Abstract
The objective of these studies was to evaluate the inclusion of a microbial muramidase (MUR) in the diets of broiler chickens on the growth performance, intestinal permeability (IP), total blood carotenoid content, apparent ileal digestibility (AID), and foot pad dermatitis (FPD). In Experiment 1, a total of 1,000 one-day-old chicks were placed in floor-pens with reused litter, and randomly distributed into 4 treatments with 10 replicates each. Treatments were a basal diet (control), or basal diet supplemented with 15,000; 25,000 or 35,000 LSU (F)/kg of MUR. Feed intake (FI), body weight gain (BWG), and feed conversion ratio (FCR) were evaluated at d 21 and 43. Intestinal permeability was evaluated on d 35 by FITC-d, and FPD and AID on d 43. In Experiment 2, a total of 800 one-day-old chicks were placed in floor-pens with fresh litter, and randomly distributed into 4 treatments with 8 replicates each. Treatments were a basal diet (control), or basal diet supplemented with 25,000 or 35,000 LSU (F)/kg of MUR, and a fourth group where the basal diet was supplemented with enramycin. The birds were induced to a mild intestinal challenge. Feed intake, BWG, and FCR were evaluated on d 21 and d 42, and total blood concentration of carotenoids was evaluated on d 28. In experiment 1, 35,000 LSU (F)/kg of MUR promoted the best FCR (P < 0.05). Muramidase supplementation linearly increased the AID of dry matter, ash, and fat (P < 0.01), and regardless of the dose, MUR decreased the IP (P < 0.05). In Experiment 2, the supplementation of 35,000 LSU (F)/kg of MUR improved BWG and FCR in the entire cycle (1–42 d) and increased the concentration of carotenoids in the blood on d 28 compared to the control group (P < 0.05). These studies show that MUR improves growth performance of broilers by improving intestinal permeability, digestibility of dry matter, ash and fat, absorption of carotenoids, and reducing FPD.
Key words: carotenoids, intestinal permeability, muramidase, peptidoglycan, welfare
INTRODUCTION
Dietary supplementation with exogenous enzymes is a common practice in animal nutrition (Bedford and Cowieson, 2012). Enzymes play an important role in making undigestible fractions of the diet available for absorption by the animal and reducing antinutritional factors of ingredients. The supplementation of enzymes can optimize the nutritional value of the diets, thus reducing feed cost, improving the growth performance of animals, and reducing the environmental pollution (Adeola and Cowieson, 2011; Kiarie et al., 2013; Meale et al., 2014).
Most of the feed enzymes in the market (e.g., phytases, carbohydrases, proteases) target substrates in the feed ingredients (Adeola and Cowieson, 2011). However, another category of enzymes, such as muramidase (MUR), target components present in the intestinal lumen and may also be considered a feed additive (Cooper et al., 2014; Long et al., 2016, Sais et al., 2020). Muramidases (EC 3.2.1.17) are enzymes produced by animals, plants, and microorganisms with high specificity to hydrolyze peptidoglycans (PGN), the major structural components of the bacterial cell wall (Morgavi et al., 1994; Vidal et al., 2005; Sytwala et al., 2015). Peptidoglycans are complex structures, formed by repeated N-acetylmuramic acid sequences connected by β-1-4 bonds with N-acetyl glucosamine (Phillips, 1966; Alcorlo et al., 2017). With the rapid bacteria cell wall recycling, PGN fragments are constantly being released in the gastrointestinal tract (GIT) and accumulate in the intestinal lumen (Lee and Hase, 2014). These PGN can also be recognized by immune cells and trigger inflammatory processes (Broom and Kogut, 2018). The consequence of the accumulation of PGN from bacterial cell debris on the intestinal absorption remains uncertain. However, Goodarzi Boroojeni et al. (2019) evaluating nutrient digestibility and intestinal histology of broilers fed with or without MUR, suggested that PGN could impair nutrient digestion and absorption. The main role of microbial MUR, also known as lysozyme or N-acetylmuramidase, is to act on the complex PGN structures, cleaving the β − 1,4 bonds between N-acetylmuramic acid and N-acetyl glucosamine (Chipman and Sharon, 1969). Therefore, MUR could reduce the load of PGN in the lumen, allowing an effective absorption of dietary nutrients.
Studies have shown the safety of microbial MUR inclusion in broiler diets. Lichtenberg et al. (2017) evaluated different inclusions of microbial MUR in broiler diets and did not observe toxic effects even when using high dietary concentrations (450,000 LSU(F)/kg), but instead obtained an enhancement in performance with the addition of the enzyme. Also, improvements in the GIT functions (digestion and absorption efficiency) and performance were observed with dietary inclusion of MUR on broilers (Sais et al., 2020). The effective functionality of the GIT depends on several factors and must be able to support an effective barrier function, mucus layer development, regulated immune response, stable microbiome, host metabolism and energy generation, and waste secretions (Turner, 2009; Mwangi et al., 2010; Oakley et al., 2014; Oakley and Kogut, 2016; Celi et al., 2017; Kogut, 2019).
To the extent of our knowledge, there are no studies published in the literature demonstrating the effect of the MUR evaluated herein in broiler chickens undergoing an intestinal challenge. Therefore, to better understand the mechanism of action of MUR, multiple approaches were applied in the 2 studies presented with the objective to evaluate different dietary inclusion concentrations of MUR on the GIT functionality. The measurements included: intestinal permeability, carotenoids absorption, apparent ileal digestibility (AID), and the consequences on the growth performance, and foot pad dermatitis (FPD) in broiler chickens subjected, or not, to a mild intestinal challenge.
MATERIALS AND METHODS
Experiment 1
Animals and Experimental Design
The animal trial was conducted at the poultry experimental unit of Federal University of Paraná, in Curitiba, Paraná, Brazil. The study was performed in accordance with the Ethics Committee for the Use of Animals of Federal University of Paraná (protocol 089/2017).
The chicks were obtained from a commercial hatchery (BRF S.A Brasil Foods, Castro/Paraná). A fraction corresponding to 10% of the total of animals was weighed to calculate the average weight of the flock and the chicks with the average weight of 37.2 ± 5 g were selected for the study. A total of 1,000 one-day-old male broilers chickens (Cobb500) were randomly divided into 4 treatments, 10 replicate pens per treatment and 25 birds per pen. The experimental treatments consisted of a basal diet formulated for each feeding phase that differed in the concentration of inclusion of MUR (Table 1). The treatment groups were a basal diet without MUR, or basal diet supplemented with 15,000; 25,000; or 35,000 LSU (F)/kg of feed of MUR.
Table 1.
Ingredients and nutritional composition of the diets in starter, grower, and finisher phases of broilers in Experiments 1 and 2.
| Ingredients, g kg−1 as fed | Starter1–21 d | Grower22–35 d | Finisher36–43 d | Starter1–21 d | Grower22–35 d | Finisher36–42 d |
|---|---|---|---|---|---|---|
| Experiment 1 | Experiment 2 | |||||
| Corn | 553.13 | 606.86 | 606.14 | 546.96 | 557.06 | 549.46 |
| Soybean meal | 388.0 | 336.0 | 314.0 | 333.2 | 292.3 | 262.7 |
| Rice bran | - | - | - | 30.0 | 60.0 | 90.0 |
| Meat and bone meal | - | - | - | 40.0 | 26.4 | 26.4 |
| Salt | 4.8 | 4.3 | 4.1 | 3.3 | 3.5 | 3.5 |
| Choline chloride | 0.8 | 0.6 | 0.5 | - | - | - |
| Soybean oil | 24.0 | 28.0 | 45.0 | 34.1 | 47.3 | 55.0 |
| Dicalcium phosphate | 10.0 | 6.8 | 4.5 | - | - | - |
| Limestone | 12.0 | 10.8 | 9.9 | 3.8 | 4.9 | 4.5 |
| DL-methionine | 2.97 | 2.67 | 2.48 | 3.10 | 2.80 | 2.60 |
| L-lysine | 0.91 | 0.59 | 0.03 | 1.90 | 2.10 | 2.20 |
| L-threonine | 0.10 | 0.09 | 0.14 | 0.50 | 0.50 | 0.50 |
| Vitamin premix1 | 1.50 | 1.50 | 1.00 | 1.50 | 1.50 | 1.50 |
| Mineral premix2 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Phytase3 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Xylanase4 | 0.05 | 0.05 | 0.05 | - | - | - |
| Ethoxyquin5 | 0.10 | 0.10 | 0.10 | - | - | - |
| Apo-ester6 | 0.04 | 0.04 | 0.00 | 0.04 | 0.04 | 0.04 |
| Inert7 | 1.00 | 1.00 | 1.40 | 1.00 | 1.00 | 1.00 |
| Celite™8 | 0.00 | 0.00 | 10.0 | - | - | - |
| Total | 1,000 | 1,000 | 1,000 | 1,000 | 1,000 | 1,000 |
| Calculated composition (% DM basis) | ||||||
| AMEn, kcal/kg | 3,000 | 3,100 | 3,200 | 3,100 | 3,200 | 3,250 |
| Crude Protein | 22.0 | 20.0 | 19.0 | 22.0 | 20.0 | 19.0 |
| Calcium | 0.94 | 0.80 | 0.70 | 0.90 | 0.77 | 0.75 |
| Available phosphorus | 0.47 | 0.40 | 0.35 | 0.47 | 0.40 | 0.40 |
| Digestible lysine | 1.20 | 1.05 | 0.95 | 1.20 | 1.10 | 1.05 |
| Digestible methionine | 0.60 | 0.55 | 0.52 | 0.60 | 0.55 | 0.52 |
| Digestible threonine | 0.78 | 0.71 | 0.68 | 0.78 | 0.71 | 0.68 |
| Digestible tryptophan | 0.26 | 0.23 | 0.22 | 0.23 | 0.21 | 0.20 |
| Analyzed composition (% DM basis) | ||||||
| Crude protein | - | - | 18.70 | - | - | - |
| Calcium | - | - | 0.72 | - | - | - |
| Total phosphorus | - | - | 0.39 | - | - | - |
One kg of vitamin premix contains: Vit. A (all-trans retinol/retynil acetate/retynil palmitate/β-carotene): 9,000,000 IU/kg; Vit. D3 2,500,000 IU/kg; Vit. E (dl-α-tocopheryl acetate/dl-α-tocopherol/d-α-tocopherol): 20,000 IU/kg; Vit. K3 2,500 mg/kg; Vit. B1 2,000 mg/kg; Vit. B2 6,000 mg/kg; Pantothenic acid 12 g/kg; Vit. B6 3,000 mg/kg; Vit. B12 15,000 mcg/kg; Nicotinic acid 35 g/kg; Folic acid 1,500 mg/kg; Biotin 100 mg/kg; Selenium 250 mg per kg of premix.
Mineral premix: Iron 100 g/kg; Cooper 20 g/kg; Manganese 130 g/kg; Cobalt 2,000 mg/kg; Zinc 130 mg/kg; Iodine 2,000 mg per kg of premix.
Phytase (RONOZYME ® HiPhos GT is a microbial 6-phytase expressed through the use of synthetic genes in Apergillus oryzae with phytase activity of 10000 phytase units (FYT) per g. One phytase unit is defined as the amount of enzyme that releases 1 µmol of inorganic phosphate under standard conditions (0.25 M acetate buffer pH 5.5, 37°C and 5 mmol sodium phytate., DSM Nutritional Products Ltd).
Xylanase (T RONOZYME® WX is a preparation of endo‐1,4‐beta‐xylanase produced with a genetically modified strain of Aspergillus oryzae. The xylanase enzymatic activity in xylanase units (FXU), defined as ‘the amount of enzyme which liberates 7.8 μmol of reducing sugars (xylose equivalents) from azo‐wheat arabinoxylan per minute at pH 6.0 and 50°C’., DSM Nutritional Products Ltd);
Ethoxyquin 66.6% (Antioxidant IMPEXQUIN®, produced by Imprextraco, Brazil)
Carophyl yellow (10% Apo-ester; DSM Nutritional Products Ltd, Kaiseraugst, Switzerland).
Inert (kaolin, used as a vehicle, chemically inert inorganic mineral produced by Minasilicio Gma Mineradora Ltda, Minas Gerais, Brazil), and used to replace the muramidase enzyme.
Celite™ (Indigestible ash, used as a undigestible marker to measure digestibility).
Diets were based on corn-soybean meal, and all the diets had inclusion of phytase (1,000 FYT/kg; RONOZYME HiPhos GT, DSM Nutritional Products) and xylanase (100 FXU/kg; RONOZYME WX 2000 CT, DSM Nutritional Products), to simulate a commercial diet. Diets were divided into 3 phases: starter (1–21 d), grower (22–35 d), and finisher (36–43 d) and supplied in mash form. The MUR activity and enzymatic recovery of each feeding phase feeding are shown in Table 2. The enzymatic inclusion was made replacing the inert component properly in each experimental diet.
Table 2.
Muramidase activity (LSU (F)/kg) and recovery (%) from the feed in starter, grower, and finisher phases in Experiments 1 and 2.
| Phase feeding, Exp. 1 | MUR1 doses LSU (F)/kg |
|||||
|---|---|---|---|---|---|---|
| 0 | 15,000 | 25,000 | 35,000 | |||
| Starter (1–21 d) | ||||||
| MUR activity LSU(F)/kg | 1,721 | 15,751 | 24,648 | 34,458 | ||
| MUR recovery (%) | 0 | 105 | 99 | 99 | ||
| Grower (22–35 d) | ||||||
| MUR activity LSU(F)/kg | 592 | 13,859 | 25,652 | 33,653 | ||
| MUR recovery (%) | 0 | 92 | 103 | 96 | ||
| Finisher (36–43 d) | ||||||
| MUR activity LSU(F)/kg | 454 | 11,556 | 21,900 | 31,027 | ||
| MUR recovery (%) | 0 | 77 | 88 | 89 | ||
| Phase feeding, Exp. 2 | MUR1 doses LSU (F)/kg |
|||||
|---|---|---|---|---|---|---|
| 0 | 25,000 | 35,000 | ||||
| Starter (1–21 d) | ||||||
| MUR activity LSU(F)/kg | 1,504 | 27,458 | 41,824 | |||
| MUR recovery (%) | 0 | 109 | 119 | |||
| Grower (22–35 d) | ||||||
| MUR activity LSU(F)/kg | 1,505 | 21,047 | 32,889 | |||
| MUR recovery (%) | 0 | 84 | 94 | |||
| Finisher (36–42 d) | ||||||
| MUR activity LSU(F)/kg | 1,585 | 20,013 | 29,752 | |||
| MUR recovery (%) | 0 | 80 | 85 | |||
Muramidase (gene coding Muramidase 007 obtained from the fungus Acremonium alcalophilum (strain 114.92), Novozymes A/S (Bagsvaerd, Denmark) were included according to the treatments (0, 15,000 LSU (F)/kg, 25,000 LSU (F)/kg and 35,000 LSU (F)/kg).
Chickens were placed in floor pens with reused wood shaving litter (3rd flock) with a density of 12 birds/m². Water and feed were supplied ad libitum, by using nipple drinkers and tubular feeders. Thermometers were placed in different points of the poultry house, and the temperature was controlled by opening or closing the side curtains. Until 21 d of age, electric brooders with thermostat control were used to maintain the ideal temperature for the starter phase. Temperature and light control followed the standards established by the breed management guide (Cobb-Vantress, 2012).
Muramidase
The enzyme Muramidase 007 (Balancius) used for this study was produced by Novozymes A/S (Bagsvaerd, Denmark) as described by Lichtenberg et al. (2017). The gene coding for Muramidase 007 is from the fungus Acremonium alcalophilum (strain 114.92), and its activity is expressed in MUR units LSU(F). Assessing the effects of this enzyme on animals and in the environment, Lichtenberg et al. (2017) concluded that, Muramidade 007 is a toxicologically inert compound and well tolerated by broilers and causes no harm to the environment.
Performance Measurements
Feed intake (FI), body weight gain (BWG), and feed conversion ratio (FCR) were measured at 21 and 43 d of age. Mortality and weight of dead animals were recorded daily to calculate mortality and adjust FI and FCR.
Apparent Ileal Digestibility
At d 43, two birds per replicate were randomly selected and euthanized by cervical dislocation. After the dissection, the content from the distal half part of the ileum was collected and stored in microcentrifuge tubes. Samples from the same pen were pooled, homogenized, and immediately frozen (−18°C). The digesta was then freeze-dried for further analysis of nutrient composition and calculation of AID of dry matter (DM), crude protein (CP), fat, ash, and ileal digestible energy (IDE). Celite at 1% was used as the indigestible marker in the finisher diets in all treatment groups. The DM content was obtained by oven drying the samples at 105°C for 16 h. The CP (method 954.01), fat (method 954.02) and ash (method 942.05) were analyzed according to the methodology described by AOAC, 1995. Gross energy of the samples was determined in a calorimetric bomb (Ika Werke C2000 Control Oxygen Bomb Calorimeter, Ika-Werke GmbH & Co., Staufen, Germany). Acid insoluble ash (AIA) was used as an internal digestibility marker using the method described by Van Keulen and Young (1977). Based on the results, the AID was calculated according to the following formula (Sens et al., 2021):
where IF (indigestibility factor) is the ratio between the AIA content in the diet and the AIA in the excreta or ileal digesta.
The ileal digestible energy (IDE) values were calculated using the formula (Sens et al., 2021): IDE (kcal/kg DM) = Crude energy (CE) of the diet – (CE of the ileal digesta × ileum IF). The % of digestibility of the nutrients was transformed to coefficient of digestibility (g/kg).
Intestinal Permeability Model
Intestinal mucosal permeability was evaluated through the passage of fluorescein isothiocyanate dextran (FITC-d) into the blood. The test was performed as previously described (Vicuña et al., 2015). Briefly, at 35 d of age, 2 birds per replicate (total of 20 samples per treatment) were randomly selected and received 1.1 mg of FITC-d 3–5 kD (SIGMA, São Paulo, Brazil) by oral gavage. Blood samples were drawn by brachial venipuncture 2.5 h later, and serum fluorescence intensity was determined in a plate fluorometer (Jenna Scientific, Sao Paulo, Brazil). A standard dilution curve of the reagent was used to determine the FITC-d concentration in the sample. The higher the serum FITC-d level, the higher the intestinal permeability is.
Foot Pad Dermatitis
Foot pad dermatitis was evaluated on d 43. Nine birds per pen were randomly selected to score FPD, totaling 90 birds per treatment. The evaluation of FPD was done according to the method described by the poultry assessment protocol Welfare Quality® (2009). A scale of 0 to 4 was used, where score 0 represented absence of lesions or dermatitis, 1 very mild evidence, 2 mild evidence of dermatitis, 3–4 clear evidence of dermatitis. For data analysis, we calculated the percentage of animals affected by FPD in each treatment. Birds with scores of 0 and 1 were considered without dermatitis, as these scores may not affect welfare, or carcass condemnation, and animals with scores 2, 3, and 4 were considered with accentuated lesions.
Statistical Analysis
Performance-related parameters were submitted to one-way ANOVA and orthogonal polynomial contrasts to examine linear and quadratic effects of the increasing enzyme inclusion. For performance data, the floor pen was considered the experimental unit. All the analyzed parameters were considered significantly different at P ≤ 0.05. Intestinal permeability data were analyzed by one-way ANOVA and Tukey's test was used for pairwise comparison between groups (P < 0.05). Additionally, intestinal permeability was also assessed considering the difference between treatments that received MUR (regardless the dose) vs. the control group. Data were log-transformed for adjustment to Gaussian distribution and compared by Student´s t test (P ≤ 0.05). The average of FPD lesion score was analyzed by using Wilcoxson's test and the means separated by Dunn's test. Additionally, the frequency of lesion scores was analyzed using the categorical platform on JMP Pro (16.0).
Experiment 2
Animals and Experimental Design
The study was carried out at the DSM Animal Nutrition Center, in Mairinque, São Paulo, Brazil, in agreement with the ethical guidelines on use of experimental animals and approved by DSM Ethics Committee on Animal Experimentation protocol number 001/16.
A total of 800 one-day-old males Ross 308, were randomly divided into 4 treatments, 8 replicate pens per treatment and 25 birds per replicate. Each pen had 2.2 m and was covered with fresh litter. The birds were raised from 1 to 42 d of age, in a conventional Brazilian poultry house. The barn was equipped with electric and gas heaters, fans, and side curtains to allow air exchange and control of the temperature. The temperature in the barn environment was adjusted throughout the trial according to the breed management guide (Aviagen, 2014). Water and feed were supplied ad libitum. The treatments were a basal diet (control group), or the basal diet supplemented with 25,000 or 35,000 LSU(F) of MUR/kg of feed, and a fourth group where the basal diet was supplemented with enramycin at 10 ppm during starter and grower and 5 ppm during the finisher feeding phase. The MUR used in the present study was the same as described in Experiment 1, and the activity and enzymatic recovery of each feeding phase is shown in Table 2.
A three-phase feeding program was used (starter: d 0–21, grower: d 21–35, and finisher: d 35–42), and the feed was supplied in mash form. All diets included 1,000 FYT of phytase (RONOZYME HiPhos GT) and 4 ppm of Apo-ester (CAROPHYLL yellow) as a biomarker. Diets were based on corn and soybean meal, rice bran, and meat and bone meal (Table 1). Rice bran and meat and bone meal were included in all diets to promote a mild intestinal challenge. Studies have shown that broiler diets with high amounts of cereals rich in non-starch polysaccharides (NSPs), can increase digesta viscosity and mucus production, decrease passage rate and increase the susceptibility of necrotic enteritis (Shojadoost et al., 2012). Additionally, the supplementation of animal by-products meal can also predispose the proliferation and establishment of Clostridium perfringens pathogenic strains in the gut (Shojadoost et al., 2012; Pereira et al., 2015).
In addition to the diet changes, at d 2, all the birds received an anticoccidial vaccine at 15 times the recommended dose (Bio-Coccivet R - Laboratório Biovet Brazilian Laboratory, containing strains of Eimeria acervulina, E. brunetti, E. maxima, E. necatrix, E. praecox, E. tenella, and E. mitis, isolated from a commercial poultry production in Brazil and grown in birds’ SPF – Specific Pathogen Free) by oral gavage. The main objective of this challenge was to combine necrotic enteritis predisposing factors to cause a mild intestinal challenge.
Performance Measurements
Feed intake, BWG, and FCR were recorded at 21 and 42 d of age. Mortality and weight of dead animals were recorded daily to calculate mortality and adjusted FI and FCR. Chickens were weighed individually on d 42 to determine uniformity, and the percentage of birds within the range of ± 10% of the average weight was calculated.
Carotenoid Determination
At d 28, blood samples were collected from 20 birds per treatment to measure total carotenoid concentration in the blood as an indicator of intestinal integrity and nutrients absorption. Total carotenoid concentration in the whole blood was determined by a commercial portable photometer, iCheck CAROTENE using iEx CAROTENE reagent vials (BioAnalytGmbH, Teltow, Germany). Carotenoids in blood plasma has been shown to be a sensitive biomarker of intestinal disruption caused by coccidiosis (Conway et al., 1993; Rochell et al., 2016).
Statistical Analysis
The data were submitted to one-way ANOVA and in case of significance, the means were pairwise compared by Tukey's test (P ≤ 0.05). Pen and bird were considered as the experimental unit for performance and carotenoid determination, respectively.
RESULTS
Experiment 1
Growth Performance
The growth performance at 21 and 43 d are shown in Table 3. From 1 to 21 d, MUR supplementation influenced FI, wherein broilers that received the highest MUR inclusion level (35,000 LSU (F)/kg) had the lowest FI than birds fed with MUR 15,000 and 25,000 LSU (F)/kg, but similar to the control group. Additionally, a quadratic response (P = 0.01) was observed. The treatments had no effects on BWG or FCR from d 21 to 43. From 1 to 43 d, the supplementation of MUR did not show effect on the BWG and FI (P > 0.05); however, a quadratic response (P = 0.01) for MUR supplementation was observed for FCR (Table 4). The FCR was lower (P = 0.0004) in animals supplemented with the highest dose of MUR (35,000 LSU (F)/kg) when compared to the birds from other groups.
Table 3.
Body weight gain (BWG, g), feed intake (FI, g), and feed conversion ratio (FCR) of broilers supplemented with increasing concentrations of muramidase from 1 to 21 and 1 to 43 d. Experiment 1.
| MuramidaseLSU1 (F)/kg of feed | BWG, g | FI, g | FCR g/g | BWG, g | FI, g | FCR g/g |
|---|---|---|---|---|---|---|
| 1 to 21 d | 1 to 43 d | |||||
| 0 | 689 | 1,026 ab | 1.493 | 2,537 | 4,334 | 1.694 b |
| 15,000 | 722 | 1,054 a | 1.438 | 2,572 | 4,367 | 1.688 b |
| 25,000 | 719 | 1,061a | 1.458 | 2,567 | 4,360 | 1.688 b |
| 35,000 | 695 | 973 b | 1.418 | 2,617 | 4,311 | 1.633 a |
| CV, % | 6.63 | 6.41 | 4.77 | 4.12 | 3.23 | 2.28 |
| P-value ANOVA | 0.33 | 0.01 | 0.11 | 0.48 | 0.84 | 0.004 |
| Linear | 0.74 | 0.21 | 0.12 | 0.75 | 0.14 | 0.013 |
| Quadratic | 0.18 | 0.012 | 0.10 | 0.66 | 0.34 | 0.014 |
abDifferent letters in the same column differ by Tukey test (P < 0.05).
LSU(F): muramidase unit.
Quadratic effect; FI = 1023,07 + 6,12216x-0,210757*dose²; R² = 19.94%.
Linear effect; FCR = 1.70539 – 0.00000146*dose; R² = 26.57%.
Quadratic effect; FCR = 1.69245 + 0.0000017587*dose - 9.50031E-11*dose²; R² = 38.71%.
Table 4.
Apparent ileal digestibility (g/kg) of dry matter (DM), crude protein (CP), fat, ash, and the ileal digestible energy (IDE) of broilers at 43 d supplemented with increasing concentrations of muramidase. Experiment 1.
| Muramidase LSU1 (F)/kg of feed | DM (g/kg) | CP (g/kg) | Fat (g/kg) | Ash (g/kg) | IDE (kcal/kg) |
|---|---|---|---|---|---|
| 0 | 698.8 | 799.5 | 652.0 b | 367.8 c | 3,253.4 |
| 15,000 | 716.9 | 814.9 | 697.6 b | 409.8 bc | 3,233.6 |
| 25,000 | 714.3 | 817.6 | 756.9 a | 435.0 ab | 3,355.4 |
| 35,000 | 731.8 | 810.2 | 758.6 a | 488.0 a | 3,281.6 |
| CV, % | 4.12 | 2.68 | 6.80 | 12.30 | 4.04 |
| P-value ANOVA | 0.13 | 0.31 | 0.01 | 0.01 | 0.20 |
| Linear | 0.032 | 0.24 | 0.013 | 0.014 | 0.30 |
| Quadratic | 0.90 | 0.14 | 0.48 | 0.44 | 0.57 |
abcDifferent letters in the same column differ by Tukey test (P < 0.05).
LSU(F): muramidase unit.
Linear significance; g/kg DM = 699.52 + 0.84*dose; R2 = 13%.
Linear significance; g/kg Fat = 654.25 + 3.31*dose; R² = 44%.
Linear significance; g/kg Ash = 362.66 + 3.31*dose; R² = 42%.
Apparent Ileal Digestibility
The inclusion of microbial MUR in the feed linearly increased the ileal digestibility of DM (g/kg DM = 699.52 + 0.84*dose; R2 = 13%; P = 0.03), fat (g/kg Fat = 654.25 + 3.31*dose; R² = 44%; P = 0.01), and ash (g/kg Ash = 362.66 + 3.31*dose; R² = 42%; P < 0.01; Table 4). However, no significant differences were observed for the digestibility of crude protein or ileal digestible energy (P > 0.05; Table 4).
Intestinal Permeability
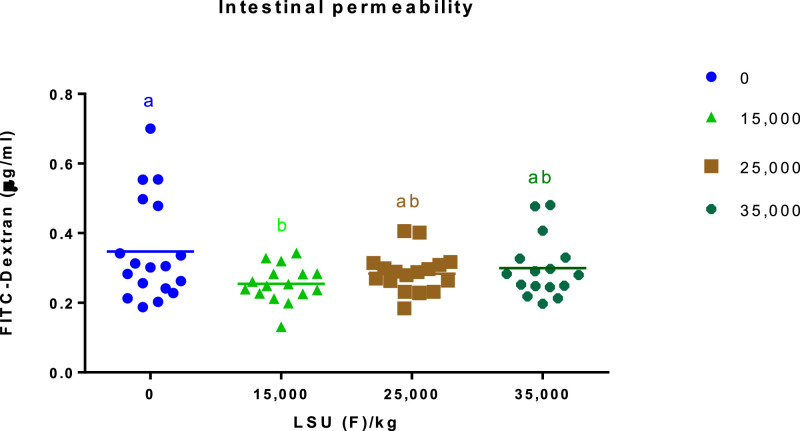
The supplementation of MUR at 15,000 LSU (F)/kg decreased the intestinal permeability when compared to the birds from the non-supplemented group (P < 0.05; Figure 1). However, the intestinal permeability of birds from the MUR 15,000 LSU group was statistically similar to those fed with MUR 25,000 and 35,000 LSU (F)/kg. Data were also assessed as treatments with MUR inclusion vs. control. Regardless of the dose used, the enzyme inclusion improved the intestinal permeability when compared to the non-supplemented group (Student´s t test, P < 0.05).
Figure 1.
Intestinal permeability, according to the FITC-d (µm/mL) concentration in the blood of birds at 35 d. Experiment 1. Different letters between the columns of the graphic differs by Student´s t test (P < 0.05) LSU (F)/kg: Muramidase unit.
Foot Pad Dermatitis
At the end of the experimental period, FPD occurrence was higher in non-supplemented birds (P = 0.05) than in birds supplemented with MUR at the 25,000 LSU (F)/kg (Table 5).
Table 5.
Presence (scores 2, 3, or 4) or absence (scores 0 or 1) of foot pad dermatitis (FPD) in broilers at d 43 supplemented with increasing concentrations of muramidase. Experiment 1.
| Muramidase LSU1 (F)/kg of feed | Mean score | Score 0 | Score 1 | Score 2 | Score 3 | P value |
|---|---|---|---|---|---|---|
| 0 | 0.84 a | 50% | 22% | 21% | 7% | a |
| 15,000 | 0.57 ab | 69% | 11% | 14% | 6% | a |
| 25,000 | 0.48 b | 63% | 28% | 7% | 2% | b |
| 35,000 | 0.59 ab | 62% | 21% | 12% | 4% | ab |
| P-value | 0.05 | <0.05 | ||||
abDifferent letters in the same column differ by Tukey test (P < 0.05).
LSU(F): muramidase unit.
Experiment 2
The experiment was performed successfully and, in despite the mild challenge conditions, the birds performed according to the breeder guidelines. Mortality varied between 11 and 13% for all the treatments, without significant differences among treatments. The analyses of MUR activity in the diets confirmed the proper addition of the enzyme within the range of the expected values ± 20%.
Growth Performance
The results of growth performance parameters are shown in Table 6. At 21 d of age, there were no differences between the treatments for any of the variables. However, during the entire experimental period, from 1 to 42 d of age, broilers fed diets supplemented with 35,000 LSU (F) / kg of MUR showed higher BWG (P = 0.03) when compared to the control or enramycin groups. Additionally, the supplementation of MUR, regardless of the dose, improved the FCR by 5.4% compared to the control group. Flock uniformity at d 42 varied from 80.8% (control) to 86.4% (enramycin), and no differences were found between treatments.
Table 6.
Body weight gain (BWG, g), feed intake (FI, g), feed conversion ratio (FCR g/g), and uniformity (Unif, %) of broilers supplemented with different concentrations of muramidase and enramycin from 1 to 21 and 1 to 42 days of age. Experiment 2.
| Treatment | BWG, g | FI, g | FCR g/g | BWG, g | FI, g | FCR g/g | Unif, % |
|---|---|---|---|---|---|---|---|
| 1 to 21 d | 1 to 42 d | ||||||
| Control | 903 | 1,251 | 1.390 | 3,265 b | 5,350 | 1.680 a | 80.8 |
| 25,000 MUR1 | 937 | 1,244 | 1.340 | 3,320 ab | 5,150 | 1.590 b | 81.4 |
| 35,000 MUR2 | 919 | 1,217 | 1.330 | 3,397 a | 5,220 | 1.590 b | 86.2 |
| Enramycin | 920 | 1,216 | 1.330 | 3,265 b | 5,110 | 1.610 ab | 86.4 |
| CV, % | 3.43 | 4.57 | 4.65 | 2.46 | 4.58 | 3.14 | 11.51 |
| P-value | 0.23 | 0.55 | 0.26 | 0.03 | 0.24 | 0.009 | 0.51 |
abDifferent letters in the same row differ by Tukey test (P < 0.05).
Abbreviation: CV, coefficient of variation.
Muramidase 007 at 25,000 LSU(F)/kg and at 35,000 LSU(F)/kg of feed.
Total Carotenoids
The total carotenoids concentration in the whole blood is shown in Table 7. Broilers fed diets supplemented with MUR 35,000 LSU (F)/kg showed higher (4.38 mg/L; P = 0.007) concentration of carotenoids in the blood on d 28 compared to the control treatment (3.57 mg/L). However, it was statistically similar to the broilers fed a diet supplemented with enramycin (4.28 mg/L) or 25,000 LSU (F)/kg of MUR (3.97 mg/L) (Table 7).
Table 7.
Plasma carotenoid concentration (mg/L) of broiler chickens supplemented with different concentrations of muramidase and enramycin at 28 d. Experiment 2.
| Treatments | Control | 25,000 MUR1 | 35,000 MUR2 | Enramycin | P-value | CV% |
|---|---|---|---|---|---|---|
| Carotenoids | 3.57 b | 3.97 ab | 4.38 a | 4.28 a | 0.007 | 21.03 |
Different letters in the same row differ by Tukey test (P < 0.05).
Abbreviation: CV, coefficient of variation.
Muramidase 007 at 25,000 LSU(F)/kg and at 35,000 LSU(F)/kg of feed.
DISCUSSION
In the present studies, the inclusion of microbial MUR in diets of broiler chickens linearly improved the FCR of the birds during the entire experimental period (Experiment 1), and the addition of 35,000 LSU (F)/kg of MUR improved BWG and FCR of chickens undergoing an intestinal challenge (Experiment 2). Furthermore, the supplementation of MUR linearly improved the AID of dry matter, fat, and ash, decreased the intestinal permeability of the birds (Experiment1) and increased the total blood concentration of carotenoids (Experiment 2). Similar results on digestibility have been obtained in previous studies conducted with European type of diets (Lichtenberg et al., 2017; Goodarzi Boroojeni et al., 2019; Sais et al., 2020). Therefore, the current studies, performed in Brazil, confirm the positive effects of MUR on the feed efficiency of broilers chickens, regardless of the type of diet used, and in the presence or absence of an intestinal challenge.
Effective GIT functionality is usually accompanied by optimal digestion and absorption. Results presented herein (Experiment 1) showed that increasing the dietary supplementation of microbial MUR resulted in better DM, fat, and ash digestibility. The results partially agree with Sais et al. (2020), wherein broilers supplemented with microbial MUR had in increased ileal digestibility of energy, dry matter, and organic matter at 35 d of study. Similarly, Goodarzi Boroojeni et al. (2019) reported that the supplementation of dietary MUR linearly improved AID of protein, fat, and phosphorus in broilers. The authors also suggested that MUR could synergistically enhance phytase activity (Goodarzi Boroojeni et al., 2019). Therefore, the benefits of MUR on the digestibility of nutrients may be due to its effect on the intestinal health and function, and by acting synergistically with other feed enzymes.
The cohesion between intestinal epithelial cells, mainly promoted by tight junctions, is crucial to maintain an effective intestinal barrier, that reduces host exposure to pathogenic microorganisms, microbial compounds and toxins, and nutritional antigens (Kogut et al., 2018). The failure of this barrier allows bacteria, bacterial compounds, and other antigens to translocate to the blood (Hietbrink et al., 2009; Teirlynck et al., 2011). This translocation can generate systemic immune responses (Song et al., 2014; Chen et al., 2015; Williams et al., 2015), and systemic bacterial infections (Ilan, 2012; Seki and Schnabl, 2012) that may impair broiler performance (Jiang et al., 2010). The dietary supplementation of enzymes and their beneficial effects, other than digestibility improvement, have been highlighted in a publication by Dal Pont et al. (2022). In fact, it was shown in the present studies that the supplementation of MUR improved the intestinal barrier function, as measured by the passage of FITC-d from the intestinal lumen to the blood. The methodology using FITC-d, which has a high molecular weight structure that cannot be absorbed in normal circumstances, has been previously described as an effective method to access intestinal integrity (Brandl et al., 2009; Yan et al., 2009; Vicuña et al., 2015; Liu et al., 2021). Therefore, the results obtained herein suggest that MUR supplementation conserved the structure of tight junctions and prevented paracellular translocation of undesirable molecules from the intestinal lumen to the blood stream.
The improvement promoted by MUR on the membrane integrity, digestion and absorption of certain nutrients is probably due to its action on hydrolyzing bacterial PGN. Peptidoglycans, originating from bacterial wall renewal, cell turnover or bacterial lysis is constantly shed on the intestinal lumen and it is considered a normal process (Johnson et al., 2013). However, the accumulation of microbial cellular debris could create a layer on the intestinal epithelia, impairing the proper absorption of nutrients (Lee and Hase, 2014; Goodarzi Boroojeni et al., 2019). Bacterial cell walls have rigid structures formed by repeated N-acetylmuramic acid sequences connected by β-1-4 bonds with N-acetyl glucosamine that are difficult to break (Alcorlo et al., 2017). Moreover, PGN can be recognized by immune cells as a microbe associated molecular pattern (MAMP) that can trigger inflammation when recognized by toll-like receptors 2 present in the cell membrane of immune cells (Broom and Kogut, 2018). It may result in the expression of pro-inflammatory mediators, anti-apoptotic factors, antimicrobial peptides, and a protective response to the infecting microbe (Carpenter and O'Neill, 2007; Lee and Kim, 2007; Takeuchi and Akira, 2010). The lysis of PGN by MUR, therefore, may improve nutrient absorption, reduce inflammation, and redirect nutrient towards growth of the animals.
The supplementation of MUR also increased total blood concentration of carotenoids in broilers undergoing a mild intestinal challenge (Experiment 2), which is another indication of improved GIT functionality by MUR. Carotenoid absorption is negatively correlated with intestinal damage. Studies have shown a reduction of carotenoid concentration in the plasma of chickens under Eimeria acervulina infection which has increased correlation with the dose of infection and, consequently, a decrease in intestinal integrity (Conway et al., 1993; Rochell et al., 2016). Carotenoids are fat soluble micronutrients that are supplied by the diet and may be used as intestinal absorption biomarker. Sais et al. (2020) observed an increase on fat digestibility and plasma vitamin A concentration in broilers fed MUR.
Footpad dermatitis is a condition of inflammation and necrotic lesions on the plantar surface of the feet of birds (Greene et al., 1985). Depending on the degree of these lesions, it can cause pain in the animals (Michel et al., 2012), impairing their mobility. Thus, the reduced access to food and water, can negatively affect the performance of the birds. Some studies have shown the association of more severe FPD scores with the presence of hock dermatitis and dermatitis of the skin on the chest (Haslam et al., 2007; Allain et al., 2009; de Jong et al., 2014). This may, in turn, lead to reduced carcass quality of the broilers and an increased number of downgraded carcasses at the processing plant. The quality of the litter is directly correlated to the presence of FPD. It can be expected that the prevalence of severe FPD is accompanied by other negative effects on welfare and productivity caused by deteriorated litter quality. Moreover, FPD can increase in occurrence and severity when the litter material is reused, especially if it not properly treated (Meluzzi et al., 2008). In the present study, we observed a reduction in FPD with MUR dietary supplementation. We hypothesize that the improvement of nutrient digestibility, and better intestinal integrity observed with MUR supplementation may reduce excreta moisture, improve litter quality, and welfare of birds.
CONCLUSIONS
The results obtained from these 2 studies clearly demonstrated that broilers supplemented with MUR presented an enhanced growth performance, nutrient digestibility, and better intestinal functionality as measured by the plasma concentrations of FITC-d and carotenoids. These beneficial effects show that MUR helped the birds to cope with the imposed intestinal challenge and may, at least partially, explain the reduction in FPD incidence in animals supplemented with MUR.
DISCLOSURES
LCB, CB, VBF, RLU, and EPC are employed by DSM Nutritional Product. The other authors declare no conflict of interest. The studies were funded by DSM Nutritional Products.
REFERENCES
- Adeola O., Cowieson A.J. Board-invited review: opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J. Anim. Sci. 2011;89:3189–3218. doi: 10.2527/jas.2010-3715. [DOI] [PubMed] [Google Scholar]
- Alcorlo M., Martínez-Caballero S., Molina R., Hermoso J.A. Carbohydrate recognition and lysis by bacterial peptidoglycan hydrolases. Curr. Opin. Struct. Biol. 2017;44:87–100. doi: 10.1016/j.sbi.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Allain V., Mirabito L., Arnould C., Colas M., Bouquin S.Le, Lupo C., Michel V. Skin lesions in broiler chickens measured at the slaughterhouse: relationships between lesions and between their prevalence and rearing factors. Br. Poult. Sci. 2009;50:407–417. doi: 10.1080/00071660903110901. [DOI] [PubMed] [Google Scholar]
- AOAC . Official Methods of Analysis. 16th ed. Association of Official Analytical Chemists; Washington, DC: 1995. [Google Scholar]
- Aviagen . Aviagen Limited Newbridge Midlothian; Scotland, UK: 2014. Ross Broiler Management Handbook. [Google Scholar]
- Bedford M.R., Cowieson A.J. Exogenous enzymes and their effects on intestinal microbiology. Anim. Feed Sci. Technol. 2012;173:76–85. [Google Scholar]
- Brandl K., Rutschmann S., Li X., Du X., Xiao N., Schnabl B., Brenner D.A., Beutler B. Enhanced sensitivity to DSS colitis caused by a hypomorphic Mbtps1 mutation disrupting the ATF6-driven unfolded protein response. Proc. Natl. Acad. Sci. U. S. A. 2009;106:3300–3305. doi: 10.1073/pnas.0813036106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broom L.J., Kogut M.H. Inflammation: friend or foe for animal production? Poult. Sci. 2018;97:510–514. doi: 10.3382/ps/pex314. [DOI] [PubMed] [Google Scholar]
- Carpenter S., O'Neill L.A. How important are Toll-like receptors for antimicrobial responses? Cell Microbiol. 2007;9:1891–1901. doi: 10.1111/j.1462-5822.2007.00965.x. [DOI] [PubMed] [Google Scholar]
- Celi P., Cowieson A.J., Fru-Nji F., Steinert R.E., Kluenter A.M., Verlhac V. Gastrointestinal functionality in animal nutrition and health: new opportunities for sustainable animal production. Anim. Feed Sci. Technol. 2017;234:88–100. [Google Scholar]
- Chen J., Tellez G., Richards J.D., Escobar J. Identification of potential biomarkers for gut barrier failure in broiler chickens. Front. Vet. Sci. 2015;2:14. doi: 10.3389/fvets.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipman D.M., Sharon N. Mechanism of lysozyme action. Science. 1969;165:454–465. doi: 10.1126/science.165.3892.454. [DOI] [PubMed] [Google Scholar]
- Cobb-Vantress . Cobb-Vantress Inc.; Siloam Springs, AR: 2012. Cobb Broiler Management Guide. [Google Scholar]
- Conway D.P., Sasai A.K., Gaafar B.S.M., Smothersc C.D. Effects of different levels of oocyst inoculation of Eimeria acervulina, E. tenella, and E. maxima on plasma constituents, packed cell volume, lesion scores, and performance in chickens. Avian Dis. 1993;37:118–123. [PubMed] [Google Scholar]
- Cooper C.A., Maga E.A., Murray J.D. Consumption of transgenic milk containing the antimicrobials lactoferrin and lysozyme separately and in conjunction by 6-week-old pigs improves intestinal and systemic health. J. Dairy Res. 2014;81:30–37. doi: 10.1017/S0022029913000575. [DOI] [PubMed] [Google Scholar]
- Dal Pont G.C., Eyng C., Bortoluzzi C., Kogut M.H. In: Gut Microbiota, Immunity, and Health in Production Animals, the Microbiomes of Humans, Animals, Plants, and the Environment. Kogut M.H., Zhang G., editors. Springer Nature; Switzerland AG: 2022. Enzymes and gut health in monogastric animals: effects beyond digestibility. Ed. [Google Scholar]
- de Jong I.C., Gunnink H., van Harn J. Wet litter not only induces footpad dermatitis but also reduces overall welfare, technical performance, and carcass yield in broiler chickens. J. Appl. Poult. Res. 2014;23:51–58. [Google Scholar]
- Goodarzi Boroojeni F., Männer K., Rieger J., Calvo E.P., Zentek J. Evaluation of a microbial muramidase supplementation on growth performance, apparent ileal digestibility, and intestinal histology of broiler chickens. Poult. Sci. 2019;98:2080–2086. doi: 10.3382/ps/pey556. [DOI] [PubMed] [Google Scholar]
- Greene J.A., McCracken R.M., Evans R.T. A contact dermatitis of broilers -clinical and pathological findings. Avian. Pathol. 1985;14:23–38. doi: 10.1080/03079458508436205. [DOI] [PubMed] [Google Scholar]
- Haslam S.M., Knowles T.G., Brown S.N., Wilkins L.J., Kestin S.C., Warriss P.D., Nicol C.J. Factors affecting the prevalence of foot pad dermatitis, hock burn and breast burn in broiler chicken. Br. Poult. Sci. 2007;48:264–275. doi: 10.1080/00071660701371341. [DOI] [PubMed] [Google Scholar]
- Hietbrink F., Besselink M.G.H., Renooij W., De Smet M.B.M., Draisma A., Van Der Hoeven H., Pickkers P. Systemic inflammation increases intestinal permeability during experimental human endotoxemia. Shock. 2009;32:374–378. doi: 10.1097/SHK.0b013e3181a2bcd6. [DOI] [PubMed] [Google Scholar]
- Ilan Y. Leaky gut and the liver: a role for bacterial translocation in nonalcoholic steatohepatitis. World J. Gastroenterol. 2012;18:2609–2618. doi: 10.3748/wjg.v18.i21.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z., Schatzmayr G., Mohnl M., Applegate T.J. Net effect of an acute phase response–partial alleviation with probiotic supplementation. Poult. Sci. 2010;89:28–33. doi: 10.3382/ps.2009-00464. [DOI] [PubMed] [Google Scholar]
- Johnson J.W., Fisher J.F., Mobashery S. Bacterial cell-wall recycling. Ann. N. Y. Acad. Sci. 2013;1277:54. doi: 10.1111/j.1749-6632.2012.06813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiarie E., Romero L.F., Nyachoti C.M. The role of added feed enzymes in promoting gut health in swine and poultry. Nutr. Res. Rev. 2013;26:71–88. doi: 10.1017/S0954422413000048. [DOI] [PubMed] [Google Scholar]
- Kogut M.H. The effect of microbiome modulation on the intestinal health of poultry. Anim. Feed Sci.Technol. 2019;250:32–40. [Google Scholar]
- Kogut M.H., Genovese K.J., Swaggerty C.L., He H., Broom L. Inflammatory phenotypes in the intestine of poultry: not all inflammation is created equal. Poult. Sci. 2018;97:2339–2346. doi: 10.3382/ps/pey087. [DOI] [PubMed] [Google Scholar]
- Lee W.J., Hase K. Gut microbiota-generated metabolites in animal health and disease. Nat. Chem. Biol. 2014;10:416–424. doi: 10.1038/nchembio.1535. [DOI] [PubMed] [Google Scholar]
- Lee M.S., Kim Y.J. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu. Rev. Biochem. 2007;76:447–480. doi: 10.1146/annurev.biochem.76.060605.122847. [DOI] [PubMed] [Google Scholar]
- Lichtenberg J., Perez Calvo E., Madsen K., Østergaard Lund T., Kramer Birkved F., van Cauwenberghe S., Mourier M., Wulf-Andersen L., Jansman A.J.M., Lopez-Ulibarri R. Safety evaluation of a novel muramidase for feed application. Regul. Toxicol. Pharmacol. 2017;89:57–69. doi: 10.1016/j.yrtph.2017.07.014. [DOI] [PubMed] [Google Scholar]
- Liu J., Teng P-Y., Kim W.K., Applegate T.J. Assay considerations for fluorescein isothiocyanate-dextran (FITC-d): an indicator of intestinal permeability in broiler chickens. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Y., Lin S., Zhu J., Pang X., Fang Z., Lin Y., Che L., Xu S., Li J., Huang Y., Su X., Wu D. Effects of dietary lysozyme levels on growth performance, intestinal morphology, non-specific immunity, and mRNA expression in weanling piglets. Anim. Sci. J. 2016;87:411–418. doi: 10.1111/asj.12444. [DOI] [PubMed] [Google Scholar]
- Meale S.J., Beauchemin K.A., Hristov A.N., Chaves A.V., McAllister T.A. Board-invited review: opportunities and challenges in using exogenous enzymes to improve ruminant production. J. Anim. Sci. 2014;92:427–442. doi: 10.2527/jas.2013-6869. [DOI] [PubMed] [Google Scholar]
- Meluzzi A., Fabbri C., Folegatti E., Sirri F. Survey of chicken rearing conditions in Italy: effects of litter quality and stocking density on productivity, foot dermatitis and carcass injuries. Br. Poult. Sci. 2008;49:257–264. doi: 10.1080/00071660802094156. [DOI] [PubMed] [Google Scholar]
- Michel V., Prampart E., Mirabito L., Allain V., Arnould C., Huonnic D., Bouquin S.Le, Albaric O. Histologically-validated footpad dermatitis scoring system for use in chicken processing plants. Br. Poult. Sci. 2012;53:275–281. doi: 10.1080/00071668.2012.695336. [DOI] [PubMed] [Google Scholar]
- Morgavi D.P., Sakurada M., Tomita Y., Onodera R. Presence in rumen bacterial and protozoal populations of enzymes capable of degrading fungal cell walls. Microbiology. 1994;140(Pt 3):631–636. doi: 10.1099/00221287-140-3-631. [DOI] [PubMed] [Google Scholar]
- Mwangi W.N., Beal R.K., Powers C., Wu X., Humphrey T., Watson M., Bailey M., Friedman A., Smith A.L. Regional and global changes in TCRαβ T cell repertoires in the gut are dependent upon the complexity of the enteric microflora. Dev. Comp. Immunol. 2010;34:406–417. doi: 10.1016/j.dci.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Oakley B.B., Kogut M.H. Spatial and temporal changes in the broiler chicken cecal and fecal microbiomes and correlations of bacterial taxa with cytokine gene expression. Front. Vet. Sci. 2016;3:11. doi: 10.3389/fvets.2016.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B.B., Lillehoj H.S., Kogut M.H., Kim W.K., Maurer J.J., Pedroso A., Lee M.D., Collett S.R., Johnson T.J., Cox N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014;360:100–112. doi: 10.1111/1574-6968.12608. [DOI] [PubMed] [Google Scholar]
- Pereira R., Menten J.M.M., Bortoluzzi C., Napty G.S., Longo F.A., Vittori J., Lourenço C.M., Santin E. Organic acid blend in diets of broiler chickens challenged with Clostridium perfringens. J. Appl. Poult. Res. 2015;24:387–393. [Google Scholar]
- Phillips D.C. The three-dimensional structure of an enzyme molecule. Sci. Am. 1966;215:78–93. doi: 10.1038/scientificamerican1166-78. [DOI] [PubMed] [Google Scholar]
- Rochell S.J., Parsons C.M., Dilger R.N. Effects of Eimeria acervulina infection severity on growth performance, apparent ileal amino acid digestibility, and plasma concentrations of amino acids, carotenoids, and α1-acid glycoprotein in broilers. Poult. Sci. 2016;95:1573–1581. doi: 10.3382/ps/pew035. [DOI] [PubMed] [Google Scholar]
- Sais M., Barroeta A.C., López-Colom P., Nofrarías M., Majó N., Lopez-Ulibarri R., Pérez Calvo E., Martín-Orúe S.M. Evaluation of dietary supplementation of a novel microbial muramidase on gastrointestinal functionality and growth performance in broiler chickens. Poult. Sci. 2020;99:235–245. doi: 10.3382/ps/pez466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki E., Schnabl B. Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. J. Physiol. 2012;590:447–458. doi: 10.1113/jphysiol.2011.219691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sens R.F., Bassi L.S., Almeida L.M., Rosso D.F., Teixeira L.V., Maiorka A. Effect of different doses of phytase and protein content of soybean meal on growth performance, nutrient digestibility, and bone characteristics of broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojadoost B., Vince A.R., Prescott J.F. The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: a critical review. Vet. Res. 2012;43:1–12. doi: 10.1186/1297-9716-43-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Xiao K., Ke Y.L., Jiao L.F., Hu C.H., Diao Q.Y., Shi B., Zou X.T. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poult. Sci. 2014;93:581–588. doi: 10.3382/ps.2013-03455. [DOI] [PubMed] [Google Scholar]
- Sytwala S., Gunther F., Melzig M.F. Lysozyme- and chitinase activity in latex bearing plants of genus Euphorbia–a contribution to plant defense mechanism. Plant Physiol. Biochem. 2015;95:35–40. doi: 10.1016/j.plaphy.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Teirlynck E., Gussem M.D.E., Dewulf J., Haesebrouck F., Ducatelle R., van Immerseel F. Morphometric evaluation of “dysbacteriosis” in broilers. Avian Pathol. 2011;40:139–144. doi: 10.1080/03079457.2010.543414. [DOI] [PubMed] [Google Scholar]
- Turner J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- Van Keulen J., Young B.A. Evaluation of acid-insoluble ash as a natural marker in ruminant digestibility studies. J. Anim. Sci. 1977;44:282–287. [Google Scholar]
- Vicuña E.A., Kuttappan V.A., Tellez G., Hernandez-Velasco X., Seeber-Galarza R., Latorre J.D., Faulkner O.B., Wolfenden A.D., Hargis B.M., Bielke L.R. Dose titration of FITC-D for optimal measurement of enteric inflammation in broiler chicks. Poult. Sci. 2015;94:1353–1359. doi: 10.3382/ps/pev111. [DOI] [PubMed] [Google Scholar]
- Vidal M.L., Gautron J., Nys Y. Development of an ELISA for quantifying lysozyme in hen egg white. J. Agric. Food Chem. 2005;53:2379–2385. doi: 10.1021/jf048692o. [DOI] [PubMed] [Google Scholar]
- Welfare Quality® . Welfare Quality® Consortium; Lelystad, Netherlands: 2009. Welfare Quality® Assessment Protocol for Poultry (Broilers, Laying Hens) [Google Scholar]
- Williams J.M., Duckworth C.A., Burkitt M.D., Watson A.J., Campbell B.J., Pritchard D.M. Epithelial cell shedding and barrier function: a matter of life and death at the small intestinal villus tip. Vet. Pathol. 2015;52:445–455. doi: 10.1177/0300985814559404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Kolachala V., Dalmasso G., Nguyen H., Laroui H., Sitaraman S.V., Merlin D. Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PLoS One. 2009;4:e6073. doi: 10.1371/journal.pone.0006073. [DOI] [PMC free article] [PubMed] [Google Scholar]