Abstract
Small populations with limited range are often threatened by inbreeding and reduced genetic diversity, which can reduce fitness and exacerbate population decline. One of the most extreme natural examples is the Devils Hole pupfish (Cyprinodon diabolis), an iconic and critically endangered species with the smallest known range of any vertebrate. This species has experienced severe declines in population size over the last 30 years and suffered major bottlenecks in 2007 and 2013, when the population shrunk to 38 and 35 individuals, respectively. Here, we analysed 30 resequenced genomes of desert pupfishes from Death Valley, Ash Meadows and surrounding areas to examine the genomic consequences of small population size. We found extremely high levels of inbreeding (FROH = 0.34–0.81) and an increased amount of potentially deleterious genetic variation in the Devils Hole pupfish as compared to other species, including unique, fixed loss-of-function alleles and deletions in genes associated with sperm motility and hypoxia. Additionally, we successfully resequenced a formalin-fixed museum specimen from 1980 and found that the population was already highly inbred prior to recent known bottlenecks. We thus document severe inbreeding and increased mutation load in the Devils Hole pupfish and identify candidate deleterious variants to inform management of this conservation icon.
Keywords: inbreeding, runs of homozygosity, mutation load, Devils Hole pupfish
1. Introduction
Due to declining population sizes and increasing isolation of many species from anthropogenic habitat fragmentation and climate change, understanding the extent and nature of genetic threats in small populations is essential for predicting and increasing population persistence and resiliency [1]. Small and isolated populations often suffer from inbreeding depression, the reduction in fitness caused by increased homozygosity of deleterious recessive alleles or overdominant loci that occurs when closely related individuals breed together [2,3]. Prolonged population decline can result in increased long-term extinction risk due to stochastic demographic events [4], reduced genetic variation for adaptation [5], and decreased efficacy of purifying selection to overpower drift and purge deleterious variants [6]. This reduced efficacy of purifying selection leads deleterious mutations to accumulate more readily in small populations. The burden of accumulated deleterious mutations is known as the mutation load, and can reduce individual fitness [7,8].
Many examples of reduced genetic diversity and inbreeding depression have been documented in the wild [9,10], including Florida panthers (Puma concolor) [11], Isle Royale wolves (Canis lupus) [12] and mountain gorillas (Gorilla beringei) [13]. Severe inbreeding in natural populations is increasingly being documented across a wide range of temporal scales, from ancient bottlenecks [14,15] to recent timescales [12]. Recently, historical museum specimens have been successfully leveraged to investigate temporal changes in inbreeding and genetic diversity, highlighting their utility for investigating the historical dynamics of inbreeding and mutation load in imperilled populations [16,17].
Traditionally, population health and extinction risk was assessed using putatively neutral molecular markers and pedigrees owing to positive correlations between genome-wide heterozygosity and fitness [18]. Maintaining genome-wide genetic variation is crucial to preserving adaptive potential and preventing inbreeding depression in populations of conservation interest [19] because populations with higher levels of genetic diversity tend to have higher mean fitness and reduced extinction risk [20]. However, several recent studies have suggested that summary statistics of genetic variation do not necessarily accurately reflect population size or extinction risk and that we should also use whole-genome sampling to assess functional genetic diversity and genetic load [17,21]. Genomes enable more detailed measurements of individual inbreeding depression and its genetic basis relative to pedigree-based approaches [22]. Thus, a comprehensive understanding of the evolutionary dynamics of small populations in the wild from a genomic perspective is key to understanding the fate of endangered populations and to inform conservation management.
Here, we leverage the unique evolutionary and demographic history of the iconic Devils Hole pupfish (Cyprinodon diabolis: figure 1a) to investigate how isolation and recent population decline have shaped inbreeding and mutation load. Death Valley pupfishes evolved from a common ancestor thousands of years ago when the climate was milder and the region was connected by large inland seas. Populations are now relatively isolated in small desert spring systems and several species are now considered critically endangered [23]. Devils Hole contains the most extreme conditions, including a nearly constant temperature of 34°C [24], absence of direct sunlight during the winter [25] which sharply limits primary production and nutrient availability [26], and dissolved oxygen levels near lethal limits for most fishes (2–3 ppm) [25,27]. Surrounding pupfishes in neighbouring springs occupy less hypoxic environments, such as Cyprinodon nevadensis mionectes in Jackrabbit Spring, due to greater water movement [28].
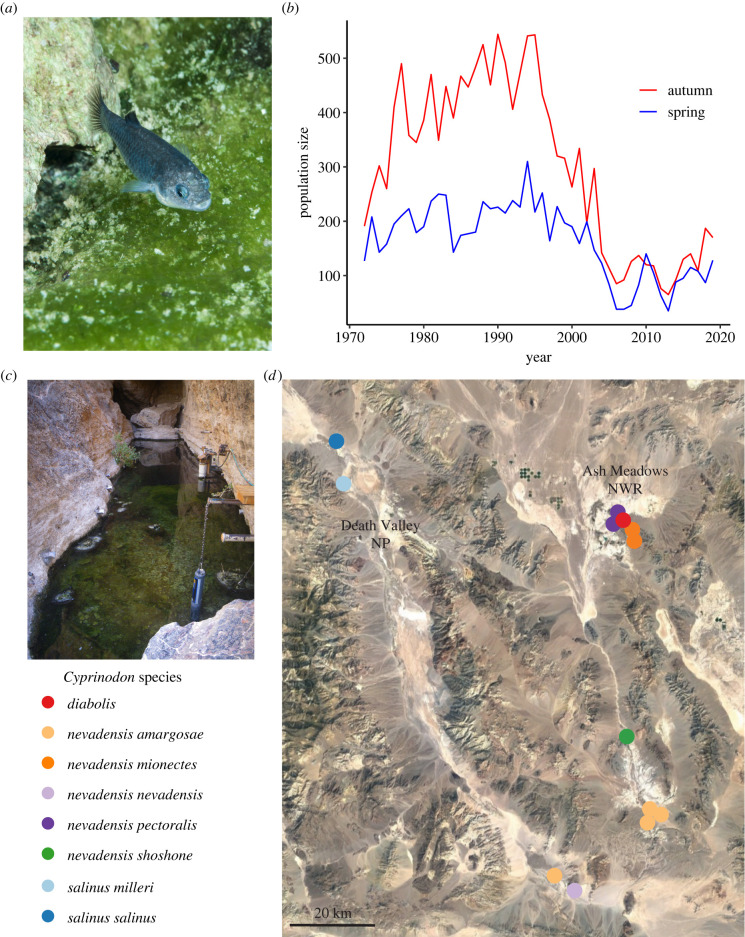
Figure 1.
Sampling locations and recent C. diabolis population decline. (a) Photo of C. diabolis by Olin Feuerbacher. (b) Biannual population census counts of C. diabolis over time. Bottlenecks in 2007 and 2013 reached 38 and 35 individuals, respectively. (c) Photo of Devils Hole by USFWS. (d) Map of Death Valley NP and Ash Meadows NWR sampling locations. (Online version in colour.)
Cyprinodon diabolis exists at one of the lowest long-term population sizes of any desert pupfish. The population has steadily declined since the late 1990s before reaching lows of 38 and 35 individuals in the spring of 2007 and 2013, respectively (figure 1b). Cyprinodon diabolis is restricted to the upper 30 m of Devils Hole, a 3.5 x 22 m water-filled cavern widely believed to be the smallest range of any vertebrate [29] (figure 1c). Population viability analyses in 2014 suggested that the median time to extinction was 26 years [30]. Although this species was previously believed to be isolated in Devils Hole for 10–20 ka (Miller [31]), more recent genome-wide estimates indicate that Devils Hole may have most recently experienced substantial admixture approximately 1–2 ka and that gene flow among these desert oases is surprisingly common [23,32,33].
The continued persistence of C. diabolis in the hottest desert on earth in one of the most inhospitable habitats for fishes is extraordinary. Despite its status as one of the world's most endangered species, genetic analyses have so far been limited to delineating phylogenetic relationships, assessing population structure, and measuring genetic diversity with reduced-representation genetic markers [23]. Here, we resequenced whole genomes of C. diabolis and several closely related Cyprinodon desert pupfishes to investigate how isolation and small population size influence inbreeding and mutation load on a genome-wide scale in this conservation icon.
2. Results
(a) . Geography and population structure
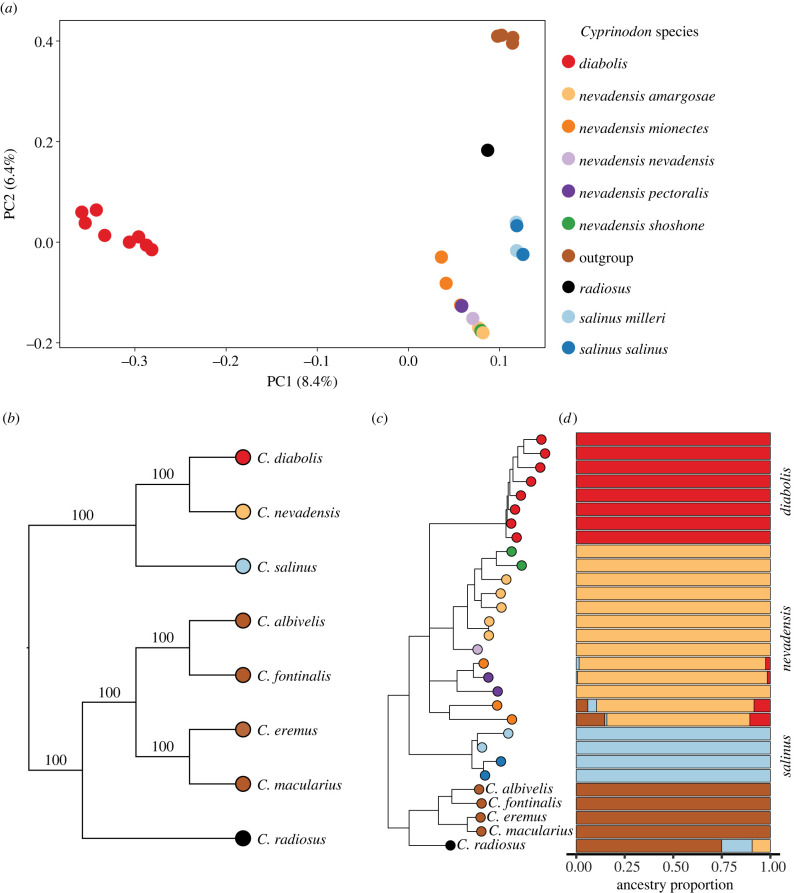
We sequenced 30 individuals (8 C. diabolis, 13 C. nevadensis, 4 C. salinus, and one individual each of C. albivelis, C. eremus, C. fontinalis, C. macularius and C. radiosus) for our analyses (figure 1d, electronic supplementary material, tables S1 and S2). After filtering for quality genotypes and exclusion of problematic samples, we retained a total of 6 295 414 SNPs with a mean coverage of 12×. We investigated genome-wide population genetic differentiation among Death Valley desert pupfishes using principal component analysis, corroborating previous results [23] (figure 2a). Devils Hole pupfish were substantially divergent from the most closely related neighbouring desert pupfish species for genome-wide mean Fst estimates (C. diabolis versus C. nevadensis = 0.34). Inference of evolutionary relationships among these populations using genome-wide SNP data under the multi-species coalescent using SVDquartets [34,35] and concatenated SNP data across all individuals using IQ-TREE v.1.6.12 [36] confirmed previous findings; C. diabolis is sister to C. nevadensis and C. salinus is sister to both [23]. Our expanded sampling of outgroups also confirmed that the Death Valley clade is most closely related to the geographically proximate Owens pupfish (C. radiosus) [37]. ADMIXTURE analyses support C. diabolis, C. nevadensis and C. salinus as distinct populations. Interestingly, this analysis infers apparent admixture within C. nevadensis from neighbouring populations as more distantly related desert pupfishes (figure 2d); this complex history of admixture may help to explain the lack of phylogenetic resolution within this group.
Figure 2.
Population structure and evolutionary relationships among desert pupfishes. (a) Principal component analysis of desert pupfishes revealing substantial population structure among species and populations. (b) Species tree estimated by SVDquartets; nonparametric bootstrap support values indicate the percentage of bootstrap replicates supporting monophyly for each clade. (c) Maximum-likelihood tree of all 30 samples estimated by IQ-TREE. Two internal branches were collapsed due to low support (ultrafast bootstrap < 95%, SH-aLRT < 80%); all other branch support was unequivocal (UFboot = 100%, SH-aLRT = 100%). (d) Ancestry proportions across individuals in Death Valley NP, Ash Meadows NWR and outgroup desert pupfishes (C. albivelis, C. eremus, C. fontinalis, C. macularius, C. radiosus) estimated from a LD-pruned SNP dataset in ADMIXTURE with k = 4. Colours in PCA, trees and ADMIXTURE designate individuals from different species or populations and correspond to the shared figure legend in (a). (Online version in colour.)
(b) . Severe inbreeding in Devils Hole pupfish
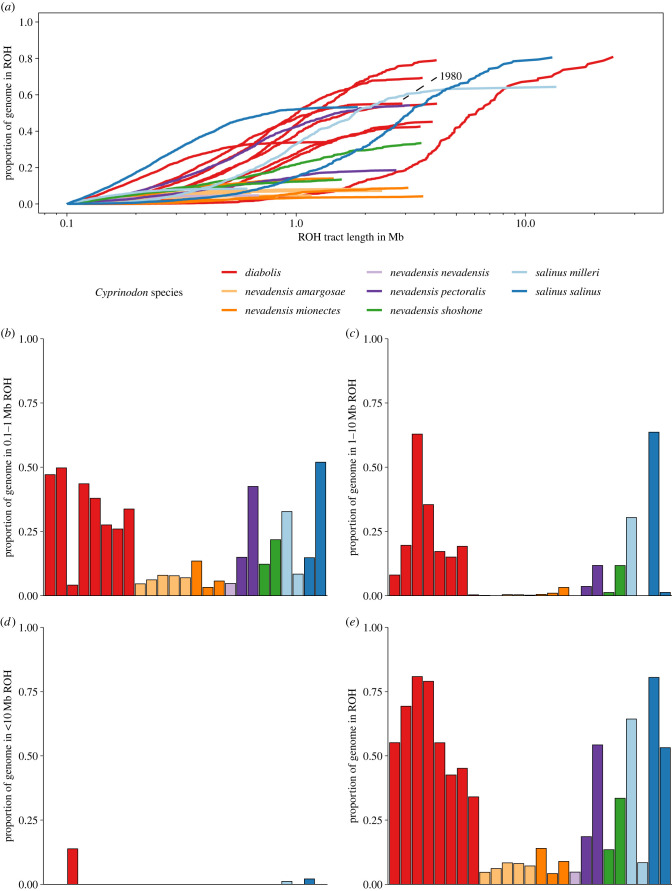
Inbreeding can be identified and quantified through runs-of-homozygosity (ROHs), which are long contiguous tracts of identical haplotypes inherited from a common ancestor [22]. We calculated FROH, an accurate measure of inbreeding, as the summed lengths of ROHs greater than 100 kb divided by the total genome size. We found that C. diabolis was highly inbred (mean FROH = 0.58), significantly exceeding the degree of inbreeding observed in C. nevadensis (mean FROH = 0.14: Tukey's HSD p = 8.38 × 10−5: figure 3). By contrast, ROHs made up less than 10% of the genome in the relatively undisturbed natural spring populations of C. nevadensis amargosae and C. nevadensis nevadensis, whereas C. nevadensis shoshone and C. nevadensis pectoralis tended to have a higher FROH than other C. nevadensis species (figure 3). These findings are consistent with small census population sizes and intensive management histories as C. nevadensis shoshone has experienced extirpation and captive breeding prior to reintroduction in the 1990s and the habitat of C. nevadensis pectoralis has undergone extensive habitat modification [38–40].
Figure 3.
Extreme inbreeding in the Devils Hole pupfish. (a) FROH measured as the cumulative fraction of the genome made up of runs of homozygosity (ROHs) at least 100 kb long. (b) Proportion of the genome in ROHs of 0.1–1 Mb for each individual. (c) Proportion of the genome in ROHs of 1–10 Mb for each individual. (d) Proportion of the genome in ROHs greater than 10 Mb for each individual. (e) Total proportion of the genome within an ROH greater than 100 kb. (Online version in colour.)
We found that the degree of inbreeding in Devils Hole has remained high, from 1980 (FROH = 0.55; n = 1) to near-present day (2008–2012: mean FROH = 0.58). This suggests that C. diabolis was already highly inbred prior to the population decline in the mid-1990s and severe bottlenecks in 2007 and 2013, during which the population size plummeted to 38 and 35 fish, respectively [30] (figure 1b).
Shorter ROHs indicate mating between distant relatives or longer-scale historical processes, whereas longer ROHs are indicative of recent inbreeding due to reduced opportunity for recombination [41]. We exploited this signature to estimate the mean number of generations back to the common ancestor of these homologous sequences using the length of ROHs and an assumed recombination rate. Populations with similar high levels of inbreeding can have ROHs that nearly span entire chromosomes [42]. Surprisingly, given the recent severe bottlenecks, we did not find many long ROHs (greater than 10 Mb) in our C. diabolis samples, barring a single exception (figure 3d). Instead, much of the cumulative inbreeding is made up of ROHs that are 0.1–1 Mb long (figure 3b), which corresponds to shared parental ancestry from 11 to 109 generations previous. Our results illustrate that extreme isolation and prolonged small population size have driven C. diabolis to become highly inbred and that much of the inbreeding occurred prior to recent bottlenecks over the course of the twentieth century.
(c) . Higher mutation load in Devils Hole pupfish
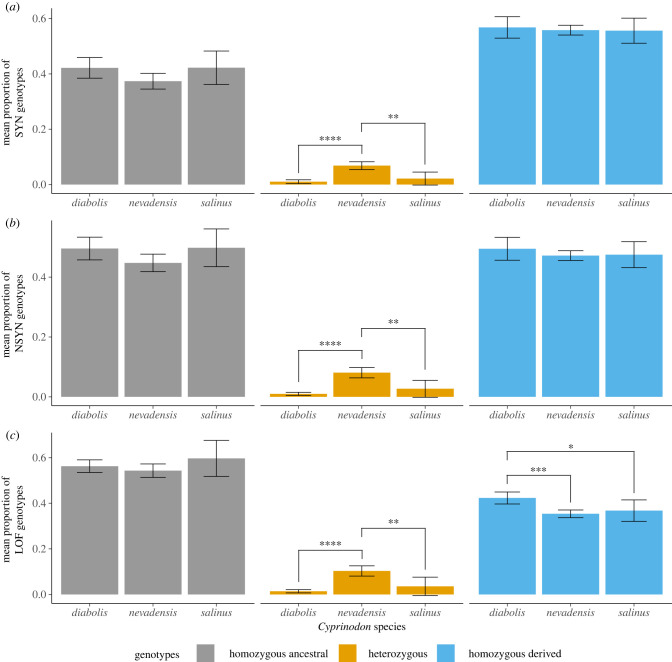
Small, inbred populations are expected to have higher frequencies and homozygosity of deleterious alleles. However, once deleterious recessive alleles are unmasked, purifying selection can also purge portions of the mutation load [43]. To assess how severe inbreeding in Devils Hole pupfish has affected mutation load, we calculated the relative proportions of homozygous ancestral, heterozygous and homozygous-derived genotypes across synonymous (SYN), non-synonymous (NSYN) and loss-of-function (LOF) mutations (figure 4; electronic supplementary material, figure S1). The number of homozygous-derived genotypes for LOF variants quantifies load under a recessive model [8,44]. Because deleterious alleles tend to be recessive [45,46], LOF homozygous-derived alleles are more likely to have a phenotypic effect that leads to a reduction in fitness.
Figure 4.
Cyprinodon diabolis suffers from uniquely high mutation load. The mean proportion of homozygous ancestral, heterozygous and homozygous-derived genotypes in C. diabolis, C. nevadensis and C. salinus with respect to the C. brontotheroides genome at segregating sites across all populations in coding regions. Variants were categorized into mutation classes as synonymous (SYN), non-synonymous (NSYN) or loss-of-function (LOF) with Snpeff. Loss-of-function mutations are defined as those that encode a premature stop codon. Error bars correspond to the 95% confidence interval, spanning the mean ± two standard errors. (Online version in colour.)
Cyprinodon diabolis had significantly lower proportions of heterozygous genotypes than C. nevadensis across all variant types (SYN: p = 1.42 × 10−5, NSYN: p = 1 × 10−5, LOF: p = 1.87 × 10−5, Tukey's HSD tests), consistent with our findings of higher inbreeding in this species. Cyprinodon diabolis also had significantly higher proportions of homozygous-derived LOF genotypes compared to both C. nevadensis (p = 6.61 × 10−4) and C. salinus (p = 0.044). Similarly, C. salinus also had significantly lower proportions of heterozygous genotypes than pooled C. nevadensis samples across all variant types (SYN: p = 2.74 × 10−3, NSYN: p = 3.71 × 10−3, LOF: p = 5.36 × 10−3, Tukey's HSD tests), likely reflecting the larger population sizes of C. nevadensis populations. There were no significant differences in the proportion of homozygous-derived genotypes for SYN and NSYN mutations among the three species.
We also assessed whether there were allele frequency differences among the three species across a comparable set of LOF alleles and found that there were no significant differences among the three species (ANOVA p = 0.134), although C. diabolis tended to have a higher mean frequency of LOF alleles at 0.63, compared to C. nevadensis = 0.54 and C. salinus = 0.49. Finally, there was no enrichment of LOF variants in ROHs for most individuals except for three C. diabolis individuals that had significantly greater proportions of LOF variants in ROHs than their respective FROH values (DHP54903: p = 1.92 × 10−3, DHP54913: p = 4.29 × 10−3, DHP54918: p = 4.71 × 10−2). One C. nevadensis amargosae individual had a significantly lower proportion of LOF variants in ROHs (CNevAma: p = 2.01 × 10−2).
(d) . Fixed variants unique to Devils Hole
Devils Hole pupfish clearly harbour a homozygous LOF mutation load greater than neighbouring desert pupfish. Thus, we focused on genetic variants most likely to be deleterious to help inform future management of this species; specifically, homozygous-derived LOF variants and deletions unique to C. diabolis, which are expected to reduce fitness by disrupting gene function.
We identified 11 predicted LOF SNPs in the form of premature stop codons, including several within genes that may affect fecundity or resistance to disease and stress (electronic supplementary material, table S3). These include cfap43, a protein involved in the structure and function of the sperm flagellum axoneme that has been implicated in male infertility [47]. Similarly, reduced sperm motility and abnormal sperm morphology were observed in Florida panthers [11] and lions [48] due to inbreeding.
We also identified 94 deletions unique to C. diabolis and focused on the 15 deletions within 2 kb of any annotated genes. Surprisingly, five of the fifteen deletions were involved in cellular responses to hypoxia (electronic supplementary material, table S4), including shifts to anaerobic metabolism, erythropoiesis, degradation of misfolded proteins and regulation of nutrient use and cell fate [49]. These hypoxia-related deletions included an 81 bp deletion in the promoter of redd1. During hypoxia, redd1 inhibits mTORC1 [50,51] through the TSC1/TSC2 tumour suppressor complex to conserve energy and prevent the accumulation of misfolded proteins. Upregulation of a homologue of redd1 (ddit4L/redd2) has been implicated in adaptation to hypoxia in shortfin mollies (Poecilia mexicana) [52] and high-altitude deer mice (Peromyscus maniculatus) (N. R. Rochette 2021, personal communication). We also found deletions in apeh, an enzyme that destroys oxidatively damaged proteins [53]; trim39, a tripartite motif involved in erythropoiesis [54] and slc25a42, a mitochondrial transporter of coenzyme A [55] associated with mitochondrial myopathy and lactic acidosis in humans [56].
3. Discussion
We documented extensive inbreeding, gene loss and mutation load in the critically endangered Devils Hole pupfish and identified a set of candidate deleterious genetic variants that can potentially inform future conservation management. We show that C. diabolis is significantly more inbred than most neighbouring desert pupfish populations. Cyprinodon diabolis was not significantly more inbred than C. salinus although our small sample size and high variability make it difficult to infer accurate levels of inbreeding in C. salinus. High levels of inbreeding are associated with elevated extinction risk [57,58] and the inbreeding in C. diabolis is equal to or more severe than levels reported so far in other isolated natural populations such as Isle Royale wolves [12], mountain gorillas [59] and Indian tigers [60]. Although we were unable to directly measure fitness, the increased inbreeding in C. diabolis likely results in a substantial reduction in fitness. Previous studies have suggested that increases in FROH have strong negative effects on fitness. For instance, in Soay sheep an increase in FROH by 10% was correlated with a 60% decline in fitness [61], whereas in helmeted honeyeaters a 9% increase in homozygosity was associated with a reduced lifetime reproductive success of 87–90% [62]. Surprisingly, C. salinus also displayed high levels of inbreeding although increased sampling is necessary to obtain reliable estimates of inbreeding.
By successfully sequencing a formalin-fixed historical museum specimen to 10× coverage (out of 8 total attempts) we discovered that inbreeding was already extensive by 1980, suggesting that C. diabolis may have an extended history of repeated population bottlenecks. Indeed, the distribution of ROH sizes suggests that a large proportion of homozygous tracts were due to inbreeding that occurred many generations prior to the recent population decline. Furthermore, C. diabolis harbours a significantly greater mutation load than either C. nevadensis or C. salinus (figure 4).
Our mutation load results support previous hypotheses that C. diabolis harbours a high mutation load due to its relative isolation and small population size [29,63]. Additionally, they are consistent with the observation of rapid allele frequency increases of C. nevadensis alleles in a C. diabolis refuge population after the accidental introduction of a few individuals [64]. Although recent studies have suggested that small, bottlenecked populations may harbour a lower mutation load due to purging [13,65,66], populations that have experienced recent severe population bottlenecks are likely to maintain a high load, because deleterious variants may reach fixation before purifying selection removes them [67]. Although our measure of mutation load does not directly measure fitness, there is empirical support that LOF mutations are on average deleterious [68].
(a) . Degradation of hypoxia and reproductive pathways in Cyprinodon diabolis
We found deleterious variants associated with reproduction and hypoxia genes. For example, we found a fixed LOF variant unique to C. diabolis in cfap43, a gene associated with sperm morphology and function. Deletions have thus far been rarely studied or quantified in conservation genetics. However, analysis of woolly mammoth genomes from different time points found that a sample dated closer to the time of extinction had accumulated more homozygous deletions than earlier ones [15], suggesting that deletions may be an understudied genetic threat to endangered populations [17].
We found numerous deletions that were unique to the Devils Hole pupfish. Of these, five were associated with hypoxia, a known environmental stressor in the system, suggesting that C. diabolis could be poorly equipped to physiologically deal with the stressful hypoxic environment in Devils Hole. Indeed, previous studies have noted that C. diabolis has low fecundity [24,69], low egg viability and juvenile survivorship [29] and lays more eggs at lower temperatures (28°C) compared to the higher constant temperature of 33°C in Devils Hole [70]. At present, we cannot rule out the possibility that some of these fixed variants are potentially the result of local adaptation; distinguishing between selection and genetic drift in small populations is extremely difficult because both processes leave similar signatures in allele frequencies [71]. One possibility is that these variants are adaptive in the unique selective environment of Devils Hole and were swept to fixation during initial colonization. Moving forward, functional genetic and eco-physiological tests will be key to understanding the full impacts of these variants. Our results highlight the importance of investigating deletions and structural variants to better understand unique genetic variation in endangered populations.
(b) . Did severe inbreeding cause the recent population decline?
The Devils Hole pupfish population began to decline in the 1990s from historical population sizes of 200–500 to less than 100 individuals for reasons still unknown (although in the most recent census the population has rebounded to 175 fish). Ecological hypotheses include declines in ostracod prey for juvenile pupfish [72,73] and changes in the dominant primary producers from Spirogyra algae to diatoms and cyanobacterial mats [74]. Furthermore, climate change may be shortening the seasonal period of optimal hatching conditions on the shallow shelf where C. diabolis spawns [75]. The stressful environment of present day Devils Hole may thus have exacerbated inbreeding depression, which typically increases under environmental stress [76,77].
Alternatively, previous studies speculated that the population has a high genetic load which led to ‘mutational meltdown’ based on the discovery of a dramatic shift towards predominantly C. nevadensis ancestry following introduction of a few C. nevadensis into a C. diabolis refuge population [64]. We estimated the effective population size of C. diabolis based on the harmonic mean of biannual census data (, ) and found that this species has an effective population size far below the suggested minimum for the maintenance of sufficient genetic variation for adaptive capacity [78,79].
(i) . Past and future management actions
Initial management of the species involved various attempts to create wild refuge populations in the 1950s–1970s prior to the landmark 1976 US Supreme Court decision mandating a minimum water level, which halted habitat reduction and population decline caused by groundwater pumping [80]. Subsequent attempts to maintain refuge populations of C. diabolis in aquaria and semi-natural outdoor pools largely failed to sustain captive breeding colonies for long periods of time [29,64]. In response to the severe population bottlenecks in the early 2000s, Ash Meadows Fish Conservation Facility was constructed in 2012 to establish a refuge population that more closely mimicked the Devils Hole habitat and the refuge colony now outnumbers the wild population. Although we relied on degraded tissue samples collected from dead pupfish retrieved from Devils Hole for genetic analyses in this study, increasing population numbers may soon allow for tissue samples or functional genetic approaches in refuge live specimens.
4. Conclusion
Our study adds to a growing number of studies that measure inbreeding and mutation load in wild populations, with a novel focus on gene deletions in a wild highly inbred population [60,66]. The demographic history and genome-wide measures of high inbreeding and mutation load in the Devils Hole pupfish suggest that the population remains in danger. While successfully sequencing formalin-fixed samples remains difficult, we were able to do so for a single C. diabolis specimen from 1980 and found that the population was likely highly inbred prior to the recent bottleneck. The increasing availability of genomic sampling spanning multiple time points for endangered species such as the Devils Hole pupfish will better inform our understanding of inbreeding, mutation load, and specific putatively deleterious variants in this system, ultimately allowing for conservation management to monitor potentially harmful variation in wild and captive populations over time. Finally, we caution that targeted genetic management of deleterious variants should not be undertaken until candidates have been verified to have fitness consequences.
5. Methods
(a) . Samples and sequencing
We sequenced 44 whole genomes including C. diabolis (n = 23), C. nevadensis (n = 13) and C. salinus (n = 4) spanning multiple independent springs, along with closely related desert pupfish species from California, Arizona and Mexico (electronic supplementary material, table S1). Of the 23 C. diabolis genomes, eight were from historical museum samples, spanning 1937–1980, while the rest were non-destructively sampled between 2008 and 2012. Given the critically endangered status of C. diabolis, NPS and USFWS staff collected and preserved dead specimens found during routine checks during this period. All other species were collected in the early 1990s [81]. Samples were sequenced on 150 PE runs using an Illumina Novaseq. Historical and several degraded C. diabolis samples were prepared using Swift 2S Turbo library kits (Swift Biosciences). All sample metadata are reported in electronic supplementary material, table S1. Fourteen samples were excluded from downstream analyses due to a low percentage of reads mapping to the reference genome (less than 70%), improperly paired reads (less than 70%), or significant amounts of missing data per individual following SNP calling (greater than 80%), presumably due to degradation (electronic supplementary material, table S2: see electronic supplementary material for additional details). Following the filtering described above to identify high-quality samples, we retained for all downstream analyses the following 30 samples: 8 C. diabolis, 13 C. nevadensis, 4 C. salinus, and five closely related desert pupfish species (C. albivelis, C. eremus, C. fontinalis, C. macularius, C. radiosus) (figure 2).
(b) . Alignment and filtering
Raw reads were mapped from all 44 individuals to the Cyprinodon brontotheroides reference genome (UCB_Cbro_1.0; total sequence length = 1 162 855 435 bp; scaffold N50 = 32 Mbp) [82] with bwa-mem (v.0.7.12) [83]. Duplicate reads were identified using MarkDuplicates and BAM indices were created using BuildBamIndex in the Picard software package (http://picard.sourceforge.net; v.2.0.1). We followed the best practices guide recommended in the Genome Analysis Toolkit (v.3.5) [84] to call and refine our single nucleotide polymorphism (SNP) variant dataset using the program HaplotypeCaller. SNPs were filtered based on the recommended hard filter criteria (QD < 2.0; FS > 60; MQ < 40; MQRankSum < −12.5; ReadPosRankSum < −8) because we lacked high-quality known variants. See electronic supplementary material for additional details. Our final dataset contained 6 295 414 SNPs.
(c) . Population structure
We pruned SNPs in strong linkage disequilibrium using the LD pruning function (–indep-pairwise 50 5 0.5) in PLINK (v.1.9) [85] leading to the retention of 1 653 597 variants. We then characterized population structure with two approaches. First, we used PLINK to conduct principal component analysis. Second, we used ADMIXTURE (v.1.3.0) [86] to assign individuals to variable numbers of population clusters (K = 1–20). We used the subset parameter in PLINK to randomly select 100 000 SNPs for analysis. We calculated genome-wide Fst between C. diabolis and C. nevadensis, based on the 6.3 million SNP dataset using the weir-fst-pop function in vcftools (v.0.1.15) [87].
(d) . Phylogenetic inference
Phylogenies were inferred at the individual and species-level from 1 653 597 LD-pruned SNPs using IQ-Tree (v.1.6.12) [36] and SVDquartets [34,35]. Full details are provided in the electronic supplementary material.
(e) . Population size
Census data for 1972–spring 2013 was acquired from Beissinger [30]. Data for subsequent counts were based on released NPS news reports. Variance effective population size was calculated as the harmonic mean of spring (NeSpring) and autumn (NeAutumn) population counts over time.
(f) . Runs of homozygosity
Runs of homozygosity (ROHs) were identified in our Death Valley samples from the full filtered set of 6 295 414 SNPs using the BCFtools/ROH command (v.1.14) [88], which uses a hidden Markov model to identify homozygous portions of the genome from genetic variation data. To calculate the cumulative fraction of the genome consisting of ROHs (FROH) for each individual, we summed the lengths of identified ROHs with length greater than 100 kb and divided by the total size of the genome (1 162 855 435 bp). We also classified ROHs into various lengths of 0.1–1 Mb, 1–10 Mb, and greater than 10 Mb and calculated the proportion of the genome that these classes of ROHs comprised in a similar fashion. Although the amount of missing data was significantly associated with our measure of FROH across all samples (electronic supplementary material, figure S2), we found no significant association between FROH and depth of coverage or missing data for C. diabolis samples alone. Furthermore, we were still able to detect low FROH in two C. nevadensis samples with large amounts of missing data. The age of ROHs was estimated as g = 100/2 × ROH length (cM), where g is the number of generations to the most recent common ancestor [41]. We assumed a generation time of 1 year [29], and an average recombination of approximately 4.6 cM/Mb (genetic map length = 5330 cM [89]; C. brontotheroides genome size = 1.16 Gb [82]).
(g) . Measuring mutation load
We categorized variants in coding regions based on their putative effect on the amino acid sequence (i.e. LOF or NSYN) and whether the alleles were derived with respect to our reference (C. brontotheroides) genome using SnpEff [90]. LOF variants were conservatively defined as SNPs that resulted in a premature stop codon [65,91], which are expected to be less prone to misannotation [92]. Unique, putatively deleterious variants were defined as being: (i) homozygous derived, (ii) a gained stop codon and (iii) present in all of our C. diabolis samples for which genotypic information was available and absent in all other non-C. diabolis samples in our dataset. See electronic supplementary material for additional details.
We did not rely on direct counts of variants or derived alleles as they are significantly positively correlated with coverage (electronic supplementary material, figure S3) and missing data (electronic supplementary material, figure S4). Instead, we measured mutation load in terms of the proportions of SYN, NSYN and LOF genotypes that were homozygous ancestral, heterozygous or homozygous-derived across species (e.g. [91]). This transformation largely corrected for the confounding effect of coverage (electronic supplementary material, figure S5) and missing data (electronic supplementary material, figure S6) on our estimates of mutation load. We assessed whether mutation loads differed among the three species using an ANOVA in R and used Tukey's HSD tests in R to test for pairwise differences among species [93]. To compare allele frequencies of LOF alleles between our three species, we first identified 62 LOF variants for which there was genotype information for at least four individuals per species and for which the variant was present in at least two of the three species, to control for differences in sampling number among species. We then calculated species-specific allele frequencies for each of these 62 LOF variants. Differences in the average allele frequency of LOF alleles were assessed using an ANOVA in R [93]. We assessed whether LOF variants were enriched in ROHs for each individual by performing binomial tests where the number of successes was defined as the number of LOF variants in ROHs, the number of trials was defined as the total number of LOF variants, and the probability of success was defined as the individual's FROH.
(h) . Unique deletions
We identified deletions that were unique to C. diabolis using DELLY (v.0.8.3) [94]. We only characterized deletions that were present in our five highest quality C. diabolis samples (DHP1980-5, DHP54903, DHP54913, DHP54917, DIAB54919), but absent in other species. Deletions that were exceptionally large and presumably artefacts were checked for accuracy in IGV and subsequently removed (n = 4). We focused primarily on deletions within 2 kb of an annotated gene. Deletions were confirmed to be unique to C. diabolis by aligning BAM files spanning the deletion and confirming their presence in C. diabolis and absence in non-C. diabolis samples. We analysed whether deletions spanned exons, introns or regulatory regions by BLASTing [95] deleted sequences against the C_variegatus-1.0 (GCF_000732505.1) assembly on Ensembl (release 102; [96]).
Acknowledgements
We thank members of the Martin lab for valuable comments and discussions; O. Feuerbacher for providing a photo of C. diabolis; T. Echelle for providing specimens of C. n. pectoralis from Indian Spring; Lee Simons, the US Fish and Wildlife Service and the National Park Service for providing C. diabolis samples and permits; and the University of California, Berkeley for computational resources. We also gratefully thank Douglas Nelson and the University of Michigan Museum of Zoology for allowing us to sample and sequence specimens from their collection (1980 specimen: UMMZ 213382).
Ethics
Pupfishes were originally collected in the Death Valley region with Endangered Species Permit PRT-769851 and Special Use Permit 56034, US Fish and Wildlife Service; Collecting Permit A9103, Death Valley National Monument, National Park Service; 6840, California, Desert District Office, Bureau of Land Management; S9494, Nevada Department of Wildlife and a memorandum of understanding with the California Department of Fish and Game.
Data accessibility
All raw genomic data are archived at the National Center for Biotechnology Information BioProject Database (Accession: PRJNA887195). Genotypic data and population census data are available through the Dryad Digital Repository: https://doi.org/10.6078/D1ND9Q [97]. Scripts are available at https://github.com/tiandavid/Cyprindon_WGS_project/.
The data are provided in electronic supplementary material [98].
Authors' contributions
D.T.: conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing—original draft, writing—review and editing; A.H.P.: formal analysis, writing—original draft, writing—review and editing; B.J.T.: resources, writing—review and editing; C.H.M.: conceptualization, data curation, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
The authors declare no competing interests.
Funding
This work was funded by the US Fish and Wildlife Service, National Park Service, National Science Foundation DEB CAREER grant no. 1749764, National Institutes of Health grant 5R01DE027052-02 and the University of California, Berkeley to CHM. D.T. was supported by a National Science Foundation Graduate Research Fellowship (DGE 1752814).
References
- 1.Frankham R. 1995. Conservation genetics. Annu. Rev. Genet. 29, 305-327. ( 10.1146/annurev.ge.29.120195.001513) [DOI] [PubMed] [Google Scholar]
- 2.Charlesworth B, Charlesworth D. 1999. The genetic basis of inbreeding depression. Genet. Res. 74, 329-340. ( 10.1017/s0016672399004152) [DOI] [PubMed] [Google Scholar]
- 3.Charlesworth D, Willis JH. 2009. The genetics of inbreeding depression. Nat. Rev. Genet. 10, 783-796. ( 10.1038/nrg2664) [DOI] [PubMed] [Google Scholar]
- 4.Melbourne BA, Hastings A. 2008. Extinction risk depends strongly on factors contributing to stochasticity. Nature 454, 100-103. ( 10.1038/nature06922) [DOI] [PubMed] [Google Scholar]
- 5.Kohn MH, Murphy WJ, Ostrander EA, Wayne RK. 2006. Genomics and conservation genetics. Trends Ecol. Evol. 21, 629-637. ( 10.1016/j.tree.2006.08.001) [DOI] [PubMed] [Google Scholar]
- 6.Kimura M. 1957. Some problems of stochastic processes in genetics. Ann. Math. Stat. 28, 882-901. ( 10.1214/aoms/1177706791) [DOI] [Google Scholar]
- 7.Agrawal AF, Whitlock MC. 2012. Mutation load: the fitness of individuals in populations where deleterious alleles are abundant. Ann. Rev. Ecol. Evol. Syst. 43, 115-135. ( 10.1146/annurev-ecolsys-110411-160257) [DOI] [Google Scholar]
- 8.Henn BM, Botigué LR, Bustamante CD, Clark AG, Gravel S. 2015. Estimating the mutation load in human genomes. Nat. Rev. Genet. 16, 333-343. ( 10.1038/nrg3931) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spielman D, Brook BW, Frankham R. 2004. Most species are not driven to extinction before genetic factors impact them. Proc. Natl Acad. Sci. USA 101, 15 261-15 264. ( 10.1073/pnas.0403809101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crnokrak P, Roff DA. 1999. Inbreeding depression in the wild. Heredity 83, 260-270. ( 10.1038/sj.hdy.6885530) [DOI] [PubMed] [Google Scholar]
- 11.Johnson WE, et al. 2010. Genetic restoration of the Florida panther. Science 329, 1641-1645. ( 10.1126/science.1192891) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson JA, Räikkönen J, Vucetich LM, Vucetich JA, Peterson RO, Lohmueller KE, Wayne RK. 2019. Genomic signatures of extensive inbreeding in Isle Royale wolves, a population on the threshold of extinction. Sci. Adv. 5, eaau0757. ( 10.1126/sciadv.aau0757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xue Y, et al. 2015. Mountain gorilla genomes reveal the impact of long-term population decline and inbreeding. Science 348, 242-245. ( 10.1126/science.aaa3952) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palkopoulou E, et al. 2015. Complete genomes reveal signatures of demographic and genetic declines in the woolly mammoth. Curr. Biol. 25, 1395-1400. ( 10.1016/j.cub.2015.04.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogers RL, Slatkin M. 2017. Excess of genomic defects in a woolly mammoth on Wrangel island. PLoS Genet. 13, e1006601. ( 10.1371/journal.pgen.1006601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bi K, Linderoth T, Vanderpool D, Good JM, Nielsen R, Moritz C. 2013. Unlocking the vault: next-generation museum population genomics. Mol. Ecol. 22, 6018-6032. ( 10.1111/mec.12516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Díez-Del-Molino D, Sánchez-Barreiro F, Barnes I, Gilbert MTP, Dalén L. 2018. Quantifying temporal genomic erosion in endangered species. Trends Ecol. Evol. 33, 176-185. ( 10.1016/j.tree.2017.12.002) [DOI] [PubMed] [Google Scholar]
- 18.Hansson B, Westerberg L. 2002. On the correlation between heterozygosity and fitness in natural populations. Mol. Ecol. 11, 2467-2474. ( 10.1046/j.1365-294x.2002.01644.x) [DOI] [PubMed] [Google Scholar]
- 19.Kardos M, Armstrong E, Fitzpatrick S, Hauser S, Hedrick P, Miller J, Tallmon DA, Funk WC. 2021. The crucial role of genome-wide genetic variation in conservation. Proc. Natl Acad. Sci. USA 118, e2104642118. ( 10.1101/2021.07.05.451163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saccheri I, Kuussaari M, Kankare M, Vikman P, Fortelius W, Hanski I. 1998. Inbreeding and extinction in a butterfly metapopulation. Nature 392, 491-494. ( 10.1038/33136) [DOI] [Google Scholar]
- 21.Teixeira JC, Huber CD. 2021. The inflated significance of neutral genetic diversity in conservation genetics. Proc. Natl Acad. Sci. USA 118, e2015096118. ( 10.1073/pnas.2015096118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kardos M, Taylor HR, Ellegren H, Luikart G, Allendorf FW. 2016. Genomics advances the study of inbreeding depression in the wild. Evol. Appl. 9, 1205-1218. ( 10.1111/eva.12414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin CH, Crawford Jacob E, Turner Bruce J, Simons Lee H. 2016. Diabolical survival in Death Valley: recent pupfish colonization, gene flow and genetic assimilation in the smallest species range on earth. Proc. R. Soc. B 283, 20152334. ( 10.1098/rspb.2015.2334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.James CJ. 1969. Aspects of the ecology of the Devil's Hole Pupfish (Cyprinodon diabolis) Wales. Las Vegas, NV: University of Nevada. [Google Scholar]
- 25.Riggs AC, Deacon JE. 2004. Connectivity in desert aquatic ecosystems: the Devils Hole story. Important scientific and cultural resources of the intermountain region [Proceedings], Las Vegas, NV, 7–9 May 2002, pp. 1-38, DRI Report No. 41210. [Google Scholar]
- 26.Wilson KP, Blinn DW, Herbst DB. 2001. The Devils Hole energetics/community relationships: Death Valley National Park, California. Two-year progress report to Death Valley National Park. Project No. 7530.
- 27.Gustafson ES, Deacon JE. 1997. .Distribution of larval Devils Hole pupfish, Cyprinodon diabolis Wales, in relation to dissolved oxygen concentration in Devil's Hole. Final report to the National Park Service, Death Valley National Park.
- 28.Madinger HL, Wilson KP, Goldstein JA, Bernot MJ. 2016. Biogeochemistry and nutrient limitation of microbial biofilms in Devils Hole. West. N. Am. Nat. 76, 53-71. ( 10.3398/064.076.0107) [DOI] [Google Scholar]
- 29.Deacon JE, Taylor FR, Pedretti JW. 1995. Egg viability and ecology of Devils Hole pupfish: insights from captive propagation. Southwest. Nat. 40, 216-223. [Google Scholar]
- 30.Beissinger SR. 2014. Digging the pupfish out of its hole: risk analyses to guide harvest of Devils Hole pupfish for captive breeding. PeerJ 2, e549. ( 10.7717/peerj.549) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller RR. 1981. Coevolution of deserts and pupfishes (genus Cyprinodon) in the American Southwest. In Fishes in North American Deserts (eds Naiman RJ, Soltz DL), pp. 39-94. New York, NY: John Wiley and Sons. See https://azgs.arizona.edu/azgeobib/coevolution-deserts-and-pupfishes-genus-cyprinodon-american-southwest-naiman-r-j-and-soltz. [Google Scholar]
- 32.Martin CH, Höhna S, Crawford JE, Turner BJ, Richards EJ, Simons LH. 2017. The complex effects of demographic history on the estimation of substitution rate: concatenated gene analysis results in no more than twofold overestimation. Proc. R. Soc. B 284, 20170537. ( 10.1098/rspb.2017.0537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin CH, Turner BJ. 2018. Long-distance dispersal over land by fishes: extremely rare ecological events become probable over millennial timescales. Proc. Biol. Sci. 285, 20172436. ( 10.1098/rspb.2017.2436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chifman J, Kubatko L. 2014. Quartet inference from SNP data under the coalescent model. Bioinformatics 30, 3317-3324. ( 10.1093/bioinformatics/btu530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chifman J, Kubatko L. 2015. Identifiability of the unrooted species tree topology under the coalescent model with time-reversible substitution processes, site-specific rate variation, and invariable sites. J. Theor. Biol. 374, 35-47. ( 10.1016/j.jtbi.2015.03.006) [DOI] [PubMed] [Google Scholar]
- 36.Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32, 268-274. ( 10.1093/molbev/msu300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller RR, Pister EP. 1971. Management of the Owens pupfish, Cyprinodon radiosus, in Mono County, California. Trans. Am. Fish. Soc. 100, 502-509. () [DOI] [Google Scholar]
- 38.Castleberry D, Williams JE, Sato GM, Hopkins TE, Brasher A, Parker MS. 1990. Status and Management of Shoshone Pupfish, Cyprinodon nevadensis shoshone (Cyprinodontidae), at Shoshone Spring, Inyo County, California. Bull. Southern California Acad. Sci. 89, 19-25. [Google Scholar]
- 39.Weissenfluh D. 2010. Conservation of Cyprinodon nevadensis pectoralis and three endemic aquatic invertebrates in an artificial desert spring refuge located in Ash Meadows National Wildlife Refuge, Nevada. Thesis. See https://ttu-ir.tdl.org/handle/2346/ETD-TTU-2010-12-1207. [Google Scholar]
- 40.Swift CC, Haglund RT, Ruiz M, Fisher RN. 1993. The status and distribution of the freshwater fishes of southern California. Bull. South. Calif. Acad. Sci. 92, 101-172. [Google Scholar]
- 41.Thompson EA. 2013. Identity by Descent: Variation in Meiosis, Across Genomes, and in Populations. Genetics 194, 301-326. ( 10.1534/genetics.112.148825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kardos M, et al. 2018. Genomic consequences of intensive inbreeding in an isolated wolf population. Nat. Ecol. Evol. 2, 124-131. ( 10.1038/s41559-017-0375-4) [DOI] [PubMed] [Google Scholar]
- 43.Hedrick PW, Garcia-Dorado A. 2016. Understanding Inbreeding Depression, Purging, and Genetic Rescue. Trends Ecol. Evol. 31, 940-952. ( 10.1016/j.tree.2016.09.005) [DOI] [PubMed] [Google Scholar]
- 44.Henn BM, et al. 2016. Distance from sub-Saharan Africa predicts mutational load in diverse human genomes. Proc. Natl Acad. Sci. USA 113, E440-E449. ( 10.1073/pnas.1510805112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simmons MJ, Crow JF. 1977. Mutations affecting fitness in drosophila populations. Annu. Rev. Genet. 11, 49-78. ( 10.1146/annurev.ge.11.120177.000405) [DOI] [PubMed] [Google Scholar]
- 46.Agrawal AF, Whitlock MC. 2011. Inferences about the distribution of dominance drawn from yeast gene knockout data. Genetics 187, 553-566. ( 10.1534/genetics.110.124560) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coutton C, et al. 2018. Mutations in CFAP43 and CFAP44 cause male infertility and flagellum defects in Trypanosoma and human. Nat. Commun. 9, 686. ( 10.1038/s41467-017-02792-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wildt DE, Bush M, Goodrowe KL, Packer C, Pusey AE, Brown JL, Joslin P, O'Brien SJ. 1987. Reproductive and genetic consequences of founding isolated lion populations. Nature 329, 328-331. ( 10.1038/329328a0) [DOI] [Google Scholar]
- 49.Lee P, Chandel NS, Simon MC. 2020. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 21, 268-283. ( 10.1038/s41580-020-0227-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, Witters LA, Ellisen LW, Kaelin WG. 2004. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 18, 2893-2904. ( 10.1101/gad.1256804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeYoung MP, Horak P, Sofer A, Sgroi D, Ellisen LW. 2008. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev. 22, 239-251. ( 10.1101/gad.1617608) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barts N. 2020. Comparative physiology and the predictability of evolution in extreme environments. Thesis, Kansas State University, Manhattan, KS. [Google Scholar]
- 53.Gogliettino M, Riccio A, Balestrieri M, Cocca E, Facchiano A, D'Arco TM, Tesoro C, Rossi M, Palmieri G. 2014. A novel class of bifunctional acylpeptide hydrolases–potential role in the antioxidant defense systems of the Antarctic fish Trematomus bernacchii. FEBS J. 281, 401-415. ( 10.1111/febs.12610) [DOI] [PubMed] [Google Scholar]
- 54.Yergeau DA, Cornell CN, Parker SK, Zhou Y, Detrich HW. 2005. bloodthirsty, an RBCC/TRIM gene required for erythropoiesis in zebrafish. Dev. Biol. 283, 97-112. ( 10.1016/j.ydbio.2005.04.006) [DOI] [PubMed] [Google Scholar]
- 55.Fiermonte G, Paradies E, Todisco S, Marobbio CMT, Palmieri F. 2009. A novel member of solute carrier family 25 (SLC25A42) is a transporter of coenzyme A and adenosine 3′,5′-diphosphate in human mitochondria. J. Biol. Chem. 284, 18 152-18 159. ( 10.1074/jbc.M109.014118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shamseldin HE, Smith LL, Kentab A, Alkhalidi H, Summers B, Alsedairy H, Xiong Y, Gupta VA, Alkuraya FS. 2016. Mutation of the mitochondrial carrier SLC25A42 causes a novel form of mitochondrial myopathy in humans. Hum. Genet. 135, 21-30. ( 10.1007/s00439-015-1608-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ralls K, Ballou JD, Templeton A. 1988. Estimates of lethal equivalents and the cost of inbreeding in mammals. Conserv. Biol. 2, 185-193. [Google Scholar]
- 58.Laikre L, Ryman N. 1991. Inbreeding depression in a captive wolf (Canis lupus) population. Conserv. Biol. 5, 33-40. [Google Scholar]
- 59.van der Valk T, Díez-Del-Molino D, Marques-Bonet T, Guschanski K, Dalén L. 2019. Historical genomes reveal the genomic consequences of recent population decline in eastern gorillas. Curr. Biol. 29, 165-170.e6. ( 10.1016/j.cub.2018.11.055) [DOI] [PubMed] [Google Scholar]
- 60.Khan A, et al. 2021. Genomic evidence for inbreeding depression and purging of deleterious genetic variation in Indian tigers. Proc. Natl Acad. Sci. USA 118, e2023018118. ( 10.1073/pnas.2023018118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stoffel MA, Johnston SE, Pilkington JG, Pemberton JM. 2021. Genetic architecture and lifetime dynamics of inbreeding depression in a wild mammal. Nat. Commun. 12, 2972. ( 10.1038/s41467-021-23222-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harrisson KA, et al. 2019. Lifetime fitness costs of inbreeding and being inbred in a critically endangered bird. Curr. Biol. 29, 2711-2717.e4. ( 10.1016/j.cub.2019.06.064) [DOI] [PubMed] [Google Scholar]
- 63.Chernoff B. 1985. Population dynamics of the Devils Hole pupfish. Environ. Biol. Fishes 13, 139-147. ( 10.1007/BF00002582) [DOI] [Google Scholar]
- 64.Martin AP, Echelle AA, Zegers G, Baker S, Keeler-Foster CL. 2012. Dramatic shifts in the gene pool of a managed population of an endangered species may be exacerbated by high genetic load. Conserv. Genet. 13, 349-358. ( 10.1007/s10592-011-0289-7) [DOI] [Google Scholar]
- 65.Robinson JA, Brown C, Kim BY, Lohmueller KE, Wayne RK. 2018. Purging of strongly deleterious mutations explains long-term persistence and absence of inbreeding depression in island foxes. Curr. Biol. 28, 3487-3494.e4. ( 10.1016/j.cub.2018.08.066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grossen C, Guillaume F, Keller LF, Croll D. 2020. Purging of highly deleterious mutations through severe bottlenecks in Alpine ibex. Nat. Commun. 11, 1001. ( 10.1038/s41467-020-14803-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van der Valk T, Manuel Md, Marques-Bonet T, Guschanski K. 2021. Estimates of genetic load suggest frequent purging of deleterious alleles in small populations. bioRxiv 696831. ( 10.1101/696831) [DOI] [Google Scholar]
- 68.Eyre-Walker A, Keightley PD. 2007. The distribution of fitness effects of new mutations. Nat. Rev. Genet. 8, 610-618. ( 10.1038/nrg2146) [DOI] [PubMed] [Google Scholar]
- 69.Liu RK. 1969. The comparative behavior of allopatric species (Teleostei-Cyprinodontidae: Cyprinodon). Thesis, University of California, Los Angeles, Los Angeles, CA. [Google Scholar]
- 70.Burg GC, et al. 2019. Care and propagation of captive pupfish from the genus Cyprinodon: insight into conservation. Environ. Biol. Fishes 102, 1015-1024. ( 10.1007/s10641-019-00887-2) [DOI] [Google Scholar]
- 71.Leigh DM, Lischer HEL, Guillaume F, Grossen C, Günther T. 2021. Disentangling adaptation from drift in bottlenecked and reintroduced populations of Alpine ibex. Mol. Ecol. Resour. 21, 2350-2363. ( 10.1111/1755-0998.13442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilson KP, Blinn DW. 2007. Food web structure, energetics, and importance of allochthonous carbon in a desert cavernous limnocrene: Devils Hole, Nevada. West. N. Am. Nat. 67, 185-198. ( 10.3398/1527-0904(2007)67[185:FWSEAI]2.0.CO;2) [DOI] [Google Scholar]
- 73.Minckley CO, Deacon JE. 1975. Foods of the Devil's Hole Pupfish, Cyprinodon diabolis (Cyprinodontidae). Southwest. Nat. 20, 105-111. ( 10.2307/3670016) [DOI] [Google Scholar]
- 74.Bernot M, Wilson K. 2012. Spatial and temporal variation of dissolved oxygen and ecosystem energetics in Devils Hole, Nevada. West. N. Am. Nat. 72, 265-275. [Google Scholar]
- 75.Hausner MB, Wilson KP, Gaines DB, Suárez F, Scoppettone GG, Tyler SW. 2014. Life in a fishbowl: prospects for the endangered Devils Hole pupfish (Cyprinodon diabolis) in a changing climate. Water Resour. Res. 50, 7020-7034. ( 10.1002/2014WR015511) [DOI] [Google Scholar]
- 76.Armbruster P, Reed DH. 2005. Inbreeding depression in benign and stressful environments. Heredity 95, 235-242. ( 10.1038/sj.hdy.6800721) [DOI] [PubMed] [Google Scholar]
- 77.Fox CW, Reed DH. 2011. Inbreeding depression increases with environmental stress: an experimental study and meta-analysis. Evolution 65, 246-258. ( 10.1111/j.1558-5646.2010.01108.x) [DOI] [PubMed] [Google Scholar]
- 78.Franklin IR. 1980. Evolutionary change in small populations. In Conservation biology: an evolutionary–ecological perspective), pp. 135-150. Sunderland, MA: Sinauer Associates, U.S.A. [Google Scholar]
- 79.Traill LW, Bradshaw CJA, Brook BW. 2007. Minimum viable population size: a meta-analysis of 30 years of published estimates. Biol. Conserv. 139, 159-166. ( 10.1016/j.biocon.2007.06.011) [DOI] [Google Scholar]
- 80.Cappaert v. United States, 426 US 128 (1976).
- 81.Duvernell DD, Turner BJ. 1998. Evolutionary genetics of Death Valley pupfish populations: mitochondrial DNA sequence variation and population structure. Mol. Ecol. 7, 279-288. ( 10.1046/j.1365-294X.1998.00347.x) [DOI] [Google Scholar]
- 82.Richards EJ, McGirr JA, Wang JR, John MES, Poelstra JW, Solano MJ, O'Connell DC, Turner BJ, Martin CH. 2021. A vertebrate adaptive radiation is assembled from an ancient and disjunct spatiotemporal landscape. Proc. Natl Acad. Sci. USA 118, e2011811118. ( 10.1073/pnas.2011811118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li H. 2013. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv:1303.3997 [q-bio]
- 84.DePristo MA, et al. 2011. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 43, 491-498. ( 10.1038/ng.806) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Purcell S, et al. 2007. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alexander DH, Novembre J, Lange K. 2009. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 19, 1655-1664. ( 10.1101/gr.094052.109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Danecek P, et al. 2011. The variant call format and VCFtools. Bioinformatics 27, 2156-2158. ( 10.1093/bioinformatics/btr330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Narasimhan V, Danecek P, Scally A, Xue Y, Tyler-Smith C, Durbin R. 2016. BCFtools/RoH: a hidden Markov model approach for detecting autozygosity from next-generation sequencing data. Bioinformatics 32, 1749-1751. ( 10.1093/bioinformatics/btw044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.St. John ME, Dunker JC, Richards EJ, Romero S, Martin CH. 2021. Parallel genetic changes underlie integrated craniofacial traits in an adaptive radiation of trophic specialist pupfishes. bioRxiv 2021.07.01.450661. ( 10.1101/2021.07.01.450661) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cingolani P, Platts A, Wang LL, Coon M, Nguyen T, Wang L, Land SJ, Lu X, Ruden DM. 2012. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff. Fly (Austin) 6, 80-92. ( 10.4161/fly.19695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Robinson JA, Ortega-Del Vecchyo D, Fan Z, Kim BY, vonHoldt BM, Marsden CD, Lohmueller KE, Wayne RK. 2016. Genomic flatlining in the endangered island fox. Curr. Biol. 26, 1183-1189. ( 10.1016/j.cub.2016.02.062) [DOI] [PubMed] [Google Scholar]
- 92.de Valles-Ibáñez G, Hernandez-Rodriguez J, Prado-Martinez J, Luisi P, Marquès-Bonet T, Casals F. 2016. Genetic load of loss-of-function polymorphic variants in great apes. Genome Biol. Evol. 8, 871-877. ( 10.1093/gbe/evw040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.R Core Team. 2021. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See https://www.R-project.org/. [Google Scholar]
- 94.Rausch T, Zichner T, Schlattl A, Stütz AM, Benes V, Korbel JO. 2012. DELLY: structural variant discovery by integrated paired-end and split-read analysis. Bioinformatics 28, i333-i339. ( 10.1093/bioinformatics/bts378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol. 215, 403-410. ( 10.1016/S0022-2836(05)80360-2) [DOI] [PubMed] [Google Scholar]
- 96.Howe KL, et al. 2021. Ensembl 2021. Nucleic Acids Res. 49, D884-D891. ( 10.1093/nar/gkaa942) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tian D, Patton AH, Turner BJ, Martin CH. 2022. Severe inbreeding, increased mutation load and gene loss-of-function in the critically endangered Devils Hole pupfish. Dryad Digital Repository. ( 10.6078/D1ND9Q) [DOI] [PMC free article] [PubMed]
- 98.Tian D, Patton AH, Turner BJ, Martin CH. 2022. Severe inbreeding, increased mutation load, and gene loss-of-function in the critically endangered Devil's Hole pupfish. Figshare. ( 10.6084/m9.figshare.c.6260113) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Tian D, Patton AH, Turner BJ, Martin CH. 2022. Severe inbreeding, increased mutation load and gene loss-of-function in the critically endangered Devils Hole pupfish. Dryad Digital Repository. ( 10.6078/D1ND9Q) [DOI] [PMC free article] [PubMed]
- Tian D, Patton AH, Turner BJ, Martin CH. 2022. Severe inbreeding, increased mutation load, and gene loss-of-function in the critically endangered Devil's Hole pupfish. Figshare. ( 10.6084/m9.figshare.c.6260113) [DOI] [PMC free article] [PubMed]
Data Availability Statement
All raw genomic data are archived at the National Center for Biotechnology Information BioProject Database (Accession: PRJNA887195). Genotypic data and population census data are available through the Dryad Digital Repository: https://doi.org/10.6078/D1ND9Q [97]. Scripts are available at https://github.com/tiandavid/Cyprindon_WGS_project/.
The data are provided in electronic supplementary material [98].