Abstract
Purpose
To determine whether quantitative plaque characterization by using CT coronary angiography (CTCA) can discriminate between type 1 and type 2 myocardial infarction.
Materials and Methods
This was a secondary analysis of two prospective studies (ClinicalTrials.gov registration nos. NCT03338504 [2014–2019] and NCT02284191 [2018–2020]) that performed blinded quantitative plaque analysis on findings from CTCA in participants with type 1 myocardial infarction, type 2 myocardial infarction, and chest pain without myocardial infarction. Logistic regression analyses were performed to identify predictors of type 1 myocardial infarction.
Results
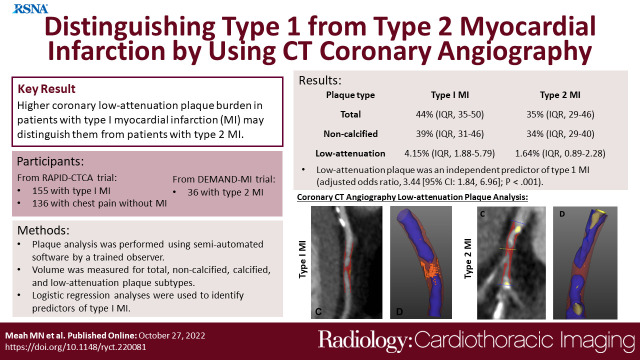
Overall, 155 participants (mean age, 64 years ± 12 [SD]; 114 men) and 36 participants (mean age, 67 years ± 12; 19 men) had type 1 and type 2 myocardial infarction, respectively, and 136 participants (62 years ± 12; 78 men) had chest pain without myocardial infarction. Participants with type 1 myocardial infarction had greater total (median, 44% [IQR: 35%–50%] vs 35% [IQR: 29%–46%]), noncalcified (39% [IQR: 31%–46%] vs 34% [IQR: 29%–40%]), and low-attenuation (4.15% [IQR: 1.88%–5.79%] vs 1.64% [IQR: 0.89%–2.28%]) plaque burdens (P < .05 for all) than those with type 2. Participants with type 2 myocardial infarction had similar low-attenuation plaque burden to those with chest pain without myocardial infarction (P = .4). Low-attenuation plaque was an independent predictor of type 1 myocardial infarction (adjusted odds ratio, 3.44 [95% CI: 1.84, 6.96]; P < .001), with better discrimination than noncalcified plaque burden and maximal area of coronary stenosis (C statistic, 0.75 [95% CI: 0.67, 0.83] vs 0.62 [95% CI: 0.53, 0.71] and 0.61 [95% CI: 0.51, 0.70] respectively; P ≤ .001 for both).
Conclusion
Higher low-attenuation coronary plaque burden in patients with type 1 myocardial infarction may help distinguish these patients from those with type 2 myocardial infarction.
Keywords: Ischemia/Infarction, CT Angiography, Quantitative CT
Clinical trial registration nos. NCT03338504 and NCT02284191
Supplemental material is available for this article.
© RSNA, 2022
Keywords: Ischemia/Infarction, CT Angiography, Quantitative CT
Summary
There may be important differences in the plaque composition of patients with type 1 and type 2 myocardial infarction, which can be quantified at CT coronary angiography.
Key Points
■ Patients presenting with type 1 myocardial infarction have greater burdens of total (44% [IQR: 35%–50%] vs 35% [IQR: 39%–46%]), noncalcified (39% [IQR: 31%–46%] vs 34% [IQR: 29%–40%]), and low-attenuation (4% [IQR: 2%–6%] vs 2% [IQR: 1%–2%]) plaque than those with type 2 myocardial infarction (P < .001 for all).
■ Low-attenuation plaque burden provided strong discrimination between type 1 and type 2 myocardial infarction, independent of the severity of coronary stenosis or clinical characteristics (adjusted odds ratio, 3.44 [95% CI: 1.84, 6.95]; P < .001).
■ Quantitative plaque analysis may help differentiate between patients with type 1 and type 2 myocardial infarction.
Introduction
According to the Fourth Universal Definition of Myocardial Infarction Consensus Document, myocardial infarction is diagnosed when a patient has evidence of acute myocardial injury in the setting of myocardial ischemia (1). Type 1 myocardial infarction is caused by atherosclerotic plaque disruption, coronary thrombosis, and vessel occlusion. In contrast, type 2 myocardial infarction occurs when there is myocardial oxygen supply and demand mismatch induced by numerous pathophysiologic precipitants (2). Differentiating between type 1 and type 2 myocardial infarction is a common clinical conundrum that can be difficult to resolve (3), particularly as both can occur in the presence or absence of obstructive coronary artery disease (4). Proper differentiation is important because treatment strategies vary substantially, and the correct diagnosis can have major implications for patient outcomes (5). For example, in type 1 myocardial infarction, dual antiplatelet therapy plays an important role in the treatment and prevention of recurrent coronary atherothrombotic events, whereas in type 2 myocardial infarction, dual antiplatelet therapy may be harmful, especially when occult bleeding and anemia are contributing factors. Thus, a noninvasive test that can distinguish between type 1 and type 2 myocardial infarction would be of major clinical benefit.
CT coronary angiography (CTCA) can noninvasively help assess both the severity of coronary artery stenosis and the characteristics of coronary atherosclerotic plaque. The necrotic core of high-risk atherosclerotic plaque is thought to be the pathologic precursor to type 1 myocardial infarction, and with the advent of quantitative CT plaque analysis, the presence and extent of low-attenuation plaque features can now be quantified (6–8). CT-defined high-risk low-attenuation plaque can be used to risk stratify patients with coronary artery disease and is one of the strongest predictors of future myocardial infarction (9). While this prognostic value in patients with stable coronary artery disease has been established, the diagnostic potential of quantitative plaque analysis in patients with acute chest pain is less clear. Identification and quantification of high-risk low-attenuation plaque at CT prior to revascularization may provide an alternative diagnostic approach that could help distinguish between type 1 and type 2 myocardial infarction.
In this study, we aimed to describe the differences in CT-defined coronary atherosclerosis in patients with acute chest pain and to determine whether quantitative CT angiographic measures of plaque composition and burden can help differentiate between type 1 and type 2 myocardial infarction.
Materials and Methods
Study Participants
Participants included in this study had been recruited into two prospective clinical studies evaluating the role of CTCA in the diagnosis of acute coronary syndromes and type 2 myocardial infarction. A detailed description of the inclusion and exclusion criteria can be found in Appendix E1 (supplement). The studies were both registered on ClinicalTrials.gov and approved by the South East Scotland Research Ethics Committee (reference nos. 14/SS/1096 and 17/SS/0078). Both studies were conducted in accordance with the Declaration of Helsinki, and written informed consent was obtained from all participants.
The Rapid Assessment of Potential Ischemic Heart Disease with CT Coronary Angiography (RAPID-CTCA) study was an open prospective multicenter randomized controlled parallel group trial that recruited 1748 patients who presented to the emergency department with suspected or provisionally diagnosed acute coronary syndrome in 37 centers across the United Kingdom between 2014 and 2019 (ClinicalTrials.gov registration no. NCT02284191, Appendix E1 [supplement]). Patients were required to have symptoms consistent with cardiac ischemia, together with at least one of the following: abnormal findings at 12-lead electrocardiography, a history of prior ischemic heart disease, or an elevated plasma cardiac troponin concentration (10). Of the 887 participants who were randomized to CTCA, 767 underwent completed imaging. In total, 422 scans were available and consecutively analyzed, comprising all scans from the top five recruitment centers and a quality assurance cohort of 121 scans obtained from all participating sites. In RAPID-CTCA, the clinical diagnosis was not independently adjudicated but was assigned by the attending clinician. To avoid potential diagnostic misclassification, patients with myocardial injury of uncertain cause or with unstable angina were a priori excluded, and only those with either type 1 myocardial infarction or chest pain without myocardial infarction were included.
The Determining the Mechanism of Myocardial Injury and Role of Coronary Disease in Type 2 Myocardial Infarction (DEMAND-MI) study was a prospective single-center observational cohort of 100 patients with a clinical diagnosis of type 2 myocardial infarction, which aimed to determine the mechanism of myocardial injury and the role of coronary artery disease (ClinicalTrials.gov registration no. NCT03338504, Appendix E1 [supplement]) (11). DEMAND-MI enrolled participants with evidence of acute myocardial injury (defined as a rise and/or fall in cardiac troponin concentration), clinical symptoms of myocardial ischemia or signs of myocardial ischemia on a 12-lead electrocardiogram, and objective evidence of myocardial oxygen supply or demand imbalance, consistent with a clinical diagnosis of type 2 myocardial infarction. All participants underwent extensive imaging to confirm the diagnosis of type 2 myocardial infarction, including systematic coronary and structural imaging with invasive coronary angiography or CTCA and cardiac MRI or echocardiography. The final diagnosis was adjudicated by consensus of an expert panel. Every participant who underwent CTCA was included in the current analysis. Patients were recruited between 2018 and 2020.
CT Angiography and Quantitative Plaque Analysis
CT imaging was performed by using CT scanners with 64 or more multidetector rows according to the study protocol (ClinicalTrials.gov registration nos. NCT03338504 and NCT02284191) (12). Reconstructions of contrast-enhanced images were performed on the best phase in mid diastole or end systole according to established techniques (13). CT angiography data sets were anonymized and exported in a Digital Imaging and Communications in Medicine (ie, DICOM) format to allow quantitative measurement of plaque subtypes. Plaque analysis was performed using semiautomated software (Autoplaque version 2.5; Cedars-Sinai Medical Center) by an observer with 3 years of experience in quantitative plaque analysis (M.N.M.) blinded to study of origin and diagnosis. This method has excellent observer agreement, even in patients with advanced coronary disease, and has been validated against intravascular US (6,14,15).
Coronary artery centerlines were extracted in a semiautomated fashion for each major artery and any tributary larger than 2 mm in diameter with visually observed disease. A region of interest was placed in the aorta to define blood pool attenuation. Coronary artery segments were defined manually according to Society of Cardiovascular Computed Tomography guidance, using side branches to mark progression from proximal to mid and distal segments (16,17). Segments with visible disease were manually identified, and vessel wall and plaque constituents were automatically determined using scan-specific thresholds with manual adjustments made as required. Area stenosis was calculated automatically and refers to the maximal area of stenosis on a per-participant level. Stented segments and graft insertion points were excluded from analysis, in line with previously described methods (6). Scans with image quality that was too poor to complete quantitative plaque analysis were excluded.
Plaque volume was measured (in millimeters cubed) for total, noncalcified, calcified, and low-attenuation plaque subtypes. Calcified and noncalcified plaque were determined using scan-specific thresholds based on attenuation in the thoracic aorta (15), and low-attenuation plaque was defined with a fixed attenuation of less than 30 HU (18). To normalize for differences in vessel volume, plaque burden was calculated by dividing plaque volume by the vessel volume of segments assessed and multiplying by 100.
Statistical Analysis
Categorical variables are presented as numbers and percentages. Continuous variables are presented as medians with IQRs in parentheses or as means ± SDs, when normally distributed. Statistical significance was assessed using Wilcoxon rank sum test, Pearson χ2 test, and Fisher exact test, as appropriate. Logistic regression analysis was performed with participants who had a diagnosis of either type 1 or type 2 myocardial infarction to determine the odds ratios with 95% CIs of type 1 myocardial infarction. Multivariable regression models were constructed using a priori selection, adjusting for age, sex, history of smoking, hypertension, hyperlipidemia, maximal area stenosis, and individual plaque subtype burdens. Plaque burdens for each subtype were log transformed for this analysis (log2 of 1 plus the plaque variable). Receiver operating characteristic curves were created to assess the discrimination of patients with type 1 myocardial infarction and type 2 myocardial infarction (pROC package version 1.17.0 in R; R Foundation for Statistical Computing), and differences between curves were compared by using DeLong test (19,20). Akaike information criteria (AIC) and log likelihood were calculated to determine which multivariable model offered the best fit for predicting type 1 myocardial infarction versus type 2 myocardial infarction. Statistical significance was defined as a two-sided P value less than .05. All statistical analysis was performed using R, version 4.0.2.
Results
Study Participants
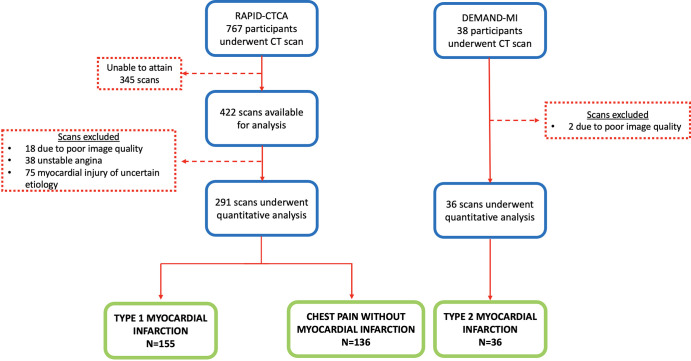
In total, 460 CT scans were assessed, of which 20 were excluded due to poor image quality, 75 due to a diagnosis of myocardial injury of uncertain cause, and 38 due to a diagnosis of unstable angina (Fig 1). The final study sample included 327 participants: 155 participants (mean age, 64 years ± 12; 114 men) had type 1 myocardial infarction, 36 (mean age, 67 years ± 12; 19 men) had type 2 myocardial infarction, and 136 (mean age, 62 years ± 12; 78 men) had chest pain without infarction (Table 1). The subgroup of participants from the RAPID-CTCA trial was representative of the overall trial participants (Table E1 [supplement]).
Figure 1:
Substudy diagram shows the screening and final study participants. DEMAND-MI = Determining the Mechanism of Myocardial Injury and Role of Coronary Disease in Type 2 Myocardial Infarction, RAPID-CTCA = Rapid Assessment of Potential Ischemic Heart Disease with CT Coronary Angiography.
Table 1:
Baseline Characteristics of Study Participants
Clinical Characteristics of Study Participants
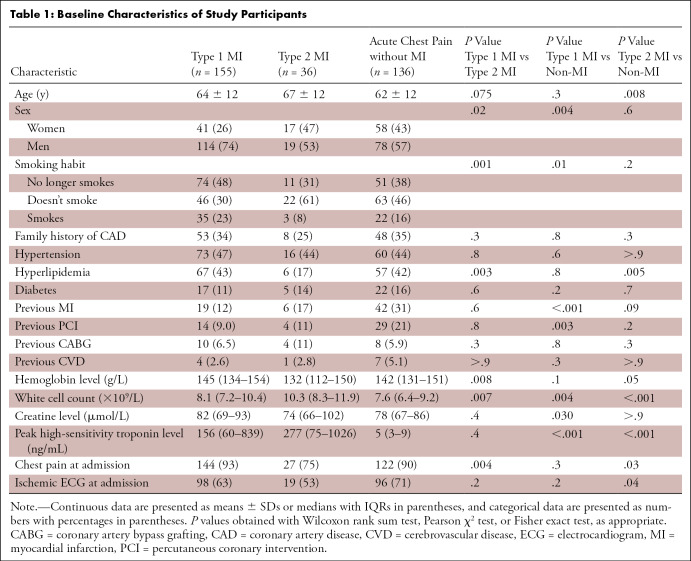
Participants with type 1 myocardial infarction were less likely to be female or to have undergone coronary artery bypass grafting and more likely to have a history of smoking or hyperlipidemia. All other risk factors for myocardial infarction were similar between cohorts (Table 1). Compared with those who had type 2 myocardial infarction, participants with type 1 myocardial infarction had higher hemoglobin concentrations and lower white cell counts but similar maximal high-sensitivity cardiac troponin concentrations. More participants with type 1 myocardial infarction than those with type 2 myocardial infarction presented with chest pain (144 of 155 [93%] vs 27 of 36 [75%], P = .004), but similar proportions had abnormal electrocardiograms at presentation (98 of 155 [63%] vs 19 of 36 [53%], P = .2). The three most common mechanisms of supply-demand imbalance in participants with type 2 myocardial infarction were tachyarrhythmia (18 of 36 [50%]), anemia (six of 36 [17%]), and hypoxia (five of 36 [14%]; Table E2 [supplement]).
In general, patients with chest pain without myocardial infarction had a similar profile to those with myocardial infarction (Table 1). The three most common discharge diagnoses in those presenting with chest pain in the absence of myocardial infarction included chest pain of uncertain cause (58 of 136 [43%]), gastrointestinal causes (21 of 136 [15%]), and musculoskeletal pain (20 of 136 [15%]; Table E2 [supplement]).
CTCA Findings
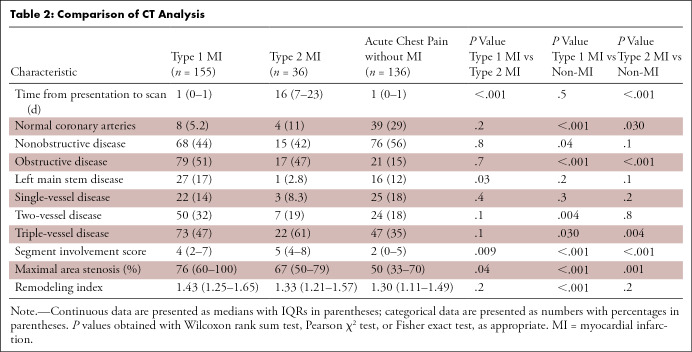
CTCA examinations were performed within a median interval of 1 day (IQR: 0–1 days) in patients with type 1 myocardial infarction and those with chest pain in the absence of myocardial infarction, and within 16 days (IQR: 7–23 days) after presentation in participants with type 2 myocardial infarction. Participants with type 1 myocardial infarction had similar proportions of normal, nonobstructive, and obstructive coronary artery disease compared with patients with type 2 myocardial infarction (Table 2), but there was more disease in the left main stem of those with type 1 myocardial infarction (P = .03). Compared with those without myocardial infarction, participants with type 1 myocardial infarction were more likely to have obstructive (P < .001) and two- or three-vessel coronary disease (P ≤ .03 for both). This was similarly the case when participants without myocardial infarction were compared with those with type 2 myocardial infarction, who were more likely to have obstructive and three-vessel coronary disease (P ≤ .004 for both).
Table 2:
Comparison of CT Analysis
Plaque Characteristics
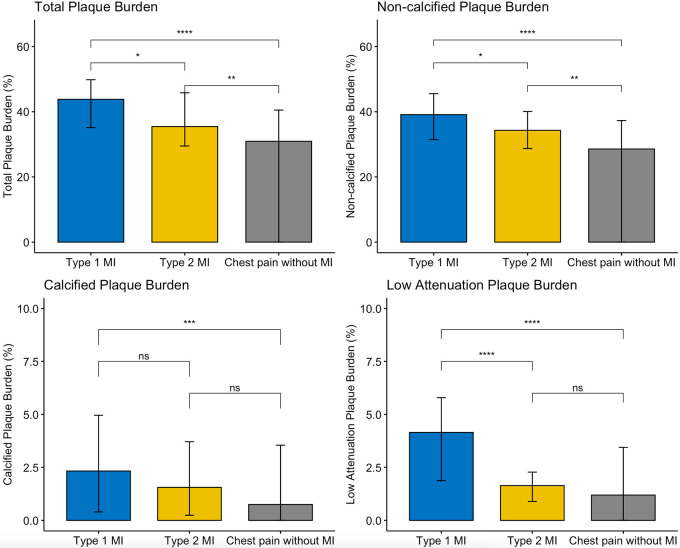
Compared with those with type 2 myocardial infarction, participants with type 1 myocardial infarction had greater maximal area stenosis and total, noncalcified, and low-attenuation plaque, but there was no evidence of a difference in calcified plaque burden (Fig 2, Table E3 [supplement]). Respective plaque volume measurements demonstrated similar relationships (Table E4 [supplement]). Participants with chest pain without myocardial infarction had lower maximal area stenosis and coronary plaque burdens compared with those with type 1 or type 2 myocardial infarction, although the burdens of calcified and low-attenuation plaque were similar to those with type 2 myocardial infarction (Table 2, Fig 2).
Figure 2:
Comparison of plaque burden subtypes in patients with type 1 myocardial infarction, type 2 myocardial infarction, and acute chest pain without myocardial infarction. Histograms (medians ± IQRs) comparing burden of plaque subtypes demonstrate that participants with type 1 myocardial infarction had higher burdens of total, noncalcified, and low-attenuation plaque burden. MI = myocardial infarction, ns = not significant, * = P < .05, ** = P < .01, *** = P < .001, **** = P < .0001.
Predictors of Type 1 Myocardial Infarction
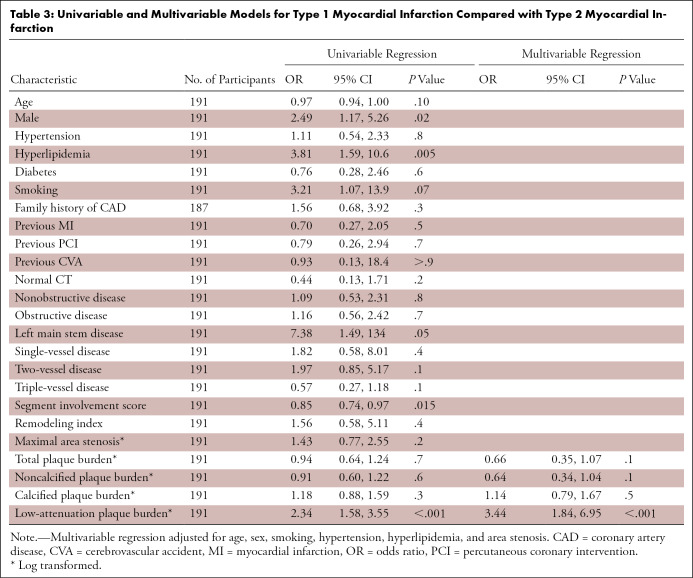
On univariable logistic regression analysis of all patients with myocardial infarction (type 1 or type 2), male sex and hyperlipidemia were associated with an increased likelihood of type 1 myocardial infarction (P < .02 for all, Table 3). On univariable analysis of imaging assessments, only low-attenuation plaque burden was associated with an increased likelihood of type 1 myocardial infarction (odds ratio, 2.34 [95% CI: 1.58, 3.55]; P < .001; Table 3). On multivariable analysis, low-attenuation plaque burden remained an independent predictor of type 1 myocardial infarction (adjusted odds ratio, 3.44 [95% CI: 1.84, 6.95]; P < .001; Table 3).
Table 3:
Univariable and Multivariable Models for Type 1 Myocardial Infarction Compared with Type 2 Myocardial Infarction
Receiver operating characteristic curves illustrate the discrimination of area stenosis, noncalcified plaque burden, and low-attenuation plaque burden in patients with type 1 myocardial infarction or type 2 myocardial infarction (Fig 3). In this restricted sample, noncalcified plaque had a C statistic of 0.62 (95% CI: 0.53, 0.71) and maximal area stenosis of 0.61 (95% CI: 0.51, 0.70). Low-attenuation plaque burden appeared to better distinguish between type 1 and type 2 myocardial infarction when compared with both these measures, with a C statistic of 0.75 (95% CI: 0.67, 0.83; P ≤ .001 for both). Similarly, the multivariable model including low-attenuation plaque offered a better fit for the data (AIC, 167.5) when compared with noncalcified plaque burden (AIC, 179.7) or maximal area stenosis (AIC, 180.0; Table E5 [supplement]). The specificity with which a type 1 myocardial infarction could be detected from a population that also included patients with type 2 myocardial infarction appeared to increase with larger burdens of low-attenuation plaque (Table E6 [supplement]).
Figure 3:
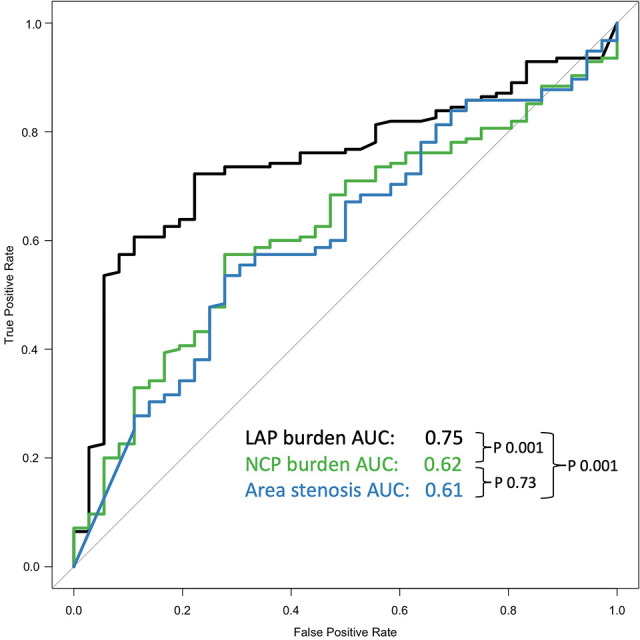
Predictors of type 1 myocardial infarction. Receiver operating characteristic curves compare ability of low-attenuation plaque (LAP; black) burden, noncalcified plaque (NCP; green) burden, and maximal area stenosis (blue) to discriminate between type 1 and type 2 myocardial infarction. There was a low-attenuation plaque burden C statistic of 0.75 (95% CI: 0.67, 0.83), noncalcified plaque burden C statistic of 0.62 (95% CI: 0.53, 0.71), and maximal area stenosis C statistic of 0.61 (95% CI: 0.51, 0.70). AUC = area under the receiver operating characteristic curve.
Discussion
In this secondary analysis of two prospective clinical studies of patients with acute chest pain, we demonstrate that quantitative coronary artery plaque characteristics at CTCA differ between patients with type 1 and type 2 myocardial infarction. Specifically, low-attenuation plaque burden distinguished between these two distinct pathologic conditions independent of maximal area stenosis and standard clinical characteristics (adjusted odds ratio, 3.44 [95% CI: 1.84, 6.96]; P < .001). These findings suggest that quantitative CT plaque analysis holds promise in discriminating between type 1 and type 2 myocardial infarction and may have the potential to inform the clinical management of patients with myocardial infarction of uncertain cause (Fig 4).
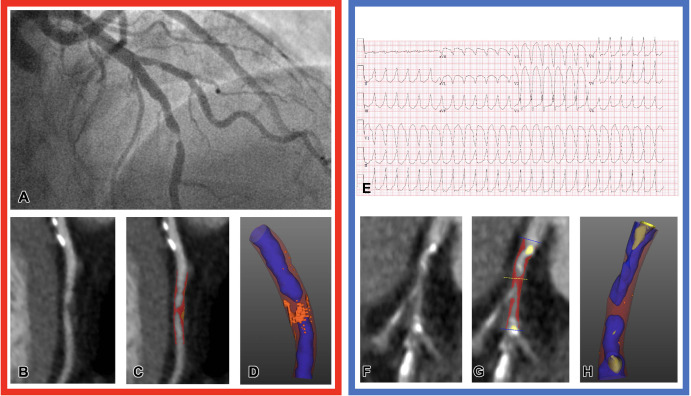
Figure 4:
Representative images of CT plaque analysis demonstrate differences between type 1 and type 2 myocardial infarction. Left panel: Images in a 42-year-old man diagnosed with type 1 myocardial infarction. (A) Image from invasive angiography demonstrates severe stenosis in the distal left anterior descending artery. (B) CT coronary angiogram, curved planar reformation, (C) quantitative plaque analysis, and (D) three-dimensional quantitative plaque analysis demonstrate a high burden of low-attenuation plaque. Right panel: Images in a 74-year-old man diagnosed with type 2 myocardial infarction. (E) Electrocardiogram demonstrates broad-complex tachycardia consistent with ventricular tachycardia. (F) CT coronary angiogram, curved planar reformation, (G) quantitative plaque analysis, and (H) three-dimensional quantitative plaque analysis demonstrate a low burden of low-attenuation plaque. Both participants have obstructive coronary artery disease detected with CT coronary angiography. Quantitative plaque analysis demonstrates clear differences, with a much higher burden of low-attenuation plaque in the participant presenting with type 1 myocardial infarction compared with the participant presenting with type 2.
Type 1 myocardial infarction is defined by acute atherosclerotic plaque rupture or erosion leading to coronary thrombosis and luminal obstruction (1,21). The lipid-rich necrotic core is central to the pathogenesis of type 1 myocardial infarction and correlates closely to CT-defined low-attenuation plaque (8,15,18,22). Consistent with this, our study demonstrates a higher burden of low-attenuation plaque at quantitative CT in patients with type 1 myocardial infarction compared with those with type 2 myocardial infarction or chest pain without infarction, a finding which has not, to our knowledge, been established in the literature. These findings are logical and intuitive because low-attenuation lipid-rich necrotic plaque is the primary driver for type 1 myocardial infarction, and our cohort is unique in that CT scans were completed prior to invasive angiography and revascularization.
With the widespread adoption of high-sensitivity cardiac troponin level testing, diagnosis of myocardial injury and infarction has increased (23), and up to 20% of patients with an elevated cardiac troponin concentration are now diagnosed with type 2 myocardial infarction (3,24,25). However, plasma cardiac biomarkers have a limited ability to discriminate between type 1 and type 2 myocardial infarction (26). This is due in part to the complex nature of type 2 myocardial infarction, which often occurs in a heterogeneous group of patients with multiple comorbidities and can have an unclear cause or pathophysiology (27–29). Infarction may occur due to unmet myocardial oxygen demand (tachyarrhythmia, hypertrophy) or a reduction in myocardial oxygen supply (anemia, hypotension, hypoxia) (2,30). While this often occurs in the presence of obstructive coronary artery disease, it may also occur when myocardial demand outstrips supply, even in the absence of flow limitation or obstruction. This begs the question, “How do we know if obstructive coronary artery disease with myocardial infarction is due to plaque rupture or a supply and demand mismatch?” In our study, the presence of obstructive coronary artery disease was not sufficient to distinguish between type 1 and type 2 myocardial infarction. However, many patients with type 2 myocardial infarction had substantial coronary artery disease but a reduced burden of low-attenuation plaque. This raises the possibility that CTCA with quantitative plaque characterization may help in the evaluation of patients with myocardial infarction of uncertain cause. In those with reduced low-attenuation plaque, clinicians may be reassured that plaque disruption and type 1 myocardial infarction are unlikely.
Irrespective of the presence of nonobstructive or obstructive coronary artery disease, it can be challenging to confirm whether and where acute plaque rupture or erosion has occurred. The culprit lesion is often misascribed at invasive angiography, with discordance between the treated lesion and the territory of infarction in up to half of cases (31). While invasive adjunctive coronary imaging, such as optical coherence tomography or intravascular US, can assist in diagnosing acute plaque disruption, the cost, necessary expertise, and inability to cross or image the majority of lesions with severe disease are barriers to widespread adoption in routine clinical practice (28). In contrast, CT angiography can provide a global assessment of coronary atherosclerotic plaque that includes the vessel wall and plaque, can assess critically stenosed lesions, and is not merely limited to assessing the luminal stenosis. The identification of low-attenuation plaque may also provide an opportunity to intensify the application of more advanced preventative therapies to reduce recurrent events.
Our study reported on a third group of patients who presented with chest pain but without evidence of myocardial injury or infarction. Acute chest pain is one of the most common reasons for individuals to present to the emergency department, and patients with a prior history of coronary heart disease are more likely to attend (32). It has previously been reported that patients presenting with acute chest pain without myocardial infarction have a higher burden of plaque compared with asymptomatic individuals (33). Moreover, due to inclusion criteria, our study sample was enriched for patients with a prior history of coronary heart disease. Despite this enhanced prevalence of coronary artery disease, patients without myocardial infarction had a low burden of low-attenuation plaque, equivalent to those with type 2 myocardial infarction. This reinforces the study conclusion that coronary artery disease in type 2 myocardial infarction is predominantly stable, and myocardial infarction has occurred due to a supply and demand imbalance. It also suggests that a high burden of low-attenuation plaque is more specific to those with type 1 myocardial infarction.
Our study had several important limitations. First, our study population was pooled from two different studies with differing study designs; as such, unmeasured confounders may impact the differences in plaque burden and characteristics. To limit ascertainment bias, patients were consecutively recruited, and image analysis was performed in a single core laboratory blinded to the trial of origin and clinical diagnosis. Image acquisition protocols were also identical, as both trials were designed and led by the same investigators. We confirmed that our cohort of patients with type 1 myocardial infarction was representative of the overall trial population, and every patient with type 2 myocardial infarction who underwent CT was included. This minimized any case selection bias. Moreover, because the diagnosis of type 2 myocardial infarction can be heterogeneous, its categorization was carefully and independently adjudicated by an expert panel following systematic and comprehensive cardiac imaging, including echocardiography and cardiac MRI. Despite this, the total number of patients with an adjudicated diagnosis of type 2 myocardial infarction who underwent CT was relatively modest (n = 36), limiting our ability to draw strong conclusions. Additionally, the diagnosis of type 1 myocardial infarction was determined by site investigators, which might have led to some misclassification. Finally, although a semiautomated process, quantification of plaque subtype can take up to 20 minutes, which may limit clinical use of this approach. Further automation and machine learning algorithms could improve the rapidity and practical application of plaque quantification (34).
In conclusion, quantitative CTCA helped identify important differences between the plaque characteristics of patients with type 1 and type 2 myocardial infarction. Future prospective studies are needed to validate this technique and investigate whether the differences could be used to differentiate between these distinct pathologic conditions in clinical practice.
Acknowledgments
Acknowledgements
We would like to thank all the RAPID-CTCA and DEMAND-MI investigators.
D.D. and M.C.W. are co–senior authors.
M.N.M. (no. FS/19/46/34445), E.T. (no. FS/CRTF/20/24086), A.R.C. (no. FS/16/75/32533), N.L.M. (nos. FS/16/14/32023, RG/20/10/34966, RE/18/5/34216), M.C.W. (nos. FS/ICRF/20/26002, FS/11/014, CH/09/002), and D.E.N. (nos. CH/09/002, RG/16/10/32375, RE/18/5/34216) are supported by the British Heart Foundation (BHF). A.B. and R.W. are supported by a clinical research training fellowship (nos. MR/V007254/1 and MR/V007017/1) from the Medical Research Council. D.D. is supported by National Institutes of Health/National Heart, Lung, and Blood Institute grants (nos. 1R01HL148787-01A1 and 1R01HL151266). The Edinburgh Clinical Research Facilities and Edinburgh Imaging facility are supported by the National Health Service Research Scotland through National Health Service Lothian Health Board. The RAPID-CTCA trial was funded by the National Institute for Health Research Health Technology Assessment Programme (no. 13/04/108). The DEMAND-MI study was funded by the BHF (no. FS/16/75/32533). A.R.C. receives additional support from the Academy of Medical Sciences (no. SGL021/1075).
Data sharing: Data generated or analyzed during the study are available from the corresponding author by request.
Disclosures of conflicts of interest: M.M. Author is a British Heart Foundation (BHF) clinical research fellow at the University of Edinburgh, a BHF grant (grant no. FS/19/46/34445) has supported author’s work since November 2019. A.B. Support for the present manuscript from the BHF (grant no. FS/16/75/32533). E.T. No relevant relationships. A.R.C. No relevant relationships. M.D. No relevant relationships. J.D.H. No relevant relationships. J.C. No relevant relationships. C.T. No relevant relationships. R.W. Clinical training research fellowship from the Medical Research Council UK (no. MR/V007017/1). A.G. Participation on advisory board for Abbott for brain and cardiac biomarkers, fees paid to author’s institution. M.R.D. No relevant relationships. C.R. No relevant relationships. N.C. Unrestricted research grants from Boston Scientific, Haemonetics, Beckmann Coulter, and HeartFlow; speakers fees from Abbott and Boston Scientific; travel sponsorship from Biosensors, Abbott, Edwards, and Medtronic. A.K. No relevant relationships. D.F. No relevant relationships. N.L.M. BHF grants (grant nos. CH/F/21/90010, RG/20/10/34966, and RE/18/5/34216) paid to author’s institution; payment or honoraria for lectures from Abbott Diagnostics and Siemens Healthineers; payment for participation on advisory boards for LumiraDX, Roche Diagnostics, and Siemens Healthineers; receipt of reagent from Siemens Healthineers to support research. P.J.S. Grants from Siemens Medical Systems and National Institutes of Health, paid to author’s institution; royalties or licenses from Cedars-Sinai Medical Systems, paid to author and author’s institution; consulting fees from IBA; several patents planned and pending, not related to this field of work, via Cedars-Sinai; vice-president of Society of Nuclear Medicine. D.E.N. Supported by the BHF (grant nos. CH/09/002, RG/16/10/32375, and RE/18/5/34216) and is the recipient of a Wellcome Trust Senior Investigator award (no. WT103782AIA); author is a co-applicant and co-author on the RAPID-CTA trial funded by the National Institute for Health (NIH) Research Health Technology Assessment Programme (no. 13/04/108) and the DEMAND-MI study funded by the BHF (FS/16/75/32533). D.D. NIH/National Heart, Lung, and Blood Institute grants (nos. 1R01HL148787-01A1 and 1R01HL151266); software royalties from Cedars-Sinai Medical Center, outside the current work. M.C.W. Supported by the BHF (grant no. FS/ICRF/20/26002); author has given talks for Canon Medical Systems and Siemens Healthineers; member of the board of directors of Society of Cardiovascular Computed Tomography; president-elect of the education committee of the British Society of Cardiovascular Imaging; president-elect of the European Society of Cardiovascular Radiology; Radiology: Cardiothoracic Imaging editorial board member.
Abbreviations:
- AIC
- Akaike information criteria
- CTCA
- CT coronary angiography
- DEMAND-MI
- Determining the Mechanism of Myocardial Injury and Role of Coronary Disease in Type 2 Myocardial Infarction
- RAPID-CTCA
- Rapid Assessment of Potential Ischemic Heart Disease with CT Coronary Angiography
References
- 1. Thygesen K , Alpert JS , Jaffe AS , et al . Fourth universal definition of myocardial infarction (2018) . Eur Heart J 2019. ; 40 ( 3 ): 237 – 269 . [DOI] [PubMed] [Google Scholar]
- 2. Sandoval Y , Jaffe AS . Type 2 Myocardial Infarction: JACC Review Topic of the Week . J Am Coll Cardiol 2019. ; 73 ( 14 ): 1846 – 1860 . [DOI] [PubMed] [Google Scholar]
- 3. Wereski R , Kimenai DM , Taggart C , et al . Cardiac troponin thresholds and kinetics to differentiate myocardial injury and myocardial infarction . Circulation 2021. ; 144 ( 7 ): 528 – 538 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chapman AR , Shah ASV , Lee KK , et al . Long-term outcomes in patients with type 2 myocardial infarction and myocardial injury . Circulation 2018. ; 137 ( 12 ): 1236 – 1245 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hung J , Roos A , Kadesjo E , et al . Performance of the GRACE 2.0 score in patients with type 1 and type 2 myocardial infarction . Eur Heart j 2021. ; 42 ( 26 ): 2552 – 2561 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meah MN , Singh T , Williams MC , et al . Reproducibility of quantitative plaque measurement in advanced coronary artery disease . J Cardiovasc Comput Tomogr 2021. ; 15 ( 4 ): 333 – 338 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Øvrehus KA , Schuhbaeck A , Marwan M , et al . Reproducibility of semi-automatic coronary plaque quantification in coronary ct angiography with sub-msv radiation dose . j Cardiovasc Comput Tomogr 2016. ; 10 ( 2 ): 114 – 120 . [DOI] [PubMed] [Google Scholar]
- 8. Virmani R , Burke AP , Kolodgie FD , Farb A . Vulnerable plaque: the pathology of unstable coronary lesions . J Interv Cardiol 2002. ; 15 ( 6 ): 439 – 446 . [DOI] [PubMed] [Google Scholar]
- 9. Chang HJ , Lin FY , Lee SE , et al . Coronary atherosclerotic precursors of acute coronary syndromes . J Am Coll Cardiol 2018. ; 71 ( 22 ): 2511 – 2522 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gray A , Roobottom C , Smith J , et al . Early computed tomography coronary angiography in patients with suspected acute coronary syndrome: randomised controlled trial . BMJ . ( 2021. ); 374 : n2106 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bularga A , Hung J , Daghem M , et al . Coronary artery and cardiac disease in patients with type 2 myocardial infarction: a prospective cohort study . Circulation 2022. ; 145 ( 16 ): 1188 – 1200 . [Published correction appears in Circulation 2022;145(16):e841.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gray AJ , Roobottom C , Smith JE , et al . The RAPID-CTCA trial (Rapid Assessment of Potential Ischaemic Heart Disease with CTCA) - a multicentre parallel-group randomised trial to compare early computerised tomography coronary angiography versus standard care in patients presenting with suspected or confirmed acute coronary syndrome: study protocol for a randomised controlled trial . Trials 2016. ; 17 ( 1 ): 579 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Celeng C , Vadvala H , Puchner S , et al . Defining the optimal systolic phase targets using absolute delay time for reconstructions in dual-source coronary CT angiography . Int J Cardiovasc Imaging 2016. ; 32 ( 1 ): 91 – 100 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tzolos E , McElhinney P , Williams MC , et al . Repeatability of quantitative pericoronary adipose tissue attenuation and coronary plaque burden from coronary CT angiography . J Cardiovasc Comput Tomogr 2021. ; 15 ( 1 ): 81 – 84 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dey D , Schepis T , Marwan M , Slomka PJ , Berman DS , Achenbach S . Automated three-dimensional quantification of noncalcified coronary plaque from coronary CT angiography: comparison with intravascular US . Radiology 2010. ; 257 ( 2 ): 516 – 522 . [DOI] [PubMed] [Google Scholar]
- 16. Raff GL , Abidov A , Achenbach S , et al . SCCT guidelines for the interpretation and reporting of coronary computed tomographic angiography . J Cardiovasc Comput Tomogr 2009. ; 3 ( 2 ): 122 – 136 . [DOI] [PubMed] [Google Scholar]
- 17. Leipsic J , Abbara S , Achenbach S , et al . SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee . J Cardiovasc Comput Tomogr 2014. ; 8 ( 5 ): 342 – 358 . [DOI] [PubMed] [Google Scholar]
- 18. Matsumoto H , Watanabe S , Kyo E , et al . Standardized volumetric plaque quantification and characterization from coronary CT angiography: a head-to-head comparison with invasive intravascular ultrasound . Eur Radiol 2019. ; 29 ( 11 ): 6129 – 6139 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Robin X , Turck N , Hainard A , et al . pROC: an open-source package for R and S+ to analyze and compare ROC curves . BMC Bioinformatics 2011. ; 12 : 77 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ruopp MD , Perkins NJ , Whitcomb BW , Schisterman EF . Youden Index and optimal cut-point estimated from observations affected by a lower limit of detection . Biom J 2008. ; 50 ( 3 ): 419 – 430 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heusch G , Gersh BJ . The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: a continual challenge . Eur Heart J 2017. ; 38 ( 11 ): 774 – 784 . [DOI] [PubMed] [Google Scholar]
- 22. Erlinge D , Maehara A , Ben-Yehuda O , et al . Identification of vulnerable plaques and patients by intracoronary near-infrared spectroscopy and ultrasound (PROSPECT II): a prospective natural history study . Lancet 2021. ; 397 ( 10278 ): 985 – 995 . [DOI] [PubMed] [Google Scholar]
- 23. McCarthy CP , Raber I , Chapman AR , et al . Myocardial injury in the era of high-sensitivity cardiac troponin assays: a practical approach for clinicians . JAMA Cardiol 2019. ; 4 ( 10 ): 1034 – 1042 . [DOI] [PubMed] [Google Scholar]
- 24. Chapman AR , Adamson PD , Shah ASV , et al . High-sensitivity cardiac troponin and the universal definition of myocardial infarction . Circulation 2020. ; 141 ( 3 ): 161 – 171 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ola O , Akula A , De Michieli L , et al . Clinical impact of high-sensitivity cardiac troponin t implementation in the community . J Am Coll Cardiol 2021. ; 77 ( 25 ): 3160 – 3170 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nestelberger T , Boeddinghaus J , Lopez-Ayala P , et al . Cardiovascular biomarkers in the early discrimination of type 2 myocardial infarction . JAMA Cardiol 2021. ; 6 ( 7 ): 771 – 780 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chapman AR , Adamson PD , Mills NL . Assessment and classification of patients with myocardial injury and infarction in clinical practice . Heart 2017. ; 103 ( 1 ): 10 – 18 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. DeFilippis AP , Chapman AR , Mills NL , et al . Assessment and treatment of patients with type 2 myocardial infarction and acute nonischemic myocardial injury . Circulation 2019. ; 140 ( 20 ): 1661 – 1678 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Neumann JT , Sörensen NA , Rübsamen N , et al . Discrimination of patients with type 2 myocardial infarction . Eur Heart J 2017. ; 38 ( 47 ): 3514 – 3520 . [DOI] [PubMed] [Google Scholar]
- 30. Wereski R , Kimenai DM , Bularga A , et al . Risk factors for type 1 and type 2 myocardial infarction . Eur Heart J 2022. ; 43 ( 2 ): 127 – 135 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heitner JF , Senthilkumar A , Harrison JK , et al . Identifying the infarct-related artery in patients with non-st-segment-elevation myocardial infarction . Circ Cardiovasc Interv 2019. ; 12 ( 5 ): e007305 . [DOI] [PubMed] [Google Scholar]
- 32. Stepinska J , Lettino M , Ahrens I , et al . Diagnosis and risk stratification of chest pain patients in the emergency department: focus on acute coronary syndromes. A position paper of the Acute Cardiovascular Care Association . Eur Heart J Acute Cardiovasc Care 2020. ; 9 ( 1 ): 76 – 89 . [DOI] [PubMed] [Google Scholar]
- 33. de Knegt MC , Linde JJ , Fuchs A , et al . Relationship between patient presentation and morphology of coronary atherosclerosis by quantitative multidetector computed tomography . Eur Heart J Cardiovasc Imaging 2019. ; 20 ( 11 ): 1221 – 1230 . [DOI] [PubMed] [Google Scholar]
- 34. Hong Y , Commandeur F , Cadet S , et al . Deep learning-based stenosis quantification from coronary CT angiography . In: Angelini ED , Landman BA , eds . Proceedings of SPIE: medical imaging 2019—image processing . Vol 10949 . Bellingham, Wash: : International Society for Optics and Photonics; , 2019. ; 109492I . [DOI] [PMC free article] [PubMed] [Google Scholar]