Abstract
TP53-mutated myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) form a distinct group of myeloid disorders with dismal outcomes. TP53-mutated MDS and AML have lower response rates to either induction chemotherapy, hypomethylating agent–based regimens, or venetoclax-based therapies compared with non–TP53-mutated counterparts and a poor median overall survival of 5 to 10 months. Recent advances have identified novel pathogenic mechanisms in TP53-mutated myeloid malignancies, which have the potential to improve treatment strategies in this distinct clinical subgroup. In this review, we discuss recent insights into the biology of TP53-mutated MDS/AML, current treatments, and emerging therapies, including immunotherapeutic and nonimmune-based approaches for this entity.
Significance:
Emerging data on the impact of cytogenetic aberrations, TP53 allelic burden, immunobiology, and tumor microenvironment of TP53-mutated MDS and AML are further unraveling the complexity of this disease. An improved understanding of the functional consequences of TP53 mutations and immune dysregulation in TP53-mutated AML/MDS coupled with dismal outcomes has resulted in a shift from the use of cytotoxic and hypomethylating agent–based therapies to novel immune and nonimmune strategies for the treatment of this entity. It is hoped that these novel, rationally designed combinations will improve outcomes in this area of significant unmet need.
INTRODUCTION
TP53 is a tumor suppressor gene that encodes for the transcription factor p53, appropriately coined the “guardian of the genome.” TP53 is the most frequently mutated gene across all human cancers and carries an adverse prognosis with suboptimal responses to conventional therapies across multiple cancer types (1). Response to cytotoxic chemotherapy is highly dependent on the presence of intact p53 to enable the induction of apoptosis (2, 3). Consequently, TP53-mutated cancers respond poorly to cytotoxic chemotherapy. Despite being one of the most studied genes since its initial discovery about 40 years ago, it has so far been considered “undruggable.” Similar to many TP53-mutated malignancies, TP53-mutated myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) remain long-standing therapeutic challenges, with a dismal median survival of 5 to 10 months, irrespective of therapies used (4–6). In the last few years, some of the novel immune-harnessing and p53 structure-modulating agents have demonstrated encouraging early clinical activity in TP53-mutated AML/MDS, and are now being advanced in phase II/III registration studies. In this review, we summarize the key biological implications of TP53 mutations, their prognostic relevance to MDS and AML, and outcomes with currently approved therapies, and we discuss current and future directions for drug development for TP53-mutated AML/MDS.
TP53 MUTATION AND CANCER
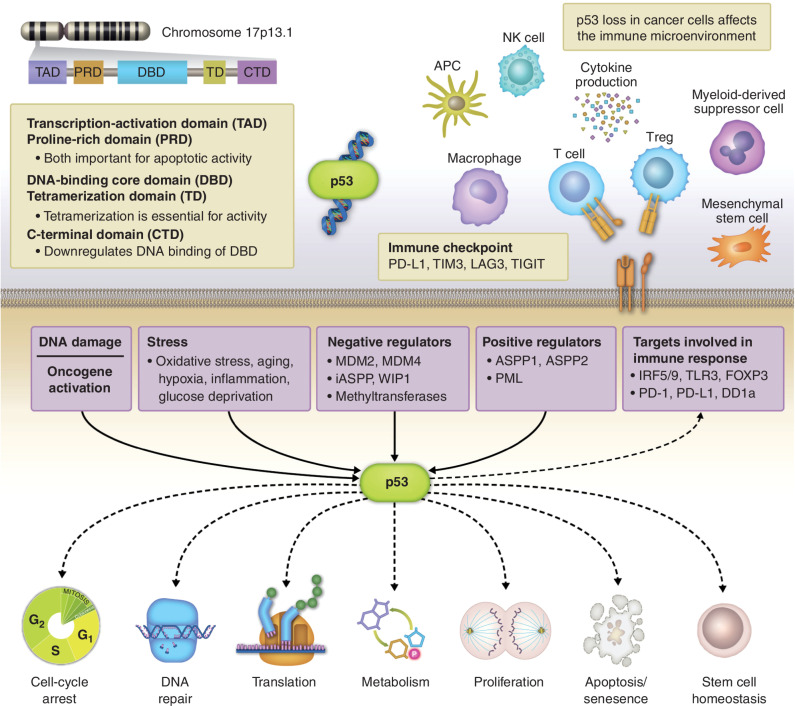
TP53 is a 20-kb gene located on chromosome 17p13.1, which codes for at least 15 different isoforms and has two paralogs, p63 and p73, with similar structures and overlapping but distinct functions and upstream pathways (7). It presides over a highly connected intracellular hub involving multiple signal transduction pathways and consequently is affected by and in turn regulates numerous cellular processes. Some of the major functions of p53 include the regulation of genomic stability, cell cycling, proliferation, differentiation, apoptosis, senescence, autophagy, metabolism, and stem cell homeostasis throughout human life, highlighting the central role of this pathway in the healthy state (Fig. 1; refs. 8, 9).
Figure 1.
Different subunits of the p53 are coded by a gene located on chromosome 17p13.1. p53 resides over a highly connected hub involving multiple signal transduction pathways, including DNA damage response, oncogene activation, cellular stress, and its positive and negative regulators. In turn, p53 regulates numerous key cellular processes including cell cycling, genomic stability, cell metabolism, differentiation, proliferation, apoptosis, senescence, and others. In addition, downstream signaling through p53 influences the tumor microenvironment through a direct effect on several immunologic targets. APC, antigen-presenting cell; NK, natural killer; Treg, regulatory T cell.
More than 90% of cancer-related TP53 mutations have structural losses of both alleles, and most result in loss or decreased function of genes in the p53 regulatory network, many of which are critical for growth arrest, routine apoptosis, and suppressing neoplasia (10). Mutations in TP53 can be somatic or germline, can be contact or structural, and based on their functional consequences can be divided into the most frequent complete or partial loss of function to rarely silent or potentially gain of function (1, 11, 12). A majority of TP53 hotspot mutations lead to loss of function, causing an inability to trigger p21, downregulation of genes associated with apoptosis, and upregulation of proteins involved in cell-cycle progression (e.g., cyclin B1, cyclin E1, FOXM1, CDK1) and those involved in DNA damage response (CHK2, MSH6; ref. 10). However, the gain-of-function hypothesis has been challenged by elegant work demonstrating a dominant-negative effect of missense TP53 mutations leading to the disruption of activity of the remaining wild-type p53 after tetramerization (13, 14). This was further supported by clinical analysis showing lack of a more aggressive phenotype, a similar comutational landscape, and comparable clinical outcomes and response to therapy between patients harboring missense and truncating TP53 mutations, throwing doubt on the gain-of-function hypothesis. Seventy percent of all TP53 mutations are nonhotspot mutations, and out of those, around 30% of the mutations, for example, those involving E180 and R181, while tumorigenic, behave very differently from p53-null and hotspot mutations (15). These partial loss-of-function mutant p53 proteins can retain 10% to 50% of transcriptional activity compared with wild-type p53, and accumulation of these mutants can rescue the transcriptional apoptosis defect and sensitize leukemia cells to chemotherapy (15). In contrast, mutations in other tumor suppressors, such as RB1 and VHL, more homogenously lead to no protein expression at all (16).
More recently, it has been noted that TP53 mutations also modulate diverse aspects of the innate and adaptive immune systems. Loss or dysfunction of p53 in solid tumors promotes tumor immune tolerance through downregulating antigen presentation, decreasing Toll-like receptor–mediated apoptosis, and increasing PD-L1 expression (17). However, mutant p53 also favorably modulates immune response by increasing NF-κB activity, increasing tumor-associated macrophage infiltration, eliciting B-cell response, and activating T cells—effects that potentially could be modulated with therapeutic intent (17). The differential impact of cytotoxic therapy on TP53-mutated cancer cells and TP53 wild-type immune cells in the tumor microenvironment further adds to the stochastic complexity of these immune interactions and may affect cytokine production, immune synapse formation between antigen-presenting cells and T cells, and T-cell fate (18–20). With these diverse effects on various components of both the adaptive and innate immune systems, p53 is increasingly being recognized as a “guardian of immune integrity” (21).
TP53 MUTATION IN MDS AND AML
Clonal hematopoiesis is noted in the blood of 2% to 6% of patients with cancer, including clonal TP53 variants that could represent a precursor lesion in diverse malignancies (22, 23). TP53 abnormalities occur in nearly 5% to 10% of patients with de novo MDS and AML (24–26). This frequency is much lower than several other solid tumors—for example, uterine carcinosarcoma, esophageal adenocarcinoma, and lung squamous cell cancers in which TP53 alterations are noted in more than 80% of cases. However, the frequency in AML/MDS goes up to 20% to 40% in older patients or those with therapy-related myeloid malignancies (6, 27). The frequency of TP53 abnormalities further increases to 70% to 80% in patients with complex karyotype and in patients with loss of chromosome 17/17p, 5/5q, or 7/7q (28, 29). Therapy for a previous cancer, including radiation or chemotherapy, does not directly induce TP53 mutations. Rather, preexisting progenitors that carry mutant TP53 and are resistant to DNA damage expand under selective pressure from radiation or chemotherapy to give rise to TP53-mutated AML/MDS later in life (5, 30, 31). Although more than 70% of TP53 abnormalities are missense substitutions clustering within the DNA-binding domain, diverse genetic aberrations in TP53 with complex and varied functional consequences have been described in MDS and AML (1). These include chromosomal alterations leading to allelic gains or losses or frameshift insertions or deletions. The impact of these disruptions ranges from partial loss of function to complete loss of function (1, 26, 27). Among TP53-mutated MDS, “multihit” involvement with more than one genomic and/or chromosome 17 abnormality is noted in the majority of patients, including multiple mutations in 24% of patients, mutations with concomitant deletions in 22% of patients, and mutations with concomitant copy-number loss of heterozygosity in 21% of patients (26). Notably, recent data strongly support that TP53 mutations, particularly multihit, results in similarly poor clinical outcomes, regardless of whether classified as MDS or AML, arguing for a revised TP53 mutant myeloid entity encompassing both MDS and AML if the blast count is 10% to 19% (MDS/AML) or AML with mutated TP53 if blasts are 20% to recognize this highly adverse-risk myeloid pathology (32–35).
Multihit TP53-mutated MDS/AML often represents a distinct stem cell disorder with a paucity of comutations in other myeloid malignancy–related genes, with comutations occurring in less than 25% of cases (36). This is consistent with TP53 mutations being early truncal events in the MDS/AML pathogenesis in such cases, and consequently multihit TP53 mutations or biallelic defects evolve to become dominant clones conferring resistance to current standard therapies and therefore carry a worse prognosis (26). Monoallelic TP53 mutations (33%) on the other hand frequently have comutations in other genes, most commonly TET2 (29%), SF3B1 (27%), ASXL1 (16%), and DNMT3A (16%), and are likely to be late subclonal events with varying impacts on outcomes (26). As accurate multihit analysis requires the determination of the allelic state by loss-of-heterozygosity mapping, clinically available conventional and cytogenetic techniques currently do not capture all biallelic patients. However, a reasonable determination of multihit state can be made if there is the presence of more than one TP53 mutation, TP53 mutation(s) in the setting of a missing chromosome 17p locus, or a variant allele frequency (VAF) >50%, which are 75% concordant with copy-neutral loss-of-heterozygosity variants (26). Nuclear p53 accumulation assessed by IHC may also serve as a surrogate for TP53 mutation and copy-number status (37). Recent reports further show that blast count does not distinguish clinical course, and patients with TP53 mutation with complex karyotype have similarly dismal outcomes irrespective of the initial diagnosis of AML or MDS or the baseline bone marrow blast percentage (32, 33). As a result, the International Consensus Classification has categorized TP53-mutated MDS with excess blasts and TP53-mutated AML as a group of high-risk myeloid neoplasms harboring TP53 mutations to facilitate clinical trial conduct and regulatory approval for new drugs targeting this patient population. Chromothripsis, or chromosome shattering, is a catastrophic event leading to extensive chromosomal rearrangement (38). Chromothripsis serves as an additional adverse-risk biological characteristic associated with TP53 mutation and complex karyotype in AML/MDS. Such massive shattering and reassembly of chromosomes correlates with genomic instability and defines a subset of complex karyotype AML/MDS with even worse outcomes (39, 40).
In a recent survey of more than 500 TP53-mutant AML cases, three quarters harbored a missense variant, most commonly R248, R273, and Y220, with other variants, such as TP53 deletion as well as frameshift and nonsense alterations being less common. It was also found that TP53 copy-number loss was extremely prevalent—identified in 70% of AML cases with a concomitant TP53 abnormality (37). AML survival appeared worse for patients who had either a concomitant TP53 mutation and TP53 copy-number loss or when multiple TP53 mutations were present. It is possible that certain TP53 hotspot variants confer a biological fitness advantage, especially if the restraining effect of the wild-type allele is also lost. Alternatively, deletion of chromosome 17p may result in an allelic loss of other haploinsufficient tumor suppressors that may further enhance the oncogenic potential of mutant TP53 via p53 independent mechanisms (41). Experimental CRISPR/Cas9 genome modeling has demonstrated that human AML cell lines expressing TP53missense/+ have a competitive growth advantage in vivo over haploinsufficient TP53+/− isogenic lines, suggesting a dominant-negative effect (13). TP53missense/− cells, however, were also competitively more potent than TP53missense/+ cells with the wild-type allele retained, consistent with clinical observations in which p53 loss of heterozygosity is often selected for at the time of clinical progression, including after venetoclax-based therapy (42). The biological dominance of TP53 missense variants in AML supports the ongoing therapeutic search for new compositions with therapeutic potential to revert aberrant p53 protein function to normal.
TP53 mutational burden has also emerged as a significant prognostic factor in AML and MDS, with a correlation with response to certain standard therapies. A VAF over 6% is associated with inferior overall survival (OS) and progression-free survival in lower-risk MDS. In high-risk MDS (HR-MDS), increasing VAF strongly correlates with risk of complex cytogenetics, and a VAF >40% was an independent covariate for poor OS (43, 44). These data were validated in a larger cohort that showed that the hazard of death increased by 1.02 per 1% increase in VAF among all MDS (45). In patients with newly diagnosed AML with monoallelic TP53 mutations, an increasing VAF (<20% vs. 20%–40% vs. >40%) did not affect the response rates or the overall dismal survival with hypomethylating agent (HMA)–based therapies, with or without venetoclax, but an increasing VAF was associated with progressively lower response rates and inferior OS in the context of cytarabine-based regimens (46, 47).
p53 also plays a vital role in the normal function and homeostasis of hematopoietic stem cells (HSC) and the bone marrow microenvironment. During normal hematopoiesis, intact p53 mediates the quiescence of HSCs and preservation of genomic stability. Loss or dysfunction of p53 leads to enhanced self-renewal of HSCs, and other supporting oncogenic aberrations can lead to their transformation into leukemia stem cells (LSC; ref. 36). p53 is activated in response to DNA damage with consequent transcriptional activation of several genes, resulting in DNA repair or cell-cycle arrest and apoptosis (2). An impaired apoptosis pathway likely contributes to resistance to cytotoxic chemotherapy or venetoclax-based therapies in multihit TP53-mutated MDS/AML (46, 48, 49). Haploinsufficiency of genes located on chromosome 5q—for example, CSNK1A1, EGR1, APC—cooperate with loss of or mutations in TP53 to confer a survival advantage in HSCs (50, 51). Degradation of the remaining CK1α leading to increased p53-mediated apoptosis is the key mechanism of benefit with lenalidomide in MDS with del(5q) (52). Expansion of preexisting clones or emergence of new clones with TP53 mutations consequently contributes to treatment failure and disease progression in lower-risk MDS with del(5q) treated with lenalidomide (53, 54). Other notable genomic associations with TP53-mutated MDS/AML include amplifications involving EPOR/JAK2 in patients with acute erythroid leukemia, which is characterized by multihit TP53 mutations (55, 56). Germline mutations in ERCC excision repair 6 like 2 (ERCC6L2) have been linked to genomic instability and somatic TP53 mutations leading to AML with erythroid differentiation (57).
Poor outcomes with available therapies prompted investigations into the immune architecture and cytokine milieu of TP53-mutated MDS/AML, with the goal of identifying potential immunotherapeutic approaches. TP53-mutated MDS and AML have an enrichment of immunoinhibitory checkpoints including PD-L1 on HSCs, TIM3 on myeloid-derived suppressor cells (MDSC), and LAG3 and TIGIT on bulk bone marrow blasts (20, 58, 59). Furthermore, TP53-mutated MDS and AML have an immune-dampened microenvironment with upregulation of FoxP3 transcription, an increase in ICOShi (activated) regulatory T cells and PD-1lo MDSCs, a decrease in OX40+ cytotoxic T cells and ICOS+ and 4-1BB+ natural killer cells, as well as marked impairment of CD3−CD28-stimulated T cells to secrete immune-effector Th1 cytokines (polyfunctionality; refs. 20, 58, 60). IFNγ signaling is well recognized as a major driver of response to immune-checkpoint inhibition in solid tumors. Although studies in TP53-mutated AML show that IFNγ signaling may be a biomarker of response to the CD123 × CD3ε dual-affinity receptor targeting (DART) antibody flotetuzumab, there is debate about whether the increased IFNγ signal is a reflection of T-cell fitness in the tumor microenvironment or a sequela of increased inflammation in response to cell death after chemotherapy causing heightened IFNγ production (20, 60). Although bulk RNA analysis of bone marrow has shown high IFNγ signaling before therapy in TP53-mutated AML responders to flotetuzumab, single-cell CD3–CD28-stimulated T-cell cytokine profiling has suggested decreased IFNγ and Th1 cytokine secretion by T cells in newly diagnosed and relapsed or refractory (R/R) TP53-mutated AML (20, 60). In addition, TP53-mutated AML showed upregulation of proinflammatory Th17 genes, NF-κB, PI3K–AKT signaling, and other markers of immune senescence. One could postulate that these aspects may not only affect response to standard therapies but also potentially abrogate the development of a robust graft-versus-leukemia effect (20).
In summary, these data point toward a profound immune dysregulation, with features of immunosenescence with an overall immune-evasive phenotype, which could potentially be leveraged to develop novel immunotherapy approaches for TP53-mutated MDS/AML.
CURRENT THERAPIES FOR TP53-MUTATED MDS AND AML
HMAs are the current standard approach for newly diagnosed HR-MDS and offer an overall response rate (ORR) of 17% to 77% [encompassing complete remission (CR), marrow complete remission (mCR), partial response (PR), and hematologic improvement (HI)] in patients with TP53-mutated MDS, with International Working Group (IWG) CR in 10% to 25%, and a median OS of 8.2 to 12.4 months, with one study reporting an ORR of 100% (n = 9) with the 10-day regimen of decitabine (45, 61, 62). In MDS, TP53 deletions are associated with significantly lower response rates to HMAs, and TP53 VAF more than 40% confer significantly worse outcomes with a median OS of 4.1 to 7.7 months with HMA therapy (Table 1; refs. 29, 45). In a large cohort of patients with MDS and oligoblastic AML who underwent sequential genomic testing during HMA therapy, TP53 mutation was a strong negative predictor with a median OS of 9.7 months (HR, 2.33; P = 0.001). Importantly, a clearance of TP53 mutations (i.e., to VAF of <5%) was a strong predictor of improved outcomes to HMA therapy, particularly in patients who were bridged to allogeneic stem cell transplantation (allo-SCT; HR 0.28; P = 0.001; ref. 44).
Table 1.
Currently available therapies and selected emerging therapies for TP53-mutated AML and MDS
| Agent/regimen | Study phase | Population | TP53-mutated pts | Response | CR rate | Median OS (months) | Reference |
|---|---|---|---|---|---|---|---|
| AML | |||||||
| Azacitidine or decitabine | II; retrospective | ND AML | 22 | CR/CRi 22%–38% | 13%–22% | 2.1–7.3 | (63–66) |
| Venetoclax + azacitidine or 5-day decitabine | Ib/II, III | ND AML | 36, 54 | CR/CRi 47%, 41% | NR, 20% | 4.9–7.2 | (70, 71) |
| Venetoclax + 10-day decitabine | II; post hoc | ND AML | 26 | ORR 77% | 48% | 5.4 | (126) |
| Magrolimab + azacitidine | Ib | ND AML | 72 | CR/CRi 49% | 33% | 10.8 | (82, 127) |
| Magrolimab + venetoclax + azacitidine | Ib/II | ND AML | 14 | ORR 86% | 64% | NR | (84) |
| Eprenetapopt + azacitidine | Ib/II | ND AML | 18 | ORR 33% | 17% | 10.4 | (97) |
| Sabatolimab + HMA | Ib | ND AML | 5 | CR/CRi 40% | 20% | DOR 6.4 | (107) |
| SGN-CD33A ± HMA | I/II | ND AML | 7 | CR/CRi 86% | NR | NA | (128) |
| Nivolumab + intensive chemotherapy | Post hoc | ND AML | 4 | ORR 50% | NA | NA | (67) |
| Intensive chemotherapy | Retrospective | ND AML | various | ORR 47%–55% | 45%–55% | 6.8–8.8 | (6, 64) |
| Low-intensity chemotherapy | Retrospective | ND AML | various | ORR 14%–50% | 36% | 6.7–9.0 | (6, 63, 64) |
| Flotetuzumab | I/II | R/R AML | 15 | ORR 60% | 47% | 4.0 | (90) |
| Nivolumab + azacitidine | II | R/R AML | 26 | ORR 23% | NA | NA | (110) |
| Venetoclax + 10-day decitabine | II; post hoc | R/R AML | 24 | ORR 46% | 19% | 4.5 | (126) |
| MDS | |||||||
| Azacitidine or decitabine | Post hoc | MDS | various | ORR 39%–100% | 1%–32% | 9.4–12.4 | (61, 64) |
| Eprenetapopt + azacitidine | Ib/II | MDS | 40 | ORR 73% | 50% | 10.8 | (96) |
| Sabatolimab + HMA | Ib | MDS | 14 | ORR 71% | 29% | OS NR (DOR 21.5) | (107) |
| Magrolimab + azacitidine | IB | MDS | 25 | ORR 68% | 40% | 16.3 | (83) |
Abbreviations: CRi, CR with incomplete hematologic recovery; DOR, duration of response; NA, not applicable; ND, newly diagnosed; NR, not reported; ORR, overall response rate, defined as sum of all responses per the IWG criteria or European LeukemiaNet (ELN2017) criteria; pts, patients.
In TP53-mutated AML, first-line therapy with low-intensity chemotherapies—for example, HMAs or low-dose cytarabine–based regimens—demonstrated an ORR of 14% to 62% with a median OS of 2.1 to 8.1 months. The rates of response with the 5-day versus 10-day regimen of decitabine were similar (29% vs. 47%, P = 0.40) in a single-institution randomized study (6, 63–66). Intensive chemotherapy-based approaches offered similar outcomes with an ORR of 47% to 55% and a median OS of 6.8 to 10.1 months, often with more toxicities, longer hospital stays, and prolonged myelosuppresion (6, 63, 64, 67). Baseline TP53 VAF was prognostic for response to cytarabine-based regimens with VAF >40% associated with an inferior CR and CR with incomplete hematologic recovery (CRi) rate of 35% and median OS of 4.7 months compared with a CR/CRi rate of 79% and median OS of 7.3 months in patients with TP53 VAF ≤40% (47). TP53 VAF, however, did not seem to affect response rates and median OS in the context of HMA-based regimens for AML, unlike the trend observed in TP53-mutated MDS with HMA (47).
TP53 mutations confer resistance to venetoclax-based regimens in AML through alterations in mitochondrial homeostasis by inhibiting mitochondrial stress response and increasing oxidative phosphorylation (68). Leukemia cells with TP53 loss have an increased threshold for BAX/BAK activation, and although this can be suppressed initially by venetoclax, over time they are able to escape BCL-2 inhibition due to competitive advantage (49). HMA with venetoclax did show encouraging responses in first-line, TP53-mutated, poor cytogenetic risk AML, with a CR/CRi rate of 41% (CR rate of 20%) versus a CR/CRi rate of 17% (CR rate of 11%) with HMA alone, as noted in subset analysis from the phase IB study of HMA with venetoclax and the VIALE-A trial (46, 48, 69–71). However, the median OS in older/unfit patients with AML treated with venetoclax and HMA was 6.5 months, which was similar to the 6.7 months with HMA alone. Given prior data suggesting 10-day decitabine may have a specific benefit in TP53-mutated AML, one study combining decitabine for 10 days with venetoclax showed a CR/CRi rate of 57% (CR rate 37%) but a median OS of only 5.2 months (46). A high 60-day mortality rate of 26% was observed with decitabine plus venetoclax, mainly due to refractory disease, and contributed to poor long-term OS. Nonetheless, venetoclax may still have a role in combination with novel therapies in TP53-mutated AML, harnessing independent mechanisms of synergy. Combined inhibition of BCL-2 and MCL1 as well as blockade of extrinsic and intrinsic apoptotic pathways may also offer a novel approach that preclinically appears to be effective against TP53-mutated AML (49, 72).
ROLE OF ALLO-SCT IN TP53-MUTATED AML
Multiple analyses have shown that patients with TP53-mutated AML/MDS harbor an 80% to 90% higher risk of relapse and death after allo-SCT compared with TP53 wild-type patients (25, 73, 74). A majority of these relapses and death following allo-SCT occur in patients with concomitant chromosome 17 abnormality or complex karyotype, leading to multihit disease (75). However, among patients with TP53-mutated AML, allo-SCT in first remission (CR1) can reduce the risk of relapse by up to 80% and risk of death by up to 70% (47). However, only a minority of patients with TP53-mutated AML, regardless of age or fitness, are able to proceed to allo-SCT in CR1, ranging from 0 to 33% across different published series, with lower response rates, poor count recovery, increased rates of early mortality, and early relapse being the predominant barriers to allo-SCT in this population (46, 47, 66). A case could be made for limiting allo-SCT only in TP53-mutated patients with AML who achieve at least a morphologic remission (i.e., <5% marrow blasts), as outcomes in patients not in morphologic remission before allo-SCT are poor in general and even more inferior in TP53-mutated patients. Clearance of TP53 mutation prior to allo-SCT has been shown to be a favorable prognostic marker, and patients who achieve TP53 mutation clearance or <5% by next-generation sequencing should be strongly considered for transition to allo-SCT in otherwise suitable candidates (76).
Although augmented reduced-intensity conditioning with fludarabine/amsacrine/cytarabine-busulphan has not been shown to improve outcomes over a fludarabine-based reduced-intensity conditioning regimen, a myeloablative conditioning regimen has been shown to improve survival over reduced-intensity conditioning in patients with AML with measurable residual disease (MRD; refs. 77, 78). Even with allo-SCT in TP53-mutated MDS and AML, the risk of relapse remains very significant and long-term survival remains low at less than 20% (28, 29). Nevertheless, allo-SCT still appears to offer the best chances of improving outcomes and achieving long-term survival in appropriately selected patients, with up-front noncytotoxic strategies to attain remissions without severe toxicities, early transition to allo-SCT in suitable candidates, close peritransplant monitoring for TP53-mutated clones, and the use of rational maintenance therapies after transplant to improve outcomes in TP53-mutated patients (75). To this end, novel mutant p53–directed therapies such as eprenetapopt in combination with azacitidine have shown promising results as maintenance therapy after allo-SCT. In patients with TP53-mutated AML/MDS following allo-SCT, this combination showed a median relapse-free survival of 14.5 months and a median OS of 20.6 months, which compared favorably with historical expectations (79).
EMERGING STRATEGIES FOR TP53-MUTATED MDS AND AML
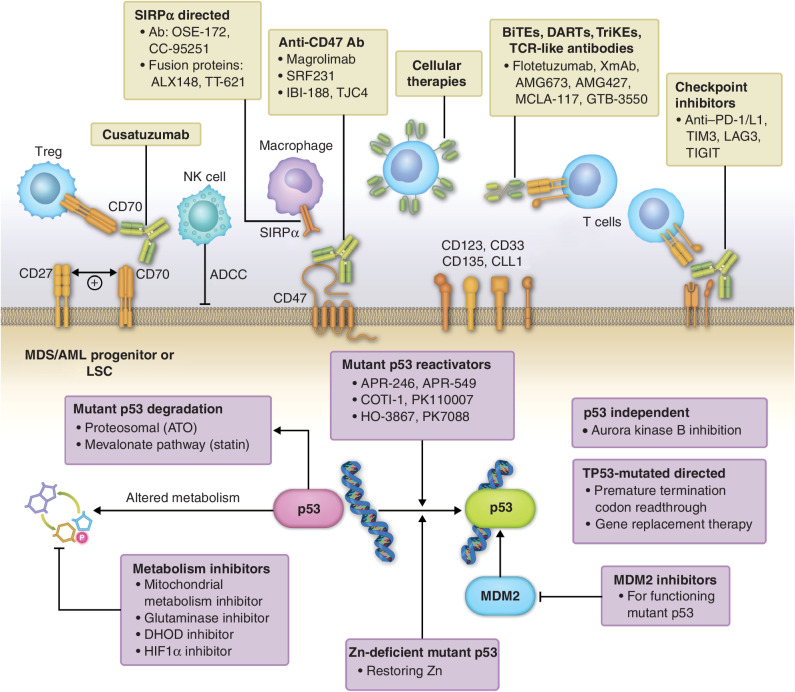
Recent progress in immunotherapeutics and mutant p53–directed approaches offer the hope of potentially improving outcomes in these patients (Fig. 2; ref. 80). In this section, we discuss emerging data with four promising agents in this space, namely, magrolimab, flotetuzumab, sabatolimab, and eprenetapopt, and have briefly described other emerging strategies with potential for the field of TP53-mutated MDS/AML (Table 2).
Figure 2.
Novel therapies for TP53-mutated MDS and AML. Cell-extrinsic immunotherapeutic approaches include targeting cell-surface markers including LSC markers, macrophage and T-cell checkpoints, bispecific engagers, and adoptive cellular therapies including unmodified and chimeric antigen receptor–modified cells. Cell-intrinsic approaches include mutant p53 reactivators, mutant p53 degraders, metabolism-targeting agents, GSPT1 degraders, and others. Ab, antibody; ADCC, antibody-dependent cell-mediated cytotoxicity; BiTE, bispecific T-cell engager; NK, natural killer; TCR, T-cell receptor; Treg, regulatory T cell; TriKE, trispecific killer cell engager.
Table 2.
Ongoing clinical trials of interest for TP53-mutated MDS and AML
| AML | Phase | Disease | Identifier |
|---|---|---|---|
| Magrolimab + azacitidine vs. venetoclax + azacitidine OR intensive chemotherapy (ENHANCE-2) | III | ND TP53-mutated AML only | NCT04778397 |
| Azacitidine + venetoclax ± magrolimab (ENHANCE-3) | III | ND AML (including TP53-mutated) | NCT05079230 |
| Magrolimab + venetoclax + azacitidine | I/II | ND and R/R AML | NCT04435691 |
Multiarm study:
|
I/II | ND, R/R, and postinduction maintenance AML | NCT04778410 |
| Decitabine + cytarabine + arsenic trioxide | II | ND AML | NCT03381781 |
| Sabatolimab + venetoclax + azacitidine | ND AML | NCT04150029 | |
| APR-246 + venetoclax + azacitidine | I | ND AML | NCT04214860 |
| CC-90009 + venetoclax + azacitidine | ND and R/R AML | NCT04336982 | |
| Gamma-delta T cells | I | MRD-positive AML | NCT05001451 |
| NK cells | I | R/R AML | NCT04220684 |
| NCT04023071 | |||
| NCT04623944 | |||
| AML/MDS | |||
| CAR-T cells targeting CD123, CD33, CD135, CLL1-CD33, NKG2D receptor, Lewis Y | I | R/R AML, high-risk myeloid neoplasms | NCT03018405 |
| NCT01864902 | |||
| NCT02159495 | |||
| NCT03795779 | |||
| APR-246 + azacitidine | II | Posttransplant AML, MDS maintenance | NCT03931291 |
| Magrolimab + azacitidine | I/II | ND and R/R AML, ND and R/R MDS | NCT03248479 |
| MDS | |||
| APR-246 ± azacitidine | III | ND TP53-mutated MDS only | NCT03745716 |
| Magrolimab ± azacitidine (ENHANCE-1) | III | ND HR-MDS | NCT04313881 |
| Sabatolimab, hypomethylating agent (STIMULUS) | II, III | ND HR-MDS, CMML | NCT03946670 |
| NCT04266301 | |||
| APR-548 + azacitidine | I | ND MDS | NCT04638309 |
Abbreviations: CAR, chimeric antigen receptor; CMML, chronic myelomonocytic leukemia; ND, newly diagnosed; NK, natural killer.
Magrolimab
CD47 is an integrin-associated antiphagocytic protein that is overexpressed in cancer cells and correlates with poor outcomes in AML. It binds to the signal receptor protein-α (SIRPα) on macrophages and dendritic cells and enables immune evasion by inhibiting prophagocytic receptors like complement receptor 3, Fc receptors, and SLAMF7 from initiating phagocytosis (81). Magrolimab (Hu5F9-G4) is a first-in-class humanized IgG4 monoclonal antibody against CD47 and prompts cancer cell phagocytosis by macrophages through disruption of the CD47–SIRPα inhibitory checkpoint, thereby blocking the “don't eat me signal.” CD47 is also an LSC marker, and targeting CD47 can potentially eliminate LSCs while sparing normal HSCs. Preclinical studies showed synergism between azacitidine and magrolimab in AML cell lines, and this combination was tested in a phase Ib trial that enrolled older/unfit patients with newly diagnosed AML ineligible for induction therapy and newly diagnosed intermediate- to high-risk MDS. Among older/unfit patients with TP53-mutated AML treated on this trial (n = 72), azacitidine with magrolimab showed an ORR of 49% (n = 35/72) and a CR rate of 33% (n = 24/72; ref. 82). The median duration of response (DOR) was 8.7 months, and the median OS was 10.8 months (82). In 25 patients with TP53-mutated MDS enrolled, the combination led to an ORR of 68%, a CR rate of 40%, and a median OS of 16.3 months (83). Magrolimab with venetoclax and azacitidine was evaluated in patients with newly diagnosed TP53-mutated AML (n = 14), with an ORR of 86% with a CR rate of 64%, an MRD-negative rate of 55%, and robust clearance of TP53-mutated clones in eight of nine CR/CRi patients (VAF sensitivity 1%; ref. 84). Other anti-CD47–targeted therapies in phase I/II clinical trials include lemzoparlimab, TTI-621, TTI-622, ALX148, SL-172154 (SIRPα-Fc-CD40L), etc., with many trials having cohorts for patients with TP53 mutations (85).
Flotetuzumab
CD123 serves as the receptor for IL3, and its downstream signaling promotes hematopoietic progenitor cell proliferation through activation of the PI3K/MAPK pathway and upregulation of antiapoptotic proteins (86). CD123 is differentially expressed in about 90% of patients with AML, and overexpression on AML blasts is associated with inferior outcomes (87, 88). Flotetuzumab is a CD123 × CD3ε DART molecule that mediates T-cell activation and proliferation, resulting in the eradication of CD123-expressing primary AML blasts in vitro and in vivo (86, 89). Flotetuzumab was evaluated in a phase I/II study in R/R AML, enriched for patients with AML with primary induction failure or early relapse (within 6 months of response; ref. 90). Among patients with TP53-mutated R/R AML, the ORR was 47% (n = 7/15) with an encouraging median OS of 10.3 months in responding patients (20). The relatively short durability of response outside of patients who were bridged quickly to allo-SCT remains a challenge with a DOR of 2 to 5 months in nontransplanted patients.
CD123 expression did not correlate with response or cytokine release syndrome with flotetuzumab. Transcriptomic analysis suggested that an IFNγ-enriched, immune-infiltrated tumor microenvironment predicted response to flotetuzumab, and an immunosuppressed tumor microenvironment could be rejuvenated by flotetuzumab through T cell–driven mechanisms (90). Specifically among TP53-mutated patients, higher bulk RNA expression of FOXP3, PD-1, and inflammatory chemokines correlated with a response along with CD8B and IFNG (20, 90). Vibecotamab (XmAb14045) is another CD123 × CD3 bispecific T-cell engager (BiTE) that showed a modest ORR of 14% (n = 7/51) in R/R AML (91). Multiple CD33-directed BiTEs are currently in the dose-escalation phase and have yielded modest responses in R/R AML. There are several other bispecific antibody platforms targeting CD123, CD33, CD135, CLEC12A, as well as novel natural killer (NK) cell–directed bispecific engager and trispecific engagers in early clinical development and if found to be effective and safe may be interesting to evaluate for TP53-mutated AML given their potential mutation-agnostic mechanism of actions.
Eprenetapopt
Eprenetapopt (APR-246) is a first-in-class agent that binds covalently to cysteine residues in the core DNA domain of mutant p53 and is postulated to cause refolding and restoration of an active wild type–like conformation and function of p53 (16). Other proposed mechanisms of this class of agents include induction of cell death via reactive oxygen species, ferroptosis, depletion of deoxyribonucleotides, and triggering of unfolded protein responses through depletion of antioxidants (92–95). Two studies evaluated eprenetapopt with azacitidine in newly diagnosed adults with HMA-naïve low- to high-risk MDS, AML, and MDS/myeloproliferative neoplasm (MPN; refs. 96, 97). In a pooled analysis of the two trials, significantly higher rates of CR were noted in patients with isolated TP53 mutations (CR rate of 52% vs. 30%), and in patients with biallelic TP53 mutation or complex karyotype (CR rate of 49% vs. 8%; ref. 98). Additionally, patients with complete or partial remission and/or clearing TP53 mutation (VAF sensitivity 1%) and proceeding to allo-SCT had favorable outcomes with the median OS not reached. In the overall AML, MDS, MPN population, IHC of bone marrow mononuclear cells showing more than 10% staining for p53 was associated with a higher CR rate (66% vs. 13%, P = 0.01; ref. 96). Reduction of mutant TP53 VAF below 0.1% was associated with improved OS (not reached vs. 10.7 months, P = 0.05; ref. 97). However, in a randomized trial in newly diagnosed patients with TP53-mutated MDS, azacitidine with eprenetapopt versus azacitidine with placebo did not meet the primary endpoint in spite of a numerically improved CR rate (33% vs. 22%, P = 0.13; refs. 99, 100). Preliminary results of a triple combination of eprenetapopt in combination with venetoclax and azacitidine in previously untreated TP53-mutated AML (n = 30) showed a CR/CRi rate of 53% and a CR rate of 37%, and accrual is ongoing (101). A next-generation oral p53 reactivator, APR-548, is currently under preclinical development. Mutant-specific p53 activators, such as PC14586 for p.Y220C, are currently under investigation for solid tumors (NCT04585750; ref. 102).
Sabatolimab
The potential for immunotherapeutic agents to act in a p53-agnostic manner and potentially circumvent some of the p53-associated resistance mechanisms, as well as growing insights into immune microenvironmental remodeling by TP53-mutant AML/MDS, has led to an increasing interest in evaluating other immunotherapies in TP53-mutant AML/MDS. TIM3 is another checkpoint that forms part of a coinhibitory receptor module expressed on exhausted T cells and is preferentially overexpressed on MDS/AML LSCs (103, 104). TIM3 is involved in an autocrine signaling loop via galectin-9, which promotes LSC renewal, and antibodies blocking TIM3 could therefore selectively eradicate AML LSCs (105, 106). Sabatolimab (MBG453) is a humanized, high-affinity IgG4-targeting TIM3 being evaluated in solid tumors and hematologic malignancies. A phase Ib trial evaluated sabatolimab with HMA in newly diagnosed patients with HR-MDS by the Revised International Prognostic Scoring System (IPSS-R; n = 53) or AML unfit for intensive therapy (n = 48; ref. 107). The adverse event profile of the combination was consistent with that of HMA alone with few and mostly lower-grade immune-related adverse events noted. In patients with HR-MDS, this combination demonstrated an ORR of 57% (CR rate 20%) and a median DOR of 17.1 months. Among patients with newly diagnosed AML, this combination yielded a CR/CRi rate of 30%, a CR rate of 25%, and a median DOR of 12.6 months. Specifically, in patients with HR-MDS with adverse-risk mutations TP53, RUNX1, and ASXL1, the CR/mCR rate was 43% and the median DOR was encouraging at 21.5 months in 10 of 14 responders. In patients with newly diagnosed TP53-mutant AML, the CR/CRi was 40% with a median DOR of 6.4 months.
OTHER IMMUNOTHERAPEUTIC APPROACHES
SIRPα-directed therapies to the macrophage ligand SIRPα offer another approach to disrupt the CD47–SIRPα immune checkpoint and modulate MDSCs. These agents may potentially mitigate on-target adverse effects of anti-CD47 antibody (e.g., anemia). Such therapies including anti-SIRPα antibodies (e.g., OSE-172 and CC-95251) and SIRPα fusion proteins (e.g., ALX148 and TT-621) are currently in phase I trials, with ALX418 and TTI-621 being evaluated in combination with HMA in MDS and in combination with HMA with venetoclax in AML.
Immune-checkpoint inhibitor–based regimens have overall yielded modest results in MDS/AML so far. The initial report with single-agent ipilimumab yielded a CR in 42% of patients (n = 5/12) with relapsed AML after allo-SCT, generating a great deal of excitement for this field in AML and MDS (108). Blockade of PD-1 or PD-1 and CTLA-4 with azacitidine or high-dose cytarabine in all R/R AML yielded modest CR/CRi rates of 14% to 36% in patients. The median OS was 6.3 to 10.5 months, with an ORR of 23% in TP53-mutated R/R AML in these PD-1–based combinations (109, 110). In the first-line setting, nivolumab with idarubicin and cytarabine yielded a CR/CRi of 50% in patients with TP53-mutated AML (n = 4/8; ref. 67). Unfortunately, no significant improvement in CR/CRi rates or in OS in first-line higher-risk MDS (n = 84) or first-line older/unfit AML (n = 129) was noted in a randomized, first-line phase II study of azacitidine with or without the anti–PD-L1 antibody durvalumab, resulting in tempered enthusiasm and uncertain future for PD-1/PD-L1/CTLA-4–based therapies in myeloid malignancies (111, 112).
Cellular therapy approaches have been challenging to develop due to the hostile milieu of the bone marrow niche in AML (80). Chimeric antigen receptor (CAR) T-cell therapies directed at myeloid antigens, including CD33, CD38, CD70, CD123, CD135, CD371, CLL1, FLT3, TIM3, LILRB4, NKG2D, Lewis Y, and others, are still in early development, with modest responses ranging from isolated blast count reductions to brief CR/CRi in up to 50% of patients in the dose-escalation cohorts (28, 113). One second-generation CAR-T targeting CLL1 has shown promising outcomes in pediatric AML with CR/CRi in six of eight patients without any grade 3/4 cytokine release syndrome or immune effector cell–associated neurotoxicity syndrome (114). Although CLL1 is not expressed in HSCs, its expression on granulocytes and monocytes led to associated neutropenia, which resolved only after the eradication of CLL1 CAR-T cells. Novel approaches to safely improve CAR-T efficacy through targeting multiple antigens with novel gating strategies, enhancing fitness and in vivo persistence, overcoming the immunosuppressive microenvironment, and developing allogeneic CAR-based approaches will hopefully lead to better cellular therapies for AML (115). Development of T-cell receptor–like antibodies against mutant p53 and the potential for engineering similar adoptive T-cell approaches are in early preclinical development (116, 117).
Off-the-shelf modified NK cell–based approaches have shown early promise in R/R AML with no dose-limiting toxicities or cytokine release syndrome, immune effector cell–associated neurotoxicity syndrome, or graft-versus-host disease. In a phase I trial of FT516/538 (an induced pluripotent stem cell–derived high-affinity, noncleavable CD16 expressing NK cell) in 12 patients with R/R AML with a median of three prior lines of therapy, the ORR was 42% with durable remissions in two patients lasting >6 months without subsequent interventions after NK infusions (118). If successful, such strategies may find an important role in traditionally difficult-to-treat molecular and cytogenetic subsets such as TP53, RUNX1, and inv3q and other subsets of AML/MDS. Such approaches may be especially attractive in patients with low-burden disease, MRD+ disease, or potentially as maintenance after AML therapy or after allo-SCT in high-risk patients in remission, as these patients are likely to have a more favorable tumor microenvironment potentially not rendered deranged by the presence of high-volume aberrant myeloid cells. Other similar adoptive cellular therapies rapidly entering the clinic for AML/MDS include gamma-delta T cells, and invariant NKT cells are currently in preclinical development (refs. 119–121).
OTHER NONIMMUNOLOGIC APPROACHES
COTI-2 is a thiosemicarbazone compound with effects like eprenetapopt. It binds to mutant p53 and reverses conformation to a wild-type form, thus restoring DNA-binding function and normalizing wild-type p53 target gene expression (16). It can also act independently through inducing DNA damage, causing replication stress, activating AMP-activated protein kinase, and inhibiting the mTOR pathway. It showed acceptable safety in a phase I trial in gynecologic malignancies (NCT02433626; ref. 122). Other similar mutant p53 reactivators including PK110007, HO-3867, and PK7088 are in various stages of development.
Other miscellaneous approaches with potential application to TP53-mutated MDS/AML include arsenic trioxide–based approaches to induce proteasomal degradation of mutant p53 (arsenic trioxide has been shown to structurally stabilize p53 mutants and transcriptionally rescue a subset of mutants through a cryptic allosteric site; ref. 123), statin-based approaches to promote mutant p53 degradation via inhibition of the mevalonate pathway, and restoring zinc to zinc-deficient p53 mutants (16, 27, 124, 125). Future approaches directed toward TP53 mutations may include promotion of premature termination codon readthrough enabling the production of full-length p53 and gene replacement therapies (16, 27).
In addition, rational combinations or sequential approaches of previously mentioned strategies with the integration of allo-SCT as a part of the continuum of therapy may be needed to improve response durability and survival of TP53-mutated MDS and AML.
CONCLUSION
Four decades of cumulative discoveries have brought us to what is hopefully the cusp of important breakthroughs in the field of TP53-mutated cancers, with many of these efforts culminating in clinical trials being initiated in myeloid malignancies. With the increasing recognition of TP53-mutated MDS and AML as distinct stem cell disorders, we are beginning to better understand the diverse genetic and immune landscape of TP53 alterations, their functional consequences on both the tumor and the immune microenvironment, and the heterogenous nature of TP53 mutations with varied prognostic consequences. Clearly, it is now well recognized that TP53-mutant MDS/AML disease represents a singular entity with poor outcomes necessitating dedicated clinical interventions with the hope of developing and optimizing the first TP53-specific agents. Encouraging early results of novel innate and adaptive immunotherapeutic approaches and mutant p53 reactivators in combination with HMA with or without venetoclax are showing encouraging efficacy that needs to be confirmed in randomized registration studies. If successful, new questions will emerge regarding predictive biomarkers, time and role of allo-SCT, resistance mechanisms, side effect management, and optimal combination and sequencing strategies as well as maintenance applications of such novel strategies with the eventual hope of improving survival in this extremely difficult patient population.
Acknowledgments
A. Maiti received support through the American Society of Clinical Oncology Young Investigator Award from the Conquer Cancer Foundation. This work was supported in part by the MD Anderson Cancer Center Support Grant (CCSG) CA016672, the MD Anderson Cancer Center Leukemia SPORE CA100632, the Charif Souki Cancer Research Fund, and generous philanthropic contributions to the MD Anderson Moon Shots Program. Figures were created with BioRender.com. The authors thank Jordan Pietz, MA, CMI, of the MD Anderson Cancer Center for assistance with the illustration.
Authors’ Disclosures
N.G. Daver reports grants and personal fees from Gilead, Pfizer, AbbVie, Shattuck Labs, Bristol Myers Squibb, Kite, Genentech, Daiichi Sankyo, Astellas, Novartis, Immunogen, Hanmi, and Servier and grants from Fate and KAHR Therapeutics outside the submitted work. A. Maiti reports other support from BioSight, Sanofi, and Astex Pharmaceuticals outside the submitted work. T.M. Kadia reports grants and personal fees from AbbVie, Jazz, and Genentech, personal fees from Agios and Daichii Sankyo, and grants from Amgen and Astellas outside the submitted work. R. Majeti reports grants from Gilead Sciences outside the submitted work; a patent for magrolimab pending, issued, licensed, and with royalties paid from Gilead Sciences to Stanford University; is on the board of directors for CircBio and advisory boards for Kodikaz Therapeutic Solutions, Syros Pharmaceuticals, and TenSixteen Bio; is an inventor on a number of patents related to CD47 cancer immunotherapy licensed to Gilead Sciences; receives research support from Gilead Sciences and CircBio; and is a cofounder of and equity holder in CircBio, Pheast Therapeutics, MyeloGene, and RNAC Therapeutics. A.H. Wei reports other support from the Walter and Eliza Hall Institute of Medical Research (WEHI) during the conduct of the study, as well as other support from AbbVie, Servier, Bristol Myers Squibb, Syndax, Astex, AstraZeneca, and Amgen outside the submitted work. D.A. Sallman reports other support from Aprea and Gilead during the conduct of the study, as well as other support from Agios, Servier, Jazz, AbbVie, Incyte, Bristol Myers Squibb, Intellia, Curis, Novartis, Janssen, and Shattuck Labs outside the submitted work. No disclosures were reported by the other authors.
References
- 1. Sabapathy K, Lane DP. Therapeutic targeting of p53: all mutants are equal, but some mutants are more equal than others. Nat Rev Clin Oncol 2018;15:13–30. [DOI] [PubMed] [Google Scholar]
- 2. Lakin ND, Jackson SP. Regulation of p53 in response to DNA damage. Oncogene 1999;18:7644–55. [DOI] [PubMed] [Google Scholar]
- 3. Joerger AC, Fersht AR. The tumor suppressor p53: From structures to drug discovery. Cold Spring Harb Perspect Biol 2010;2:a000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bowen D, Groves MJ, Burnett AK, Patel Y, Allen C, Green C, et al. TP53 gene mutation is frequent in patients with acute myeloid leukemia and complex karyotype, and is associated with very poor prognosis. Leukemia 2009;23:203–6. [DOI] [PubMed] [Google Scholar]
- 5. Wong TN, Ramsingh G, Young AL, Miller CA, Touma W, Welch JS, et al. Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature 2015;518:552–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kadia TM, Jain P, Ravandi F, Garcia-Manero G, Andreef M, Takahashi K, et al. TP53 mutations in newly diagnosed acute myeloid leukemia: clinicomolecular characteristics, response to therapy, and outcomes. Cancer 2016;122:3484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Olivier M, Eeles R, Hollstein M, Khan MA, Harris CC, Hainaut P. The IARC TP53 database: new online mutation analysis and recommendations to users. Hum Mutat 2002;19:607–14. [DOI] [PubMed] [Google Scholar]
- 8. Levine AJ. p53: 800 million years of evolution and 40 years of discovery. Nat Rev Cancer 2020;20:471–80. [DOI] [PubMed] [Google Scholar]
- 9. Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature 2000;408:307–10. [DOI] [PubMed] [Google Scholar]
- 10. Donehower LA, Soussi T, Korkut A, Liu Y, Schultz A, Cardenas M, et al. Integrated analysis of TP53 gene and pathway alterations in The Cancer Genome Atlas. Cell Rep 2019;28:1370–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Freed-Pastor WA, Prives C. Mutant p53: one name, many proteins. Genes Dev 2012;26:1268–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol 2010;2:a001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boettcher S, Miller PG, Sharma R, McConkey M, Leventhal M, Krivtsov AV, et al. A dominant-negative effect drives selection of TP53 missense mutations in myeloid malignancies. Science 2019;365:599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giacomelli AO, Yang X, Lintner RE, McFarland JM, Duby M, Kim J, et al. Mutational processes shape the landscape of TP53 mutations in human cancer. Nat Genet 2018;50:1381–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klimovich B, Merle N, Neumann M, Elmshäuser S, Nist A, Mernberger M, et al. p53 partial loss-of-function mutations sensitize to chemotherapy. Oncogene 2022;41:1011–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Duffy MJ, Synnott NC, O'Grady S, Crown J. Targeting p53 for the treatment of cancer. Semin Cancer Biol 2022;79:58–67. [DOI] [PubMed] [Google Scholar]
- 17. Blagih J, Buck MD, Vousden KH. p53, cancer and the immune response. J Cell Sci 2020;133:jcs237453. [DOI] [PubMed] [Google Scholar]
- 18. Guo G, Yu M, Xiao W, Celis E, Cui Y. Local activation of p53 in the tumor microenvironment overcomes immune suppression and enhances antitumor immunity. Cancer Res 2017;77:2292–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Levine AJ. P53 and the immune response: 40 years of exploration—a plan for the future. Int J Mol Sci 2020;21:541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vadakekolathu J, Lai C, Reeder S, Church SE, Hood T, Lourdusamy A, et al. TP53 abnormalities correlate with immune infiltration and associate with response to flotetuzumab immunotherapy in AML. Blood Adv 2020;4:5011–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muñoz-Fontela C, Mandinova A, Aaronson SA, Lee SW. Emerging roles of p53 and other tumour-suppressor genes in immune regulation. Nat Rev Immunol 2016;16:741–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xie M, Lu C, Wang J, McLellan MD, Johnson KJ, Wendl MC, et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med 2014;20:1472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen S, Wang Q, Yu H, Capitano ML, Vemula S, Nabinger SC, et al. Mutant p53 drives clonal hematopoiesis through modulating epigenetic pathway. Nat Commun 2019;10:5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. The Cancer Genome Atlas Research Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 2013;368:2059–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lindsley RC, Saber W, Mar BG, Redd R, Wang T, Haagenson MD, et al. Prognostic mutations in myelodysplastic syndrome after stem-cell transplantation. N Engl J Med 2017;376:536–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bernard E, Nannya Y, Hasserjian RP, Devlin SM, Tuechler H, Medina-Martinez JS, et al. Implications of TP53 allelic state for genome stability, clinical presentation and outcomes in myelodysplastic syndromes. Nat Med 2020;26:1549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barbosa K, Li S, Adams PD, Deshpande AJ. The role of TP53 in acute myeloid leukemia: challenges and opportunities. Genes Chromosomes Cancer 2019;58:875–88. [DOI] [PubMed] [Google Scholar]
- 28. Hunter AM, Sallman DA. Current status and new treatment approaches in TP53 mutated AML. Best Pract Res Clin Haematol 2019;32:134–44. [DOI] [PubMed] [Google Scholar]
- 29. Hunter AM, Sallman DA. Targeting TP53 mutations in myelodysplastic syndromes. Hematol Oncol Clin North Am 2020;34:421–40. [DOI] [PubMed] [Google Scholar]
- 30. Bolton KL, Ptashkin RN, Gao T, Braunstein L, Devlin SM, Kelly D, et al. Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat Genet 2020;52:1219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saiki R, Momozawa Y, Nannya Y, Nakagawa MM, Ochi Y, Yoshizato T, et al. Combined landscape of single-nucleotide variants and copy number alterations in clonal hematopoiesis. Nat Med 2021;27:1239–49. [DOI] [PubMed] [Google Scholar]
- 32. Weinberg OK, Siddon AJ, Madanat Y, Gagan J, Arber DA, Dal Cin P, et al. TP53 mutation defines a unique subgroup within complex karyotype de novo and therapy-related MDS/AML. Blood Adv 2022;6:2847–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grob T, Sanders MA, Kavelaars FG, Rijken M, Gradowska PL, et al. Molecular characterization of mutant TP53 acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood 2022;139:2347–54. [DOI] [PubMed] [Google Scholar]
- 34. Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, et al. Diagnosis and management of AML in adults: 2022 ELN recommendations from an international expert panel. Blood 2022;140:1345–77. [DOI] [PubMed] [Google Scholar]
- 35. Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, et al. The International Consensus Classification of myeloid neoplasms and acute leukemias: integrating morphological, clinical, and genomic data. Blood 2022;140:1200–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sill H, Zebisch A, Haase D. Acute myeloid leukemia and myelodysplastic syndromes with TP53 aberrations: a distinct stem cell disorder. Clin Cancer Res 2020;26:5304–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tashakori M, Kadia T, Loghavi S, Daver N, Kanagal-Shamanna R, Pierce S, et al. TP53 copy number and protein expression inform mutation status across risk categories in acute myeloid leukemia. Blood 2022;140:58–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang CZ, Spektor A, Cornils H, Francis JM, Jackson EK, Liu S, et al. Chromothripsis from DNA damage in micronuclei. Nature 2015;522:179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rücker FG, Dolnik A, Blätte TJ, Teleanu V, Ernst A, Thol F, et al. Chromothripsis is linked to TP53 alteration, cell cycle impairment, and dismal outcome in acute myeloid leukemia with complex karyotype. Haematologica 2018;103:e17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Iacobucci I, Wen J, Meggendorfer M, Choi JK, Shi L, Pounds SB, et al. Genomic subtyping and therapeutic targeting of acute erythroleukemia. Nat Genet 2019;51:694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu Y, Chen C, Xu Z, Scuoppo C, Rillahan CD, Gao J, et al. Deletions linked to TP53 loss drive cancer through p53-independent mechanisms. Nature 2016;531:471–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. DiNardo CD, Tiong IS, Quaglieri A, MacRaild S, Loghavi S, Brown FC, et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood 2020;135:791–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Belickova M, Vesela J, Jonasova A, Pejsova B, Votavova H, Merkerova MD, et al. TP53 mutation variant allele frequency is a potential predictor for clinical outcome of patients with lower-risk myelodysplastic syndromes. Oncotarget 2016;7:36266–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sallman DA, Komrokji R, Vaupel C, Cluzeau T, Geyer SM, McGraw KL, et al. Impact of TP53 mutation variant allele frequency on phenotype and outcomes in myelodysplastic syndromes. Leukemia 2016;30:666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Montalban-Bravo G, Kanagal-Shamanna R, Benton CB, Class CA, Chien KS, Sasaki K, et al. Genomic context and TP53 allele frequency define clinical outcomes in TP53-mutated myelodysplastic syndromes. Blood Adv 2020;4:482–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kim K, Maiti A, Loghavi S, Pourebrahim R, Kadia TM, Rausch CR, et al. Outcomes of TP53-mutant acute myeloid leukemia with decitabine and venetoclax. Cancer 2021;127:3772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Short NJ, Montalban-Bravo G, Hwang H, Ning J, Franquiz MJ, Kanagal-Shamanna R, et al. Prognostic and therapeutic impacts of mutant TP53 variant allelic frequency in newly diagnosed acute myeloid leukemia. Blood Adv 2020;4:5681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Venugopal S, Shoukier M, Konopleva M, Dinardo CD, Ravandi F, Short NJ, et al. Outcomes in patients with newly diagnosed TP53-mutated acute myeloid leukemia with or without venetoclax-based therapy. Cancer 127:3541–51. [DOI] [PubMed] [Google Scholar]
- 49. Thijssen R, Diepstraten ST, Moujalled D, Chew E, Flensburg C, Shi MX, et al. Intact TP-53 function is essential for sustaining durable responses to BH3-mimetic drugs in leukemias. Blood 2021;137:2721–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schneider RK, Ademà V, Heckl D, Järås M, Mallo M, Lord AM, et al. Role of casein kinase 1A1 in the biology and targeted therapy of del(5q) MDS. Cancer Cell 2014;26:509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stoddart A, Fernald AA, Wang J, Davis EM, Karrison T, Anastasi J, et al. Haploinsufficiency of del(5q) genes, Egr1 and Apc, cooperate with Tp53 loss to induce acute myeloid leukemia in mice. Blood 2014;123:1069–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Krönke J, Fink EC, Hollenbach PW, MacBeth KJ, Hurst SN, Udeshi ND, et al. Lenalidomide induces ubiquitination and degradation of CK1α in del(5q) MDS. Nature 2015;523:183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jädersten M, Saft L, Smith A, Kulasekararaj A, Pomplun S, Göhring G, et al. TP53 mutations in low-risk myelodysplastic syndromes with del(5q) predict disease progression. J Clin Oncol 2011;29:1971–9. [DOI] [PubMed] [Google Scholar]
- 54. Lodé L, Ménard A, Flet L, Richebourg S, Loirat M, Eveillard M, et al. Emergence and evolution of TP53 mutations are key features of disease progression in myelodysplastic patients with lower-risk del(5q) treated with lenalidomide. Haematologica 2018;103:e143–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Takeda J, Yoshida K, Nakagawa MM, Nannya Y, Yoda A, Saiki R, et al. Amplified EPOR/JAK2 genes define a unique subtype of acute erythroid leukemia. Blood Cancer Discov 2022;3:410–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Montalban-Bravo G, Benton CB, Wang SA, Ravandi F, Kadia T, Cortes J, et al. More than 1 TP53 abnormality is a dominant characteristic of pure erythroid leukemia. Blood 2017;129:2584–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Douglas SPM, Siipola P, Kovanen PE, Pyörälä M, Kakko S, Savolainen ER, et al. ERCC6L2 defines a novel entity within inherited acute myeloid leukemia. Blood 2019;133:2724–8. [DOI] [PubMed] [Google Scholar]
- 58. Sallman DA, McLemore AF, Aldrich AL, Komrokji RS, McGraw KL, Dhawan A, et al. TP53 mutations in myelodysplastic syndromes and secondary AML confer an immunosuppressive phenotype. Blood 2020;136:2812–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Williams P, Basu S, Garcia-Manero G, Hourigan CS, Oetjen KA, Cortes JE, et al. The distribution of T-cell subsets and the expression of immune checkpoint receptors and ligands in patients with newly diagnosed and relapsed acute myeloid leukemia. Cancer 2019;125:1470–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Daver N, Kantarjian H, Mackay S, Flynn B, Basu S, Garcia-Manero G, et al. Polyfunctionality determined by single-cell proteomics of bone marrow-derived CD4 T cells from patients with acute myeloid leukemia identifies patients responding to anti–PD-1-based therapy and discovers profound T cell defect in mutant TP53 disease [abstract]. In: Proceedings of the American Association for Cancer Research Annual Meeting 2019; 2019 Mar 29–Apr 3; Atlanta, GA. Philadelphia (PA): AACR; Cancer Res 2019;79(13 Suppl):Abstract nr LB-222. [Google Scholar]
- 61. Montalban-Bravo G, Takahashi K, Garcia-Manero G. Decitabine in TP53-mutated AML [comment]. N Engl J Med 2017;376:796–7. [DOI] [PubMed] [Google Scholar]
- 62. Al-Issa K, Sekeres MA, d Nielsen A, Jha B, Przychodzen BP, Aly M, et al. TP53 mutations and outcome in patients with myelodysplastic syndromes (MDS). Blood 2016;128:4336. [Google Scholar]
- 63. Boddu P, Kantarjian H, Ravandi F, Garcia-Manero G, Borthakur G, Andreeff M, et al. Outcomes with lower intensity therapy in TP53-mutated acute myeloid leukemia. Leuk Lymphoma 2018;59:2238–41. [DOI] [PubMed] [Google Scholar]
- 64. Bewersdorf JP, Shallis RM, Gowda L, Wei W, Hager K, Isufi I, et al. Clinical outcomes and characteristics of patients with TP53-mutated acute myeloid leukemia or myelodysplastic syndromes: a single center experience. Leuk Lymphoma 2020;61:2180–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Short NJ, Kantarjian HM, Loghavi S, Huang X, Qiao W, Borthakur G, et al. Treatment with a 5-day versus a 10-day schedule of decitabine in older patients with newly diagnosed acute myeloid leukaemia: a randomised phase 2 trial. Lancet Haematol 2018;6:e29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Welch JS, Petti AA, Miller CA, Fronick CC, O'Laughlin M, Fulton RS, et al. TP53 and decitabine in acute myeloid leukemia and myelodysplastic syndromes. N Engl J Med 2016;375:2023–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ravandi F, Assi R, Daver N, Benton CB, Kadia T, Thompson PA, et al. Idarubicin, cytarabine, and nivolumab in patients with newly diagnosed acute myeloid leukaemia or high-risk myelodysplastic syndrome: a single-arm, phase 2 study. Lancet Haematol 2019;6:e480–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nechiporuk T, Kurtz SE, Nikolova O, Liu T, Jones CL, D'Alessandro A, et al. The TP53 apoptotic network is a primary mediator of resistance to BCL2 inhibition in AML cells. Cancer Discov 2019;9:910–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med 2020;383:617–29. [DOI] [PubMed] [Google Scholar]
- 70. Pollyea DA, Pratz KW, Wei AH, Pullarkat VA, Jonas BA, Recher C, et al. Outcomes in patients with poor-risk cytogenetics with or without TP53 mutations treated with venetoclax combined with hypomethylating agents. Blood 2021;138:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019;133:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Maiti A, Andreeff M, Konopleva MY. Beyond BCL-2 inhibition in acute myeloid leukemia: other approaches to leverage the apoptotic pathway. Clin Lymphoma Myeloma Leuk 2021;21:S3–6. [DOI] [PubMed] [Google Scholar]
- 73. Della Porta MG, Gallì A, Bacigalupo A, Zibellini S, Bernardi M, Rizzo E, et al. Clinical effects of driver somatic mutations on the outcomes of patients with myelodysplastic syndromes treated with allogeneic hematopoietic stem-cell transplantation. J Clin Oncol 2016;34:3627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yoshizato T, Nannya Y, Atsuta Y, Shiozawa Y, Iijima-Yamashita Y, Yoshida K, et al. Genetic abnormalities in myelodysplasia and secondary acute myeloid leukemia: impact on outcome of stem cell transplantation. Blood 2017;129:2347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Loke J, Labopin M, Craddock C, Cornelissen JJ, Labussière-Wallet H, Wagner-Drouet EM, et al. Additional cytogenetic features determines outcome in patients allografted for TP53 mutant acute myeloid leukemia. Cancer 2022;128;2922–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hunter AM, Komrokji RS, Yun S, Al Ali N, Chan O, Song J, et al. Baseline and serial molecular profiling predicts outcomes with hypomethylating agents in myelodysplastic syndromes. Blood Adv 2021;5:1017–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Craddock C, Jackson A, Loke J, Siddique S, Hodgkinson A, Mason J, et al. Augmented reduced-intensity regimen does not improve postallogeneic transplant outcomes in acute myeloid leukemia. J Clin Oncol 2021;39:768–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hourigan CS, Dillon LW, Gui G, Logan BR, Fei M, Ghannam J, et al. Impact of conditioning intensity of allogeneic transplantation for acute myeloid leukemia with genomic evidence of residual disease. J Clin Oncol 2020;38:1273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mishra A, Tamari R, DeZern AE, Byrne MT, Gooptu M, Chen YB, et al. Eprenetapopt plus azacitidine following allogeneic hematopoietic stem cell transplantation for TP53-mutant AML and MDS. J Clin Oncol 2022Jul 11 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 80. Daver N, Alotaibi AS, Bücklein V, Subklewe M. T-cell-based immunotherapy of acute myeloid leukemia: current concepts and future developments. Leukemia 2021;35:1843–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Veillette A, Tang Z. Signaling regulatory protein (SIRP)α-CD47 blockade joins the ranks of immune checkpoint inhibition. J Clin Oncol 2019;37:1012–4. [DOI] [PubMed] [Google Scholar]
- 82. Daver NG, Vyas P, Kambhampati S, Al Malki MM, Larson R, Asch A, et al. Tolerability and efficacy of the first-in-class anti-CD47 antibody magrolimab combined with azacitidine in frontline patients with TP53-mutated acute myeloid leukemia: phase 1b results. HemaSphere 2022;6:33–4. Abstr S132. [Google Scholar]
- 83. Sallman DA, Al Malki MM, Asch AS, Wang ES, Jurcic JG, et al. Magrolimab in combination with azacitidine for patients with untreated higher-risk myelodysplastic syndromes (HR MDS): 5F9005 phase 1b study results. HemaSphere 2022;6:67–8. Abstr S166. [Google Scholar]
- 84. Daver N, Konopleva M, Maiti A, Kadia TM, DiNardo CD, Loghavi S, et al. Phase I/II study of azacitidine (AZA) with venetoclax (VEN) and magrolimab (Magro) in patients (pts) with newly diagnosed older/unfit or high-risk acute myeloid leukemia (AML) and relapsed/refractory (R/R) AML. Blood 2021;138Suppl 1:371. Abstr 616. [Google Scholar]
- 85. Haddad F, Daver N. An update on immune-based therapies in acute myeloid leukemia: 2021 and beyond! Adv Exp Med Biol 2021;1342:273–95. [DOI] [PubMed] [Google Scholar]
- 86. Al-Hussaini M, Rettig MP, Ritchey JK, Karpova D, Uy GL, Eissenberg LG, et al. Targeting CD123 in acute myeloid leukemia using a T-cell–directed dual-affinity retargeting platform. Blood 2016;127:122–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Testa U, Riccioni R, Militi S, Coccia E, Stellacci E, Samoggia P, et al. Elevated expression of IL-3Rα in acute myelogenous leukemia is associated with enhanced blast proliferation, increased cellularity, and poor prognosis. Blood 2002;100:2980–8. [DOI] [PubMed] [Google Scholar]
- 88. Vergez F, Green AS, Tamburini J, Sarry JE, Gaillard B, Cornillet-Lefebvre P, et al. High levels of CD34+CD38low/−CD123+ blasts are predictive of an adverse outcome in acute myeloid leukemia: a Groupe Ouest-Est des Leucémies Aiguës et Maladies du Sang (GOELAMS) study. Haematologica 2011;96:1792–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chichili GR, Huang L, Li H, Burke S, He L, Tang Q, et al. A CD3xCD123 bispecific DART for redirecting host T cells to myelogenous leukemia: preclinical activity and safety in nonhuman primates. Sci Transl Med 2015;7:289ra82. [DOI] [PubMed] [Google Scholar]
- 90. Uy GL, Aldoss I, Foster MC, Sayre PH, Wieduwilt MJ, Advani AS, et al. Flotetuzumab as salvage immunotherapy for refractory acute myeloid leukemia. Blood 2021;137:751–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ravandi F, Bashey A, Stock W, Foran JM, Mawad R, Egan D, et al. Complete responses in relapsed/refractory acute myeloid leukemia (AML) patients on a weekly dosing schedule of vibecotamab (XmAb14045), a CD123 × CD3 T cell-engaging bispecific antibody; initial results of a phase 1 study. Blood 2020;136:4–5.32614961 [Google Scholar]
- 92. Tessoulin B, Descamps G, Moreau P, Maïga S, Lodé L, Godon C, et al. PRIMA-1Met induces myeloma cell death independent of p53 by impairing the GSH/ROS balance. Blood 2014;124:1626–36. [DOI] [PubMed] [Google Scholar]
- 93. Birsen R, Larrue C, Decroocq J, Johnson N, Guiraud N, Gotanegre M, et al. APR-246 induces early cell death by ferroptosis in acute myeloid leukemia. Haematologica 2022;107:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Peng X, Zhang MQZ, Conserva F, Hosny G, Selivanova G, Bykov VJN, et al. APR-246/PRIMA-1MET inhibits thioredoxin reductase 1 and converts the enzyme to a dedicated NADPH oxidase. Cell Death Dis 2013;4:e881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Teoh PJ, Bi C, Sintosebastian C, Tay LS, Fonseca R, Chng WJ. PRIMA-1 targets the vulnerability of multiple myeloma of deregulated protein homeostasis through the perturbation of ER stress via p73 demethylation. Oncotarget 2016;7:61806–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sallman DA, DeZern AE, Garcia-Manero G, Steensma DP, Roboz GJ, Sekeres MA, et al. Eprenetapopt (APR-246) and azacitidine in TP53-mutant myelodysplastic syndromes. J Clin Oncol 2021;39:1584–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Cluzeau T, Sebert M, Rahmé R, Cuzzubbo S, Lehmann-Che J, Madelaine I, et al. Eprenetapopt plus azacitidine in TP53-mutated myelodysplastic syndromes and acute myeloid leukemia: a phase II study by the Groupe Francophone des Myélodysplasies (GFM). J Clin Oncol 2021;39:1575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sallman DA, Komrokji RS, DeZern AE, Sebert M, Garcia-Manero G, Rahmé R, et al. Long-term follow-up and combined phase 2 results of eprenetapopt (APR-246) and azacitidine (AZA) in patients with TP53 mutant myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia (AML). Blood 2021;138:246.34292322 [Google Scholar]
- 99. Results of primary endpoint from phase 3 trial of eprenetapopt in TP53 mutant myelodysplastic syndromes (MDS). Aprea Therapeutics. 2020. [cited 2021 Sep 14]. Available from: https://ir.aprea.com/news-releases/news-release-details/aprea-therapeutics-announces-results-primary-endpoint-phase-3/.
- 100. A partial clinical hold on myeloid malignancy programs. Aprea Therapeutics. 2021. [cited 2021 Sep 14]. Available from: https://ir.aprea.com/news-releases/news-release-details/aprea-therapeutics-announces-partial-clinical-hold-myeloid/.
- 101. Garcia-Manero G, Goldberg AD, Winer ES, Altman JK, Fathi AT, Odenike O, et al. Phase I and expansion study of eprenetapopt (APR-246) in combination with venetoclax (VEN) and azacitidine (AZA) in TP53-mutant acute myeloid leukemia (AML). Blood 2021;138:3409. [Google Scholar]
- 102. Dumbrava E, Johnson M, Tolcher A, Shapiro G, Thompson JA, El-Khoueiry AB, et al. First-in-human study of PC14586, a small molecule structural corrector of Y220C mutant p53, in patients with advanced solid tumors harboring a TP53 Y220C mutation. In J Clin Oncol 40: 16s, 2022. (suppl; abstr 3003). [Google Scholar]
- 103. Wolf Y, Anderson AC, Kuchroo VK. TIM3 comes of age as an inhibitory receptor. Nat Rev Immunol 2020;20:173–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Das M, Zhu C, Kuchroo VK. Tim-3 and its role in regulating anti-tumor immunity. Immunol Rev 2017;276:97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kikushige Y, Miyamoto T, Yuda J, Jabbarzadeh-Tabrizi S, Shima T, S Takayanagi, et al. A TIM-3/Gal-9 autocrine stimulatory loop drives self-renewal of human myeloid leukemia stem cells and leukemic progression. Cell Stem Cell 2015;17:341–52. [DOI] [PubMed] [Google Scholar]
- 106. Kikushige Y, Shima T, ichiro TS, Urata S, Miyamoto T, Iwasaki H, et al. TIM-3 is a promising target to selectively kill acute myeloid leukemia stem cells. Cell Stem Cell 2010;7:708–17. [DOI] [PubMed] [Google Scholar]
- 107. Brunner AM, Esteve J, Porkka K, Knapper S, Traer E, Scholl S, et al. Efficacy and safety of sabatolimab (MBG453) in combination with hypomethylating agents (HMAs) in patients (Pts) with very high/high-risk myelodysplastic syndrome (vHR/HR-MDS) and acute myeloid leukemia (AML): final analysis from a phase Ib study. Blood 2021;138:244. [Google Scholar]
- 108. Davids MS, Kim HT, Bachireddy P, Costello C, Liguori R, Savell A, et al. Ipilimumab for patients with relapse after allogeneic transplantation. N Engl J Med 2016;375:143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Koshy AG, Daver NG, Fathi AT. A new era of immuno-oncology in acute myeloid leukemia – antibody-based therapies and immune checkpoint inhibition. Best Pract Res Clin Haematol 2020;33:101220. [DOI] [PubMed] [Google Scholar]
- 110. Daver N, Garcia-Manero G, Basu S, Boddu PC, Alfayez M, Cortes JE, et al. Efficacy, safety, and biomarkers of response to azacitidine and nivolumab in relapsed/refractory acute myeloid leukemia: a nonrandomized, open-label, phase II study. Cancer Discov 2019;9:370–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zeidan AM, Boss I, Beach C, Copeland WB, Thompson E, Fox BA, et al. Azacitidine and durvalumab in first-line treatment of elderly patients with acute myeloid leukemia. Blood Adv 2021;6:2219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zeidan AM, Boss IW, Beach C, Copeland WB, Thompson EG, Fox BA, et al. A randomized phase 2 trial of azacitidine ± durvalumab as first-line therapy for higher-risk myelodysplastic syndromes. Blood Adv 2021;6:2207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Epperly R, Gottschalk S, Velasquez MP. A bump in the road: how the hostile AML microenvironment affects CAR T cell therapy. Front Oncol 2020;10:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Zhang H, Bu C, Peng Z, luo m, Li C. The efficacy and safety of anti-CLL1 based CAR-T cells in children with relapsed or refractory acute myeloid leukemia: a multicenter interim analysis. J Clin Oncol 39:15s, 2021. (suppl; abstr 10000). [DOI] [PubMed] [Google Scholar]
- 115. Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol 2020;17:147–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Deniger DC, Pasetto A, Robbins PF, Gartner JJ, Prickett TD, Paria BC, et al. T-cell responses to TP53 “hotspot” mutations and unique neoantigens expressed by human ovarian cancers. Clin Cancer Res 2018;24:5562–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Low L, Goh A, Koh J, Lim S, Wang CI. Targeting mutant p53-expressing tumours with a T cell receptor-like antibody specific for a wild-type antigen. Nat Commun 2019;10:5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Fate therapeutics announces encouraging interim phase 1 data for iPSC-derived NK cell programs in relapsed/refractory acute myeloid leukemia. GlobeNewswire News Room. 2021 [cited 2021 Sep 14]. Available from: https://www.globenewswire.com/en/news-release/2021/05/13/2229432/24675/en/Fate-Therapeutics-Announces-Encouraging-Interim-Phase-1-Data-for-iPSC-derived-NK-Cell-Programs-in-Relapsed-Refractory-Acute-Myeloid-Leukemia.html.
- 119. Minculescu L, Marquart HV, Ryder LP, Andersen NS, Schjoedt I, Friis LS, et al. Improved overall survival, relapse-free-survival, and less graft-vs.-host-disease in patients with high immune reconstitution of TCR gamma delta cells 2 months after allogeneic stem cell transplantation. Front Immunol 2019;10:1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Klyuchnikov E, Badbaran A, Massoud R, Fritsche-Friedland U, Janson D, Ayuk F, et al. Enhanced immune reconstitution of γδ T cells after allogeneic stem cell transplantation overcomes the negative impact of pretransplantation minimal residual disease-positive status in patients with acute myelogenous leukemia. Transplant Cell Ther 2021;27:841–50. [DOI] [PubMed] [Google Scholar]
- 121. Zhu Y, Smith DJ, Zhou Y, Li YR, Yu J, Lee D, et al. Development of hematopoietic stem cell-engineered invariant natural killer T cell therapy for cancer. Cell Stem Cell 2019;25:542–57. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Westin SN, Nieves-Neira W, Lynam C, Salim KY, Silva AD, Ho RT, et al. Safety and early efficacy signals for COTI-2, an orally available small molecule targeting p53, in a phase I trial of recurrent gynecologic cancer [abstract]. In: Proceedings of the American Association for Cancer Research Annual Meeting 2018; 2018 Apr 14–18; Chicago, IL. Philadelphia (PA): AACR; Cancer Res 2018;78(13 Suppl): Abstract nr CT033. [Google Scholar]
- 123. Chen S, Wu JL, Liang Y, Tang YG, Song HX, Wu LL, et al. Arsenic trioxide rescues structural p53 mutations through a cryptic allosteric site. Cancer Cell 2021;39:225–39. [DOI] [PubMed] [Google Scholar]
- 124. Kaymak I, Maier CR, Schmitz W, Campbell AD, Dankworth B, Ade CP, et al. Mevalonate pathway provides ubiquinone to maintain pyrimidine synthesis and survival in p53-deficient cancer cells exposed to metabolic stress. Cancer Res 2020;80:189–203. [DOI] [PubMed] [Google Scholar]
- 125. Freed-Pastor W, Prives C. Targeting mutant p53 through the mevalonate pathway. Nat Cell Biol 2016;18:1122–4. [DOI] [PubMed] [Google Scholar]
- 126. DiNardo CD, Maiti A, Rausch CR, Pemmaraju N, Naqvi K, Daver NG, et al. 10-day decitabine with venetoclax for newly diagnosed intensive chemotherapy ineligible, and relapsed or refractory acute myeloid leukaemia: a single-centre, phase 2 trial. Lancet Haematol 2020;7: e724–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Sallman DA, Asch AS, Al Malki MM, Lee DJ, Donnellan WB, Marcucci G, et al. The first-in-class anti-CD47 antibody magrolimab (5F9) in combination with azacitidine is effective in MDS and AML patients: ongoing phase 1b results. Blood 2019;134Suppl 1:569. Abstr 637. [Google Scholar]
- 128. Walter RB. Investigational CD33-targeted therapeutics for acute myeloid leukemia. Expert Opin Investig Drugs 2018;27:339–48. [DOI] [PubMed] [Google Scholar]