Abstract
Tracheal tumors are rare diseases. They can cause narrowing of a central airway, a severe respiratory distress, and death. The objective of this case series is to highlight the role of rigid bronchoscopy in diagnosing and treating carina masses which are difficult to remove surgically. Tumor excision was performed by the rigid bronchoscopic intervention. Additional treatment was administered according to the diagnosis of each individual patient. After the procedure, patients’ symptoms were improved and stenotic central airways were reopened. Rigid bronchoscopy can be a good therapeutic option to reestablish airway patency and a bridge treatment for further definitive treatment.
Keywords: Rigid bronchoscopy, Carina mass, Bridging intervention
List of abbreviation
- APC
Argon plasma coagulation
- CT
Computed tomography
- FEV1
Forced expiratiory volume in the first second
- FVC
Forced vital capacity
1. Introduction
Primary tumors of the trachea can be benign or malignant; they account for fewer than 0.1% of tumors [1]. These neoplasms are often slow-growing and have an insidious onset until the airway becomes critically narrowed [2]. Because tracheal tumors can cause serious dyspnea due to severe obstruction of the central airway, clinicians must be aware of their initial manifestations, natural history, and recommended management [3]. The presenting symptoms are related to the location of the mass within the trachea. Complete surgical resection is recommended to treat primary malignant tracheal tumors. However, it is difficult to reconstruct when the carina is involved, and the mortality and morbidity are high. Therefore, it requires an experienced medical center with skilled surgeons [4]. Thus, rigid bronchoscopic intervention can be an effective modality to reduce morbidity and mortality in these patients. Moreover, it can be used as a bridging therapy to maintain airway patency and provide an opportunity for definitive therapeutic modalities [5]. Herein, we retrospectively reviewed three cases of patients with carina mass diagnosed and treated using rigid bronchoscopy. The latter two cases were reported previously [6,7].
2. Case 1
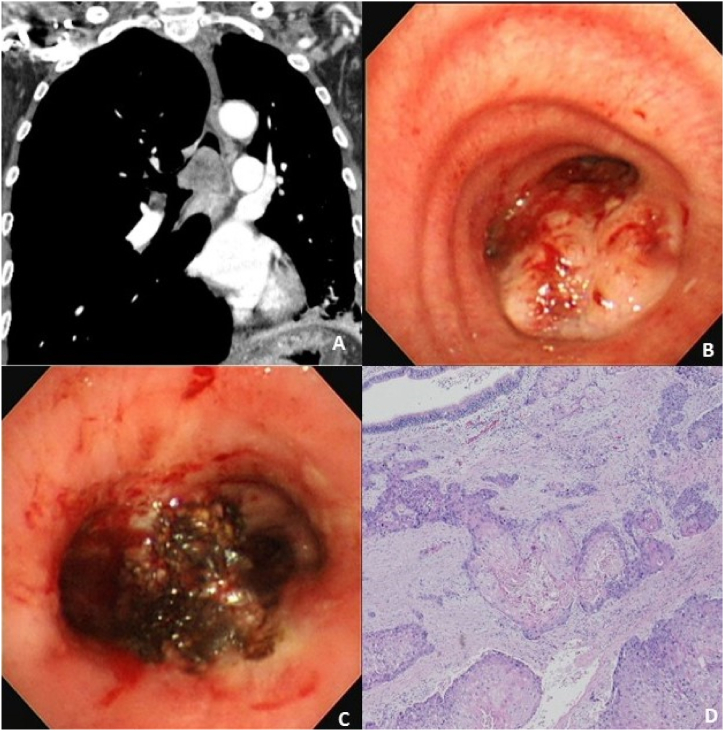
A 72-year-old male was transferred to our institute due to progressive respiratory acidosis with oxygen saturation at 85% despite the administration of 15L oxygen with a mask. He was intubated at the emergency room in our hospital. His chest computed tomography (CT) showed a 3.7-cm enhancing polypoid mass in the lower trachea, several enlarged mediastinal lymph nodes, and a consolidation in the left lower lobe (Fig. 1 A). His initial laboratory findings presented as an inflammatory condition, including an elevated white blood cell count of 25700/μl with 93.4% of neutrophils, 10.12 mg/dL of C-reactive protein, and 6.29 ng/mL of procalcitonin. He was admitted to the intensive care unit, and mechanical ventilation was applied. Broad spectrum antibiotics were prescribed to treat his pneumonia. To remove the carina mass and secure a patent airway, he underwent a rigid bronchoscopic intervention under general anesthesia. There was a huge hypervascular mass in his carina, obstructing both right and left main bronchi (Fig. 1 B). Tumor removal was performed using the tip of the rigid bronchoscope and rigid forceps. Argon plasma coagulation (APC) was applied on the tumor bed before and after the rigid bronchoscopic removal for control of bleeding and ablation of the residual tumor. After tumor removal, the left and right main bronchi were cleared (Fig. 1 C). Immediately after rigid bronchoscopic removal of the carinal mass, oxygen demand dramatically decreased, and the patient was weaned from mechanical ventilation. His pathologic findings confirmed squamous cell carcinoma (Fig. 1 D); thus, he was transferred to a lung cancer center to receive definitive treatment.
Fig. 1.
Chest computed tomography scan, bronchoscopy images, and histology of a 72-year-old man with a carinal mass
(A) Chest computed tomography showed a large enhancing mass obstructing the carina.
(B) Bronchoscopy revealed a large hypervascular tumor on the carina obstructing both right and left main bronchi.
(C) Bronchoscopy after removal of the carinal mass using the tip of rigid bronchoscope with pre- and post-application of argon plasma coagulation showed clear bilateral main bronchi.
(D) Her histology showed squamous cell carcinoma.
3. Case 2
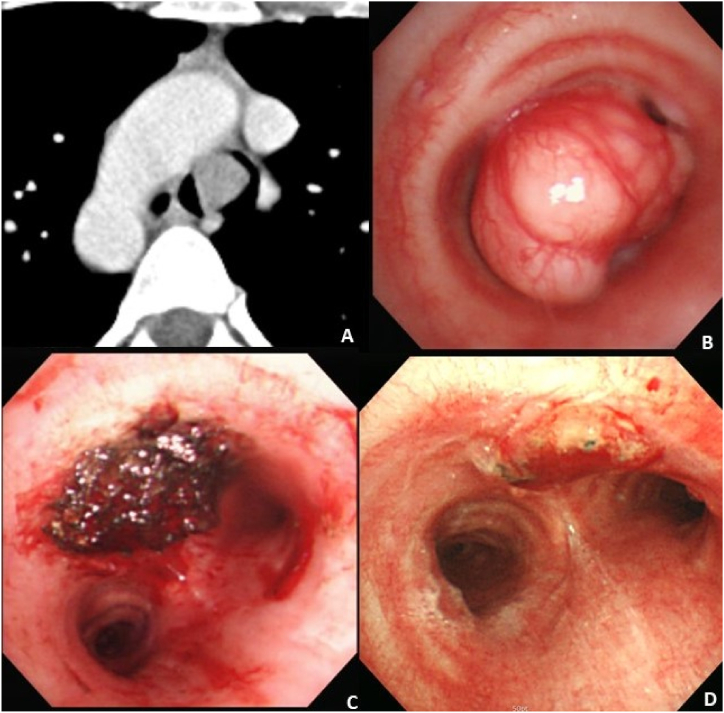
A 32-year-old female visited an allergy clinic for evaluation of intermittent dyspnea and wheezing. Her symptoms started 8 months before visiting the clinic. She was allergic to crustaceans, such as crab or shrimp, but had no history of atopic dermatitis. Her laboratory findings were normal, including 7800/μl of white blood cells, 12.8g/dL of hemoglobin, and 212k/μl of platelets. Eosinophil count was 0.5% and immunoglobulin E level was within the normal range. Her chest radiograph revealed a suspicious mass in the carina. Her chest CT scan revealed a 1.6-cm homogeneously enhancing intraluminal mass without extraluminal extension immediately superior to her carina (Fig. 2 A). Her pulmonary function test showed a forced vital capacity (FVC) of 0.94 L (25% of predicted), forced expiratory volume in the first second (FEV1) of 0.31 L (11% of predicted), and FEV1/FVC of 32%. A rigid bronchoscopy was performed under general anesthesia, and it revealed a narrow-stalked tumor obstructing her distal trachea (Fig. 2 B). The tumor was removed with the tip of a rigid bronchoscope. APC was applied before and after the tumor removal for hemostasis and ablation of the residual tumor (Fig. 2 C). There was no immediate complication, such as bleeding, hemothorax, or pneumothorax. Biopsy revealed a pleomorphic adenoma. Her pulmonary function test result dramatically improved after the rigid bronchoscopic tumor removal; the results included an FVC of 3.26 L (88% of predicted), FEV1 of 2.81 L (96% of predicted), and FEV1/FVC of 86.2%. Follow-up bronchoscopies at 1 month and 2 years after the tumor removal showed a clear tumor bed and no evidence of tumor recurrence (Fig. 2 D).
Fig. 2.
Chest computed tomography scan and bronchoscopy images of a 32-year old woman with a carinal mass.
(A) Chest computed tomography revealed a 1.6 cm enhancing mass on the carina.
(B) Bronchoscopy revealed a narrow stalked tumor on the carina.
(C) Bronchoscopy after removal of the carinal mass using the tip of rigid bronchoscope with pre- and post-application of argon plasma coagulation showed a clear tracheal lumen.
(D) There was no residual tumor or local recurrence on her follow-up bronchoscopy after one month of rigid bronchoscopic tumor removal.
4. Case 3
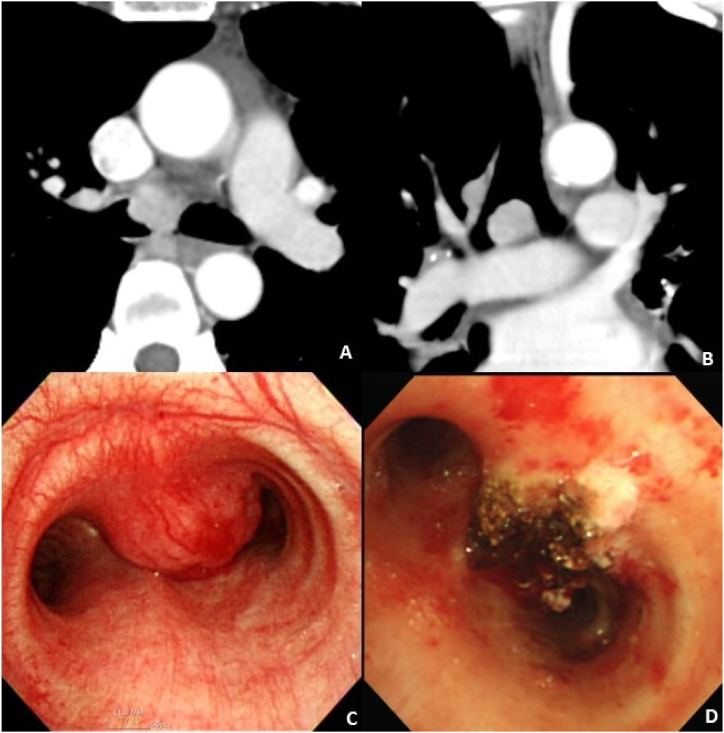
A 47-year-old female visited our hospital due to a 1-month history of blood-tinged sputum. Her chest CT showed a 1.5-cm nodular enhancing mass in her carina (Fig. 3 A). She did not have dyspnea, cough, sputum, fever, chilliness, night sweat, or weight loss. There were no crackles, wheezing, or stridor upon chest auscultation. Laboratory findings were normal. Pulmonary function test showed mild obstructive lung defect (FVC of 3.21 L [90% of predicted], FEV1 of 2.28 L [76% of predicted], and FEV1/FVC of 71.03%). Flexible bronchoscopy was performed to evaluate the protruding mass, which obstructed 80% of the carina. The biopsy examination was suggestive of a plasma cell neoplasm, possibly plasmacytoma. Positron emission tomography-CT was performed to evaluate the malignancy, and it showed a 1.9-cm hypermetabolic lesion in the tracheal bifurcation, suggestive of high probability of lung malignancy (Fig. 3 B). Rigid bronchoscopy was performed to confirm the diagnosis and maintain the central airway. A mass of approximately 2 cm was observed from the carina to the right main bronchus (Fig. 3 C). The tumor surface was smooth, hypervascular, and bled easily upon touching. APC was applied to the base of the tumor, and the tumor was removed with the tip of the rigid bronchoscope and forceps. However, the tumor was not removed completely due to its wide base. After removal, APC was applied again to the base of the tumor for hemostasis and ablation of the residual tumor. A biopsy examination confirmed it as plasmacytoma. She was referred to hematology for further evaluation. Serum and urine electrophoresis showed negative results. Serum immunoglobulin and free light chains were normal. Approximately 3% plasma cells were found in bone marrow aspirates. The patient received a final diagnosis of solitary extramedullary plasmacytoma.
Fig. 3.
Chest computed tomography scan and bronchoscopy images of a 47-year old woman with a carinal mass.
(A and B) Chest computed tomography showed a 1.9cm sized enhancing nodule on the carina
(C) Bronchoscopy revealed a wide based tumor on the carina.
(D) Bronchoscopy after removal of the carinal mass using the tip of rigid bronchoscope with pre- and post-application of argon plasma coagulation showed a widened right main bronchus.
5. Discussion
Signs and symptoms of tracheal tumors vary widely. The tracheal lumen has a large functional reserve, and tumors are asymptomatic until they occlude 50–75% of the lumen diameter [1]. Tumors in the carina and/or distal trachea are diagnosed late due to less frequent symptoms in the early stage of disease. Most malignant tumors in these regions are diagnosed in the advanced stages and are no longer candidates for surgical resection. Moreover, surgical resection of these tumors is extremely challenging; hence it has a high rate of complications and requires high levels of surgical expertise. Rigid bronchoscopic intervention could be a useful alternative treatment for these tumors and can immediately relieve symptoms. The combination of biopsy forceps and the tip of the rigid bronchoscope to “core out” tumors has been very effective for opening an obstructed airway [8]. This could be also applicable to patients with respiratory failure due to a tracheal tumor. The first case described in our report received intubation due to progressive respiratory acidosis, but rigid bronchoscopic tumor removal immediately improved oxygenation and allowed waning from mechanical ventilation.
Some patients with tracheal tumors often have dyspnea on exertion or new onset of wheezing, which is frequently misdiagnosed as asthma. The second patient in our report visited the allergy department due to intermittent wheezing. However, asthma treatment did not improve the symptoms, and the tumor was detected on the patient's chest CT.
Tracheal tumors can present various symptoms. Hemoptysis could be one such initial symptom. The third patient in our report presented with blood tinged sputum, which lasted for 1 month. Such non-specific symptoms and signs can cause delayed diagnosis of tracheal tumors. A chest CT and bronchoscopy could be the best tools to detect these tumors.
Up to 90% of primary tracheal tumors are malignant. Squamous cell carcinoma and adenoid cystic carcinoma account for approximately two-thirds of these. A complete surgical resection can cure benign and malignant tumors and achieve long-term survival [1]. Malignant tumors involving the carina and/or distal trachea are usually diagnosed at an advanced stage and are not candidates for surgical resection. Therefore a rigid bronchoscopic intervention could be the best alternative treatment for these patients, not only for palliation, but also for giving a better opportunity to try other definitive modalities [5]. Rigid bronchoscopy could be a life-saving intervention and bridging therapy for further definitive treatment of patients with carinal tumors.
6. Conclusion
Carina masses are rare and fatal, but are difficult to remove surgically. Using rigid bronchoscopy, the obstructed airway can be opened by excising the carina mass. This procedure can increase the patient's survival by providing an opportunity for definitive treatment.
Funding
This study was supported by a grant (BCRI2022-00200) of Chonnam National University Hospital Biomedical Research Institute. The fund did not have any role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Data availability statement
The datasets generated during the current study are available from the corresponding author on reasonable quest.
Patient consent statement
Patients has provided informed consent for publication of the cases.
Declaration of competing interest
The authors have no conflicts of interest to disclose.
Contributor Information
Jae-Kyeong Lee, Email: oceanbeachsf@naver.com.
Bo-Gun Kho, Email: imdrkgb@gmail.com.
Tae-Ok Kim, Email: tokim07@gmail.com.
Hong-Joon Shin, Email: 99nausicca@naver.com.
Yu-Il Kim, Email: kyionly@jnu.ac.kr.
Sung-Chul Lim, Email: lscmd@jnu.ac.kr.
Yoo-Duk Choi, Email: drydchoi@hanmail.net.
Yong-Soo Kwon, Email: yskwon@jnu.ac.kr.
References
- 1.Macchiarini P. Primary tracheal tumours. Lancet Oncol. Jan 2006;7(1):83–91. doi: 10.1016/s1470-2045(05)70541-6. [DOI] [PubMed] [Google Scholar]
- 2.Wood D.E. Management of malignant tracheobronchial obstruction. Surg. Clin. Jun 2002;82(3):621–642. doi: 10.1016/s0039-6109(02)00025-7. [DOI] [PubMed] [Google Scholar]
- 3.Allen M.S. Malignant tracheal tumors. Mayo Clin. Proc. Jul 1993;68(7):680–684. doi: 10.1016/s0025-6196(12)60604-1. [DOI] [PubMed] [Google Scholar]
- 4.Costantino C.L., Geller A.D., Wright C.D., et al. Carinal surgery: a single-institution experience spanning 2 decades. J. Thorac. Cardiovasc. Surg. May 2019;157(5):2073–2083. doi: 10.1016/j.jtcvs.2018.11.130. e1. [DOI] [PubMed] [Google Scholar]
- 5.Jeon K., Kim H., Yu C.M., et al. Rigid bronchoscopic intervention in patients with respiratory failure caused by malignant central airway obstruction. J. Thorac. Oncol. May 2006;1(4):319–323. [PubMed] [Google Scholar]
- 6.Sim D.W., Oh I.J., Kim K.S., Choi Y.D., Kwon Y.S. Pleomorphic adenoma of the trachea. Journal of bronchology & interventional pulmonology. Jul 2014;21(3):230–233. doi: 10.1097/lbr.0000000000000076. [DOI] [PubMed] [Google Scholar]
- 7.Woo Park C., Kim W., Jae Oh I., Sik Kim K., Duk Choi Y., Soo Kwon Y. Solitary extramedullary plasmacytoma presenting as an endobronchial mass. Intern. Med. (Tokyo) 2013;52(18):2113–2116. doi: 10.2169/internalmedicine.52.0572. [DOI] [PubMed] [Google Scholar]
- 8.Mathisen D.J., Grillo H.C. Endoscopic relief of malignant airway obstruction. Ann. Thorac. Surg. Oct 1989;48(4):469–473. doi: 10.1016/s0003-4975(10)66842-7. discussion 473-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable quest.