Abstract
Objective
Prevention of fragility fractures is one of the public health priorities worldwide, whilst the incidence of osteoporotic vertebral compression fractures (OVCF) continues to rise and lacks the corresponding accurate prediction model. This study aimed to screen potential causes and risk factors for primary non‐traumatic osteoporotic vertebral compression fractures (NTOVCF) in the elderly by characterizing a patient population with NTOVCF and comparing it with a population of osteoporotic patients.
Methods
Between January 2013 and January 2022, 208 elderly patients with unequivocal evidence of bone fragility manifested as painful NTOVCF were enrolled, and compared with 220 patients with osteoporosis and no fractures. The demographic data, bone turnover markers, blood routine, serum biochemical values, and radiological findings were investigated. Differences between the fracture and non‐fracture groups were analyzed, and variables significant in univariate analysis and correlation analysis were included in the logistic analysis to build the risk prediction model for osteoporotic vertebral fractures. Univariate analysis using student's t‐tests for continuous variables or a chi‐squared test for categorical variables was conducted to identify risk factors.
Results
No significant differences were revealed regarding age, gender, BMI, smoking, alcohol consumption, blood glucose, propeptide of type I procollagen (P1NP), and N‐terminal middle segment osteocalcin (N‐MID) (P > 0.05). Parathyroid Hormone (PTH), 25(OH)D, serum albumin (ALB), hemoglobin (HB), bone mineral density (BMD), and cross‐sectional area (CSA) of the paraspinal muscle in the fracture group were significantly lower than those in the control group; however, b‐C‐terminal telopeptide of type I collagen (β‐CTX), total cholesterol (TC), high‐density lipoprotein cholesterol (HDL‐C), non‐prostatic acid phosphatase (NACP), and fatty degeneration ratio (FDR) were significantly higher than those in the control group (P < 0.05). Logistic regression analysis showed that ALB, HB, CSA, and BMD were negatively correlated with NTOVCF, while β‐CTX, HDL‐C, NACP, and FDR were positively correlated with NTOVCF.
Conclusion
Decreased physical activity, anemia, hypoproteinemia, imbalances in bone metabolism, abnormal lipid metabolism, and degenerative and decreased muscle mass, were all risk factors for OVCF in the elderly, spontaneous fractures may be the consequence of cumulative declines in multiple physiological systems over the lifespan. Based on this risk model, timely detection of patients with high OVCF risk and implementation of targeted preventive measures is expected to improve the effect of fracture prevention.
Keywords: Elderly population, Osteoporosis, Osteoporotic vertebral compression fracture, Risk prediction model
Screening potential causes for primary OVCF in the elderly by characterizing a patient population with OVCF and comparing it with osteoporotic patients. The demographic data, bone turnover markers, serum biochemical values, and radiological findings were investigated. Variables significant were included in the logistic analysis to build the risk prediction model.
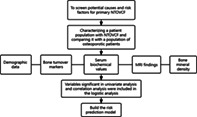
Introduction
Osteoporotic vertebral compression fracture (OVCF) is the most common complication of osteoporosis, which can lead to acute or chronic pain, impaired activities of daily living, disfigurement, psychological distress, and diminished lifespan. 1 Prevention of osteoporotic fracture is one of the public health priorities worldwide, which is also the major goal in the treatment of osteoporosis. 2 , 3 Presently, it is generally accepted that bone loss and falls are chief risk factors for OVCF, therefore, prevention measures mainly focus on drug therapy for osteoporosis and fall prevention in this category. 4 , 5 However, the prevention scheme for OVCF has not achieved the ideal goal: in the USA an estimated 1.5 million suffer osteoporotic fragility fractures each year; similarly, in the UK, epidemiological studies hypothesize that one in two women and one in five men aged over 50 years will suffer an osteoporotic fracture in their lifetime. 6 , 7 At the same time, clinically, non‐traumatic osteoporotic fractures (NTOVCF) are pathological fractures mainly attributed to the vulnerability and mechanical fragility of osteoporotic bone, and hardly ever accompanied by energy damage, therefore, many patients do not pay enough attention until complications arrive, consequently delaying treatment. 8 , 9
With a worldwide aging population, prevention of osteoporosis‐related fractures is becoming increasingly important over time, and the first step to prevent fractures is to screen and identify potential causes and risk factors for fragile fractures. The osteoporosis self‐assessment tool for Asians (OSTA), could assess the risk of osteoporosis based on age and weight, which also had the ability to predict OVCF as reported. Clinical results show that OSTA had an area under the curve (AUC) of 0.618 in identifying patients with OVCF (cutoff < −1, sensitivity 32.3%, specificity 92.3%). 10 , 11 However, this prediction tool based on two demographic factors is mediocre and not accurate enough to identify high‐risk factors for fractures. Fracture risk assessment according to the T‐score of bone mineral density (BMD), may not be accurate enough, because BMD can only reflect bone mass but not bone quality and bone microarchitecture. The risk calculators (FRAX®), using clinical risk factors and calculating the 10 year probability of fracture, could effectively predict the hip fracture when combined with BMD, but are complex and limited in predicting the risk of other fractures, especially for OVCF, because the circumstances leading to OVCF are frequently controversial and unknown. 3 , 6 , 12 , 13 In addition to BMD and bone turnover markers (BTM), 14 previous studies also reported that serum albumin, 15 serum cholesterol level, 16 and lumbar muscle mass 17 were associated with osteoporotic fractures. Moreover, a previous study had shown that OVCFs were related to frailty, and conversely, frailty was further worsened after OVCFs due to the deficit accumulation being greater. 18 Importantly, as previous studies reported, approximately 47% of the OVCF events occurred spontaneously or in otherwise unclear circumstances. 19 , 20
Taken together, the clinical onset of non‐traumatic OVCF in the elderly is hidden and the identification of significant risk factors is crucial because they are robust predictors of future fractures. The predictive value of clinical factors for NTOVCF has not been extensively studied. The current study voluntarily chose a stringent definition of NTOVCF to better screen and identify potential causes and risk factors of osteoporotic fracture, since the only difference between osteoporosis and NTOVCF is whether a vertebral fracture occurs. The purpose of this retrospective study was: (i) to explore whether lifestyle, blood tests, serum biochemical indicators, bone turnover markers (BTM), BMD, and lumbar muscle status were potential predictors of NTOVCF; and (ii) to establish a risk prediction model for NTOVCF, thereby providing reliable methods for the prevention of osteoporosis‐related fractures.
Methods
Study Design and Participants
This single‐center retrospective study was approved by the institutional review board and ethics committee of the research institution (No[2020]150).
The inclusion criteria were as follows: (i) patients with primary non‐traumatic osteoporotic vertebral compression fractures occur spontaneously without explicit external forces, including falls, drops, impacts, and other factors (NTOVCF group); (ii) those osteoporotic cases admitted because of lumbago, nerve compression, or other spinal problems but no history of any fractures (control group); (iii) age ≥ 60 years old; (iv) lumbar BMD T‐score ≤ −2.5; and (v) Chinese patients. The exclusion criteria were as follows: (i) neoplasms of the vertebral column; (ii) history of vertebral fracture, spinal surgery, and low back soft tissue injury or surgery; (iii) any co‐morbidity or chronic diseases that could significantly affect bone or soft tissue metabolism (for example, diabetes, liver and kidney disease, chondromalacia, thyroid disorders, ankylosing spondylitis, diffuse idiopathic skeletal hyperostosis, or connective tissue disease); (iv) history of certain drug use (hormonal drugs, anti‐osteoporosis drugs, or diet pills); (v) severe cardiopulmonary diseases or coagulation dysfunction; and (vi) incomplete clinical data.
The medical records, clinical manifestations, laboratory results, and imaging examinations of 208 patients (143 women, 65 men; mean age, 73.5 years) presenting with NTOVCF were extracted, analyzed, and compared with osteoporotic control cases (136 women, 84 men; mean age, 73.1 years). Plain radiographs were the routine examination item of this study, which can preliminarily judge whether the patients with osteoporosis and ostealgia have fractures and other skeletal diseases. In addition, it could help locate the vertebral fracture, and MRI can further exclude those old vertebral compression fractures. Basic information, blood test results, and radiological data of the participants were searched through the Hospital Information System records and the PACS system. All the data and files were reviewed and collected by one researcher but measured and analyzed by another single‐blinded researcher. The following data were collected: age, gender, body mass index (BMI), smoking and alcohol consumption, diabetes mellitus, Parathyroid Hormone (PTH), propeptide of type I procollagen (P1NP), b‐C‐terminal telopeptide of type I collagen (β‐CTX), N‐terminal middle segment osteocalcin (N‐MID), alkaline phosphatase (ALP), acid phosphatase (ACP), non‐prostatic acid phosphatase (NACP), 25(OH)D, uric acid (UA), Calcium (Ca), Phosphorus (P), Magnesium (Mg), serum albumin (ALB), total protein (TP), total cholesterol (TC), triglyceride (TG), high‐density lipoprotein cholesterol (HDL‐C), low‐density lipoprotein cholesterol (LDL‐C), blood glucose (Glu), red blood cell (RBC), hemoglobin (HB), white blood cell (WBC), platelet and lumbar BMD (T‐score) measured.
BMD Evaluation
The BMD (T‐score) was measured at the lumbar spine (L1–4), total hip, and femoral neck areas using the Lunar iDXA (GE Healthcare, Chicago, IL, USA); Fractured vertebrae were excluded from the measurement, and patients with a T‐score of−2.5 or less in the lumbar or femoral neck were diagnosed with osteoporosis. For the current study, the T‐score of lumbar spines was extracted as a potential factor for the NTOVCF predictive model. The severity of osteoporosis was graded according to the T‐score: first‐degree −2.5 to −3.5, second‐degree −3.5 to −4.0, and third‐degree −4.0 and below.
Assessment of Blood Indicators
Venous blood was drawn in the morning from participants who had fasted for 8 h. Serum ALP, ACP, NACP, ALB, TP, UA, Ca, P, Mg, TC, TG, HDL‐C, and LDL‐C were examined using AU5800 automatic biochemistry (Beckman Coulter, Pasadena, CA, USA). Serum P1NP, β‐CTX, N‐MID, and 25(OH)D were measured by Cobas 6000 analyzer series (Roche, Basel, Switzerland). Nutritional status was graded regarding serum ALB levels: levels above 35 g/L were defined as normal, while mild malnutrition ranged from 30 to 35 g/L, moderate from 25 to 30 g/L, and 25 g/L and below were severe malnutrition. Besides, HB can reflect anemia and, to some extent, the nutritional status of patients. In this study, serum HB of 110 g/L and above, 90–110 g/L, 60–90 g/L, and below 60 g/L were accordingly classified as a normal, mild, moderate, and severe deficiency, respectively. Meanwhile, since β‐CTX and P1NP were tightly representative markers of bone‐resorbing osteoclast and bone‐forming osteoblast, respectively, by calculating the ratio of β‐CTX to P1NP (CPR), the relative activity of osteoclastic action and osteogenesis was analyzed.
Image Analyses
Magnetic resonance imaging (MRI) examinations were completed using the 3.0T (Siemens Healthineers, Erlangen, Germany) scanner. T2‐weighted images, parallel to the inferior endplate of the L3 vertebral body, were selected for analysis. The cross‐sectional area (CSA) of the bilateral multifidus and erector spinae, and vertebral body size (VB) were separately outlined with the graphic cursor and measured on images using the hospital PACS digital imaging system (Fig. 1). The fatty degeneration ratio (FDR) of the paraspinal muscle was analyzed and calculated using the ImageJ software for Windows (ImageJ version 1.53k, National Institutes of Health, Bethesda, MD, USA). After the images were transferred to ImageJ, the gray‐scale ranges for CSA of paraspinal muscle on both sides and subcutaneous fat (SCF) were presented as histograms, then the overlapping area of CSA and SCF grayscale ranges were produced on the gray‐scale histograms (Fig. 1), which indicated the amount of fatty degeneration within the CSA. FDR was formulated by the number of pixels in the overlap area divided by the total number of pixels in the CSA. 21 Lumbar muscle mass (LMM) was calculated by multiplying CSA with (1‐FDR), while lumbar muscle fat content (LMF) was calculated by multiplying CSA with FDR. The degrees of paraspinal muscular fatty degeneration was graded as mild, moderate, and severe grades as the FDR < 10%, 10%–50%, and > 50%, respectively. 22 The researcher who assessed the images was blinded to the clinical and demographic data of the patients.
FIG. 1.
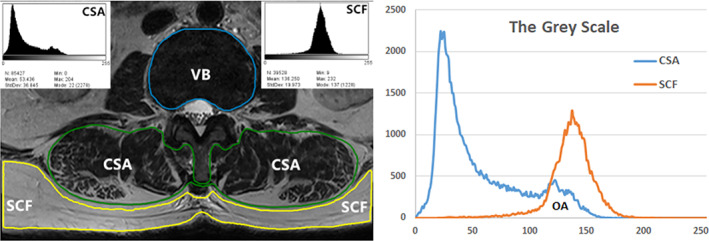
The cross‐sectional area (CSA) of the paraspinal muscle and vertebral body size (VB) were separately outlined. The fatty degeneration ratio (FDR) of the paraspinal muscle was calculated using the ImageJ, and the gray‐scale ranges for CSA and subcutaneous fat (SCF) were presented as histograms, then the overlapping area (OA) of CSA and SCF grayscale ranges were produce, which indicated the amount of fatty degeneration within the CSA. FDR was formulated by the number of pixels in the overlap area divided by the total number of pixels in the CSA
Lifestyle
Lifestyles of patients, such as smoking, alcohol intake, activities of daily living, and Calcium tablets and vitamin D3 tablets taken, were compared between the two groups. Concerning smoking and drinking, “Never” referred to non‐smoker or non‐drinker, “Previous” referred to former smokers or previous drinkers who had already quit smoking or drinking, whereas “Present” referred to those with daily smoking or alcohol consumption habits. The activities of daily living (ADL) before admission were evaluated using the Barthel Index, which is a recognized and simple scoring tool used to evaluate basic ADL functions and the level of physical performance. 23 The ADL assessment includes eating, dressing, washing, bathing, going to the toilet, and functional movements include turning over, sitting up from bed, transferring, walking, driving wheelchairs, going up and down stairs; any item that could not meet the needs of daily living was directly identified as insufficient daily activities (“Underactivity”), while “Normal” referred to sufficient activity to meet those needs. In terms of Calcium and vitamin D3 supplements, “Daily use” was defined as patients regularly taking calcium once a day (600 mg) or vitamin D3 tablets daily (125IU); “Irregular taken” referred to those who take their medication irregularly and sometimes forget it, and “Never” represented those who had never taken medication supplements.
Statistical Analysis
The SPSS 24.0 for Windows (IBM, Armonk, NY, USA) was used for statistical analysis, and values of P < 0.05 were considered to indicate statistical significance. Continuous variables were presented as mean ± standard deviation (SD), and as numbers or percentages for categorical data. Univariate analysis using student's t‐tests for continuous variables or a chi‐squared test for categorical variables was conducted to identify risk factors. The correlations between clinical parameters and the presence or absence of vertebral fractures in 428 participants were analyzed with the Spearman correlation coefficient. The NTOVCF‐related variables, the statistical significances that had been verified by both the univariate analysis and correlation analysis (P < 0.05), were identified as candidate independent factors to build the binary logistic regression model for risk prediction, and the logistic regression analysis was performed using a stepwise approach to determine independent predictors of the occurrence of vertebral fractures. The capacities of each significant clinical parameter to predict NTOVCF were assessed with a receiver operating characteristic (ROC) curve analysis. As an accuracy index, the AUC was used to evaluate the diagnostic performance of the variable; besides, specificity, sensitivity, positive and negative predictive values, and diagnostic efficiency, were evaluated for each diagnostic method. To identify potential collinearity between variables, collinearity tests were performed. To verify the validity of the risk model, this study randomly screened and selected 50 elderly patients with primary osteoporosis and another 50 patients with primary OVCF, and extracted the data and information required for verification. The validation of the new model was carried out by comparing it to T‐score‐based prediction, the OSTA risk tool, and the FRAX system (http://www.sheffield.ac.uk/FRAX), and the comparison of ROC curves was conducted.
Results
The demographic characteristics and differences in lifestyle of the participants were shown in Table 1. No significant differences were found between groups regarding gender, age, region, menopausal age, BMI, smoking, alcohol consumption, Calcium taken, and vitamin D3 supplement. However, the rate of the participants who achieved sufficient daily physical activity in the fractured group was significantly lower than that in the control group (16.8% vs. 36.8%) (P < 0.001).
TABLE 1.
Comparison of the demographic characteristics and lifestyles between groups
| Variables | NTOVCF (n = 208) | Control (n = 220) | P value | |
|---|---|---|---|---|
| Age (years) a | 73.9 ± 4.6 | 73.1 ± 3.6 | 0.101 | |
| Gender (female/male) b | 143/65 | 136/84 | 0.133 | |
| Menopausal age (years) a | 49.5 ± 3.0 | 50.0 ± 2.9 | 0.221 | |
| BMI (kg/m2) a | 22.97 ± 1.82 | 23.11 ± 3.42 | 0.606 | |
| Region of the patient (n) b | Northern female | 34 (16.3%) | 40 (18.2%) | 0.315 |
| Northern male | 13 (6.3%) | 19 (8.6%) | ||
| Southern female | 109 (52.4%) | 96 (43.6%) | ||
| Southern male | 52 (25.0%) | 65 (29.5%) | ||
| Cigarette smoking b | Never | 158 | 165 | 0.355 |
| Previous | 39 | 36 | ||
| Present | 11 | 19 | ||
| Alcohol drinking b | Never | 151 | 148 | 0.286 |
| Previous | 48 | 65 | ||
| Present | 9 | 7 | ||
| Daily activity b | Underactivity | 173 | 139 | 0.000 |
| Normal | 35 | 81 | ||
| Calcium tablets taken b | Daily use | 33 | 25 | 0.392 |
| Irregular taken | 56 | 61 | ||
| Never | 119 | 134 | ||
| Vitamin D3 supplement b | Daily use | 3 | 7 | 0.404 |
| Irregular taken | 21 | 18 | ||
| Never | 184 | 195 | ||
Notes: The North–South boundary refers to the Qinling‐Huaihe line.
Abbreviation: BMI, body mass index.
Values were expressed as mean ± SD and evaluated by the student's t‐test.
Values were expressed as numbers and compared using the Chi‐square test.
Differences in BMD, Serum Bone Turnover Markers, and Related Hormones
Compared with the control group, the levels of β‐CTX (0.89 ± 0.65 vs. 0.57 ± 0.30 ng/ml), ACP (8.82 ± 1.23 vs 8.21 ± 1.11 U/L), and NACP (4.71 ± 0.70 vs 4.13 ± 0.61U/L) were significantly higher in NTOVCF group (P < 0.001). However, the serum PTH and 25(OH)D in the NTOVCF group were 28.31 ± 17.28 pg./ml and 21.93 ± 8.39 ng/ml, significantly lower than the 38.11 ± 20.17 pg./ml and 24.82 ± 8.50 ng/ml in the control group (P < 0.001). There were no significant differences between groups regarding the levels of P1NP, ALP, N‐MID, Ca, P, Mg, and PACP. For analyzing the relative strength of the bone formation and bone resorption, the β‐CTX/P1NP ratio was significantly higher in the NTOVCF group than in the control group (0.02509 ± 0.02586 vs 0.01184 ± 0.0060, P < 0.001). The average of the BMD T‐score in the NTOVCF group was significantly lower than that in the control group (−3.48 ± 0.47 vs −3.07 ± 0.44, P < 0.001), and the proportions of patients with second‐and third‐degree osteoporosis in NTOVCF group were significantly higher than those in the control group (41.3% and 14.9% vs 16.4% and 4.1%, respectively, P < 0.001). (Table 2 and Fig. 2A,B).
TABLE 2.
Comparison of the laboratory results and biochemical indices between groups
| Variables | NTOVCF(n = 208) | Control 2 (n = 220) | P value |
|---|---|---|---|
| RBC (×1012/L) a | 4.19 ± 0.60 | 4.41 ± 0.60 | 0.000 |
| HB (g/L) a | 119.95 ± 16.85 | 129.86 ± 15.11 | 0.000 |
| Normal (n) a | 152 (75.2%) | 197 (89.5%) | 0.000 |
| Mild deficiency (n) a | 40 (19.8%) | 22 (10.0%) | |
| Moderate deficiency (n) b | 16 (7.9%) | 1 (0.5%) | |
| Severe deficiency (n) b | 0 | 0 | |
| WBC (×109/L) b | 6.70 ± 1.73 | 6.62 ± 1.86 | 0.618 |
| Platelet (×109/L) b | 249.52 ± 75.31 | 238.60 ± 72.51 | 0.128 |
| TP (g/L) b | 63.89 ± 5.92 | 65.76 ± 6.07 | 0.001 |
| ALB (g/L) b | 34.23 ± 3.93 | 37.90 ± 4.07 | 0.000 |
| Normal (n) a | 83 (39.9%) | 176 (80.0%) | 0.000 |
| Mild malnutrition (n) a | 84 (40.4%) | 40 (18.2%) | |
| Moderate malnutrition (n) b | 39 (18.8%) | 4 (1.8%) | |
| Severe malnutrition (n) b | 2 (1.0%) | 0 | |
| UA (umol/L) b | 317.40 ± 81.89 | 328.17 ± 82.33 | 0.176 |
| Glu (mmol/L) b | 5.63 ± 1.33 | 5.52 ± 1.26 | 0.407 |
| TC (mmol/L) b | 5.26 ± 1.21 | 4.99 ± 0.86 | 0.008 |
| TG (mmol/L) b | 1.52 ± 0.58 | 1.60 ± 0.67 | 0.427 |
| HDL‐C (mmol/L) b | 1.39 ± 0.39 | 1.19 ± 0.26 | 0.000 |
| LDL‐C (mmol/L) b | 3.17 ± 0.96 | 3.19 ± 0.72 | 0.806 |
Abbreviations: ALB, serum albumin; Glu, blood glucose; HB, hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; RBC, red blood cell; TC, total cholesterol; TP, total protein; TG, triglyceride; WBC, white blood cell.
Notes: Values were expressed as numbers and compared using a chi‐square test.
Values were expressed as mean ± SD and evaluated by the student's t‐test.
FIG. 2.
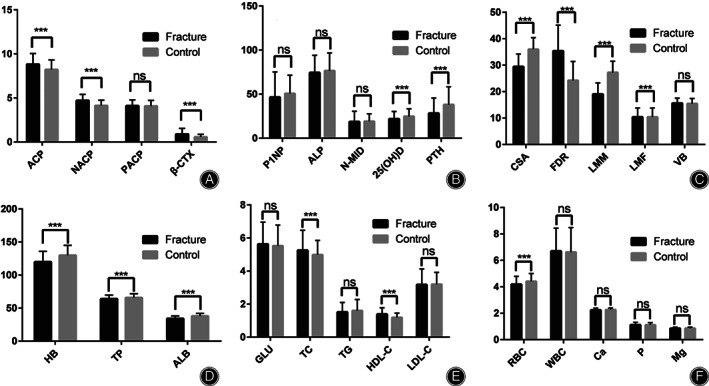
(A and B) showed the differences in serum bone turnover markers and related hormones. MRI findings were demonstrated in (C), and blood routine and serum biochemical indicators were shown in (D–F). Statistical differences were expressed as P < 0.05 (*), P < 0.01 (**), and P < 0.001 (***), besides, “ns” meant no significant difference. Parathyroid Hormone (PTH, pg./ml), 25(OH)D (ng/ml), propeptide of type I procollagen (P1NP, ng/ml), b‐C‐terminal telopeptide of type I collagen (β‐CTX, ng/ml), N‐terminal middle segment osteocalcin (N‐MID, ng/ml), alkaline phosphatase (ALP, U/L), acid phosphatase (ACP, U/L), non‐prostatic acid phosphatase (NACP, U/L), prostatic acid phosphatase (PACP, U/L), bone mineral density (BMD, T‐score); Cross‐sectional area (CSA, cm2), vertebral body size (VB, cm2), fatty degeneration ratio (FDR, %), lumbar muscle mass (LMM, cm2), lumbar muscle fat content (LMF, cm2); Red blood cell (RBC, ×1012/L), hemoglobin (HB, g/L), white blood cell (WBC, ×109/L). serum albumin (ALB, g/L), total protein (TP, g/L), total cholesterol (TC, mmol/L), triglyceride (TG, mmol/L), high‐density lipoprotein cholesterol (HDL‐C, mmol/L), low‐density lipoprotein cholesterol (LDL‐C, mmol/L), blood glucose (Glu, mmol/L), calcium (Ca, mmol/L), phosphorus (P, mmol/L), magnesium (Mg, mmol/L)
Magnetic Resonance Imaging Measurements
The average of VB was 15.59 ± 1.95 cm2 in the NTOVCF group and similar to the 15.59 ± 1.95 cm2 of the control group (P > 0.05), which also meant that imaging measurements included in the comparison such as FDR, CSA, LMM, and LMF, were comparable. The values of CSA and LMM of the paraspinal muscle (erector spinae and multifidus) were significantly less in the NTOVCF group than in the control group (29.45 ± 4.76 and 19.04 ± 4.24 cm2 vs 35.96 ± 4.43 and 27.24 ± 4.22 cm2, P < 0.001), while the average of LMF in the NTOVCF group were 10.41 ± 3.39 cm2, significantly higher than the 8.72 ± 2.78 cm2 of the control group (P < 0.001). The degree of lumbar muscular fat degeneration in the NTOVCF group was notably severe than in the control (35.38 ± 9.73 vs 24.24 ± 7.16, P < 0.001), and the proportion of severe degeneration in the NTOVCF group was 12.5%, significantly higher than the 1.8% of the control group (p < 0.001) (Table 3 and Fig. 2C).
TABLE 3.
Comparison of the levels of BTMs and BMD between groups
| Variables | NTOVCF (n = 208) | Control (n = 220) | P value |
|---|---|---|---|
| P1NP (ng/ml) a | 46.58 ± 28.57 | 50.75 ± 20.67 | 0.086 |
| β‐CTX (ng/ml) a | 0.89 ± 0.65 | 0.57 ± 0.30 | 0.000 |
| N‐MID (ng/ml) a | 18.72 ± 11.81 | 19.16 ± 8.51 | 0.664 |
| ALP (U/L) a | 74.38 ± 19.72 | 76.57 ± 20.11 | 0.257 |
| ACP (U/L) a | 8.82 ± 1.23 | 8.21 ± 1.11 | 0.000 |
| NACP (U/L) a | 4.71 ± 0.70 | 4.13 ± 0.61 | 0.000 |
| PACP (U/L) a | 4.11 ± 0.67 | 4.08 ± 0.63 | 0.656 |
| PTH (pg/ml) a | 28.31 ± 17.28 | 38.11 ± 20.17 | 0.000 |
| 25(OH)D (ng/ml) a | 21.93 ± 8.39 | 24.82 ± 8.50 | 0.000 |
| Calcium (mmol/L) a | 2.24 ± 0.15 | 2.26 ± 0.14 | 0.092 |
| Phosphorus (mmol/L) a | 1.12 ± 0.19 | 1.11 ± 0.17 | 0.643 |
| Magnesium (mmol/L) a | 0.85 ± 0.09 | 0.87 ± 0.08 | 0.135 |
| β‐CTX/P1NP (%) a | 2.51 ± 2.59 | 1.18 ± 0.60 | 0.000 |
| BMD (T‐score) a | −3.48 ± 0.47 | −3.07 ± 0.44 | 0.000 |
| First‐degree (n) b | 91 (43.8%) | 175 (79.5%) | 0.000 |
| Second‐degree (n) b | 86 (41.3%) | 36 (16.4%) | |
| Third‐degree (n) b | 31 (14.9%) | 9 (4.1%) |
Abbreviations: β‐CTX, b‐C‐terminal telopeptide of type I collagen; ACP, acid phosphatase; ALP, alkaline phosphatase; BMD, bone mineral density; NACP, non‐prostatic acid phosphatase; N‐MID, N‐terminal middle segment osteocalcin; PACP, prostatic acid phosphatase; P1NP, propeptide of type I procollagen; PTH, Parathyroid Hormone.
Notes: Values were expressed as mean ± SD and evaluated by the student's t‐test.
Values were expressed as numbers and compared using a Chi‐square test.
The Differences in the Blood Test and Biochemical Indices
The levels of TP and ALB in the NTOVCF group (63.89 ± 5.92 g/L and 34.23 ± 3.93 g/L, respectively) were significantly lower than those in the control group (65.76 ± 6.07 and 37.90 ± 4.07, respectively) (P = 0.001 and P < 0.001). Similar results were observed regarding RBC and HB, 4.19 ± 0.60 × 1012/L and 119.95 ± 16.85 g/L in the NTOVCF group, while 4.41 ± 0.60 × 1012/L and 129.86 ± 15.11 g/L in the control group, respectively (P < 0.001). In terms of nutritional status, the proportion of normal, mild, moderate, and severe malnutrition in the NTOVCF group was 39.9%, 40.4%, 18.8%, and 1.0% respectively, which were significantly more severe than 80.0%, 18.2%, 1.8% and 0 in the control group (P < 0.001). Meanwhile, patients with a normal level, mild, and moderate deficiencies of HB accounted for 75.2%, 19.8%, and 7.9% of the NTOVCF group, respectively; in the control group, the proportions were 89.5%, 10.0%, 35%, and 0.5%, respectively (P < 0.001). For lipid metabolism, serum TC and HDL‐C in NTOVCF patients were 5.26 ± 1.21 mmol/L and 1.39 ± 0.39 mmol/L, significantly higher than those in the control, 4.99 ± 0.86 mmol/L, and 1.19 ± 0.26 mmol/L, respectively (P = 0.008 and 0.000); as for the analysis of TG and LDL‐C, no differences were evidenced for NTOVCF group in comparison with the control. There were no significant differences between groups as to the levels of WBC, platelet, Glu, and UA (P > 0.05) (Table 4, Fig. 2D,E,F).
TABLE 4.
Comparison of MRI imaging measurements between the two groups
| Variables | NTOVCF (n = 208) | Control (n = 220) | P value |
|---|---|---|---|
| CSA (cm2) a | 29.45 ± 4.76 | 35.96 ± 4.43 | 0.000 |
| VB (cm2) a | 15.59 ± 1.95 | 15.43 ± 1.99 | 0.392 |
| LMM (cm2) a | 19.04 ± 4.24 | 27.24 ± 4.22 | 0.000 |
| LMF (cm2) a | 10.41 ± 3.39 | 8.72 ± 2.78 | 0.000 |
| FDR (%) a | 35.38 ± 9.73 | 24.24 ± 7.16 | 0.000 |
| Mild degeneration (n) b | 0 | 0 | 0.000 |
| Moderate degeneration (n) b | 182 (87.5%) | 216 (98.2%) | |
| Severe degeneration (n) b | 26 (12.5%) | 4 (1.8%) |
Abbreviations: CSA, cross‐sectional area; FDR, fatty degeneration ratio; LMF, lumbar muscle fat content; LMM, lumbar muscle mass; VB, vertebral body size.
Notes: Values were expressed as mean ± SD and evaluated by the student's t‐test.
Values were expressed as numbers and compared using a Chi‐square test.
Correlations among BTMs, BMD, Lifestyles, Blood and Serum Parameters, MRI Measurements, and the Occurrence of NTOVCF
The occurrence of NTOVCF had significant correlations with TP, ALB, RBC, HB, 25 (OH)D, PTH, P1NP, BMD, β‐CTX, ACP, NACP, TC, HDL‐C, CSA, LMF, LMM, FDR and activities of daily living (all P values were <0.05). However, fractures had no obvious correlation with age, BMI, WBC, platelet, P1NP, N‐MID, ALP, Ca, P, Mg, smoking, alcohol drinking, and VB. The correlation analysis showed that the levels of 25 (OH)D, PTH, P1NP, TP, ALB, RBC, HB, LMM, CSA, BMD, and activities of daily living were negatively correlated with the occurrence of NTOVCF, while β‐CTX, β‐CTX/P1NP, ACP, NACP, TC, HDL‐C, LMF, and FDR were positively correlated with the occurrence of NTOVCF. (The detailed correlations were shown in Table 5).
TABLE 5.
NTOVCF related BTMs, BMD, biochemical indicators, imaging measurements
| Variables | Occurrence of NTOVCF | |
|---|---|---|
| R 2 | P value | |
| P1NP (ng/ml) | −0.131** | 0.007 |
| β‐CTX (ng/ml) | 0.277** | 0.000 |
| β‐CTX/P1NP (%) | 0.368** | 0.000 |
| 25(OH)D (ng/ml) | −0.157** | 0.001 |
| PTH (pg/ml) | −0.288** | 0.000 |
| TP (g/L) | −0.132** | 0.006 |
| ALB (g/L) | −0.420** | 0.000 |
| ALB degree (n)* | 0.429** | 0.000 |
| TC (mmol/L) | 0.157** | 0.001 |
| HDL‐C (mmol/L) | 0.284** | 0.000 |
| RBC (×1012/L) | −0.190** | 0.000 |
| HB (g/L) | −0.283** | 0.000 |
| HB degree (n)* | 0.220** | 0.000 |
| ACP (U/L) | 0.244** | 0.000 |
| NACP (U/L) | 0.405** | 0.000 |
| BMD (T‐score) | −0.410** | 0.000 |
| BMD degree (n)* | 0.369** | 0.000 |
| Daily activity (n)* | −0.225** | 0.000 |
| FDR (%) | 0.598** | 0.000 |
| FDR degree (n)* | 0.209** | 0.000 |
| CSA (cm2) | −0.613** | 0.000 |
| LMM (cm2) | −0.729** | 0.000 |
| LMF (cm2) | 0.289** | 0.000 |
Abbreviations: β‐CTX, b‐C‐terminal telopeptide of type I collagen; ALB, serum albumin; AUC, area under the ROC curve; BMD, bone mineral density; CI, confidence interval; CSA, cross‐sectional area; DE, diagnostic efficiency; FDR, fatty degeneration ratio; HB, hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LMF, lumbar muscle fat; LMM, lumbar muscle mass; NACP, non‐prostatic acid phosphatase; NPV, negative predictive value; P1NP, propeptide of type I procollagen; PPV, positive predictive value; PTH, Parathyroid Hormone; R 2, correlation coefficient; TC, total cholesterol.
Notes: All P values were calculated with the Spearman correlation analysis. **P value < 0.01 was considered the correlation was particularly significant.
The Performances of the Related Factors in Predicting the Occurrence of NTOVCF
Receiver operating characteristic curves of β‐CTX, P1NP, 25(OH)D, PTH, ALB, TC, HDL‐C, HB, NACP, BMD, activities of daily living, FDR, and CSA were shown in Fig. 3, where the cut‐off value, specificity and sensitivity, positive and negative predictive values, and diagnostic efficiency of each factor was demonstrated. Among these biomarkers, CSA showed the highest AUC (0.854, 95% confidence interval: 0.817–0.886) for predicting the occurrence of NVOVCF; both CSA and FDR had AUC values exceeding 0.8, meanwhile, all of the AUC values of β‐CTX/P1NP, ALB, ALB degree, NACP, and BMD were larger than 0.7 (Table 6 and Fig. 3).
FIG. 3.
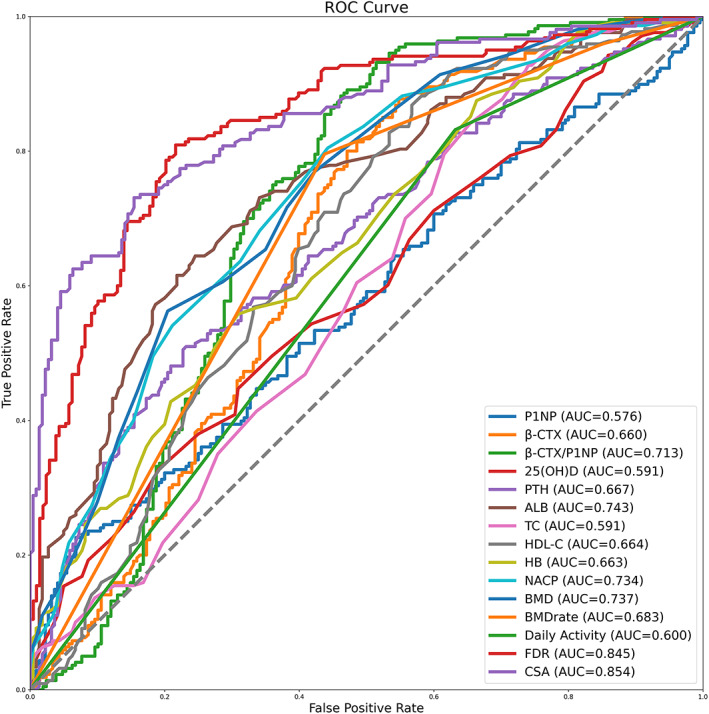
The performances of the related factors in predicting the occurrence of NTOVCF. Receiver operating characteristic curves (ROC) of hemoglobin (HB), serum albumin (ALB), total cholesterol (TC), high‐density lipoprotein cholesterol (HDL‐C), propeptide of type I procollagen (P1NP), b‐C‐terminal telopeptide of type I collagen (β‐CTX), non‐prostatic acid phosphatase (NACP), bone mineral density (BMD), fatty degeneration ratio (FDR), cross‐sectional area (CSA), and activities of daily living (daily activity) were shown. Area under curve (AUC)
TABLE 6.
The performances of the related factors in predicting the occurrence of NTOVCF
| Variables | AUC (95% CI) | P value | Cut‐off value | Sensitivity (%, 95% CI) | Specificity (%, 95% CI) | PPV (%, 95% CI) | NPV (%, 95% CI) | DE (%, 95% CI) |
|---|---|---|---|---|---|---|---|---|
| P1NP (ng/ml) | 0.576 (0.527–0.623) | 0.0064 | 23.94 | 22.6 (17.1–28.9) | 93.2 (89.0–96.1) | 75.8 (64.4–84.4) | 56.0 (54.0–58.0) | 58.9 (54.1–63.6) |
| β‐CTX (ng/ml) | 0.660 (0.613–0.705) | <0.0001 | 0.899 | 45.2 (38.3–52.2) | 87.7 (82.6–91.8) | 77.7 (70.3–83.6) | 62.9 (59.7–65.9) | 67.1 (62.4–71.5) |
| β‐CTX/P1NP (%) | 0.713 (0.667–0.755) | <0.0001 | 1.7092 | 53.9 (46.8–60.8) | 88.2 (83.2–92.1) | 81.2 (74.6–86.3) | 66.9 (63.4–70.2) | 71.5 (67.0–75.7) |
| 25(OH)D (ng/ml) | 0.591 (0.543–0.638) | 0.0009 | 20 | 44.7 (37.8–51.7) | 69.1 (62.5–75.1) | 57.8 (51.6–63.7) | 56.9 (53.2–60.6) | 57.2 (52.4–62.0) |
| PTH (pg/ml) | 0.667 (0.620–0.711) | <0.0001 | 24.6 | 51.0 (44.0–57.9) | 76.8 (70.7–82.2) | 67.5 (61.2–73.2) | 62.4 (58.6–66.0) | 64.3 (59.5–68.8) |
| ALB (g/L) | 0.743 (0.699–0.784) | <0.0001 | 35.6 | 64.4 (57.5–70.9) | 75.5 (69.2–81.0) | 71.3 (65.8–76.2) | 69.2 (64.8–73.2) | 70.1 (65.5–74.4) |
| ALB degree (n)† | 0.715 (0.669–0.757) | <0.0001 | 0 | 60.1 (53.1–66.8) | 80.0 (74.1–85.1) | 74.0 (68.1–79.1) | 68.0 (63.9–71.7) | 70.3 (65.8–74.6) |
| TC (mmol/L) | 0.591 (0.542–0.638) | <0.001 | 5.7 | 34.6 (28.2–41.5) | 84.1 (78.6–88.7) | 67.3 (59.0–74.6) | 57.6 (54.8–60.4) | 60.0 (55.2–64.7) |
| HDL‐C (mmol/L) | 0.664 (0.617–0.709) | <0.0001 | 1.44 | 43.3 (36.4–50.3) | 86.8 (81.6–91.0) | 75.6 (68.1–81.8) | 61.8 (58.7–64.8) | 65.7 (60.9–70.1) |
| HB (g/L) | 0.663 (0.616–0.708) | <0.0001 | 123 | 55.8 (48.7–62.6) | 69.1 (62.5–75.1) | 63.0 (57.5–68.3) | 62.3 (58.1–66.3) | 62.6 (57.8–67.2) |
| HB degree (n)† | 0.586 (0.537–0.633) | <0.0001 | 0 | 26.9 (21.0–33.5) | 89.6 (84.7–93.3) | 70.9 (60.9–79.2) | 56.4 (54.1–58.7) | 59.1 (54.3–63.8) |
| NACP (U/L) | 0.734 (0.689–0.775) | <0.0001 | 4.6 | 55.8 (48.7–62.6) | 80.5 (74.6–85.5) | 73.0 (66.8–78.4) | 65.8 (62.0–69.4) | 68.5 (63.8–72.8) |
| BMD (T‐score) | 0.737 (0.692–0.778) | <0.0001 | −3.5 | 56.3 (49.2–63.1) | 79.6 (73.6–84.7) | 72.2 (66.1–77.6) | 65.8 (61.9–69.5) | 68.2 (63.6–72.6) |
| BMD degree (n)† | 0.683 (0.636–0.727) | <0.0001 | 1 | 56.3 (49.2–63.1) | 79.6 (73.6–84.7) | 72.2 (66.1–77.6) | 65.8 (61.9–69.5) | 68.2 (63.6–72.6) |
| Daily avctivity (n)† | 0.600 (0.552–0.647) | <0.0001 | 0 | 83.2 (77.4–88.0) | 36.8 (30.4–43.6) | 55.5 (52.5–58.3) | 69.8 (62.0–76.6) | 59.3 (54.5–64.0) |
| FDR (%) | 0.845 (0.807–0.878) | <0.0001 | 28.49 | 78.4 (72.1–83.8) | 80.9 (75.1–85.9) | 79.5 (74.6–83.7) | 79.8 (75.2–83.8) | 79.7 (75.5–83.4) |
| FDR degree (n)† | 0.553 (0.505–0.601) | <0.0001 | 2 | 12.5 (8.3–17.8) | 98.2 (95.4–99.5) | 86.7 (69.8–94.8) | 54.3 (52.9–55.6) | 56.5 (51.7–61.3) |
| CSA (cm2) | 0.854 (0.817–0.886) | <0.0001 | 32.33 | 73.1 (66.5–79.0) | 84.6 (79.1–89.1) | 81.7 (76.5–86.0) | 76.9 (72.5–80.7) | 79.0 (74.8–82.7) |
Abbreviations: β‐CTX, b‐C‐terminal telopeptide of type I collagen; ALB, serum albumin; AUC, area under the ROC curve; BMD, bone mineral density; CI, confidence interval; CSA, cross‐sectional area; DE, diagnostic efficiency; FDR, fatty degeneration ratio; HB, hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; NACP, non‐prostatic acid phosphatase; NPV, negative predictive value; P1NP, propeptide of type I procollagen; PPV, positive predictive value; R 2, correlation coefficient; TC, total cholesterol.
Note: Data were presented as value (95% CI) or % (95% CI). p value <0.05 was considered a statistical difference.
Binary Logistic Regression Analysis and Risk Prediction Model for NTOVCF
The NTOVCF‐related variables, the statistical significances that had been verified by both the univariate analysis and correlation analysis (P < 0.05), were identified as candidate independent factors to build the logistic regression model. Since LMR and FDR were complementary, and LMF and LMM were calculated by multiplying FDR and LMR with CSA, respectively, only FDR and CSA were included in the binary regression analysis to avoid subsequent collinear problems. What is more, in the correlation analysis, strong correlations were found between those factors of the same practical significance such as RBC and HB, TP and ALB, and ACP and NACP (R 2 = 0.533, 0.626, and 0.895, respectively, and all the P values were <0.001), therefore, only the factors with higher correlation coefficients with NTOVCF were included in the regression analysis. After comprehensive consideration, β‐CTX/P1NP, 25(OH)D, PTH, ALB, TC, HDL‐C, BMD, HB, NACP, BMD, daily activity, FDR, and CSA were included in the final binary logistic regression analysis. Due to their small contribution to the prediction of NTOVCF, factors such as TC, PTH, 25(OH)D, and daily activity were not included in the final risk model. Logistic regression analysis showed that inadequate ALB, HB, BMD, and CSA, as well as increased β‐CTX/P1NP, NACP, HDL‐C, and FDR were risk factors for NTOVCF. (Table 7). Accordingly, the following risk prediction model for NTOVCF was obtained:
To verify the validity of the risk model, this study further screened and included 50 elderly patients with primary osteoporosis and another 50 patients with primary OVCF, and extracted the data and information required for verification. The validation of the new model was carried out by comparing it to T‐score‐based prediction, the OSTA risk tool [], and the FRAX system (http://www.sheffield.ac.uk/FRAX), and the comparison of ROC curves was conducted and demonstrated in Fig. 4. The AUC of the novel prediction model for NTOVCF was 0.979 (SE 0.013, 95%CI 0.928–0.997), significantly superior to the 0.720 (SE 0.050, 95%CI 0.621–0.805) of BMD, better than the 0.728 (SE 0.051, 95%CI 0.629–0.812) of FRAX, and far outperformed the 0.680 (SE 0.054, 95%CI 0.579–0.769) of OSTA (p < 0.001). The R 2 value that represented overall model fitness was 0.833, the sensitivity (%) was 89.42 (84.4–93.3), and the specificity (%) was 95.45 (91.8–97.8) for the predicting of fractures. All these parameters reflected the good performance of the risk prediction model obtained based on this study.This study excluded patients with a history of fractures, which would affect the calculation of FRAX values, so in order to better evaluate the superiority of this new model over the FRAX system, a previously published AUC of FRAX for predicting OVCF 24 was also used and compared with the AUC of the new model. As reported, the AUC of the FRAX system for predicting OVCF was 0.796 (0.768–0.823), and the sensitivity and specificity were 74.85% and 78.52%, respectively. Among the risk prediction models tested in this study, the new model had the highest discriminating ability to identify OVCF, followed by FRAX, BMD, and OSTA tools.
TABLE 7.
Binary logistic regression analysis of the factors influencing the occurrence of NTOVCF
| Variables | β | SE | Wald | OR (95% CI) | P |
|---|---|---|---|---|---|
| ALB (g/L) | −0.158 | 0.051 | 9.780 | 0.854 (0.773–0.943) | 0.002 |
| HB (g/L) | −0.064 | 0.014 | 19.621 | 0.938 (0.912–0.965) | <0.001 |
| HDL‐C (mmol/L) | 1.905 | 0.631 | 9.118 | 6.720 (1.951–23.142) | 0.003 |
| β‐CTX/P1NP (%) | 0.721 | 0.244 | 8.761 | 2.057 (1.276–3.315) | 0.003 |
| NACP (U/L) | 1.325 | 0.311 | 18.121 | 3.763 (2.044–6.926) | <0.001 |
| BMD (T‐score) | −1.620 | 0.426 | 14.475 | 0.198 (0.086–0.456) | <0.001 |
| FDR (%) | 0.153 | 0.027 | 32.175 | 1.166 (1.105–1.229) | <0.001 |
| CSA (cm2) | −0.383 | 0.059 | 41.682 | 0.682 (0.607–0.766) | <0.001 |
Abbreviations: ALB, serum albumin; β‐CTX, b‐C‐terminal telopeptide of type I collagen; BMD, bone mineral density; β, regression coefficient; CI, Confidence interval; CPR, Ratio of β‐CTX/P1NP; CSA, cross‐sectional area; FDR, fatty degeneration ratio; HB, hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; NACP, non‐prostatic acid phosphatase; OR, odds ratio; P1NP, propeptide of type I procollagen; SE, standard error.
Note: Data were presented as value or value (95% CI). p value < 0.05 was considered a statistical difference.
FIG. 4.
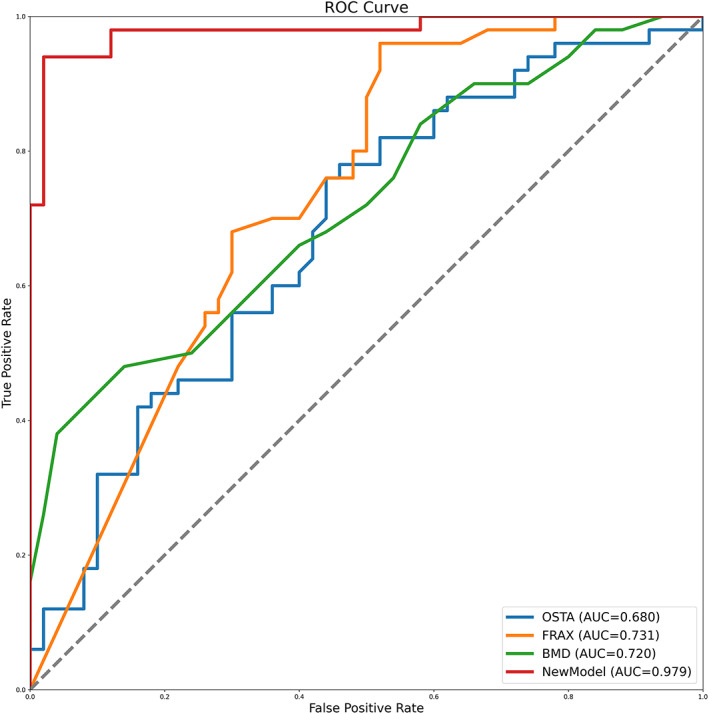
Comparing the receiver operating characteristic curves (ROC) of the new model to those of BMD T‐score‐based prediction and OSTA tool. Area under curve (AUC), bone mineral density (BMD), osteoporosis self‐assessment tool for Asians (OSTA)
Discussion
The results of this study suggested that nutritional deficiencies, anemia, hypoproteinemia, decreased physical activity, imbalances in bone metabolism, abnormal lipid metabolism, lacking micronutrients, and degenerative and decreased muscle mass, were all risk factors for fragility fractures in the elderly. As implied by the findings, the spontaneous fractures may be consequences of cumulative declines in multiple physiological systems over the lifespan, so from this perspective, this study also helped understand why still there has been a high incidence of vertebral fractures even after taking falls prevention and other preventive measures against fragile fractures.
Frailty
Frailty has been the most problematic expression of population aging, more seriously, with the aggravation of aging worldwide, the incidence of fragile fractures continues to increase, accompanied by a sharp increase in the demand for medical resources and a substantial increase in social health costs. 25 , 26 Worse still, the incidence of osteoporosis and related fractures is grossly underestimated and underdiagnosed. There are numerous modalities existing to visualize the suspected fractures, but identifying or predicting vertebras that are about to fracture is extremely difficult and poorly researched. 27 , 28 Instead, importantly, screening out relevant risk factors and potential causative factors is a more effective approach to predict fractures and intervene in advance accordingly. For the elderly population, many factors can lead to frailty and subsequent fragile fractures. Frailty is a pathologic accelerated decline in strength, homeostatic function, and physiologic reserves; thus, it is considered a significant predictor of OVCFs in older adults. 29 , 30 However, frailty is intrinsically inter‐related with skeletal muscle, immune, endocrine, and other organ systems, and approaches used to measure it are wide variation; therefore, the current study has better classified the included indicators and evaluated the correlation between specific indicators and fractures, and constructed the risk model on this basis.
Serum Albumin and Hemoglobin
Although there was no age difference between the two groups of elderly patients, the serum albumin and hemoglobin concentrations were significantly lower in fracture patients, and the proportions of patients with severe hypoalbuminemia and anemia were significantly higher. As previous studies reported, decreased hemoglobin was associated with an increased risk of fragility fractures in women 31 and non‐vertebral fractures in men. 32 Patients with vertebral fractures had lower serum albumin levels. 33 In this study, albumin and hemoglobin had an AUC of 0.743 (0.699–0.784) and 0.663 (0.616–0.708) for predicting the fragile fracture, respectively. Anemia and hypoalbuminemia can reflect nutritional deficiencies in patients and are also related to patients' physical function and activity intensity, and maintenance of physical fitness. Moreover, as independent risk factors, anemia and hypoproteinemia increase the likelihood of frailty. Lower serum albumin is negatively connected with muscle strength. The deficiency of hemoglobin results in poor muscle oxygenation, decreased muscle strength, and inadequate physical activity, which are also important parameters of frailty. 34 , 35
Bone Mineral Density and Bone Turnover Biomarkers
The indispensable role of BMD in predicting the risk of NTOVCF has been well recognized in this study, and the consequences of fractures are partly attributed to the poor quality of the fragile bone. In the United States, osteoporosis screening is recommended for elders to identify individuals with abnormal values of BMD and a higher risk of related fragility fractures. 36 The BMD measurements play an important role in assessing bone mass and predicting the risk of fractures. However, as research progresses, there are different views and recommendations with hot debates. The most controversial issue is the discrepancy between bone density and incidence of fracture, where the role of bone quality is more emphasized in the prediction of fractures. 37 The BMD measurements could not sufficiently evaluate the microarchitecture and mineral composition of the bone microenvironment, which remain critical in evaluating the strength of the bone. Moreover, the BTMs are also important components of the risk prediction model for osteoporotic fractures. Having the ability to reflect the activities of osteogenesis and osteoclasts, the BTMs are relatively stable in serum and are considered the ideal serum biomarkers for evaluating bone metabolism. 38 This study reveals pathological bone metabolism in patients with fragility fractures, where the low‐transformation state of bone metabolism has been altered and the markers of bone metabolism are out of balance. β‐CTX, is a collagenous bone resorption indicator, its concentrations in fractured individuals were significantly higher than those of osteoporotic patients. However, no obvious difference was detected between groups regarding P1NP, a marker used to reflect the activity of osteoblasts. The aforementioned results of β‐CTX/P1NP represent the strength of osteoclastic activity relative to osteogenic activity. The ratio was significantly higher in the fractured population than in the control and had a high predictive efficiency for fracture. The AUC value of β‐CTX/P1NP in the prediction of fracture was 0.713 (0.667–0.755), and its sensitivity and specificity were 53.9 (46.8–60.8) and 88.2 (83.2–92.1) respectively at the optimal critical value. Qu et al. also found that the level of β‐CTX was significantly higher in fractured older women than in the non‐fracture group, and they considered the high level of β‐CTX as a risk factor for osteoporotic fracture. 38 However, another study supported the usage of P1NP as a predictor for bone density and reported nothing was correlated with β‐CTX. 39 There were no statistical differences in the levels of P1NP, ALP, and N‐MID between the two groups in this study, but found that NTOVCF patients had higher levels of NACP, which can be used to assist in the diagnosis of bone metabolism diseases. The marker of NACP also reflected the imbalanced bone microenvironment and confirmed the abnormal bone metabolism.
Lifestyles
PTH and 25(OH)D are closely related to the serum level of Ca and P, and both play integral roles in bone homeostasis. The current study did not identify differences in Ca and P metabolism between the two groups, but there were significant differences in the levels of PTH and 25(OH)D. Relative deficiencies of PTH and 25(OH)D were present in the fracture group, but the deficiencies were not severe and might have little determinant effect on the occurrence of fractures, so they were not included in the final regression model. Furthermore, the lack of difference in serum Ca concentration indicated that supplementation with Ca and 25(OH)D may have little effect on the likelihood of fragile fractures.
Skeletal Muscle Conditions
This study has confirmed that decreased lumbar muscle mass and muscle fat degeneration are important risk factors and predictors of osteoporotic fractures. Previous studies name this muscle condition sarcopenia, 40 , 41 a state of progressive loss of muscle mass and decrease in strength and function with age. The conceptual definition has been widely accepted but how to measure and quantify it varies and lacks consensus. To accurately analyze qualitative and quantitative differences in lumbar muscles between groups, MRI images were used for measurement. Furthermore, in this study, the MRI‐based measurements are independent and sensitive predictors of the risk for NTOVCF. As the results show, a significantly higher FDR and LMF were demonstrated in the fractured group and characterized by a smaller CSA and LMM as well. A similar phenomenon was confirmed in the findings of Tokeshi et al., 42 except that the case screening scheme included all OVCF participants without restrictions on the mechanism of injury. As Jeon et al. reported, in patients with OVCFs, paraspinal muscle fatty degeneration was a risk factor for progressive vertebral collapse. 43 Wang et al. also found that sarcopenia was an independent predictive factor of osteoporotic vertebral refracture. 44 Even after vertebral augmentation procedures for the treatment of OVCF, patients with sarcopenia still face a higher risk of postoperative mortality. 45 Paraspinal muscles are crucial for maintaining normal spinal alignment, and the loss of muscle mass and increase of fatty degeneration severely affect spinal balance and damage to muscle strength, consequently, leading to a state of vulnerability and increasing the risk of fragility fractures. This study demonstrated FDR and CSA could predict the risk of fragility fractures in elderly osteoporotic patients and outperform the BMD T‐score based risk prediction tool, with AUC of 0.845(0.807–0.878), 0.854(0.817–0.886) and 0.737(0.692–0.778) for OVCF, respectively. Even though BMD remains the gold standard in the diagnosis of osteoporosis and prediction of related fractures, as demonstrated in this study, the predictive power of both FDR and CSA are independent and even greater than that of BMD (T‐score). From this perspective, there is a need to reconsider BMD indexes as the ideal predictors of fragile fractures. In addition to FDR, lipid metabolism markers HDL‐C also played a role in the risk model, with a higher HDL‐C value suggesting a higher risk of fractures. The finding was unexpected and the underlying mechanism was unclear. This may be because HDL‐C contains a high amount of cholesterol, which is negatively correlated with BMD, thus resulting in a high risk of succeeding related fractures. 46
Activities of Daily Living
This study has confirmed that sufficient physical daily activities are beneficial to the occurrence of orthopedic fractures and lacking activity is correlated with a higher risk of OVCF. Adequate exercise could strengthen the lumbar muscles, improve physical function, and assist in maintaining coordination and balance of the skeletal muscle system, thereby significantly reducing the risk of fractures. 47 , 48 Additionally, adequate exercise will inevitably lead to sufficient skeletal muscle volume and strength, which can achieve a protective effect for fragile fractures as shown above. This study failed to identify the differences in lifestyles between groups regarding Ca, P, and Mg, cigarette smoking, and alcohol drinking. Qi et al. also found similar levels of Ca and P, 49 but in another study, serum Ca and P in the OVCF group were significantly lower than in the control group. 50 The study of Bae et al. also found no difference in the habits of smoking or drinking, 51 even though both factors have been reported to have a connection with the decrease of BMD. 52 However, these meaningless results may be caused by the limitation of the sample size.
Strengths and Limitations
The current study revealed the risk factors and potential causes for the occurrence of non‐traumatic osteoporotic vertebral fractures in the elderly and innovatively established a risk model to predict the incidence of NTOVCF, which could potentially be used as a risk prediction scheme for other vertebral fragility fractures as well. Compared with T‐score‐based prediction, the OSTA risk tool, and the FRAX® system, the biggest advantage of this risk prediction model is that it has a strong fragility fracture prediction performance because this study comprehensively analyzed the indicators of imaging examinations, laboratory tests, and demographics. With high sensitivity and specificity, the novel model had the ability to directly calculate the possibility of vertebral fractures and provided corresponding prevention and treatment recommendations for the elderly population during physical examinations and health checks. Considering the good performance of the FRAX tool in predicting the possibility of hip fractures, this study provides a reliable and stable prediction model for osteoporotic vertebral fractures and enriches the prediction tools for fragility fractures in the Chinese elderly population.
Though with a high discriminating ability to predict fractures, in clinical practice, the risk model incorporates eight fracture‐related factors and can be tedious and time‐consuming to use. Also, participants were collected and recruited from a single hospital, and could not be fully representative of the whole population. The design of this retrospective study itself was a limitation. This study only tested the superiority of the constructed model over other existing risk tools through internal cohort validation, which was based on the small sample size brought about by the strict inclusion and exclusion criteria of this study. However, this study included as many samples as possible in the internal validation, which improved statistical power and model stability to some extent. Constructing an external independent validation cohort of the model and conducting a multi‐center study will further strengthen the findings. This part of the study is already underway and the results will be announced in due course.
Conclusion
This study summarizes the typical characteristics of fragility fracture patients: nutritional deficiencies, muscle degeneration and strength loss, lack of daily exercise, decreased bone mass, and a state of frailty. In addition to bone turnover markers, serum albumin, lipid metabolism and paraspinal muscle condition were also important factors in predicting osteoporotic fractures; ALB, HB, CSA, and BMD were protective factors of NTOVCF, while β‐CTX, NACP, TC, HDL‐C, and FDR were risk factors. Based on the results of this study, a novel model capable of accurately predicting the probability of osteoporotic fracture risk was obtained. Targeted prevention and treatment are expected to reduce the incidence of fragile fractures.
Author Contributions
Zhenxing Wen: conception, design, acquisition of data, analysis and interpretation of data, drafting, and final approval. Xiaoyi Mo: acquisition of data, analysis, and interpretation of data, drafting, critical revision, and final approval. Shengli Zhao: imaging evaluation and measurement, acquisition of data, software, and final approval. Zhichao Qi: acquisition of data, interpretation of data, drafting, and final approval. Dan Fu: software, validation, critical revision, and final approval. Shifeng Wen: conceptualization, interpretation of data, software, critical revision, and final approval. Wing Hoi Cheung: grammatical modification, critical revision, and final approval. Bailing Chen: conceptualization, methodology, project administration, drafting, validation, supervision, and final approval. All authors read and approved the final manuscript.
Funding Information
The present study was supported by grants from the National Natural Science Foundation of China (No. 31570976), and the Science and Technology Program of Guangzhou (No. 201604020148). The content is solely the responsibility of the authors and does not represent any official views.
Ethics Statement
This study was approved by the ethics committee of The First Affiliated Hospital, Sun Yat‐sen University (No[2020]150), and all methods were performed in accordance with the Declaration of Helsinki and its later amendments or comparable ethical standards. Since the study was retrospective, the informed consent was exempt according to relevant regulations by the Institutional Review Board of The First Affiliated Hospital, Sun Yat‐sen University.
Zhenxing Wen, Xiaoyi Mo, and Shengli Zhao these authors contributed equally to this work.
Contributor Information
Shifeng Wen, Email: 93071@163.com.
Wing Hoi Cheung, Email: louischeung@cuhk.edu.hk.
Bailing Chen, Email: chenbl96@mail.sysu.edu.cn.
Data Availability Statement
Research‐related data generated and/or analyzed during the current study are not publicly available due to the limitations of data sharing in the clinical research center of The First Affiliated Hospital, Sun Yat‐sen University, but are available from the corresponding author on reasonable request.
References
- 1. Kendler DL, Bauer DC, et al. Vertebral fractures: clinical importance and management. Am J Med. 2016;129(2):221–10. [DOI] [PubMed] [Google Scholar]
- 2. Gambacciani M, Levancini M. Management of postmenopausal osteoporosis and the prevention of fractures. Panminerva Med. 2014;56(2):115–31. [PubMed] [Google Scholar]
- 3. Yong EL, Logan S. Menopausal osteoporosis: screening, prevention and treatment. Singapore Med J. 2021;62(4):159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miller PD. Management of severe osteoporosis. Expert Opin Pharmacother. 2016;17(4):473–88. [DOI] [PubMed] [Google Scholar]
- 5. Kerschan‐Schindl K. Prevention and rehabilitation of osteoporosis. Wiener Medizinische Wochenschrift (1946). 2016;166(1–2):22–7. [DOI] [PubMed] [Google Scholar]
- 6. Clynes MA, Harvey NC, Curtis EM, Fuggle NR, Dennison EM, Cooper C. The epidemiology of osteoporosis. Br Med Bull. 2020;133(1):105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnston CB, Dagar M. Osteoporosis in older adults. Med Clin North Am. 2020;104(5):873–84. [DOI] [PubMed] [Google Scholar]
- 8. Kanis JA, Cooper C, Rizzoli R, Reginster JY. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2019;30(1):3–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McCarthy J, Davis A. Diagnosis and management of vertebral compression fractures. Am Fam Physician. 2016;94(1):44–50. [PubMed] [Google Scholar]
- 10. Koh LK, Sedrine WB, Torralba TP, et al. A simple tool to identify asian women at increased risk of osteoporosis. Osteoporos Int. 2001;12(8):699–705. [DOI] [PubMed] [Google Scholar]
- 11. Subramaniam S, Chan CY, Soelaiman IN, et al. The performance of osteoporosis self‐assessment tool for Asians (OSTA) in identifying the risk of osteoporosis among Malaysian population aged 40 years and above. Arch Osteoporos. 2019;14(1):117. [DOI] [PubMed] [Google Scholar]
- 12. Lekamwasam S. The diversity of fracture risk assessment tool (FRAX)‐based intervention thresholds in Asia. Osteoporosis and Sarcopenia. 2019;5(4):104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chanplakorn P, Lertudomphonwanit T, Daraphongsataporn N, Sritara C, Jaovisidha S, Sa‐Ngasoongsong P. Development of prediction model for osteoporotic vertebral compression fracture screening without using clinical risk factors, compared with FRAX and other previous models. Arch Osteoporos. 2021;16(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao S, Mo X, Wen Z, et al. Declining serum bone turnover markers are associated with the short‐term positive change of lumbar spine bone mineral density in postmenopausal women. Menopause. 2022;29(3):335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fisher A, Srikusalanukul W, Fisher L, Smith PN. Lower serum P1NP/βCTX ratio and hypoalbuminemia are independently associated with osteoporotic nonvertebral fractures in older adults. Clin Interv Aging. 2017;12:1131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang Y, Dai J, Zhong W, Hu C, Lu S, Chai Y. Association between serum cholesterol level and osteoporotic fractures. Front Endocrinol. 2018;9:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang CWC, Tseng IJ, Yang SW, Lin YK, Chan WP. Lumbar muscle volume in postmenopausal women with osteoporotic compression fractures: quantitative measurement using MRI. Eur Radiol. 2019;29(9):4999–5006. [DOI] [PubMed] [Google Scholar]
- 18. Walters S, Chan S, Goh L, Ong T, Sahota O. The prevalence of frailty in patients admitted to hospital with vertebral fragility fractures. Curr Rheumatol Rev. 2016;12(3):244–7. [PubMed] [Google Scholar]
- 19. Kato S, Demura S, Kurokawa Y, et al. Correlation between osteoporotic vertebral fracture and abdominal trunk muscle strength in middle‐aged and older women. Arch Osteoporos. 2019;14(1):106. [DOI] [PubMed] [Google Scholar]
- 20. Freitas SS, Barrett‐Connor E, Ensrud KE, et al. Rate and circumstances of clinical vertebral fractures in older men. Osteoporos Int. 2008;19(5):615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jun HS, Kim JH, Ahn JH, et al. The effect of lumbar spinal muscle on spinal sagittal alignment: evaluating muscle quantity and quality. Neurosurgery. 2016;79(6):847–55. [DOI] [PubMed] [Google Scholar]
- 22. Kader DF, Wardlaw D, Smith FW. Correlation between the MRI changes in the lumbar multifidus muscles and leg pain. Clin Radiol. 2000;55(2):145–9. [DOI] [PubMed] [Google Scholar]
- 23. Ryg J, Engberg H, Mariadas P, et al. Barthel index at hospital admission is associated with mortality in geriatric patients: a Danish nationwide population‐based cohort study. Clin Epidemiol. 2018;10:1789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. An N, Lin JS, Fei Q. Beijing friendship hospital osteoporosis self‐assessment tool for elderly male (BFH‐OSTM) vs fracture risk assessment tool (FRAX) for identifying painful new osteoporotic vertebral fractures in older Chinese men: a cross‐sectional study. BMC Musculoskelet Disord. 2021;22(1):596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim HJ, Park S, Park SH, et al. Prevalence of frailty in patients with osteoporotic vertebral compression fracture and its association with numbers of fractures. Yonsei Med J. 2018;59(2):317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li G, Papaioannou A, Thabane L, Cheng J, Adachi JD. Frailty change and major osteoporotic fracture in the elderly: data from the global longitudinal study of osteoporosis in women 3‐year Hamilton cohort. J Bone Miner Res. 2016;31(4):718–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hernlund E, Svedbom A, Ivergård M, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos. 2013;8(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Keevil VL, Romero‐Ortuno R. Ageing well: a review of sarcopenia and frailty. Proc Nutr Soc. 2015;74(4):337–47. [DOI] [PubMed] [Google Scholar]
- 30. Kojima G. Frailty as a predictor of fractures among community‐dwelling older people: a systematic review and meta‐analysis. Bone. 2016;90:116–22. [DOI] [PubMed] [Google Scholar]
- 31. Lee EA, Shin DW, Yoo JH, Ko HY, Jeong SM. Anemia and risk of fractures in older Korean adults: a nationwide population‐based study. J Bone Miner Res. 2019;34(6):1049–57. [DOI] [PubMed] [Google Scholar]
- 32. Valderrábano RJ, Lee J, Lui LY, et al. Older men with anemia have increased fracture risk independent of bone mineral density. J Clin Endocrinol Metab. 2017;102(7):2199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van der Jagt‐Willems HC, van Hengel M, et al. Why do geriatric outpatients have so many moderate and severe vertebral fractures? Exploring prevalence and risk factors. Age Ageing. 2012;41(2):200–6. [DOI] [PubMed] [Google Scholar]
- 34. Mailliez A, Guilbaud A, Puisieux F, Dauchet L, Boulanger É. Circulating biomarkers characterizing physical frailty: CRP, hemoglobin, albumin, 25OHD and free testosterone as best biomarkers. Results of a meta‐analysis. Exp Gerontol. 2020;139:111014. [DOI] [PubMed] [Google Scholar]
- 35. Pires Corona L, Drumond Andrade FC, de Oliveira Duarte YA, Lebrao ML. The relationship between anemia, hemoglobin concentration and frailty in Brazilian older adults. J Nutr Health Aging. 2015;19(9):935–40. [DOI] [PubMed] [Google Scholar]
- 36. Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US preventive services task force recommendation statement. JAMA. 2018;319(24):2521–31. [DOI] [PubMed] [Google Scholar]
- 37. Ehresman J, Schilling A, Yang X, et al. Vertebral bone quality score predicts fragility fractures independently of bone mineral density. Spine J. 2021;21(1):20–7. [DOI] [PubMed] [Google Scholar]
- 38. Vasikaran S, Eastell R, Bruyère O, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22(2):391–420. [DOI] [PubMed] [Google Scholar]
- 39. Azizieh FY, Shehab D, Al Jarallah K, Mojiminiyi O, Gupta R, Raghupathy R. Circulatory pattern of cytokines, adipokines and bone markers in postmenopausal women with low BMD. J Inflamm Res. 2019;12:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Larsson L, Degens H, Li M, et al. Sarcopenia: aging‐related loss of muscle mass and function. Physiol Rev. 2019;99(1):427–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cruz‐Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tokeshi S, Eguchi Y, Suzuki M, et al. Relationship between skeletal muscle mass, bone mineral density, and trabecular bone score in osteoporotic vertebral compression fractures. Asian Spine Journal. 2021;15(3):365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jeon I, Kim SW, Yu D. Paraspinal muscle fatty degeneration as a predictor of progressive vertebral collapse in osteoporotic vertebral compression fractures. Spine J. 2022;22(2):313–20. [DOI] [PubMed] [Google Scholar]
- 44. Wang WF, Lin CW, Xie CN, et al. The association between sarcopenia and osteoporotic vertebral compression refractures. Osteoporos Int. 2019;30(12):2459–67. [DOI] [PubMed] [Google Scholar]
- 45. Bayram S, Akgül T, Adıyaman AE, Karalar Ş, Dölen D, Aydoseli A. Effect of sarcopenia on mortality after percutaneous vertebral augmentation treatment for osteoporotic vertebral compression fractures in elderly patients: a retrospective cohort study. World Neurosurg. 2020;138:e354–60. [DOI] [PubMed] [Google Scholar]
- 46. Ghorabi S, Shab‐Bidar S, Sadeghi O, Nasiri M, Khatibi SR, Djafarian K. Lipid profile and risk of bone fracture: a systematic review and meta‐analysis of observational studies. Endocr Res. 2019;44(4):168–84. [DOI] [PubMed] [Google Scholar]
- 47. Deng D, Lian Z, Cui W, Liang H, Xiao L, Yao G. Function of low back muscle exercise: preventive effect of refracture analysis of postoperative vertebral fractures. Der Orthopade. 2019;48(4):337–42. [DOI] [PubMed] [Google Scholar]
- 48. Ogawa T, Sueyoshi Y, Taketomi S, Chijiiwa N. Factors associated with skeletal muscle mass increase by rehabilitation in older adults with vertebral compression fractures. J Aging Phys Act. 2022;30(1):12–7. [DOI] [PubMed] [Google Scholar]
- 49. Qi H, Xue J, Gao J, Zhang Y, Sun J, Wang G. Changes of bone turnover markers and bone tissue content after severe osteoporotic vertebral compression fracture. Med Sci Monit. 2020;26:e923713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ying P, Gu M, Jiang X, et al. Serum calcium‐phosphorus product for predicting the risk of osteoporotic vertebral compression fractures in elderly patients: a retrospective observational study. J Orthop Res. 2022;17(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bae IS, Kim JM, Cheong JH, Han MH, Ryu JI. Association between cerebral atrophy and osteoporotic vertebral compression fractures. PloS One. 2019;14(11):e0224439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lu Y, Di YP, Chang M, et al. Cigarette smoke‐associated inflammation impairs bone remodeling through NFκB activation. J Transl Med. 2021;19(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research‐related data generated and/or analyzed during the current study are not publicly available due to the limitations of data sharing in the clinical research center of The First Affiliated Hospital, Sun Yat‐sen University, but are available from the corresponding author on reasonable request.


