Abstract
BACKGROUND
Respiratory syncytial virus (RSV) is a prevalent cause of lower respiratory tract infections. It may be associated with hepatocellular involvement, as indicated by increased liver enzymes aspartate aminotransferase and alanine transaminase (ALT).
AIM
To evaluate the rate of increased liver enzyme levels in children with acute bronchiolitis and correlate them with clinical, laboratory, and radiological variables.
METHODS
The study was a retrospective review of the medical records of children who presented with acute bronchiolitis when admitted to the Pediatric Department, Salmaniya Medical Complex, the Kingdom of Bahrain, between 2019 and 2020. We collected the demographic data, the clinical presentation, the laboratory and radiological findings, and the clinical outcomes. We compared the patients with elevated liver enzymes to those with normal levels at the time of presentation and at follow-up.
RESULTS
We included 166 (57.8%) of 287 patients with acute bronchiolitis who fulfilled the inclusion criteria. Ninety-three (56%) patients were males. The median age at presentation was 3.4 (interquartile range 1.1 to 12.4) mo. Fifty-four (28%) patients tested positive for RSV, which was confirmed in 15 of them (28%) by PCR. Laboratory findings of 161 patients tested at presentation showed high ALT levels in 14 (8.7%) patients and normal ALT in 147 (91.3%). Coagulation profiles were measured in 46 (27.7%) of 166 patients. High prothrombin time was present in 15 (32.6%), a high international normalized ratio was present in 13 (28.3%), and high activated partial thromboplastin time was present in three (6.5%). Thrombin time was elevated in nine (27.3%) of 33 patients. Five (21.7%) of 23 patients with available radiological data had hepatomegaly; one of them had findings suggestive of fatty infiltration. High ALT had a significant association with lengthy hospital stays (P < 0.05) and positive urine culture (P < 0.05). Seventy (42.2%) patients had documented follow-up with liver function tests over a median follow-up period of 10.2 (IQR, 2.4-23.3) mo. Total serum protein and serum globulin levels were normalized at the follow-up time, with a significant P value of < 0.05.
CONCLUSION
This study showed a low prevalence of liver function involvement in patients with acute bronchiolitis with a benign course. However, there was a rising trend in ALT during follow-up. Prolonged hospital stay and positive urine cultures were associated with elevated liver enzymes.
Keywords: Children, Acute bronchiolitis, Liver function tests, Respiratory syncytial virus
Core Tip: How frequent is hepatic involvement in children with acute bronchiolitis? Furthermore, what are the predicted factors? To answer these questions, we conducted this retrospective study. Despite the low prevalence of impaired liver function in patients with acute bronchiolitis and the benign course, there was a rising trend in alanine aminotransferase levels during follow-up. Elevated liver enzymes were linked to an extended hospital stay and positive urine cultures. Therefore, children with acute bronchiolitis should be monitored and followed up with liver function tests.
INTRODUCTION
Respiratory syncytial virus (RSV) is one of the most common causes of lower respiratory tract infections in children[1]. By the age of two years, most children have had at least one episode of RSV bronchiolitis[2]. It is a single-stranded RNA virus belonging to the Paramyxovirus family that affects 4-5 million children annually, leading to more than 125000 pediatric admissions annually in the United States[3,4]. Premature infants and infants with chronic lung disease or congenital heart diseases have much higher morbidity and mortality due to acute bronchiolitis[2].
RSV has been found to affect the gastrointestinal system in addition to respiratory tract infections. It has been associated with hepatocellular involvement, causing an increase in liver enzymes, including aspartate aminotransferase (AST) and alanine transaminase (ALT). Several types of research have proven that the elevation of transaminases is associated with more severe cases of RSV in children[1,5]. The hepatocellular involvement is reported to be due to different mechanisms, for example, high viral load causing dispersion into the systemic circulation, which can lead to acute hepatitis, hepatic congestion, or ischemia[5].
Data on hepatic involvement in children with acute bronchiolitis are scarce. There have only been a few reports describing this issue[1,3]. Unfortunately, there are no previously published data regarding hepatic involvement in children with acute bronchiolitis in the Kingdom of Bahrain. In this study, we aimed to assess the rate of impaired liver functions in children with acute bronchiolitis and to detect the predicted clinical, radiological, and laboratory variables.
MATERIALS AND METHODS
Study design and setting
In this study, we retrospectively reviewed the electronic medical records of all children with a clinical diagnosis of acute bronchiolitis admitted to the pediatric department at Salmaniya Medical Complex (SMC), the Kingdom of Bahrain, between September 1, 2019, and February 29, 2020.
Study participants
The study included children aged five years or less admitted to the hospital with the clinical diagnosis of acute bronchiolitis who had a reported nasopharyngeal swab for RSV direct antigen detection, PCR test, and liver function tests (LFTs). Children were diagnosed with acute bronchiolitis according to the diagnostic criteria of the American Academy of Pediatrics[6]. Signs and symptoms suggestive of bronchiolitis include running nose, tachypnea, cough, wheezing, rales, and indications of increased respiratory effort such as nasal flaring, grunting, and intercostal and/or subcostal retractions. These are the necessary criteria for clinical diagnosis. We did not routinely rely on radiographic or laboratory results to confirm acute bronchiolitis diagnoses[6].
Data collection
We collected patients' demographic information, including age at the time of the study, age at presentation, sex, nationality, gestational age, clinical presentation, and length of hospital stay. Fever was diagnosed with a rectal temperature of 38 °C (100.4 °F) or an axillary temperature of 37.5 °C (99.5 °F)[7]. In our hospital, the patient's temperature was measured rectally if the patient's age was below one year or axillary in those older than one year after adding 0.5 °C to the measured temperature.
The results of laboratory investigations included complete blood count, LFTs, blood, urine, and cerebrospinal fluid (CSF) culture, coagulation profile, and direct detection of RSV antigen in the nasopharyngeal swabs. We also gathered the results of the respiratory microbial serology panel (immunoglobulin G and M) for Mycoplasma pneumonia, Legionella pneumophilia, Coxiella burnetii, Chlamydia pneumonia, RSV, adenovirus, influenza A and B, parainfluenza, Cytomegalovirus, and Epstein-Barr virus and hepatitis profile. Senior radiologists reported and documented radiological findings from abdominal ultrasound, and computed tomography (CT) scans. Medical treatments included antibiotics and steroids. Patient outcomes and complications were also documented.
Statistical analysis
The data were entered into an Excel 2010 worksheet and then transferred to Statistical Package for the Social Sciences (SPSS) version 21 for statistical analysis. Demographic data are presented as frequencies and percentages. Continuous variables are presented as mean and standard deviation (SD) if found to be normally distributed or as a median and interquartile range (IQR) if not normally distributed. We divided the patients according to LFT results into two groups: group 1 included patients with hepatic involvement and group 2 with normal LFTs. High and average values were defined based on the ALT level being the best enzyme that reflects liver injury. An elevated ALT level of more than 41 U/L was considered high, while an ALT of less than or equal to 41 U/L was considered average. We compared the two groups regarding the demographic data, the clinical presentation (presence of fever, cough, and other symptoms and signs), laboratory results (blood, urine, and CSF cultures, complete blood count, and RSV swab for direct antigen detection and PCR test), radiological results (ultrasound and CT when performed), antibiotic and steroid use, and the clinical outcomes. Children with a single viral infection were compared with those with multiple infections in terms of their mean ALT level. The Chi-Square and Fisher's tests were used to compare categorical variables. Student's t-tests or Mann-Whitney U-tests were used to compare continuous variables. P value < 0.05 was considered statistically significant. The confidence interval was set at 95%.
Ethical approval
We performed the current study in accordance with the Helsinki declaration. The study was ethically approved by The Research and Research Ethics Committee of the Salmaniya Medical Complex, Government Hospitals, Kingdom of Bahrain (with IRB number: 27130220). As the study is retrospective without exposure of patient personal data, we did not require patient consent.
RESULTS
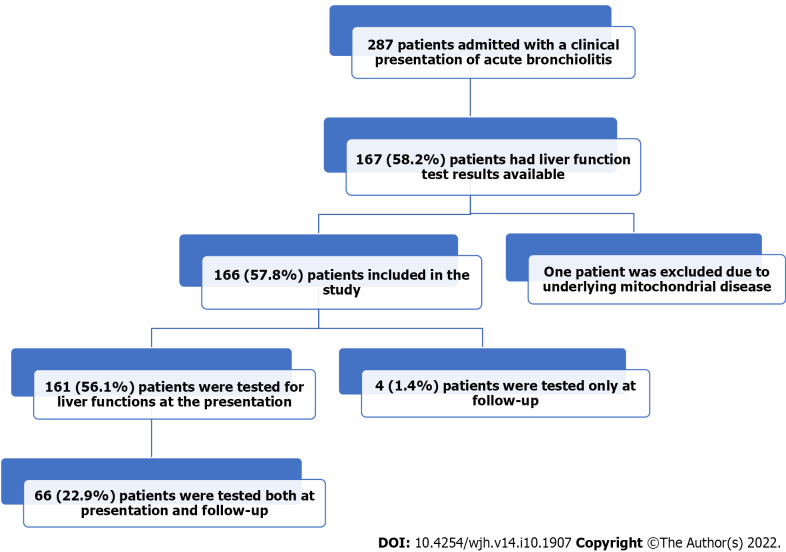
Figure 1 shows the flow chart of the study. We studied 287 infants admitted with the clinical diagnosis of acute bronchiolitis. One hundred and sixty-seven patients (58.2%) had LFT results available in their medical records. One patient was excluded due to his underlying mitochondrial myopathy and elevated gamma-glutamyl transferase. Of the remaining 166 (57.8%) patients, 161 (56.1%) were tested for liver function at presentation, 66 (22.9%) were tested both at presentation and follow-up LFTs, and four (1.4%) patients only at follow-up.
Figure 1.
The study flow chart describes hospitalized infants with acute bronchiolitis.
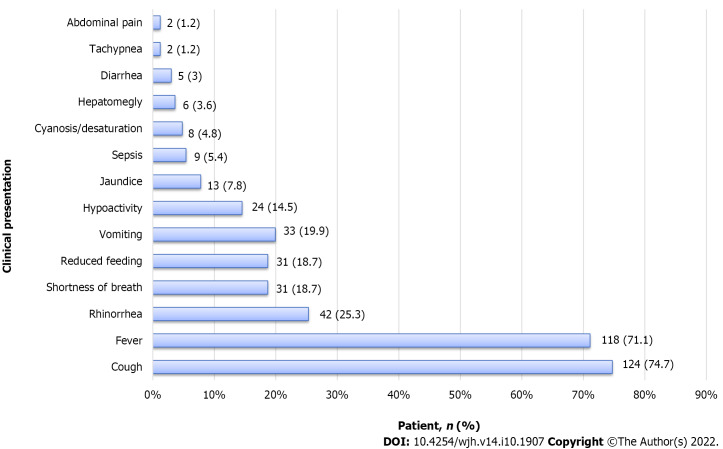
Table 1 shows the demographic data of the included patients. Ninety-three (56%) were males; 117 (70%) infants were Bahraini, and 49 (29.5%) were not Bahraini (seven from Yemen, five from Pakistan, four from India, one from Indonesia, Jordan, Saudi Arabia, Libya, and the Philippines, and 28 without nationality data). Most patients presented with cough (n = 124, 74.7%), followed by fever (n = 118, 71.1%), and other signs of severe respiratory distress such as shortness of breath (n = 31, 18.7%), cyanosis (n = 8, 4.8%), and tachypnea (n = 2, 1.2%) as shown in Figure 2. Thirty-three (19.9%) patients had vomiting, 31 (18.7%) had reduced feeding, 13 (7.8%) had jaundice, 6 (3.6%) were found to have clinical hepatomegaly, and 2 (1.2%) had abdominal pain.
Table 1.
Demographic data of children with acute bronchiolitis (n = 166)
|
Demographic data
|
No. of patients (%)
|
|
| Sex | Male | 93 (56) |
| Female | 73 (44) | |
| Nationality | Bahraini | 117 (70.5) |
| Other | 49 (29.5) | |
| Gestational age (n = 140) | Term | 111 (79.3) |
| Preterm | 29 (20.7) | |
| Age at presentation (mo), median (IQR) | 3.4 (1.1-12.4) | |
| Current age (mo), median (IQR) | 30.3 (28.31-39.8) | |
| Length of stay (d), median (IQR) | 6.0 (3.0-11.0) | |
Values are presented as n (%) for categorical variables and median (interquartile range) for continuous variables. IQR: Interquartile range.
Figure 2.
Clinical characteristics of 166 infants admitted with the diagnosis of acute bronchiolitis.
The results of the laboratory investigations are shown in Table 2. One hundred and sixteen (69.9%) patients were anemic; 83 (50%) had leukocytosis; six (3.6%) had leukopenia; 74 (44.6%) had thrombocytosis; and eight (4.8%) patients had thrombocytopenia. ALT levels were high in 14 (8.7%) patients and normal in 147 (91.3%) patients. 46 of 166 patients (27.7%) had documented data regarding their coagulation profile. High prothrombin time (PT) was found in 15 (32.6%), high international normalized ratio (INR) was found in 13 (28.3%), and high activated partial thromboplastin time (APTT) was found in three patients (6.5%). Fibrinogen levels were tested in 34 (20.5%) patients; 16 of them (47.1%) had low levels. Thrombin time was tested in 33 (19.9%); nine of them (27.3%) had prolonged thrombin time. Random glucose levels were available for 54 (32.5%) patients; five (3%) were found to have hyperglycemia, and two (1.2%) had hypoglycemia. Lactate dehydrogenase was determined in six (3.6%) patients; three of them (50%) had high levels. The iron level was available for nine (5.4%) patients; seven (77.7%) of them had low levels. C-reactive protein (CRP) levels were high in 116 (69.9%) patients, while 50 (30.1%) patients had normal levels.
Table 2.
Results of laboratory investigations in the 166 children who presented with acute bronchiolitis
|
Variable
|
Normal range
|
Mean
|
SD
|
Median
|
Minimum
|
Maximum
|
Tested patients (%)
|
| White blood cell count (× 109/L) | 3.6-9.6 | 10.8 | 5.4 | 9.7 | 0.80 | 27.8 | 166 (100) |
| Hemoglobin level (g/dL) | 12.0-14.5 | 11.5 | 2.3 | 11.0 | 5.7 | 20.0 | 166 (100) |
| Platelet count (× 109/L) | 150-400 | 410 | 179.7 | 383.5 | 14.5 | 971.0 | 166 (100) |
| Total serum protein (g/L) | 64-82 | 57.8 | 8.7 | 58.0 | 25.0 | 96.0 | 161 (97) |
| Serum albumin (g/L) | 38-54 | 41.5 | 10.7 | 41.0 | 17.0 | 143.0 | 161 (97) |
| Serum globulin (g/L) | 15-30 | 18.0 | 10.7 | 17.0 | 6.0 | 140.0 | 161 (97) |
| Total bilirubin (µmol/L) | 5-21 | 37.4 | 61.2 | 10.0 | 2.0 | 482.0 | 161 (97) |
| Direct bilirubin (µmol/L) | 0-5 | 12.0 | 14.4 | 11.0 | 1.0 | 122.0 | 71 (42.8) |
| Indirect bilirubin (µmol/L) | < 18 | 56.0 | 46.0 | 27.0 | 1.0 | 454.0 | 71 (42.8) |
| Alkaline phosphatase (U/L) | 150-420 | 242.6 | 141.7 | 198.0 | 58.0 | 841.0 | 161 (97) |
| Alanine aminotransferase (U/L) | < 41 | 25.6 | 30.2 | 19.0 | 6.0 | 247.0 | 161 (97) |
| Gamma glutamyl transferase (U/L) | < 18 | 63.2 | 80.0 | 32.0 | 6.0 | 526.0 | 145 (87.3) |
| Prothrombin time | 10-14 | 13.7 | 2.8 | 12.9 | 9.5 | 25.2 | 46 (27.7) |
| INR | 0.6-1.2 | 1.1 | 0.3 | 1.1 | 0.79 | 2.2 | 46 (27.7) |
| PTT (sec) | 28-43 | 28.2 | 9.7 | 26.8 | 13.2 | 63.3 | 46 (27.7) |
| Fibrinogen (mg/dl) | 217-496 | 285.9 | 213.8 | 233.8 | 0.0 | 1115.2 | 34 (20.48) |
| Thrombin time (sec) | 15.6 -18.4 | 17.9 | 4.1 | 17.3 | 4.1 | 13.0 | 33 (19.87) |
| Random blood sugar (mmol/L) | 3.6-8.9 | 5.7 | 1.4 | 5.2 | 4.0 | 10.0 | 53 (32) |
| Ammonia (µmol/L) | 11-35 | 80.6 | 61.2 | 62.0 | 7.0 | 230.0 | 11 (6.6) |
| Lactic acid (mmol/L) | 0.5-2.2 | 3.1 | 3.3 | 1.5 | 1.0 | 10.0 | 7 (4.2) |
| Lactate dehydrogenase (units/L) | 250-650 | 1030.1 | 1281.9 | 531.5 | 3.0 | 3509.0 | 6 (3.6) |
| Iron profile (µg/dL) | 11.6-31.3 | 9.4 | 5.7 | 8.0 | 4.0 | 19.0 | 9 (5.4) |
| CRP (mg/L) | 0.3 | 26.8 | 39.2 | 9.5 | 10.0 | 297.0 | 166 (100) |
SD: Standard deviation; INR: International normalized ratio; PTT: Partial thromboplastin time; CRP: C-reactive protein.
Of the 137 (82.5%), patients who were checked for bacterial co-infections, 20 (14.6%) patients were confirmed to have bacterial infections. Twelve (8.7%) patients had positive blood cultures; 7 (5.2%) had positive urine cultures, and one (0.7%) had positive CSF culture by lumbar puncture.
The hepatitis profile was conducted for two patients and was found to be negative. EBV was tested in seven patients; five were positive for IgG, indicating past infection, and two showed negative results. Six patients were tested for CMV, three had normal results, and three had reactive IgG and non-reactive IgM, indicating previous infection.
Of 163 patients who were tested for RSV via nasopharyngeal swabs, 124 (74.7%) were tested by rapid antigen test only, one (0.6%) by PCR alone, and 38 (23%) patients were tested by both. Positive RSV results were found in 54 (28%) patients; 15 of them (28%) were confirmed by PCR. A respiratory microbial serology panel was accomplished in 42 (25.3%) infants; 14 of them (33.3%) had positive findings. Four (29%) of the 14 infants were positive for more than one virus. As for IgG, it was positive in six (14.3%) patients tested for adenovirus, six (14.3%) with RSV, three (7%) with influenza B, and one (2%) with influenza A and parainfluenza. IgM was positive in one (2%) patient with adenovirus, RSV, influenza B, and Mycoplasma pneumonia, respectively.
Radiological findings were available for 23 patients; 21 (12.7%) underwent abdominal ultrasound, and two (1.2%) had abdominal CT. Abdominal ultrasound results were normal in 12 (57%) patients, and abnormal in nine (43%) patients. Five patients (23.8%) had hepatomegaly: One of them had mild diffuse increased parenchymal echogenicity suggestive of fatty infiltration; one patient had an unvisualized gall bladder; one patient had subhepatic free fluid and dilated bowel loops, and one had a slightly edematous gallbladder wall. Abdominal CT in one patient showed a cyst between the liver and kidney, and the other patient had a normal CT. Both patients who had CT scans had an associated underlying disease. The first patient was a seven-year-old female with retroperitoneal neuroblastoma, and a CT scan was performed as a follow-up for her previous condition. She had chemotherapy that was stopped years before she had acute bronchiolitis. Her original medical condition did not affect her liver function as her ALT levels were normal at the time of admission and on follow-up. The second patient was a four-year-old male diagnosed with polycystic kidney disease, and the CT scan was performed as he was scheduled for a nephrectomy. His CT report did not show any cystic lesions in the liver, and he was also admitted with normal ALT (22 U/L). All the infants were managed with normal saline and bronchodilator inhalation. Antibiotics were given to 128 (77.1%) patients. The antibiotics were cefotaxime (n = 61, 46.9%), ampicillin (n = 47, 36.1%), gentamycin (n = 20, 15.3%), ceftriaxone and clindamycin (n = 9, 6.9% each), amoxicillin/clavulanic acid (n = 8, 6.1%), vancomycin (n = 7, 5.3%), clarithromycin (n = 5, 3.8%), metronidazole, meropenem and ceftazidime (n = 4, 3.1% each), piperacillin/tazobactam (n = 3, 2.3%), and ciprofloxacin, rifampicin, and sulfamethoxazole/trimethoprim (n = 1, 0.7% each). Some patients received a combination of antibiotics. In these patients, paracetamol was the antipyretic given as syrup, suppositories, or intravenously, and they were rarely given ibuprofen.
The outcome was documented for all hospitalized patients. Eleven patients (6.70%) required admission to the pediatric intensive care unit (PICU), eight (4.80%) required total parenteral nutrition, five (3.01%) had a surgical procedure during their admission (tracheostomy was performed in two infants, while insertion of a central line, intercostal drain tube, and nasojejunal tube were performed in one infant each). Three infants (1.8%) needed endotracheal intubation with ventilatory support. Unfortunately, nine infants (5.4%) died. No patients developed fulminant hepatic failure or required liver biopsy or liver transplantation.
A comparison between group 1 (high ALT level) and group 2 (average ALT level) is shown in Table 3. Patients with high ALT levels had extended hospital stays (P = 0.025) and were found to have more frequent positive urine cultures (P = 0.030) than those with normal ALT levels. Other parameters such as nationality, sex, gestational age, age at presentation, current age, white blood cell count, platelet count, other LFT variables, coagulation profile, positive RSV swabs, positive blood and CSF cultures, antibiotic use, complications, PICU admission, mortality, and outcomes were not significantly different between the two groups.
Table 3.
Comparison of patients with and without liver function test abnormalities
| Variable |
Alanine aminotransferase level (n = 161)
|
P value | ||
|
High (> 41 U/L), 14 (8.7)
|
Normal (≤ 41 U/L), 147 (91.3)
|
|||
| Sex | Female | 9 (64.3) | 61(41.5) | 0.157a |
| Male | 5 (35.7) | 86 (58.5) | ||
| Nationality | Bahraini | 9 (64.3) | 104 (70.7) | 0.760a |
| Non-Bahraini | 5 (35.7) | 43 (29.3) | ||
| Gestational age (n = 135) | Term | 8 (57.1) | 98 (66.7) | 0.683 |
| Preterm | 1 (7.1) | 28 (19.0) | ||
| Age at presentation (mo), mean ± SD | 10.1 ± 12.8 | 10.6 ± 17.3 | 0.535b | |
| Age at the time of study (mo), mean ± SD | 37.5 ± 13.2 | 37.8 ± 17.5 | 0.394b | |
| Length of hospital stay (d), mean ± SD | 18.0 ± 19.3 | 13.4 ± 41.1 | 0.025b | |
| History of fever | 9 (7.9) | 105 (92.1) | 0.552a | |
| White blood cell count (× 109/L), mean ± SD | 12.2 ± 7.0 | 10.6 ± 5.3 | 0.764b | |
| Hemoglobin (g/dL), mean ± SD | 11.6 ± 2.1 | 11.5 ± 2.4 | 0.796b | |
| Platelet count (× 109/L), mean ± SD | 335.4 ± 194.8 | 417.6 ± 178.5 | 0.109b | |
| Total serum protein (g/L), mean ± SD | 56.6 ± 7.3 | 57.9 ± 8.9 | 0.895b | |
| Serum globulin (g/L), mean ± SD | 16.71 ± 3.69 | 18.1 ± 11.2 | 0.801b | |
| Serum albumin (g/L), mean ± SD | 39.86 ± 5.89 | 41.63 ± 11.1 | 0.971b | |
| Total bilirubin (µmol/L), mean ± SD | 29.4 ± 41.2 | 38.11 ± 62.81 | 0.794b | |
| Direct bilirubin (µmol/L), (n = 71), mean ± SD | 24.1 ± 39.9 | 10.5 ± 5.7 | 0.722b | |
| Indirect bilirubin (µmol/L), (n = 71), mean ± SD | 22.5 ± 17.9 | 62.0 ± 75.1 | 0.184b | |
| Alkaline phosphatase (U/L), mean ± SD | 271.9 ± 217.2 | 239.9 ± 133.1 | 0.935b | |
| Gamma-glutamyl transferase (U/L), (n = 145), mean ± SD | 92.4 ± 88.2 | 60.6 ± 79.0 | 0.080b | |
| Prothrombin time, (n = 46), mean ± SD | 13.2 ± 1.6 | 13.8 ± 3.0 | 0.809b | |
| INR, (n = 46), mean ± SD | 1.1 ± 0.1 | 1.2 ± 0.3 | 0.831b | |
| PTT, (n = 46), mean ± SD | 25.5 ± 4.9 | 28.8 ± 10.4 | 0.579b | |
| Fibrinogen, (n = 34), mean ± SD | 326.4 ± 387.2 | 277.3 ± 166.4 | 0.581b | |
| Thrombin time, (n = 33), mean ± SD | 21.4 ± 7.3 | 16.9 ± 2.0 | 0.131b | |
| Random blood glucose, (n = 52), mean ± SD | 6.0 ± 1.0 | 6.0 ± 1.0 | 0.905b | |
| Positive blood culture (n = 127) | 2 (14.3) | 10 (6.8) | 0.278a | |
| Positive urine culture (n = 88) | 3 (21.4) | 4 (2.7) | 0.030a | |
| Positive cerebrospinal fluid culture (n = 23) | 0 (0.0) | 1.0 (0.7) | 1.000a | |
| Positive RSV test (n = 159) | 6 (42.9) | 46 (31.3) | 0.356a | |
| Antibiotic use (n = 157) | 11 (78.6) | 115 (78.2) | 1.000a | |
| Complications | 2 (14.3) | 28 (19) | 1.000a | |
| Admission to the intensive care unit | 1 (7.1) | 10 (6.8) | 1.000a | |
| Mortality | 1 (7.1) | 8 (5.4) | 0.569a | |
Fisher’s exact test was used for categorical variables.
Mann-Whitney U test was used for continuous variables.
Values are presented as n (%) or mean ± SD. Bold indicates a statistically significant difference with P < 0.05. SD: Standard deviation; INR: International normalized ratio; PTT: Partial thromboplastin time; RSV: Respiratory syncytial virus.
In this study, 13 (7.8%) patients tested positive for more than one viral infection. There was no significant difference between children with single viral infection and those with multiple infections in mean ALT level (25 U/L ± 30 U/L vs 23 U/L ± 17 U/L, respectively) (P = 0.872).
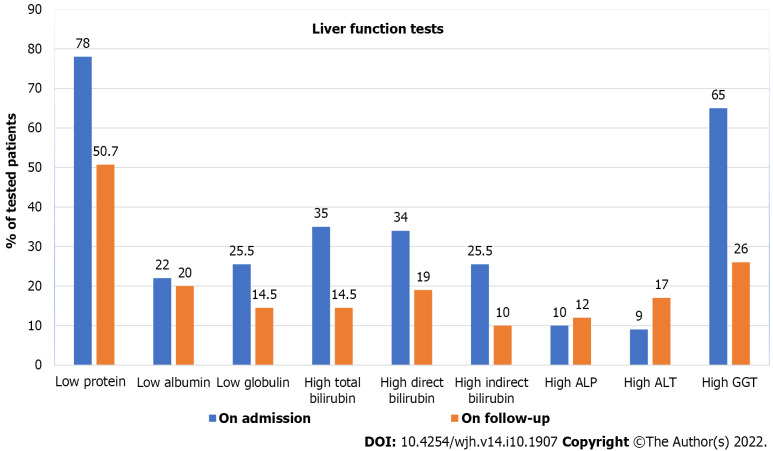
Of the 166 patients, 70 (42.2%) were followed up for LFTs with a median follow-up period of 10.2 (IQR, 2.4-23.3) mo. A comparison between LFTs at the time of presentation and at the time of follow-up is shown in Figure 2 and Table 4. It was found that only total serum protein and serum globulin levels were normalized at the follow-up time with a significant P value of 0.008. However, no significant changes were found in other LFTs. Of 72 patients with available data on ALT levels at the follow-up time, 11 (15.3%) still had high levels (Figure 3).
Table 4.
Comparison of patients with acute bronchiolitis who were tested for liver enzymes at the time of presentation and at the time of follow up
|
Variable
|
Normal range
|
n
|
At the time of the presentation
|
At the time of follow-up
|
P
valuea
|
| Total serum protein (g/L) | 64-82 | 65 | 59.0 ± 11.0 | 62.0 ± 11.0 | 0.011 |
| Serum albumin (g/L) | 38-54 | 65 | 42.0 ± 9.0 | 42.5 ± 7.0 | 0.467 |
| Serum globulin (g/L) | 15-30 | 65 | 18.0 ± 5.0 | 20.0 ± 6.0 | 0.008 |
| Total bilirubin (µmol/L) | 5-21 | 64 | 37.0 ± 49.0 | 28.0 ± 91.0 | 0.376 |
| Direct bilirubin (µmol/L) | 0-5 | 16 | 18.0 ± 28.0 | 51.0 ± 157.0 | 0.322 |
| Indirect bilirubin (µmol/L) | < 18 | 15 | 56.0 ± 46.0 | 35.0 ± 50.0 | 0.103 |
| Alkaline phosphatase (U/L) | 150-420 | 64 | 240.0 ± 143.0 | 242.0 ± 121.0 | 0.897 |
| Alanine aminotransferase (U/L) | < 41 | 61 | 31.0 ± 43.0 | 42.0 ± 88.0 | 0.371 |
| Gamma-glutamyl transferase (U/L) | < 18 | 50 | 61.0 ± 75.0 | 41.0 ± 91.0 | 0.234 |
P value was calculated by the paired t-test.
Figure 3.
Comparison of liver function tests in infants admitted with acute bronchiolitis at admission and during follow-up. ALP: Alkaline phosphatase; ALT: Alanine aminotransferase; GGT: Gamma-glutamyl transferase.
These patients were further investigated to exclude other causes of ALT elevation. Eight were diagnosed at the follow-up time with other underlying diseases, which may have been related to their elevated ALT. The first patient had Dandy-Walker syndrome, skeletal dystrophy, and ventricular septal defect; the second patient had tracheomalacia, severe combined immune deficiency (SCID), and hypothyroidism; while the third patient had polycystic kidney disease on hemodialysis; the fourth patient had isolated SCID; the fifth patient had cystic fibrosis; the sixth patient had progressive familial intrahepatic cholestasis; the seventh patient had biliary atresia with cirrhosis and ascites, and the eighth patient had intestinal volvulus with gangrene. One patient died from an unknown cause, and two patients had no apparent medical reason for elevated ALT.
DISCUSSION
This study showed that symptoms and signs of hepatic involvement, such as jaundice and hepatomegaly, are infrequent among patients hospitalized for acute bronchiolitis. Jaundice was found in 7.8% (n = 16 out of 166) of our patients, while hepatomegaly was observed in only 3.6% (n = 6/166). Jaundice was also reported by Nadal et al[8] in a seven-month-old infant who presented with acute bronchiolitis and cholestatic jaundice. RSV was detected in his liver biopsy. Al-Maskari et al[3] and Kirin et al[9] reported two infants with acute bronchiolitis and hepatomegaly; one was 11 mo, and one was 13 mo. However, these patients were pooled from case reports with weak evidence. With a rigorous literature review, we found that the current study was the first to investigate the prevalence of hepatomegaly and jaundice in a large cohort of children with acute bronchiolitis.
In the present study, ALT levels were high in 8.7% of our patients. This percentage is considered low compared to several previous studies. The Oh et al[10] study showed that 16% of the RSV-positive children had high ALT levels. Thorburn et al[5] showed that 19% of ventilated children with acute bronchiolitis had high transaminase levels. In 2002, Eisenhut et al[11] studied 55 ventilated patients with RSV bronchiolitis. They found that 46% had high transaminase levels (ALT and/or AST). Two years later, in 2004, Eisenhut et al[12] published a follow-up study and reported a higher percentage of elevated transaminase levels, reaching up to 49%. Giordano et al[1] also reported three male children hospitalized for mild to moderate RSV bronchiolitis. All their patients' laboratory tests showed elevated levels of transaminases (ALT/AST). The differences in the percentage of patients with elevated ALT levels between the current study and other studies could be attributed to differences in the patients included, as most other studies focused on sick, ventilated patients admitted to the PICU. Consequently, high transaminase levels could reflect the disease severity[5].
Virus-induced, ischemic, or hypoxic hepatitis are the possible causes of increased transaminase levels in patients with RSV bronchiolitis[5]. Similar to the other Paramyxoviridae, RSV can attach to specific receptors on the non-epithelial cell surfaces to infect them. RSV can be detected and isolated from the liver, human myocardial tissue, and cerebrospinal fluid[11]. Direct liver invasion by RSV in an immunocompetent infant has been documented by the successful isolation and culture of RSV from liver tissue obtained by biopsy[8]. The higher the transaminase levels are, the higher the viral load, causing spillover of the virus, which spreads from the lungs into the systemic circulation with subsequent extrapulmonary manifestations[12]. RSV infection was the causative agent in 28% of our patients with acute bronchiolitis. However, we did not find significant differences in the ALT levels between RSV-positive patients and RSV-negative. On the other hand, Do et al[13] found a high prevalence of elevated AST in isolated RSV infections. Moreover, Oh et al reported that children with RSV infection had the highest prevalence of elevated ALT levels (16%) compared to other viruses[10].
In the current study, patients with high ALT levels had extended hospital stays (P = 0.025) compared to those with average ALT levels. Similarly, Oh et al[10] 's study showed that children with the highest elevated ALT had more extended hospital stays than those with normal ALT levels. However, Thorburn et al[5] reported that ventilation duration and the length of PICU admission were significantly longer (P < 0.05 for both) in patients with elevated AST but not with elevated ALT. Eisenhut et al[11] also found a significantly longer duration of ventilation in patients with elevated transaminases (P = 0.02).
This study observed an abnormal coagulation profile, such as high PT, high INR, high APTT, low fibrinogen, and prolonged thrombin time in 32.6%, 28.3%, 6.5%, 47.1%, and 27.3%, respectively. Eisenhut et al[11], Bakalli[14], and Lee et al[15] noted a deranged coagulation profile affecting PT, INR, APTT, and fibrinogen. In addition, Al-Maskari et al[3] also noted similar findings, yet this coagulopathy recovered within one week with supportive management.
The current study found that disturbed glucose homeostasis was infrequent, as only 3.0% of our patients developed hyperglycemia, and 1.2% had hypoglycemia. Fares et al[16] studied 49 patients admitted with acute bronchiolitis and found that hyperglycemia was not frequent. They related this finding to the non-standardized timing of blood glucose analysis. On the other hand, Branco et al[17] studied 50 children. They showed that hyperglycemia was frequent in children with bronchiolitis who required mechanical ventilation, as 98% of the patients had peak glucose levels higher than or equal to 6.1 mmol/L and 72% had peak glucose higher than or equal to 8.3 mmol/L.
In this study, all the patients were managed with steam and bronchodilator inhalation. Unfortunately, we found that bronchodilators are still used in cases of acute bronchiolitis in our hospital despite most of the studies and guidelines recommendations, including that of the American Academy of Pediatrics (AAP), showing that their use was ineffective in reducing hospitalization rate, oxygen requirement, mechanical ventilation, the duration of illness, or hospital stay[16]. This behavior can be explained by the limited therapeutic options that can be delivered to children with acute bronchiolitis, which puts the physician under pressure to give inhaled bronchodilators even if they are aware of the recommendations[18].
In the present study, confirmed bacterial co-infection was found in 14.6% of the patients. Bacterial co-infections, especially urinary tract infections, were associated with elevated ALT levels in the current study (P 0.030 =). Thorburn et al[5] also showed that 32% of their patients had bacterial co-infection with elevated transaminase levels (AST and/or ALT). However, Fares et al[16] found no difference between patients with isolated RSV infection and those with bacterial co-infection with elevated liver enzymes. Despite the low rate of confirmed bacterial co-infections (14.6%) in the current study, there was a high rate of antibiotic use in children with acute bronchiolitis (77%). However, this rate was comparable to the previously reported rates in infants with severe bronchiolitis (79.9%)[19]. Unnecessary antibiotic use in infants with acute bronchiolitis is common due to challenging differentiation between infants with isolated viral infections and those with invasive bacterial co-infections[19]. The antibiotic prescription was common in infants with high CRP levels, even though these high levels could not predict the presence of alveolar condensation on chest X-rays[20]. Some radiological findings of acute bronchiolitis and elevated CRP levels might deceive physicians. These findings might push physicians to use antibiotics unnecessarily in a viral-induced disease. However, all the patients in this study were tested for liver function at presentation, even before any medications were given to them. Some antibiotics and antipyretics can cause impaired liver function. Accordingly, the decision to use antibiotics in infants with acute bronchiolitis should be based on more meticulous evidence, such as positive tracheal aspirate, blood, urine, or cerebrospinal fluid cultures[21]. In this study, abdominal ultrasound was not performed as a routine investigation for patients with acute bronchiolitis. It was only performed in 12.7% of patients. However, 43% of them showed positive findings. Nonetheless, Giordano et al[1] described three cases of acute bronchiolitis. All had normal abdominal ultrasound findings.
In the current study, 6.7% of patients required PICU admission compared to 5.1% in the study by Papoff et al[7]. Thorburn et al[5] focused on patients admitted to the PICU with acute bronchiolitis to establish a relationship between transaminase levels and disease severity. They discovered that patients with elevated transaminase levels require more ventilation and spend more time in the PICU. Eisenhut et al[11] also confirmed the same observation.
Although no patient in this study deteriorated into fulminant hepatic failure, Al-Maskari et al[3] reported that an 11-month-old female infant with RSV-induced acute bronchiolitis developed acute fulminant hepatic failure and hepatic encephalopathy. Bakalli also described a one-month-old boy with RSV-induced acute bronchiolitis who was admitted to the PICU with liver failure. He related the presence of liver dysfunction to the effects of the initial tissue hypoperfusion and the consequences of shock[14].
The limitations of the study
As this was a retrospective study, some demographic and laboratory data of some patients were missing. In this research, we looked at the presence or absence of fever as a clinical presentation, but we did not specify the degree of fever as this was not the aim of the study. In addition, the hepatitis profile was available for some but not all the patients included in the study, which could impact the study's results, as hepatic involvement by other viruses could not be entirely excluded. Antibiotic use or the presence of bacterial co-infections could also be a factor in increased liver enzymes. Nevertheless, most of the patients in this study received antibiotics after LFTs. Another confounder in patients with high ALT on follow-up is the 10-mo duration, which is long enough for complete recovery from a viral illness such as acute bronchiolitis. Looking for alternative causes for this ALT elevation is essential as a new illness with another viral infection, which is common in children and unrelated to the original infection, might be the cause.
Moreover, the number of published studies that have attempted to find an association between acute bronchiolitis and hepatic function involvement is minimal, making comparing this study's findings with those of other studies challenging. Another significant limitation is that this is a single center-based study, so we cannot generalize our data. Despite these limitations, we think this study's findings are significant, being the first to investigate this issue in the Kingdom of Bahrain.
CONCLUSION
This study showed a low prevalence of liver function impairment in patients with acute bronchiolitis. Even those with hepatic impairment had a benign course, as most of them improved liver function with time. However, there was a rising trend in ALT during follow-up. Extended hospital stays and the presence of urinary tract infections were linked to elevated hepatic enzyme levels. Therefore, further studies are needed to assess the relationship between disease severity and liver function involvement.
ARTICLE HIGHLIGHTS
Research background
Acute bronchiolitis caused by Respiratory syncytial virus (RSV) is the most common type of lower respiratory tract infection of viral etiology. It is occasionally associated with hepatocellular involvement, as indicated by the increase in liver enzymes, including aspartate aminotransferase and alanine transaminase.
Research motivation
Due to the limited data on liver involvement in acute bronchiolitis, we were motivated to study the prevalence of liver involvement in RSV-induced acute bronchiolitis and the associated factors.
Research objectives
To assess the frequency of impaired liver functions in infants with acute bronchiolitis and to detect the predicted clinical, radiological, and laboratory variables.
Research methods
We retrospectively reviewed demographic data, clinical presentation, laboratory results, radiological findings, and outcomes of infants with acute bronchiolitis admitted to the pediatric department, Salmaniya Medical Complex, Kingdom of Bahrain, collected from medical records in 2019-2020. Infants with high liver enzymes were compared to those with normal levels at the time of presentation and at follow-up.
Research results
One hundred sixty-six (57.8%) out of 287 patients with acute bronchiolitis fulfilled the inclusion criteria. Ninety-three (56%) patients were males. The median age at presentation was 3.4 (interquartile range 1.1 to 12.4) mo. Fifty-four (28%) patients tested positive for RSV, which was confirmed by PCR in 15 of them (28%). High ALT levels were found in 14 (8.7%) patients and ALT was normal in 147 (91.3%). Coagulation profiles were measured in 46 (27.7%) of 166 patients. High PT was present in 15 (32.6%), high INR was present in 13 (28.3%), and high APTT was present in three (6.5%). Thrombin time was elevated in nine (27.3%) of 33 patients. Five (21.7%) of 23 patients with available radiological data had hepatomegaly; one of them had findings suggestive of fatty infiltration. High ALT was significantly associated with lengthy hospital stay (P < 0.05) and a positive urine culture (P < 0.05). Seventy (42.2%) patients had documented follow-up with liver function tests over a median follow-up period of 10.2 (IQR, 2.4 -23.3) mo. Total serum protein and serum globulin levels were normalized at the follow-up time, with a significant P value of < 0.05.
Research conclusions
This study showed a low prevalence of liver function involvement in patients with acute bronchiolitis with a benign course. However, there was a rising trend in ALT during follow-up. Prolonged hospital stay and positive urine cultures were associated with elevated liver enzymes.
Research perspectives
A proper evaluation of liver function during acute bronchiolitis is needed. Both diagnostic and therapeutic approaches are required to alleviate hepatic involvement in children with acute bronchiolitis.
ACKNOWLEDGEMENTS
We gratefully acknowledge all health care providers and colleagues who provided the required care for infants with acute bronchiolitis in the pediatric, pathology, and radiology departments of Salmaniya Medical Complex, Kingdom of Bahrain.
Footnotes
Institutional review board statement: The study was ethically approved by the Research and Research Ethics Committee for Governmental Hospitals, Salmaniya Medical Complex, Bahrain (Approval No. 27130220).
Informed consent statement: Consent was not needed as the study was retrospective without exposure of the patient's data.
Conflict-of-interest statement: The authors declare no conflict of interest for this article.
STROBE statement: The authors have read the STROBE Statement—checklist of items, and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: June 14, 2022
First decision: August 18, 2022
Article in press: October 10, 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Bahrain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Shabrawi MH, Egypt; Jha P, United States S-Editor: Chen YL L-Editor: Webster JR P-Editor: Chen YL
Contributor Information
Hasan M Isa, Department of Pediatrics, Salmaniya Medical Complex, Manama 12, Bahrain; Department of Pediatrics, Arabian Gulf University, Manama 26671, Bahrain.
Asma Z Hasan, Department of Pediatrics, Sulwan Psychiatric Hospital, Manama 973, Bu Quwah, Bahrain.
Sara I Khalifa, Department of Pediatrics, Salmaniya Medical Complex, Manama 12, Bahrain.
Sana S Alhewaizem, Department of Pediatrics, Dream Reem Medical Center, Muharraq 50573, Bahrain.
Abdulrahman D Mahroofi, Department of Pediatrics, King Hamad University Hospital, Muharraq 24343, Bahrain.
Fatema N Alkhan, Department of Pediatrics, Salmaniya Medical Complex, Manama 12, Bahrain.
Mohammed Al-Beltagi, Department of Pediatrics, Faculty of Medicine, Tanta University, Tanta 31527, Algharbia, Egypt; Department of Pediatrics, University Medical Center, King Abdulla Medical City, Arabian Gulf University, Manama 26671, Bahrain; Department of Pediatrics, University Medical Center, Dr. Sulaiman Al-Habib Medical Group, Bahrain, Manama 26671, Bahrain. mbelrem@hotmail.com.
Data sharing statement
Data are available upon reasonable request.
References
- 1.Giordano S, Di Gangi M, Failla MC, Bruno L, Falcone V, Dones P. Respiratory syncytial virus bronchiolitis and hypertransaminasemia. Infez Med . 2018;26:81–84. [PubMed] [Google Scholar]
- 2.Piedimonte G, Perez MK. Respiratory syncytial virus infection and bronchiolitis. Pediatr Rev . 2014;35:519–530. doi: 10.1542/pir.35-12-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Maskari N, Mohsin J, Al-Maani A, Al-Macki N, Al-Ismaili S. Atypical Presentations of Respiratory Syncytial Virus Infection: Case Series. Sultan Qaboos Univ Med J . 2016;16:e86–e91. doi: 10.18295/squmj.2016.16.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, Auinger P, Griffin MR, Poehling KA, Erdman D, Grijalva CG, Zhu Y, Szilagyi P. The burden of respiratory syncytial virus infection in young children. N Engl J Med . 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thorburn K, Fulton C, King C, Ramaneswaran D, Alammar A, McNamara PS. Transaminase levels reflect disease severity in children ventilated for respiratory syncytial virus (RSV) bronchiolitis. Sci Rep . 2018;8:1803. doi: 10.1038/s41598-018-20292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silver AH, Nazif JM. Bronchiolitis. Pediatr Rev . 2019;40:568–576. doi: 10.1542/pir.2018-0260. [DOI] [PubMed] [Google Scholar]
- 7.Papoff P, Moretti C, Cangiano G, Bonci E, Roggini M, Pierangeli A, Scagnolari C, Antonelli G, Midulla F. Incidence and predisposing factors for severe disease in previously healthy term infants experiencing their first episode of bronchiolitis. Acta Paediatr. 2011;100:e17–e23. doi: 10.1111/j.1651-2227.2011.02181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nadal D, Wunderli W, Meurmann O, Briner J, Hirsig J. Isolation of respiratory syncytial virus from liver tissue and extrahepatic biliary atresia material. Scand J Infect Dis . 1990;22:91–93. doi: 10.3109/00365549009023125. [DOI] [PubMed] [Google Scholar]
- 9.Kirin BK, Topić RZ, Dodig S. Hepatitis during respiratory syncytial virus infection--a case report. Biochem Med (Zagreb) . 2013;23:112–116. doi: 10.11613/BM.2013.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oh JS, Choi JS, Lee YH, Ko KO, Lim JW, Cheon EJ, Lee GM, Yoon JM. The Relationships between Respiratory Virus Infection and Aminotransferase in Children. Pediatr Gastroenterol Hepatol Nutr . 2016;19:243–250. doi: 10.5223/pghn.2016.19.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenhut M, Thorburn K. Hepatitis associated with severe respiratory syncytial virus-positive lower respiratory tract infection. Scand J Infect Dis . 2002;34:235. doi: 10.1080/00365540110077191. [DOI] [PubMed] [Google Scholar]
- 12.Eisenhut M, Thorburn K, Ahmed T. Transaminase levels in ventilated children with respiratory syncytial virus bronchiolitis. Intensive Care Med . 2004;30:931–934. doi: 10.1007/s00134-004-2236-2. [DOI] [PubMed] [Google Scholar]
- 13.Do LA, Bryant JE, Tran AT, Nguyen BH, Tran TT, Tran QH, Vo QB, Tran Dac NA, Trinh HN, Nguyen TT, Le Binh BT, Le K, Nguyen MT, Thai QT, Vo TV, Ngo NQ, Dang TK, Cao NH, Tran TV, Ho LV, Farrar J, de Jong M, van Doorn HR. Respiratory Syncytial Virus and Other Viral Infections among Children under Two Years Old in Southern Vietnam 2009-2010: Clinical Characteristics and Disease Severity. PLoS One . 2016;11:e0160606. doi: 10.1371/journal.pone.0160606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakalli I. Liver Dysfunction in Severe Sepsis from Respiratory Syncytial Virus. J Pediatr Intensive Care . 2018;7:110–114. doi: 10.1055/s-0037-1612609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee Y, Yi DY, Lee YM, Choi SY, Choi YJ, Lee KJ. A Multicenter Study of Real-world Practice for Management of Abnormal Liver Function Tests in Children with Acute Infectious Diseases. J Korean Med Sci . 2021;36:e310. doi: 10.3346/jkms.2021.36.e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fares M, Mourad S, Rajab M, Rifai N. The use of C-reactive protein in predicting bacterial co-Infection in children with bronchiolitis. N Am J Med Sci . 2011;3:152–156. doi: 10.4297/najms.2011.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Branco RG, Tasker RC. Glycemic level in mechanically ventilated children with bronchiolitis. Pediatr Crit Care Med . 2007;8:546–550. doi: 10.1097/01.PCC.0000288712.67749.45. [DOI] [PubMed] [Google Scholar]
- 18.Isa HM, Mohroofi AD, Alkhan FN, Hasan AZ, Alkubisi MM, Alhewaizem SS, Khalifa SI, Alromaihi NG. C-Reactive Protein Levels in Children with Acute Bronchiolitis. Int J Pediatr . 2022;2022:1311936. doi: 10.1155/2022/1311936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogoina D. Fever, fever patterns and diseases called 'fever'--a review. J Infect Public Health . 2011;4:108–124. doi: 10.1016/j.jiph.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Alejandre C, Balaguer M, Guitart C, Torrús I, Felipe A, Launes C, Cambra FJ, Jordan I. Procalcitonin-guided protocol decreased the antibiotic use in paediatric patients with severe bronchiolitis. Acta Paediatr . 2020;109:1190–1195. doi: 10.1111/apa.15148. [DOI] [PubMed] [Google Scholar]
- 21.Desmarest M, Aupiais C, Le Gal J, Tourteau L, Le Coz J, de Paepe E, Titomanlio L, Faye A. [Value of procalcitonin for infants with bronchiolitis in an emergency department] Arch Pediatr . 2017;24:1060–1066. doi: 10.1016/j.arcped.2017.08.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.