Summary
Background
Post COVID-19 condition (PCC) is defined as symptoms lasting more than 12 weeks after developing COVID-19. Evidence of mitochondrial dysfunction has been reported in peripheral blood mononuclear cells obtained from patients with COVID-19. We hypothesized that PCC is caused by prolonged mitochondrial dysfunction. Given that coenzyme Q10 (CoQ10) can improve mitochondrial function, we examined whether high-dose CoQ10 can reduce the number and/or severity of PCC-related symptoms.
Methods
In this placebo-controlled, double-blind, 2 × 2 crossover interventional trial, participants were recruited from two centres at Aarhus University Hospital and Gødstrup Hospital, Denmark. They were randomly assigned to receive either oral capsules of CoQ10 in a dose of 500 mg/day or placebo for 6 weeks, with crossover treatment after a 4-week washout period. The ED-5Q and a PCC-symptom specific questionnaire were completed by the participants at 5 visits during the 20-week study period. The primary endpoint was the change in the number and/or severity of PCC-related symptoms after the 6-week intervention compared to placebo. Participants who completed the two-dosing period were included in the primary analysis, while all participants receiving one dose were included in safety assessment.
Findings
From May 25th, 2021, to September 22nd, 2021, 121 participants underwent randomization, and 119 completed both dosing periods – 59 and 60 in group A and B, respectively. At baseline, the mean PCC-related symptom score was 43.06 (95% CI: 40.18; 45.94), and the mean EQ-5D health index was 0.66 (95% CI: 0.64; 0.68). The difference between CoQ10 and placebo was not significant with respect to either the change in EQ-5D health index (with a mean difference of 0.01; 95% CI: −0.02; 0.04; p = 0.45) or the change in PCC-related symptom score (with a mean difference of −1.18; 95% CI: −3.54; 1.17; p = 0.32).
Interpretation
Based on self-reported data, CoQ10 treatment does not appear to significantly reduce the number or severity of PCC-related symptoms when compared to placebo. However, we observed a significant spontaneous improvement on both scores regardless of treatment during 20 weeks observation.
Funding
Placebo and CoQ10 capsules were provided by Pharma Nord, and the trial was supported by grants from the Novo Nordisk Foundation (NNF21OC0066984). This trial is registered with EudraCT, 2020-005961-16 and ClinicalTrials.gov, NCT04960215. The trial is completed.
Research in context.
Evidence before this study
We searched on PubMed on Dec 2nd, 2020, with no limitations by starting date or language, with the terms “Long COVID-19”, “Persistent COVID-19”, “post-COVID syndrome”, “chronic COVID-19”, “Long Haulers”, “Chronic fatigue syndrome”, “mitochondria,” “oxidative stress” and “Coenzyme Q10” and “Ubiquinone”. Systematic terminology in the field of post COVID-19 condition (PCC) was not yet established, complicating the initial research in databases.
We found several papers describing PCC, but at this early stage of recognizing the disorder, we found only few articles addressing a possible pathogenesis. Some papers stated that PCC may be caused by changes in cellular metabolism, and one study draw comparison between the symptomatology described in PCC and in myalgic encephalomyelitis (also termed chronic fatigue syndrome). We further identified studies, which showed that Coenzyme Q10 had significant effect on fatigue in myalgic encephalomyelitis and increased survival in patients suffering from heart insufficiency.
We found no randomized controlled trials exploring the effect of a pharmacologic intervention on patient reported symptoms. As PCC is based on subjective reporting, no animal studies had been conducted. Later more and larger studies characterizing PCC emerged, alongside studies in acute COVID-19 describing impaired mitochondrial dysfunction.
Added value of this study
Based on WHO-estimates, millions of patients worldwide suffer from post COVID-19 condition, but no evidence of an effective medical treatment has yet been published. This study is the first randomized, controlled trial investigating an effect of a medical product in post COVID-19 condition. It adds knowledge to a scarcely described field and to the current effort of demasking the biological mechanism behind post COVID-19 condition.
Implications of all the available evidence
Our findings suggest that Coenzyme Q10 should not be recommended as treatment PCC. Further studies on host cell metabolism in patients with PCC are required to illuminate the mitochondrial involvement in this disorder.
Introduction
As of May 2022, more than 500 million confirmed cases of SARS-CoV-2 have been reported, with an unparalleled impact on global society.1 Importantly, many reports describe the presence of persistent, debilitating symptoms in COVID-19 survivors, reflecting the lasting clinical effects of SARS-CoV-2 infection.2 Post COVID-19 condition (PCC, also known as long COVID) is defined as a diverse range of multi-organ symptoms lasting at least three months from the onset of COVID-19.3, 4, 5 Up to 32% of COVID-19 patients report experiencing these persistent symptoms after 90 days.6 Although several hypotheses regarding the pathogenesis of PCC have been suggested, the underlying mechanism remains poorly understood.7
Mitochondria play an essential role in cellular homeostasis and are involved in regulating both innate and adaptive immunity.8 Acquired mitochondrial dysfunction can be triggered by environmental factors and often leads to metabolic dysregulation and chronic illness, causing numerous symptoms and involving multiple organ systems, particularly organs and tissues with high energy demand such as the brain, skeletal muscle, and cardiac muscle.9,10 Interestingly, the predominant characteristics of PCC include fatigue, neurological symptoms, muscle weakness, and dyspnea, thus overlapping in symptomatology with impaired mitochondrial respiration.5,11
Recent studies suggest that the inflammatory response in acute COVID-19 may compromise mitochondrial function. Evidence of mitochondrial dysfunction, altered mitochondrial morphology, and altered cellular metabolism with increased glycolysis has thus been reported in peripheral blood mononuclear cells obtained from patients with COVID-19.12, 13, 14, 15 Coenzyme Q10 (CoQ10) is a natural occurring, endogenously produced vitamin-like compound that plays a key role in cellular bioenergetics by serving as an electron transporter in the mitochondrial membrane and is essential to aerobic respiration.16,17 Based on the multi-organ symptomatology of PCC and studies showing impaired cellular mitochondrial metabolism in patients infected with SARS-CoV-2, we hypothesized that the fatigue, dyspnea, neurological symptoms, and muscle symptoms in patients who develop PCC are caused by chronic mitochondrial dysfunction. We therefore investigated whether treating patients with high-dose CoQ10 can reduce the number and/or severity of PCC-related symptoms, providing a potential therapeutic strategy.
Methods
Study design and participants
This study was an investigator-initiated, randomized, double-blind, placebo-controlled interventional clinical trial with a crossover design. The trial was conducted at the Department of Infectious Diseases at Aarhus University Hospital, Denmark. Participants were recruited from the PCC Outpatient Clinic at Aarhus University Hospital and Gødstrup Hospital, Denmark, directly or by letter invitation. In Denmark, patients are eligible for referral to a specialized PCC Outpatient Clinic if they have more than two persisting symptoms 12 weeks after COVID-19. The following inclusion criteria were applied: >18 years of age; ability to give written informed consent; a history of SARS-CoV-2 infection based on either a PCR test or antibody test; and PCC-related symptoms that were diagnosed by a specialized infectious disease physician at the PCC Outpatient Clinic and are not attributable to any other comorbidity or condition. In addition, we excluded patients who presented with symptoms associated with acute COVID-19, hypersensitivity to any components in the study drugs, reduced liver and/or kidney function, or current pregnancy.
The study protocol was approved by the Danish Medicines Agency, the Danish Data Protection Agency, and the Central Denmark Regional Committee on Health Research Ethics (Protocol version 1.3, ID: M-2020-329-20) and conducted in accordance with the principles of the Declaration of Helsinki. Each patient provided written informed consent before any study procedures.
Randomization and masking
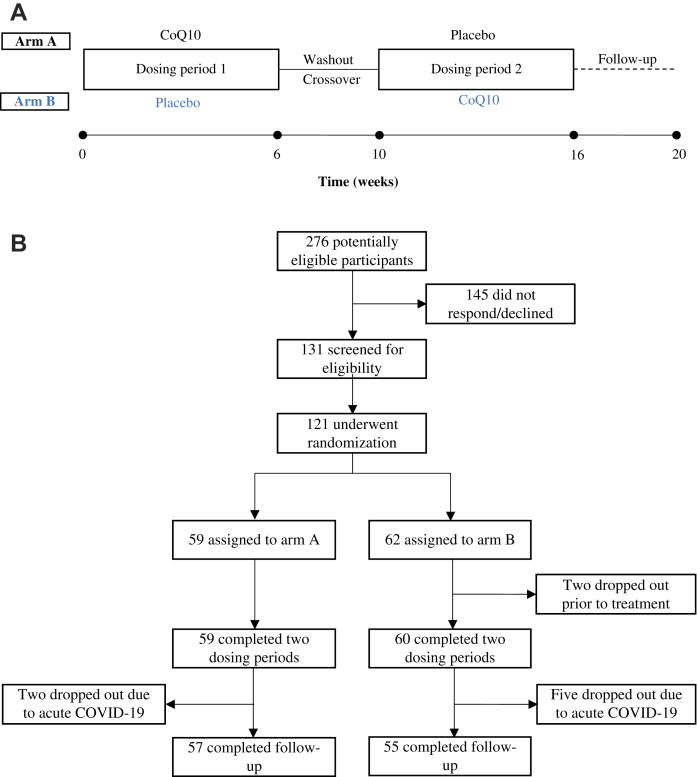
Eligible patients were randomized to either arm A or arm B (Fig. 1). Allocation lists were generated at a 1:1 ratio using permuted blocks with random varying sizes of four and eight. Proper concealment of randomization was obtained by the use of an external randomization service (Clinical Trial Unit, Department of Clinical Medicine, Aarhus University, Denmark), who created the computer-generated sequence. The allocation list was stored in an electronic database with a concealed de-identification code, in case premature blinding was needed. This process was logged and monitored. No pre-randomizing stratification occurred. Allocation lists were obtained by the pharmaceutical supplier for preparation of the study medicine without the involvement of trial personnel. Upon inclusion, participants were granted study-ID chronologically and received appertaining prepared study medicine. The placebo capsules were specifically created to resemble capsules of CoQ10 and were undistinguishable. The packs of study medicine were labelled with study-ID and dosing period. Thus, the allocation was concealed for participants and all trial personnel.
Fig. 1.
Outline of the study design and trial profile. a) Schematic illustration of the study design. The solid circles indicate the five timepoints for completing the questionnaires. b) Flow diagram showing the number of participants at each stage of the trial.
Trial procedures
The 20-week study period consisted of 5 visits in total. The patients assigned to arm A received CoQ10 capsules for 6 weeks, and the patients assigned to arm B received placebo capsules containing soy oil for 6 weeks. After a 4-week washout period, the patients were allocated to the opposite treatment regimen for a second 6-week dosing period; the patients then had a final follow-up visit 4 weeks after the second dosing period (Fig. 1A). At each visit, the participants completed two questionnaires addressing organ-specific symptoms and quality of life (the complete questionnaires are provided in the Supplementary Appendix). CoQ10 has been thoroughly tested in numerous clinical trials at doses ranging from 50 to 3000 mg/day, generally showing a low incidence of adverse events. The recommended daily dose for CoQ10 supplementation ranges from 30 to 200 mg/day.18 We chose a dose of 500 mg/day, which was administered as five 100-mg doses per day in order to maximize intestinal absorption. All study-related data were collected and managed using REDCap electronic data capture tools, hosted at Aarhus University, Denmark.19
Outcomes
The primary endpoint was the change in the number and/or severity of PCC-related symptoms after six weeks of CoQ10 treatment or placebo compared to baseline, measured as a symptom score and a health index. When the study was designed, no objective analysis was available for evaluating the severity of PCC; therefore, we developed a scoring system using two questionnaires, the EQ-5D and a PCC-specific questionnaire. The EQ-5D is a validated questionnaire that provides a generic measure of health status. We used the descriptive part of this questionnaire, which assesses health based on the following five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression.20 Each dimension has a 5-tier response ranging from a score of 1 (no problems) to a score of 5 (extreme problems). A country-specific health state index score ranging from 0 to 1 can then be derived from the responses, with 0 representing the worst imaginable health and 1 representing optimal health. The PCC-specific questionnaire consists of 32 questions based on the available literature and prevalent symptoms reported in a Danish cohort of patients with PCC.21 Each response is divided into five levels of severity ranging from 0 to 4 (corresponding to the lowest severity to the highest severity), yielding a total PCC-related symptom score with a maximum of 128 points.
Safety and tolerability of CoQ10 were assessed for all participants who received at least one dose, and adverse events were scored in accordance with the Common Terminology Criteria for Adverse Events (CTCAE), v5.0. For safety analyses, the number and percentage of subjects who experienced one or more adverse events were summarized by severity, and the potential relationship between the adverse events and the study drug was assessed.
Statistical analysis
No previous data regarding the effect of CoQ10 for use in the treatment PCC were available to guide our determination of the sample size needed for this study. The power calculation was therefore based on assumptions derived from data collected in Denmark using the PCC-specific questionnaire.21 Assuming a minimal detectable difference in mean symptom score of 10 points with a standard deviation of 20 points and a 5% significance level, number of participants needed was 106.
A health state index score was calculated using the country-specific values obtained from the EQ-5D questionnaire.22 To analyze the treatment effect of CoQ10 versus placebo and to quantify the period and sequence effects, we used a linear mixed-effects model with sequence, period, and treatment as two-level, fixed factors and participants as a random factor. This method was applied on the data from both the PCC and the EQ-5D questionnaire.
The PCC-specific questionnaire is divided into seven clusters of questions representing various organ systems; the same model was therefore used to compare CoQ10 versus placebo regarding the change in total symptom score in each cluster, performed as a post-hoc analysis. Statistical analyses were performed using Stata (v17). The statistical approach was modified slightly before performing any analyses of the data (Supplementary Appendix, page 8). There were no other modifications of the protocol and no amendments that affected recruitment or conduct were made.
The trial was monitored by the Danish Good Clinical Practice Unit, there was no specific data monitoring committee. The trial is registered with EudraCT, 2020-005961-16 and ClinicalTrials.gov, NCT04960215, and is complete.
Role of the funding source
Placebo and CoQ10 capsules were provided by Pharma Nord, and the trial was supported by grants from the Novo Nordisk Foundation (NNF21OC0066984). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
From May, 2021, to September, 2021, a total of 121 participants enrolled in the study and were randomly assigned to either arm A or arm B (Fig. 1B); however, two participants assigned to arm B dropped out of the study prior to the first dosing period. The baseline demographics of the remaining 59 and 60 participants in arm A and arm B, respectively, are summarized in Table 1. At baseline, the participants in the two arms differed significantly with respect to baseline PCC symptom score and EQ-5D health index, reflecting a slightly higher level of PCC severity among the participants in arm A. Aside from this small difference, the groups were well balanced. Of the 119 participants, 117 (98.3%) were Caucasian. Eighteen out of 119 participants were admitted to the hospital during the course of acute COVID-19. None of them were admitted to an intensive care unit and none received dexamethasone or remdesivir for acute COVID-19. All participants were in a follow-up course at a specialized PCC outpatient clinic and had 2.57 contacts to a PPC physician on average at time of inclusion. Regarding adherence to study medicine, the participants missed no capsules in 40% of dosing regimens (238 in total) and had an average of 7 capsules missed per dosing regimen (each consisting of 210 capsules).
Table 1.
Patient characteristics at baseline.
| Total cohort (n = 119) | Arm A (n = 59) | Arm B (n = 60) | |
|---|---|---|---|
| Age, years - median (range) | 49.0 (22–70) | 50 (25–64) | 48.5 (22–70) |
| Sex | |||
| Female - n (%) | 89 (74.8) | 44 (74.6) | 45 (75.0) |
| Male - n (%) | 30 (25.2) | 15 (25.4) | 15 (25.0) |
| BMI – mean (SD) | 28.08 (5.8) | 28.45 (6.1) | 27.71 (5.5) |
| BMI <25 - n (%) | 42 (35.3) | 21 (35.6) | 21 (35.0) |
| BMI 25–30 - n (%) | 37 (18.6) | 20 (33.9) | 17 (45.0) |
| BMI >30 - n (%) | 40 (33.6) | 18 (30.5) | 22 (36.7) |
| Smoking status - n (%) | |||
| Current | 7 (5.9) | 3 (5.1) | 4 (6.7) |
| Never | 76 (63.9) | 39 (66.1) | 37 (61.7) |
| Former | 36 (30.2) | 17 (28.8) | 19 (31.7) |
| Hospital admission during acute COVID-19 - n (%) | 18 (15.1) | 10 (17.0) | 8 (13.3) |
| Interval between COVID-19 onset and enrollment, days - mean (SD) | 288.55 (119.7) | 282.78 (117.3) | 294.23 (122.8) |
| CCI - n (%) | |||
| 0 points | 60 (50.4) | 29 (49.2) | 31 (51.7) |
| 1 point | 40 (33.6) | 22 (37.3) | 18 (30.0) |
| 2 points | 16 (13.5) | 7 (11.9) | 9 (15.0) |
| 3 points | 2 (1.7) | 1 (1.7) | 1 (1.7) |
| 4 points | 1 (0.8) | 0 | 1 (1.7) |
| SARS-CoV-2 vaccination status at enrollment - n (%) | |||
| Fully vaccinated | 101 (84.9) | 53 (89.8) | 48 (80.0) |
| Partially vaccinated | 12 (10.1) | 4 (6.8) | 8 (13.3) |
| Unvaccinated | 6 (5.0) | 2 (3.4) | 4 (6.7) |
| PCC symptom score at baseline - mean (SD) | 43.06 (15.9) | 46.35 (16.8) | 39.8 (14.4)∗ |
| EQ-5D index at baseline - mean (SD) | 0.66 (0.1) | 0.63 (0.1) | 0.69 (0.10)† |
∗p = 0.02 and †p < 0.001 versus arm A.
BMI, body mass index; CCI, Charlson Comorbidity Index; PCC, post COVID-19 condition; SD, standard deviation.
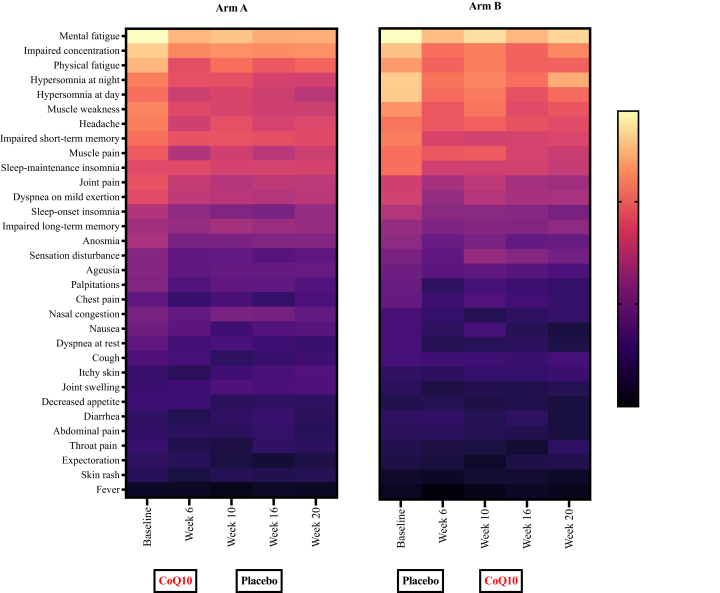
For the PCC-specific questionnaire, the mean symptom score at baseline was 43.06 (95% CI: 40.18; 45.94), 46.35 (95% CI: 41.98; 50.73), and 39.80 (95% CI: 36.07; 43.53) for all 119 participants, the 59 participants in arm A, and the 60 participants in arm B, respectively. As noted above, the difference at baseline between arm A and arm B was statistically significant (p = 0.02) (Table 1). The most prevalent symptoms at baseline were concentration difficulties, mental fatigue, physical fatigue, headache, and muscle weakness (Table S1). A heatmap illustrating the PCC-related symptoms throughout the study is shown for the participants in arms A and B in Fig. 2. Depicting the distribution of severity of all the symptoms of the PCC specific questionnaire, we observed, that the participants in both arms slightly improve in almost every symptom from baseline to week 6. This trend was specifically pronounced for mental fatigue, impaired concentration, hypersomnia and muscle weakness. There is no clear pattern associated with treatment regimen.
Fig. 2.
Heatmap of PCC-specific symptom scores throughout the study period. Heatmap of mean PPC-specific symptom scores for each visit for the participants in arm A (left) and arm B (right). The questionnaire contains 32 questions regarding PCC symptoms, which were graded for severity from 0 to 4 (higher scores indicate more severe symptoms). Boxes at the bottom indicate the treatment periods.
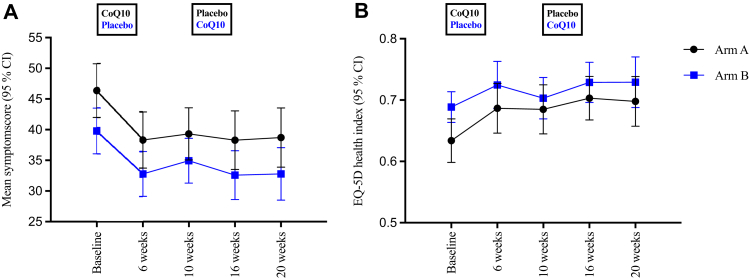
On average, the symptom scores were reduced by 5.18 points (95% CI: 3.40; 6.95) after the six-week treatment with CoQ10, compared to a reduction of 4.04 points (95% CI: 2.13; 5.96) after receiving placebo (Fig. 3A). After adjusting for sequence and period, the mean difference in the change in symptom scores between CoQ10 and placebo was −1.18 (95% CI: −3.54; 1.17), indicating that on average the reduction in symptom score was 1.18 points larger after CoQ10 treatment compared to placebo; however, this difference was not significant (p = 0.32). In contrast, we found a significant period effect; specifically, the mean difference in symptom scores between baseline and week six was −5.85 points (95% CI: −8.21;−3.48; p < 0.001), indicating that the participants in both arms improved significantly regardless of the treatment regimen in the first treatment period.
Fig. 3.
Time course of PCC-specific symptom scores and EQ-5D health index. Time course of mean PCC-specific symptom scores (a) and mean EQ-5D health index scores (a) reported by the participants in arm A (black circles) and arm B (blue squares) at the indicated time points. The boxes indicate treatment regimen at the two treatment periods.
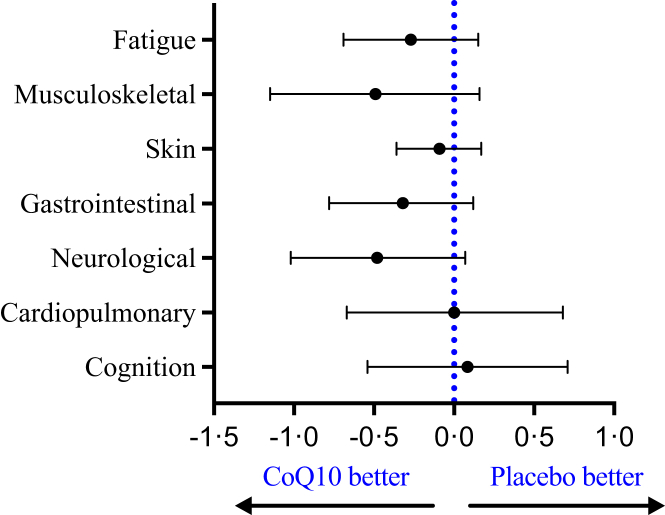
The PCC-specific questionnaire is divided into seven clusters of organ-specific symptoms covering cognition, the cardiopulmonary system, neurological symptoms, the gastrointestinal system, skin, the musculoskeletal system, and fatigue. We therefore performed a post-hoc analysis to compare the effects of CoQ10 with the effects of placebo (Fig. 4). Although our analysis revealed no significant differences, the largest difference was a reduction in neurological symptoms (with a mean difference of −0.48; 95% CI: −1.02; 0.07) and musculoskeletal symptoms (with a mean difference of −0.49; 95% CI: −1.15; 0.16).
Fig. 4.
Results of the mixed-effects model analysis for seven symptom groups derived from the PCC-specific questionnaire. The mean regression coefficients (slopes) with a 95% confidence interval are plotted to visualize the difference in the change in symptom scores between CoQ10 and placebo. A negative slope indicates a larger reduction in the symptom score with CoQ10 compared to placebo; the dotted vertical line at 0.0 indicates no difference between CoQ10 and placebo. None of the estimated difference in the symptom groups reached statistical significance.
Addressing the EQ-5D questionnaire, the estimated mean health index at baseline was 0.66 (95% CI: 0.64; 0.68), 0.63 (95% CI: 0.60; 0.67), and 0.69 (95% CI: 0.66; 0.71) for all 119 participants, the 59 participants in arm A, and the 60 participants in arm B, respectively (Fig. 3B). The most prominently affected dimension was ability to perform usual activities, with 116 participants (97.5%) reporting experiencing problems at any level of severity and 61 participants (51.3%) reporting either severe or extreme problems. The second most affected dimension was pain/discomfort, with 108 participants (90.8%) reporting pain/discomfort at any level and 22 participants (18.5%) reporting severe or extreme pain/discomfort (Figure S1 and Table S2).
The estimated mean improvement in health index score was 0.04 (95% CI: 0.02; 0.06) and 0.03 (95% CI: 0.006; 0.05) after six weeks of CoQ10 treatment or placebo, respectively. After adjusting for period and sequence effect in the linear mixed-effects model, the estimated difference was 0.01 (95% CI: −0.02; 0.04), which was not statistically significant (p = 0.40). We observed an insignificantly larger increase in health index in the first treatment period (0.02 (95% CI: −0.01; 0.05), p = 0.12) and in group A (0.005 (95% CI: −0.02; 0.03), p = 0.80).
A total of 295 adverse events were registered throughout the study period; 148 of these adverse events were either new or exacerbated symptoms. Overall, 19 adverse events were considered to be related to CoQ10 (Tables S3 and S4); four of these events were grade 2, and 15 were grade 1. These 19 treatment-related adverse events were registered in 13 participants; thus, 11% of the 119 participants who had study medicine intake experienced an adverse event that was related to CoQ10.
Discussion
To the best of our knowledge, this is the first randomized, placebo-controlled crossover study designed to test whether a registered medical product can reduce the number and/or severity of PCC-related symptoms. Overall, we found no significant difference between the effects of high-dose CoQ10 and placebo on the participants’ overall health status or PCC-related symptoms. However, several points warrant discussion. First, we found no significant difference between CoQ10 and placebo with respect to the change in EQ-5D health index. Second, we found that an additional 1.18 points were deducted from the PCC-related symptom score when participants received CoQ10 versus placebo. Third, we examined seven groups of symptoms in order to identify whether CoQ10 has any organ-specific benefits; although none of the results were statistically significant, the largest beneficial effects of CoQ10 were observed for neurological symptoms. Taken together, and despite observing a slight trend towards CoQ10 having potentially beneficial effects, we found no overall significant effects of CoQ10 compared to placebo.
Interestingly, we found a significant 5.85-point reduction in the mean PCC-related symptom scores between baseline and the second visit, regardless of the treatment regimen. One possible explanation for this finding is that the participants’ acknowledgment of PCC-related symptoms and entering a clinical interventional study may have contributed to a placebo-driven relief of their symptoms. In addition, we found that the entire cohort had a significant overall improvement in PCC-related symptom scores (from 42.63 to 35.80) and EQ-5D health index (from 0.66 to 0.71) between baseline and the final follow-up time point at week 20; these improvements were driven primarily by the initial improvement in both arms. However, because we were unable to show any apparent treatment effect of CoQ10 compared to placebo, this change may have been attributed—at least in part—to spontaneous improvement during the 20-week study period.23
Most participants were enrolled several months after the acute episode of COVID-19. A possible explanation for our negative results could be that mitochondrial dysfunction is established very early during –and post-COVID-19, and CoQ10 dosing is effectuated too late in the course of PCC to reverse a potential dysfunction. Thus, it cannot be excluded that CoQ10 would significantly benefit patients if administered at an earlier point of time in the course of PCC or even during acute COVID-19. Another explanation may be that a treatment period of six weeks is too short to establish effect of CoQ10. Finally, the average skipped/missed capsule per dosing regimen were seven. This is viewed as a reflection of real practice and patient adherence, thus not affecting the overall interpretation of the study results.
Our results are based solely on self-reported data collected using questionnaires; thus, our results may have been affected by recall bias.24 The participants were not disturbed when completing the questionnaires, receiving help from health-care personnel only when needed, thereby minimizing interviewer bias and an incorrect interpretation of the questions. Importantly, however, because PCC-related symptoms can fluctuate considerably, it is possible that the questionnaire provided a snapshot of the participant's health status on that day and may not have fully represented the entire period since the previous visit.25 This effect may be reflected by the residual standard deviation of 9.19 (95% CI: 8.08; 10.45) in the PCC mixed-effects model analysis, which describes an extremely high within-subject variation regardless of treatment regime. Finally, an important limitation to this study is the lack of plasma CoQ10-measurements, making it difficult to draw final conclusions on treatment effect as no biochemical monitoring was included.
A strength of using a crossover design is that two treatments (i.e., CoQ10 and placebo) can be compared within the same patient, eliminating between-subject variability. This design also decreases the number of individuals needed in a clinical trial. Our power calculation indicated that 106 individuals were required, and 119 participants completed both dosing regimens. On the other hand, a caveat of this design is the possibility of a carry-over effect; however, we attempted to minimize this possibility by including a four-week washout period, which is 14 times longer than the 48-h biological half-life of CoQ10. The most frequent symptoms reported by our participants were mental fatigue, concentration difficulties, muscle weakness, and headache, thus our cohort reflects the symptomatology currently described in literature on PCC.4,5 In addition, the mean EQ-5D health index at baseline was 0.66, which is consistent with studies conducted in Norway and the US, which reported mean health indices of 0.61 and 0.65, respectively.26,27 Overall, we conclude that our patient cohort adequately reflects the general population of patients with PCC. There is increasing evidence that PCC also is prevalent among children. As in adults, the mechanism is unknown, but may be at least partly different from the pathogenesis in adults due to the different acute clinical course.28 It should be noted that the results of this study performed on adults cannot be translated to treatment considerations in children.
The pathogenesis of PCC is still poorly understood. Among some suggestions are persistent systemic inflammation, venous thromboembolisms, autoimmunity and metabolic disturbances.7 It is also a possibility that this complex, multisymptomatic condition is a result of more than one harmful mechanism, making a single treatment intervention, as in this study, insufficient. Thus, it should be noted, that CoQ10 would only benefit patients who suffer from PCC due to mitochondrial dysfunction or a condition caused by excessive oxidative stress. It may therefore be reasonable to study CoQ10 intervention alongside other treatment regimens. For future investigations of CoQ10 as a treatment for PCC, the following could be prioritized: administration of CoQ10 early in the course of illness, long treatment period, inclusion of patients with evidence of mitochondrial dysfunction, plasma-monitoring of CoQ10 to verify intestinal uptake and an objective clinical measure of improvement.
Our results do not provide evidence to either support or reject the hypothesis that mitochondrial dysfunction causes PCC; thus, further studies are needed in order to clarify the role of mitochondria in PCC. The concept of “post-viral fatigue” has been described—and debated—in the medical community for nearly a century.29 Although several infectious agents—particularly viruses—undoubtedly have the ability to have prolonged, debilitating effects, the underlying pathophysiology remains poorly understood; thus, no intervention-based treatments are currently available.30 Aside from revealing the obvious, urgent need to treat the millions of patients with PCC, the ongoing COVID-19 pandemic provides a valuable opportunity to study the general phenomenon of post-viral fatigue. Identifying the pathogenic features of PCC would represent a major step toward managing all types of patients with post-infectious symptoms. In this respect, both in vivo and in vitro studies characterizing the immunological and mitochondrial profiles of patients with PCC, as well as the effects on host cell metabolism, are needed in order to close this important knowledge gap.
Contributors
L.Ø., S.L., and L.V.K. designed the study. L.V.K., J.A., and S.L. selected the questionnaires and outcome measures. K.S.H. performed the clinical studies, collected patient data, and performed the statistical analysis with S.L. K.S.H., S.L., and L.V.K. wrote the manuscript. B.S.C., T.H.M., J.A. and L.Ø. critically revised the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. The main text has been edited for grammar, spelling, punctuation and sentence structure by Curtis F. Barrett from English Editing Solutions.
Data sharing statement
Individual de-identified participant data will be shared following the publication of the endpoints as outlined in this paper. Data to be shared includes de-identified data points in published, peer-reviewed articles. Additional, related documents will also be available (study protocol, informed consent form, statistical analysis plan). Data will become available following publication with no planned end date. Access to the data sharing will be given to researchers who provide a methodologically sound proposal for any type of analysis and requires IRB/Ethics committee approval (if applicable). Proposal should be addressed to larsoest@rm.dk.
Declarations of interests
We declare no competing interests.
Acknowledgments
Novo Nordisk Foundation (NNF21OC0066984) and PharmaNord funded this study.
We thank our project nurses Yordanos Yehdego, Ane Søndergaard, and Malene Østergaard; we are particularly grateful to nurse Sandra Schieber for assistance administering the investigational drugs and for logistical assistance. We thank physicians Nina Breinholt Stærke and Vibeke Klastrup for their assistance with the study visits. We also thank Johan Palmfeldt and Rikke Katrine Jentoft Olsen for their assistance in selection of therapy. Finally, we thank the MULTICOV Study Group.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2022.100539.
Contributor Information
Kristoffer S. Hansen, Email: krthas@rm.dk.
Steffen Leth, Email: stefleth@rm.dk.
Appendix A. Supplementary data
References
- 1.WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/
- 2.Rubin R. As their numbers grow, COVID-19 “long haulers” stump experts. JAMA. 2020;324:1381–1383. doi: 10.1001/jama.2020.17709. [DOI] [PubMed] [Google Scholar]
- 3.WHO A clinical case definition of post COVID-19 condition by a Delphi consensus. 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 [DOI] [PMC free article] [PubMed]
- 4.Nalbandian A., Sehgal K., Gupta A., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez-Leon S., Wegman-Ostrosky T., Perelman C., et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11:16144. doi: 10.1038/s41598-021-95565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C., Haupert S.R., Zimmermann L., Shi X., Fritsche L.G., Mukherjee B. Global prevalence of post COVID-19 condition or long COVID: a meta-analysis and systematic review. J Infect Dis. 2022 doi: 10.1093/infdis/jiac136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crook H., Raza S., Nowell J., Young M., Edison P. Long covid - mechanisms, risk factors, and management. BMJ. 2021;374 doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 8.Mills E.L., Kelly B., O'Neill L.A.J. Mitochondria are the powerhouses of immunity. Nat Immunol. 2017;18:488–498. doi: 10.1038/ni.3704. [DOI] [PubMed] [Google Scholar]
- 9.Gorman G.S., Chinnery P.F., DiMauro S., et al. Mitochondrial diseases. Nat Rev Dis Primers. 2016;2:16080. doi: 10.1038/nrdp.2016.80. [DOI] [PubMed] [Google Scholar]
- 10.Filler K., Lyon D., Bennett J., et al. Association of mitochondrial dysfunction and fatigue: a review of the literature. BBA Clin. 2014;1:12–23. doi: 10.1016/j.bbacli.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang L., Yao Q., Gu X., et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398:747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ajaz S., McPhail M.J., Singh K.K., et al. Mitochondrial metabolic manipulation by SARS-CoV-2 in peripheral blood mononuclear cells of patients with COVID-19. Am J Physiol Cell Physiol. 2021;320:C57–C65. doi: 10.1152/ajpcell.00426.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medini H., Zirman A., Mishmar D. Immune system cells from COVID-19 patients display compromised mitochondrial-nuclear expression co-regulation and rewiring toward glycolysis. iScience. 2021;24:103471. doi: 10.1016/j.isci.2021.103471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flynn R.A., Belk J.A., Qi Y., et al. Discovery and functional interrogation of SARS-CoV-2 RNA-host protein interactions. Cell. 2021;184:2394–2411.e16. doi: 10.1016/j.cell.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romão P.R., Teixeira P.C., Schipper L., et al. Viral load is associated with mitochondrial dysfunction and altered monocyte phenotype in acute severe SARS-CoV-2 infection. Int Immunopharmacol. 2022;108 doi: 10.1016/j.intimp.2022.108697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ernster L., Dallner G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta Mol Basis Dis. 1995;1271:195–204. doi: 10.1016/0925-4439(95)00028-3. [DOI] [PubMed] [Google Scholar]
- 17.Bhagavan H.N., Chopra R.K. Coenzyme Q10: absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic Res. 2006;40:445–453. doi: 10.1080/10715760600617843. [DOI] [PubMed] [Google Scholar]
- 18.Hidaka T., Fujii K., Funahashi I., Fukutomi N., Hosoe K. Safety assessment of coenzyme Q10 (CoQ10) Biofactors. 2008;32(1-4):199–208. doi: 10.1002/biof.5520320124. [DOI] [PubMed] [Google Scholar]
- 19.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herdman M., Gudex C., Lloyd A., et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leth S., Gunst J.D., Mathiasen V., et al. Persistent symptoms in patients recovering from COVID-19 in Denmark. Open Forum Infect Dis. 2021;8 doi: 10.1093/ofid/ofab042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen C.E., Sørensen S.S., Gudex C., Jensen M.B., Pedersen K.M., Ehlers L.H. The Danish EQ-5D-5L value set: a hybrid model using cTTO and DCE data. Appl Health Econ Health Policy. 2021;19:579–591. doi: 10.1007/s40258-021-00639-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tran V.T., Porcher R., Pane I., Ravaud P. Course of post COVID-19 disease symptoms over time in the ComPaRe long COVID prospective e-cohort. Nat Commun. 2022;13(1):1812. doi: 10.1038/s41467-022-29513-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi B.C., Pak A.W. A catalog of biases in questionnaires. Prev Chronic Dis. 2005;2(1):A13. [PMC free article] [PubMed] [Google Scholar]
- 25.Ziauddeen N., Gurdasani D., O'Hara M.E., et al. Characteristics and impact of long Covid: findings from an online survey. PLoS One. 2022;17(3) doi: 10.1371/journal.pone.0264331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lerum T.V., Aaløkken T.M., Brønstad E., et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur Respir J. 2021;57(4):2003448. doi: 10.1183/13993003.03448-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han J.H., Womack K.N., Tenforde M.W., et al. Associations between persistent symptoms after mild COVID-19 and long-term health status, quality of life, and psychological distress. Influenza Other Respir Viruses. 2022;16(4):680–689. doi: 10.1111/irv.12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buonsenso D., Munblit D., de Rose C., et al. Preliminary evidence on long COVID in children. Acta Paediatr. 2021;110:2208–2211. doi: 10.1111/apa.15870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jenkins R. Epidemiology: lessons from the past. Br Med Bull. 1991;47:952–965. doi: 10.1093/oxfordjournals.bmb.a072523. [DOI] [PubMed] [Google Scholar]
- 30.Hickie I., Davenport T., Wakefield D., et al. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. Br Med J. 2006;333:575–578. doi: 10.1136/bmj.38933.585764.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.