Highlights:
-
•
Septal area is a major cholinergic neuronal aggregate involved in Parkinson’s disease relevant to cognitive and motor functions.
-
•
We manually segmented septal nuclei of PD patients and healthy subjects of 100 people.
-
•
Unlike other subcortical grey structure, Septal nuclei significantly shrinks in PD.
-
•
Septal degeneration is associated with motor dysfunction in PD.
Keywords: Parkinson’s disease, Subcortical grey, Septal nuclei, MRI
Abstract
Objective
Parkinson’s disease (PD) mainly affects basal ganglia including septal nuclei. Septal nuclei have extensive cholinergic connections with thalamus and brain stem nuclei. We hypothesized that the degeneration of septal nuclei has an impact on dopaminergic (motor) and non-dopaminergic (cognitive) symptoms in PD.
Method
Clinical and MRI data of 80 patients with Parkinson’s disease and 20 healthy controls (HC) with a structural magnetic resonance imaging (MRI) were selected from their first visit from PPMI database. Septal nuclei were manually segmented from T1W images according to previously established anatomical criteria. In addition, subcortical structures such as thalamus, amygdala, hippocampus, caudate, putamen, pallidum and accumbens were automatically segmented.
Results
Volume of septal nuclei in the patients with PD was decreased in comparison with controls. These changes were independent of volume changes in other subcortical grey structure in PD. In addition, we found a correlation between motor components of unified Parkinson’s disease rating scale (UPDRS) and volume of septal nuclei in PD. Other clinical measures such as olfactory test, upper extremity function (mobility) performance, total UPDRS, lower extremity function (mobility) performance, and cognitive function were significantly more in PD group than in control. No correction was found between cognitive function and volume of septal nuclei.
Conclusion
We concluded that septal nuclei is distinctly affected in PD and is strongly associated with motor impairment. This may be a modulatory effect of cholinergic system on dopaminergic and glutamergic system. It is suggested that volume of septal nuclei may be a useful biomarker in PD diagnosis and monitoring.
1. Introduction
Parkinson’'s disease is a progressive neurodegenerative disorder, characterized by bradykinesia, rigidity, and tremor. These problems mainly stem from pathological changes in the basal ganglia and are often related to dopaminergic function. However, PD is also associated with postural instability, depression, and cognitive impairment, which are not responsive to dopaminergic therapy but directly affect the quality of life of these patients [1]. Several studies showed association of cortical atrophy (and not subcortical grey structures) with cognitive impairment in PD [2], [3], [4], [5]. Structural changes in the subcortical regions and their contribution to motor dysfunction have drawn less attention.
One of the important, but least studied, subcortical grey structures in PD is septal nuclei. Septal nuclei are part of the functional network that regulates learning, memory, pleasure, reward, movement, and cognition. Septal nuclei receive bilateral connections from olfactory bulb, hippocampus, amygdala, hypothalamus, and thalamus [6], [7]. Septal region contains ample cholinergic neurons [8]. Mesulam divided cholinergic neurons into the medial septal nucleus (Ch1), the nucleus of the vertical limb of the diagonal band of Broca (Ch2), the nucleus of the horizontal limb of the DBB (Ch3) and the nucleus basalis of Meynert (Ch4). Ch1 and Ch2 provide cholinergic input to the hippocampal complex, Ch3 to the olfactory bulb and Ch4 to the neocortex and amygdala.[8] Several cholinergic drugs have been shown to improve cognition in PD [9], [10]. Cholinergic system is in close relationship with dopaminergic system [11] and there are evidence to believe this system may contribute to global impairment of motor dysfunction in PD too [12], [13], [14].
The aim of this study was to investigate structural changes of septal nuclei in comparison to other subcortical grey structures in PD and its relationship with PD symptoms to elucidate the relationship between these changes and PD symptomatology.
2. Materials and methods
2.1. MRI subjects
Data used in the preparation of this article were obtained from the Parkinson’s Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org. The data were obtained between 2011 and 2013. Details of data acquisition has been explained in PPMI website (https://www.ppmi-info.org/study-design). In brief, high resolution T1-weighted scans were acquired sagittally on a 3 T Siemens scanner with MPRAGE sequence, TE = 3.0 ms, TR = 2300 ms, TI = 900 ms, flip angle = 9.0°, matrix = 240.0 × 256.0 × 176.0, voxel size 1 mm isotropic, Pulse Sequence = GR/IR. In addition, all participants underwent medical, psychiatric, neuropsychological testing and neurological assessments as per PPMI protocol (https://www.ppmi-info.org/study-design). These include Clock Drawing Test, Geriatric Depression Scale (GDS), University of Pennsylvania Smell Identification Test (UPSIT), Epworth Sleepiness Scale (ESS), Benton Judgment of Line Orientation (JLO), Upper Extremity Function, Lower Extremity Function, UPDRS, Schwab and England ADL, Autonomic Score (SCOPA-AUT), Cognition Function-Short Form, Communication-Short Form, Boston Naming Test, Trail making A and B, State-Trait Anxiety Inventory (STAI), Hopkins Verbal Learning Test-Recall/Delayed Recall/Retention/Recognition, Lexical Fluency Test, Montreal Cognitive Assessment Test (MoCA), Semantic Fluency Test, Symbol Digit Modalities Test, and Rapid Eye Movement Sleep Behaviour Disorder Questionnaire (RBD).
We accessed PPMI data base on 17/1/2020. Subjects were selected consecutively from their first visit and based on the following criteria:
-
1-
Diagnosis of PD
-
2-
No comorbidity
-
3-
MRI with 1 mm isotropic resolution
-
4-
Having at least 95 % of behavioural and clinical data
-
5-
Healthy subjects matched age and sex with PD patients
3. Segmentation of septal nuclei and other subcortical grey structures
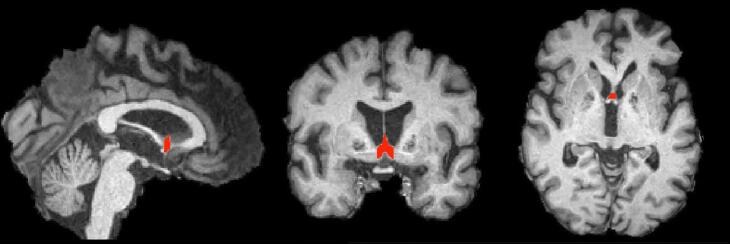
Septal nuclei were manually segmented and according to previously described anatomical criteria [15] which includes Ch1 (Medial septal nucleus) and Ch2 (Vertical limb of the diagonal band nucleus) cell groups. The anterior septal boundary was defined as the most anterior coronal slice in which both globus pallidi were visible and grey matter at the base of the septum pellucidum was present. The superior extent of septal nuclei was defined by the plane where the membranous septum pellucidum widened into septal nuclei. Lateral boundaries were defined by parallel sagittally oriented planes through the most inferior and medial aspect of each lateral ventricle. When a slice was anterior to the crossing fibres of the anterior commissure, the inferior boundary was the base of the brain. When the crossing fibres of the anterior commissure were fully visible, they served as the inferior boundary. The posterior boundary was defined by the following rule: A slice was considered to contain septal grey matter if it met at least 2 of the following 3 criteria: (1) the T1-weighted signal intensity indicates the presence of some grey matter (rather than pure white matter intensity); (2) presence of the crossing fibres of the anterior commissure; and (3) lack of cerebrospinal fluid (CSF) space in the centre of the septal region (corresponding to CSF space between columns of the fornix) [15] Fig. 1.
Fig. 1.
A typical example of manual segmentation of Septal nuclei.
For segmentation of other subcortical structures automated method using FIRST (part of FMRIB Software Library) was used [16]. The reason that septal nuclei was not automatically segmented was that this method is often inaccurate due to poor grey/white matter contrast in the region and scattered cellular distribution in the region. This is particularly true when there a severe atrophy exists. FIRST is a model-based segmentation/registration tool. The shape/appearance models used in FIRST are constructed from manually segmented images. The manual labels are parameterized as surface meshes and modelled as a point distribution model. This involves multiple steps including removal of non-brain tissue, affine registration to the MNI 152 (Montreal Neurological Institute) space at 1 mm resolution and finally segmentation based on shape models and voxel intensities [16]. Once these structures were segmented, their volume was calculated.
The volumetric data was analysed using IBM SPSS Statistics (Version 25.0.0). We used Kolmogorov–Smirnov test to ensure that the data has a normal distribution. Groups comparison (healthy controls vs Parkinson) was carried out using ANCOVA, controlling for age and sex as covariates of no interest. Secondly, Pearson correlation was used to explore the relationship between volume of septal nuclei with volume of other subcortical structures, and with clinical scores. Level of significance was defined at p < 0.05, correcting for multiple comparisons using Bonferroni’s correction when necessary.
4. Results
Subjects consisted of 80 patients with PD (mean age: 59.75 ± 10.29) and 20 normal control subjects (mean age: 60.15 ± 11.12). Within the PD group there were some missing data in clock drawing, upper extremity function, lower extremity function, cognition function, communication, Boston naming, trail making A and B, and lexical fluency. These missing data had a random pattern of distribution and were<5 % of cases.
Groups comparison showed no significant differences in sex, age, year of education and handedness (Table 1). Age was significantly correlated with the volume of thalami, nuclei accumbens and right hippocampus. Mean volume of septal nuclei were significantly (p < 0.0001) lower in PD patients (226.2 ± 76.4) than in control group (327.3 ± 63.4). Pearson correlation analysis did not show any significant relationship (after Bonferroni correction) between volume of septal nuclei and volume of other subcortical grey structures, however, there was a trend of correlation with the volume of left amygdala (see Table 2).
Table 1.
Demographic information.
| Variables | Healthy Controls | Parkinson's disease | p-value |
|---|---|---|---|
| Number of subjects | 20 | 80 | |
| Age, y | 60.15 ± 11. 12 | 59.75 ± 10.29 | 0.88 |
| Sex, M/F | 15/5 | 54/26 | 0.52 |
| Education, y | 16.25 ± 2.173 | 15.45 ± 2.66 | 0.22 |
| Handedness (R/L/Mixed) | 17/2/1 | 71/5/4 | 0.84 |
| Disease duration, y | NA | 6.31 ± 7.24 | NA |
| UPDRS III | 1.10 ± 1.97 | 19.31 ± 8.89 | 0.000 |
| MOCA | 28.20 ± 1.10 | 27.59 ± 2.26 | 0.24 |
| LEDD (mg) | NA | 253.41 ± 188.11 | NA |
*LEDD = Levodopa equivalent daily dosage; NA = not available.
Table 2.
Group comparison of volumes of subcortical grey structures and their relation with volume of septal nuclei.
|
Septal nucleus vs subcortical structures |
HC vs PD | |||
|---|---|---|---|---|
| Regions of interest | r | P | Mean ± SD | P |
| Right Globus pallidus | 0.19 | 0.05 | HC:1863.0 ± 190.6 PD:1892.7 ± 227.5 |
0.59 |
| Left Globus pallidus | 0.16 | 0.10 | HC:1834.9 ± 235.2 PD:1905.8 ± 236.5 |
0.24 |
| Right Caudate nucleus | 0.22 | 0.02 | HC:3586.8 ± 394.2 PD:3512.4 ± 369.7 |
0.43 |
| Left Caudate nucleus | 0.20 | 0.04 | HC:3513.1 ± 290.5 PD:3406.7 ± 330.6 |
0.19 |
| Right Thalamus | 0.17 | 0.09 | HC:7852.3 ± 614.0 PD:8006.8 ± 620.9 |
0.32 |
| Left Thalamus | 0.17 | 0.09 | HC:8128.2 ± 681.8 PD:8144.6 ± 606.4 |
0.92 |
| Right Putamen | 0.17 | 0.09 | HC:5032.8 ± 447.9 PD:4942.9 ± 481.6 |
0.45 |
| Left Putamen | 0.19 | 0.05 | HC:5043.7 ± 496.3 PD:4857.6 ± 462.4 |
0.11 |
| Right Nucleus accumbens | 0.21 | 0.03 | HC:410.3 ± 104.4 PD:403.4 ± 132.7 |
0.83 |
| Left Nucleus accumbens | 0.09 | 0.33 | HC:483.9 ± 134.7 PD:492.5 ± 149.4 |
0.81 |
| Right Hippocampus | 0.17 | 0.09 | HC:3944.1 ± 510.8 PD:3955.4 ± 478.0 |
0.93 |
| Left Hippocampus | 0.09 | 0.33 | HC:3831.4 ± 482.5 PD:3865.8 ± 458.1 |
0.76 |
| Right Amygdala | 0.07 | 0.44 | HC:1538.7 ± 302.3 PD:1567.4 ± 256.6 |
0.67 |
| Left Amygdala | 0.28 | 0.005 | HC:1506.7 ± 203.1 PD:1488.1 ± 213.9 |
0.73 |
Level of significance set at p < 0.003 after Bonferroni correction.
PD patients had significantly lower score on UPSIT, upper and lower extremity score, and UPDRS. Global Cognition performance was also lower in PD than in control. There was also a trend in Geriatric Depression Score in PD when compared with controls (Table 3). Handedness had no significant impact on the volume of septal nuclei and other subcortical grey structures in HC (Rt: 333.54 ± 15.95 vs Lt: 283 ± 28, p = 0.41) or in PD (Rt: 225 ± 54 ± 9.14 vs Lt: 226.8 ± 37.83, p = 0.79) group. Predominance of tremor or rigidity did not have any significant effect on the volume of septal nuclei or on the volume of any other subcortical grey structure in this study.
Table 3.
Behavioural measures and their relationship with the volume of septal nuclei.
|
Correlation with septal nucleus volume |
Group comparison HC vs PD |
|||
|---|---|---|---|---|
| r | p | Mean ± SD | p | |
| The Clock Drawing Test | 0.24 | 0.08 | HC:6.78 ± 0.67; PD:5.96 ± 1.63 |
0.15 |
| The Geriatric Depression Scale (GDS) | −0.14 | 0.15 | HC:0.90 ± 1.55; PD:2.63 ± 2.78 |
0.009 |
| The University of Pennsylvania Smell Identification Test (UPSIT) | 0.23 | 0.02 | HC:33.85 ± 4.60; PD:21.59 ± 8.57 | 0.000 |
| The Epworth Sleepiness Scale (ESS) | −0.22 | 0.03 | HC:5.30 ± 3.37; PD:6.18 ± 3.39 |
0.30 |
| Disease Duration | −0.11 | 0.34 | PD:6.31 ± 7.24 | – |
| The Benton Judgment of Line Orientation (JLO) | −0.10 | 0.88 | HC:27.10 ± 3.21 PD:25.75 ± 3.91 |
0.16 |
| Upper Extremity Function (Mobility) | 0.23 | 0.05 | HC: 40.00 ± 0.00 PD:35.65 ± 5.36 | 0.000 |
| Lower Extremity Function (Mobility) | 0.25 | 0.03 | HC:40.00 ± 0.00 PD:35.68 ± 5.24 | 0.000 |
| UPDRS part Ι | −0.25 | 0.01 | HC:2.45 ± 2.76 PD:5.14 ± 3.60 |
0.002 |
| UPDRS part II | −0.37 | 0.000 | HC:0.30 ± 0.66 PD:5.73 ± 4.06 |
0.000 |
| UPDRS part III | 0.33 | 0.001 | HC:1.10 ± 1.97 PD:19.31 ± 8.89 | 0.000 |
| UPDRS Total | 0.37 | 0.000 | HC:3.85 ± 3.30 PD:30.18 ± 13.52 | 0.000 |
| Schwab and England ADL | 0.13 | 0.26 | HC:100 ± 0 PD:94.19 ± 5.05 |
– |
| Autonomic score (SCOPA-AUT) | −0.25 | 0.01 | HC:5.85 ± 4.22 PD:8.90 ± 5.85 |
0.03 |
| Cognition Function-Short Form | 0.27 | 0.02 | HC:20.00 ± 0.00 PD:17.92 ± 2.45 | 0.000 |
| Communication-Short Form | 0.31 | 0.009 | HC:24.73 ± 0.64 PD:22.70 ± 3.08 |
0.03 |
| Boston Naming Test | 0.08 | 0.50 | HC:103.20 ± 32.81 PD:99.33 ± 34.24 |
0.74 |
| Trail making A | −0.08 | 0.49 | HC:38.50 ± 12.71 PD:41.33 ± 20.85 |
0.68 |
| Trail making B | 0.01 | 0.93 | HC:91.30 ± 37.26 PD:97.91 ± 53.89 |
0.09 |
| State-Trait Anxiety Inventory (STAI) | −0.13 | 0.19 | HC:59.80 ± 15.71 PD:67.00 ± 17.64 |
0.91 |
| Hopkins Verbal Learning Test-Recall | −0.082 | 0.42 | HC:47.00 ± 12.37 PD:47.33 ± 12.39 |
0.43 |
| Hopkins Verbal Learning Test-Delayed Recall | −0.07 | 0.49 | HC:43.00 ± 13.98 PD:45.44 ± 12.01 |
0.50 |
| Hopkins Verbal Learning Test-Retention | −0.04 | 0.66 | HC:44.20 ± 14.09 PD:46.13 ± 10.73 |
0.25 |
| Hopkins Verbal Learning Test-Recognition | −0.16 | 0.12 | HC:39.45 ± 16.02 PD:43.44 ± 13.29 |
0.49 |
| Lexical fluency Test | −0.07 | 0.57 | HC:43.10 ± 15.38 PD:40.12 ± 12.16 |
0.49 |
| Montreal Cognitive Assessment Test (MoCA) | 0.08 | 0.40 | HC:28.20 ± 1.10 PD:27.59 ± 2.26 |
0.24 |
| Semantic fluency Test | −0.07 | 0.49 | HC:48.45 ± 10.24 PD:49.76 ± 11.17 |
0.63 |
| Symbol Digit Modalities Test | 0.14 | 0.15 | HC:48.05 ± 10.32 PD:42.40 ± 10.39 |
0.03 |
| Rapid eye movement Sleep behavior Disorder Questionnaire (RBD) | −0.23 | 0.01 | HC:2.65 ± 1.75 PD:4.49 ± 2.81 |
0.007 |
| Levodopa Equivalent Daily Dose (LEDD) | 0.004 | 0.97 | HC: – PD:253.41 ± 188.10 |
– |
There was a trend with the volume of the right hippocampus (p = 0.09) but this difference did not survive after Bonferroni correction. There was no significant difference in the volume of septal nuclei and other subcortical grey structures in PD patient whose symptoms started on the right versus those whose symptoms started on the left.
Correlation of volume of subcortical grey structures with clinical symptoms showed a significant relationship between the volume of left thalamus and Symbol Digit Modalities score (r = 0.34, p < 0.0001). There was similar relationship with the volume of right thalamus, but the level of significance did not survive after Bonferroni correction (set at 0.0017). Also, there were negative correlations between the volume of thalami and trail making B score (p < 0.006).
5. Discussion
5.1. Volume change in septal nuclei and other subcortical grey structures
We studied volume of septal nuclei as measured on coronal MRI views of the brain of PD patients. This measurement corresponds with cholinergic groups of CH1 and CH2 in the septal region. We found that septal nuclei atrophy in PD and that this degeneration is distinct and unrelated to changes in other subcortical grey structures such as hippocampus, amygdala, thalamus, and basal ganglia.
Previous studies showed involvement of septal nuclei in Alzheimer's disease [15], affective disorders [17], and schizophrenia [18]. Butler et al. [19] studied septal nuclei (CH1 and CH2) in temporal lobe epilepsy and found that it was enlarged in these patients when compared with control group. In a review study, Callen et al. showed that the mean volume of the septal region (CH1 and CH2), which was obtained automatically, decreased in Alzheimer's disease compared to healthy controls [20]. Paradoxically, Butler et al. showed that MCI patient who were destined to develop AD had larger septal nuclei than those who did not [15]. These observations may suggest that neurons in the septal region play a compensatory role in response to neurodegenerative changes in Alzheimer’s disease in a way that they initially enlarge but undergo atrophy at the later disease stages [15].
We observed remarkable reduction of the volume of septal nuclei in PD compared to healthy controls. This change was unrelated to the volume changes in other subcortical structures. As septal nuclei are densely populated with cholinergic neurons, it is suggested that the main culprit contributing to this atrophy are cholinergic neurons (CH1 contains 10 % of cholinergic neurons and CH2 contains 70 % of cholinergic neurons). Pathology of cholinergic neurons in PD is well proven [21], however, lack of relationship between degeneration of these cholinergic neurons and other grey subcortical structures in our study suggest a distinct vulnerability of septal nuclei in PD pathology.
In addition to the septal nuclei, nucleus basalis of Meynert [22] and pedunculopontine nucleus (laterodorsal tegmental complex) [23] contain cholinergic neurons that play important roles in PD pathophysiology. Loss of cholinergic neurons in nucleus basalis of Meynert have been associated with cognitive impairment [24] and gait problem [25]. The latter is further supported by a clinical study showing association of atrophy of nucleus basalis of Meynert with gait disturbance even after deep brain stimulation [13]. In addition, a recent study showed decrease in myelination in the connections emerging from substantia nigra, nucleus basalis of Meynert, amygdala, hippocampus, and midbrain in patients with PD [26].
Whilst our findings on the reduction of septal nuclei in PD is consistent with previous findings, [27] we did not that this reduction was related to cognitive impairment. This difference could be either due to our study being underpowered in comparison to Barret et al, or the difference in anatomical segmentation of the septal area. We manually segmented the septal nuclei only but Barret et al used voxel-based morphometry and practically detected a larger area of the basal forebrain [27]. In addition, our cohort was relatively younger that other studies and our PD patients did not have significant global cognitive impairment. A previous study showed that memory problem in PD occurs only when there are medial temporal atrophy and cholinergic depletion per se was not enough to cause memory deficit [28]. In our cohort there was no significant cognitive deficit in PD group when compared with control. This may have lead to lack a linear correlation between cognitive impairment and volume of septal nuclei as there was not enough variability in the cognitive impairment in PD group. It is possible that if we did have a cohort with a more severe and variable cognitive impairment, a linear correlation would have been discovered. In comparison, motor dysfunction was more variable and prominent in this cohort, which is likely the reason such correlation was observed statistically.
6. Septal nuclei and clinical symptoms
We found strong correlation between motor impairment and volume of septal nuclei. This is likely to be due to direct effect of septal connections with pedunculopontine nucleus (PPN). PPN plays a significant role in motor symptoms of PD and is currently a therapeutic target for deep brain stimulation in PD [29]. PPN is located in the pons and is divided into two parts pars compacta with 80–90 % cholinergic neurones and pars dissipatus with mostly glutamergic neurones [30]. PPN has rich and direct connections with substantia nigra, subthalamic nucleus, cerebellum, thalamus and motor cortices through which it exerts a substantial role in motor control [31]. Absence of dopaminergic response to improve motor functions particularly freezing and gait problem is thought to be attributed to cholinergic deficit of PPN [14]. In this context, septal degeneration may be a contributing factor in cholinergic deficit of PPN leading to gait and balance difficulty in PD. A recent meta-analysis showed that acetylcholinesterase inhibitors might improve gait variability in Parkinson disease [12]. Putting together, there are emerging evidence of the effect of cholinergic system on global motor function particularly gait and balance in PD, however, this effect is most likely to be modulatory in nature than direct effect on motor control.
The volume of septal nuclei may also be useful in prediction of functional deficit in PD. This, however, requires a prospective study to identify the value of this radiomic measure in the diagnosis and prognosis of PD. Lower and upper extremity function scores were equally higher in healthy controls than in PD, suggesting that septal degeneration has a global impact on locomotion and is not limb specific.
As expected, [32], [33] olfactory score was lower in PD patients than in healthy controls, however, this did not correlate with the volume of septal region. This further suggests that cholinergic and dopaminergic neurodegeneration may be independent processes, although there are well recognized anatomical connections between olfactory tubercle and septal nuclei [34].
Cognitive function was significantly decreased in PD comparing to normal control. This is consistent with previous reports [2], [3], [5]. The time of onset of cognitive impairment varies in relation to other symptoms of the disease and its rate of progression. Approximately 40 % of people at early-stage of PD have some form of cognitive impairment which increases the risk of disease progression. These impairments may include problems in planning, working memory, decision making and executive function which are all associated with frontal lobe-striatum loop and is dopamine dependent [35]. Other symptoms such as attention deficit, semantic function, and visual-spatial abilities are related to cholinergic projections and are not affected by dopamine depletion in the brain [24], [36].
There are two limitations in this study; firstly, the number of subjects is relatively small. It is likely that some of the measures remained non-significant due to low study power. Secondly, manual segmentation of septal region requires careful consideration of boundaries which may be unclear in some patients particularly patients with severe septal atrophy. Automated segmentation methods would probably remove inter-researchers’ variability but may not improve accuracy of segmentation. It is suggested that in future studies these points are considered.
CRediT authorship contribution statement
Niloufar Kamalkhani: Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft. Mojtaba Zarei: Conceptualization, Project administration, Resources, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Data used in the preparation of this article were obtained from the Parkinson's Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data. “PPMI – a public-private partnership – is funded by the Michael J. Fox Foundation for Parkinson’s Research funding partners 4D Pharma, Abbvie, Acurex Therapeutics, Allergan, Amathus Therapeutics, ASAP, Avid Radiopharmaceuticals, Bial Biotech, Biogen, BioLegend, Bristol-Myers Squibb, Calico, Celgene, Dacapo Brain Science, Denali, The Edmond J. Safra Foundaiton, GE Healthcare, Genentech, GlaxoSmithKline, Golub Capital, Handl Therapeutics, Insitro, Janssen Neuroscience, Lilly, Lundbeck, Merck, Meso Scale Discovery, Neurocrine Biosciences, Pfizer, Piramal, Prevail, Roche, Sanofi Genzyme, Servier, Takeda, Teva, UCB, Verily, and Voyager Therapeutics.”
References
- 1.Bohnen N.I., Yarnall A.J., Weil R.S., Moro E., Moehle M.S., Borghammer P., Bedard M.A., Albin R.L. Cholinergic system changes in Parkinson's disease: emerging therapeutic approaches. Lancet Neurol. 2022;21(4):381–392. doi: 10.1016/S1474-4422(21)00377-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zarei M., Ibarretxe-Bilbao N., Compta Y., Hough M., Junque C., Bargallo N., Tolosa E., Martí M.J. Cortical thinning is associated with disease stages and dementia in Parkinson's disease. J. Neurol. Neurosurg. Psychiatry. 2013;84(8):875–882. doi: 10.1136/jnnp-2012-304126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ibarretxe-Bilbao N., Zarei M., Junque C., Marti M.J., Segura B., Vendrell P., Valldeoriola F., Bargallo N., Tolosa E. Dysfunctions of cerebral networks precede recognition memory deficits in early Parkinson's disease. Neuroimage. 2011;57(2):589–597. doi: 10.1016/j.neuroimage.2011.04.049. [DOI] [PubMed] [Google Scholar]
- 4.IbarretKe-Bilbao N., Junque C., Tolosa E., Marti M., Valldeoriola F., Bargallo N., Zarei M. Impaired decision-making and recognition of emotions in Parkinson's disease: a neuroimaging study of the orbitofrontal cortex T201. Eur. J. Neurol. 2008;15 [Google Scholar]
- 5.Ibarretxe-Bilbao N., Junque C., Tolosa E., Marti M.J., Valldeoriola F., Bargallo N., Zarei M. Neuroanatomical correlates of impaired decision-making and facial emotion recognition in early Parkinson’s disease. Eur. J. Neurosci. 2009;30(6):1162–1171. doi: 10.1111/j.1460-9568.2009.06892.x. [DOI] [PubMed] [Google Scholar]
- 6.DeFrance J.F., Yoshihara H., Chronister R.B. Electrophysiological studies of the septal nuclei: I. The lateral septal region. Exp. Neurol. 1976;53(2):399–419. doi: 10.1016/0014-4886(76)90081-9. [DOI] [PubMed] [Google Scholar]
- 7.DeFrance J.F., Yoshihara H., Chronister R.B. Electrophysiological studies of the septal nuclei. II. The medial septal region. Exp. Neurol. 1978;58(1):14–31. doi: 10.1016/0014-4886(78)90117-6. [DOI] [PubMed] [Google Scholar]
- 8.Mesulam M.M., Mufson E.J., Levey A.I., Wainer B.H. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J. Comp. Neurol. 1983;214(2):170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- 9.Baik K., Kim S.M., Jung J.H., Lee Y.H., Chung S.J., Yoo H.S., Ye B.S., Lee P.H., Sohn Y.H., Kang S.W., Kang S.Y. Donepezil for mild cognitive impairment in Parkinson's disease. Sci. Rep. 2021;11(1):4734. doi: 10.1038/s41598-021-84243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bohnen N.I., Teipel S.J. Cholinergic forebrain density loss in Parkinson disease: More than just cognitive changes. Neurology. 2018;90(18):823–824. doi: 10.1212/WNL.0000000000005408. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez-Catasus C.A., Bohnen N.I., D'Cruz N., Muller M. Striatal acetylcholine-dopamine imbalance in Parkinson disease: in vivo neuroimaging study with dual-tracer PET and dopaminergic PET-informed correlational tractography. J. Nucl. Med. 2022;63(3):438–445. doi: 10.2967/jnumed.121.261939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J.H., Huang T.W., Hong C.T. Cholinesterase inhibitors for gait, balance, and fall in Parkinson disease: a meta-analysis. NPJ Parkinsons Dis. 2021;7(1):103. doi: 10.1038/s41531-021-00251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilkins K.B., Parker J.E., Bronte-Stewart H.M. Gait variability is linked to the atrophy of the Nucleus Basalis of Meynert and is resistant to STN DBS in Parkinson's disease. Neurobiol. Dis. 2020;146 doi: 10.1016/j.nbd.2020.105134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bohnen N.I., Kanel P., Zhou Z., Koeppe R.A., Frey K.A., Dauer W.T., Albin R.L., Muller M. Cholinergic system changes of falls and freezing of gait in Parkinson's disease. Ann. Neurol. 2019;85(4):538–549. doi: 10.1002/ana.25430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butler T., Harvey P., Deshpande A., Tanzi E., Li Y., Tsui W., Silver C., Fischer E., Wang X., Chen J., Rusinek H., Pirraglia E., Osorio R.S., Glodzik L., de Leon M.J. Basal forebrain septal nuclei are enlarged in healthy subjects prior to the development of Alzheimer's disease. Neurobiol. Aging. 2018;65:201–205. doi: 10.1016/j.neurobiolaging.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patenaude B., Smith S.M., Kennedy D.N., Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56(3):907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brisch R., Bernstein H.G., Dobrowolny H., Krell D., Stauch R., Trubner K., Steiner J., Ghabriel M.N., Bielau H., Wolf R., Winter J., Kropf S., Gos T., Bogerts B. A morphometric analysis of the septal nuclei in schizophrenia and affective disorders: reduced neuronal density in the lateral septal nucleus in bipolar disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2011;261(1):47–58. doi: 10.1007/s00406-010-0119-9. [DOI] [PubMed] [Google Scholar]
- 18.Brisch R., Bernstein H.G., Krell D., Stauch R., Trubner K., Dobrowolny H., Kropf S., Bielau H., Bogerts B. Volumetric analysis of septal region in schizophrenia and affective disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2007;257(3):140–148. doi: 10.1007/s00406-006-0697-8. [DOI] [PubMed] [Google Scholar]
- 19.Butler T., Zaborszky L., Wang X., McDonald C.R., Blackmon K., Quinn B.T., DuBois J., Carlson C., Barr W.B., French J., Kuzniecky R., Halgren E., Devinsky O., Thesen T. Septal nuclei enlargement in human temporal lobe epilepsy without mesial temporal sclerosis. Neurology. 2013;80(5):487–491. doi: 10.1212/WNL.0b013e31827f0ed7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Callen D.J., Black S.E., Gao F., Caldwell C.B., Szalai J.P. Beyond the hippocampus: MRI volumetry confirms widespread limbic atrophy in AD. Neurology. 2001;57(9):1669–1674. doi: 10.1212/wnl.57.9.1669. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Lloret S., Barrantes F.J. Deficits in cholinergic neurotransmission and their clinical correlates in Parkinson's disease. NPJ Parkinsons. Dis. 2016;2:16001. doi: 10.1038/npjparkd.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mesulam M.M., Geula C. Nucleus basalis (Ch4) and cortical cholinergic innervation in the human brain: observations based on the distribution of acetylcholinesterase and choline acetyltransferase. J. Comp. Neurol. 1988;275(2):216–240. doi: 10.1002/cne.902750205. [DOI] [PubMed] [Google Scholar]
- 23.Heckers S., Geula C., Mesulam M.M. Cholinergic innervation of the human thalamus: dual origin and differential nuclear distribution. J. Comp. Neurol. 1992;325(1):68–82. doi: 10.1002/cne.903250107. [DOI] [PubMed] [Google Scholar]
- 24.Mattila P.M., Roytta M., Lonnberg P., Marjamaki P., Helenius H., Rinne J.O. Choline acetytransferase activity and striatal dopamine receptors in Parkinson's disease in relation to cognitive impairment. Acta Neuropathol. 2001;102(2):160–166. doi: 10.1007/s004010100372. [DOI] [PubMed] [Google Scholar]
- 25.Stein J.F. Akinesia, motor oscillations and the pedunculopontine nucleus in rats and men. Exp. Neurol. 2009;215(1):1–4. doi: 10.1016/j.expneurol.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 26.Boshkovski T., Cohen-Adad J., Misic B., Arnulf I., Corvol J.C., Vidailhet M., Lehericy S., Stikov N., Mancini M. The myelin-weighted connectome in Parkinson's disease. Mov. Disord. 2022;37(4):724–733. doi: 10.1002/mds.28891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barrett M.J., Sperling S.A., Blair J.C., Freeman C.S., Flanigan J.L., Smolkin M.E., Manning C.A., Druzgal T.J. Lower volume, more impairment: reduced cholinergic basal forebrain grey matter density is associated with impaired cognition in Parkinson disease. J. Neurol. Neurosurg. Psychiatry. 2019;90(11):1251–1256. doi: 10.1136/jnnp-2019-320450. [DOI] [PubMed] [Google Scholar]
- 28.Schmitz T.W., Nathan Spreng R. Alzheimer's Disease Neuroimaging, Basal forebrain degeneration precedes and predicts the cortical spread of Alzheimer's pathology. Nat. Commun. 2016;7:13249. doi: 10.1038/ncomms13249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamani C., Lozano A.M., Mazzone P.A., Moro E., Hutchison W., Silburn P.A., Zrinzo L., Alam M., Goetz L., Pereira E., Rughani A., Thevathasan W., Aziz T., Bloem B.R., Brown P., Chabardes S., Coyne T., Foote K., Garcia-Rill E., Hirsch E.C., Okun M.S., Krauss J.K. Pedunculopontine nucleus region deep brain stimulation in parkinson disease: surgical techniques, side effects, and postoperative imaging. Stereotact. Funct. Neurosurg. 2016;94(5):307–319. doi: 10.1159/000449011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.French I.T., Muthusamy K.A. A review of the pedunculopontine nucleus in Parkinson's disease. Front. Aging Neurosci. 2018;10:99. doi: 10.3389/fnagi.2018.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez-Gonzalez C., Bolam J.P., Mena-Segovia J. Topographical organization of the pedunculopontine nucleus. Front. Neuroanat. 2011;5:22. doi: 10.3389/fnana.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ibarretxe-Bilbao N., Junque C., Marti M.J., Valldeoriola F., Vendrell P., Bargallo N., Zarei M., Tolosa E. Olfactory impairment in Parkinson's disease and white matter abnormalities in central olfactory areas: a voxel-based diffusion tensor imaging study. Mov. Disord. 2010;25(12):1888–1894. doi: 10.1002/mds.23208. [DOI] [PubMed] [Google Scholar]
- 33.Ansari K.A., Johnson A. Olfactory function in patients with Parkinson's disease. J. Chronic Dis. 1975;28(9):493–497. doi: 10.1016/0021-9681(75)90058-2. [DOI] [PubMed] [Google Scholar]
- 34.Kohler C., Eriksson L.G. An immunohistochemical study of somatostatin and neurotensin positive neurons in the septal nuclei of the rat brain. Anat. Embryol. (Berl.) 1984;170(1):1–10. doi: 10.1007/BF00319452. [DOI] [PubMed] [Google Scholar]
- 35.Siepel F.J., Bronnick K.S., Booij J., Ravina B.M., Lebedev A.V., Pereira J.B., Gruner R., Aarsland D. Cognitive executive impairment and dopaminergic deficits in de novo Parkinson's disease. Mov. Disord. 2014;29(14):1802–1808. doi: 10.1002/mds.26051. [DOI] [PubMed] [Google Scholar]
- 36.Hilker R., Thomas A.V., Klein J.C., Weisenbach S., Kalbe E., Burghaus L., Jacobs A.H., Herholz K., Heiss W.D. Dementia in Parkinson disease: functional imaging of cholinergic and dopaminergic pathways. Neurology. 2005;65(11):1716–1722. doi: 10.1212/01.wnl.0000191154.78131.f6. [DOI] [PubMed] [Google Scholar]