Abstract
The apical membrane antigen 1 (AMA-1) family is a promising family of malaria blood-stage vaccine candidates that have induced protection in rodent and nonhuman primate models of malaria. Correct conformation of the protein appears to be essential for the induction of parasite-inhibitory responses, and these responses appear to be primarily antibody mediated. Here we describe for the first time high-level secreted expression (over 50 mg/liter) of the Plasmodium vivax AMA-1 (PV66/AMA-1) ectodomain by using the methylotrophic yeast Pichia pastoris. To prevent nonnative glycosylation, a conservatively mutagenized PV66/AMA-1 gene (PV66Δglyc) lacking N-glycosylation sites was also developed. Expression of the PV66Δglyc ectodomain yielded similar levels of a homogeneous product that was nonglycosylated and was readily purified by ion-exchange and gel filtration chromatographies. Recombinant PV66Δglyc43–487 was reactive with conformation-dependent monoclonal antibodies. With the SBAS2 adjuvant, Pichia-expressed PV66Δglyc43–487 was highly immunogenic in five rhesus monkeys, inducing immunoglobulin G enzyme-linked immunosorbent assay titers in excess of 1:200,000. This group of monkeys had a weak trend showing lower cumulative parasite loads following a Plasmodium cynomolgi infection than in the control group.
Plasmodium vivax and Plasmodium falciparum are the two most important human malaria parasites. P. vivax, although causing less mortality than P. falciparum, has an enormous socioeconomic impact, particularly in South America and Asia. The difficulties in the treatment of P. falciparum malaria due to the spread of chloroquine resistance seem likely to occur for P. vivax in the future (23). The most cost-effective measure to control infectious diseases like malaria is a vaccine, and effective malaria vaccines are still not available.
Accumulated data, including those from nonhuman primate (5, 9) and rodent (1, 7) studies, have indicated that the apical membrane antigen 1 (AMA-1) family of molecules are targets for protective immune responses. In all Plasmodium species reported to date, with the exception of P. falciparum (26), AMA-1 is synthesized de novo as a 66-kDa protein. Analysis of disulfide bond arrangements (17) and intraspecies sequence polymorphism due to point mutations (22, 25, 31) reveals clustering of mutations in particular domains of the molecule. Despite this, between species there is considerable conservation of primary and predicted secondary amino acid structures, and evidence to date indicates that protection invoked by AMA-1 is directed at conformational epitopes (1, 5, 7, 10) located in the AMA-1 ectodomain (1). Immunization with reduced AMA-1 fails to induce parasite-inhibitory antibodies (1, 9), and so far only those monoclonal antibodies (MAbs) that recognize reduction-sensitive conformational AMA-1 epitopes have been shown to inhibit parasite multiplication in vitro for Plasmodium knowlesi (8, 30) and P. falciparum (20). This indicates that for an AMA-1 vaccine the correct conformation will be critical. Because eukaryotic expression systems are likely to produce this material directly, we have focused on production of vaccine-quality P. vivax AMA-1 (PV66/AMA-1) (4) by using the methylotrophic yeast Pichia pastoris.
P. pastoris is rapidly becoming a very popular tool for the heterologous expression of recombinant proteins due to the ease with which it can be manipulated and the high expression levels of recombinant proteins that have been reported (reviewed in reference 6). In addition, P. pastoris generally fails to hyperglycosylate recombinant proteins (15, 32), although hyperglycosylation has been reported (28), and proper folding of recombinant proteins can be expected in this eukaryotic expression system. For high-level production of PV66/AMA-1, as a first step towards clinical testing of this protein, we have exploited the P. pastoris secretion expression system. We have characterized the recombinant protein and determined its immunogenicity in a nonhuman primate model with an adjuvant that is being used in clinical trials. We have also analyzed the boosting effect on the immune system of a live parasite challenge subsequent to PV66/AMA-1 immunization, using Plasmodium cynomolgi, a parasite of macaque monkeys that is phylogenetically closely grouped with P. vivax.
MATERIALS AND METHODS
Cloning of PV66/AMA-1 and generation of recombinant P. pastoris.
Recombinant DNA procedures were performed as described by Sambrook et al. (27). The P. pastoris (GS115) expression kit (Invitrogen, Leek, The Netherlands) was used to prepare recombinant P. pastoris clones expressing the PV66/AMA-1 ectodomain (residues 43 to 487) as a secreted protein. Using DNA for (i) the wild-type gene and (ii) a nonglycosylated mutagenized version of the gene (see below) from P. vivax (Sal I strain), the region selected for expression was amplified by PCR with primers A (5′-CGGGATCCTACCGTTGAG-3′) (nucleotides [nt] 127 to 138 and an additional BamHI restriction site) and B (5′-CGGGATCCTATAGTAGCATCTG-3′) (complementary to nt 1450 to 1461 with an additional BamHI restriction site) by using Ultma DNA polymerase (Perkin-Elmer, Foster City, Calif.). The resulting 1,354-bp PCR products were phosphorylated, agarose gel purified by using the Wizard PCR Preps DNA purification system (Promega, Leiden, The Netherlands), and cloned into the dephosphorylated SmaI site of the P. pastoris shuttle vector pHIL-S1 (Invitrogen). Cloned products were fully sequenced by double-stranded DNA protocols with Sequenase enzyme (U.S. Biochemicals, Cleveland, Ohio).
Plasmids pHIL-S1/PV6643–487 and pHIL-S1/PV66Δglyc43–487 (mutagenized form lacking N-glycosylation sites [see below]) were digested with BglII and used to transfect P. pastoris GS115 by electroporation according to the manual for the P. pastoris expression kit. Transfected cells were plated on MD plates (1.34% Yeast Nitrogen Base minus amino acids [Difco, Detroit, Mich.], 1% dextrose, 0.4 mg of biotin [Sigma, St. Louis, Mo.] per liter), and colonies were allowed to grow for 4 days at 30°C. Individual colonies were patched in duplicate on plates containing either 1% dextrose (MD) or 0.5% methanol (MM) as the carbon source, incubated for 2 days at 30°C, and analyzed for protein expression. Colonies growing on dextrose but not, or only slowly, on methanol (Muts phenotype), were picked for further analysis of PV66/AMA-1 expression (18).
Site-directed mutagenesis.
The three consensus sequences for N-linked glycosylation present in the PV66/AMA-1 Sal I strain were mutagenized by using the pAlter II kit (Promega) according to the manufacturer’s protocol and the mutagenesis primers PVm1 (5′-GATCAAAATTCGAACTACAGACACCC-3′) (nt 520 to 545), PVm2 (5′-CCAGATAAAGATGAAAGCT-3′) (nt 667 to 685), and PVm3 (5′-GAGCGCATTTCCCAGAGTACCTGCAAC-3′) (nt 1309 to 1335). Mutations were confirmed by double-stranded DNA sequencing, and a clone containing all three mutations was designated PV66Δglyc.
Protein analysis.
For small-scale induction experiments, P. pastoris clones were grown for 2 days at 30°C in 10 ml of BMGY (1% yeast extract, 2% peptone, 1.34% Yeast Nitrogen Base, 1% glycerol, 0.4 mg of biotin per liter, 0.1 M K-phosphate, pH 6.0) in 50-ml Falcon tubes with vigorous shaking. Cells were harvested by low-speed centrifugation, resuspended in 4 ml of BMMY (BMGY with glycerol replaced by 0.5% methanol), and cultured for an additional 3 days. Cells were harvested, and the culture supernatants were analyzed for the presence of PV66/AMA-1 by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (11) on a 10% gel, using the Miniprotean II system (Bio-Rad, Veenendaal, The Netherlands). Gels were stained with Coomassie brilliant blue and scanned with a Bio-Rad model GS-690 densitometer, and purity was estimated by using Molecular Analyst version 2.1 software (Bio-Rad).
Deglycosylation.
Proteins were deglycosylated by using N-glycosidase F (Boehringer, Mannheim, Germany). PV66/AMA-143–487 present in 25 μl of culture supernatant was denatured by boiling for 10 min in 0.2% SDS–1% β-mercaptoethanol and then incubated overnight at 37°C with 0.2 U of N-glycosidase F in a buffer consisting of 20 mM NaPi (pH 6.8), 10 mM EDTA, 0.1% SDS, 0.5% Nonidet P-40, and 0.5% β-mercaptoethanol (final concentrations). Alternatively, cells were cultured as outlined above, and expression of PV66/AMA-143–487 was induced in the presence of tunicamycin (1 to 100 μg/ml) (Sigma). Samples were analyzed throughout by SDS-PAGE.
Mid-scale production of PV66/AMA-1.
For mid-scale production of PV66/AMA-143–487 and PV66Δglyc43–487, recombinant P. pastoris was cultured in 1-liter baffled flasks (250 ml of BMGY per flask) for 48 h at 30°C with vigorous shaking (final optical density at 600 nm, ≅20). Cells were harvested and resuspended in BMMY at a final optical density at 600 nm of 120 to 160 and then cultured (120 ml per 1-liter baffled flask) for 72 h at 30°C with vigorous shaking. Methanol was added to a final concentration of 0.5% every 24 h. After low-speed centrifugation, the cleared culture supernatant was stored at −80°C until use. Alternatively, P. pastoris was grown in aerobic chemostat cultures. Chemostat cultivation was performed at 30°C in 2-liter Applikon laboratory fermentors with working volumes of 1.5 liters and at a dilution rate of 0.03 h−1. The mineral medium contained the following, per liter of demineralized water: (NH4)2SO4, 5 g; KH2PO4, 10 g; MgSO4 · 7H2O, 2.5 g; EDTA, 150 mg; ZnSO4 · 7H2O, 45 mg; CoCl2 · 6H2O, 3 mg; MnCl2 · 4H2O, 10 mg; CuSO4 · 5H2O, 3 mg; CaCl2 · 2H2O, 45 mg; FeSO4 · 7H2O, 30 mg; NaMoO4 · 2H2O, 4 mg; H3BO3, 10 mg; and KI, 1.0 mg. Filter-sterilized vitamins were added to give the following final concentrations: biotin, 0.5 mg/liter; calcium pantothenate, 10 mg/liter; nicotinic acid, 10 mg/liter; inositol, 250 mg/liter; thiamine · HCl, 10 mg/liter; pyridoxine · HCl, 10 mg/liter; and para-aminobenzoic acid, 2 mg/liter. Glycerol and methanol were added to concentrations of 80 and 10 g/liter, respectively. The pH of the cultures was controlled at 6.0 by an Applikon ADI 1020 biocontroller unit, using 25% (wt/vol) ammonia, which also served as a nitrogen source. A 4% (wt/vol) autoclaved solution of the antifoaming agent Struktol J673 (Struktol Co., Stow, Ohio) in water was added at the same rate as the ammonia titrant. Cultures were stirred at 1,000 rpm and sparged with sterile air (2 liters/min). The dissolved oxygen concentration in the cultures was continuously monitored with an Ingold oxygen electrode and remained above 15% of air saturation. The effluent from four chemostat cultures was collected at 4°C. Supernatants of these cultures typically contained about 80 mg of the product per liter. After removal of the biomass by microfiltration, the culture supernatant was concentrated by ultrafiltration and stored at −80°C until purification.
Protein purification.
Culture supernatants were extensively dialyzed against phosphate-buffered saline (PBS) and then directly used for immunization of rats for MAb production. For material to immunize rhesus monkeys, the supernatant was concentrated by 70% ammonium sulfate precipitation. Pellets were resolubilized in PBS to 5% of the original volume, dialyzed at 4°C extensively against 25 mM Tris-HCl containing 0.03% NaN3 (pH 7.6), and fractionated by ion-exchange fast protein liquid chromatography (Pharmacia, Uppsala, Sweden) on a DEAE 650M column (TosoHaas, Stuttgart, Germany), with 1 mg of total protein loaded per ml of gel. Elution with a linear NaCl gradient (0 to 0.5 M) was monitored at 280 nm, and peak fractions were analyzed by SDS-PAGE, using the Phast System (Pharmacia). Additional purification was obtained by size-exclusion fast protein liquid chromatography on a HiLoad 16/60 Superdex 75 column (Pharmacia) with PBS as the solvent. Peak fractions were analyzed by SDS-PAGE; those containing PV66Δglyc43–487 were pooled and the protein content was estimated by using the Bio-Rad protein assay with bovine serum albumin as a standard. Pooled material was stored aliquoted at −80°C.
MAb production and rabbit immunization.
Rat MAbs were prepared by standard protocols with the Y3 cell line as fusion partner (3) for cells isolated from the spleens of rats (male Lou/M, 8 to 12 weeks old) that had been immunized four times at 2-week intervals with dialyzed culture supernatant, induced in the presence of tunicamycin, containing 10 μg of PV66/AMA-143–487 in polyalphaolephine (Behringwerke, Marburg, Germany) adjuvant (13). Screening and selection of double-cloned cell lines producing the MAbs were performed by enzyme-linked immunosorbent assay (ELISA) and by indirect immunofluorescence assay (IFA) on P. vivax (ONG) schizont-infected erythrocytes that had been obtained from an infected Aotus azarae boliviensis monkey. Rabbits were immunized (25 μg per immunization; one subcutaneous [s.c.] immunization with Freund complete adjuvant followed by three s.c. immunizations with Freund incomplete adjuvant, spaced 4 weeks apart) with material either from PV66Δglyc43–487 expression cultures (rabbit 2) or from control human serum albumin (HSA) expression cultures (rabbit 3).
In vitro inhibition of P. vivax invasion assay.
P. vivax Chesson strain blood-stage parasites were obtained from infected A. azarae boliviensis monkeys when parasitemia was between 0.25 and 1.0% and the parasites were at the mature trophozoite stage of development. Parasites were isolated by density gradient centrifugation as previously described (20). In vitro cultures enriched with Aotus reticulocytes obtained from an uninfected monkey to yield a final 3% hematocrit with starting parasitemias of 2.8% (experiment 1) and 0.5% (experiment 2) were incubated under conditions previously described (19). Triplicate 100-μl cultures containing either no added immunoglobulin G (IgG) or purified and extensively dialyzed IgG at 2.0 mg/ml obtained 6 weeks after the final immunization of rabbits 2 and 3 (see above) were harvested 25 h later, and thin films were prepared and Giemsa stained. After masking, parasitemia determinations were made by counting ring-stage parasites.
Immunization of rhesus monkeys.
Ten rhesus monkeys (Macaca mulatta) of either sex, 3 to 4 years of age and weighing 3 to 6 kg, were randomly allocated to two groups of five animals. Both groups were immunized intramuscularly three times at weeks 0, 4, and 11 (at two sites) with material that had been freshly formulated with 500 μl of the adjuvant SBAS2 (29). Group I (monkeys BCP, N6B, V8T, 16B, and VH2) received three immunizations with 100 μg of purified PV66Δglyc43–487. In order to control for the effects of non-PV66Δglyc43–487 components that copurified, group II (monkeys VH9, VOG, AJW, VC6, and 47B) was immunized with a pool of material that was fractionated by exactly the same protocol but that was prepared from P. pastoris cultures of a clone expressing HSA at high levels (2). In this way the same non-PV66Δglyc43–487 components were expected to be present in both preparations at the same concentrations.
Infection with P. cynomolgi.
In order to determine whether live parasite infection after immunization induced a specific boost of the PV66/AMA-1 antibody response, 4 weeks after the third immunization heparinized blood from a donor rhesus monkey infected with P. cynomolgi M strain parasites was diluted in RPMI to contain 5 × 105 parasites/ml, and 1 ml of this was injected intravenously into all animals from groups I and II. Blood was obtained by finger prick on days 5, 6, 7, 8, 9, 11, 13, and 15 after infection, and determinations of parasitemia were made on duplicate Giemsa-stained thin blood films. To cure the disease, all animals were subsequently drug treated (orally with pyrimethamine [1 mg/kg] on three consecutive days). Statistical comparisons between the groups were by a two-tailed, unpaired Student t test.
Masking.
The determination of parasitemia and IFA titers and all rhesus monkey handling were performed by individuals who were unaware of the experimental group to which animals had been assigned.
ELISA and IFA.
ELISA was performed in triplicate on serum samples in 96-well flat-bottomed microtiter plates coated with 0.4 μg of purified PV66Δglyc43–487 per ml by published methods (16). The secondary antibody for rhesus monkey sera was goat anti-human IgG conjugated to alkaline phosphatase (Pierce, Rockford, Ill.). IFA was performed as previously described (8) with schizont-infected erythrocytes isolated either from an infected Aotus monkey (P. vivax ONG), from infected rhesus monkeys (P. cynomolgi M and P. knowlesi Nuri), or from in vitro culture (P. falciparum 7G8). Secondary antibodies were fluorescein isothiocyanate-conjugated goat anti-human IgG (heavy plus light chains) (Kirkegaard & Perry, Gaithersburg, Md.).
T-lymphocyte proliferation assay.
Peripheral blood mononuclear cells were isolated by using lymphocyte separation medium (Organon Teknica, Durham, N.C.) and resuspended at 4 × 106 cells/ml in RPMI 1640–10% fetal calf serum (Life Technologies, Inchinnan, United Kingdom) containing 40 μg of gentamicin per ml. Triplicates were prepared in 96-well round-bottomed plates (Costar, Cambridge, Mass.) containing 2 × 105 cells per well in the presence of 1 μg of purified PV66Δglyc43–487 per ml, or medium only, in a total volume of 100 μl of complete medium. After being cultured for 72 h, the cells were pulsed with 0.5 μCi of [3H]thymidine (1.0 Ci/mmol) (Amersham, Buckinghamshire, United Kingdom) for 18 h and harvested on glass fiber filters (Packard, Groningen, The Netherlands). [3H]thymidine incorporation was determined by scintillation counting. Background levels of lymphoproliferation in medium-only controls were comparable in the two groups. Proliferative responses were expressed as stimulation indices (SIs), which represent the ratio between the mean proliferation after stimulation and the mean proliferation of medium-only controls.
Animal use.
All experimental animal work in this study was carried out under protocols approved by the independent Institutional Animal Care and Use Committee.
Nucleotide sequence accession number.
The GenBank nucleotide sequence accession number for P. vivax PV66/AMA-1 (Sal I strain) is Y16950.
RESULTS
PV66/AMA-143–487 is expressed at high levels in P. pastoris.
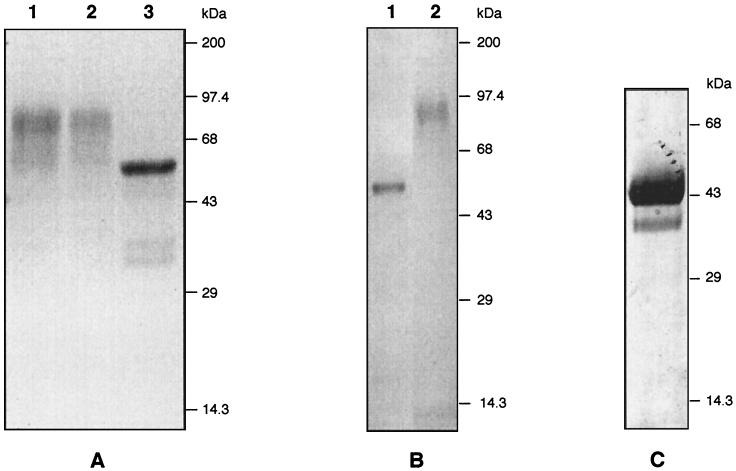
The PV66/AMA-1 gene fragment encoding the ectodomain (amino acids 43 to 487) was cloned into the P. pastoris pHIL-S1 vector, which contains the acid phosphatase signal sequence for secretion of the recombinant protein. After transfection, phenotype selection (Muts), and expression screening (18), 10 PV66/AMA-1 expression-positive P. pastoris clones were analyzed by SDS-PAGE for expression of PV66/AMA-143–487. All of these clones showed high-level expression of a protein in the culture supernatant (routinely 50 mg/liter in small-scale induction experiments as determined by scanning densitometry of Coomassie blue-stained gels) with a heterogeneous molecular mass (Mw) of 50 to 90 kDa (Fig. 1A, lane 1). The expected Mw of PV66/AMA-143–487 is 50 kDa, and, since PV66/AMA143–487 contains three possible N-glycosylation sites, it was possible that the higher Mw might be due to N glycosylation. Hyperglycosylation is not common in P. pastoris (reviewed in reference 6) but has been reported for some other heterologous expressed proteins (28). Treatment of the secreted PV66/AMA-143–487 protein with N-glycosidase F reduced the Mw to the expected 50 kDa (Fig. 1A, lane 3), demonstrating N glycosylation of the protein. The conditions used fully denature the protein prior to deglycosylation and thus do not provide material appropriate for vaccine testing. Tunicamycin treatment was partially effective at blocking glycosylation only at a high concentration (100 μg/ml), but this significantly reduced levels of protein expression (not shown). Because of the complications for the production of homogeneous material due to unwanted glycosylation and because of the unknown effects that such glycosylation may have on the conformation of PV66/AMA-1, we used site-directed mutagenesis to develop a variant that exploited the lack of conservation of N-glycosylation sites in Plasmodium AMA-1. For two of the three potential N-glycosylation sites in the P. vivax Sal I strain AMA-1 sequence, residues present in the equivalent position for P. falciparum AMA-1 were introduced (Ser178 was replaced by Asn178, and Asn226 was replaced by Asp226). The third glycosylation site, Asn441-Ser442-Thr443, is present in all published AMA-1 sequences except that of Plasmodium chabaudi, where Asn441 is replaced by Glu441 (21). This substitution was also introduced to yield PV66Δglyc43–487.
FIG. 1.
SDS-PAGE analysis of PV66/AMA-1 expression in P. pastoris. (A) N-glycosidase F digestion of secreted recombinant PV66/AMA-143–487. PV66/AMA-143–487, present in the culture supernatant after methanol induction for 72 h, was digested with N-glycosidase F. Samples representing 10 μl of culture supernatant were analyzed. Lanes: 1, culture supernatant; 2, mock N-glycosidase F digestion; 3, N-glycosidase F-digested material. (B) Expression of PV66Δglyc43–487. Ten microliters of culture supernatant, at 48 h postinduction, was analyzed. Lanes: 1, PV66Δglyc43–487; 2, wild-type PV66/AMA-143–487. (C) Purified mid-scale-produced PV66Δglyc43–487. PV66Δglyc43–487 was purified by ion-exchange and gel filtration chromatographies from a mid-scale culture and chemostat fermentor supernatant. Ten micrograms of purified material was loaded on a nonreducing SDS gel. All gels were stained with Coomassie brilliant blue.
Small-scale and mid-scale expression of this mutagenized version, PV66Δglyc43–487, in P. pastoris resulted in secretion of a homogeneous nonglycosylated protein showing little degradation (Fig. 1B, lane 1). Estimated expression levels, based on densitometry of Coomassie blue-stained gels, were also 50 mg/liter in small-scale and mid-scale cultures. Aerobic chemostat cultures resulted in expression levels of approximately 80 mg/liter, and slightly higher levels of degradation products were observed (data not shown).
Mutagenized PV66Δglyc43–487 expressed in P. pastoris attains the natural conformation.
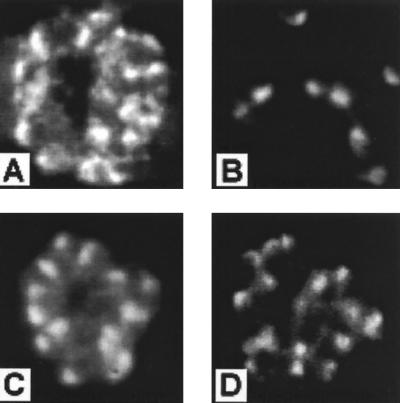
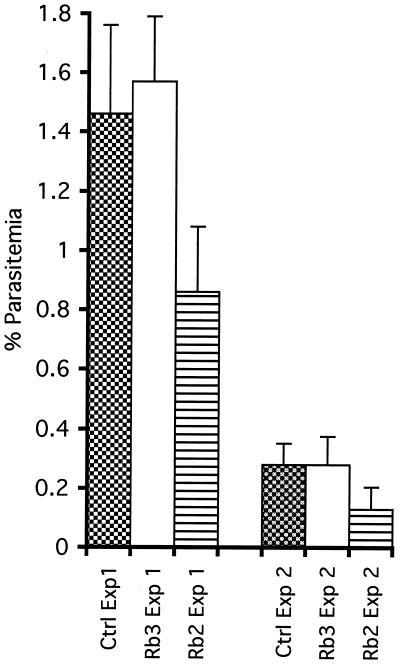
Two rat MAbs, 3A9 and 5G2, developed against PV66/AMA-143–487 expressed in the presence of tunicamycin were selected based on reactivity with native P. vivax conformational epitopes that were lost on Western blotting under reducing conditions. The antibodies did not react with PV66/AMA-143–487 expressed by Escherichia coli but were reactive with the native protein on immunofluorescence with P. vivax schizont-infected erythrocytes and merozoites (Fig. 2A and B), revealing an apical merozoite staining typical of AMA-1 (24, 26). MAb 3A9 was also reactive with schizont-infected erythrocytes from P. cynomolgi, P. knowlesi, and P. falciparum, whereas 5G2 reacted only with P. cynomolgi. The PV66/AMA-143–487 and PV66Δglyc43–487 proteins were equally reactive with these MAbs by ELISA (not shown), indicating that the mutagenesis had not interfered with the structure of the protein. This was confirmed by using antibodies raised in rabbit 2 against PV66Δglyc43–487, which were reactive by ELISA with recombinant PV66/AMA-143–487 (not shown) and, more importantly were also reactive by IFA with mature schizonts (Fig. 2C), free merozoites, and young ring stages of P. vivax. Preimmunization serum from rabbit 2 and serum obtained following immunization of rabbit 3 were nonreactive in IFA. In addition, in two separate experiments purified rabbit total IgG from animals immunized with PV66Δglyc43–487, but not that from rabbits immunized with the control HSA preparation, inhibited P. vivax invasion in vitro (Fig. 3), indicating that the mutagenesis did not alter functionally important, antibody-targetable structures of the protein.
FIG. 2.
Distribution of staining on immunofluorescence analysis of culture supernatants by MAbs 5G2 (A) and 3A9 (B) and of serum from rabbit 2 (diluted 1:1000) (C) and rhesus monkey VH2 (diluted 1:500) (D). Merozoites of P. vivax ONG strain parasites developing within schizont-infected erythrocytes (A, C, and D) and as free merozoites following release from schizonts (B) show the typical apically restricted pattern of staining consistent with AMA-1 localization in rhoptries (24). Identical patterns of distribution were seen for all serum samples from rhesus monkeys immunized with PV66Δglyc43–487.
FIG. 3.
Results of assays to evaluate in vitro inhibitory effects of IgG isolated after immunization of rabbit 2 (PV66Δglyc43–487) and rabbit 3 (control HSA preparation). Ring-stage parasitemias are shown at 25 h after initiation of invasion cultures in two separate experiments (Exp 1 and Exp 2) in which no extra IgG was present (Ctrl) and in which IgG at 2.0 mg/ml from rabbit 2 (Rb2) and rabbit 3 (Rb3) was present. The standard deviations for triplicate samples are shown.
PV66Δglyc43–487/SBAS2 is highly immunogenic in nonhuman primates.
For immunogenicity studies, mid-scale and chemostat-produced culture supernatants were combined and purified by ion-exchange and size exclusion chromatographies. Purity was assessed (by scanning densitometry of Coomassie blue-stained SDS-polyacrylamide gels) to be approximately 95%, with the protein running as a single band with an apparent Mw of 43 kDa under nonreducing conditions (Fig. 1C). Analysis by Western blotting with rabbit polyclonal antibodies against PV66Δglyc43–487 showed that the vast majority of the material elsewhere on the gel was degraded PV66Δglyc43–487 protein, as none of the reactive bands were present in the identically prepared HSA control material (not shown). Under reducing conditions, approximately 80% of the purified PV66Δglyc43–487 protein runs as a single band, and additional discrete degradation products are observed, which presumably are held together by disulfide bridges (data not shown). This purified material was formulated with SBAS2 adjuvant. Rhesus monkeys have been widely used as a model for evaluating immunogenicity, so this species was selected for these studies.
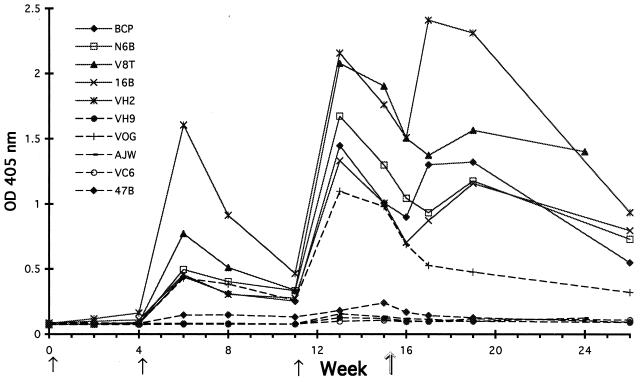
Sera obtained from group I and group II rhesus monkeys 2 weeks after the third immunization (week 13) were assessed for PV66/Δglyc43–487-specific antibody by ELISA. All group I animals showed strong responses to PV66Δglyc43–487, with end titers of 1:200,000 and above. Group II animals had been immunized with the same fraction of P. pastoris expression supernatant except that the expression clone was HSA in place of PV66Δglyc43–487. All of these animals except VOG had very low ELISA titers against the purified PV66Δglyc43–487 fraction. Western blotting of all preimmune and week 13 rhesus monkey sera indicated that the response in VOG was directed at copurifying, non-PV66Δglyc43–487-related material and also confirmed that all group I animals had strong PV66Δglyc43–487-specific antibody responses (data not shown). The development of antibody responses during the immunization period and after P. cynomolgi infection is shown in Fig. 4. Boosting of the responses is evident after the second and third immunizations. It is notable that this boost is also seen after infection, but a stable antibody level is not maintained. Only in monkey VOG was no boosting effect seen after infection, supporting the idea that the response seen in VOG was to nonmalarial molecules in the ELISA coating preparation that copurified with PV66Δglyc43–487. All group I animals showed significant lymphoproliferative responses (SI values of 2.3, 2.4, 2.4, 2.5, and 5.4) in peripheral blood samples taken at week 13. Control group II animals had SI values of 0.5, 0.9, 1.0, 1.1, and 2.4 (monkey VC6) at 13 weeks.
FIG. 4.
Specific IgG levels in immunized rhesus macaques. Serum samples obtained over a 26-week period were tested at a 1:5,000 dilution in a PV66Δglyc43–487 IgG ELISA. Time points for immunization are indicated by solid arrows, and the day of infection with the P. cynomolgi M strain is indicated by the outline arrow. OD, optical density.
By IFA on serum samples taken at week 13, all group I sera were positive and all group II sera were negative at a 1:400 dilution on P. vivax schizont-infected erythrocytes. Immunofluorescence patterns were typical of AMA-1-directed responses (Fig. 2D) in showing punctate apical merozoite staining which often could be resolved into a double dot, presumed to represent the two rhoptries. End point IFA titers were determined for 16B and VH2, the two monkeys having, respectively, the lowest and highest ELISA reactivities at week 13. Both had IFA end point titers of 1:12,800 measured against P. vivax and P. cynomolgi schizont-infected erythrocytes.
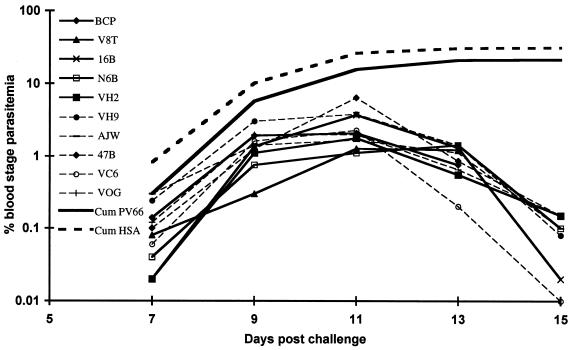
The infection with P. cynomolgi was instigated in order to evaluate the effect of a live parasite challenge on the specific PV66/AMA-1 immune response. The time course for parasitemia is shown for individual monkeys and as a cumulative parasitemia for each of the two groups in Fig. 5. Because schizont-infected erythrocytes for this parasite can sequester from the circulation, time points for sampling were selected at 48-h periods when the great majority of the parasites were at the ring or young trophozoite stage. Although animals in both groups developed substantial parasitemias, there was an unexpected trend suggesting that there may be some weak P. cynomolgi-inhibitory effects evoked by PV66/AMA-1 immunization, particularly early in the infection. This trend is most evident at day 7, where there is a P value of 6 (t test) for the difference between the two groups; this level of significance decreases with time.
FIG. 5.
Parasitemias of individual monkeys following P. cynomolgi infection in groups immunized with PV66/AMA-1 (solid lines) or an equivalent fraction from an HSA expression clone (dashed lines). Also shown are the cumulative (Cum) parasitemias for the entire group of PV66/AMA-1-immunized animals and for HSA-immunized animals.
DISCUSSION
AMA-1 is one of the most promising components for a malaria blood-stage vaccine identified to date. MAbs reactive with AMA-1 and capable of inhibiting erythrocyte invasion in vitro recognize disulfide bond-dependent conformational epitopes (9, 30), and only the properly folded protein confers protection (1, 7, 10). We therefore considered it likely that a eukaryotic expression system will most readily produce AMA-1 material directly qualified for vaccine evaluation. In this study we show that the eukaryotic expression system P. pastoris can be utilized to secrete high levels of conformationally intact PV66/AMA-143–487. To our knowledge this is the first report of PV66/AMA-1 protein production in any system. High-level production and simple downstream processing procedures result in less expensive products, and inexpensive vaccines are urgently needed for use in developing countries.
Initial expression in P. pastoris of the PV66/AMA-1 ectodomain resulted in secretion of a heterogeneous product not ideally suited for vaccine studies. Expression of a mutagenized form of PV66/AMA-1, where N-glycosylation sites had been conservatively mutagenized, resulted in secretion of a product that migrates with a discrete Mw on SDS-PAGE and is easily purified to approximately 95% homogeneity. During mid-scale production and in a slightly enhanced form during chemostat fermentation, some degradation of the recombinant protein seems to occur. Preliminary results from nonoptimized fed-batch fermentation at a 15-liter scale have yielded expression levels approaching 1 g/liter but also higher levels of degradation (26a). This suggests that improvements can be made to the fed-batch fermentation protocol. In addition, a good manufacturing practice (GMP) protocol will need to be developed to obtain large amounts of highly homogeneous, well-defined vaccine material.
The use of PV66Δglyc43–487 necessitated comparison with PV66/AMA-143–487 and with native PV66/AMA-1 of parasite origin. Two MAbs recognizing two different conformational epitopes were strongly reactive with all three forms of the protein, suggesting that the PV66Δglyc43–487 conformation was broadly comparable with the native form, and the conformational integrity of this material was confirmed by analysis of rabbit antibodies after immunization. As a prelude to further development of this material for possible clinical testing, the immunogenicity of PV66Δglyc43–487 in rhesus monkeys was assessed, as a common model for human responses. We were also interested to determine whether exposure to live parasites would boost the AMA-1-specific response. However, P. vivax does not infect rhesus monkeys. P. cynomolgi has been closely clustered with P. vivax in phylogenetic analysis based on small-subunit rRNA sequencing (14). The two AMA-1 sequences differ at 13% of the amino acid residues (13). We therefore assumed that sufficient epitopes would be shared by the two AMA-1 species to determine whether a boosting effect was induced by infection.
Rhesus monkey responses to PV66Δglyc43–487 formulated with SBAS2 showed the high immunogenicity of the protein in this particular formulation. Both B- and T-cell responses were evident in all monkeys after three immunizations, and antibodies were fully reactive with parasite-derived AMA-1 as shown by the positive IFA results. For all but one group I monkey, peak IgG values were reached after the third immunization. One monkey reached peak values only after exposure to live parasites. For all group I monkeys these peak IgG levels were maintained only short term. However, all animals showed a temporary increase in specific IgG levels as a result of exposure to live parasite infection. This is important, as parasite-inhibitory AMA-1 responses are antibody dependent (1, 5, 7, 10), and a correlation has been demonstrated between antibody levels and in vivo protection (1). Although specific T-cell lymphoproliferative SIs in rhesus monkeys are generally low, after three immunizations peripheral blood lymphocytes from all animals in group I contained specifically proliferating T cells upon stimulation with PV66Δglyc43–487. One animal from group II, VC6, also showed a significant SI on stimulation with PV66 at week 13. This suggests that at a cellular level VC6 mounted a response against the non-PV66 components shared by the two immunizing preparations.
Early in the course of P. cynomolgi infection, there is a weak trend suggestive of a parasite-inhibitory effect in the animals immunized with PV66/AMA-1. If this effect is real, one interpretation is that small amounts of functional antibody are cross-reactive between the parasite species, and these antibodies become limiting in the system as parasite growth outstrips antibody production. If this is the case, the effect may be accentuated because of the heterologous challenge system used in this study.
In this study we have shown that high levels of expression of PV66/AMA-1 can be obtained in a yeast expression system, that a mutagenized, nonglycosylated variant of PV66/AMA-1 is correctly folded and evokes antibodies that recognize the native parasite, and that in rabbits this antigen can induce parasite-inhibitory antibodies as assessed in vitro. This mutagenized form has been used to immunize rhesus monkeys with an adjuvant that is currently in clinical trials as an adjuvant to other malarial proteins. The monkeys developed high specific antibody levels and were primed to respond to live parasite infection. Future work on the evaluation of PV66/AMA-1 as a potential vaccine component will include an evaluation of the in vitro inhibitory activities of the antibodies induced in rhesus monkeys against homologous and heterologous strains of P. vivax. Due to the difficulties of in vitro cultivation of P. vivax, this study will be run in parallel with an evaluation of PV66/AMA-1-induced protection against P. vivax in saimiri monkeys which is currently being planned.
ACKNOWLEDGMENTS
We are grateful to W. E. Collins for providing parasite stocks, to J. W. Barnwell for initial screening of MAbs, to N. Garçon and J. Cohen of SmithKline Beecham Biologicals for the generous gift of formulated PV66/AMA-1/SBAS2, and to J. P. van Dijken for stimulating discussions. M. Brandhorst, K. A. Bangma, D. Maljers, and M. Zomerdijk are acknowledged for excellent technical assistance.
This work was supported in part by the European Commission, grants CT 92-0147 and CT 94-0275 from the STD-3 programme of DG XII.
REFERENCES
- 1.Anders R F, Crewther P E, Edwards S, Margetts M, Matthew M L S M, Pollock B, Pye D. Immunisation with recombinant AMA-1 protects mice against infection with Plasmodium chabaudi. Vaccine. 1998;16:240–247. doi: 10.1016/s0264-410x(97)88331-4. [DOI] [PubMed] [Google Scholar]
- 2.Barr K A, Hopkins S A, Sreekrishna K. Protocol for efficient secretion of HSA developed from Pichia pastoris. Pharm Eng. 1992;12:48–51. [Google Scholar]
- 3.Bazin H, editor. Rat hybridomas and rat monoclonal antibodies. Boca Raton, Fla: CRC Press, Inc.; 1990. [Google Scholar]
- 4.Cheng Q, Saul A. Sequence analysis of the apical membrane antigen 1 (AMA-1) of Plasmodium vivax. Mol Biochem Parasitol. 1994;65:183–187. doi: 10.1016/0166-6851(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 5.Collins W E, Pye D, Crewther P E, Vandenberg K L, Galland G G, Sulzer A J, Kemp D J, Edwards S J, Coppel R L, Sullivan J S, Morris C L, Anders R F. Protective immunity induced in squirrel monkeys with recombinant apical membrane antigen-1 of Plasmodium fragile. Am J Trop Med Hyg. 1994;51:711–719. doi: 10.4269/ajtmh.1994.51.711. [DOI] [PubMed] [Google Scholar]
- 6.Cregg J M, Vedvick T S, Raschke W C. Recent advances in the expression of foreign genes in Pichia pastoris. Biotechnology. 1993;11:905–910. doi: 10.1038/nbt0893-905. [DOI] [PubMed] [Google Scholar]
- 7.Crewther P E, Matthew M L S M, Flegg L H, Anders R H. Protective immune responses to apical membrane antigen 1 of Plasmodium chabaudi involve recognition of strain-specific epitopes. Infect Immun. 1996;64:3310–3317. doi: 10.1128/iai.64.8.3310-3317.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deans J A, Alderson T, Thomas A W, Mitchell G H, Lennox E S, Cohen S. Rat monoclonal antibodies which inhibit the in vitro multiplication of Plasmodium knowlesi. Clin Exp Immunol. 1982;49:297–309. [PMC free article] [PubMed] [Google Scholar]
- 9.Deans J A, Jean W C. Structural studies on a putative protective Plasmodium knowlesi merozoite antigen. Mol Biochem Parasitol. 1987;26:155–166. doi: 10.1016/0166-6851(87)90139-3. [DOI] [PubMed] [Google Scholar]
- 10.Deans J A, Knight A M, Jean W C, Waters A P, Cohen S, Mitchell G H. Vaccination trials in rhesus monkeys with a minor, invariant, Plasmodium knowlesi 66 kD merozoite antigen. Parasite Immunol. 1988;10:535–552. doi: 10.1111/j.1365-3024.1988.tb00241.x. [DOI] [PubMed] [Google Scholar]
- 11.Doucet J P, Trifaro J M. A discontinuous and highly porous SDS-polyacrylamide slab-gel system of high resolution. Anal Biochem. 1988;168:265–271. doi: 10.1016/0003-2697(88)90317-x. [DOI] [PubMed] [Google Scholar]
- 12.Dutta S, Malhotra P, Chauhan V S. Sequence analysis of apical membrane antigen-1 (AMA-1) of Plasmodium cynomolgi bastianelli. Mol Biochem Parasitol. 1995;73:267–270. doi: 10.1016/0166-6851(95)00112-e. [DOI] [PubMed] [Google Scholar]
- 13.Enders B, Hundt E, Bernhardt D, Schorlemmer H, Weinmann E, Küpper H. Comparative studies on the influence of different adjuvants on serum conversion after immunization with tetanus toxoid and recombinant malarial and viral antigens in mice and monkeys. Modern approaches to new vaccines. Cold Spring Harbor Symp Quant Biol. 1990;90:29–45. [Google Scholar]
- 14.Escalante A A, Ayala F J. Phylogeny of the malarial genus Plasmodium, derived from rRNA gene sequences. Proc Natl Acad Sci USA. 1994;91:11373–11377. doi: 10.1073/pnas.91.24.11373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grinna L S, Tschopp J F. Size distribution and general structural features of N-linked oligosaccharides from the methylotrophic yeast Pichia pastoris. Yeast. 1989;5:107–115. doi: 10.1002/yea.320050206. [DOI] [PubMed] [Google Scholar]
- 16.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- 17.Hodder A N, Crewther P E, Matthew M L S M, Reid G E, Moritz R L, Simpson R J, Anders R F. The disulfide bond structure of Plasmodium apical membrane antigen-1. J Biol Chem. 1996;271:29446–29452. doi: 10.1074/jbc.271.46.29446. [DOI] [PubMed] [Google Scholar]
- 18.Kocken C H M, Thomas A W. Rapid screening and mapping of conformational epitopes expressed in the secretion expression system Pichia pastoris. Anal Biochem. 1996;239:111–113. doi: 10.1006/abio.1996.0299. [DOI] [PubMed] [Google Scholar]
- 19.Kocken C H M, van der Wel A, Rosenwirth B, Thomas A W. Plasmodium vivax: in vitro anti-parasitic effect of cyclosporins. J Exp Parasitol. 1996;84:439–443. doi: 10.1006/expr.1996.0132. [DOI] [PubMed] [Google Scholar]
- 20.Kocken C H M, van der Wel A M, Dubbeld M A, van de Rijke F M, van Gemert G J, van der Linde X, Bannister L H, Janse C, Waters A P, Thomas A W. Precise timing of expression of a Plasmodium falciparum derived transgene in P. berghei is a critical determinant of subsequent subcellular localisation. J Biol Chem. 1998;273:15119–15124. doi: 10.1074/jbc.273.24.15119. [DOI] [PubMed] [Google Scholar]
- 21.Marshall V M, Peterson M G, Lew A W, Kemp D J. Structure of the apical membrane antigen-1 (AMA-1) of Plasmodium chabaudi. Mol Biochem Parasitol. 1989;37:281–284. doi: 10.1016/0166-6851(89)90160-6. [DOI] [PubMed] [Google Scholar]
- 22.Marshall V M, Zhang L, Anders R F, Coppel R L. Diversity of the vaccine candidate AMA-1 of Plasmodium falciparum. Mol Biochem Parasitol. 1996;77:109–113. doi: 10.1016/0166-6851(96)02583-2. [DOI] [PubMed] [Google Scholar]
- 23.Murphy G S, Basri H, Purnomo, Andersen E M, Bangs M J, Mount D L, Gorden J, Lal A A, Purwokusomo A R, Harjosuwarno S. Vivax malaria resistant to treatment and prophylaxis with chloroquine. Lancet. 1993;341:96–100. doi: 10.1016/0140-6736(93)92568-e. [DOI] [PubMed] [Google Scholar]
- 24.Narum D L, Thomas A W. Differential localisation of full-length and processed forms of PF83/AMA-1, an apical membrane antigen of Plasmodium falciparum merozoites. Mol Biochem Parasitol. 1994;67:59–68. doi: 10.1016/0166-6851(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 25.Oliveira D A, Udhayakumar V, Bloland P, Shi Y P, Nahlen B L, Oloo A J, Hawley W E, Lal A A. Genetic conservation of the Plasmodium falciparum apical membrane antigen-1 (AMA-1) Mol Biochem Parasitol. 1996;76:333–336. doi: 10.1016/0166-6851(95)02548-0. [DOI] [PubMed] [Google Scholar]
- 26.Peterson M G, Marshall V M, Smythe J A, Crewther P E, Lew A, Silva A, Anders R F, Kemp D J. Integral membrane protein located in the apical complex of Plasmodium falciparum. Mol Cell Biol. 1989;9:3151–3154. doi: 10.1128/mcb.9.7.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26a.Pronk, J. T., and C. H. M. Kocken. Unpublished observation.
- 27.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 28.Scorer C A, Buckholz R G, Clare J J, Romanos M A. The intracellular production and secretion of HIV-1 envelope protein in the methylotrophic yeast Pichia pastoris. Gene. 1993;136:111–119. doi: 10.1016/0378-1119(93)90454-b. [DOI] [PubMed] [Google Scholar]
- 29.Stoute J A, Slaoui M, Heppner D G, Momin P, Kester K E, Desmons P, Wellde B T, Garçon N, Krzych U, Marchand M, Ballou W R, Cohen J D. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. N Engl J Med. 1997;336:86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 30.Thomas A W, Deans J A, Mitchell G H, Alderson T, Cohen S. The Fab fragments of monoclonal IgG to a merozoite surface antigen inhibit Plasmodium knowlesi invasion of erythrocytes. Mol Biochem Parasitol. 1984;13:187–199. doi: 10.1016/0166-6851(84)90112-9. [DOI] [PubMed] [Google Scholar]
- 31.Thomas A W, Waters A P, Carr D. Analysis of variation in PF83, an erythrocytic merozoite vaccine candidate antigen of Plasmodium falciparum. Mol Biochem Parasitol. 1990;42:285–288. doi: 10.1016/0166-6851(90)90172-i. [DOI] [PubMed] [Google Scholar]
- 32.Tschopp J F, Sverlow G, Kosson R, Craig W, Grinna L. High-level secretion of glycosylated invertase in the methylotrophic yeast Pichia pastoris. Biotechnology. 1987;5:1305–1308. [Google Scholar]