Abstract
Glucose has previously been shown to increase the in vitro phagocytosis of unopsonized Pseudomonas aeruginosa by freshly explanted murine peritoneal macrophages (PM) and cultivated alveolar macrophages (AM). This study examined the effect of glucose on the same phagocytosis process in human AM in order to determine whether this phenomenon is conserved among species. Freshly explanted human AM phagocytosed unopsonized P. aeruginosa at a low level (2 bacteria/macrophage/30 min), whereas mouse AM ingested a negligible number of P. aeruginosa (0.01 bacterium/macrophage/30 min). Glucose had no effect on this or other phagocytic processes in freshly explanted mouse or human AM. However, following in vitro cultivation for 72 h, human AM phagocytosed three to four times more unopsonized P. aeruginosa than did freshly explanted cells, but only in the presence of glucose. This glucose-inducible phagocytic response had also been observed in cultivated murine AM. Although similar increases were also detected for the phagocytosis of latex particles and complement-coated sheep erythrocytes by cultivated human AM, these processes were not glucose dependent. The lack of response to glucose in freshly explanted mouse AM was attributed to insufficient glucose transport; however, freshly explanted human AM exhibited significant facilitative glucose transport activity that was inhibitable by cytochalasin B and phloretin. Taken together, these results suggest that the process of glucose-inducible phagocytosis of unopsonized P. aeruginosa is conserved among macrophages from different species, including humans, and that AM, but not PM, required cultivation for this glucose effect to occur. Glucose transport by AM appears to be necessary but not sufficient for phagocytosis of unopsonized P. aeruginosa.
Pseudomonas aeruginosa is an opportunistic, gram-negative bacterial pathogen commonly found in immunocompromised individuals, in burn patients, and in the airways of the lungs of cystic fibrosis (CF) patients (3). Once established, P. aeruginosa infection is rarely eradicated in patients with CF despite antimicrobial therapies, and it continues to be a major cause of morbidity and mortality. The means by which P. aeruginosa evades host defenses to establish infection in the lungs of CF patients is under intense investigation. Several hypotheses have been proposed to explain the prevalence of P. aeruginosa in the lungs of CF patients, including a relationship to the genetic defect of the CF transmembrane conductance regulator (11, 17–20, 22) and the survival strategies of P. aeruginosa (7, 23). The main initial defender of the lower airways against airborne infections is the alveolar macrophage (AM) (8). Elucidating their role in the clearance of P. aeruginosa may be of critical importance to our understanding of the initial events that result in the survival and growth of this bacterium in the lower respiratory tract of the CF patient. AM provide the first line of defense against infection of the respiratory tract; the early recruitment of polymorphonuclear leukocytes (PMN) to the lower airway in CF may represent a breach of this normally robust host defense. Since AM reside in an environment with low complement and immunoglobulin concentrations (13, 26), it is probable that they utilize nonopsonic instead of Fc or complement receptor-mediated phagocytosis as their initial primary mechanism for eliminating microorganisms such as P. aeruginosa from the airways.
Our previous studies have indicated that the phagocytosis of unopsonized P. aeruginosa by freshly explanted murine resident and thioglycollate-elicited peritoneal macrophages (PM) is a glucose-dependent process (24). This phenomenon is unique in that glucose has no effect on the macrophage phagocytosis of several other respiratory bacterial pathogens, antibody- or complement-coated sheep erythrocytes, zymosan, or latex particles (24), providing a possible explanation for the success of P. aeruginosa as a respiratory pathogen. In striking contrast to PM, freshly explanted mouse AM could not phagocytose bound P. aeruginosa even in the presence of glucose. The difference in response to glucose in these two types of macrophages was attributed to the absence of significant glucose transport activity in mouse AM. This was suggested by the finding that when mouse AM acquire glucose transport activity following a 48-h in vitro cultivation period, they can also ingest unopsonized P. aeruginosa in a glucose-dependent manner (6). These results suggest that mouse AM are incapable of ingesting unopsonized P. aeruginosa unless they are cultured and acquire the capacity to transport exogenous glucose. Whereas the process of nonopsonic phagocytosis of P. aeruginosa by rodent AM has been characterized (1, 6, 24), that of human cells has not yet been reported. The principal aim of this study was to determine the phagocytic response of human AM to a challenge of unopsonized P. aeruginosa in the presence of glucose, thereby gaining an understanding of our preliminary studies in the context of human respiratory defenses.
MATERIALS AND METHODS
Alveolar macrophages.
Approval for the bronchoscopy and bronchoalveolar lavage procedures was obtained from the Institution Review Board of Ethics in Human Experimentation at the University of Sherbrooke. All volunteers were healthy and provided signed informed consent prior to participating in the study. Bronchoalveolar lavages were performed on healthy volunteers by previously established protocols (4). Cells from the fluids obtained were washed twice with phosphate-buffered saline (50 mM sodium phosphate–0.15 M NaCl, pH 7.2) by centrifugation at 200 × g and resuspended in 1 ml of complete medium (Dulbecco’s modified Eagle’s medium containing penicillin [100 U/ml], streptomycin [50 μg/ml], l-glutamine [10 mM], MgCl2 [1 mM], and 10% autologous serum). Cell viability was determined by exclusion of trypan blue dye, and phenotype was determined by staining cells with Wright stain. Mouse AM were prepared according to procedures described previously (6).
Adherence property of human alveolar macrophages.
The adherence of human AM to untreated Thermanox plastic coverslips (13 mm in diameter; Nunc Inc., Naperville, Ill.) for 1 h at 37°C and 5% CO2 was compared to their adherence to glass coverslips precoated with 50 μl of 0.01% polylysine, 2% gelatin, 0.004% fibronectin, or human autologous or heterologous serum. With the exception of sera, all coating agents were obtained from Sigma Chemical Company (St. Louis, Mo.). Cells from bronchoalveolar lavage fluids (106/ml) with or without 1 mM MgCl2 were plated onto coverslips (100 μl/coverslip) in 24-well Falcon tissue culture plates (Becton Dickinson & Co., Franklin Lakes, N.J.) and incubated for 1 h at 37°C and 5% CO2. Additional complete medium (0.5 ml) was added, and the cells either were allowed to adhere for at least a further 3 h prior to phagocytosis assays or were incubated for 1 to 3 days in order to determine the adherence of cultivated human AM on coated coverslips. The percentage of adherent cells was calculated by multiplying 100% by the ratio of the number of cells remaining on the coverslips to the total number of cells plated. A representative field of each coverslip was counted for this purpose.
Phagocytosis assays.
The various phagocytic particles were prepared as described previously (1, 24), but phagocytosis assays were modified as follows: (i) the particle incubation time was reduced from 1 h to 30 min, and (ii) extracellular uningested sheep erythrocytes and P. aeruginosa P1 were lysed with 55 and 50% phosphate-buffered saline, respectively, instead of water for 2 min and 10 s. Following phagocytosis assays, the coverslips were air dried and mounted on microscope slides with either Entellan (Merck, Darmstadt, Germany) or Omnimount (National Diagnostics, Atlanta, Ga.) mounting medium. The number of particles ingested by human AM was determined by visually examining Giemsa-stained cells and counting internalized particles with the aid of a light microscope. At least 60 macrophages per coverslip were scored. Each sample was assayed in triplicate, and the experiments were repeated two to three times with different AM preparations. Data were expressed as means ± standard errors of the means. Student’s t test for independent means was used to evaluate data, and P values of <0.05 were deemed significant. In cases in which no bacteria were ingested, the ability of human AM to bind P. aeruginosa was investigated as described previously (1).
Glucose transport assay.
Adherent human AM were carefully rinsed twice with a HEPES-buffered saline solution (HBS) consisting of 140 mM NaCl, 2.4 mM MgSO4, 5 mM KCl, 1 mM CaCl2, and 20 mM sodium HEPES, pH 7.4. Glucose uptake was measured indirectly with radiolabelled 2-deoxy-d-glucose (NEN, Markham, Ontario, Canada). Briefly, human AM were incubated for 10 min at 37°C with 10 μM 2-deoxy-d-glucose and tritium-labelled 2-deoxy-d-glucose (1.0 μCi/ml) in a total volume of 0.5 ml of HBS/well. Nonspecific 2-deoxy-d-glucose counts were determined by inclusion of 10 μM cytochalasin B in duplicate assay mixtures. Following the incubation, the wells were rinsed twice with HBS and then the cells were solubilized in 0.5 ml of 1 mM NaOH for 45 min. Cell-associated radioactivity was quantified by liquid scintillation counting, using an aqueous liquid scintillant (Amersham, Little Chalfont, Buckinghamshire, United Kingdom) and a model LS 6800 liquid scintillation counter (Beckman, Irvine, Calif.). Specific facilitated glucose transport activity was determined by subtracting nonspecific counts, obtained in the presence of cytochalasin B, from total counts, obtained in the absence of cytochalasin B. An aliquot of the standard assay mixture (10 μl) was measured for radioactivity to determine the relationship between radioactivity of the 2-deoxyglucose and the amount of 2-deoxy-d-glucose transported. Under the conditions stated above, approximately 100 cpm is equivalent to the transport of 1 pmol of 2-deoxy-d-glucose. Glucose transport is expressed as the average of triplicate measurements, in units of picomoles per minute per 105 cells.
RESULTS
Cells from bronchoalveolar lavage fluid.
Bronchoalveolar lavage fluids from eight different individuals were used for phagocytosis and glucose transport assays. The cell populations of the lavage fluids were fairly similar. A representative cell population consisted mainly of macrophages (85 to 90%) and lymphocytes (8 to 11%), with a small percentage of eosinophils (0.3 to 3.5%) and neutrophils (0 to 1.2%) but no basophils. Similar differential cell counts for bronchoalveolar lavage fluids from healthy, nonsmoking, young volunteer subjects have been reported previously (5). Cell recovery ranged from 3 × 106 to 10 × 106, with an average of 5.8 × 106 per lavage. Cell viability was between 66 and 96%, averaging about 80%. AM were found to be positive for nonspecific esterase.
Adherence properties of mouse and human AM.
Murine AM were isolated from lymphocytes, erythrocytes, and other contaminating cells in peritoneal exudates and bronchoalveolar lavage fluids by their ability to adhere to glass coverslips. They remained on glass coverslips for the duration of the phagocytosis assay period. This characteristic allowed them to be purified without subjecting them to conventional cell panning or sorting methods and facilitated the assessment of phagocytic activity. In contrast, human AM appeared to adhere to glass more loosely than their murine counterparts. As a result, few cells were left on the glass coverslips after the phagocytosis assay procedures. Consequently, in one experiment, human AM were tested for their adherence to glass coverslips coated individually with a variety of agents that can enhance cell adhesion, as well as to untreated plastic coverslips (Fig. 1). With the exception of glass coverslips treated with human fibronectin, precoating did not appear to enhance the adherence of human AM to glass coverslips. Surprisingly, there was a significant decrease in adherence of human AM to glass coverslips precoated with bovine fibronectin or gelatin plus MgCl2 after a 3-day incubation. This decline in adherence was not observed with the other treatments.
FIG. 1.
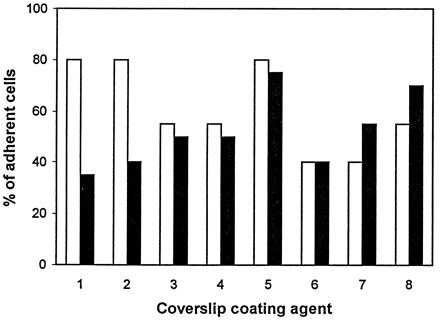
Adherence of human AM to glass coverslips with coating agents was determined after 1 day (open bars) and after 3 days (closed bars) in culture. The coating agents used were bovine fibronectin (1), gelatin plus Mg2+ (2), polylysine plus Mg2+ (3), human heterologous serum (4), human fibronectin (5), human autologous serum (6), and human autologous serum plus Mg2+ (7); human AM were also plated onto plastic coverslips without any coating agent but in the presence of Mg2+ ions (8).
Although fibronectin could be used to increase the adherence of freshly explanted human AM to glass coverslips, it would not be useful for cultivation of these cells. Furthermore, fibronectin has been shown to enhance macrophage phagocytosis of P. aeruginosa, and that would have complicated our analysis of the glucose effect on nonopsonic phagocytosis (15). Since both human and mouse AM adhered well to untreated plastic coverslips, we therefore compared the phagocytic abilities of mouse and human AM following their adherence to plastic coverslips. Adherent human AM, unlike mouse AM, could not be maintained in culture in medium containing fetal calf serum. However, they remained viable and phagocytic for at least 6 days in medium containing autologous serum.
Particle phagocytosis by human AM.
Adherent AM were tested for their ability to phagocytose various particles on the day of explantation (day 0) and on day 3. The photomicrographs in Fig. 2 demonstrate the phagocytic ability of freshly explanted adherent human AM. Initial phagocytosis assays indicated that P. aeruginosa particles ingested by human AM were not as discernible as those ingested by murine AM. This difference may be due to the more rapid degradation of P. aeruginosa in human AM, which can generate substantial quantities of reactive oxygen intermediates following ingestion of bacteria (10). It was necessary to shorten the time period allotted for P. aeruginosa phagocytosis by human AM to ensure that the particles could be counted accurately by visual examination of stained macrophages. Although tedious to perform, this assessment is very reliable for distinguishing unbound, bound, and ingested particles. The number of particles ingested per cell appeared small, relative to the inoculum, because the assay was carried out for 30 min. This does not reflect inefficient phagocytosis since, if given enough incubation time, the AM could ingest many more of the available particles. A wide range of phagocytic ability, irrespective of particle type, was detected, which probably reflects the known heterogeneity of AM isolated from bronchoalveolar lavage fluids.
FIG. 2.
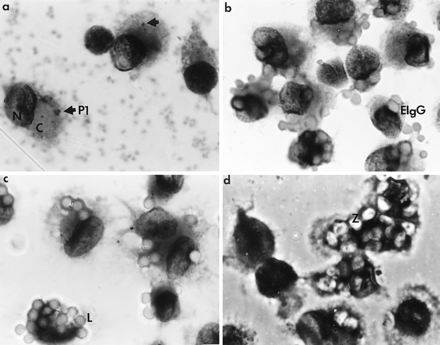
Particle phagocytosis by freshly explanted human AM. Cells from bronchoalveolar lavage fluids were plated onto plastic coverslips. Adherent AM were tested for their ability to ingest unopsonized P. aeruginosa P1 (a), sheep erythrocytes coated with rabbit anti-sheep erythrocyte IgG antibodies (EIgG) (b), 3-μm-diameter latex particles (L) (c), and zymosan (Z) (d). Macrophage morphology was preserved, with the Giemsa-stained nucleus (N) and cytoplasm (C) being clearly defined. Poor ingestion of P. aeruginosa was observed. Due to incomplete lysis of lysozyme-treated bacteria, extracellular P. aeruginosa cells appeared as lightly stained fuzzy dots (background in photomicrograph a). Ingested bacteria appeared as dark rods (arrow in photomicrograph a). Magnification, ×1,667.
The phagocytic activities of freshly explanted human AM and those that had been cultured for 3 days are shown in Fig. 3a. Freshly explanted human AM (day 0 AM) ingested similar numbers of zymosan, latex particles, and immunoglobulin G (IgG)-coated sheep erythrocytes (2.6 to 3.2 particles/macrophage). Complement-coated sheep erythrocytes were also ingested, albeit in smaller numbers than the other particles (0.8 particle/macrophage). Human AM cultured for 3 days (day 3 AM) ingested more latex and complement-coated particles but not significantly more IgG-coated erythrocytes or zymosan particles than day 0 AM. More importantly, glucose had no effect on the phagocytosis of any of these particles (data not shown). On the contrary, the phagocytosis of P. aeruginosa by day 3 human AM increased three- to fourfold in the presence of glucose. This glucose-stimulated three- to fourfold increase in phagocytosis was not evident in day 0 human AM, which ingested 2.4 and 1.6 P. aeruginosa cells/macrophage in the presence and absence of glucose, respectively (Fig. 3a).
FIG. 3.
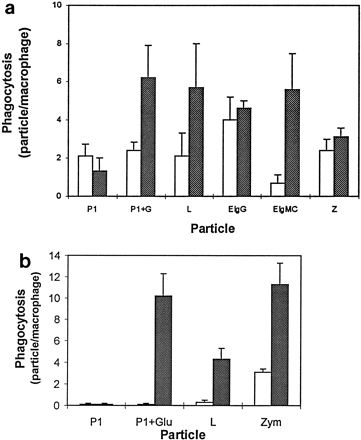
(a) Phagocytic responses of human AM isolated from bronchoalveolar lavage fluids. Freshly explanted AM (open bars) and those cultivated for 3 days (cross-hatched bars) were tested for their ability to ingest P. aeruginosa in the absence of glucose (P1) or in the presence of 10 mM glucose (P1+G), 3-μm-diameter latex particles (L), sheep erythrocytes coated with either rabbit anti-sheep erythrocyte IgG antibodies (EIgG) or rabbit anti-sheep erythrocyte IgM antibodies plus complement C3 (EIgMC), or zymosan (Z). Sixty macrophages from each coverslip were scored for the number of ingested particles, and phagocytosis is expressed as the number of particles per macrophage. Data are the means of results from two to three separate experiments in which triplicate determinations were performed for each sample. Each bar represents the standard error of the mean. (b) Phagocytosis of P. aeruginosa by freshly explanted mouse AM (open bars) and those cultivated for 3 days (cross-hatched bars) in the absence (P1) or the presence (P1+Glu) of 10 mM glucose was determined as for human AM to compare the effects of glucose on these cells. Ingestion of 3-μm-diameter latex particles (L) and zymosan (Z) was used to monitor the phagocytic competence of mouse AM.
There were two notable differences between human (Fig. 3a) and mouse (Fig. 3b) AM. One difference was that cultivated murine AM showed increased ingestion of zymosan while cultured human AM did not. The other difference was in phagocytosis of P. aeruginosa; fresh human, but not murine, AM ingested a small number of P. aeruginosa in the presence or absence of glucose, but glucose enhancement was dramatic with both cell types after in vitro cultivation.
Facilitated glucose transport activity of human AM.
It has been shown that freshly explanted mouse AM transport negligible amounts of glucose, and this glucose transport activity increases in day 3 cells (6). Due to the importance of glucose in the phagocytic response of AM toward P. aeruginosa, the glucose transport activities of day 0 and 3 human AM were measured. As shown in Fig. 4, human AM transported glucose in a cytochalasin B-inhibitable manner on days 0, 3, and 6, and there was no significant difference between them in the amounts of glucose transported. Phloretin, which inhibits facilitative but not active glucose transport, also reduced transport of 2-deoxy-d-glucose by human AM, by about 81% (data not shown). The glucose transport rate of 1.5 pmol/min/105 human AM was comparable to those measured with mouse PM and cultivated mouse AM. The number of glucose molecules transported into each cell would be 9 × 106 per min. In mouse PM, glucose triggered phagocytosis of bound P. aeruginosa cells within 10 to 15 min following its addition to the assay medium. If in vivo human AM were able to respond to glucose in the same way as mouse PM, then only 15 to 23 pmol of glucose would be needed to activate P. aeruginosa phagocytosis by 105 human AM.
FIG. 4.
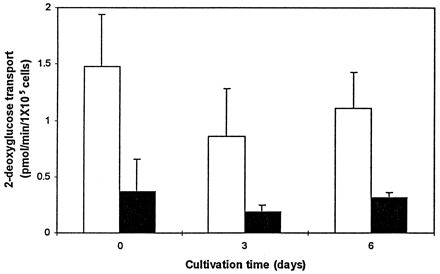
Glucose transport by human AM. The uptake of [3H]2-deoxy-d-glucose by freshly explanted AM and those cultivated for 3 or 6 days was determined in the absence (open bars) or the presence (closed bars) of cytochalasin B. Data are means ± standard errors of the means of values from three separate experiments in which duplicate determinations were performed for each sample.
DISCUSSION
In our previous report, we provided evidence that glucose could activate phagocytosis of P. aeruginosa but not other particles in rodent macrophages, and we hypothesized that it might be possible to stimulate human AM to phagocytose P. aeruginosa in vivo if they behave similarly to rodent macrophages (6). Several differences between mouse and human AM were revealed in this study. The minor differences, such as adherence to glass and water sensitivity, resulted in changes in our phagocytosis assay procedures, which were established previously for rodent macrophages. Nevertheless, the results obtained with freshly explanted and cultivated mouse AM which had adhered to plastic (Fig. 3b) were similar to previously published data on glass-adhered cells (6).
The major and unexpected differences were that freshly explanted human AM, but not their mouse equivalents, could phagocytose P. aeruginosa, albeit poorly, in a glucose-independent manner (Fig. 3a) and that they exhibited facilitative glucose transport (Fig. 4). It is not known whether the former difference is due to a subpopulation of AM that is present in the human but not the mouse lung or a species-specific AM response to unopsonized P. aeruginosa. Subpopulations of AM have been identified with monoclonal antibodies (25), so that the nature of human AM subpopulations could be further investigated to determine if phagocytic competence correlates with cell phenotype. Despite their differences, the efficiency of P. aeruginosa phagocytosis was increased significantly by glucose in both the cultivated mouse and human AM (Fig. 3). Similar glucose-inducible phagocytosis of unopsonized P. aeruginosa was also demonstrable in cultivated rat and sheep AM (data not shown). The species conservation of this process suggests a common mechanism by which macrophages interact with and phagocytose unopsonized P. aeruginosa. However, it is clear that in human AM, a glucose-independent mechanism is also involved in the phagocytosis of P. aeruginosa.
The changes in AM during cultivation that would enable them to respond to glucose and phagocytose P. aeruginosa are as yet unclear. One of the changes that may affect this process is an increase in the affinity or number of a phagocytic receptor(s) for P. aeruginosa during cultivation, but this cannot be addressed at present because the receptor(s) is incompletely characterized. Our P. aeruginosa binding assays did not indicate any significant difference in the numbers of P. aeruginosa bound to freshly explanted and cultivated human AM. Therefore, binding of P. aeruginosa to human AM and phagocytosis of this bacterium by these cells appear to be two independent events, as was the case for mouse macrophages. In contrast, upregulation of receptors upon cultivation was most likely responsible for the increase in the rate of phagocytosis of latex particles and complement-coated sheep erythrocytes in cultivated human AM and in the ingestion of latex and zymosan particles in cultivated mouse AM (Fig. 3). However, unlike the nonopsonic phagocytosis of P. aeruginosa, glucose has no effect on these phagocytic processes.
The acquisition of glucose responsiveness by AM during cultivation may differ depending on species. In mouse AM, glucose responsiveness coincides with increased facilitative glucose transport, whereas in human AM there was no significant increase in glucose transport during cultivation (Fig. 4). The lack of glucose transport in freshly explanted mouse AM is a characteristic not shared by human, guinea pig (9), or rabbit (12) AM. Analyses of glucose-dependent phagocytosis of P. aeruginosa by mouse thioglycollate-elicited PM demonstrated that glucose has to be transported by these cells and then metabolized via glycolysis to trigger P. aeruginosa phagocytosis. It is possible that freshly explanted human AM cannot utilize the transported glucose for the phagocytosis of P. aeruginosa as effectively as do PM. PM have a high rate of glucose utilization via glycolysis (16), and interestingly, their cultivation is not required for glucose to trigger P. aeruginosa phagocytosis. In freshly explanted rabbit AM, glycolytic enzyme activities are three- to fourfold lower than those of rabbit PM due to their preferential use of oxidative phosphorylation rather than glycolysis for metabolic energy (21). Further investigations are in progress to determine the mechanism of the glucose effect on nonopsonic phagocytosis of P. aeruginosa by AM.
Poor phagocytosis of unopsonized P. aeruginosa by both rodent and human AM has been documented (2, 6, 14). In normal, healthy individuals, bactericidal factors, mucociliary clearance, and immune responses are operative in the upper respiratory tract for the prevention of bacterial infections, and phagocytic clearance of P. aeruginosa by pulmonary AM may not be critical. However, in CF patients, all of these antibacterial defense mechanisms may be dysfunctional or compromised, thereby predisposing these individuals to bacterial infections. Resistance of P. aeruginosa to phagocytic clearance by human AM may explain, at least in part, the persistence of this opportunist in the lower airways of CF patients. Increasing phagocytic uptake of P. aeruginosa by AM could therefore be beneficial for CF patients. Our results indicate that the process of nonopsonic phagocytosis of P. aeruginosa by cultivated human AM may be stimulated by glucose. By extrapolating the requirements for triggering this process in mouse PM, we hypothesize that human AM can be stimulated to ingest P. aeruginosa in vivo if an activator of glycolysis is delivered along with glucose to these cells. Phagocytosis studies with freshly explanted human AM and liposomes encapsulated with glucose and fructose 2,6-bisphosphate, an activator of phosphofructose kinase which catalyzes the rate-limiting step in glycolysis, are currently under way and should enable us to test this hypothesis. Insights into the glucose-induced triggering mechanism involved in the phagocytosis of unopsonized P. aeruginosa by macrophages, which is conserved among several species, including humans, may provide more opportunities to modulate macrophage defense against P. aeruginosa infections in susceptible hosts.
Since AM provide the first line of defense against respiratory tract infection, any weaknesses could be exploited by potential pathogens to enable infection to be established. Once the initial step in infection (evasion of AM phagocytosis) is taken, the second line of defense (PMNs) will be recruited. Since patients with CF have an exuberant, and destructive, PMN inflammatory response, preventing this response from being initiated is particularly important and is one of the objectives of antibacterial therapy in this chronic progressive disease. By bolstering AM phagocytic function, this goal might ultimately be achieved.
ACKNOWLEDGMENTS
S.Y.C.W. and L.M.G. contributed equally to this work.
We thank Jocelyn Labbe and Ms. Ginette Bilodeau for excellent technical assistance in the preparation of AM from human bronchoalveolar lavage fluids and autologous human sera.
This work was supported by grants from Polydex Ltd., The Medical Research Council of Canada, The Canadian Bacterial Diseases Network, and The Canadian Cystic Fibrosis Foundation’s SPARxI program.
REFERENCES
- 1.Barghouthi S, Everett K D E, Speert D P. Nonopsonic phagocytosis of Pseudomonas aeruginosa requires facilitated transport of d-glucose by macrophages. J Immunol. 1995;154:3420–3428. [PubMed] [Google Scholar]
- 2.Berger M, Norvell T M, Tosi M F, Emancipator S N, Konstan M W, Schreiber J R. Tissue-specific Fcγ and complement receptor expression by alveolar macrophages determines relative importance of IgG and complement in promoting phagocytosis of Pseudomonas aeruginosa. Pediatr Res. 1994;35:68–77. doi: 10.1203/00006450-199401000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Botzenhart K, Doring G. Ecology and epidemiology of Pseudomonas aeruginosa. In: Campa M, Bendinelli M, Friedman H, editors. Pseudomonas aeruginosa as an opportunistic pathogen. New York, N.Y: Plenum Press; 1993. pp. 1–13. [Google Scholar]
- 4.Cantin A, Allard C, Begin R. Increased alveolar plasminogen activator in early asbestosis. Am Rev Respir Dis. 1989;139:604–609. doi: 10.1164/ajrccm/139.3.604. [DOI] [PubMed] [Google Scholar]
- 5.Ettensohn D B, Jankowski M J, Duncan P G, Lalor P A. Bronchoalveolar lavage in the normal volunteer subject. I. Technical aspects and intrasubject variability. Chest. 1988;94:275–280. doi: 10.1378/chest.94.2.275. [DOI] [PubMed] [Google Scholar]
- 6.Everett K D E, Barghouthi S, Speert D P. In vitro culture of murine peritoneal and alveolar macrophages modulates phagocytosis of Pseudomonas aeruginosa and glucose transport. J Leukoc Biol. 1996;59:539–544. doi: 10.1002/jlb.59.4.539. [DOI] [PubMed] [Google Scholar]
- 7.Galloway D R. Role of exotoxins in the pathogenesis of P. aeruginosa infections. In: Campa M, Bendinelli M, Friedman H, editors. Pseudomonas aeruginosa as an opportunistic pathogen. New York, N.Y: Plenum Press; 1993. pp. 107–127. [Google Scholar]
- 8.Goldstein E, Lippert W, Warshauer D. Pulmonary alveolar macrophages: defender against bacterial infection of the lung. J Clin Investig. 1974;54:519–528. doi: 10.1172/JCI107788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gudewicz P W. The effects of cortisone therapy on lung macrophage host defence function and glucose metabolism. Circ Shock. 1981;8:95–103. [PubMed] [Google Scholar]
- 10.Kemmerich B, Rossing T H, Pennington J E. Comparative oxidative microbicidal activity of human blood monocytes and alveolar macrophages and activation by recombinant gamma interferon. Am Rev Respir Dis. 1987;136:266–269. doi: 10.1164/ajrccm/136.2.266. [DOI] [PubMed] [Google Scholar]
- 11.Levine S J, Larivee P, Logun C, Angus C W, Ognibene F P, Shelhamer J H. Tumor necrosis factor-α induces mucin hypersecretion and MUC-2 gene expression by human airway epithelial cells. Am J Respir Cell Mol Biol. 1995;12:196–204. doi: 10.1165/ajrcmb.12.2.7865217. [DOI] [PubMed] [Google Scholar]
- 12.Low R B, Bulman C A. Substrate transport by the pulmonary alveolar macrophage. Effects of smoke components. Am Rev Respir Dis. 1977;116:423–431. doi: 10.1164/arrd.1977.116.3.423. [DOI] [PubMed] [Google Scholar]
- 13.Low R B, Davis G S, Giancola M S. Biochemical analyses of bronchoalveolar lavage fluids of healthy human volunteer smokers and nonsmokers. Am Rev Respir Dis. 1978;118:863–875. doi: 10.1164/arrd.1978.118.5.863. [DOI] [PubMed] [Google Scholar]
- 14.Martin T R, Rubens C E, Wilson C B. Lung antibacterial defense mechanisms in infant and adult rats: implications for the pathogenesis of group B streptococcal infections in the neonatal lung. J Infect Dis. 1988;157:91–100. doi: 10.1093/infdis/157.1.91. [DOI] [PubMed] [Google Scholar]
- 15.Mork T, Hancock R E W. Mechanisms of nonopsonic phagocytosis of Pseudomonas aeruginosa. Infect Immun. 1993;61:3287–3293. doi: 10.1128/iai.61.8.3287-3293.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newsholme P, Curi R, Gordon S, Newsholme E A. Metabolism of glucose, glutamine, long-chain fatty acids and ketone bodies by murine macrophages. Biochem J. 1986;239:121–125. doi: 10.1042/bj2390121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pier G B, Grout M, Zaidi T S, Olsen J C, Johnson L G, Yankaskas J R, Goldberg J B. Role of mutant CFTR in hypersusceptibility of cystic fibrosis patients to lung infections. Science. 1996;271:64–67. doi: 10.1126/science.271.5245.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Regnis J A, Robinson M, Bailey D L, Cook P, Hooper P, Chan H-K, Gonda L, Bautovich G, Bye P T P. Mucociliary clearance in patients with cystic fibrosis and in normal subjects. Am J Respir Crit Care Med. 1994;150:66–71. doi: 10.1164/ajrccm.150.1.8025774. [DOI] [PubMed] [Google Scholar]
- 19.Rommens J M, Iannuzzi M C, Kerem B, Drumm M L, Melmer B, Dean M, Rozmahel R, Cole J L, Kennedy D, Hidaka N, Zsiga M, Buchwald M, Riordan J R, Tsui L C, Collins F S. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- 20.Saiman L, Prince A. Pseudomonas aeruginosa pili bind to asialoGM1 which is increased on the surface of cystic fibrosis epithelial cells. J Clin Investig. 1993;92:1875–1880. doi: 10.1172/JCI116779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simon L M, Robin E D, Phillips J R, Acevedo J, Axline S G, Theodore J. Enzymatic basis for bioenergetic differences of alveolar versus peritoneal macrophages and enzyme regulation by molecular O2. J Clin Investig. 1977;59:443–448. doi: 10.1172/JCI108658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith J J, Travis S M, Greenberg E P, Welsh M J. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 23.Speert D P. Pseudomonas aeruginosa infections in patients with cystic fibrosis. In: Baltch A L, Smith R P, editors. Pseudomonas aeruginosa infections and treatment. New York, N.Y: Marcel Dekker, Inc.; 1994. pp. 183–236. [Google Scholar]
- 24.Speert D P, Gordon S. Phagocytosis of unopsonized Pseudomonas aeruginosa by murine macrophages is a two-step process requiring glucose. J Clin Investig. 1992;90:1085–1092. doi: 10.1172/JCI115924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spiteri M A, Clarke S W, Poulter L W. Isolation of phenotypically and functionally distinct macrophage subpopulations from human bronchoalveolar lavage. Eur Respir J. 1992;5:717–726. [PubMed] [Google Scholar]
- 26.Valeyre D, Soler P, Basset G, Loisear P, Pre J, Turbie P, Battesti J P, Georges R. Glucose, K+, and albumin concentrations in alveolar milieu of normal humans and pulmonary sarcoidosis patients. Am Rev Respir Dis. 1991;143:1096–1101. doi: 10.1164/ajrccm/143.5_Pt_1.1096. [DOI] [PubMed] [Google Scholar]


