Abstract
Objective
Neuropathic pain (NP) plays an important role in patients with knee osteoarthritis (KOA). However, the prevalence of NP at different treatment stages including outpatient, awaiting and after total knee arthroplasty (TKA) have not been compared. The understanding of this issue and identify risk factors can help physicians develop individualized strategies to manage the pain of KOA. Therefore, the aim of the study is to investigate the prevalence and risk factors of NP at different treatment stages of KOA.
Methods
Patients diagnosed as KOA between August 2016 and August 2020 were enrolled in this cross‐sectional study and divided into three groups according to treatment stage, including outpatient stage, awaiting TKA stage (pre‐TKA) and after TKA stage (post‐TKA). A numeric rating scale (NRS) and PainDETECT questionnaire were used to evaluate nociceptive pain and NP. Patient demographics, radiological assessments using Kellgren–Lawrence (K‐L) grade, and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores were analyzed. Data analysis and statistics were processed using SPSS 20.0 and examined by ANOVA with/without Bonferroni correction or Kruskal–Wallis test. A chi‐square test was used to determine cross‐table data and calculate the odds ratio (OR) value.
Results
Of the 921 patients, the prevalence of possible and likely NP was 17.5% (56/320) and 2.5% (8/320) in the pre‐TKA group compared with 3.4% (8/233) and 0.4% (1/233) in the outpatient group and 1.4% (5/368) and 0.5% (2/368) in the post‐TKA group, respectively. In the pre‐TKA group, higher NRS (NRS >3; OR = 10.65, 95% CI: 3.25–34.92, P < 0.001) and WOMAC pain score (score > 10; OR = 4.88, 95% CI: 2.38–10.01, P < 0.001) conferred an increased risk of unclear pain. Age, gender, BMI and K‐L grade showed no significant differences among the unlikely, possible and likely NP groups.
Conclusion
Prevalence of NP is different at stages of out‐patient, awaiting and after TKA in patients with KOA. Patients awaiting TKA have the highest prevalence of NP compared with patients in outpatient and post‐TKA groups. In the patients waiting for TKA, higher NRS (NRS >3) and WOMAC pain scores (score > 10) are risk factors of NP.
Keywords: Arthroplasty replacement knee, Osteoarthritis knee, Pain, Prevalence
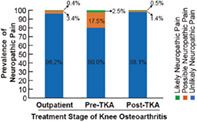
This image shows the prevalence of neuropathic pain in different treatment stages of knee osteoarthritis. The prevalence of possible and likely NP was 17.5% (56/320) and 2.5% (8/320) in the pre‐TKA group compared with 3.4% (8/233) and 0.4% (1/233) in the outpatient group and 1.4% (5/368) and 0.5% (2/368) in the post‐TKA group, respectively. The results suggest that surgeon needs to pay more attention to neuropathic pain when patients are waiting for TKA and may need to further treat this type of pain.
Introduction
Pain is the major symptom and the reason that urges patients to seek help from doctors in patients with knee osteoarthritis (KOA) and is the leading cause for dissatisfaction after total knee arthroplasty (TKA). 1 , 2 , 3 However, accurately treating this pain is still challenge due to the unclear mechanisms of pain. 4 , 5 Recently, neuropathic pain (NP) was found to play an important role and widely existed in patients with KOA and after TKA. 4 Therefore, in the view of personalized treatment strategies, it is essential to know the prevalence of NP especially in different treatment stages such as outpatient, awaiting or after TKA.
Several studies have investigated the prevalence of NP in different cohorts of KOA and after TKA. Hochman et al. first reported 28% of KOA individuals had NP symptoms in a community cohort using modified PainDETECT questionnaire. 6 Güngör et al. showed up to half of patients with primary KOA had NP. 7 Another study by Power et al. investigated patients with end‐stage hip and knee OA and reported 35.6% of women and 27.7% of men suffered possible or likely NP. 8 In addition, Hasegawa et al. find that possible NP existed in 22.2% patients before TKA and decreased to 3.3% after TKA. 9 The increasing data on the reported prevalence of NP vary from 5.4% to 28% 6 , 10 , 11 , 12 in patients with KOA and 0% to 15.3% of patients after TKA. 13 , 14 , 15 However, the prevalence of NP at different treatment stages, including the outpatient stage and the stages of awaiting and after TKA has not been compared in one cohort. The understanding of the prevalence of NP symptoms at different treatment stages can not only help clinicians be aware of NP based on the characteristics of the population, but also lead to the treatment of pain in a personalized strategic manner according to the prevalence of NP.
Moreover, to identify patients with NP is also a challenge for treatment of pain of KOA. However, only a few of research studies have focused on risk factors related to NP that may help clinicians find patients with NP at different treatment stages. In a cross‐sectional community study focusing on knee pain, NP showed associations with several factors, including fibromyalgia, widespread pain, nodal osteoarthritis, injury, pain catastrophizing and fatigue. 16 Hasegawa et al. reported that patients with moderate‐to‐severe or unclear pain after TKA had malignment and lower knee society knee scores. 17 As the risk factors for NP are poorly understood, it is necessary to explore risk factors associated with NP to better identify the patients and develop treatment strategies at different treatment stages.
Therefore, the aim of this study was to investigate: (i) the prevalence in patients at different treatment stages, including outpatient, awaiting and after TKA stages; and (ii) to identify possible risk factors of NP.
Materials and Methods
Ethics Statement
The protocol of this cross‐sectional study was approved by the ethics committees of 2nd affiliated hospital of the Wenzhou Medical University (LCKY2019‐174). Informed consent was obtained from all patients.
Inclusion and Exclusion Criteria
The inclusion criteria were: (i) patients diagnosed with primary KOA according the American College of Rheumatology criteria 18 ; and (ii) the major evaluation indicators included level of NP and nociceptive pain and range of motion (ROM).
The exclusion criteria included the following: (i) inflammatory arthritis (i.e., rheumatoid arthritis (RA), spondylarthritis and gout); (ii) autoimmune disease (i.e., connective tissue disorders); (iii) previous trauma; (iv) symptoms of spinal disease; (v) joint replacement operation in the other knee; (vi) cognitive disorders; (vii) fibromyalgia; and (viii) septic arthritis.
In total, three groups in different treatment stages were analyzed in this study, including the outpatient stage (outpatient‐KOA group) and awaiting TKA (pre‐TKA group) and after TKA (post‐TKA group) stages. The outpatient‐KOA group included the patients coming to our outpatient department and diagnosed with KOA according to the above criteria. The pre‐TKA group included patients waiting for TKA due to KOA, while the post‐TKA group contained patients who had TKA due to KOA between August 2016 and August 2018 in our department.
Data Collection
A questionnaire including patient demographics, knee function and pain evaluation was answered by the recruited patients. Patient demographics were recorded, including age and gender, in all three groups. BMI was recorded in the pre‐TKA group.
Knee Function
The function of the knee was evaluated using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score in the outpatient‐KOA group and the pre‐TKA group. 19 The WOMAC score included three subscales related to pain, stiffness and function. The higher the WOMAC score, the worse the joint function.
Radiological Evaluation
The radiological grade of KOA was evaluated by the authors according to the Kellgren–Lawrence (K‐L) grading scale (0 = none, 1 = doubtful, 2 = minimal, 3 = moderate, 4 = severe) in the outpatient‐KOA group and pre‐TKA group. 20 For the post‐TKA group, the K‐L grade of the knee before the operation was also recorded.
Pain Evaluation
NP was measured according to the PainDETECT questionnaire. 21 This questionnaire is widely used in NP evaluation related to KOA and contains questions related to pain‐affected sensory symptoms, including burning pain, paresthesia, mechanical allodynia, spontaneous pain attacks, thermal hyperalgesia, numbness, and pressure hyperalgesia. Each type of pain was classified across five grades from none (0), hardly noticed (1), slightly (2), moderately (3), strongly (4) and very strongly (5). This questionnaire also includes questions about the frequency and radiation of pain to describe the features of pain. Patients with a total score from 19 to 38 were considered likely to have NP, whereas a total score between 13 and 18 indicated ambiguous pain. Patients with a total score range from 0 to 12 were considered unlikely to have NP. Nociceptive pain was evaluated using a numeric rating scale (NRS) and the WOMAC pain score was used to measure function‐related pain.
Statistical Analysis
Data analysis and statistics were processed using SPSS 20.0 (IBM Corp., Armonk, NY, USA). Normally distributed data were described as the mean and standard deviation. Nonnormally distributed data were reported as the mean and 95% confidence interval (CI). The comparison of values was examined by ANOVA with/without Bonferroni correction or Kruskal–Wallis test depending on the results of the normality test and Levene's test. A chi‐square test was used to determine cross‐table data and calculate the odds ratio (OR) value. Correlation coefficients were determined by Spearman's rank correlation test using two‐tailed P values. P < 0.05 was set as indicating a significant difference.
Results
Patient Characteristics in the Three Different Groups
A total of 921 patients between August 2018 and August 2020 were investigated in this study, including 233, 320, and 368 in the outpatient‐KOA, pre‐TKA and post‐TKA groups, respectively. Patient demographics, knee function based on the WOMAC scores and radiological assessments is illustrated in Table 1. It was not surprising that the age of the outpatient‐KOA group was 6.75 and 7.92 years younger than that in the pre‐TKA and post‐TKA groups (P < 0.001). No difference was found for gender (P = 0.401). However, knee function based on WOMAC scores was significantly worse in the pre‐TKA group than in the outpatient‐KOA group (P < 0.001). In addition, the proportion of patients with different K‐L grades was significantly different between the groups (P < 0.001). Moreover, in the outpatient‐KOA group, most of the KOA patients were in K‐L grade 2 (51.1%), while 66.9% and 81.3% of pre‐TKA and post‐TKA patients, respectively, were in K‐L grade 4.
TABLE 1.
Demographic and radiological assessment in the outpatient, pre‐TKA and post‐TKA groups
| Outpatient‐KOA (n = 233) | Pre‐TKA (n = 320) | Post‐TKA (n = 368) | |
|---|---|---|---|
| Age (year) | 62.94 ± 10.68 | 69.69 ± 7.55 | 70.86 ± 7.08 |
| Male/Female | 64/169 | 93/227 | 118/250 |
| Follow up (months) | / | / | 20.54 ± 3.87 |
| WOMAC | 20.67 ± 15.72 | 37.95 ± 13.23 | / |
| K‐L grade | |||
| 1 | 62 (26.6%) | 0 (0%) | 0 (0%) |
| 2 | 119 (51.1%) | 41 (12.8%) | 23 (6.2%) |
| 3 | 38 (16.3%) | 65 (20.3%) | 46 (12.5%) |
| 4 | 14 (6.0%) | 214 (66.9%) | 299 (81.3%) |
Abbreviations: K‐L grade, Kellgren–Lawrence grade; KOA, knee osteoarthritis; TKA, total knee arthroplasty; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Prevalence of Neuropathic Pain Was Significantly Different in the Outpatient, Pre‐TKA and Post‐TKA Groups
To evaluate the prevalence of NP in the different groups, all of the participating patients answered a survey that included the PainDETECT questionnaire and an NRS during the study. In total, we identified 11 patients likely having NP and 69 patients possibly having NP. The number of patients and the prevalence of NP in the different groups is illustrated in Table 2. In general, the three groups had significantly different NP scores (P < 0.001). The highest NP score existed in the pre‐TKA group and dramatically decreased after TKA. Correspondingly, the pre‐TKA group had the highest proportion of patients possibly having (17.5%) and likely having (2.5%) NP. However, the prevalence of possible and likely NP in the outpatient‐KOA and post‐TKA groups was relatively low. Furthermore, we hypothesized that NP may be an unclear pain factor and found that the proportion of patients with unclear pain was significantly different in the pre‐TKA group compared with the outpatient‐KOA and post‐TKA groups (P < 0.001). In the post‐TKA group, there were 111/368 (30.2%) patients who still had persistent pain whose NRS scores ranged from 1 to 4. In patients with persistent pain (NRS≥1), the percentages of possible and likely NP patients were 4.50% (5/111) and 1.80% (2/111), respectively. In summary, NP existed in the patients with KOA, but the prevalence was significantly different across the treatment stages.
TABLE 2.
The prevalence of neuropathic pain in the outpatient, pre‐TKA and post‐TKA groups
| Op‐OA (n = 233) | Pre‐TKA (n = 320) | Post‐TKA (n = 368) | |
|---|---|---|---|
| NP Score (mean, 95% CI) | 3.63 (3.15–4.10) | 7.93 (7.43–8.42) | 1.29 (1.01–1.56) |
| NP Score | |||
| None (<12) | 224 | 256 | 361 |
| Possible (12–18) | 8 | 56 | 5 |
| Likely (>19) | 1 | 8 | 2 |
| Percentage of unlikely NP pain | 96.2% | 80.0% | 98.1% |
| Percentage of possible NP pain | 3.4% | 17.5% | 1.4% |
| Percentage of Likely NP pain | 0.4% | 2.5% | 0.5% |
Abbreviations: CI, confidence interval; NP, neuropathic pain; OA, osteoarthritis; TKA, total knee arthroplasty.
To further explore the relationship between the NP score and other pain measurements, NRS and WOMAC pain scores were compared among the three different groups using the Kruskal‐Wallis test. As shown in Figure 1, the highest NRS occurred in the pre‐TKA group compared with the outpatient group, but was dramatically lower in the post‐TKA group. Moreover, the WOMAC pain scores were higher in the pre‐TKA group than in the outpatient group. In addition, we found that the PainDETECT scores were highly correlated with the NRS scores (r = 0.704, P < 0.001) and WOMAC pain scores (r = 0.504, P < 0.001). In summary, the NRS and WOMAC pain scores showed similar trends as NP in different groups and had a significant association with the PainDETECT score.
Fig. 1.
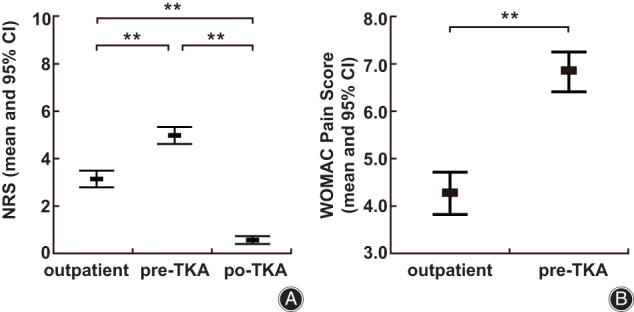
Comparison of NRS and WOMAC pain scores among the outpatient, pre‐TKA and post‐TKA groups. (A). Comparison of NRS scores among the outpatient, pre‐TKA and post‐TKA groups. (B). Comparison of WOMAC pain scores between the outpatient and pre‐TKA groups. ** p < 0.01, * p < 0.05
Risk Factors of Neuropathic Pain in the Pre‐TKA Group
Due to the low prevalence of NP in the outpatient group and post‐TKA group, we suggest that it may be not necessary to regularly screen for NP in these patients. However, in patients waiting for TKA, NP screening may be necessary, and giving anti‐NP medication in these patients may be helpful for relieving the pain.
To identify the patients with differing potential for developing NP, the correlated risk factors of the pre‐TKA group were analyzed using the Spearman test, and the OR was calculated using the chi‐square test. The characteristics of the patients in the unlikely NP, possible NP and likely NP groups are shown in Table 3. We found no significant differences in age (P = 0.308), gender (P = 0.059), BMI (P = 0.343) or K‐L grade (P = 0.476) among these groups. However, significantly different NRS (P < 0.001) and WOMAC pain (P < 0.001) scores were found among the three groups. Moreover, knee function measured using the WOMAC score also showed significant differences among the groups (P < 0.001). We hypothesized that the possible and likely NP groups were more representative of an unclear pain group than the unlikely NP group was. We found that compared with patients with lower NRS (NRS≤3), the OR value of NP was 10.65 (95% CI: 3.25–34.92, P < 0.001) in patients with higher NRS (NRS >3). In addition, compared with the lower WOMAC pain score group (WOMAC pain score ≤ 10), the OR value of NP was 4.881 (95% CI: 2.38–10.01, P < 0.001) in the higher WOMAC pain score group (WOMAC pain score > 10). Furthermore, compared with patients with lower WOMAC scores (WOMAC score ≤ 45), the OR value of NP was 2.44 (95% CI: 1.37–4.33, p = 0.002) in patients with higher WOMAC scores (WOMAC score > 45). In summary, due to the relatively high proportion of NP in the pre‐TKA group, we suggest evaluating NP in patients waiting for TKA, especially those with high NRS (NRS >3) and WOMAC pain (WOMAC pain score > 10) scores, because giving anti‐NP medication may be helpful for relieving their pain during the waiting stage.
TABLE 3.
Patient characteristics based on different levels of potential neuropathic pain in the pre‐TKA group
| Unlikely (<12) n = 256 | Possible (12–18) n = 56 | Likely (>18) n = 8 | |
|---|---|---|---|
| Age (years) | 70.09 ± 7.26 | 68.27 ± 8.44 | 66.88 ± 9.28 |
| Male/Female | 81/175 | 9/47 | 3/5 |
| BMI (kg/m2) | 25.83 ± 3.22 | 26.73 ± 3.81 | 25.77 ± 3.96 |
| WOMAC | 36.42 ± 12.45 | 43.20 ± 15.10 | 50.25 ± 8.07 |
| NRS | 4.34 (4.11–4.58) | 6.50 (6.04–6.90) | 6.75 (4.92–8.58) |
| WOMAC pain | 6.33 (5.99–6.67) | 8.54 (7.59–9.48) | 10.88 (8.71–13.04) |
| K‐L grade | |||
| 1 | 0 | 0 | 0 |
| 2 | 28 (10.9%) | 12 (21.4%) | 1 (12.5%) |
| 3 | 55 (21.5%) | 9 (16.1%) | 1 (12.5%) |
| 4 | 173 (67.6%) | 35 (62.5%) | 6 (75.0%) |
Abbreviations: BMI, body mass index; K‐L grade, Kellgren–Lawrence grade; NRS, numeric rating scale; TKA, total knee arthroplasty;WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Discussion
Our study suggests that different prevalence of NP exists in the three treatment stages of KOA, including outpatient stage, awaiting and after TKA stages. Patients awaiting TKA have the highest prevalence of NP compared with patients in outpatient and after TKA groups. To our knowledge, this is the first report to compare the prevalence of NP in three treatment stages of KOA. In addition, we identify higher NRS (NRS >3) and WOMAC pain scores (score > 10) are risk factors of NP in the pre‐TKA group. Our findings also add evidence to a growing body of literature that NP contributes to a portion of KOA pain.
Prevalence of Neuropathic Pain in outpatients' Stage
Our research found a relative lower prevalence of NP (0.4%) in outpatients than the previous reports. The prevalence of NP in KOA patients in the outpatient department or community has been reported in several studies. The lowest prevalence (5.4%) was reported by Ohtori et al., while the highest prevalence (33.3%) was reported in Oteo‐Alvaro et al.'s research. 11 , 22 A meta‐analysis showed that 23% of KOA patients had symptoms of NP. 12 The large range of prevalence shows the great heterogeneity encountered in KOA patients. Potential explanations for the different prevalence of NP between our research and other studies include the following: (i). differential recruitment practices (depending on the people investigated, outpatient or community setting 6 , 11 ); (ii). differential inclusion and exclusion criteria (inclusion criteria such as knee pain patients or patients diagnosed as KOA; exclusion criteria such as whether to exclude patients with possibly related diseases, such as lumbar disease or fibromyalgia); and (iii). ethnic and cultural differences between regions and countries (for example, the lowest prevalence of NP in previous reports occurred in an East Asian country 11 ). In the context with varied prevalence rates of NP, we suggest that cross‐domain and multinational research using unified standards should be conducted in the future. However, based on our results, we suggest that it is not necessary to use neuropathic tools to regularly screen patients with KOA in the outpatient department.
Prevalence of Neuropathic Pain in the Stage Awaiting TKA
There is a limited number of studies focused on the prevalence of NP in patients during the awaiting TKA period, and the reported prevalence has shown a wide range of differences. In our study, 2.5% of patients likely had NP, while 17.5% of patients possibly had NP in the pre‐TKA group. This result is in the range of previous research (1.0%–30%) and showed a relatively low prevalence of NP. In one study, after investigating 96 patients during the awaiting TKA period, Phillips et al. reported that only one in 96 (1.0%) patients had a PainDETECT score greater than 19. 14 In contrast, Kurien et al. found that NP existed in 30% of patients before TKA. 23 However, compared with the prevalence in the outpatient department patients, the prevalence of NP in patients during the pre‐TKA period was dramatically higher in our study. Because the waiting time for TKA varies from months to years across different hospitals, we suggest that screening for NP in patients waiting for TKA and providing anti‐NP medication may be useful to relieve their pain during the waiting period.
Prevalence of Neuropathic Pain in the Stage after TKA
Our study confirms that although TKA is effective in relieving pain, a certain proportion of patients still endure persistent pain, and NP contributes to persistent with pain after TKA. In our study, only 0.5% of patients after TKA suffered NP symptoms. This prevalence is comparable with previous reports which indicate the prevalence of NP after TKA varied from 0%–15% in different reports depending on the follow‐up time and population investigated. For example, Albayrak et al. reported that 15.3% of patients likely have NP, the prevalence increased up to 22.9% in the severe pain patients (NRS >3), 15 while Hasegawa et al. reported 9% of patients after TKA endure unclear pain, although no patients met the criteria for likely NP according to the PainDETECT questionnaire in Japan. 17 A review estimated that the prevalence of NP ranges from 5.2% to 13% in the 6 month postoperative period. 24 Compared with these results, our study demonstrated a relatively low prevalence of NP after TKA and similar rate as Hasegawa et al.'s research. Despite the fact that medications for anti‐NP have been demonstrated to be useful for the treatment of persistent pain after TKA in several reports, 25 , 26 due to the low prevalence of NP after TKA, we suggest that it is not necessary to regularly screen for NP in these patients.
Risk Factors of Neuropathic Pain in Patients with KOA
Several risk factors of NP have been identified in KOA patients. Hochman et al. found the pain intensity, presence of referring back/hip pain, number of painful joints and one or more self‐reported neurological conditions were risk factors of NP in KOA patients. 6 Albayrak et al. reported that being widowed, having a low education level, being a housewife, having employment that required physical effort, having presurgical pain at rest and having presurgical restricted walking distance were risk factors of NP after TKA. 15 Moreover, pain intensity and PainDETECT scores have been strongly correlated in several studies. 11 , 14 , 27 , 28 , 29 In line with their results, we also found that the NRS and WOMAC pain scores had high correlations with NP, and the patients with high NRS and WOMAC pain scores had a higher risk of NP. Therefore, due to the strong correlation between pain intensity and NP, we suggest evaluating NP in patients with high NRS and WOMAC pain scores. In the future, the more risk factors should be investigated to help clinicians identify these patients.
Strengths and Limitations
This study has some limitations. First, we did not investigate the length of time of knee pain, which limited further analyses with correlations of NP and time because central sensitization may depend on the duration of persistent pain. Therefore, the time issue should be considered in future research. Second, the changes in NP before and after TKA in the same patients was not recorded in this study. Whether NP existing before TKA affects the development of NP after TKA remains unclear. This should be clarified in future research.
Our study has implications for both clinical practice and future research. Compared with medications treating nociceptive pain, the treatment strategy for NP is different and includes antidepressant, antiepileptic, topical anesthetic, and opioid agents. 30 However, because the prevalence of NP is different depending on the treatment stages of KOA, clinicians should consider NP features in different treatment stages and develop a personalized pain treatment strategy, especially in patients waiting for TKA with higher NRS and WOMAC pain scores. In addition, the mechanisms of NP should be further elucidated, which may help to treat NP in these patients.
Conclusion
In summary, this study demonstrates that different prevalence rates of NP occur in patients at three treatment stages and higher NRS or WOMAC pain scores are important risk factors for NP. This finding may lead to personalized treatment strategies for KOA. In the future, considering the coexistence of nociceptive pain and NP in KOA patients, we suggest conducting clinical trials to find out whether treatment based on the features of pain is more effective than traditional treatment strategies.
Author Contributions
Study conception and design: LL, MD and PF. Collection and assembly of data: LL, ZHZ, HLZ, YYL, LHX, YZ, MD and PF. SPSS statistical analysis: LL, MD and PF. Analysis and interpretation of data: LL, MD and PF. Manuscript: LL, MD and PF. All authors approved the final version to be published.
Funding Information
This work was supported by Wenzhou Municipal Science and Technology Bureau funding (Y20210053).
Acknowledgment
We gratefully acknowledge statistics assistance from Dr. Qun Wang.
Co‐first authors: Li LI and Zhaohui Zeng.
Contributor Information
Ming Deng, Email: dengming1983@whu.edu.cn.
Pei Fan, Email: fanpei@wmu.edu.cn.
References
- 1. Katz J, Arant K, Loeser R. Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA. 2021;325:568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hunter D, Bierma ZS. Osteoarthritis. Lancet. 2019;393:1745–59. [DOI] [PubMed] [Google Scholar]
- 3. Lin YY, Chen XY, Li L, Li Z, Zhang Y, Fan P. Comparison of patient satisfaction between medial pivot prostheses and posterior‐stabilized prostheses in total knee arthroplasty. Orthop Surg. 2020;12:836–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thakur M, Dickenson AH, Baron R. Osteoarthritis pain: nociceptive or neuropathic? Nat Rev Rheumatol. 2014;10:374–80. [DOI] [PubMed] [Google Scholar]
- 5. da Costa BR, Reichenbach S, Keller N, Nartey L, Wandel S, Jüni P, et al. Effectiveness of non‐steroidal anti‐inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta‐analysis. Lancet. 2017;390:e21–33. [DOI] [PubMed] [Google Scholar]
- 6. Hochman JR, Gagliese L, Davis AM, Hawker GA. Neuropathic pain symptoms in a community knee OA cohort. Osteoarthr Cartil. 2011;19:647–54. [DOI] [PubMed] [Google Scholar]
- 7. Güngör DU, Demir A, Toraman N. Neuropathic pain in knee osteoarthritis. Adv Rheumatol. 2021;61:67. [DOI] [PubMed] [Google Scholar]
- 8. Power JD, Perruccio AV, Gandhi R, Veillette C, Davey JR, Syed K, et al. Neuropathic pain in end‐stage hip and knee osteoarthritis: differential associations with patient‐reported pain at rest and pain on activity. Osteoarthr Cartil. 2018;26:363–9. [DOI] [PubMed] [Google Scholar]
- 9. Hasegawa M, Tone S, Naito Y, Sudo A. Possible neuropathic pain in patients with osteoarthritis of the knee before and after total knee arthroplasty. J Pain Res. 2021;14:3011–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dimitroulas T, Duarte RV, Behura A, Kitas GD, Raphael JH. Neuropathic pain in osteoarthritis: a review of pathophysiological mechanisms and implications for treatment. Semin Arthritis Rheum. 2014;44:145–54. [DOI] [PubMed] [Google Scholar]
- 11. Ohtori S, Orita S, Yamashita M, Ishikawa T, Ito T, Shigemura T, et al. Existence of a neuropathic pain component in patients with osteoarthritis of the knee. Yonsei Med J. 2012;53:801–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. French HP, Smart KM, Doyle F. Prevalence of neuropathic pain in knee or hip osteoarthritis: a systematic review and meta‐analysis. Semin Arthritis Rheum. 2017;47:1–8. [DOI] [PubMed] [Google Scholar]
- 13. Valdes AM, Suokas AK, Doherty SA, Jenkins W, Doherty M. History of knee surgery is associated with higher prevalence of neuropathic pain‐like symptoms in patients with severe osteoarthritis of the knee. Semin Arthritis Rheum. 2014;43:588–92. [DOI] [PubMed] [Google Scholar]
- 14. Phillips JR, Hopwood B, Arthur C, Stroud R, Toms AD. The natural history of pain and neuropathic pain after knee replacement: a prospective cohort study of the point prevalence of pain and neuropathic pain to a minimum three‐year follow‐up. Bone Joint J. 2014;96:1227–33. [DOI] [PubMed] [Google Scholar]
- 15. Albayrak I, Apiliogullari S, Erkocak OF, Kavalci H, Ozerbil OM, Levendoglu F. Total knee arthroplasty due to knee osteoarthritis: risk factors for persistent postsurgical pain. J Natl Med Assoc. 2016;108:236–43. [DOI] [PubMed] [Google Scholar]
- 16. Fernandes G, Valdes A, Walsh D, Zhang W, Doherty M. Neuropathic‐like knee pain and associated risk factors: a cross‐sectional study in a UK community sample. Arthritis Res Ther. 2018;20:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hasegawa M, Tone S, Naito Y, Wakabayashi H, Sudo A. Prevalence of persistent pain after total knee arthroplasty and the impact of neuropathic pain. J Knee Surg. 2019;32:1020–3. [DOI] [PubMed] [Google Scholar]
- 18. Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–49. [DOI] [PubMed] [Google Scholar]
- 19. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–40. [PubMed] [Google Scholar]
- 20. Kellgren JH, Lawrence JS. Radiological assessment of osteo‐arthrosis. Ann Rheum Dis. 1957;16:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Freynhagen R, Baron R, Gockel U, Tolle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22:1911–20. [DOI] [PubMed] [Google Scholar]
- 22. Oteo‐Alvaro A, Ruiz‐Iban MA, Miguens X, Stern A, Villoria J, Sánchez‐Magro I. High prevalence of neuropathic pain features in patients with knee osteoarthritis: a cross‐sectional study. Pain Pract. 2015;15:618–26. [DOI] [PubMed] [Google Scholar]
- 23. Kurien T, Arendt‐Nielsen L, Petersen KK, Graven‐Nielsen T, Scammell BE. Preoperative neuropathic pain‐like symptoms and central pain mechanisms in knee osteoarthritis predicts poor outcome 6 months after total tnee replacement surgery. J Pain. 2018;19:1329–41. [DOI] [PubMed] [Google Scholar]
- 24. Drosos GI, Triantafilidou T, Ververidis A, Agelopoulou C, Vogiatzaki T, Kazakos K. Persistent post‐surgical pain and neuropathic pain after total knee replacement. World J Orthop. 2015;6:528–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang G, Bi L, Li X, Li Z, Zhao D, Chen J, et al. Efficacy and safety of duloxetine in Chinese patients with chronic pain due to osteoarthritis: a randomized, double‐blind, placebo‐controlled study. Osteoarthr Cartil. 2017;25:832–8. [DOI] [PubMed] [Google Scholar]
- 26. Buvanendran A, Kroin JS, Della Valle CJ, Kari M, Moric M, Tuman KJ. Perioperative oral pregabalin reduces chronic pain after total knee arthroplasty: a prospective, randomized, controlled trial. Anesth Analg. 2010;110:199–207. [DOI] [PubMed] [Google Scholar]
- 27. Moss P, Benson HAE, Will R, Wright A. Patients with knee osteoarthritis who score highly on the PainDETECT questionnaire present with multimodality hyperalgesia, increased pain, and impaired physical function. Clin J Pain. 2018;34:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Golob M, Markovic I, Zovko N, Šakić D, Gudelj‐Gračanin A, Morović‐Vergles J. Do we pay enough attention to neuropathic pain in knee osteoarthritis patients? Acta Med Croatica. 2018;57:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roubille C, Raynauld JP, Abram F, Paiement P, Dorais M, Delorme P, et al. The presence of meniscal lesions is a strong predictor of neuropathic pain in symptomatic knee osteoarthritis: a cross‐sectional pilot study. Arthritis Res Ther. 2014;16:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9:807–19. [DOI] [PubMed] [Google Scholar]


