Abstract
Immunological dysfunctions in eating disorders have recently gained increasing scientific attention. Furthermore, the reciprocal association between anorexia and autoimmune diseases is of particular interest and suggests a role of autoimmunity in the pathogenesis of eating disorders. Anorexia nervosa (AN) and autoimmune diseases are linked by a bidirectional relationship based on common immunopathological mechanisms. In this review, in addition to reporting the numerous cases described in which autoimmune disorders are associated with anorexia or vice versa, we summarize the many aspects of this relationship between the immune system (IS) and AN. We describe how the microbiota affects the IS, disrupts gut‐brain communication, and possibly triggers eating disorders. We also describe the shared immunological pathways of autoimmune and eating disorders and in particular the occurrence of disrupted T cell tolerance and autoantibodies in AN. The described observations represent the starting point for possible, future research directions.
Keywords: anorexia, autoimmunity, immunity, microbiome, neuropsychoimmunology
Anorexia nervosa and autoimmune diseases are linked by a bidirectional relationship based on common immunopathological mechanisms. A possible link is the intricate mechanisms that intertwine the gut microbiome, nervous system and immune system: microbiota affects the immune system and disrupts the gut‐brain communication.
1. INTRODUCTION
Anorexia nervosa (AN) is a worrying and severe psychiatric disease diagnosed when these three conditions are met: significantly low body weight in relation to age and sex, developmental trajectory and physical health of the patient, and an intense fear of weight gain and a disturbed body perception. 1 Medical complications affecting all body systems are frequently associated with increased mortality. 2 In adolescence, AN is the third most frequent chronic disturbance and has the highest mortality among all psychiatric conditions. The disease occurs worldwide among males and females of all ages and is associated with a mortality risk increased by five times or more. Its overall incidence over the past decades is considered stable. In persons aged <15 years incidence has increased, but it is unclear whether this increase depends on earlier age of onset or earlier detection. AN has a lifetime prevalence rate of up to 0.3% among males and 4% among females 3 and is also associated with a high incidence of coexisting psychiatric conditions, treatment resistance, and suicidal risk. 4 We still know little about the exact etiology of AN and this limits the possibility of developing new therapeutic approaches. Understanding the biological correlates of the disease that contribute to the development and maintenance of the disorder is crucial for possible targeted interventions. 5 , 6 , 7 , 8 , 9 A new unifying vision of eating disorders comes to us from psycho‐neuro‐immuno‐endocrinology, which integrates knowledge derived from the psychological and biomedical sciences. 10
Psychological theories of anorexia reigned for generations, 11 but now researchers are working to untangle the biology of the disease and a succession of discoveries suggests that the biological roots of the disease run deep. Curiously, some psychiatric disorders have been linked to excessive activation of the immune system (IS). The release of soluble inflammatory mediators and the mobilization of immune cells can also be stimulated by adequate psychological stressors. 12 Both functional and structural brain alterations 13 such as brain volume loss, astrocyte reduction, and inflammation 14 are commonly related to AN. We have a lot of evidence of how the IS, along with neurochemical and hormonal responses, can significantly influence central nervous system (CNS) stress circuitry, especially through inflammation molecules. It is intuitive that the brain and the IS are the ones mainly responsible for regulating relations with the external environment and have evolved together with the aim of preserving the internal homeostasis of the organism. Therefore, these are systems strictly interconnected in a bidirectional way allowing a balanced and safe relationship of the organism with the environment. 12 AN is associated with a mild inflammatory state, alterations in the immune response, the production of autoantibodies, and occurs during autoimmune diseases, but this is also true in the opposite sense: those affected by autoimmune disorders present similar pathophysiological conditions and can develop anorexia. The aim of our work is to bring together the current knowledge on the neuroimmune interaction in AN with new interesting therapeutic perspectives as opened up by the hypothesis of autoimmune mechanisms.
2. THE BIDIRECTIONAL RELATIONSHIP BETWEEN THE IS AND AN
The existence of a relationship between IS and eating disorders is indubitable and many studies have dealt with it, 10 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 however, these have several limitations, their results are often conflicting and many aspects are still to be investigated. The IS is dysregulated in anorexic patients as strongly suggested by the proinflammatory cytokines profile and other markers of central and peripheral inflammation. Inflammatory and autoimmune diseases are interestingly associated with the risk of developing bulimic syndromes. The relationship between IS and nutrition can be studied from two opposite points of view. This relationship was initially investigated by considering the effect on the IS of an inadequate nutritional intake, but subsequently, it became evident that conditions of primary or secondary dysregulation of the immune state (such as in the course of infections or autoimmune diseases) induce anorexia. Malnutrition deprives the IS of several components essential to generating an effective immune response. 17 Immunocompetence and nutritional status are closely linked. 18 The most consistent alterations are observed in the phagocyte function, complement system, cytokine production, mucosal secretory antibody response, antibody affinity, and cell‐mediated immunity. 17 Immune changes and increased risk of infection seem to be associated with chronicity of AN. 19 Immune system changes in AN (Figure 1) were reported to be not so severe as those observed in malnutrition secondary to somatic diseases. 20 Leukopenia, polycythemia, and thrombocytopenia are reported in adolescent and young adult males with AN. 30 Peripheral blood (PB) of anorexic subjects shows elevated levels of proinflammatory cytokines. A mild inflammatory status characterizes AN with an increase of tumor necrosis factor (TNF), interleukin(IL)‐1α, and IL‐6 5 together with modulation of cellular components of the adaptative and innate IS. Interleukin‐6 and other cytokines have a leading role in the anorexia of aging, linked to apoptosis. 31 , 32 Recently Tyszkiewicz‐Nwaforet al. showed that the fasting levels of interleukins‐1 (IL‐1), 6 (IL‐6), and 8 (IL‐8), C‐X‐C Motif Chemokine Ligand 1 (CXCL1), C‐X‐C Motif Chemokine Ligand 9 (CXCL9), metalloproteinases 8 (MMP8) and 9 (MMP9), cathepsin S (CTSS), fibroblast growth factor 2 (FGF2), and granzyme B (GrB) are diminished in the initial phase of restrictive AN in adolescents in the first year of the disease. 20 These data suggest the absence of a pro‐inflammatory state in these subjects. Furthermore, they show that some immune‐related protein alterations may be associated with changed neuropeptides, primarily leptin, its receptors, and resistin. NK cells and neutrophils are reduced in numbers while neutrophilic chemotaxis and adherence are impaired. PB CD4/CD8 T cell ratios are increased as CD8+ T cells show to be diminished among overall T cells. 21 , 22 No changes in the proportion of regulatory T cells (Tregs) are described in AN. Studies on T cell proliferation do not show univocal data as it can be equivalent, increased, or reduced, depending on the mitogens used. Regarding the number and percentage of B cells in anorexic subjects, there do not seem to be significant variations compared to healthy controls. Only Elegido et al. 21 reported an increase of B cells in anorexic adolescents, but with comparable percentages. We can learn more about the cellular B compartment by studying the composition of the B cell compartment on the basis of maturation immunophenotype. The efficiency of the IS in subjects suffering from AN has also been studied through the evaluation of the response to vaccines, 33 but no significant differences emerged with respect to the normal weight population both with respect to the flu vaccine 34 and H1N1 vaccination. 35 Subsequently, the view from the opposite perspective developed, that is how immunological alterations could generate anorexia, known to be one of the most characteristic symptoms in the course of several diseases, derived mainly from pharmacological treatment and inflammatory processes. 23 Even if we are still very far from understanding them, there is more and more evidence suggesting a relevant role of immunological mechanisms in the genesis of AN. A potential primary immunologic defect contributing to the development of AN is conceivable. 36 Control of the immune function of the neuroendocrine system is recognized and several studies support the relevance of immunoendocrine factors in the aetiopathogenesis of AN. 24 The crucial role of the interaction between neurotransmitters, neuropeptides, and cytokines in the pathogenesis of AN has been suggested since 1996. 25 Increased risk of developing eating disorders in association with infections has been shown. Anorexia can initially be part of the body's defensive armamentarium and then becomes an independent disease. The remodeling of the intestinal microbiota is among the pathophysiological hypotheses. 26 It seems that the dysbiosis associated with infections is similar to that observed in the course of AN, however, it is yet to be defined whether the infection represents a significant trigger along with others in subjects predisposed for genetic and/or epigenetic reasons. 27 Changes in neurotransmitters, neuropeptides, and cytokines and their interactions have a leading role in the pathophysiology of anorexia. The fundamental role of cytokines in the modulation of inflammation and controlling infections is acknowledged, as well as in the regulation of neurotransmitter systems, neuroplasticity, and neuroendocrine functioning. 20 , 28 In conclusion, a dysregulated IS seems characteristic of AN while it is becoming increasingly intriguing to consider that the IS may actually be causal in the pathogenesis and maintenance of AN.
FIGURE 1.
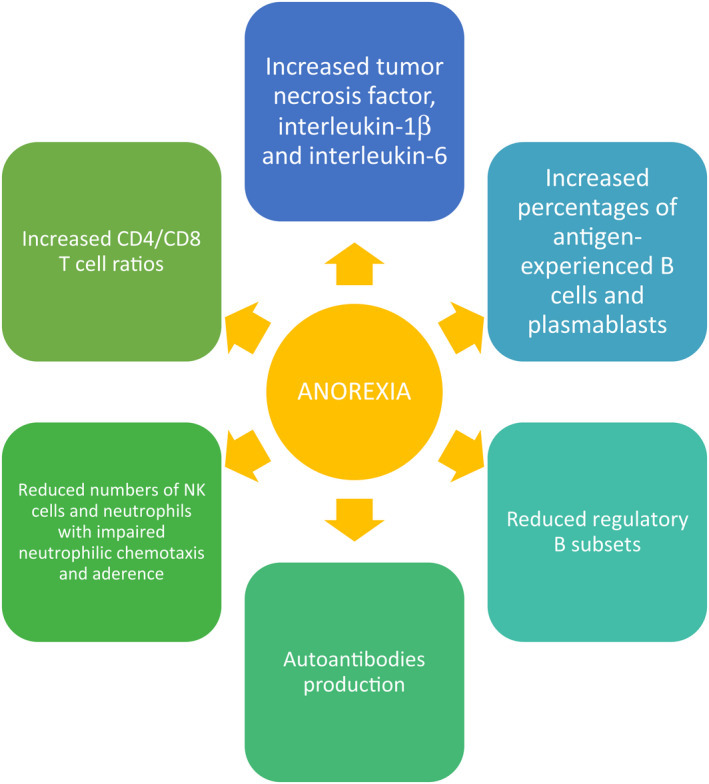
Immune changes associated with anorexia nervosa
3. THE MICROBIOTA AFFECTS THE IS, DISRUPTS THE GUT‐BRAIN COMMUNICATION, AND POSSIBLY TRIGGERS EATING DISORDERS
The gut microbiome has a leading role in regulating metabolism, gastrointestinal symptomatology, appetite, behavior, and mood, and one of healthy individuals, may diverge from that of AN patients for a number of potential avenues. 37 The intricate mechanisms that intertwine the gut microbiome, nervous system, and IS are implicated in the pathophysiology of eating disorders. 29 Recent studies suggested that the brain‐gut‐microbiota axis is a complex system that plays a crucial role in the occurrence and development of central nervous system diseases. 38 Through several interactions, the microbiota may impact both autoimmune diseases and AN. These include innate and adaptive immunity, epigenetic alteration of protein expression, proteostasis, hematopoiesis, coagulation, hormone secretion, autonomic nerve system, and metabolism. 39 Several observations demonstrated that via the gut‐brain axis the microbiota acts on the neurobiological, inflammatory, and immune pathways implicated in AN and comorbidities. The synthesis of neurotransmitters by the intestinal microflora is essential in the gut‐brain interplay. The study of microbiota is a research field of increasing interest and it was shown that a contribution to the development of AN could come from the association of a specific genetic susceptibility with dysbiosis. Perturbations of the intestinal microbiota that could represent disease biomarkers have been identified in multiple psychiatric disorders. 40 Furthermore, recent stroke research suggested that a specific set of inflammatory microbiota exists in central and peripheral organs and can serve as a disease biomarker and a therapeutic target. 41 In the pathophysiology of AN, dysbiosis disrupts proper communication between the gut and the brain, promoting gut inflammation, altering intestinal permeability, and triggering immune reactions in the regulation center of hunger/satiety. Microbiota changes alter the hunger/satiety regulatory system, mood, and the hypothalamic–pituitary–adrenalaxis. The gut microbiota changes significantly in quantity, quality, and composition in subjects with AN following the changes in weight. Assimilation and accumulation of calories from food and changes in the IS are a consequence of the reduced intestinal microbial biodiversity. 42 Lower alpha diversity is observed during weight loss with the increase of Enterobacteriaceae, Actinobacteria, Methanobrevibactersmithii, and Bacteroides and a reduction of short‐chain fatty acids (SCFAs) and Firmicutes. Weight recovery corresponds an increase of SCFAs and recovery of the Firmicutes/Bacteroides ratio. 43 Interestingly, Escherichia coli produces large quantities of caseinolytic peptidase B (ClpB) which has a role in the stimulation and autoimmune response, 44 , 45 capable of modifying feeding behavior by acting on the melanocortin system. In AN, altered microbiota produces neuropeptide‐like proteins able to stimulate endogenous satiety and appetite hormones and induce an immunoglobulin cross‐reaction. This is the case of autoantibodies against the α‐melanocyte‐stimulating hormone (α‐MSH) acting directly on the arcuate nucleus and on the regulation of appetite. The increase in anti‐α‐MSH antibodies observed in subjects with AN correlates with psychopathological symptoms and high stress. 43 Changes in the gut microbiome and IS can serve as biomarkers of increased risk of developing AN as well as maintaining and exacerbating incorrect eating habits. 46 The role of the microbiota and its action on the IS in the pathogenesis of various diseases is ultimately evident. Changes in the microbiota and some specific immunological profiles can become valuable biomarkers of disease and useful therapeutic targets.
4. DISRUPTED T CELL TOLERANCE AND AUTOANTIBODIES IN AN
A recent study, showing the presence of immune dysregulation in psychiatric disorders, speculates on the role of autoimmunity in these conditions. In particular, it was underlined as eating disorders, similar to several other psychiatric conditions, are associated with the presence of autoantibodies. 15 , 47 A relatively large number of brain antigens are implicated in autoimmune diseases 48 and this suggests evidence of a possible co‐evolution of IS and CNS. Thus, in several autoimmune diseases including rheumatoid arthritis, psoriasis, multiple sclerosis or diabetes, enzymes or receptors for the neurotransmitters GABA (GAD65) and acetylcholine (AchR) are targeted, as well as tyrosine hydroxylase, laminin, and myelin. It is intriguing that only a small subset of an enormity of possible antigens are recognized to be responsible for such autoimmune conditions and that it is easy to find them in large amounts in the CNS. It can be hypothesized that many of these super autoantigens capable of overcoming the regular inhibitory limitations of the IS are hosted in the CNS and it is suggestive to think that this is due to the evolutionary pressures that have bi‐directionally shaped the immunity‐brain cooperation. It is also conceivable that, at least in part, our communities, increasingly interconnected, and our growing social ties have driven the co‐evolution of the IS and of the brain, while the unmet social needs represent some of the new stressors of our psychosocial world. The increase in immunological defensive strategies and their complexity has also increased the possibility of weaknesses for the onset of possible complex pathologies. Acres MJ et al. 49 have suggested autoimmunity underlying AN. They hypothesized the production of autoantibodies directed against regulatory peptides and hypothalamic neurons, cross‐reacting with microbial antigens, due to a delayed exposure to common microorganisms. These autoantibodies would induce an appetite disorder with reduced food intake. In subjects with AN, the presence of IgG, IgA, and IgM autoantibodies against several appetite‐regulating peptides is detected. The latter show antigenic homologies with the gut microbiome and a positive correlation were found between AN and the presence of autoantibodies to alpha‐melanocyte stimulating hormone (α‐MSH). IgM antibody levels against α‐MSH are increased in patients with eating disorders compared to controls. 16 However, the study reports the lack of data on the prevalence in adults with eating disorders of cerebrospinal fluid/serum autoantibodies. 15 So the regulation of eating behavior depends on the gut‐brain‐adipose tissue (AT) peptides and the presence of neutralizing autoantibodies may result in AN. 50 Psychiatric disorders have been associated with the presence of neural autoantibodies, although the significance of this association often remains difficult to explain. 15 , 51 Autoantibodies, directed against key appetite‐regulating peptide hormones or neuropeptides, were found in healthy subjects while psychopathological traits of people affected by eating disorders correlate with the amount and affinities of autoantibodies against anorexigenic and orexigenic neuropeptides. The development of AN could be triggered by access to the brain centers of these high‐affinity autoantibodies. 52 Furthermore, an immuno‐reactivity toward primate hypothalamic neurons in serum from AN subjects was found by Escelsior et al. 53 Diet has an impact on autoimmunity. 54 Increased gut permeability to food antigens and the potential reduction of oral tolerance could justify the presence of a mild inflammatory state and the increased possibility of developing autoimmunity. 19 Even small alterations in the intrinsic signaling programs of B lymphocytes are sufficient to trigger a systemic autoimmune process through the disruption of T cell tolerance. The increase in autoreactive mature B lymphocytes is a consequence of altered signaling programs of B cells that modulate their negative or positive selection. 55 AN patients show increased percentages of antigen‐experienced B cells and plasmablasts while regulatory B cell subgroups are reduced. Furthermore, these latter cells have a strong relationship with body composition suggesting their leading role in the immunopathogenetic mechanism of AN. These alterations in B lymphocyte subsets may at least partially explain the production of autoantibodies. 19
5. THE SHARED IMMUNOLOGICAL PATHWAYS OF AUTOIMMUNE AND EATING DISORDERS
A high prevalence of autoimmune diseases is reported among patients with eating disorders. 53 Raevuori et al. 56 believe that shared immunological pathways link autoimmune and eating diseases (Figure 2) and that, at least in a subset of subjects, autoimmunity triggers and maintains the eating disorder. The association between various autoimmune diseases and anorexia is described (Table 1). Hyla‐Klekot et al. 57 described a case of associated anorexia and juvenile systemic lupus erythematosus. Like other authors before them, they suggest a common origin of the two diseases on the basis of their coexistence and the resolution of anorexia as a consequence of the immunosuppressive treatment. Other similar cases have been described in the past and AN was considered a possible onset manifestation of lupus erythematosus. 58 , 59 , 60 , 61 Anorexia could also be one of the presenting symptoms of lupus with gastrointestinal involvement. 62 Celiac and Inflammatory bowel diseases may trigger the development of eating disorders. 63 , 64 , 65 Coexistence of Hashimoto's thyroiditis and AN are also reported. 66 , 67 Dell'Osso et al. 68 recently reported the association of Behçet's Syndrome with AN.
FIGURE 2.
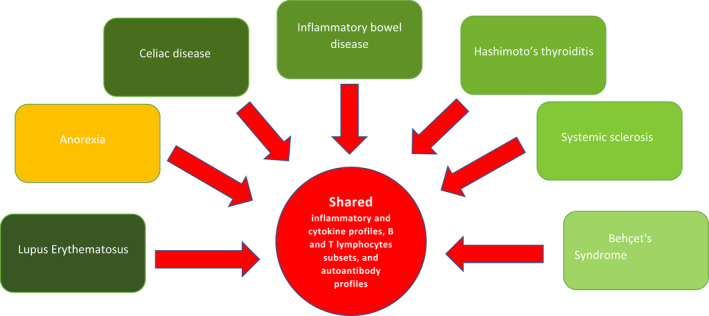
Common immunological changes of AN with autoimmune diseases. The link between eating disorders and autoimmune diseases is based on shared immunological mechanisms: inflammatory and cytokine profiles, B and T lymphocytes subsets, and autoantibody profiles. Moreover, the interplay between the gut microbiome, immune regulation, and sex hormones thus provides one potential, the complex mechanism underlying eating disorders and explains the partly shared etiopathogenesis of eating disorders and autoimmune diseases.
TABLE 1.
The references listed in the table highlight the association between various autoimmune diseases and anorexia
| Hyla‐Klekot L. et al. | Anorexia nervosa and juvenile lupus erythematosus in a 16‐year‐old female patient—common disease origin or random coincidence? | Cent Eur J Immunol | 2021 |
| Toulany A. et al. | Chicken or the egg: anorexia nervosa and systemic lupus erythematosus in children and adolescents. | Pediatrics | 2014 |
| Bambery P. et al. | Anorexia nervosa in a patient with systemic lupus erythematosus. | Rheumatol Int | 1987 |
| Sloan D. et al. | Anorexia nervosa complicates systemic lupus erythematosus (SLE). | Ir Med J | 1998 |
| Dalbeth N. and Callan M. | Arthritis and anorexia? | Lancet | 2002 |
| Trapani S. et al. | Gastrointestinal and hepatic involvement in pediatric systemic lupus erythematosus. | Clin Exp Rheumatol | 2021 |
| Tokatly Latzer I. et al. | Disordered eating behaviors in adolescents with celiac disease. | Eat Weight Disord | 2020 |
| Ilzarbe L. et al. | Inflammatory bowel disease and eating dsisorders: A systematized review of comorbidity. | J Psychosom Res | 2017 |
| Blanchet C. and Luton JP. | Anorexie mentale et maladie de Crohn: intrications et difficultés diagnostiques. Trois cas [Anorexia nervosa and Crohn disease: diagnostic intricacies and difficulties. 3 cases]. | Presse Med | 2002 |
| Pehlivantürk Kızılkan M. et al. | An adolescent boy with comorbid anorexia nervosa and hashimoto thyroiditis. | J Clin Res Pediatr Endocrinol | 2016 |
| Smalls‐Mantey A. et al. | Hypothyroidism due to Hashimoto's thyroiditis masked by anorexia nervosa. | Int J Eat Disord | 2015 |
| De Martinis M. et al. | Raynaud's phenomenon and nailfold capillaroscopic findings in anorexia nervosa. | Curr Med Res Opin | 2018 |
| Dell'Osso L. et al. | Subthreshold autism spectrum in a patient with anorexia nervosa and Behçet's syndrome. | Case Rep Psychiatry | 2020 |
AN patients with Raynaud's phenomenon could show an early scleroderma pattern by studying their microcirculation by videocapillaroscopy. 69 , 70 Association of scleroderma and anorexia have been reported 71 and the presence of a common clinical and capillaroscopic picture between systemic sclerosis 72 and AN is very intriguing. These observations strengthen the hypothesis of shared pathogenetic mechanisms that could also trigger each other. These disease associations can also lead to misdiagnosis. In both conditions, AN and autoimmune diseases, inflammatory and cytokine profiles, B and T lymphocyte subsets, and common autoantibody profiles are reported. Several other shared factors, such as being a female, abnormal levels of estrogen preponderance, metabolic changes mediated by adipokines such as leptin and adiponectin, elevated cytokines, and lower abundance or diversity of intestinal microbiota, potentially influence the relationship between AN and autoimmunity. 73 , 74 , 75 Furthermore, cortisol levels are dysregulated in AN and the same molecule is often included in the therapeutic regimen for autoimmune diseases. Moreover, a genetic overlap was suggested between psychiatric disorders and several autoimmune disturbances. 76 Dysregulation of the inflammatory system characterizes autoimmune diseases and AN, with an increase of pro‐inflammatory cytokines. In the latter, an alteration of the neurotrophic system is also observed. 77 All the cases described above, support the hypothesis of common pathophysiology between autoimmune diseases and eating disorders, which represent a mutual alert and each confers an increased risk for the other. 78 During the COVID‐19 pandemic, there was a significant increase in the number of cases of AN 79 , 80 , 81 and also in the development of autoimmune phenomena. 82 , 83 , 84 Similar to other viruses, SARS‐CoV‐2 seems capable of triggering autoimmune reactions. 85 The dysregulation of the IS induced by COVID‐19 induces the development of autoimmune phenomena even if related mechanisms are not yet known. 86 Without diminishing the importance of psychosocial factors, it could therefore be hypothesized that this increase in the burden of EDs could also be linked to the increase in self‐reactivity of an imbalanced IS as a consequence of the infection with SARS‐CoV‐2.
6. CONCLUSIONS
AN and autoimmune diseases are linked by a bidirectional relationship based on common immunopathological mechanisms (Figure 3). There is evidence that those suffering from autoimmune diseases can develop anorexia and vice versa it would seem that those suffering from AN could develop an autoimmune disease. The presence of a mild inflammatory state, the alterations of the immune response, and the production of autoantibodies would be the common pathogenetic substrate for these conditions. It is an intriguing topic full of suggestions. We summarized and highlighted current knowledge to provide impulse and amplify the stimulus for new research. To date there is a complete lack of systematic studies able to precisely define the common immunopathogenetic mechanisms and the links between autoimmunity and eating disorders and establish the mutual risk of passing from one disease to the other. Several data further encourage a reconceptualization of AN as a psycho‐neuro‐endocrine‐immune disorder. Elucidating the immune component is a critical direction for future research, and paying attention to both neuro‐psychiatric and immune components may be key to improving outcomes.
FIGURE 3.
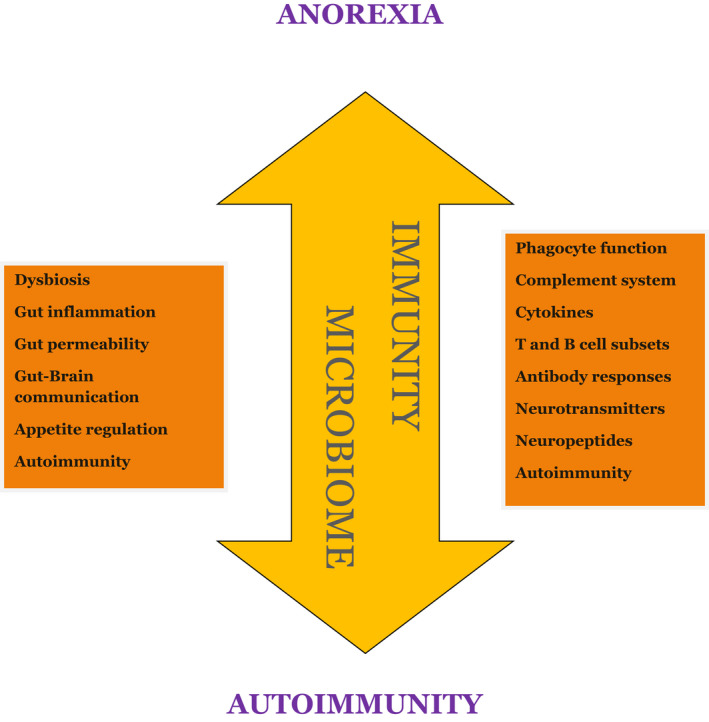
There is a bidirectional relationship between autoimmunity and anorexia nervosa: autoimmune diseases and eating disorders give each other a mutual increase in risk. Altered immunity and dysbiosis have leading roles in these processes.
AUTHOR CONTRIBUTIONS
M.M.S., L.M.M, L.G., and M.D.M. contributed equally to the work. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
ACKNOWLEDGMENTS
The authors would like to thank Prof. Andrea Dean Bucci for her English language assistance.
Sirufo MM, Magnanimi LM, Ginaldi L, De Martinis M. Anorexia nervosa and autoimmune comorbidities: A bidirectional route? CNS Neurosci Ther. 2022;28:1921‐1929. doi: 10.1111/cns.13953
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Mitchell JE, Peterson CB. Anorexia nervosa. N Engl J Med. 2020;382(14):1343‐1351. doi: 10.1056/NEJMcp1803175 [DOI] [PubMed] [Google Scholar]
- 2. Gibson D, Workman C, Mehler PS. Medical complications of anorexia nervosa and bulimia nervosa. Psychiatr Clin North Am. 2019;42(2):263‐274. doi: 10.1016/j.psc.2019.01.009 [DOI] [PubMed] [Google Scholar]
- 3. van Eeden AE, van Hoeken D, Hoek HW. Incidence, prevalence and mortality of anorexia nervosa and bulimia nervosa. Curr Opin Psychiatry. 2021;34(6):515‐524. doi: 10.1097/YCO.0000000000000739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Westmoreland P, Krantz MJ, Mehler PS. Medical complications of anorexia nervosa and bulimia. Am J Med. 2016;129(1):30‐37. doi: 10.1016/j.amjmed.2015.06.031 [DOI] [PubMed] [Google Scholar]
- 5. Dalton B, Leppanen J, Campbell IC, et al. A longitudinal analysis of cytokines in anorexia nervosa. Brain Behav Immun. 2020;85:88‐95. doi: 10.1016/j.bbi.2019.05.012 [DOI] [PubMed] [Google Scholar]
- 6. Hay P. Current approach to eating disorders: a clinical update. Intern Med J. 2020;50(1):24‐29. doi: 10.1111/imj.14691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gorrell S, Murray SB. Eating disorders in males. Child Adolesc Psychiatr Clin N Am. 2019;28(4):641‐651. doi: 10.1016/j.chc.2019.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Samuels KL, Maine MM, Tantillo M. Disordered eating, eating disorders, and body image in midlife and older women. Curr Psychiatry Rep. 2019;21(8):70. doi: 10.1007/s11920-019-1057-5 [DOI] [PubMed] [Google Scholar]
- 9. Mangweth‐Matzek B, Hoek HW. Epidemiology and treatment of eating disorders in men and women of middle and older age. Curr Opin Psychiatry. 2017;30(6):446‐451. doi: 10.1097/YCO.0000000000000356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sirufo MM, Ginaldi L, De Martinis M. PeripheralVascularAbnormalities in anorexia nervosa: a psycho‐neuro‐immune‐metabolic connection. Int J Mol Sci. 2021;22(9):5043. doi: 10.3390/ijms22095043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Couzin‐Frankel J. Rethinking anorexia. Science. 2020;368(6487):124‐127. doi: 10.1126/science.368.6487.124 [DOI] [PubMed] [Google Scholar]
- 12. Hayley S, Sun H. Neuroimmune multi‐hit perspective of coronaviral infection. J Neuroinflammation. 2021;18(1):231. doi: 10.1186/s12974-021-02282-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Jorge MC, Rukh G, Williams MJ, Gaudio S, Brooks S, Schiöth HB. Genetics of anorexia nervosa: an overview of genome‐wide association studies and emerging biological links. J Genet Genomics. 2022;49(1):1‐12. doi: 10.1016/j.jgg.2021.09.005 [DOI] [PubMed] [Google Scholar]
- 14. Seitz J, Trinh S, Kogel V, Beyer C. Brain volume loss, astrocyte reduction, and inflammation in anorexia nervosa. Adv Neurobiol. 2021;26:283‐313. doi: 10.1007/978-3-030-77375-5_12 [DOI] [PubMed] [Google Scholar]
- 15. Hansen N, Lipp M, Vogelgsang J, et al. Autoantibody‐associated psychiatric symptoms and syndromes in adults: a narrative review and proposed diagnostic approach. Brain Behav Immun. Health. 2020;9:100154. doi: 10.1016/j.bbih.2020.100154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fetissov SO, Harro J, Jaanisk M, Järv A, Podar I, Allik J. Autoantibodies against neuropeptides are associated with psychological traits in eating disorders. Proc Natl Acad Sci USA. 2005;102:14865‐14870. doi: 10.1073/pnas.0507204102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marcos A, Nova E, Montero A. Changes in the immune system are conditioned by nutrition. Eur J Clin Nutr. 2003;57(Suppl 1):S66‐S69. doi: 10.1038/sj.ejcn.1601819 [DOI] [PubMed] [Google Scholar]
- 18. Montero A, López‐Varela S, Nova E, Marcos A. The implication of the binomial nutrition‐immunity on sports women's health. Eur J Clin Nutr. 2002;56(Suppl 3):S38‐S41. doi: 10.1038/sj.ejcn.1601483 [DOI] [PubMed] [Google Scholar]
- 19. Freff J, Schwarte K, Bröker L, et al. Alterations in B cell subsets correlate with body composition parameters in female adolescents with anorexia nervosa. Sci Rep. 2021;11(1):1125. doi: 10.1038/s41598-020-80693-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tyszkiewicz‐Nwafor M, Jowik K, Paszynska E, Dutkiewicz A, Słopien A, Dmitrzak‐Weglarz M. Expression of immune‐related proteins and their association with neuropeptides in adolescent patients with anorexia nervosa. Neuropeptides. 2021;27(91):102214. doi: 10.1016/j.npep.2021.102214 [DOI] [PubMed] [Google Scholar]
- 21. Elegido A, Graell M, Andrés P, Gheorghe A, Marcos A, Nova E. Increased naive CD4+ and B lymphocyte subsets are associated with body mass loss and drive relative lymphocytosis in anorexia nervosa patients. Nutr Res. 2017;39:43‐50. doi: 10.1016/j.nutres.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 22. Mustafa A, Ward A, Treasure J, Peakman M. T lymphocyte subpopulations in anorexia nervosa and refeeding. Clin Immunol Immunopathol. 1997;82:282‐289. doi: 10.1006/clin.1996.4310 [DOI] [PubMed] [Google Scholar]
- 23. López Plaza B, Bermejo López LM. Nutrición y trastornos del sistema inmune [Nutrition and immune system disorders]. Nutr Hosp. 2017;34(Suppl 4):68‐71. doi: 10.20960/nh.1575 [DOI] [PubMed] [Google Scholar]
- 24. Słotwińska SM, Słotwiński R. Immune disorders in anorexia. Cent Eur J Immunol. 2017;42(3):294‐300. doi: 10.5114/ceji.2017.70973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holden RJ, Pakula IS. The role of tumor necrosis factor‐alpha in the pathogenesis of anorexia and bulimia nervosa, cancer cachexia and obesity. Med Hypotheses. 1996;47(6):423‐438. doi: 10.1016/s0306-9877(96)90153-x [DOI] [PubMed] [Google Scholar]
- 26. Sirufo MM, De Pietro F, Catalogna A, Ginaldi L, De Martinis M. The microbiota‐bone‐allergy interplay. Int J Environ Res Public Health. 2021;19(1):282. doi: 10.3390/ijerph19010282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Galmiche M, Achamrah N, Déchelotte P, Ribet D, Breton J. Role of microbiota‐gut‐brain axis dysfunctions induced by infections in the onset of anorexia nervosa. Nutr Rev. 2021;80:381‐391. doi: 10.1093/nutrit/nuab030 [DOI] [PubMed] [Google Scholar]
- 28. Vezzani A, Viviani B. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology. 2015;96(Pt A):70‐82. doi: 10.1016/j.neuropharm.2014.10.027 [DOI] [PubMed] [Google Scholar]
- 29. Butler MJ, Perrini AA, Eckel LA. The role of the gut microbiome, immunity, and neuroinflammation in the pathophysiology of eating disorders. Nutrients. 2021;13(2):500. doi: 10.3390/nu13020500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. J Silla KE, Brigham KS, Goldstein M, Misra M, Singhal V. Clinical, biochemical, and hematological characteristics of community‐dwelling adolescent and young adult males with anorexia nervosa. Int J Eat Disord. 2021;54(12):2213‐2217. doi: 10.1002/eat.23622 [DOI] [PubMed] [Google Scholar]
- 31. Roubenoff R. Catabolism of aging: is it an inflammatory process? Curr Opin Clin Nutr Metab Care. 2003;6(3):295‐299. doi: 10.1097/01.mco.0000068965.34812.62 [DOI] [PubMed] [Google Scholar]
- 32. De Martinis M, Franceschi C, Monti D, Ginaldi L. Apoptosis remodeling in immunosenescence: implications for strategies to delay ageing. Curr Med Chem. 2007;14(13):1389‐1397. doi: 10.2174/092986707780831122 [DOI] [PubMed] [Google Scholar]
- 33. Xiao K, Gillissie ES, Lui LMW, et al. Immune response to vaccination in adults with mental disorders: a systematic review. J Affect Disord. 2022;304:66‐77. doi: 10.1016/j.jad.2022.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Armstrong‐Esther CA, Lacey JH, Crisp AH, Bryant TN. An investigation of the immune response of patients suffering from anorexia nervosa. Postgrad Med J. 1978;54(632):395‐399. doi: 10.1136/pgmj.54.632.395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zastrow A, Schnitzler P, Eckerle I, Herzog W, Friederich HC. Immunogenicity and safety of H1N1 vaccination in anorexia nervosa–results from a pilot study. Int J Eat Disord. 2012;45(1):146‐149. doi: 10.1002/eat.20908 [DOI] [PubMed] [Google Scholar]
- 36. Gibson D, Mehler PS. Anorexia nervosa and the immune system‐a narrative review. J Clin Med. 2019;8(11):1915. doi: 10.3390/jcm8111915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ruusunen A, Rocks T, Jacka F, Loughman A. The gut microbiome in anorexia nervosa: relevance for nutritional rehabilitation. Psychopharmacology (Berl). 2019;236(5):1545‐1558. doi: 10.1007/s00213-018-5159-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li XJ, You XY, Wang CY, et al. Bidirectional brain‐gut‐microbiota axis in increased intestinal permeability induced by central nervous system injury. CNS Neurosci Ther. 2020;26(8):783‐790. doi: 10.1111/cns.13401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tonomura S, Ihara M, Friedland RP. Microbiota in cerebrovascular disease: a key player and future therapeutic target. J Cereb Blood Flow Metab. 2020;40(7):1368‐1380. doi: 10.1177/0271678X20918031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nikolova VL, Hall MRB, Hall LJ, Cleare AJ, Stone JM, Young AH. Perturbations in gut microbiota composition in psychiatric disorders: a review and meta‐analysis. JAMA Psychiatry. 2021;78(12):1343‐1354. doi: 10.1001/jamapsychiatry.2021.2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kingsbury C, Shear A, Heyck M, et al. Inflammation‐relevant microbiome signature of the stroke brain, gut, spleen, and thymus and the impact of exercise. J Cereb Blood Flow Metab. 2021;41(12):3200‐3212. doi: 10.1177/0271678X211039598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roubalová R, Procházková P, Papežová H, Smitka K, Bilej M, Tlaskalová‐Hogenová H. Anorexia nervosa: gut microbiota‐immune‐brain interactions. Clin Nutr. 2020;39(3):676‐684. doi: 10.1016/j.clnu.2019.03.023 [DOI] [PubMed] [Google Scholar]
- 43. Carbone EA, D'Amato P, Vicchio G, De Fazio P, Segura‐Garcia C. A systematic review on the role of microbiota in the pathogenesis and treatment of eating disorders. Eur Psychiatry. 2020;64(1):e2. doi: 10.1192/j.eurpsy.2020.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Breton J, Legrand R, Akkermann K, et al. Elevated plasma concentrations of bacterial ClpB protein in patients with eating disorders. Int J Eat Disord. 2016;49:805‐808. doi: 10.1002/eat.22531 [DOI] [PubMed] [Google Scholar]
- 45. Fetissov SO, Legrand R, Lucas N. Bacterial protein mimetic of peptide hormone as a new class of protein‐based drugs. Curr Med Chem. 2019;26:546‐553. doi: 10.2174/0929867324666171005110620 [DOI] [PubMed] [Google Scholar]
- 46. Metwaly A, Reitmeier S, Haller D. Microbiome risk profiles as biomarkers for inflammatory and metabolic disorders. Nat Rev Gastroenterol Hepatol. 2022;19:383‐397. doi: 10.1038/s41575-022-00581-2 [DOI] [PubMed] [Google Scholar]
- 47. Hoffmann C, Zong S, Mané‐Damas M, Molenaar P, Losen M, Martinez‐Martinez P. Autoantibodies in neuropsychiatric disorders. Antibodies (Basel). 2016;5(2):9. doi: 10.3390/antib5020009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Diamond B, Honig G, Mader S, Brimberg L, Volpe BT. Brain‐reactive antibodies and disease. Annu Rev Immunol. 2013;31:345‐385. doi: 10.1146/annurev-immunol-020711-07504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Acres MJ, Heath JJ, Morris JA. Anorexia nervosa, autoimmunity and the hygiene hypothesis. Med Hypotheses. 2012;78(6):772‐775. doi: 10.1016/j.mehy.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 50. Smitka K, Papezova H, Vondra K, Hill M, Hainer V, Nedvidkova J. The role of "mixed" orexigenic and anorexigenic signals and autoantibodies reacting with appetite‐regulating neuropeptides and peptides of the adipose tissue‐gut‐brain axis: relevance to food intake and nutritional status in patients with anorexia nervosa and bulimia nervosa. Int J Endocrinol. 2013;2013:483145. doi: 10.1155/2013/483145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hansen N, Luedecke D, Malchow B, et al. Autoantibody‐associated psychiatric syndromes in children: link to adult psychiatry. J Neural Transm (Vienna). 2021;128(6):735‐747. doi: 10.1007/s00702-021-02354-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Smitka K, Prochazkova P, Roubalova R, et al. Current aspects of the role of autoantibodies directed against appetite‐regulating hormones and the gut microbiome in eating disorders. Front Endocrinol (Lausanne). 2021;19(12):613983. doi: 10.3389/fendo.2021.613983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Escelsior A, Cogorno L, Sukkar SG, et al. Anti‐hypothalamus autoantibodies in anorexia nervosa: a possible new mechanism in neuro‐physiological derangement? Eat Weight Disord. 2022. doi: 10.1007/s40519-022-01388-5. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gershteyn IM, Burov AA, Miao BY, Morais VH, Ferreira LMR. Immunodietica: interrogating the role of diet in autoimmune disease. Int Immunol. 2020;32(12):771‐783. doi: 10.1093/intimm/dxaa054 [DOI] [PubMed] [Google Scholar]
- 55. Rawlings DJ, Metzler G, Wray‐Dutra M, Jackson SW. Altered B cell signalling in autoimmunity. Nat Rev Immunol. 2017;17(7):421‐436. doi: 10.1038/nri.2017.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Raevuori A, Haukka J, Vaarala O, et al. The increased risk for autoimmune diseases in patients with eating disorders. PLoS One. 2014;9(8):e104845. doi: 10.1371/journal.pone.0104845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hyla‐Klekot L, Wolny A, Janas‐Kozik M, Koszutski T. Anorexia nervosa and juvenile lupus erythematosus in a 16‐year‐old female patient ‐ common disease origin or random coincidence? Cent Eur J Immunol. 2021;46(1):127‐132. doi: 10.5114/ceji.2021.104326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Toulany A, Katzman DK, Kaufman M, Hiraki LT, Silverman ED. Chicken or the egg: anorexia nervosa and systemic lupus erythematosus in children and adolescents. Pediatrics. 2014;133(2):e447‐e450. doi: 10.1542/peds.2012-3048 [DOI] [PubMed] [Google Scholar]
- 59. Bambery P, Malhotra S, Kaur U, Chadda R, Deodhar SD. Anorexia nervosa in a patient with systemic lupus erythematosus. Rheumatol Int. 1987;7(4):177‐179. doi: 10.1007/BF00270367 [DOI] [PubMed] [Google Scholar]
- 60. Sloan D, Gallagher S, Walsh N. Anorexia nervosa complicating systemic lupus erythematosus (SLE). Ir Med J. 1998;91(3):97. [PubMed] [Google Scholar]
- 61. Dalbeth N, Callan M. Arthritis and anorexia? Lancet. 2002;360(9342):1300. doi: 10.1016/S0140-6736(02)11320-1 Erratum in: Lancet 2003;361(9354):352. [DOI] [PubMed] [Google Scholar]
- 62. Trapani S, Rubino C, Simonini G, Indolfi G. Gastrointestinal and hepatic involvement in paediatric systemic lupus erythematosus. Clin Exp Rheumatol. 2021;39(4):899‐906. [PubMed] [Google Scholar]
- 63. Tokatly Latzer I, Lerner‐Geva L, Stein D, Weiss B, Pinhas‐Hamiel O. Disordered eating behaviors in adolescents with celiac disease. Eat Weight Disord. 2020;25(2):365‐371. doi: 10.1007/s40519-018-0605-z [DOI] [PubMed] [Google Scholar]
- 64. Ilzarbe L, Fàbrega M, Quintero R, et al. Inflammatory bowel disease and eating disorders: a systematized review of comorbidity. J Psychosom Res. 2017;102:47‐53. doi: 10.1016/j.jpsychores.2017.09.006 [DOI] [PubMed] [Google Scholar]
- 65. Blanchet C, Luton JP. Anorexie mentale et maladie de Crohn: intrications et difficultés diagnostiques. Trois cas [Anorexia nervosa and Crohn disease: diagnostic intricacies and difficulties. 3 cases]. Presse Med. 2002;31(7):312‐315. [PubMed] [Google Scholar]
- 66. Pehlivantürk Kızılkan M, Kanbur N, Akgül S, Alikaşifoğlu A. An adolescent boy with comorbid anorexia nervosa and Hashimoto thyroiditis. J Clin Res Pediatr Endocrinol. 2016;8(1):92‐95. doi: 10.4274/jcrpe.2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Smalls‐Mantey A, Steinglass J, Primack M, Clark‐Hamilton J, Bongiovi M. Hypothyroidism due to Hashimoto's thyroiditis masked by anorexia nervosa. Int J Eat Disord. 2015;48(7):932‐935. doi: 10.1002/eat.22420 [DOI] [PubMed] [Google Scholar]
- 68. Dell'Osso L, Carpita B, Cremone IM, et al. Subthreshold autism spectrum in a patient with anorexia nervosa and Behçet's syndrome. Case Rep Psychiatry. 2020;2020:6703979. doi: 10.1155/2020/6703979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. De Martinis M, Sirufo MM, Ginaldi L. Raynaud's phenomenon and nailfold capillaroscopic findings in anorexia nervosa. Curr Med Res Opin. 2018;34(3):547‐550. doi: 10.1080/03007995.2017.1417828 [DOI] [PubMed] [Google Scholar]
- 70. Sirufo MM, Magnanimi LM, Ginaldi L, De Martinis M. Letter to the editor: anorexia nervosa, immunity and autoimmunity. Autoimmun Rev. 2022;21(4):103040. doi: 10.1016/j.autrev.2022.103040 [DOI] [PubMed] [Google Scholar]
- 71. Kaplan AS, Katz M. Eating disorders and connective tissue disease. Etiologic and treatment considerations. Psychosomatics. 1992;33(1):105‐108. doi: 10.1016/S0033-3182(92)72028-3 [DOI] [PubMed] [Google Scholar]
- 72. De Martinis M, Ciccarelli F, Sirufo MM, Ginaldi L. An overview of environmental risk factors in systemic sclerosis. Expert Rev Clin Immunol. 2016;12(4):465‐478. doi: 10.1586/1744666X.2016.1125782 [DOI] [PubMed] [Google Scholar]
- 73. Shoenfeld Y, Tincani A, Gershwin ME. Sex gender and autoimmunity. J Autoimmun. 2012;38(2–3):J71‐J73. doi: 10.1016/j.jaut.2011.12.007 [DOI] [PubMed] [Google Scholar]
- 74. Irelli A, Sirufo MM, D'Ugo C, Ginaldi L, De Martinis M. Sex and gender influences on cancer immunotherapy response. Biomedicine. 2020;8(7):232. doi: 10.3390/biomedicines8070232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. De Martinis M, Sirufo MM, Suppa M, Di Silvestre D, Ginaldi L. Sex and gender aspects for patient stratification in allergy prevention and treatment. Int J Mol Sci. 2020;21(4):1535. doi: 10.3390/ijms21041535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hedman A, Breithaupt L, Hübel C, et al. Bidirectional relationship between eating disorders and autoimmune diseases. J Child Psychol Psychiatry. 2019;60(7):803‐812. doi: 10.1111/jcpp.12958 [DOI] [PubMed] [Google Scholar]
- 77. Keeler JL, Patsalos O, Chung R, et al. Short communication: serum levels of brain‐derived neurotrophic factor and association with pro‐inflammatory cytokines in acute and recovered anorexia nervosa. J Psychiatr Res. 2022;150:34‐39. doi: 10.1016/j.jpsychires.2022.03.031 [DOI] [PubMed] [Google Scholar]
- 78. Hommer RE, Swedo SE. Anorexia and autoimmunity: challenging the etiologic constructs of disordered eating. Pediatrics. 2017;140(6):e20173060. doi: 10.1542/peds.2017-3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Goldberg L, Ziv A, Vardi Y, et al. The effect of COVID‐19 pandemic on hospitalizations and disease characteristics of adolescents with anorexia nervosa. Eur J Pediatr. 2022;181:1767‐1771. doi: 10.1007/s00431-021-04350-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zipfel S, Schmidt U, Giel KE. The hidden burden of eating disorders during the COVID‐19 pandemic. Lancet Psychiatry. 2022;9(1):9‐11. doi: 10.1016/S2215-0366(21)00435-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lin JA, Hartman‐Munick SM, Kells MR, et al. The impact of the COVID‐19 pandemic on the number of adolescents/young adults seeking eating disorder‐related care. J Adolesc Health. 2021;69(4):660‐663. doi: 10.1016/j.jadohealth.2021.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Winchester NE, Calabrese C, Calabrese L. The intersection of COVID‐19 and autoimmunity: what is our current understanding? Pathog Immun. 2021;6:31‐54. doi: 10.20411/pai.v6i1.417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sirufo MM, Magnanimi LM, Ginaldi L, De Martinis M. Eating disorders, COVID‐19 and autoimmunity. Pediatr Ann. 2022;51(8):e299. doi: 10.3928/19382359-20220706-01 [DOI] [PubMed] [Google Scholar]
- 84. Sirufo MM, Magnanimi LM, Ginaldi L, De Martinis M. Diabetes, eating disorders, autoimmunity and the Covid‐19 pandemic. Acta Diabetol. 2022;59(8):1125‐1126. doi: 10.1007/s00592-022-01912-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Smatti MK, Cyprian FS, Nasrallah GK, Al Thani AA, Almishal RO, Yassine HM. Viruses and autoimmunity: a review on the potential interaction and molecular mechanisms. Viruses. 2019;11:762. doi: 10.3390/v11080762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gracia‐Ramos AE, Martin‐Nares E, Hernández‐Molina G. New onset of autoimmune diseases following COVID‐19 diagnosis. Cell. 2021;10(12):3592. doi: 10.3390/cells10123592 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


