Abstract
Background:
Intensity-modulated radiation therapy (IMRT) beams traverse nontarget normal structures not irradiated during three-dimensional conformal RT (3D-CRT) for head and neck cancer (HNC). This study estimates the doses and toxicities to nontarget structures during IMRT.
Materials and Methods:
Oropharyngeal cancer IMRT and 3D-CRT cases were reviewed. Dose–volume histograms (DVH) were used to evaluate radiation dose to the lip, cochlea, brainstem, occipital scalp, and segments of the mandible. Toxicity rates were compared for 3D-CRT, IMRT alone, or IMRT with concurrent cisplatin. Descriptive statistics and exploratory recursive partitioning analysis were used to estimate dose “breakpoints” associated with observed toxicities.
Results:
A total of 160 patients were evaluated for toxicity; 60 had detailed DVH evaluation and 15 had 3D-CRT plan comparison. Comparing IMRT with 3D-CRT, there was significant (p ≤ 0.002) nonparametric differential dose to all clinically significant structures of interest. Thirty percent of IMRT patients had headaches and 40% had occipital scalp alopecia. A total of 76% and 38% of patients treated with IMRT alone had nausea and vomiting, compared with 99% and 68%, respectively, of those with concurrent cisplatin. IMRT had a markedly distinct toxicity profile than 3D-CRT. In recursive partitioning analysis, National Cancer Institute’s Common Toxicity Criteria adverse effects 3.0 nausea and vomiting, scalp alopecia and anterior mucositis were associated with reconstructed mean brainstem dose >36 Gy, occipital scalp dose >30 Gy, and anterior mandible dose >34 Gy, respectively.
Conclusions:
Dose reduction to specified structures during IMRT implies an increased beam path dose to alternate nontarget structures that may result in clinical toxicities that were uncommon with previous, less conformal approaches. These findings have implications for IMRT treatment planning and research, toxicity assessment, and multidisciplinary patient management.
Keywords: IMRT, Head-and-neck cancer, Toxicity, Nausea and vomiting, Mandible osteonecrosis
INTRODUCTION
Intensity-modulated radiation therapy (IMRT) is an attractive treatment strategy for head and neck cancer (HNC) because dose distributions conform to tumor topography while simultaneously limiting the normal tissue volume exposed to relatively high radiation doses (1). Decrements in specific toxicities (e.g., xerostomia), however, have lead some to assume that IMRT leads to a global reduction in toxicity as compared with 3-dimensional conformal radiation therapy (3D-CRT) techniques. This may explain, in part, the rapid growth in IMRT use in the Unites States, especially for HNC treatment (2). Actual experience, however, reveals that IMRT may not reduce common HNC RT toxicities (e.g., rates of acute high-grade mucositis, need for therapeutic feeding tubes) (3) and may, in fact, result in additional toxicities that were uncommon during 3D-CRT and apparently unique to IMRT.
IMRT plans for HNC typically implement approximately nine radiation fields, some of which traverse nontarget tissues that in the prior two-dimensional and 3D-CRT eras would not have been directly irradiated (Fig. 1). In our experience, some patients undergoing IMRT for HNC experienced toxicities in these nontarget areas that were not typical with traditional 3D-CRT, specifically mucositis in anterior oral structures (lip and tongue tip), occipital scalp alopecia, headache, and nausea and vomiting (even when RT was given without concurrent chemotherapy). These toxicities were rare in the previous 3D-CRT era (4, 5). Careful examination of beam path doses in delivered plans also showed that IMRT dose to the anterior alveolus could be much higher than in 3D-CRT plans. This raised concern because dental oncologists typically do not scrutinize the anterior teeth as closely as those more posterior for preradiation prophylaxis.
Fig. 1.

Comparison of nontarget beam paths in intensity-modulated radiotherapy (top) vs. conventional three-dimensional technique (bottom).
As a result of these observations, this hypothesis-generating study was undertaken with the following specific aims: (1) quantification of observed toxicity to noncontoured structures of interest; (2) dosimetric evaluation of the delivered dose to those structures; (3) determination of potential dose differentials of those structures in IMRT vs. 3D-CRT plans; and (4) generation of dose/toxicity threshold values through exploratory recursive partitioning-based analysis to generate hypotheses for future testing.
MATERIALS AND METHODS
Medical records from a consecutive series of patients who underwent IMRT for oropharyngeal cancer at The University of Texas M. D. Anderson Cancer Center between September 2002 and November 2006 were retrospectively reviewed. Patients were identified from a departmental database and were included if they received definitive IMRT or IMRT with concurrent chemotherapy. The year 2002 was chosen as a start date because treatment techniques between 2002 and 2006 were relatively uniform among our group and followed an approximately 2-year previous experience with IMRT (6). The study subjects were restricted to patients with oropharyngeal cancer owing to numerical frequency, and relative similarity in IMRT technical parameters, setups, beam paths, and prescribed dose ranges. Chart review and waiver of consent were approved by the institutional review board of The University of Texas M. D. Anderson Cancer Center, and individual patient confidentiality was maintained.
Digital Imaging and Communications in Medicine-RT treatment plans for individual patients were extracted from archival media and reconstructed. Previously noncontoured structures of interest were then delineated on the reconstructed plans for subsequent reevaluation using reconstructed dose–volume histograms (DVH). Specified structures of interest included the lower lip; anterior, mid, and posterior segments of the maxilla and mandible; the middle ear; cochlea; brainstem; and occipital scalp (Fig. 2). A formally defined contouring technique was used to achieve consistent contouring and to minimize the introduction of variability in the delineation process. Contouring was performed by one specialist, and the quality was verified by another expert physician. Contouring, DVH generation, and virtual plan analysis were done with the ADAC Pinnacle planning system (Phillips Medical Systems, Bothell, WA).
Fig. 2.
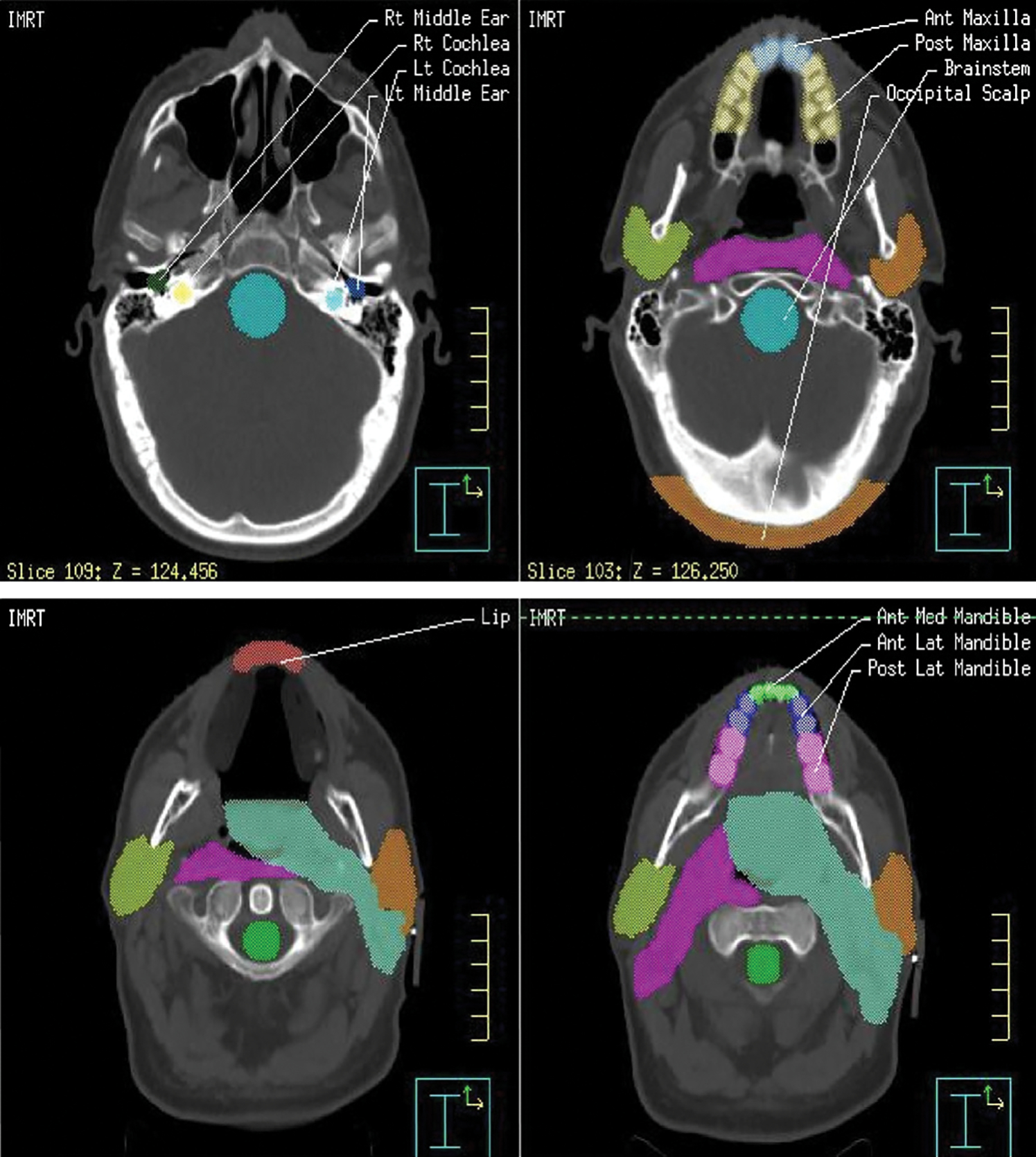
Representative computed tomography slices with contours of the lip, maxilla, mandible, middle ear, cochlea, brainstem, and occipital scalp.
Toxicity data were extracted from standard weekly management visit forms, on which toxicity had been graded according to the National Cancer Institute’s Common Toxicity Criteria (v3.0) for hair loss, mucositis, nausea, and vomiting. These forms include maximum toxicity grades but do not necessarily specify the exact location of toxic effects such as mucositis or alopecia. The managing physicians’ dictated weekly notes were also reviewed for additional toxicity data.
Rates of nausea and vomiting were compared between patients given IMRT and patients given concurrent IMRT and cisplatin at 100 mg/m2. Descriptive statistics were generated and data analyzed with SPSS 12.0 (SPSS Inc., Chicago, IL) for Windows. Two-tailed Pearson chi-square tests were used to compare frequencies between groups where applicable.
To evaluate the comparative dose to previously noncontoured structures of interest, IMRT DVH parameters were calculated and plans (with unaltered target volumes and structures) of 15 patients were “replanned” with a three-field 3D-CRT technique for internal consistency (7). Statistical analysis confirmed that this represented a sufficient comparative sample size based on the significant differences so that additional patient comparisons were not necessary. Interim statistical analysis of cohort (conventional vs. IMRT) dose to specified structures of interest was performed by Wilcoxon rank-order nonparametric comparison (owing to sample size). A modified Bonferroni correction to account for the large number of comparisons and the comparative loss of power associated with nonparametric analyses was used to preclude inadvertent type I error (8); in this specific case, between-cohort rank-order differentials would be statistically significant at a p value of ≤ 0.0023.
Exploratory analysis for future prospective hypothesis-driven efforts was done using tree-based classification/recursive partitioning analysis (RPA) with JMP 6.0 (SAS Institute, Cary, NC). RPA is a nonparametric, nonlinear graphical data-mining technique that does not depend on a priori identification of fitting parameters or prediction variables (9). We used RPA to generate hypotheses about dose/toxicity relationships because the sample size and the relative infrequency of any given toxic effect rendered the data set insufficiently robust to fit an absolute dose–response curve for toxicity. Categorical RPA analysis was performed by using dosimetry parameters derived from DVHs. A binary toxicity variable was created (toxicity/no toxicity), and dose “breakpoints” associated with differences in probability of toxicity at specified dose levels were computed.
RESULTS
A total of 160 consecutive patients were included in this retrospective review for analysis of toxicity (Table 1). IMRT plans were reevaluated in 60 patients for whom archival data were readily available to determine dose to the additional structures of interest. Patient characteristics are shown in Table 1. The median age was 58 years. Eighty-seven percent were men, and 75% had Stage IVa disease. Staging of patients by T- and N-stage are shown in Table 2. The most common chemotherapy regimen used in patients receiving concurrent chemotherapy was high-dose cisplatin (60%). All but 15 patients were treated with once-daily fractionation schedules.
Table 1.
Patient characteristics (n = 160)
| # Pts | |
|---|---|
|
| |
| Age (years) | |
| Range | 34 – 81 |
| Median | 58 |
| Sex | |
| Male | 139 |
| Female | 21 |
| Primary site | |
| Base of tongue | 78 |
| Tonsil | 80 |
| Oropharyngeal wall | 2 |
| Treatment type | |
| Intensity-modulated radiation therapy alone | 93 |
| Concurrent cisplatin | 40 |
| Other concurrent chemo | 27 |
| Dose to primary site (Gy) | |
| 60 – 63/30 fx | 5 |
| 66/30 fx | 79 |
| 66 – 68/33 fx | 5 |
| 70/33 fx | 56 |
| 72/40 fx (concomitant boost) | 15 |
Table 2.
T and N staging (n = 160)
| T1 | T2 | T3 | T4 | Total | |
|---|---|---|---|---|---|
|
| |||||
| N0 | 3 | 13 | 7 | 3 | 26 |
| N1 | 8 | 6 | 3 | 2 | 19 |
| N2 | 34 | 49 | 11 | 13 | 107 |
| N3 | 3 | 4 | 0 | 1 | 8 |
| Total | 48 | 72 | 21 | 19 | 160 |
The overall incidence of the toxicities measured is shown in Table 3. Figure 3 shows typical examples of anterior oral ulcerative mucositis and occipital scalp IMRT-induced alopecia. Seventy-six percent and 38% of patients treated with IMRT-alone had nausea and vomiting, compared with 98% and 68%, respectively, of those also receiving concurrent cisplatin (Table 4).
Table 3.
Rates (%) of toxicities by treatment group: IMRT with or without concurrent cisplatin (100 mg/m2)
| Incidence of toxicities by treatment group (%) | ||
|---|---|---|
|
| ||
| IMRT alone | Concurrent cisplatin | |
|
| ||
| Nausea | 76 | 98 |
| Vomiting | 38 | 68 |
| Headache | 10 | 30 |
| Occipital scalp epilation | 40 | 25 |
| Moist skin desquamation | 28 | 35 |
| Anterior oral mucositis | 9 | 22 |
Abbreviation: IMRT = intensity-modulated radiation therapy.
Fig. 3.

(a) Anterior oral mucositis during intensity-modulated radiotherapy (IMRT). (b) Occipital scalp epilation after IMRT. (c) Scalp hair subsequent regrowth, same patient.
Table 4.
Percentages of patients experiencing nausea and vomiting in the IMRT or IMRT-plus-concurrent-cisplatin groups
| Toxicity grade |
|||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
|
| |||||
| Nausea* | |||||
| IMRT alone | 24 | 33 | 38 | 5 | 0 |
| Concurrent cisplatin | 2 | 22 | 58 | 18 | 0 |
| Vomiting** | |||||
| IMRT alone | 63 | 16 | 18 | 3 | 0 |
| Concurrent cisplatin | 32 | 18 | 38 | 12 | 0 |
Abbreviation: IMRT = intensity-modulated radiation therapy.
p < 0.004 based on Pearson Chi-Square test.
p < 0.04 based on Pearson Chi-Square test.
Distributional characterization of median, mean, and standard deviation of the DVH-derived median dose to each structure of interest is detailed in Table 5. Table 6 lists the calculated average maximum dose to each structure by treatment modality. Table 7 summarizes calculated differentials in the maximum dose and median voxel dose received by a given structure of interest for each patient. For every site except the posterior mandible, the calculated maximum and median voxel dose to each structure demonstrated a statistically significant rank-order differential. In RPA analysis, nausea and emesis were associated with reconstructed mean dose to the brainstem of >36 Gy (log–worth 0.27 for nausea and 0.4 for emesis, respectively). Of the 9 patients with mean reconstructed brainstem dose >36 Gy, 6 (66%) had clinically evident nausea, and all 9 (100%) reported emesis. By comparison, 26 (52%) and 41 (82%) of 50 patients exhibited nausea and emesis, respectively, at lower mean brainstem dose levels. Anterior oral cavity mucositis (Grade ≥1) was found to be more prevalent (log–worth 2.14) when anterior or lateral mandible maximum voxel doses were >33.5 Gy, with 7 of 26 patients receiving that dose experiencing detectable anterior mucositis compared with only 1 of 31 patients (3%) receiving <33.5 Gy. Alopecia of the occipital scalp was noted more frequently (log–worth 0.44) when maximum occipital scalp doses exceeded 30 Gy (48%; 21 of 43 cases) vs. <30 Gy (19%; 3 of 13 cases).
Table 5.
Means, medians, and standard deviations of median voxel dose in cGy to structures per patient (n = 15) by treatment technique
| Distributional parameter | Technique | Brainstem | Cochlea, right | Cochlea, left | Lower lip | Mandible, anterior | Mandible, middle | Mandible, posterior | Maxilla, anterior | Maxilla, posterior | Middle ear, left | Middle ear, right | Occipital scalp |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Mean | Conventional | 416.0 | 330.8 | 328.4 | 126.2 | 196.0 | 280.7 | 2,400.5 | 157.5 | 461.4 | 399.0 | 413.9 | 86.9 |
| IMRT | 2,838.4 | 2,556.5 | 2,621.7 | 2,530.9 | 3,150.9 | 3,607.1 | 4,770.4 | 2,456.6 | 3,006.2 | 2,587.5 | 2,549.0 | 1,868.4 | |
| Median | Conventional | 289.0 | 338.0 | 320.5 | 123.5 | 176.5 | 257.0 | 980.5 | 130.5 | 353.5 | 390.0 | 403.5 | 88.5 |
| IMRT | 3,249.0 | 2,606.0 | 2,882.0 | 2,464.0 | 3,288.0 | 3,495.0 | 4,506.0 | 2,582.5 | 3,129.0 | 2,684.0 | 2,486.0 | 1,735.0 | |
| SD | Conventional | 395.8 | 55.3 | 63.5 | 45.9 | 71.6 | 125.8 | 2,626.3 | 77.8 | 535.7 | 79.4 | 91.1 | 12.7 |
| IMRT | 1,154.8 | 1,123.5 | 1,136.8 | 621.4 | 791.3 | 1,048.5 | 1,170.9 | 790.9 | 920.3 | 1,044.9 | 1,146.3 | 314.0 | |
Abbreviation: IMRT = intensity-modulated radiation therapy.
Table 6.
Average of maximum voxel dose (in cGy) to noncontoured structures per patient, by treatment technique
| Structure | Conventional | IMRT |
|---|---|---|
|
| ||
| Brain stem | 3741.6 | 4590.4 |
| Cochlea, left | 426.4 | 3467.1 |
| Cochlea, right | 433.5 | 3372.3 |
| Lower lip | 226.7 | 3587.1 |
| Mandible, anterior | 752.4 | 3871.1 |
| Mandible, middle | 1124.3 | 4954.3 |
| Mandible, posterior | 4886.1 | 6149.3 |
| Maxilla, anterior | 264.7 | 3070.8 |
| Maxilla, posterior | 2894.0 | 4206.8 |
| Middle ear, left | 574.6 | 3557.3 |
| Middle ear, right | 642.3 | 3584.4 |
| Occipital scalp | 118.6 | 3453.6 |
Abbreviation: IMRT = intensity-modulated radiation therapy.
Table 7.
Rank-order comparison of conventional and IMRT maximum and median voxel dose per patient to specified structures
| Structure/region | Modality | Median highest value voxel dose per patient (cGy) | p value | Median voxel dose per patient (cGy) | p value |
|---|---|---|---|---|---|
|
| |||||
| Brain stem | Conventional | 1,695.5 | 0.002* | 289 | <0.001* |
| IMRT | 4455 | 3,249 | |||
| Cochlea, left | Conventional | 428.5 | <0.001* | 320.5 | <0.001* |
| IMRT | 5,048 | 2,882 | |||
| Cochlea, right | Conventional | 439.5 | <0.001* | 338 | <0.001* |
| IMRT | 4642 | 2,606 | |||
| Lower lip | Conventional | 213 | <0.001* | 123.5 | <0.001* |
| IMRT | 3341 | 2464 | |||
| Mandible, anterior | Conventional | 348.5 | <0.001* | 176.5 | <0.001* |
| IMRT | 4,418 | 3,288 | |||
| Mandible, middle | Conventional | 530 | <0.001* | 257 | <0.001* |
| IMRT | 5,738 | 3,495 | |||
| Mandible, posterior | Conventional | 7,050 | 0.6 (NS) | 980.5 | 0.02 (NS) |
| IMRT | 6,510 | 4506 | |||
| Maxilla, anterior | Conventional | 380 | <0.001* | 130.5 | <0.001* |
| IMRT | 3,738 | 2,582.5 | |||
| Maxilla, posterior | Conventional | 2,282 | <0.001* | 353.5 | <0.001* |
| IMRT | 4,038 | 3,129 | |||
| Middle ear, left | Conventional | 569 | <0.001* | 390 | <0.001* |
| IMRT | 4,348 | 2,684 | |||
| Middle ear, right | Conventional | 638 | <0.001* | 403.5 | <0.001* |
| IMRT | 4,650 | 2,486 | |||
| Occipital scalp | Conventional | 112.5 | <0.001* | 88.5 | <0.001* |
| IMRT | 2,464 | 1,735 | |||
Abbreviations: IMRT = intensity-modulated radiation therapy; NS = not significant.
Statistically significant after Bonferroni adjustment.
DISCUSSION
The conformal dose advantages of IMRT are well known; nevertheless, it is not without pitfalls. IMRT has solved some HNC treatment planning problems, but has created others. Although IMRT may limit the volumes of user-delineated organs at risk exposed to high-dose radiation, integral dose to the entire body is generally higher, and dose to nondelineated extra-target tissues and organs in the beam paths that would not typically be directly irradiated during traditional 3D-RT techniques may substantially increase. In this report, these potential consequential IMRT-specific toxicities are referred to as nontarget or non-CTV IMRT beam path toxicities.
Several benefits have been demonstrated for IMRT in the treatment for HNC. IMRT can reduce dose relative to the parotid salivary glands and reduce high-grade xerostomia for many, but not all patients (10–12). IMRT can reduce optic and central nervous system toxicity rates, in the treatment of tumors in or near the skull base (e.g., paranasal sinus and nasopharyngeal cancers) (13, 14). These benefits derived from IMRT result from the relative reduction of radiation dose to contoured functional normal structures, but the nature of IMRT dose delivery may in turn increase dose to other structures in the IMRT beam path, only some of which may be contoured or designated with dose limitations (15).
The multiplicity of fields and segments used in IMRT make it relatively inefficient compared with conventional therapy, because IMRT plans typically require a significant increase in monitor units (16). Some studies suggest that this would increase integral dose and thus may increase the risk for radiation-associated second malignancy (16, 17). Other studies, however, have shown that IMRT can be delivered with a minimal increase or even a possible decrease in integral dose (18).
IMRT was implemented and widely adopted before clinical trial data demonstrated full evaluation of potential associated risks (19). Only experience with IMRT led to the understanding that it could lead to unanticipated toxicity to some normal structures not in the CTV that reside within IMRT beam paths if not appropriately considered during IMRT planning (20–22). Fua et al. reported that IMRT could lead to significantly higher rates of dysphagia and prolonged feeding tube requirement than seen with the 3D-CRT technique if appropriate dose constraints for the cervical esophagus, for example, are not used (21). In contrast, investigators from the University of Michigan proposed that risk of dysphagia could be reduced by decreasing the dose to previously noncontoured structures deemed essential to normal swallowing (11, 23–25). If a structure subject to potential toxicity is not contoured and given appropriate hierarchical dose-goal rank in IMRT plans, then the dose to such normal structures and the clinical consequences of acute or late damage to these structures may not be appreciated until toxicities develop (26, 27).
Our study is the first to emphasize that nontarget normal tissues or organs in IMRT beam paths not directly irradiated in the 3D-CRT era are subject to specific potential toxicities, including anterior oral mucositis, occipital scalp hair loss, headache, nausea, and emesis, and that these are, in fact, comparatively common events in patients receiving IMRT without concurrent chemotherapy for HNC. Our data show that, despite the use of an “anterior oral” avoidance structure, the anterior oral volume often receives 35–40 Gy, and additional focal “hot spots” may exacerbate mucositis (Fig. 4). This occurred in a clinically significant proportion of patients despite a lower rate of dose accumulation (35–45 Gy/30–33 fractions) and without concurrent sensitizing chemotherapy.
Fig. 4.

Results of intensity-modulated radiotherapy “hot spots” on oral tongue mucositis.
The use of commonly accepted dose constraints may be useful for preventing the devastating chronic toxicities that represented the thrust of safety measures in the two-dimensional and 3D eras, but they may not prevent reversible but burdensome acute toxicities. These whole-organ dose limitations ignore dose–volume effects in general and more specifically the dose to specific subvolumes. A maximum 54 Gy dose to the brain stem, used as a common constraint in cooperative group clinical trials with IMRT, may reduce risk for brain necrosis but may not prevent acute nausea and vomiting resulting from radiation to specific brain regions (28). Experience in stereotactic radiosurgery, on the other hand, demonstrated that the dose to the area postrema (29, 30) in the brain stem correlated with nausea and vomiting and that limiting dose to even lower levels could reduce the incidence of those toxicities (31).
The American Society for Clinical Oncology antiemetic guidelines list RT-only for HNC as having low emetogenic potential (5). These guidelines, however, antedate IMRT (32). Our chemotherapy analysis was limited to those patients who received cisplatin at a dose of 100 mg/m2, the Radiation Therapy Oncology Group standard regimen that is also classified as highly emetogenic by American Society for Clinical Oncology guidelines (5, 28, 33). Our data show that nausea and vomiting are common events in HNC patients treated with IMRT only and that this toxicity is exacerbated by emetogenic chemotherapy to higher levels than typically reported for the same chemotherapy in HNC chemoradiation trials using 3D-CRT (34, 35). These findings suggest that antiemetic guidelines may need to be updated after the incidence of nausea and vomiting is studied on a larger cohort of patients treated at a variety of institutions (32).
We surmise that the cause of the nausea and vomiting during IMRT only is related to the dose to the brain stem, specifically the area postrema (30), for which a dose association has been implicated during stereotactic radiosurgery (31). The number of patients and events in this exploratory study were too low to establish a curve-fit dose–response relationship. Here, these and the other dose–toxicity probability thresholds were estimated by RPA analysis, which is a more flexible technique insofar as it does not rely on parametric or model fitting to achieve classification thresholds (36–38). Consequently, extrapolation of complex relational features from RPA is limited, and the dose thresholds for observed toxicity probability described herein should not be considered as absolute dose constraints, but rather as approximations or benchmarks for additional verification studies.
Our findings also showed that IMRT for HNC leads to occipital scalp alopecia (Fig. 3b). Our observation that occipital scalp hair ultimately grows back for most but not all patients is consistent with that of Ling et al., who reported that IMRT tangential beam effects could enhance radiation dermatitis depending on the planning technique (19). However, even this degree of toxicity may be mitigated by careful attention to scalp structures in the treatment planning phase (39).
Cumulatively, our dosimetric analysis reveals a substantial, even factorial, dose increase to uncontoured structures of interest using IMRT compared with conventional radiotherapy. As Table 7 suggests, even a small cohort of 3D-CRT plans was sufficient to generate a statistical significance threshold corrected for the number of cohort comparisons (p ≤ 0.0023) to IMRT. Statistically significant differences in median and maximum voxel dose per patient were observed for all clinically important structures. Consequently, it may be worthwhile to modify prescription constraints, or at the very least acknowledge conceptually the dose delivery to structures with in beam paths.
It is standard practice for dentate patients to undergo preradiation oral evaluation because tooth-bearing areas, especially in the mandible, receiving more than 40 Gy are considered at risk for subsequent complications (40). It is commonly recommended that partially erupted, nonrestorable, and periodontally compromised teeth exhibiting moderate to severe mobility or periapical infection be extracted if the affected areas are to receive such dose (41). Dental oncologists have traditionally focused on the evaluation of the more posterior teeth, namely molars and premolars, particularly in the mandible, when rendering a treatment plan for pre-RT intervention, because these were the only areas of the alveolus commonly receiving 40 Gy or more with 3D-CRT. Our results show that the IMRT dose to the more anterior mandible (incisors and canines) can commonly exceed these limits. These findings suggest that a shift in the paradigm of pre-RT dental evaluations in the IMRT era must occur to include the anterior segments of the mandible.
The lack of prospective collection and full documentation of toxicity distribution and the sample size are the main limitations of this study. The rates of nausea and vomiting reported here, for example, had been regularly recorded during weekly management visits, so are considered reliable. The rates of oral mucositis and scalp hair loss, however, had not always been documented with respect to the specific anterior oral and occipital locations, respectively, by our team, so these data probably underestimate the true rates of these toxicities. This report brings these problems to light, however, and indicates the need for them to be captured on future toxicity measures so that exact rates or occurrence, severity, and recovery can be fully elucidated in prospective evaluations.
This series focuses on toxicities in IMRT patients, without comparison to a matched non-IMRT comparison cohort. In an effort to illustrate the relative incidence of nontarget 3D-CRT beam path toxicities, a post hoc analysis was performed by surveying the records of 52 patients treated with 3D-CRT for oropharynx cancer without chemotherapy. The reported rates of alopecia (0%), tip of tongue or lip mucositis (0%), emesis (4%), nausea (4%), and headache (2%), were substantially less than those observed in the IMRT patients within this series (Fisher’s exact chi-square p < 0.001for all comparisons). This illustrates the markedly distinct toxicity profile observed with IMRT.
Observed extra-target beam path toxicities could in theory be reduced if the dose to the affected structures could be lowered sufficiently. The optimal thresholds, dose–volume effects, and planning dose goals are not fully known, however, and so require further study for elaboration. Potential solutions to reduce the beam path toxicities are as follows.
Step-and-shoot IMRT plans can be optimized with attention to these recognized areas at risk for toxicity. We typically use laryngeal, cricopharyngeal/cervical esophagus, cochlear, middle ear, anterior oral, and occipital avoidance structures. We also create an additional single contour for all nontargets referred to as a “normal tissue avoidance structure.”
The use of heterogeneity and conformality indices, and perhaps the integration of equivalent uniform dose calculations to help chose among different IMRT plans might help to improve the therapeutic ratio (42).
One technique that limits laryngeal/cricopharyngeal dose treats the upper neck and mucosal primary site with IMRT, and treats the lower neck with an anterior supraclavicular field with at least a partial midline block. Studies have shown that the match line dosimetry is excellent with <5% inhomogeneity, and that splitting the fields can shorten treatment time (27). We prefer this technique whenever possible because not using IMRT where it is not needed reduces unnecessary radiation dose to these functionally important structures when not involved by cancer (13, 14, 28).
Helical tomotherapy might improve the therapeutic index as compared with step-and-shoot IMRT technique (16, 29–31).
The relatively much lower entry dose for protons as compared to photons, the lack of dose exit because of Bragg peak, the use of fewer beams, and the ability to select their paths all lead to a substantial reduction in extra-target beam path effects for proton beam therapy as compared with IMRT (32, 33).
CONCLUSIONS
In conclusion, the use of IMRT is rapidly growing, especially for HNC. IMRT has solved several HNC treatment planning problems but creates others because of associated potential integral dose increases and clear nontarget normal tissue beam path effects. Here “uncommon” toxicities associated with IMRT for HNC that affect structures typically contoured and those not are described. This recognition suggests the approach to pre-IMRT dental evaluations requires modification, and that the emetogenic potential of IMRT be recognized especially when combined with concurrent emetogenic drugs (e.g., cisplatin). It is important to recognize that the dose to noncontoured structures at risk is either unknown or known poorly are not contoured.
Further study with more patients is required to clarify the dose–volume and partial volume relationships for these toxicities so that other solutions can be identified and integrated into IMRT treatment planning. A full understanding of the benefits and costs of IMRT is likely not yet realized. Additional unanticipated toxicities and risks associated with HNC IMRT may well emerge for which we must be watchful, so that they too can be recognized and addressed expeditiously.
Acknowledgments—
We wish to thank John W. Fan, M.D., for assistance in data collection, and James A. Lemoine for medical photography.
C.D.F. is funded by the National Institute of Biomedical Imaging and BioEngineering Multidisciplinary Training Program in Human Imaging (5T32EB000817–04).
Footnotes
Conflict of interest: none.
REFERENCES
- 1.Mohan R, Wu Q, Manning M, et al. Radiobiological considerations in the design of fractionation strategies for intensity-modulated radiation therapy of head and neck cancers. Int J Radiat Oncol Biol Phys 2000;46:619–630. [DOI] [PubMed] [Google Scholar]
- 2.Mell LK, Mehrotra AK, Mundt AJ. Intensity-modulated radiation therapy use in the US, 2004. Cancer 2005;104:1296–1303. [DOI] [PubMed] [Google Scholar]
- 3.Garden AS, Morrison WH, Wong PF, et al. Disease-control rates following intensity-modulated radiation therapy for small primary oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2007;67:438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radiation-induced emesis: A prospective observational multi-center Italian trial. The Italian Group for Antiemetic Research in Radiotherapy. Int J Radiat Oncol Biol Phys 1999;44:619–625. [DOI] [PubMed] [Google Scholar]
- 5.Kris MG, Hesketh PJ, Somerfield MR, et al. American Society of Clinical Oncology Guideline for Antiemetics in Oncology: Update 2006. J Clin Oncol 2006;24:2932–2947. [DOI] [PubMed] [Google Scholar]
- 6.Rosenthal DI, Asper JA, Barker JLJ, et al. Importance of patient examination to clinical quality assurance in head and neck radiation oncology. Head Neck 2006;28:967–973. [DOI] [PubMed] [Google Scholar]
- 7.Ang KK, Garden AS. Radiotherapy for head and neck cancers: Indications and techniques. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 8.Ludbrook J. Multiple comparison procedures updated. Clin Exp Pharmacol Physiol 1998;25:1032–1037. [DOI] [PubMed] [Google Scholar]
- 9.Hand DJ. Statistical methods in diagnosis. Stat Methods Med Res 1992;1:49–67. [DOI] [PubMed] [Google Scholar]
- 10.de Arruda FF, Puri DR, Zhung J, et al. Intensity-modulated radiation therapy for the treatment of oropharyngeal carcinoma: The Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys 2006;64:363–373. [DOI] [PubMed] [Google Scholar]
- 11.Eisbruch A, Ten Haken RK, Kim HM, et al. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys 1999;45:577–587. [DOI] [PubMed] [Google Scholar]
- 12.Kam MM, Leung SF, Zee B, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol 2007;25:4873–4879. [DOI] [PubMed] [Google Scholar]
- 13.Bristol IJ, Ahamad A, Garden AS, et al. Postoperative radiotherapy for maxillary sinus cancer: Long-term outcomes and toxicities of treatment. Int J Radiat Oncol Biol Phys 2007;68:719–730. [DOI] [PubMed] [Google Scholar]
- 14.Hoppe BS, Stegman LD, Zelefsky MJ, et al. Treatment of nasal cavity and paranasal sinus cancer with modern radiotherapy techniques in the postoperative setting—the MSKCC experience. Int J Radiat Oncol Biol Phys 2007;67:691–702. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Taylor JMG, Ten Haken RK, et al. The impact of dose on parotid salivary recovery in head and neck cancer patients treated with radiation therapy. Int J Radiat Oncol Biol Phys 2007;67:660–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meeks SL, Paulino AC, Pennington EC, et al. In vivo determination of extra-target doses received from serial tomotherapy. Radiother Oncol 2002;63:217–222. [DOI] [PubMed] [Google Scholar]
- 17.Hall EJ. Cancer caused by x-rays—a random event? Lancet Oncol 2007;8:369–370. [DOI] [PubMed] [Google Scholar]
- 18.Aoyama H, Westerly DC, Mackie TR, et al. Integral radiation dose to normal structures with conformal external beam radiation. Int J Radiat Oncol Biol Phys 2006;64:962–967. [DOI] [PubMed] [Google Scholar]
- 19.Ling CC, Yorke E, Fuks Z. From IMRT to IGRT: Frontierland or neverland? Radiother Oncol 2006;78:119–122. [DOI] [PubMed] [Google Scholar]
- 20.Amdur RJ, Li JG, Liu C, et al. Unnecessary laryngeal irradiation in the IMRT era. Head Neck 2004;26:257–264. [DOI] [PubMed] [Google Scholar]
- 21.Fua TF, Corry J, Milner AD, et al. Intensity-modulated radiotherapy for nasopharyngeal carcinoma: Clinical correlation of dose to the pharyngo-esophageal axis and dysphagia. Int J Radiat Oncol Biol Phys 2007;67:976–981. [DOI] [PubMed] [Google Scholar]
- 22.Pacholke HD, Amdur RJ, Morris CG, et al. Late xerostomia after intensity-modulated radiation therapy versus conventional radiotherapy. Am J Clin Oncol 2005;28:351–358. [DOI] [PubMed] [Google Scholar]
- 23.Feng FY, Kim HM, Lyden TH, et al. IMRT aimed at reducing dysphagia: Early dose-volume-effect relationships for swallowing structures. Int J Radiat Oncol Biol Phys 2006;66:S44–S45. [DOI] [PubMed] [Google Scholar]
- 24.Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: Early dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys 2007;68:1289–1298. [DOI] [PubMed] [Google Scholar]
- 25.Feng M, Eisbruch A. Future issues in highly conformal radiotherapy for head and neck cancer. J Clin Oncol 2007;25:1009–1013. [DOI] [PubMed] [Google Scholar]
- 26.Deasy JO, Alaly JR, Zakaryan K. Obstacles and advances in intensity-modulated radiation therapy treatment planning. Front Radiat Ther Oncol 2007;40:42–58. [DOI] [PubMed] [Google Scholar]
- 27.Wilkens JJ, Alaly JR, Zakarian K, et al. IMRT treatment planning based on prioritizing prescription goals. Phys Med Biol 2007;52:1675–1692. [DOI] [PubMed] [Google Scholar]
- 28.Radiation Therapy Oncology Group (RTOG 0522): A randomized, phase III trial of concurrent accelerated radiation and cisplatin versus concurrent accelerated radiation, cisplatin, and cetuximab (C225) for stage III and IV head and neck carcinomas. www.rtog.org [PubMed]
- 29.Miller AD. Central mechanisms of vomiting. Dig Dis Sci 1999;44:39S–43S. [PubMed] [Google Scholar]
- 30.Miller AD, Leslie RA. The area postrema and vomiting. Front Neuroendocrinol 1994;15:301–320. [DOI] [PubMed] [Google Scholar]
- 31.Alexander Er, Siddon RL, Loeffler JS. The acute onset of nausea and vomiting following stereotactic radiosurgery: Correlation with total dose to area postrema. Surg Neurol 1989;32:40–44. [DOI] [PubMed] [Google Scholar]
- 32.Urba S. Radiation-induced nausea and vomiting. J Natl Compr Canc Netw 2007;5:60–65. [DOI] [PubMed] [Google Scholar]
- 33.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 2003;349:2091–2098. [DOI] [PubMed] [Google Scholar]
- 34.Sheng K, Molloy JA, Read PW. Intensity-modulated radiation therapy (IMRT) dosimetry of the head and neck: A comparison of treatment plans using linear accelerator-based IMRT and helical tomotherapy. Int J Radiat Oncol Biol Phys 2006;65:917–923. [DOI] [PubMed] [Google Scholar]
- 35.van Vulpen M, Field C, Raaijmakers CPJ, et al. Comparing step-and-shoot IMRT with dynamic helical tomotherapy IMRT plans for head-and-neck cancer. Int J Radiat Oncol Biol Phys 2005;62:1535–1539. [DOI] [PubMed] [Google Scholar]
- 36.Grobman WA, Stamilio DM. Methods of clinical prediction. Am J Obstet Gynecol 2006;194:888–894. [DOI] [PubMed] [Google Scholar]
- 37.Cooper JS, Farnan NC, Asbell SO, et al. Recursive partitioning analysis of 2105 patients treated in Radiation Therapy Oncology Group studies of head and neck cancer. Cancer 1996;77:1905–1911. [DOI] [PubMed] [Google Scholar]
- 38.Leon X, Gich I, Orus C, et al. Comparison of the Radiation Therapy Oncology Group recursive partitioning classification and Union Internationale Contre le Cancer TNM classification for patients with head and neck carcinoma. Head Neck 2005;27:248–257. [DOI] [PubMed] [Google Scholar]
- 39.Ting J, Thomas CR, McClure JA, et al. “Alopecia-less” whole brain radiotherapy (WBRT) via IMRT: Preliminary experience and outcomes. Int J Radiat Oncol Biol Phys 2005;63:S263–S264. [Google Scholar]
- 40.Cooper JS. The oral cavity. In: Cox JD, Ang KK, editors. Radiation oncology: rationale, technique, results. St Louis: Mosby; 2003. p. 219–223. [Google Scholar]
- 41.Marx RE, Johnson RP. Studies in the radiobiology of osteoradionecrosis and their clinical significance. Oral Surg Oral Med Oral Pathol 1987;64:379–390. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Zhang X, Dong L, et al. Effectiveness of noncoplanar IMRT planning using a parallelized multiresolution beam angle optimization method for paranasal sinus carcinoma. Int J Radiat Oncol Biol Phys 2005;63:594–601. [DOI] [PubMed] [Google Scholar]


