Abstract
Sonodynamic therapy (SDT) is a promising new anti-tumor therapy that inhibits tumor growth by ultrasound activation of sonosensitizers to produce reactive oxygen species (ROS). However, the problems of hypoxia in the microenvironment within solid tumors and the effectiveness of SDT will decrease due to the little accumulation of sonosensitizers at the tumor site, as well as tumor cell tolerance, have limited the development of SDT. To overcome these problems, a core-shell structured nanoparticle (IR780/PLGA@MnO2 NPs) loaded with IR780 and manganese dioxide (MnO2) was developed as a nanocarrier to transport the sonosensitizer IR780 and the generated oxygen into the tumor tissue. The MnO2 shell layer of IR780/PLGA@MnO2 NPs can prevent the premature release of IR780 in the blood and also it can react with acidic and high H2O2, the generated oxygen can relieve tumor tissue hypoxia, and the generated Mn can enhance magnetic resonance imaging (MRI) signal intensity by acting as a contrast agent for MRI. More importantly, the released IR780 can produce ROS to kill tumor cells under ultrasound excitation. This PH-responsive and H2O2-triggered SDT based on the IR780/PLGA@MnO2NPs is an effective platform to inhibit tumor growth with negligible systemic toxicity. This work develops a multifunctional therapeutic integrated nanoplatform for breast cancer treatment, which is expected to be used in the clinic.
Keywords: sonodynamic therapy, magnetic resonance imaging, tumor hypoxia, reactive oxygen species, manganese dioxide
Introduction
Advances in cancer diagnosis and therapy have attracted immense attention in achieving accurate treatment to minimize side effects and improving the survival rate of patients. Ultrasound (US), as a mechanical wave, is widely used in clinical US imaging for cancer diagnosis and high-intensity focused US for cancer therapy (Wu et al., 2005; Wells, 2006; Hussain and Nguyen, 2014). Moreover, US could facilitate both the drug release and cellular uptake by inducing reversible cell deformation and membrane permeabilization (Ahmadi et al., 2020). In recent years, sonodynamic therapy (SDT), based on US, as a non-invasive treatment in promoting malignant tumor deterioration, has led to burgeoning research interest, which has the distinct advantages of deep penetration, low cost and low toxicity (Wood and Sehgal, 2015; Qian et al., 2016; Wan et al., 2016). SDT employs activating sonosensitizers through US and utilizes oxygen molecules of tissues into toxic reactive oxygen species (ROS), which can oxidize crucial biomacromolecules such as proteins, enzymes, DNA of tumor cells, thus inhibiting the tumor growth (Rosenthal et al., 2004; Costley et al., 2015; Zhang et al., 2019; Son et al., 2020; Wang et al., 2020). However, the organic sonosensitizer molecule hinders its further application of SDT due to low bioavailability and biological instability (Son et al., 2020). To overcome these limitations, our previous studies have found that loading the organic sonosensitizer such as IR780 or ICG onto polymeric PLGA could effectively improve the biocompatibility and biological stability (Niu et al., 2017; Wang et al., 2018; Chen et al., 2020; Huang et al., 2020; Pei et al., 2020; Xu et al., 2020).
SDT progress requires enough oxygen molecules in the process of cancer cell destruction, however, the tumor hypoxic microenvironment significantly some inherent resistances to SDT due to the abnormal proliferation of cells, vascular abnormalities, and lymphatic system dysfunction (Hockel and Vaupel, 2001; Dong et al., 2021). Moreover, the high consumption of oxygen in the process of SDT can worsen the hypoxic microenvironment of tumor tissues and conversely influences the therapeutic efficacy of SDT (Wang et al., 2020; Dong et al., 2021). Therefore, how to overcome the limitation of hypoxia in the tumor area on the low ROS-generation efficacy of SDT is a challenging subject. Several strategies have developed to relieve hypoxia, such as using perfluorocarbon to deliver additional oxygen, inhaling supply hyperbaric oxygen, and catalyzing overexpressed hydrogen peroxide (H2O2) to O2 in the tumor tissues (Song et al., 2016a; Song et al., 2016b; Zhu et al., 2018; Huang et al., 2020). Manganese dioxide (MnO2) and its various nanocomposites, as a catalase-like nanoenzyme to product oxygen by the decomposition of excess H2O2 in tumor area is the most effective way to relieve hypoxia, have been attracting increasing attention (Chu et al., 2017; Zhu et al., 2018; Zhang et al., 2020; Zhang and Ji, 2020). Thus, utilizing tumor PH- and H2O2- sensitive MnO2 to relieve hypoxia is promising way, the process of MnO2 at mild acid and excess H2O2 condition can be expressed by the following reactions (Gordijo et al., 2015):
Many studies have already manifested that nanostructured MnO2 can be act as a catalyst to react with overexpressed H2O2 in tumor microenvironment to produce O2, which could surmount hypoxia in tumor area (Zhu et al., 2018; Yang et al., 2019; Liu et al., 2021). In addition, the process of nanostructured MnO2 can generate water-soluble Mn2+ ions which can be used as an excellent magnetic resonance imaging (MRI) contrast agent for early and accurate diagnosis of cancer (Huang et al., 2017; Liu et al., 2018; Fu et al., 2019; Ding et al., 2020). Furthermore, nanostructured MnO2 can also assemble anticancer drug molecules and release them when itself degrade in an acidic environment, which could be used as “gatekeeper” carrier for drug delivery (Ma et al., 2017).
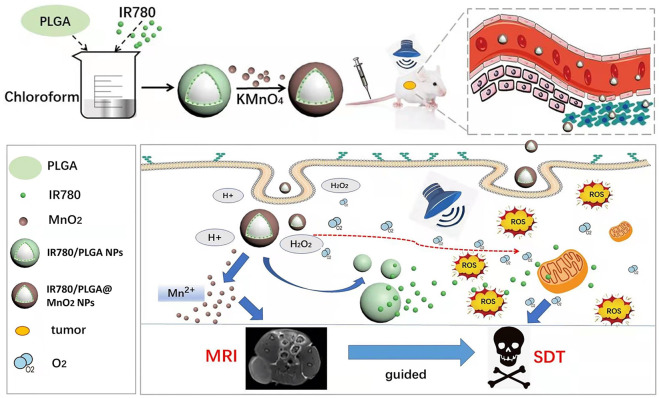
Therefore, we designed a core-shell structure multi-layer nanoparticle (IR780/PLGA@MnO2 NPs) as sonosensitizer carrier and the oxygen reservoir to enhance the efficacy of SDT and MR imaging (Figure 1). Meanwhile, the MnO2 shell layer of these IR780/PLGA@MnO2 NPs could prevent IR780 release in the blood circulation, while react with H+ and H2O2 in the tumor area, then decomposing and releasing the IR780 from IR780/PLGA@MnO2 NPs. Finally, the generation of oxygen could relieve tumor hypoxia, making the exhibit higher ROS generation efficiency even under hypoxia atmosphere, the generation of Mn2+ ions could act as the contrast agents for T1-weighted magnetic resonance imaging (MRI) for the recognition and diagnosis of tumor. Thus, the developed IR780/PLGA@MnO2 NPs would be a potential nanoplatform for controllable tumor oxygenation and MRI-guided tumor-growth suppression.
FIGURE 1.
Schematic illustration of the IR780/PLGA@MnO2 NPs used for enhance SDT and MR imaging of breast cancer.
Materials and methods
Preparation and characterization of IR780/PLGA@MnO2 NPs
Firstly, 200 mg PLGA and 6 mg IR780 was fully dissolved in 6 ml chloroform, then the above mixture was put into 30 ml PVA (4%) solution, emulsified for 2 min using an ultrasonic processor (5 s on and 5 s off), 20 ml deionized water was added into the mixture and stirred for 12 h in order to volatilize the chloroform. Then, the resulting IR780/PLGA NPs was washed with deionized water at least 3 times. To form layered manganese oxide, the prepared IR780/PLGA NPs were dispersed in 100 ml deionized water, then 300 mg potassium permanganate was added, the resulting IR780/PLGA @MnO2 NPs was stirred for 6 h in dark place, washed with deionized water at least 3 times, and kept at 4°C in dark place.
The morphology, structure and element mapping of the IR780/PLGA@MnO2 NPs was detected by Transmission electron microscopy (TEM, Hitachi, Ltd., Japan), the size and zeta potential of the NPs were tested by dynamic light scattering (DLS, ZEN3600, Malvern Instruments, United Kingdom), and the sizes of the NPs in PBS and 10% fetal bovine serum (FBS) were tested for 7 days to study the size stability. The optical properties of various NPs (PLGA NPs, IR780/PLGA NPs, IR780/PLGA@MnO2 NPs) were tested by a UV-vis-NIR spectrophotometer (UV-3600, Shimadzu, Japan). The Mn content was calculated by ICP-OES (ICP-OES730, Agilent Co. Ltd., USA). The amount of IR780 encapsulated into the IR780/PLGA@MnO2 NPs were recorded by a UV-vis-NIR spectrophotometer.
Oxygen Storage and IR780 Release from the IR780/PLGA@MnO2 NPs in Response to H2O2
A dissolved oxygen meter (AMT08, United States) was used to measure the O2 concentration of IR780/PLGA@MnO2 NPs in response to H2O2. Ten milliliters of deoxygenated water with H2O2 (25 µM), IR780/PLGA NPs or IR780/PLGA@MnO2 NPs were loaded into a glass bottle. The real-time O2 concentration in the bottle was recorded every 10 s, the IR780 release experiment of the IR780/PLGA@MnO2 NPs was recorded by a UV-vis-NIR spectrophotometer. The IR780/PLGA@MnO2 NPs were loaded into dialysis bags (1 ml) and put into different buffers, including PBS (pH 7.4) and PBS (pH 5.5) with 25 µM H2O2, PBS (pH 5.5) without 25 µM H2O2 to partially mimic the microenvironments of healthy tissue and tumor tissue. At the different time points up to 72 h, IR780 concentration of each time point was recorded according to the standard curve of IR780.
Measurement of ROS generation in vitro
SOSG was used as a fluorescent probe to measure the production of 1O2, 1 ml of IR780/PLGA@MnO2 NPs (100 μg/ml) and 1 µL of (SOSG, 5 µM) was irradiated by US transducer at an ultrasonic intensity of 1 W/cm2 with the duty cycle of 40% in different time (0, 30, 60, 90, 120, 150 or 180 s). The SOSG fluorescence was tested by a fluorescence spectrometer (Tecan, Männedorf, Switzerland) for IR780/PLGA@MnO2 NPs, H2O2 and IR780/PLGA NPs, the relative efficiency of 1O2 production was calculated by F1 (under US irradiation)/F0 (before US irradiation).
Intracellular uptake
4T1 mice breast cancer cells were cultured at 37°C in RPMI-1640 medium containing 10% (v/v) FBS, 1% (v/v) penicillin G and streptomycin, 4T1 cells (1 × 104 cells/well) were seeded on laser confocal petri dish for 24 h. Then, DiI labeled IR780/PLGA@MnO2 NPs (200 μL, 1 mg/ml) were added and co-incubated for 0, 4, 12 and 24 h, washed for 3 times in dark, fixed with 4% paraformaldehyde for 15 min and stained with DAPI for 5 min. Finally, the cells were observed by a laser confocal microscope.
Cellular reactive oxygen species generation
DCFH-DA cellular ROS assay kit was used to detect intracellular ROS production. 4T1 cells (1 × 105 cells/well) were seeded in the 6-well plates and treated with different conditions: (Hussain and Nguyen, 2014) PBS, (Wells, 2006) US, (Wu et al., 2005) IR780/PLGA NPs, (Ahmadi et al., 2020) IR780/PLGA NPs + US, (Qian et al., 2016) IR780/PLGA@MnO2 NPs, (Wood and Sehgal, 2015) IR780/PLGA@MnO2 NPs + US. The intensity of US is 1.0 W/cm2 and the time is 2 min, the NPs concentration is 1.0 mg/ml and the dosage is 200 µL. After incubation for 24 h, DCFH-DA (10 µM) was added to each well, ROS production was determined by fluorescence microscopy.
Cell viability assay
Mice breast cancer 4T1 cells and human breast cancer MCF-7 cells (5 × 103 cells/well) were seeded in a 96-well plate and cultured overnight under normoxic or hypoxic conditions, respectively. To test the cell viabilities of different IR780/PLGA@MnO2 NPs concentrations, 50 µL of IR780/PLGA@MnO2 NPs with different concentrations (1.65, 3.25, 6.25, 12.5, 25.0, and 50.0 μg/ml) were added into the each well and incubated overnight, respectively, then the cell viability was calculated with a CCK-8 cytotoxicity assay kit. To test the cell viabilities of different treatments under normoxic or hypoxic conditions, cells were randomly divided into six groups: (Hussain and Nguyen, 2014) PBS, (Wells, 2006) US, (Wu et al., 2005) IR780/PLGA NPs, (Ahmadi et al., 2020) IR780/PLGA NPs + US, (Qian et al., 2016) IR780/PLGA@MnO2 NPs, (Wood and Sehgal, 2015) IR780/PLGA@MnO2 NPs + US. The intensity of US is 1.0 W/cm2, the time is 2 min The NPs concentration is 6.25 μg/ml and the dosage is 50 µL. After 24 h of incubation, the cell viability was determined using a CCK-8 cytotoxicity assay kit.
Apoptosis assay
4T1 cells (1 × 105 cells/well) were seeded in the 6-well plates and cultured for 24 h. The cells were treated with different conditions: (Hussain and Nguyen, 2014) PBS, (Wells, 2006) US, (Wu et al., 2005) IR780/PLGA NPs, (Ahmadi et al., 2020) IR780/PLGA NPs + US, (Qian et al., 2016) IR780/PLGA@MnO2 NPs, (Wood and Sehgal, 2015) IR780/PLGA@MnO2 NPs + US. The intensity of US is 1.0 W/cm2, the time is 2 min, the NPs concentration is 1.0 mg/ml and the dosage is 200 µL. Then the cells were washed 3 times and stained with PI and DAPI, and observed by fluorescence microscopy.
Animal model
Female BALB/c mice (6 weeks old, 20 g) were obtained from the Hunan Silaike Laboratory Animal Corporation (Changsha, China). 4T1 cells (1 × 106) were injected into the mice by subcutaneous injection to establish a breast tumor model when the tumor volume reached 80 mm3. All animal experiments were approved by the Ethics Committee of the Second Xiangya Hospital of Central South University and conducted in accordance with the guidelines of the Department of Laboratory Animals of Central South University.
Biodistribution and biosafety
For evaluating the biodistribution of IR780/PLGA@MnO2 NPs, 15 tumor-bearing BALB/c mice (n = 3) were injected with 200 μL of IR780/PLGA@MnO2 NPs (1.0 mg/ml) via the tail vein (0, 4, 8, 12, 24 h before imaging) for in vivo fluorescent imaging (Ex: 745 nm, Em: 840 nm) by a Lumina IVIS Spectrum imaging system (PerkinElmer, United States). Mice of each group were sacrificed after the whole-body imaging, then the major organs and tumor were taken out for in vitro imaging and the average fluorescence intensity was further analyzed.
To further assess the biotoxicity of IR780/PLGA@MnO2 NPs in vivo, 10 tumor-bearing BALB/c mice (n = 5) were randomly divided into 2 groups: (Hussain and Nguyen, 2014) saline and (Wells, 2006) IR780/PLGA@MnO2 NPs. Mice in the two groups were injected with 200 μL of saline or IR780/PLGA@MnO2 NPs (2.0 mg/ml) via the tail vein, then the H&E stain of main organs was observed after 14 days injection.
MR imaging in vitro and in vivo
MR imaging in vitro were tested on a clinical by MRI instrument (UR770 3.0T, United imaging Ltd., China) equipped with a small animal coil. Different MnO2 concentrations (Mn concentrations: 0.00, 0.05, 0.10, 0.20, 0.30, and 0.40 mM) of the IR780/PLGA@MnO2 NPs were dispersed in different media: (Hussain and Nguyen, 2014) pH 7.4; (Wells, 2006) pH 5.5, 25 µM H2O2. Then, the different concentrations of IR780/PLGA@MnO2 NPs were added to 2 ml Eppendorf tubes for MR imaging (T1 WI). The T1 relaxation time was measured, and the T1 relaxation coefficient (r1) was calculated.
In the in vivo T1-weighted MRI experiments, 200 μL of IR780/PLGA@MnO2 NPs solution (2 mg/ml) was intravenously injected into 4T1 tumor-bearing mice. The T1-weighted MR images of tumor sites were captured before and 24 h after injection of IR780/PLGA@MnO2 NPs. Finally, the SI within the ROI of the MR images were measured.
In vivo antitumor effect
To investigate hypoxia environment in vivo, the 4T1 tumor-bearing mice (n = 5) were randomly divided with different treatments: (Hussain and Nguyen, 2014) saline, (Wells, 2006) IR780/PLGA NPs, (Wu et al., 2005) IR780/PLGA@MnO2 NPs. After 24 h of injection, immunofluorescence of an antibody against hypoxia-inducible factor-1α (HIF-1α) in the tumor was observed.
To investigate the antitumor effect in vivo, the 4T1 tumor-bearing mice (n = 5) were randomly divided with different treatments: (Hussain and Nguyen, 2014) saline, (Wells, 2006) US, (Wu et al., 2005) IR780/PLGA NPs, (Ahmadi et al., 2020) IR780/PLGA@MnO2 NPs, (Qian et al., 2016) IR780/PLGA NPs + US, (Wood and Sehgal, 2015) IR780/PLGA@MnO2 NPs + US, 200 µL of different NP solutions (2 mg/ml) was intravenously injected into the mice every 2 days. After injection for 8 h, the tumors of the mice in groups (Wells, 2006), (Qian et al., 2016), and (Wood and Sehgal, 2015) were irradiated with US (the ultrasonic intensity is 2 W/cm2 and duration is 10 min). Body weight and tumor volume were recorded every other day for 14 days. On day 15, the tumors of the mice were obtained. In addition, TUNEL and PCNA immunohistochemical staining were performed on the tumor tissues to observe tumor cell apoptosis and proliferation, respectively.
Statistical analysis
The experimental data were presented as the mean ± SD. Comparisons of two groups were analyzed by Student’s t-test and multiple groups were analyzed by two-way analysis using GraphPad Prism 8 software. p < 0.05 was considered significant.
Results and discussion
Characterization of IR780/PLGA@MnO2 NPs
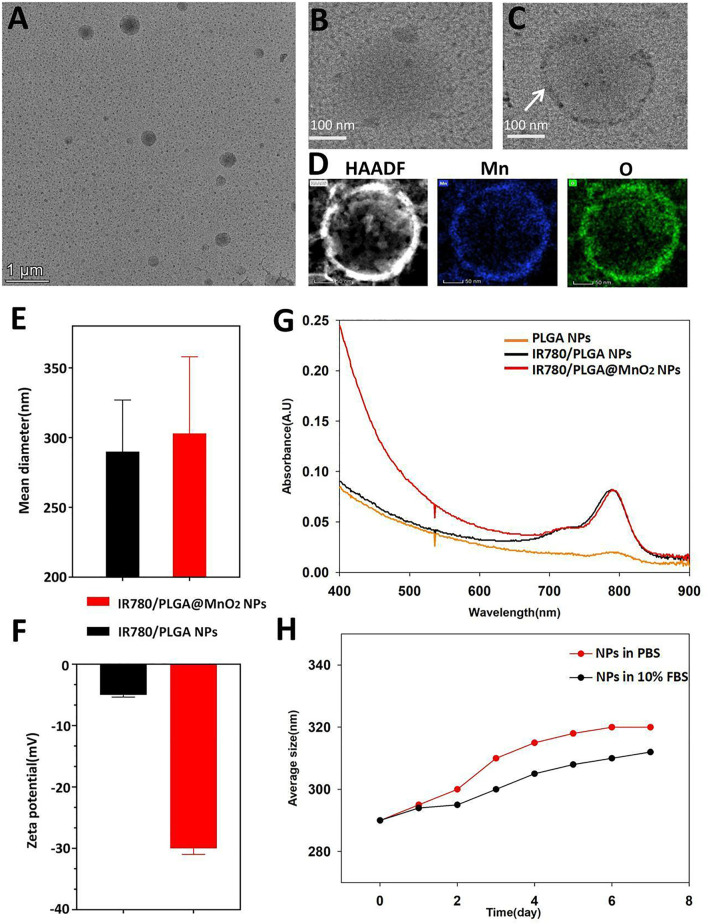
As shown in Figure 2B of the TEM image, spherical IR780/PLGA NPs nanoparticles were successfully constructed. The shell of IR780/PLGA@MnO2 NPs coating of MnO2 was proved by TEM images (Figures 2A,C). The elemental mapping of Mn and O of IR780/PLGA@MnO2 (Figure 2D). The size of the IR780/PLGA NPs was approximately 293.05 ± 5.43 nm and increased to 300.34 ± 4.56 nm after MnO2 coating (Figure 2E). The zeta potential decreased from −5.3269 mV to −30.563 mV after MnO2 coating, indicating that the IR780/PLGA@MnO2 NPs is more stable in the blood than the IR780/PLGA NPs (Figure 2F). The UV absorption spectra are shown in Figure 2G, there was an absorption peak at 780–790 nm of the IR780/PLGA@MnO2 NPs, which indicated that the PLGA/IR780@MnO2 NPs was successfully loaded the IR780. The stability of the IR780/PLGA@MnO2 NPs in PBS and 10% FBS solution were shown in Figure 2H. The entrapment efficiency of IR780 in the IR780/PLGA@MnO2 NPs was calculated to be 58.47% ± 1.48%. The Mn content in the IR780/PLGA@MnO2 NPs was approximately 0.2761 mg/ml.
FIGURE 2.
Characterization of IR780/PLGA@MnO2 NPs. (A–C) The TEM images of IR780/PLGA@MnO2 NPs (A) and IR780/PLGA NPs (B), the white arrow indicated the MnO2 on the shell of IR780/PLGA@MnO2 NPs (C). (D) The elemental mapping of Mn and O of IR780/PLGA@MnO 2. (E) Size and (F) zeta potential of IR780/PLGA NPs with and without MnO2 shell. (G) UV−vis−NIR absorption spectra of IR780/PLGA NPs, PLGA NPs, IR780/PLGA@MnO2 NPs. (H) Size distributions of IR780/PLGA@MnO2 NPs in PBS and 10% FBS for 7 days.
PH and H2O2 responsive IR780 release and responsive oxygen generation
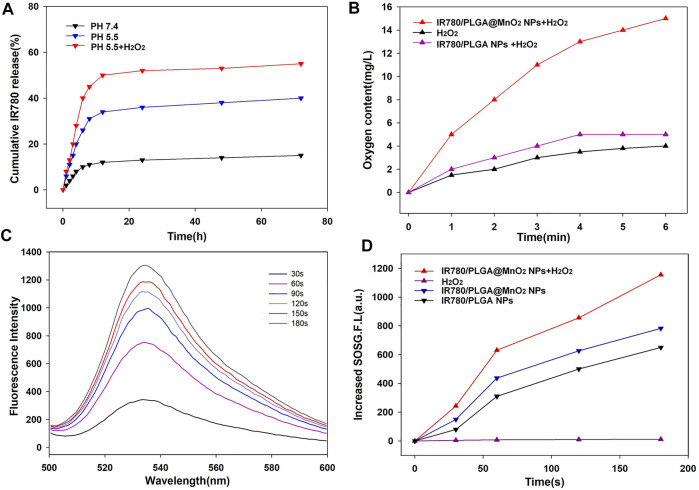
It is known that tumor microenvironment is featured with regional hypoxia, low PH and increasing H2O2 level due to insufficient blood supplement. The MnO2 can react with the increasing H+ and H2O2 in the solid tumor to produce O2 and Mn2+ (Sun et al., 2021). When the IR780/PLGA@MnO2 NPs entered the tumor tissue, MnO2 shell gradually dissolved, the IR780/PLGA NPs core exposed, and IR780 release from the IR780/PLGA@MnO2 NPs in the tumor (Figure 3A). Compared with the IR780/PLGA@MnO2 NPs in PBS (pH 7.4), the IR780/PLGA@MnO2 NPs in PBS (pH 5.5) with 25 µM H2O2 exhibited considerably higher IR780 release, and the release rate of IR780 approached 32% within 72 h. For the IR780/PLGA@MnO2 NPs in PBS (pH 5.5) without 25 µM H2O2, the drug release rate was only 20%, revealing that the IR780 release rate was in H2O2 and PH-responsive way.
FIGURE 3.
(A) The IR780 release from IR780/PLGA@MnO2 NPs at pH 7.4 or 5.5 with or without H2O2. (B) Oxygen content of IR780/PLGA MnO2 NPs with H2O2. (C) Time-dependent 1O2 generation of IR780/PLGA MnO2 NPs irradiated by US (1 MHz, 1.0 W/cm2). (D) Time-dependent fluorescence increasement of SOSG with different treatments under ultrasound irradiation.
The oxygen release profiles of IR780/PLGA@MnO2 NPs were analyzed under hypoxic conditions (Figure 3B). While the IR780/PLGA@MnO2 NPs was dispersed in the deoxygenated medium containing H2O2, the oxygen concentration in water increased from 4 to 13 mg/L water in the first 1 min. Few O2 generation was observed while the IR780/PLGA@MnO2 NPs was dispersed in the deoxygenated medium without H2O2. The oxygen concentration of the IR780/PLGA@MnO2 NPs with H2O2 group was obviously higher than that in the IR780/PLGA@MnO2 NPs without H2O2 group all the time. It means that the oxygen generation the IR780/PLGA@MnO2 NPs with H2O2 was fast and continuous.
Singlet oxygen generation capacity
Based on the efficient oxygen generation of MnO2, SOSG was used as a probe to measure the 1O2 production to assess the SDT effect of the IR780/PLGA@MnO2 NPs with US irradiation. As shown in Figure 3C, at a final IR780/PLGA@MnO2 NPs concentration of 100 μg/ml, the fluorescence intensity of SOSG increased along with the time of US irradiation (0, 60, 120, and 180 s), indicating that the 1O2 production capacity of the IR780/PLGA@MnO2 NPs is outstanding and have the potential against cancer through SDT. As shown in Figure 3D, the fluorescence intensity of SOSG was 819 at 180 s in the IR780/PLGA@MnO2 NPs without H2O2 group. Surprisingly, for the IR780/PLGA@MnO2 NPs with H2O2, the fluorescence intensity of SOSG was increased as high as 1150. Therefore, more 1O2 generation can be attributed to the increased oxygen concentration from MnO2 which react with H2O2 in the SDT process. These results demonstrated that the IR780/PLGA@MnO2 NPs can effectively generate 1O2 under US irradiation and can be used for sonodynamic treatment of cancer.
Cell uptake and reactive oxygen species determination
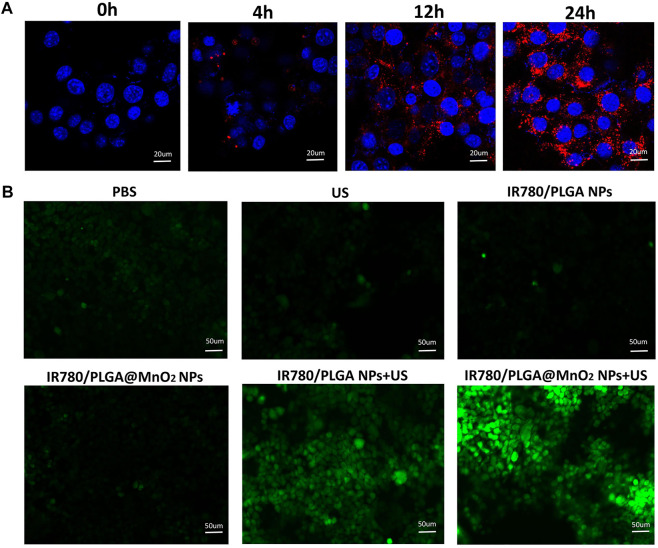
To understand the cell uptake of the IR780/PLGA@MnO2 NPs by 4T1 cells, DiI-labeled NPs was used to co-incubation with 4T1 cells for 0, 4, 12, and 24 h. As shown in Figure 4A, an obvious red fluorescence of the IR780/PLGA@MnO2 NPs was observed in 4 h and increased over time, which demonstrated that the longer co-incubation time between the IR780/PLGA@MnO2 NPs and cells, the more nanoparticles phagocytized by 4T1 cells.
FIGURE 4.
(A) Fluorescence images of 4T1 cells incubated with IR780/PLGA@MnO2 NPs for 0, 4, 12 and 24 h (scale bar: 20 μm). (B) Fluorescence microscope images of DCFH-DA stained 4T1 cells subjected to different treatments: (Hussain and Nguyen, 2014) PBS, (Wells, 2006) US, (Wu et al., 2005) IR780/PLGA NPs, (Ahmadi et al., 2020) IR780/PLGA@MnO2 NPs, (Qian et al., 2016) IR780/PLGA NPs + US, (Wood and Sehgal, 2015) IR780/PLGA@MnO2 + US (scale bar, 50 µm).
To understand intracellular oxidative stress of the IR780/PLGA@MnO2 NPs to examine the sonodynamic activity, DCFH-DA was used as a probe. As shown in Figure 4B, barely green fluorescence was observed in (Hussain and Nguyen, 2014) PBS, (Wells, 2006) US, (Wu et al., 2005) IR780/PLGA NPs and (Ahmadi et al., 2020) IR780/PLGA@MnO2 groups, which suggested that only US irradiation or only NPs cannot produce 1O2. Obvious green fluorescence was observed in (Qian et al., 2016) IR780/PLGA NPs + US and (Wood and Sehgal, 2015) IR780/PLGA@MnO2 + US groups, which suggested both the NPs and US irradiation were necessary for generation of 1O2. Comparing between groups (Qian et al., 2016) and (Wood and Sehgal, 2015), the group of IR780/PLGA@MnO2 + US irradiation has stronger green fluorescence intensity than the group of IR780/PLGA NPs + US irradiation, which suggested that with the help of MnO2 shell reacting with H2O2, the IR780/PLGA@MnO2 NPs could generate more O2 and then product more 1O2. Therefore, with loading of the shell of MnO2 to provide O2, the IR780/PLGA@MnO2 NPs have excellent properties of SDT against cancer.
In vitro biocompatibility and antitumor activity
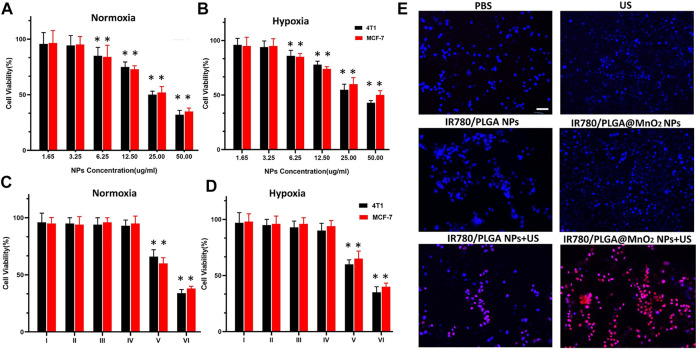
Cytotoxicity and antitumor activity of IR780/PLGA@MnO2 NPs under normoxic and hypoxic environments were measured in 4T1 cells and MCF-7 cells. According to CCK8 assay (Figures 5A,B), when the concentration of IR780/PLGA@MnO2 was 0–6.25 µg/ml, the cell survival rate of CCK8 was more than 95% under normoxic and hypoxic conditions, which suggested low toxicity of IR780/PLGA@MnO2 NPs, and 6.25 µg/ml is the appropriate concentration for further biological experiment. When the concentration increased, the cell survival rate of 4T1 cell and MCF-7 cells obviously decreased. To evaluate the antitumor activity of IR780/PLGA@MnO2 NPs through SDT, the 4T1 and MCF-7 viabilities in normoxic and hypoxic environments treated with different conditions was measured (Figures 5C,D). In the normoxic and hypoxic environments, both the 4T1 and MCF-7 cell viabilities of US group were up to 90%, indicating that US exposure (1W/cm2) is safe to cells. When the IR780/PLGA NPs and IR780/PLGA@MnO2 NPs combined with US exposure, the 4T1 cell viabilities further decreased. Treatment of IR780/PLGA@MnO2 NPs combined with US exposure results in 75% cell death, which was higher than that of IR780/PLGA NPs results in 45% cell death, suggesting that the MnO2 shell coated in IR780/PLGA@MnO2 NPs enhances more cell apoptosis through the SDT effects due to more O2 production.
FIGURE 5.
(A,B) Cell viability of 4T1 and MCF-7 cells incubated with different concentrations of IR780/PLGA@MnO2 NPs under normoxia environment (A) and hypoxia environment (B). (C,D) Cell viability of 4T1 and MCF-7 cells under different treatments: (I) PBS, (II) US, (III) IR780/PLGA NPs, (IV) IR780/PLGA@MnO2, (V) IR780/PLGA NPs + US, (VI) IR780/PLGA@MnO2 + US) in normoxia environment (C) and hypoxia environment (D). (E) Fluorescence microscope images of DAPI and PI co-stained 4T1 cells after various treatments: (Hussain and Nguyen, 2014) PBS, (Wells, 2006) US only, (Wu et al., 2005) IR780/PLGA NPs, (Ahmadi et al., 2020) IR780/PLGA@MnO2 NPs, (Qian et al., 2016) IR780/PLGA NPs + US, (Wood and Sehgal, 2015) IR780/PLGA@MnO2 + US (scale bar, 100 µm). (*p < 0.05, compared with the control group).
A live/dead staining assay was used to evaluate the therapeutic efficiency of IR780/PLGA@MnO2 NPs under US irradiation. Blue fluorescence of DAPI stained with all cells and red fluorescence of PI stained with dead cells. As shown in Figure 5E, there is bare red fluorescence in the (Hussain and Nguyen, 2014) PBS, (Wells, 2006) US, (Wu et al., 2005) IR780/PLGA NPs and (Ahmadi et al., 2020) IR780/PLGA@MnO2 NPs groups. Red fluorescence was observed when the cells treated with IR780/PLGA NPs under US irradiation. Furthermore, more red fluorescence was observed in the IR780/PLGA@MnO2 NPs under US irradiation group, which further confirmed that with the help of MnO2 shell respective to H2O2 to produce O2, the SDT efficacy of IR780 is enhanced and suggested that IR780/PLGA@MnO2 NPs is an efficient sonodynamic agent for hypoxic cancer cells under US irradiation.
Biodistribution and biosafety
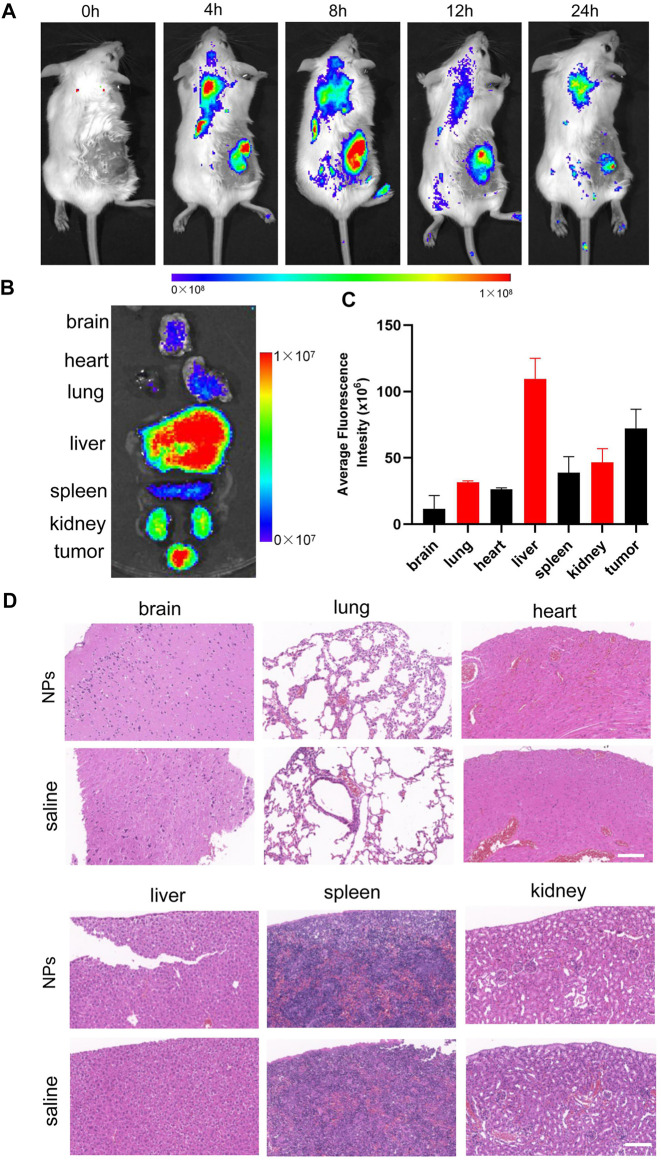
To evaluate the biodistribution of IR780/PLGA@MnO2 NPs, the in vivo fluorescent imaging at different time points were carried out. As shown in Figure 6A, before injection of the IR780/PLGA@MnO2 NPs, there were no red fluorescent signals in the tumor region of tumor bearing mice. Obvious fluorescence was observed at the tumor site after injection 4 h, arrived to the peak intensity at 8 h and maintained until 24 h. The fluorescence of the major organs and tumor after injection 8 h was shown in Figures 6B,C, the IR780/PLGA@MnO2 NPs was mainly accumulated in the tumors and the reticuloendothelial system (such as liver), which was consistent with the previous studies (Chen et al., 2020).
FIGURE 6.
(A) Biodistribution of IR780/PLGA@MnO2 NPs in tumor-bearing mice at different time points by in vivo fluorescent imaging. (B,C) Fluorescence imaging of major organs and tumor at 8 h after injection of the IR780/PLGA@MnO2 NPs. (D) H&E-staining images of major organs collected from the IR780/PLGA@MnO2 NPs and saline groups.
To further assess the biotoxicity of IR780/PLGA@MnO2 NPs in vivo, the H&E stain of main organs was observed after 14 days injection. As shown in Figure 6D, there was no apparent changes in pathological damage and inflammation lesion in the major organs (brain, heart, liver, spleen, lung, and kidney) with injection of IR780/PLGA@MnO2 NPs, which proved that IR780/PLGA@MnO2 NPs have low potential toxicity and was biosafe in vivo for further tumor SDT.
MR imaging in vitro and in vivo
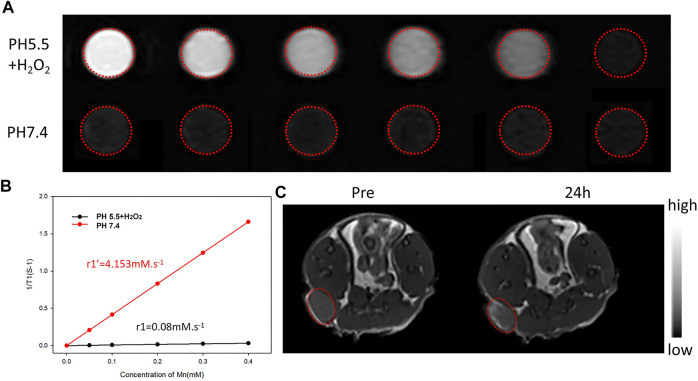
As we known, because of high spatial resolution and deep tissue penetration, MRI is widely used as an imaging modality in clinical diagnosis. Several manganese dioxide related contrast agents that respond to tumor microenvironment, such as low-pH and excess-H2O2, have been reported for enhancement MR imaging. The relaxation properties of IR780/PLGA@MnO2 NPs under different buffer in vitro were verified to investigate the MR imaging performance using a 3.0 T clinical MRI scanner. As shown in Figure 7A, in the presence of acid (pH 5.5) and H2O2, IR780/PLGA@MnO2 NPs shows that the brightness of the T1 weighted MR images increased rapidly with the increasing MnO2 concentration. As the MnO2 concentration increased, the T1 weighted MR images showed brighter. However, in the presence of the physiological environment pH 7.4, the change of the brightness was small and the increase of the T1 weighted MR images was relatively weaker. Moreover, as shown in Figure 7B, the T1 relaxation coefficient (r1) is also increased from 0.08 × 10–3 m/s (pH 7.4) to 4.1 × 10–3 m/s (pH 5.0 and H2O2), which demonstrated that IR780/PLGA@MnO2 NPs can be used as tumor microenvironment stimulate-responsive T1 MRI contrast agents.
FIGURE 7.
(A) T1-weighted MR images of IR780/PLGA@MnO2 NPs suspensions at different Mn concentrations and pH values with H2O2. (B) The longitudinal (r1) relaxation rate vs. the concentration of the IR780/PLGA@MnO2 NPs (in terms of Mn). (C) In vivo MR images of mice bearing implanted 4T1 cancer before and after 24 h of the intravenous injection of IR780/PLGA@MnO2 NPs.
Then the T1-weighted MRI of the tumor before and after 24 h injection of IR780/PLGA@MnO2 NPs in vivo was investigated. As shown in Figure 7C, compared with before injection, the tumor area turned bright after injection of IR780/PLGA@MnO2 NPs and the signal intensity (SI) in the tumor area obviously increased at 24 h after injection, which indicated that IR780/PLGA@MnO2 NPs can accumulate at the tumor site and have the capability of tumor microenvironment -activated MR imaging.
In vivo tumor hypoxia environment after different treatments
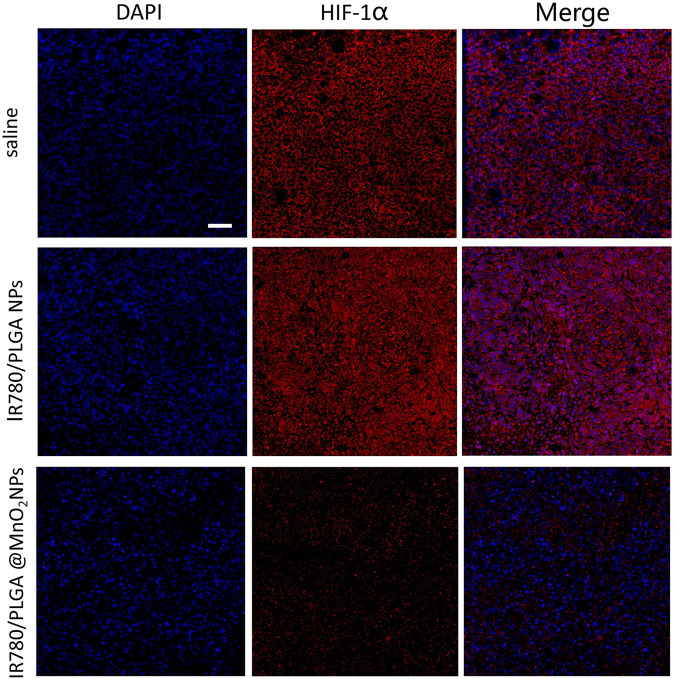
Tumor hypoxia plays an important role in SDT resistance, angiogenesis and invasiveness of tumor, the hypoxic tumor microenvironment highly express hypoxia-inducible factors (HIFs). In this study, the capability of O2 production via IR780/PLGA@MnO2 NPs to relieve tumor hypoxia in vitro were confirmed. Then, in vivo, a HIF-1α probe was used to verify the tumor hypoxia with different treatments by immunofluorescence assay. As shown in Figure 8, there was strong red fluorescence in the saline and IR780/PLGA NPs groups, which suggested obvious hypoxia tumor conditions. Comparatively, the red fluorescence of the group treated with IR780/PLGA@MnO2 NPs was much weaker, which demonstrated that tumor hypoxia was relieved by IR780/PLGA@MnO2 NPs due to O2 production by MnO2.
FIGURE 8.
Representative immunofluorescence images of tumor slices stained by the HIF-1α under different treatments (scale bar, 200 µm).
Antitumor efficacy in vivo
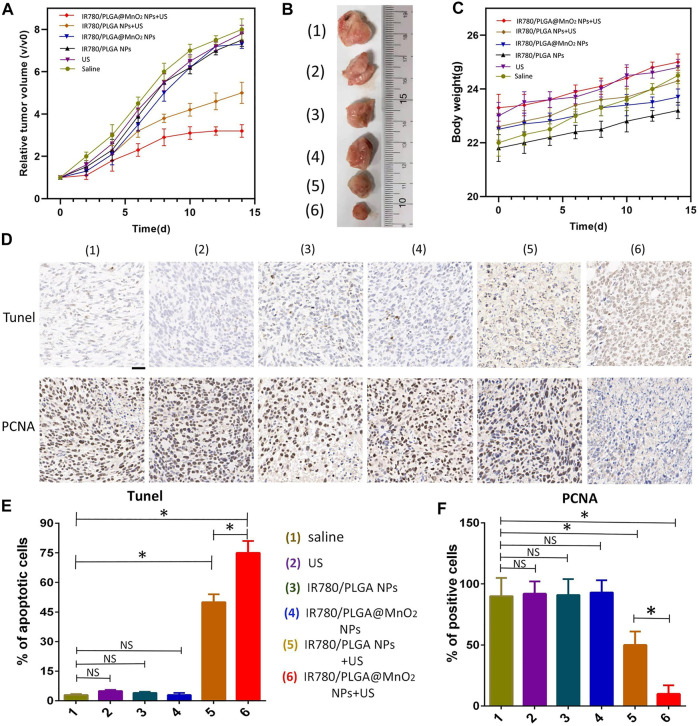
The effectiveness of SDT therapy of the IR780/PLGA@MnO2 NPs in vivo were investigated. There were 6 groups with different treatments: (Hussain and Nguyen, 2014) saline, (Wells, 2006) US irradiation, (Wu et al., 2005) IR780/PLGA NPs, (Ahmadi et al., 2020) IR780/PLGA@MnO2 NPs, (Qian et al., 2016) IR780/PLGA NPs + US, (Wood and Sehgal, 2015) IR780/PLGA@MnO2 NPs + US. As shown in Figure 9A, after 14 days of treatments, the tumor volumes increased rapidly in the saline, US irradiation, IR780/PLGA@MnO2 NPs groups with 6-, 6.5-, 7-fold increase of the average tumor volumes, respectively. The tumor volume increased 5.5-fold in the group of IR780/PLGA NPs and US irradiation. However, the growth of tumors is slowly increased in the group of IR780/PLGA@MnO2 NPs and US irradiation. Figure 9B showed photographs of tumors during the various treatments after 14 days, indicating that the IR780/PLGA@MnO2 NPs combined with US irradiation could inhibit tumor growth through SDT and that the effectiveness against tumor of IR780/PLGA NPs coated with MnO2 was better than the IR780/PLGA NPs without MnO2. As shown in Figure 9C, there was bare weight loss of the mice in the 6 groups, which implied the good biosafety of PLGA/IR780@MnO2 NPs and US. The therapeutic efficacy of SDT was further evaluated by the TUNEL and PCNA assays of the tumor tissues. As shown in Figures 9D,E, compared with the groups of (Hussain and Nguyen, 2014) saline, (Wells, 2006) US irradiation, (Wu et al., 2005) IR780/PLGA NPs and (Ahmadi et al., 2020) IR780/PLGA@MnO2 NPs, the proliferative cells with brown nuclear staining of the TUNEL assay of the (Qian et al., 2016) IR780/PLGA NPs + US group was higher, but they were lower than that in the (Wood and Sehgal, 2015) IR780/PLGA@MnO2 NPs + US group. Opposite to the TUNEL expression pattern, the proliferative cells with brown nuclear staining of PCNA expression were the lowest in the (Wood and Sehgal, 2015) IR780/PLGA@MnO2 NPs + US group (Figures 9D,F). These results indicated that the PH-responsive and H2O2-triggered SDT based on the IR780/PLGA@MnO2NPs is an effective platform to inhibit tumor growth with negligible systemic toxicity.
FIGURE 9.
(A) The relative tumor growth curves and (B) photographs of tumors during the various treatments after 14 days. (C) The weight growth curves of different groups of mice after various treatments. (D) Microscopy images of TUNEL and PCNA assays of stained tumor tissues at different treatments: (Hussain and Nguyen, 2014) Saline, (Wells, 2006) US, (Wu et al., 2005) IR780/PLGA NPs, (Ahmadi et al., 2020) IR780/PLGA@MnO2NPs, (Qian et al., 2016) IR780/PLGA NPs + US, (Wood and Sehgal, 2015) IR780/PLGA@MnO2 NPs + US (scale bar, 200 µm). (E) The relative quantification of TUNEL and (F) PCNA after different treatments. (*p < 0.05).
Conclusion
In summary, we successfully developed a multi-layer core-shell nanostructure of IR780/PLGA@MnO2 NPs with a high IR780-loading and low-leaking, which enhanced the SDT effect of 4T1 tumor bearing mice by deep penetration, H2O2-sensitive oxygen supply, under the guidance of MRI imaging. Introduction of the MnO2 shell can be react with overexpressed acidic H2O2 of tumor site to product Mn2+ for MRI and generate O2 for the improvement of hypoxia for enhanced SDT. We believe that our design of the IR780/PLGA@MnO2 NPs as a tumor microenvironment-sensitive nanoplatform provides a promising strategy for cancer diagnosis and SDT treatment, which could be further applied for cancer theranostics.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by the Ethics Committee of Second Xiangya Hospital, Central South University, China.
Author contributions
Conceptualization, CN and YX; methodology and investigation, YX, WT, MC, SC, KT, and HL; writing-original draft, YX; writing-review and editing, CN; supervision, CN; funding acquisition, YX and CN. All authors read and approved the final manuscript.
Funding
This project was funded by the National Natural Science Foundation of China (81974267), Science and Technology Innovation Program of Hunan Province (2021RC3033), Hunan Provincial Natural Science Foundation of China (2022JJ30827) and Hunan Health Commission (A202309026329).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Ahmadi A., Hosseini-Nami S., Abed Z., Beik J., Aranda-Lara L., Samadian H., et al. (2020). Recent advances in ultrasound-triggered drug delivery through lipid-based nanomaterials. Drug Discov. Today 25 (12), 2182–2200. 10.1016/j.drudis.2020.09.026 [DOI] [PubMed] [Google Scholar]
- Chen S., Huang B., Pei W., Wang L., Xu Y., Niu C. (2020). Mitochondria-targeting oxygen-sufficient perfluorocarbon nanoparticles for imaging-guided tumor phototherapy. Int. J. Nanomedicine 15, 8641–8658. 10.2147/IJN.S281649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C., Lin H., Liu H., Wang X., Wang J., Zhang P., et al. (2017). Tumor microenvironment-triggered supramolecular system as an in situ nanotheranostic generator for cancer phototherapy. Adv. Mat. 29 (23), 1605928. 10.1002/adma.201605928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costley D., Mc Ewan C., Fowley C., McHale A. P., Atchison J., Nomikou N., et al. (2015). Treating cancer with sonodynamic therapy: A review. Int. J. Hyperth. 31 (2), 107–117. 10.3109/02656736.2014.992484 [DOI] [PubMed] [Google Scholar]
- Ding B., Zheng P., Ma P., Lin J. (2020). Manganese oxide nanomaterials: Synthesis, properties, and theranostic applications. Adv. Mat. 32 (10), e1905823. 10.1002/adma.201905823 [DOI] [PubMed] [Google Scholar]
- Dong C., Hu H., Sun L., Chen Y. (2021). Inorganic chemoreactive nanosonosensitzers with unique physiochemical properties and structural features for versatile sonodynamic nanotherapies. Biomed. Mat. 16 (3), 032006. 10.1088/1748-605X/abef58 [DOI] [PubMed] [Google Scholar]
- Fu C., Duan X., Cao M., Jiang S., Ban X., Guo N., et al. (2019). Targeted magnetic resonance imaging and modulation of hypoxia with multifunctional hyaluronic acid-MnO2 nanoparticles in glioma. Adv. Healthc. Mat. 8 (10), e1900047. 10.1002/adhm.201900047 [DOI] [PubMed] [Google Scholar]
- Gordijo C. R., Abbasi A. Z., Amini M. A., Lip H. Y., Maeda A., Cai P., et al. (2015). Design of hybrid MnO2-polymer-lipid nanoparticles with tunable oxygen generation rates and tumor accumulation for cancer treatment. Adv. Funct. Mat. 25 (12), 1858–1872. 10.1002/adfm.201404511 [DOI] [Google Scholar]
- Hockel M., Vaupel P. (2001). Tumor hypoxia: Definitions and current clinical, biologic, and molecular aspects. JNCI J. Natl. Cancer Inst. 93 (4), 266–276. 10.1093/jnci/93.4.266 [DOI] [PubMed] [Google Scholar]
- Huang B., Chen S., Pei W., Xu Y., Jiang Z., Niu C., et al. (2020). Oxygen-sufficient nanoplatform for chemo-sonodynamic therapy of hypoxic tumors. Front. Chem. 8, 358. 10.3389/fchem.2020.00358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P., Qian X., Chen Y., Yu L., Lin H., Wang L., et al. (2017). Metalloporphyrin-encapsulated biodegradable nanosystems for highly efficient magnetic resonance imaging-guided sonodynamic cancer therapy. J. Am. Chem. Soc. 139 (3), 1275–1284. 10.1021/jacs.6b11846 [DOI] [PubMed] [Google Scholar]
- Hussain T., Nguyen Q. T. (2014). Molecular imaging for cancer diagnosis and surgery. Adv. Drug Deliv. Rev. 66, 90–100. 10.1016/j.addr.2013.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Feng L., Wu Y. (2021). Enzymatically synthesised MnO2 nanoparticles for efficient near-infrared photothermal therapy and dual-responsive magnetic resonance imaging. Nanoscale 13 (25), 11093–11103. 10.1039/d1nr02400k [DOI] [PubMed] [Google Scholar]
- Liu Z., Zhang S., Lin H., Zhao M., Yao H., Zhang L., et al. (2018). Theranostic 2D ultrathin MnO2 nanosheets with fast responsibility to endogenous tumor microenvironment and exogenous NIR irradiation. Biomaterials 155, 54–63. 10.1016/j.biomaterials.2017.11.015 [DOI] [PubMed] [Google Scholar]
- Ma Z., Jia X., Bai J., Ruan Y., Wang C., Li J., et al. (2017). MnO2Gatekeeper: An intelligent and O2-evolving shell for preventing premature release of high cargo payload core, overcoming tumor hypoxia, and acidic H2O2-sensitive MRI. Adv. Funct. Mat. 27 (4), 1604258. 10.1002/adfm.201604258 [DOI] [Google Scholar]
- Niu C., Xu Y., An S., Zhang M., Hu Y., Wang L., et al. (2017). Near-infrared induced phase-shifted ICG/Fe3O4 loaded PLGA nanoparticles for photothermal tumor ablation. Sci. Rep. 7 (1), 5490. 10.1038/s41598-017-06122-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei W., Huang B., Chen S., Wang L., Xu Y., Niu C. (2020). <p>Platelet-Mimicking drug delivery nanoparticles for enhanced chemo-photothermal therapy of breast cancer</p>. Int. J. Nanomedicine 15, 10151–10167. 10.2147/IJN.S285952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X., Zheng Y., Chen Y. (2016). Micro/nanoparticle-augmented sonodynamic therapy (SDT): Breaking the depth shallow of photoactivation. Adv. Mat. 28 (37), 8097–8129. 10.1002/adma.201602012 [DOI] [PubMed] [Google Scholar]
- Rosenthal I., Sostaric J. Z., Riesz P. (2004). Sonodynamic therapy--a review of the synergistic effects of drugs and ultrasound. Ultrason. Sonochem. 11 (6), 349–363. 10.1016/j.ultsonch.2004.03.004 [DOI] [PubMed] [Google Scholar]
- Son S., Kim J. H., Wang X., Zhang C., Yoon S. A., Shin J., et al. (2020). Multifunctional sonosensitizers in sonodynamic cancer therapy. Chem. Soc. Rev. 49 (11), 3244–3261. 10.1039/c9cs00648f [DOI] [PubMed] [Google Scholar]
- Song G., Liang C., Yi X., Zhao Q., Cheng L., Yang K., et al. (2016). Perfluorocarbon-loaded hollow Bi2Se3 nanoparticles for timely supply of oxygen under near-infrared light to enhance the radiotherapy of cancer. Adv. Mat. 28 (14), 2716–2723. 10.1002/adma.201504617 [DOI] [PubMed] [Google Scholar]
- Song X., Feng L., Liang C., Yang K., Liu Z. (2016). Ultrasound triggered tumor oxygenation with oxygen-shuttle nanoperfluorocarbon to overcome hypoxia-associated resistance in cancer therapies. Nano Lett. 16 (10), 6145–6153. 10.1021/acs.nanolett.6b02365 [DOI] [PubMed] [Google Scholar]
- Sun W., Yu H., Wang D., Li Y., Tian B., Zhu S., et al. (2021). MnO2 nanoflowers as a multifunctional nano-platform for enhanced photothermal/photodynamic therapy and MR imaging. Biomater. Sci. 9 (10), 3662–3674. 10.1039/d1bm00033k [DOI] [PubMed] [Google Scholar]
- Wan G. Y., Liu Y., Chen B. W., Liu Y. Y., Wang Y. S., Zhang N., et al. (2016). Recent advances of sonodynamic therapy in cancer treatment. Cancer Biol. Med. 13 (3), 325–338. 10.20892/j.issn.2095-3941.2016.0068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Chen S., Zhu Y., Zhang M., Tang S., Li J., et al. (2018). Triple-modal imaging-guided chemo-photothermal synergistic therapy for breast cancer with magnetically targeted phase-shifted nanoparticles. ACS Appl. Mat. Interfaces 10 (49), 42102–42114. 10.1021/acsami.8b16323 [DOI] [PubMed] [Google Scholar]
- Wang X., Zhong X., Gong F., Chao Y., Cheng L. (2020). Newly developed strategies for improving sonodynamic therapy. Mat. Horiz. 7 (8), 2028–2046. 10.1039/d0mh00613k [DOI] [Google Scholar]
- Wells P. N. (2006). Ultrasound imaging. Phys. Med. Biol. 51 (13), R83–R98. 10.1088/0031-9155/51/13/R06 [DOI] [PubMed] [Google Scholar]
- Wood A. K., Sehgal C. M. (2015). A review of low-intensity ultrasound for cancer therapy. Ultrasound Med. Biol. 41 (4), 905–928. 10.1016/j.ultrasmedbio.2014.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Wang Z. B., Zhu H., Chen W. Z., Zou J. Z., Bai J., et al. (2005). Feasibility of US-guided high-intensity focused ultrasound treatment in patients with advanced pancreatic cancer: Initial experience. Radiology 236 (3), 1034–1040. 10.1148/radiol.2362041105 [DOI] [PubMed] [Google Scholar]
- Xu Y., Li W., Chen S., Huang B., Pei W., Niu C. (2020). Near-Infrared responsive phase-shifted nanoparticles for magnetically targeted MR/US imaging and photothermal therapy of cancer. Front. Bioeng. Biotechnol. 8, 599107. 10.3389/fbioe.2020.599107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yang Y., Gao F., Wei J. J., Qian C. G., Sun M. J. (2019). Biomimetic hybrid nanozymes with self-supplied H(+) and accelerated O2 generation for enhanced starvation and photodynamic therapy against hypoxic tumors. Nano Lett. 19 (7), 4334–4342. 10.1021/acs.nanolett.9b00934 [DOI] [PubMed] [Google Scholar]
- Zhang L., Yi H., Song J., Huang J., Yang K., Tan B., et al. (2019). Mitochondria-targeted and ultrasound-activated nanodroplets for enhanced deep-penetration sonodynamic cancer therapy. ACS Appl. Mat. Interfaces 11 (9), 9355–9366. 10.1021/acsami.8b21968 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Xu Y., Sun D., Meng Z., Ying W., Gao W., et al. (2020). Hollow magnetic nanosystem-boosting synergistic effect between magnetic hyperthermia and sonodynamic therapy via modulating reactive oxygen species and heat shock proteins. Chem. Eng. J. 390, 124521. 10.1016/j.cej.2020.124521 [DOI] [Google Scholar]
- Zhang Z., Ji Y. (2020). Nanostructured manganese dioxide for anticancer applications: Preparation, diagnosis, and therapy. Nanoscale 12 (35), 17982–18003. 10.1039/d0nr04067c [DOI] [PubMed] [Google Scholar]
- Zhu P., Chen Y., Shi J. (2018). Nanoenzyme-augmented cancer sonodynamic therapy by catalytic tumor oxygenation. ACS Nano 12 (4), 3780–3795. 10.1021/acsnano.8b00999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.