Abstract
Background
The use of stimulant medications for treatment of ADHD has raised concern as to whether they adversely impact linear growth. Previous studies have indicated that stimulant medications may suppress growth for a short period after treatment initiation; however, more information is needed to evaluate the long-term effects on final adult stature. This mini review aims to evaluate the effect of stimulant medications on final adult height in children with ADHD.
Contents
We performed a literature review across PubMed/MEDLINE database. Only articles that included data on final adult height or near final adult height (age≥16 or 17 years) were included.
Summary
Early studies investigating the long-term impacts of stimulant medications observed growth suppression during the active treatment period, but when comparing final adult height, there was no difference between the control and ADHD groups. A recent larger comprehensive study (Multimodal Treatment of ADHD study) has suggested that the long-term use of significant doses of stimulants during childhood may compromise final adult height to a clinically significant degree when comparing adult height across three long-term patterns of stimulant treatment (Consistent, Intermittent, Negligible). The consistent use subgroup was significantly shorter than other subgroups.
Outlook
For children with ADHD, a significant long-term dose of stimulant treatment should be used with caution to avoid diminishing adult height potential. Pediatric endocrinologists should consider chronic use of stimulants as a factor contributing to reduced adult height.
Keywords: ADHD, adult height, stimulant treatment
Introduction
Attention-deficit hyperactivity disorder (ADHD) is one of the most commonly diagnosed childhood behavioral disorders with an estimated 9.4% of U.S. school-aged children and adolescents having ever received the diagnosis [1]. Boys are 2–4 times more likely to be diagnosed with ADHD than girls [1], [2], [3], [4]. Treatment includes pharmacotherapy, behavioral therapy, or a combination of the two. Stimulants constitute the medical treatment of choice and have been shown to have an excellent response rate when dosages are individualized for the patient. Methylphenidate and amphetamine are the most commonly prescribed stimulants in childhood and are deemed to be safe [5].
Although there are well-established adverse effects associated with stimulant use, the risk-benefit profile of stimulants is overall favorable. A serious adverse effect of stimulant use in children is cardiovascular risk [6], given that stimulants have both central and peripheral catecholaminergic effects leading to an increase in heart rate and blood pressure. However, the most common reason for referral to specialists is linear growth suppression. Lack of appetite and weight loss are commonly reported side effects for children on stimulants and frequently associated with poor height gain, which is commonly observed during the first and second year of stimulant administration [7, 8]. Altered appetite during stimulant treatment may be explained by altered neurotransmitter levels, such as dopamine. It is suggested that methylphenidate increases the availability of dopamine by increasing its transport to vesicles for release and inhibiting its reuptake, thereby reducing feeding behavior [9]. Interestingly, a recent study confirmed the involvement of dopaminergic neurons in a mouse model by showing that administration of methylphenidate caused a significant reduction in food intake and body weight in wild-type mice. On the contrary, ablation of selective dopaminergic neuronal circuit prevented the observed side effects confirming the involvement of dopaminergic neurons in the suppression of appetite by simulants [10]. Although suppression of appetite was proposed as a prominent reason for poor height gain, the precise pathophysiology of stimulant medications, methylphenidate treatment in particular, on growth parameters is not well characterized [7, 11].
There have been three proposed mechanisms for stimulant effect on linear growth [12]. First, as described above, stimulants can cause appetite suppression, leading to a reduction in caloric intake which negatively affects linear growth [13]. A second proposed mechanism is that increased dopamine by stimulants inhibits growth hormone secretion, at least for the first few years of treatment [14]. This mechanism is shown in animal models and cultured human pituitary cells [15]. Interestingly, a report of two newborns who received continuous dopamine infusion showed suppressed growth hormone secretion supporting the potential indirect role of stimulants on growth hormone secretion [16]. Conversely, a cross-sectional study reports no significant difference in GH, GHBP and IGF-I levels between children treated with methylphenidate for ADHD and the control children with ADHD although there are limitations of studies, such as a small sample size or various drug holiday protocols [17]. Furthermore, another prior investigation into possible methylphenidate-induced changes by Bereket et al. on specific growth parameters in prepubertal children has indicated that methylphenidate treatment did not have sustained effects on IGF-1 and IGF-BP3 levels [18]. Lastly, in vitro studies have shown that stimulants may have direct effects on growth plate chondrogenesis by suppressing chondrocyte proliferation or inhibiting sulfate uptake by cartilage; however, this has not been proven in animal models [19, 20].
Discontinuation of pharmacotherapy results in rebound growth to compensate for the stimulant-induced height loss (catch-up growth) indicating a reversible nature of growth suppression [21]. This has served as the basis for “summer holidays” in treatment where children are taken off stimulants during the summer when school is not in session, although the long-term impact of this approach has not been established [22, 23]. While some studies suggest that nonmedicated children with ADHD may be taller and heavier than children without ADHD [14], ADHD itself, without treatment, has not been known to have an impact on final adult height [23].
Even though suppression of linear growth during stimulant treatment has been frequently observed in clinic, the effects of long-term use of stimulants on final adult height have not been well established. Previous studies suggested that the effects of stimulant medication on growth may be dosage dependent, with higher doses of stimulant medication causing greater growth deficits, and that amphetamine causes more growth suppression than methylphenidate [14]. To clinicians, it is clear that the linear growth of children may be affected for a short period after the initiation of treatment, but further information on the long-term impact of stimulants on final height is needed. Therefore, for this review, we have focused on reviewing studies investigating the impact on final adult stature for children with ADHD who received stimulants during childhood.
Methods
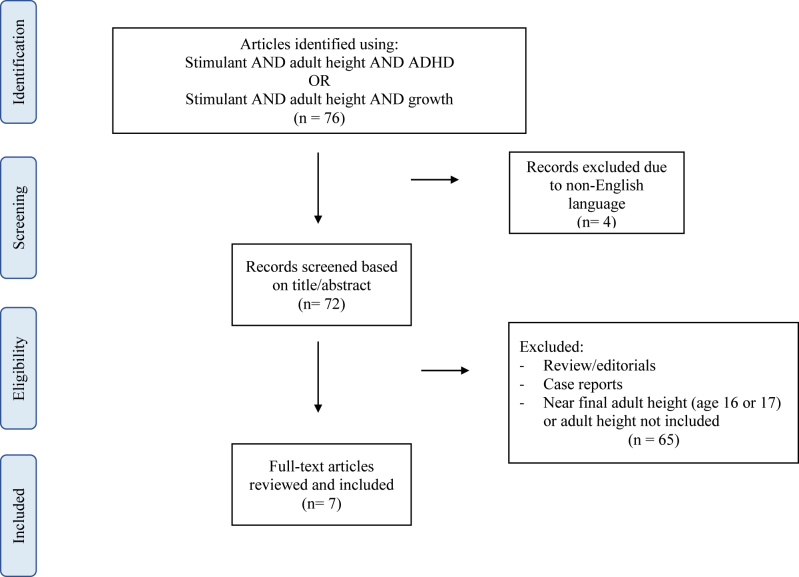
The authors performed a literature review across PubMed/MEDLINE database using the search terms “Stimulant AND adult height AND ADHD” OR “Stimulant AND adult height AND growth.” (Figure 1) Articles were all screened by the authors on the basis of title and abstract. Assessment of articles for final inclusion was based on full text review. Articles were excluded if they did not assess final adult height or near final adult height (age≥16 or 17 years). Review papers, case reports, editorials, or commentaries were excluded.
Figure 1:
Flow diagram of study selection and identification.
Impact of stimulants on adult height
The first study to evaluate the impact of stimulants on adult height was published in 1988 by Klein et al. in which 61 boys treated with at least 6 months of methylphenidate therapy during childhood were included in the case group; the control group consisted of 99 boys seen at the same medical center who were matched for race, socioeconomic status (SES), and age range [24]. An adverse impact on growth during the active treatment period was observed (Table 1). Adult height was measured at follow-up between 16 and 23 years of age in the ADHD group (mean 17.93 ± 1.4 years) and compared to the control group. When comparing the two groups at or near final adult height, there was no difference in height suggesting that a compensatory accelerated growth rate or period of growth rebound appears to occur after discontinuation of stimulant treatment in order to bring the children to their final stature. However, this study presents a significant limitation in that only 56% of probands had direct height measurements while 78% of controls had direct height measurements. The heights of the remaining subjects were obtained through self-reports or parent reports, which introduces a source of bias in precise height measurements. Additionally, 82% of methylphenidate treated children received other pharmacological treatments including dextroamphetamine, imipramine, and thioridazine, making it difficult to fully exclude any potential effects of the combination of drugs on final height. The duration of stimulant treatment was relatively short, and the study did not include subjects’ adherence to stimulants. In another study by Kramer et al. [25], 97 boys aged 4–12 years old were treated with methylphenidate for an average of 36 months. When reevaluated between 21 and 23 years of age, their final height on average did not differ from family (fathers and brothers), community (randomly selected classmates), or matched (never-medicated boys with comparable behavior problems) controls, suggesting that the impact of stimulant medication on final adult height is negligible. However, subjects in this study were treated for a short period of time and also obtained height measurement through self-reporting, presenting similar biases as the study by Klein et al. (Table 1).
Table 1:
The effect of stimulants on final adult height.
| Authors, year | Sample size, demographic: Mean age (range) | Trial design | Med, dose | Duration mean (range) | Assessment of growth method | Final height | Limitations of study |
|---|---|---|---|---|---|---|---|
| Klein et al. [22] | Subjects: n=61 males, 9.08 ± 1.4 y (6–12) at baseline, 17.93 ± 1.4 y (16–23) at final | Longitudinal observational | MPHa, mean dose=45 mg | 2.24 y (0.5–5) | Combination of direct assessment, self-report/and parents’ report |
|
|
| Controls: n=99 males matched for race, SES, age 18.94 ± 1.5 y (NR) at final | |||||||
| Kramer et al. [23] | Subjects: n=97 males, 8.2 y (4–12) at baseline, NR (21–23) at final | Longitudinal observational | MPHa, mean dose=31.2 mg | 36.2 m (1–76) | Height obtained primarily by self-report |
|
|
| Controls: n=255 family members, community, unmedicated controls, age NR | |||||||
| Biederman et al. [24] | Subjects: n=112 males, 8.4 ± 3.3 y at baseline, 21.5 ± 3.5 y at final | Longitudinal, case-control | MPH (dose NR) | Males: 7.4 ± 4.5 y (0.5–18) | Height and weight measured at baseline and at four and ten-year follow-up |
|
|
| n=96 girls, 8.7 ± 3.3 y at baseline, 21.1 ± 3.3 y at final | Females: 6.1 ± 3.8 y (0.5–16) | ||||||
| Controls: n=105 male controls, 22.3 ± 4.1 y at final | |||||||
| n=91 female controls, 22.2 ± 2.8 y at final | |||||||
| Peyre et al. [25] | Subjects: n=216, 56.5% male, 15.9 y at baseline, 35.9 y at final | Longitudinal observational | Stimulant (likely MPH) | 7.4 y (NR) | NR |
|
|
| Controls: n=591 ADHD without medication, 59.5% male, 41.01 y at final | |||||||
| n=34,652 controls without ADHD, 47.6% male, 48.4 y at final | |||||||
| Harstad et al. [21] | Subjects: n=171, 76% male, 10.2 ± 3.5 y at baseline, 26.8 ± 4.8 y at final | Retrospective chart review with prospective follow-up study | 26.2 ± 10.7 MEU (MPH equivalent unit)b | Total months: 53.0 ± 37.4 m | Height obtained from medical records and during study visits |
|
|
| Males: 54.4 ± 37.2 m | |||||||
| Controls: n=394 age and gender matched controls without ADHD, 72.6% male, 24.6 ± 5.8 y at final | Females: 48.3 ± 37.9 m | ||||||
| Swanson et al. [26] | Subjects: n=476, 78% male, 8.4 y at baseline, 24.8 year at final | Longitudinal observational | MPH (3 groups)c: Consistent (117,102 mg), inconsistent (60,567 mg), negligible users (2,153 mg) | 16 y | Weight and height assessment during clinic visit (assessment at years 2, 3, 6, 8, 10, 12, 14 and 16 y) |
|
|
| Controls: n=241 (LNCG), 80% male, 10.4 y at baseline, 24.4 y at final | |||||||
| Greenhill et al. [27] | Subjects: n=568, 78% male 8.4 y at baseline, 24.7 ± 1.31 y at final | Same as above | Same as above | Same as above | Same as above |
|
|
| Controls: n=258 (LNCG), 80% male, 10.4 y at baseline, 24.4 ± 1.36 y at final |
LNCG, local normative comparison group; Med, medication; MPH, methylphenidate; n, number; NR, not reported; SES, socioeconomic status; SD, standard deviation; y, years. aSubjects received other pharmacological agents: dextroamphetamine, imipramine, and thioridazine. bStimulant dosages converted to MEU with following formula: 20 mg methylphenidate=10 mg dextroamphetamine=56.25 mg pemoline=10 mg methamphetamine=10 mg levoamphetamine plus dextroamphetamine. cAverage 10 year dose.
More recently, Biederman et al. [26] performed the first study of stimulant effect on adult stature in a cohort including both girls and boys. Seventy-eight boys with ADHD and 68 matched controls along with 59 girls with ADHD and 56 matched controls were included in the final analysis. The mean duration of stimulant treatment was 7.4 ± 4.5 years (range: 0.5–18 years) in boys and 6.1 ± 3.8 years (range: 0.5–16 years) in girls. No significant difference in height was found at 10 year follow-up when the participants were reevaluated at age 21–22 years. Additionally, the authors found no association between duration of treatment and adult height outcome. However, the subjects were treated for a short period of time, such as 6 months, and it is not clear how the duration of treatment was determined or if patients received intermittent treatment for a long period of time. Moreover, the association between the duration of treatment stratified by the dose of stimulant and adult height outcome was not investigated (Table 1) [26]. Later, larger cohort studies were published. A study by Peyre et al. [27] looked at a cohort of patients with a lifetime diagnosis of ADHD using the 2004–2005 National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) and compared adult height among three groups: (1) ADHD treated with stimulants (n=216), (2) ADHD without stimulants (n=591), and (3) controls without ADHD (n=34,652). No significant difference was observed between the three groups or when groups were stratified by sex, and no association between duration of treatment and adult height was found. However, the study did not report on dosages, dose reductions over time, treatment discontinuation, or whether participants experienced treatment interruptions. Harstad et al. [23] similarly performed a cohort study including 340 ADHD cases and 680 controls. Eighty-two males were treated with ≥3 years of methylphenidate and 21 females received ≥3 years of stimulant treatment. On reevaluation at ages ≥18 and ≥20 years for females and males, respectively, there was no difference in adult height between ADHD cases and controls for male or female subjects and between stimulant treated and stimulant naïve ADHD male and female subjects [23]. However, the authors observed that among the 59 ADHD cases treated for ≥3 years, there was a clinically insignificant decrease in mean Z score at the end of treatment. An important limitation of this study is that the ADHD cases were not all treated with stimulant medications during adolescence, and the number of subjects who received stimulants for a longer period of time was too small. Taken together, these early studies indicate that the effect of stimulant medications on linear growth did not have an impact on final adult height. However, the limitations of these studies merit further investigation.
Recently, a larger comprehensive study was performed. Swanson et al. [28] were the first to observe a negative effect on final adult height in the Multimodal Treatment of ADHD (MTA) study (Table 1). The study originally began as a 14 month randomized clinical trial and afterward, transitioned into an observational long-term follow-up study with assessments 2–16 years after baseline. Subjects with ADHD were divided into naturalistic subgroups based on the pattern of long-term stimulant medication use (Consistent, Inconsistent, and Negligible). A minimum MPH equivalent regimen was defined as at least 10 mg/day for at least 50% of days since the previous assessment. This regimen was used to define the three subgroups in the study: Consistent (≥minimal in all intervals), inconsistent (≥minimal in some but not all intervals), and negligible (<minimal in all intervals). The control group was the local normative comparison group (LNCG) which consisted of participants recruited from the same school as ADHD cases. The authors observed that the final adult height of the ADHD group (including all subgroups of stimulant use) was 1.3 cm shorter than the LNCG. The treated group with the consistent and inconsistent pattern of stimulant use was 3 cm shorter than the subgroup with the negligible pattern. The consistent use group was 2 cm shorter than the Inconsistent group. The study indicates that consistent long-term use of medication was potentially associated with suppression of final adult height. The authors suggest that the negative impact of stimulants in the study may be a result of changes in clinical practice over the past few decades resulting in increases in the average cumulative medication dose for treatment-as-usual.
Using the same MTA group data, Greenhill et al. [29] performed a 16-year growth analysis. Groups were also divided into consistent, inconsistent, and negligible stimulant use. 568 children with ADHD combined type and 258 classmates used as the LNCG were included. While Swanson et al. investigated the adult height as the endpoint in their analyses, Greenhill et al. aimed to understand whether medication subgroup types were associated with specific growth trajectories. The height trajectories for the LNCG and Inconsistent subgroups were flat, suggesting an average growth tempo, whereas the negligible subgroup had an upward trajectory, indicating a faster-than-average growth tempo, and the consistent subgroup had a downward trajectory, indicating a growth slow down. Paired comparisons demonstrated significant subgroup differences at the endpoint with the consistent group shorter than negligible group (−4 cm), consistent shorter than inconsistent (−3 cm), consistent shorter than LNCG (−3 cm). The authors suggest that long-term consistent stimulant treatment may be associated with a reduction in adult height. This study is the first to investigate the impact of the duration and dosage of stimulants on final height of children who received stimulants during childhood using a proper stratification. This result is concerning to pediatricians and pediatric endocrinologists because the growth reduction with long-term consistent stimulant treatment can be clinically significant. Therefore, if a child already has short stature or another growth concern, this study may provide reasoning that such children should avoid a long-term consistent stimulant treatment or use a different type of treatment for their ADHD. Although the positive findings of Swanson et al. and Greenhill et al. are based on one study population, these studies have their strengths compared to previous studies that did not provide stratified outcomes based on dosage and duration of stimulant treatment. Their findings are also based on longitudinal data rather than cross-sectional data, providing a more accurate and valuable growth assessment. Future studies focusing on the duration of treatment and dosage of stimulants are required to confirm the findings.
Conclusions
Stimulants have been widely used in children with ADHD. However, there has been scarce data for the impact of stimulants on long term childhood growth and final adult height. The recent studies suggest the possibility that the dose and duration of treatment may be important for linear growth, which a long-term treatment with a significant dose of stimulants during childhood may compromise final adult height to a clinically significant degree. Based on the available recent studies, a long-term persistent use of stimulants should be used with caution in children with ADHD, especially if children already have short stature. In addition, clinicians may consider chronic use of stimulants as one factor that could reduce the final adult height.
Footnotes
Research funding: The research was funded by NIH intramural research grant.
Author contributions: NMW and EZ searched, selected, and reviewed relevant articles and wrote the manuscript with critical contents in the papers. YHJ supervised the entire review process, provided guidance, revised the manuscript, and added her expert opinion.
Competing interests: Authors state no conflict of interest.
Informed consent: Not applicable.
Ethical approval: The local Institutional Review Board deemed the study exempt from review.
References
- 1.Danielson ML, Bitsko RH, Ghandour RM, Holbrook JR, Kogan MD, Blumberg SJ. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. Children and adolescents, 2016. J Clin Child Adolesc Psychol. 2018;47:199–212. doi: 10.1080/15374416.2017.1417860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaub M, Carlson CL. Gender differences in ADHD: a meta-analysis and critical review. J Am Acad Child Adolesc Psychiatry. 1997;36:1036–45. doi: 10.1097/00004583-199708000-00023. [DOI] [PubMed] [Google Scholar]
- 3.Ramtekkar UP, Reiersen AM, Todorov AA, Todd RD. Sex and age differences in attention-deficit/hyperactivity disorder symptoms and diagnoses: implications for DSM-V and ICD-11. J Am Acad Child Adolesc Psychiatry. 2010;49:217–28.e1–3. doi: 10.1097/00004583-201003000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rucklidge JJ. Gender differences in attention-deficit/hyperactivity disorder. Psychiatr Clin North Am. 2010;33:357–73. doi: 10.1016/j.psc.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Wolraich ML, Hagan JF, Allan C, Chan E, Davison D, Earls M, et al. Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2019;144:e20192528. doi: 10.1542/peds.2019-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gould MS, Walsh BT, Munfakh JL, Kleinman M, Duan N, Olfson M, et al. Sudden death and use of stimulant medications in youths. Am J Psychiatr. 2009;166:992–1001. doi: 10.1176/appi.ajp.2009.09040472. [DOI] [PubMed] [Google Scholar]
- 7.Poulton A, Cowell CT. Slowing of growth in height and weight on stimulants: a characteristic pattern. J Paediatr Child Health. 2003;39:180–5. doi: 10.1046/j.1440-1754.2003.00107.x. [DOI] [PubMed] [Google Scholar]
- 8.Group MTAC. National institute of mental health multimodal treatment study of ADHD follow-up: changes in effectiveness and growth after the end of treatment. Pediatrics. 2004;113:762–9. doi: 10.1542/peds.113.4.762. [DOI] [PubMed] [Google Scholar]
- 9.Goldfield GS, Lorello C, Doucet E. Methylphenidate reduces energy intake and dietary fat intake in adults: a mechanism of reduced reinforcing value of food? Am J Clin Nutr. 2007;86:308–15. doi: 10.1093/ajcn/86.2.308. [DOI] [PubMed] [Google Scholar]
- 10.Han Y, Xia G, He Y, He Y, Farias M, Xu Y, et al. A hindbrain dopaminergic neural circuit prevents weight gain by reinforcing food satiation. Sci Adv. 2021;7:eabf8719. doi: 10.1126/sciadv.abf8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faraone SV, Biederman J, Morley CP, Spencer TJ. Effect of stimulants on height and weight: a review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47:994–1009. doi: 10.1097/chi.obo13e31817eoea7. [DOI] [PubMed] [Google Scholar]
- 12.Goldman RD. ADHD stimulants and their effect on height in children. Can Fam Physician. 2010;56:145–6. [PMC free article] [PubMed] [Google Scholar]
- 13.Efron D, Jarman F, Barker M. Side effects of methylphenidate and dexamphetamine in children with attention deficit hyperactivity disorder: a double-blind, crossover trial. Pediatrics. 1997;100:662–6. doi: 10.1542/peds.100.4.662. [DOI] [PubMed] [Google Scholar]
- 14.Negrao BL, Viljoen M. Stimulants and growth in children with attention-deficit/hyperactivity disorder. Med Hypotheses. 2011;77:21–8. doi: 10.1016/j.mehy.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Ishibashi M, Yamaji T. Direct effects of catecholamines, thyrotropin-releasing hormone, and somatostatin on growth hormone and prolactin secretion from adenomatous and nonadenomatous human pituitary cells in culture. J Clin Invest. 1984;73:66–78. doi: 10.1172/jci111208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Zegher F, Van Den Berghe G, Devlieger H, Eggermont E, Veldhuis JD. Dopamine inhibits growth hormone and prolactin secretion in the human newborn. Pediatr Res. 1993;34:642–5. doi: 10.1203/00006450-199311000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Toren P, Silbergeld A, Eldar S, Laor N, Wolmer L, Koren S, et al. Lack of effect of methylphenidate on serum growth hormone (GH), GH-binding protein, and insulin-like growth factor I. Clin Neuropharmacol. 1997;20:264–9. doi: 10.1097/00002826-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Bereket A, Turan S, Karaman MG, Haklar G, Ozbay F, Yazgan MY. Height, weight, IGF-I, IGFBP-3 and thyroid functions in prepubertal children with attention deficit hyperactivity disorder: effect of methylphenidate treatment. Horm Res. 2005;63:159–64. doi: 10.1159/000084683. [DOI] [PubMed] [Google Scholar]
- 19.Gumustas F, Yilmaz I, Sirin DY, Gumustas SA, Batmaz AG, Isyar M, et al. Chondrocyte proliferation, viability and differentiation is declined following administration of methylphenidate utilized for the treatment of attention-deficit/hyperactivity disorder. Hum Exp Toxicol. 2017;36:981–92. doi: 10.1177/0960327116678294. [DOI] [PubMed] [Google Scholar]
- 20.Kilgore BS, Dickinson LC, Burnett CR, Lee J, Schedewie HK, Elders MJ. Alterations in cartilage metabolism by neurostimulant drugs. J Pediatr. 1979;94:542–5. doi: 10.1016/s0022-3476(79)80007-4. [DOI] [PubMed] [Google Scholar]
- 21.Safer DJ, Allen RP, Barr E. Growth rebound after termination of stimulant drugs. J Pediatr. 1975;86:113–6. doi: 10.1016/s0022-3476(75)80720-7. [DOI] [PubMed] [Google Scholar]
- 22.Klein RG, Landa B, Mattes JA, Klein DF. Methylphenidate and growth in hyperactive children. A controlled withdrawal study. Arch Gen Psychiatr. 1988;45:1127–30. doi: 10.1001/archpsyc.1988.01800360075011. [DOI] [PubMed] [Google Scholar]
- 23.Harstad EB, Weaver AL, Katusic SK, Colligan RC, Kumar S, Chan E, et al. ADHD, stimulant treatment, and growth: a longitudinal study. Pediatrics. 2014;134:e935–44. doi: 10.1542/peds.2014-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein RG, Mannuzza S. Hyperactive boys almost grown up. III. Methylphenidate effects on ultimate height. Arch Gen Psychiatr. 1988;45:1131–4. doi: 10.1001/archpsyc.1988.01800360079012. [DOI] [PubMed] [Google Scholar]
- 25.Kramer JR, Loney J, Ponto LB, Roberts MA, Grossman S. Predictors of adult height and weight in boys treated with methylphenidate for childhood behavior problems. J Am Acad Child Adolesc Psychiatry. 2000;39:517–24. doi: 10.1097/00004583-200004000-00022. [DOI] [PubMed] [Google Scholar]
- 26.Biederman J, Spencer TJ, Monuteaux MC, Faraone SV. A naturalistic 10-year prospective study of height and weight in children with attention-deficit hyperactivity disorder grown up: sex and treatment effects. J Pediatr. 2010;157:635–40.e1. doi: 10.1016/j.jpeds.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peyre H, Hoertel N, Cortese S, Acquaviva E, Limosin F, Delorme R. Long-term effects of ADHD medication on adult height: results from the NESARC. J Clin Psychiatr. 2013;74:1123–4. doi: 10.4088/jcp.13l08580. [DOI] [PubMed] [Google Scholar]
- 28.Swanson JM, Arnold LE, Molina BSG, Sibley MH, Hechtman LT, Hinshaw SP, et al. Young adult outcomes in the follow-up of the multimodal treatment study of attention-deficit/hyperactivity disorder: symptom persistence, source discrepancy, and height suppression. J Child Psychol Psychiatry. 2017;58:663–78. doi: 10.1111/jcpp.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenhill LL, Swanson JM, Hechtman L, Waxmonsky J, Arnold LE, Molina BSG, et al. Trajectories of growth associated with long-term stimulant medication in the multimodal treatment study of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2020;59:978–89. doi: 10.1016/j.jaac.2019.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]