Abstract
Endocrine mucin-producing sweat gland carcinoma is a low-grade eyelid tumor. Small biopsies and insensitive immunohistochemistry predispose to misdiagnosis. We aimed to identify clarifying immunohistochemical markers, molecular markers, or both.
Clinicopathologic data (22 cases) were reviewed. Immunohistochemistry (insulinoma-associated protein 1, BCL-2, mucin 2 [MUC2], mucin 4, androgen receptor, β-catenin, and Merkel cell polyomavirus) and next-generation sequencing (Memorial Sloan Kettering integrated mutation profiling of actionable cancer targets, 468 genes) were performed (3 cases).
Female patients (n = 15) and male patients (n = 7) (mean age 71.8 years; range 53–88 years) had eyelid or periorbital tumors (>90%) with mucin-containing solid or cystic neuroendocrine pathology. Immunohistochemistry (insulinoma-associated protein 1, BCL2, androgen receptor, retinoblastoma-associated protein 1, and β-catenin) was diffusely positive (5/5), MUC2 partial, mucin 4 focal, and Merkel cell polyomavirus negative. Memorial Sloan Kettering integrated mutation profiling of actionable cancer targets identified 12 single-nucleotide variants and 1 in-frame deletion in 3 cases, each with DNA damage response or repair (BRD4, PPP4R2, and RTEL1) and tumor-suppressor pathway (BRD4, TP53, TSC1, and LATS2) mutations. Microsatellite instability, copy number alterations, and structural alterations were absent.
Insulinoma-associated protein 1 and MUC2 are positive in endocrine mucin-producing sweat gland carcinoma. MUC2 positivity suggests conjunctival origin. Multistep pathogenesis involving DNA damage repair and tumor-suppressor pathways may be implicated.
INTRODUCTION
Endocrine mucin-producing sweat gland carcinoma (EMPSGC) is a low-grade neuroendocrine glandular tumor of the eyelid presenting predominantly in postmenopausal women in the sixth and seventh decades of life and occasionally associated with invasive mucinous carcinoma.1–4
The clinical presentation is nonspecific. Skin lesions can mimic inflammatory or neoplastic processes affecting the eyelid and periorbital region.5,6 If the biopsy encompasses the tumor, the basaloid-cribriform pathology is pathognomonic for diagnosis, clinched with immunohistochemical confirmation of neuroendocrine differentiation. Difficulties arise with superficial biopsies showing cystic fragments or basaloid tumor cell proliferations without revealing diagnostic architecture, cytology, and combination of intratumoral and peritumoral mucinous stroma typical of EMPSGC.7,8 Molecular data on EMPSGC are scant. The pathogenesis is not understood.
Although the greatest challenges in the diagnosis and treatment of EMPSGC are performing an adequately large biopsy and recognizing the characteristic pathologic features in nonoptimal biopsies, information about etiopathogenesis, risk factors for development, and relationships with other tumors is incomplete. We studied EMPSGC cases observed at our cancer center to better elucidate sensitive and specific markers for diagnosis in incomplete biopsies, and to identify molecular and clinical features that would contextualize these tumors within current concepts of tumor biology.
METHODS
Case selection
We performed an institutional review board—approved search of pathology department archives for diagnoses of EMPSGC from 2000 to 2018. Of 32 possible EMPSGCs, 22 cases met inclusion criteria, including confirmation of labeling with neuroendocrine markers (synaptophysin, chromogranin, or both). Excluded cases included 2 scalp and chest lesions with unresolved differential diagnoses of metastatic tumor, 1 lip tumor of possible myoepithelial origin, and 7 cases with inadequate immunohistochemistry.
Clinicopathologic analysis
Clinical charts and pathologic data for 22 cases and lesional photographs for 5 patients were reviewed. Hematoxylin and eosin and immunohistochemically stained sections were rereviewed for diagnosis and for invasive mucinous carcinoma in 11 available cases. The remaining pathology data were culled from patient charts.
Immunohistochemistry
Tissue blocks were available in 5 cases. Immunohistochemical stains were performed, including insulinoma-associated protein 1 (INSM1) (A-8, 1:250; Santa Cruz), mucin 2 (MUC2) (MRQ18, prediluted; Cell Marque), mucin 4 (8G7, 1:2000; Santa Cruz), retinoblastoma-associated protein 1 (13A10, 1:50; Leica), Merkel cell polyomavirus (CM2B4, 1:150; Santa Cruz), BCL2 (124, prediluted; Ventana), and androgen receptor (SP107, 1:250; Spring Bioscience). Labeling was assessed as nuclear, cytoplasmic or membranous, and diffuse or focal. Staining intensity was graded on a scale of 0 to 4.
Molecular analysis
Memorial Sloan Kettering integrated mutation profiling of actionable cancer targets is a next-generation sequencing—based clinical assay that interrogates all coding exons of 468 genes and select introns for genomic alterations.9 FFPE samples from 3 patients were sequenced with Memorial Sloan Kettering integrated mutation profiling of actionable cancer targets without matched normals. Target coverage ranged from 272 to 865× with a mean of 536×. Presumed somatic mutations were identified with pooled normal and further filtered based on presence of a reference single nucleotide polymorphism cluster identification or maf greater than or equal to 2% in 1000 Genomes or gnomAD.10 Coverage-based copy number analysis and structural variant analysis using DELLY version 0.7.5 were followed by manual review of all calls.11 Presumptive somatic mutations were referenced to the Catalogue of Somatic Mutations in Cancer database.12 Functional analysis through hidden Markov models scores13 were analyzed to determine potential functional consequences of mutations.
RESULTS
Clinical data
Twenty-two patients, 15 women and 7 men, ranged in age from 53 to 88 years (mean 71.8 years; median 70 years) at presentation. Women were older (55–88 years; mean 77.8 years; median 70 years) versus men (53–87 years; mean 69 years; median 66 years). Tumor sites included the eyelid (17 cases; 7 upper, 8 lower, and 2 unspecified) (Fig 1, A), with 1 case each on the eyebrow, cheek, temple, “face,” and tragus (Fig 1, B). Five patients had follow-up ranging from 30 to 517 weeks (mean 173 weeks; median 73 weeks), with no recurrences after presentation to Memorial Sloan Kettering Cancer Center. For 10 patients, each identifying as White/Caucasian, additional data including body mass index, medical history, and personal and family cancer history were assessed (Table I).
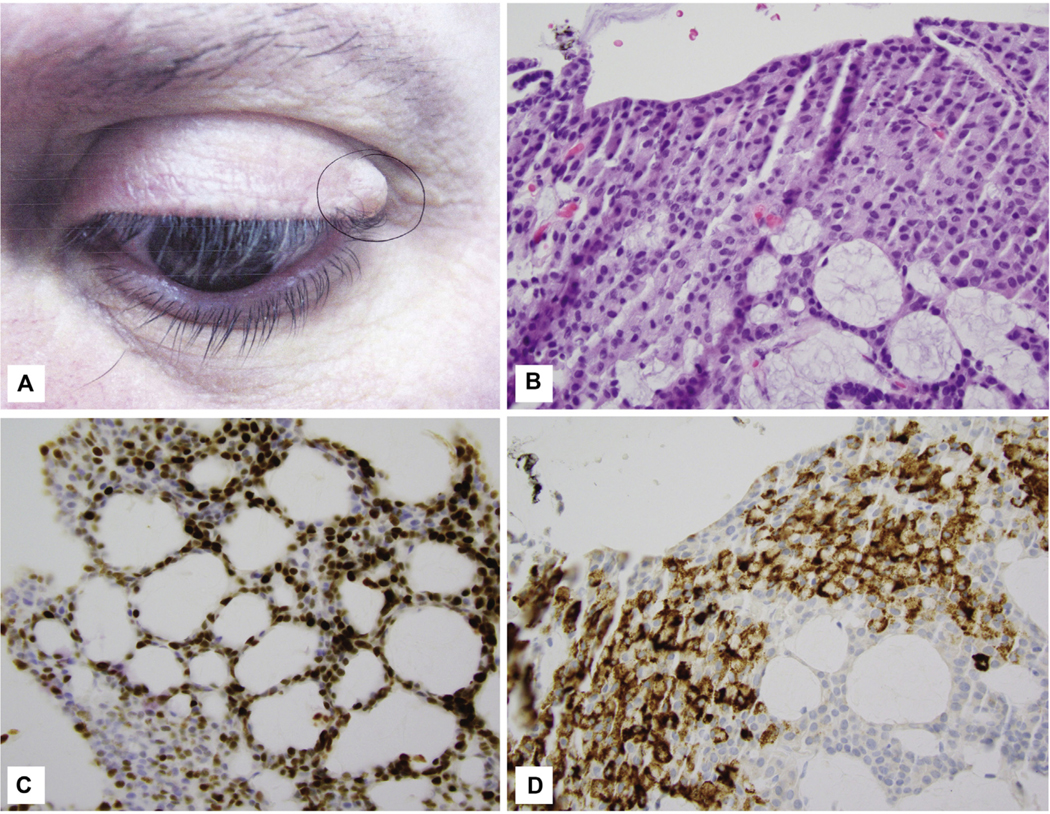
Fig 1.
A, Endocrine mucin-producing sweat gland carcinoma. Classic tumor of the left upper eyelid presenting as a flesh-colored cystic swelling. B, Endocrine mucin-producing sweat gland carcinoma. Mucinous cells in cribriform neoplasm. C, Endocrine mucin-producing sweat gland carcinoma. Most tumor cell nuclei are labeled with 4+ intensity. D, Endocrine mucin-producing sweat gland carcinoma. MUC2 labels the mucin-filled cells. (B, Hematoxylin-eosin stain; original magnification: ×400. C, Insulinoma-associated protein 1 stain; original magnification: ×400. D, MUC2 stain; original magnification: ×400.)
Table I.
Clinical data including family history
| Age, years | Sex | Site | BMI | Medical history (nonsurgical) | Cancer history | Family cancer history | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 | 83 | F | L tragus | CAD with stents, HTN, DM, appendicitis | Colon CA, endometrial CA, BCC, sebaceous adenoma | Mother uterine CA, family “extensive CA” | |
| 2 | 64 | F | R upper eyelid | 25 | Kidney stones osteoarthritis, hyperlipidemia, TMJ, hypothyroidism | BCC, SCC | Mother, brother NMSCA; maternal grandmother colon and rectal CA |
| 3 | 88 | F | L cheek (malar) | HTN, DM, hypothyroidism | Breast CA | ||
| 4 | 57 | F | R upper eyelid | 33 | Asthma, pancreatitis, neuropathy | Breast CA, “thyroid nodule” | Father esophageal CA, mother NHL and bladder CA, aunt breast CA, uncle colon CA |
| 5 | 83 | M | R lower eyelid | 38.6 | DM, HL, HTN, CAD with stent, cholecystitis | NMSCA | |
| 6 | 87 | F | L upper eyelid | 27 | TIA, arthritis | Colon CA | Mother, pancreas CA; sister, unknown type CA |
| 7 | 59 | M | L upper eyelid | 28.4 | HTN, hypothyroidism | Father, CA “multiple CA in family members” | |
| 8 | 64 | F | R lower eyelid | 48.4 | Obesity, hypothyroidism, HTN, heart murmur, cholecystitis |
Endometrial CA | Father, prostate CA |
| 9 | 87 | M | R lower eyelid | HTN, DM | |||
| 10 | 70 | F | R lower eyelid | DM, HTN, CAD with cardiac stent | |||
Mean BMI was 32.7, median 30. BMI 25 to 30 is considered overweight and BMI greater than 30 obese.14
BCC, Basal cell carcinoma; BMI, body mass index; CA, cancer; CAD, coronary artery disease; DM, diabetes mellitus; F, female; HL, hyperlipidemia; HTN, hypertension; L, left; M, male; NHL, non-Hodgkin lymphoma; NMSCA, nonmelanoma skin cancer; R, right; SCC, squamous cell carcinoma; TIA, transient ischemic attack; TMJ, temporomandibular joint.
Pathologic data
Histology was rereviewed for 10 cases. Specimens exhibited tumor islands within dermis or adipose tissue and skeletal muscle, sparing epidermis and hair follicles. Tumors were circumscribed by pink sclerotic stroma or abruptly abutted nonsclerotic dermis. Most showed a cribriform or rosetting pattern. Two solid and cystic cases showed cribriform areas focally expanding the 2-cell-thick lining of a cyst wall (Supplemental Fig 1, C and D, available via Mendeley at https://data.mendeley.com/datasets/4ctynsfk79/1). Cells were bland and round to oval, with moderate eosinophilic to amphophilic cytoplasm, finely stippled nuclear chromatin, and inconspicuous nucleoli. A second smaller population of cells showed variably more abundant illdefined, pale cytoplasm with intracellular mucin, increased in mucin-rich tumor islands (Supplemental Fig 1, C and D). Pools of extracellular mucin were present in 2 cases, each with “floating” tumor islands. Adjacent changes included a dilatated apocrine/eccrine duct (n = 1), lymphocytic folliculitis (n = 5), and follicular mucinosis (n = 1). Sparse inflammation included focal bandlike lymphocytic infiltrates (n = 3) or minimal intratumoral lymphocytic exocytosis (n = 4).
Immunohistochemistry
All 22 cases had immunohistochemical positivity documented or reidentified for synaptophysin, chromogranin, or both. Five cases had 1 of the 2 markers performed. Two cases had both performed but 1 of either was negative. Seven cases had only focal staining of 1 or both neuroendocrine markers. Estrogen receptor, progesterone receptor, and cytokeratin 7 were strongly and diffusely positive in all cases.
Immunohistochemistry was performed on 5 cases for INSM1, androgen receptor, BCL2, MUC2, mucin 4, retinoblastoma, β-catenin, and Merkel cell polyomavirus (Supplemental Table I). INSM1 showed diffuse 4+ labeling for all tumors, including 3 cases with only focal or negative labeling by other neuroendocrine markers (Supplemental Fig 1, A and B). MUC2 cytoplasmic and membranous labeling was prominent (4+ intensity) in medium/large clusters of tumor cells, and increased in mucinous areas (Supplemental Fig 1, C and D). Mucin 4 showed 2+ to 3+ intensity of scattered cells at the periphery of tumor islands. Androgen receptor and BCL2 were diffusely 4+ positive in each of 5 cases (Supplemental Fig 1, E). Retinoblastoma-associated protein 1 nuclear positivity was retained and β-catenin showed wild-type membranous staining (Supplemental Fig 1, F). Merkel cell polyomavirus was negative.
Genetic analysis
Three tumors were sequenced. On manual review of integrated mutation profiling of actionable cancer targets data, they showed in total 13 presumed somatic alterations, (3, 3, and 7 per sample) (Supplemental Table II), including 12 single-nucleotide variants (11 missense and 1 nonsense) and 1 in-frame deletion. One of 12 single nucleotide variants was a C-T substitution (0.08%), and 5 were G-A substitutions. Three mutations were referenced in the Catalogue of Somatic Mutations in Cancer. No genes showed greater than 1 mutation or were altered in greater than 1 sample.
Each sample harbored mutations in at least 1 gene implicated in DNA damage response or repair (BRD4, PPP4R2, and RTEL1), regulation of transcription/posttranscriptional processing (BRD4, RBM10, ZFHX3, and SMYD3), and in a tumor suppressor pathway (BRD4, TP53, TSC1, and LATS2). Two cases had known oncogenic or predicted high functional impact mutations.
DISCUSSION
In our molecular analysis using next-generation sequencing of 468 tumor-related genes9 to examine 3 EMPSGC cases, we found potential oncogenic mutations involving both DNA damage response or repair and tumor suppressor genes in each case. Specifically, case 1 harbored a BRD4 pP978_p980del, an in-frame deletion of bromodomain-containing protein 4, a chromatin-reader protein with a role as chromatin insulator in DNA damage response pathways (isoform B) and as a regulator of TP53-mediated transcription, previously reported in 1 metastatic adenoid cystic carcinoma of salivary gland.15 Suspect missense mutations in case 2 included PPP4R-2 T339A encoding serine/threonine-protein phosphatase 4 regulatory subunit 2 protein, involved in DNA double-strand break repair, and tumor suppressors TP53 G356R (novel) and TSC1 G1035C, reported previously in 4 adenoid cystic carcinomas.16–18 Case 3 had mutations of RTEL1 R856K encoding regulator of telomer elongation helicase I involved in DNA repair/genomic stability, and of LATS2 pN656S, encoding an intracellular kinase in the HIPPO tumor suppressor signaling pathway.
Among models of cancer evolution, a process of sequential, cumulative mutations in rapidly proliferating cells, culminating in unregulated growth, remains favored for many tumors.19 An inflammatory milieu in which DNA damage responses are impaired (eg, by methylation) to silence DNA damage repair proteins increases the probability that mutations favoring cellular proliferation are transcribed. Although mutations that unequivocally impair DNA damage responses are well characterized in mismatch repair syndromes such as Lynch syndrome (MSH2, MSH6, MLH1, and PSM2),20,21 other proteins involved in DNA damage control not linked to hereditary syndromes may occur sporadically, increasing the likelihood of replicative errors leading to mutations in tumor suppressor pathways. The cases in this study did not exhibit evidence of microsatellite instability, defined familial cancer history, or syndromic mutations in mismatch repair genes. However, distinct mutations in DNA repair and tumor suppressor pathways were present in all 3 EMPSGCs, occurring in older individuals with a risk for cumulative events, suggesting this sequence of replicative error and tumor suppressor pathway alteration as a route of tumorigenesis. One prior molecular analysis of EMPSGC showing a deletion on chromosome 6 from 6p11.2 to 6q16.1 lends tangential support to this idea.22 The authors noted possible affected tumor suppressor genes in the deleted region to include MAP3K7, SnRNA U50, and EPHA7. Deletions of 6p11 have been reported in 4 cases of carcinoid tumors23 that bear similarity to EMPSGC.
We found altered genes in our patients’ tumors that have been reported to be mutated at different sites in mucinous colorectal and gastric carcinomas (PLCG2 in case 1; IDH1, PREX2, RBM10, ZFHX3, and TSC1 in case 2; and LATS2 in case 3). Prior attempts to link EMPSGC to mucinous neoplasms of other sites on a molecular basis have failed; mutations in KRAS, GNAS, or EGFR genes commonly observed in those cases were not identified.24,25 Other molecular analysis results of EMPSGC were negative. Qin et al22 pyrosequenced 3 EMPSGC and 3 mucinous carcinomas, demonstrating wild-type BRAF. Cornejo et al26 performed next-generation sequencing on 1 EMPSGC targeting 50 genes with common somatic cancer mutations, but found no mutations, including within PIK3CA or AKT1 genes implicated in the putatively analogous papillary breast cancer. Held et al27 found no MYB-NFIB fusion or MYB amplification in 10 EMPSGCs assayed with fluorescence in situ hybridization. Only on an immunohistochemical level have possible pathogenetic protein alterations been demonstrated, and these in the setting of histologic disease “progression”; Shon and Salomao28 showed that tumor suppressor WTI remains expressed in both EMPSGC and mucinous carcinoma, supporting shared tumor origin. Held et al27 showed loss of MYB in mucinous carcinoma, supporting tumor evolution.
Five of 10 of our clinically examined patients had breast, endometrial, or colorectal carcinoma, and 7 had family histories of uterine, colorectal, breast, bladder, pancreatic, esophageal, or prostatic carcinoma. We considered a familial cancer predisposition; however, retrievable data were limited in detail, available only for a few patients, and difficult to contextualize, given the subgroup median age of 76 years at presentation and lack of identified familial cancer-related genetic alterations. However, a high body mass index (overweight or obese) was noted in 6 of 6 patients queried, suggesting a mechanism for inflammatory or hormonal mediation of EMPSGC.
Immunohistochemical analysis of our cases revealed an additional candidate in the pathogenesis of EMPSGC, INSM1. INSM1 is a transcriptional regulator with a zinc-finger DNA binding domain functional in neuroendocrine differentiation, predominantly expressed in developing mammalian neuroendocrine tissue and nervous system, hypothesized to have a role in neuroendocrine cancer progression.29 In neuroblastoma, INSM1 stabilizes N-MYC, which binds the E2 box region of INSM1 promotor, activating INSM1 expression in a positive-feedback loop, or via pI3K/AkT, cyclin D1, or β-catenin pathways. Strong INSM1 expression may indicate a similar role of this transcriptional regulator in EMPSGC, although membranous β-catenin found in our cases does not support transcriptional activation of Wnt signaling.30
As a diagnostic marker, INSM1 is sensitive and specific for neuroendocrine tumors, including gastrointestinal and pancreaticobiliary tract neuroendocrine neoplasms, small-cell lung cancer, and Merkel cell carcinoma.29 INSM1 is a nuclear stain, improving on interpretability versus the cytoplasmic stains synaptophysin and chromogranin. EMPSGC is defined by immunoreactivity with at least 1 neuroendocrine marker (synaptophysin, chromogranin, or neuron-specific enolase); however, many cases show only focal chromogranin or synaptophysin positivity (35% and 28% of positive cases, respectively).31 In all cases in our study, INSM1 showed strong nuclear reactivity and diffuse staining, suggesting a higher diagnostic sensitivity for EMPSGC.
The preference for EMPSGC for the eyelid is striking, with divergent cell types including the primitive neuroendocrine phenotype dominant within the neoplasm, begging consideration of a “stem cell niche” similar to intestinal crypts, in which multipotent cell differentiation into epithelial, mucous, neuroendocrine, and Paneth cells occurs.32,33 Basal conjunctival stem cells capable of differentiating to mucous or epithelial cells34 or a rapidly cycling transit-amplifying cell33,35 reside in all parts of the conjunctiva, including the invaginated mucous-cell-rich pseudo-glands of Henle, and, as rapidly cycling cells, may be susceptible to oncogenic mutations. A multipotent conjunctival progenitor cell would explain characteristic androgen receptor, estrogen receptor, and progesterone receptor positivity in EMPSGC.17,18,27,33 Estrogen receptor and progesterone receptor, usually exclusively positive in the Meibomian/sebaceous glands in the eyelid region,36 are known mediators of conjunctival goblet cell maturation, causing changes in the postmenopausal context.37
Conjunctival goblet cells are MUC2 positive38,39 and cytokeratin 7 positive,40 similar to EMPSGC, and they may reside in proximity to conjunctival stem cells. MUC2, a secreted/gel-forming mucin, is not expressed within skin adnexal structures, including Moll glands or lacrimal glands around the eyelids.41 Aside from EMPSGC, in cutaneous disease, MUC2 has been described within rare lesions of extramammary Paget disease of the perianal area and associated with rectal carcinoma, consistent with known expression of MUC2 in normal rectal mucosa.42 However, MUC2 is also present within excretory and striated ducts of the salivary gland apparatus and is identified in Warthin tumors and a minority of mucoepidermoid carcinoma and salivary gland adenoid cystic carcinoma.43 Multipotent precursors of MUC2-secreting cells in salivary ducts may explain the occasional occurrence of EMPSGC around the ear or cheek rather than eyelid. In either site, tumors may grow into adjacent nonneoplastic sweat glands, mimicking primary sweat duct neoplasia,2,22,28 rather than being derived from exceedingly rarely reported eyelid eccrine cysts.44
Because biopsies of EMPSGC are often suboptimal owing to location, specific and sensitive markers are critical. This study showed that MUC2 and BCL-2 are sensitive for EMPSGC. Pitfalls include rare adenoid cystic carcinoma (1 case)43 or mucoepidermoid carcinoma (15 reported cases)31 that express MUC2, but not gross cystic disease fluid protein 15, synaptophysin, chromogranin, or estrogen receptor/progesterone receptor. Although a single adenoid cystic carcinoma was reported to label for INSM1 in a study of 19 tumors,45 adenoid cystic carcinoma would also express myoepithelial markers S100, SMA, and calponin.31 INSM1, demonstrated here to be sensitive for EMPSGC, may help to rule out chromogranin/synaptophysin-positive basal cell carcinoma, which should be INSM1 negative.31,46,47 Finally, Merkel cell polyomavirus in our study was negative in all EMPSGCs, supporting its utility in excluding Merkel cell carcinoma.
Limitations of our study include the small case number and preselected next-generation sequencing panel of 468 known cancer-related genes. Mutations occurring with low minor allele frequency could have been filtered out during bioinformatic analysis. Collaborative studies are needed to expand on these findings, and to better evaluate any possible relationship with familial carcinoma that may have bearing on screening recommendations.
In summary, we suggest a multistep molecular pathogenesis of EMPSGC, considering a conjunctival site of origin to explain clinicopathologic characteristics of this rare eyelid-predominant tumor of postmenopausal women. Because this site is challenging to biopsy adequately for all cases, we suggest INSM1 as a helpful ancillary marker and suggest further analysis of INSM1 and MUC2 to distinguish EMPSGC from other regional basaloid/glandular neoplasms.
Supplementary Material
CAPSULE SUMMARY.
This article supports a tumor suppressor pathway alteration role, previously identified in 1 sequenced case, in endocrine mucin-producing sweat gland carcinoma pathogenesis, and adds new DNA damage and repair pathway alteration and novel neuroendocrine marker data.
Insulinoma-associated protein 1 and mucin 2 improve on conventional immunohistochemistry, enabling appropriate treatment for this low-grade, clinically characteristic tumor.
Funding sources:
Supported in part by the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute under award P30CA008748.
Thank you to Bruce Crilly for the assistance in putting together figures for this manuscript.
Abbreviations used:
- EMPSGC
endocrine mucin-producing sweat gland carcinoma
- INSM1
insulinoma-associated protein 1
- MUC2
mucin 2
Footnotes
IRB approval status: Not applicable.
Conflicts of interest
None disclosed.
REFERENCES
- 1.Flieder A, Koerner FC, Pilch BZ, Maluf HM. Endocrine mucin-producing sweat gland carcinoma: a cutaneous neoplasm analogous to solid papillary carcinoma of breast. Am J Surg Pathol. 1997;21(12):1501–1506. [DOI] [PubMed] [Google Scholar]
- 2.Zembowicz A, Garcia CF, Tannous ZS, Mihm MC, Koerner F, Pilch BZ. Endocrine mucin-producing sweat gland carcinoma: twelve new cases suggest that it is a precursor of some invasive mucinous carcinomas. Am J Surg Pathol. 2005;29(10): 1330–1339. [DOI] [PubMed] [Google Scholar]
- 3.Chang S, Shim SH, Joo M, Kim H, Kim YK. A case of endocrine mucin-producing sweat gland carcinoma co-existing with mucinous carcinoma - a case report. Korean J Pathol. 2010; 44(1):97–100. [Google Scholar]
- 4.Salim AA, Karim RZ, McCarthy SW, Scolyer RA. Endocrine mucin producing sweat gland carcinoma: a clinicopathological analysis of three cases. Pathology. 2012;44(6):568–571. [DOI] [PubMed] [Google Scholar]
- 5.Agni M, Raven ML, Bowen RC, et al. An update on endocrine mucin-producing sweat gland carcinoma: clinicopathologic study of 63 cases and comparative analysis. Am J Surg Pathol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meltzer OA, Joseph JM. Delayed treatment of endocrine mucin-producing sweat gland carcinoma initially diagnosed as a chalazion. JAAD Case Rep. 2019;5(9):789–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ansari AN, Bobos M, Shih S, et al. Renal cell carcinoma antigen expression in primary cutaneous endocrine mucinous carcinomas: a case series of 14 patients and review of the literature. Am J Dermatopathol. 2019;41(8):571–577. [DOI] [PubMed] [Google Scholar]
- 8.Flux K, Brenn T. Cutaneous sweat gland carcinomas with basaloid differentiation: an update with emphasis on differential diagnoses. Clin Lab Med. 2017;37(3):587–601. [DOI] [PubMed] [Google Scholar]
- 9.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17(3):251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karczewski KJ, Francioli LC, Tiao G, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581(7809):434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rausch T, Zichner T, Schlattl A, Stutz AM, Benes V, Korbel JO. DELLY: structural variant discovery by integrated paired-end and split-read analysis. Bioinformatics. 2012;28(18):i333–i339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tate JG, Bamford S, Jubb HC, et al. COSMIC: the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2019;47(D1): D941–D947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogers MF, Shihab HA, Mort M, Cooper DN, Gaunt TR, Campbell C. FATHMM-XF: accurate prediction of pathogenic point mutations via extended features. Bioinformatics. 2018; 34(3):511–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carr PR, Amitay EL, Jansen L, et al. Association of BMI and major molecular pathological markers of colorectal cancer in men and women. Am J Clin Nutr. 2020;111(3):562–569. [DOI] [PubMed] [Google Scholar]
- 15.Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23(6):703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA. The cBio Cancer Genomics Portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(10):960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho AS, Ochoa A, Jayakumaran G, et al. Genetic hallmarks of recurrent/metastatic adenoid cystic carcinoma. J Clin Invest. 2019;129(10):4276–4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White MC, Holman DM, Boehm JE, Peipins LA, Grossman M, Henley J. Age and cancer risk a potentially modifiable relationship. Am J Prev Med. 2014;46(3):S7–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138(6):2073–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walsh MD, Buchanan DD, Cummings MC, et al. Lynch syndrome-associated breast cancers: clinicopathologic characteristics of a case series from the Colon Cancer Family Registry. Clin Cancer Res. 2010;16(7):2214–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin H, Moore RF, Ho CY, Eshleman J, Eberhart CG, Cuda J. Endocrine mucin-producing sweat gland carcinoma: a study of 11 cases with molecular analysis. J Cutan Pathol. 2018. [DOI] [PubMed] [Google Scholar]
- 23.http://AtlasGeneticsOncology.org; 2020.
- 24.Li X, Sun K, Liao X, Gao H, Zhu H, Xu R. Colorectal carcinomas with mucinous differentiation are associated with high frequent mutation of KRAS or BRAF mutations, irrespective of quantity of mucinous component. BMC Cancer. 2020;20(1): 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wakejima R, Inamura K, Ninomiya H, et al. Mucinous lung adenocarcinoma, particularly referring to EGFR-mutated mucinous adenocarcinoma. Pathol Int. 2020;70(2):72–83. [DOI] [PubMed] [Google Scholar]
- 26.Cornejo KM, Hutchinson L, Meng X, O’Donnell P, Deng A. Endocrine mucin-producing sweat gland carcinoma of the eyelid: a report of a case with molecular analysis. Am J Dermatopathol. 2016;38(8):636–638. [DOI] [PubMed] [Google Scholar]
- 27.Held L, Ruetten A, Kutzner H, Palmedo G, John R, Mentzel T. Endocrine mucin-producing sweat gland carcinoma: clinicopathologic, immunohistochemical, and molecular analysis of 11 cases with emphasis on MYB immunoexpression. J Cutan Pathol. 2018. [DOI] [PubMed] [Google Scholar]
- 28.Shon W, Salomao DR. WT1 expression in endocrine mucin-producing sweat gland carcinoma: a study of 13 cases. Int J Dermatol. 2014;53(10):1228–1234. [DOI] [PubMed] [Google Scholar]
- 29.Mahalakshmi B, Baskaran R, Shanmugavadivu M, Nguyen NT, Velmurugan BK. Insulinoma-associated protein 1 (INSM1): a potential biomarker and therapeutic target for neuroendocrine tumors. Cell Oncol (Dordr). 2020;43(3): 367–376. [DOI] [PubMed] [Google Scholar]
- 30.Yin X, Farin HF, van Es JH, Clevers H, Langer R, Karp JM. Niche-independent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nat Methods. 2014;11(1):106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Immunoquery. https://www.immunoquery.com/.
- 32.Zeki SS, Graham TA, Wright NA. Stem cells and their implications for colorectal cancer. Nat Rev Gastroenterol Hepatol. 2011;8(2):90–100. [DOI] [PubMed] [Google Scholar]
- 33.Rangel-Huerta E, Maldonado E. Transit-amplifying cells in the fast lane from stem cells towards differentiation. Stem Cells Int. 2017;2017:7602951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pellegrini G, Golisano O, Paterna P, et al. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J Cell Biol. 1999; 145(4):769–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su L, Cui H, Xu C, Xie X, Chen Q, Gao X. Putative rabbit conjunctival epithelial stem/progenitor cells preferentially reside in palpebral conjunctiva. Curr Eye Res. 2011;36(9):797–803. [DOI] [PubMed] [Google Scholar]
- 36.Schroder A, Abrar DB, Hampel U, Schicht M, Paulsen F, Garreis F. In vitro effects of sex hormones in human meibomian gland epithelial cells. Exp Eye Res. 2016;151:190–202. [DOI] [PubMed] [Google Scholar]
- 37.Den S, Shimizu K, Ikeda T, Tsubota K, Shimmura S, Shimazaki J. Association between meibomian gland changes and aging, sex, or tear function. Cornea. 2006;25(6):651–655. [DOI] [PubMed] [Google Scholar]
- 38.McKenzie RW, Jumblatt JE, Jumblatt MM. Quantification of MUC2 and MUC5AC transcripts in human conjunctiva. Invest Ophthalmol Vis Sci. 2000;41(3):703–708. [PubMed] [Google Scholar]
- 39.Gipson IK. Goblet cells of the conjunctiva: a review of recent findings. Prog Retin Eye Res. 2016;54:49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krenzer KL, Freddo TF. Cytokeratin expression in normal human bulbar conjunctiva obtained by impression cytology. Invest Ophthalmol Vis Sci. 1997;38(1):142–152. [PubMed] [Google Scholar]
- 41.Chakraborty S, Bonthu N, Swanson BJ, Batra SK. Role of mucins in the skin during benign and malignant conditions. Cancer Lett. 2011;301(2):127–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuan SF, Montag AG, Hart J, Krausz T, Recant W. Differential expression of mucin genes in mammary and extramammary Paget’s disease. Am J Surg Pathol. 2001;25(12):1469–1477. [DOI] [PubMed] [Google Scholar]
- 43.Mannweiler S, Beham A, Langner C. MUC1 and MUC2 expression in salivary gland tumors and in non-neoplastic salivary gland tissue. APMIS. 2003;111(10):978–984. [DOI] [PubMed] [Google Scholar]
- 44.Jakobiec FA, Qureshi S, Zakka FR, Tu Y, Lee NG. Eyelid eccrine cyst: an exceptional lesion among dominant apocrine cysts. Ophthalmic Plast Reconstr Surg. 2017;33(5):e128–e131. [DOI] [PubMed] [Google Scholar]
- 45.Tsai HK, Hornick JL, Vivero M. INSM1 expression in a subset of thoracic malignancies and small round cell tumors: rare potential pitfalls for small cell carcinoma. Mod Pathol. 2020. [DOI] [PubMed] [Google Scholar]
- 46.Lilo MT, Chen Y, LeBlanc RE. INSM1 is more sensitive and interpretable than conventional immunohistochemical stains used to diagnose Merkel cell carcinoma. Am J Surg Pathol. 2018;42(11):1541–1548. [DOI] [PubMed] [Google Scholar]
- 47.Terada T. Expression of NCAM (CD56), chromogranin A, synaptophysin, c-KIT (CD117) and PDGFRA in normal non-neoplastic skin and basal cell carcinoma: an immunohistochemical study of 66 consecutive cases. Med Oncol. 2013;30(1):444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.