.R., Latha S., Malleshi N.G. The functional properties of popped, flaked, extruded and roller-dried foxtail millet (Setaria italica) J. Food Technol. 2004;39(9):907–915. [Google Scholar]
- Vali Pasha K., Ratnavathi C.V., Ajani J., Raju D., Manoj Kumar S., Beedu S.R. Proximate, mineral composition and antioxidant activity of traditional small millets cultivated and consumed in Rayalaseema region of south India. J. Sci. Food Agric. 2018;98(2):652–660. doi: 10.1002/jsfa.8510. [DOI] [PubMed] [Google Scholar]
- Vetriventhan M., Upadhyaya H., Anandakumar C., Senthilvel S., Parzies H., Bharathi A.…Gowda C. Assessing genetic diversity, allelic richness and genetic relationship among races in ICRISAT foxtail millet core collection. Plant Genet. Resour. 2012;10(3):214–223. [Google Scholar]
- Wen Y., Liu J., Meng X., Zhang D., Zhao G. Characterization of proso millet starches from different geographical origins of China. Food Sci. Biotechnol. 2014;23(5):1371–1377. [Google Scholar]
- Wu H.-C., Chen H.-M., Shiau C.-Y. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus) Food Res. Int. 2003;36(9-10):949–957. [Google Scholar]
- Yang X.-S., Wang L.-L., Zhou X.-R., Shuang S.-M., Zhu Z.-H., Li N., Lu P. Determination of protein, fat, starch, and amino acids in foxtail millet [Setaria italica (L.) Beauv.] by Fourier transform near-infrared reflectance spectroscopy. Food Sci. Biotechnol. 2013;22(6):1495–1500. [Google Scholar]
- Yasumatsu K., Sawada K., Moritaka S., Misaki M., Toda J., Wada T., Ishii K. Whipping and emulsifying properties of soybean products. Agric. Biol. Chem. 1972;36(5):719–727. [Google Scholar]
- Yousf N., Nazir F., Salim R., Ahsan H., Sirwal A. Water solubility index and water absorption index of extruded product from rice and carrot blend. J. Pharmacogn. Phytochem. 2017;6(6):2165–2168. [Google Scholar]
- Zhang A., Liu X., Wang G., Wang H., Liu J., Zhao W., Zhang Y. Crude fat content and fatty acid profile and their correlations in foxtail millet. Cereal Chem. 2015;92(5):455–459. [Google Scholar]
- Zhang L.Z., Liu R.H. Phenolic and carotenoid profiles and antiproliferative activity of foxtail millet. Food Chem. 2015;174:495–501. doi: 10.1016/j.foodchem.2014.09.089. [DOI] [PubMed] [Google Scholar]
- Złotek U., Mikulska S., Nagajek M., Świeca M. The effect of different solvents and number of extraction steps on the polyphenol content and antioxidant capacity of basil leaves (Ocimum basilicum L.) extracts. Saudi J. Biol. Sci. 2016;23(5):628–633. doi: 10.1016/j.sjbs.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.