Abstract
Objective:
Different cognitive development histories in schizophrenia may reflect diverse dimensions of genetic influence. Authors derived and characterized cognitive development trajectory subgroups within a schizophrenia sample and profiled the subgroups across polygenic scores (PGSs) for schizophrenia, cognition, educational attainment, and ADHD.
Method:
Demographic, clinical, and genetic data were collected at NIMH for 540 schizophrenia cases, 247 unaffected siblings and 844 controls. Cognitive trajectory subgroups were derived through cluster analysis using estimates of premorbid and current IQ. PGSs were generated using standard methods. Associations were tested using general linear models and logistic regression.
Results:
Cluster analyses identified 3 cognitive trajectory subgroups in the schizophrenia cases: pre-adolescent cognitive impairment (19%), adolescent disruption of cognitive development (44%), and cognitively stable adolescent development (37%). The four PGSs predicted 7.9% of the variance in subgroup membership (ΔX2[8]=43.83, p=6.10E-07). Subgroup characteristics converged with genetics. Cognitively stable individuals had the best adult clinical outcomes and only differed from controls on schizophrenia PGS. Those with adolescent disruption of cognitive development showed the worst symptoms after diagnosis and had the highest schizophrenia PGS and disadvantageous cognitive PGS. Individuals showing pre-adolescent impairment in cognitive and academic performance and poor adult outcome exhibited a generalized PGS disadvantage relative to controls and were the only subgroup to differ significantly on education and ADHD PGSs.
Conclusions and Relevance:
Subgroups derived based on patterns of premorbid and current IQ showed different premorbid and clinical characteristics, which converged with broad genetic profiles. Simultaneous analysis of multiple PGSs may contribute to clinical stratification in schizophrenia.
INTRODUCTION
Heterogeneity in schizophrenia and other psychotic disorders is a major challenge for understanding relevant biology and developing new treatments. Differing trajectories of development prior to illness onset are an important dimension of this heterogeneity(1), reflecting poorly understood genetic and environmental influences(2,3), and yet predicting some of the clinical, functional, and biological characteristics of affected adults(4,5). Three developmental trajectory subtypes are frequently described: one including those with lifelong history of impaired social, behavioral, and/or intellectual functioning and an insidious progression toward psychotic illness; a second encompassing individuals with a benign developmental course through adolescence followed by a relatively abrupt onset of psychotic symptoms; and a third including individuals who experience a prominent prodromal phase, beginning and progressing during adolescence before transitioning into psychosis. These distinctions have been linked to important course, symptom and outcome variables(6,7).
Cognitive ability has often served as a developmental marker in psychotic disorders. Abundant research demonstrates histories of academic difficulties, attention disorders, and cognitive testing differences in many children and adolescents who will later develop schizophrenia(8–10), as well as relatively stable cognitive performance following diagnosis(11). A strategy for using data from adult patients to identify distinct trajectories of cognitive development takes advantage of well-studied patterns on two estimates of intellectual ability: irregular word reading (e.g., the Wide Range Achievement Test [WRAT] reading subtest(12)); and full scale IQ (e.g., the Wechsler [WAIS] batteries(13)). These measures are generally commensurate in healthy adults(14). However, many people with schizophrenia diverge from the typical pattern, performing at near normal levels on word reading, while showing marked impairment on full scale IQ. This pattern is thought to reflect the maturation and crystallization of word reading skill in advance of the typical time frame for the onset of acute psychotic symptomatology – in contrast with the continuing developmental sensitivity of the skills underlying full scale IQ – and has prompted the use of word reading scores from after diagnosis as proxies for “premorbid IQ”.(14,15)
Variation in developmental history has been a target for data-driven subgrouping methods, such as cluster analysis, which parse heterogeneous psychotic disorders samples into subgroups that may be more biologically and behaviorally distinct and treatment-relevant(6,16). In 117 schizophrenia cases, Weickert et al.(17) used cluster analysis to show that current (WAIS) and premorbid (WRAT) IQ performance patterns distinguished three subgroups: one with low premorbid and current IQ suggestive of pre-adolescent cognitive development issues; one with high premorbid and current IQ indicating a more stable course of cognitive development; and one with high premorbid IQ but low current IQ, highlighting disruptions to cognitive development during adolescence, in particular. These subgroups parallel the broad developmental trajectory subtypes described earlier, reflecting distinct trajectories of cognitive development in schizophrenia (depicted schematically in Figure 1A). In studies following Weickert et al., the premorbid/current IQ subgrouping strategy has proven replicable and shown associations with clinical course, symptom profiles, and functional outcomes(18–21). Regarding biological substrates, recent studies have reported subgroup differences in intracranial and total brain volume(22,23). No studies have examined the association of premorbid/current IQ subgroups with genome-wide polygenic scores (PGSs), which aggregate the effects of thousands of common genetic markers on particular phenotypes(24,25).
Figure 1: Schematic of cognitive trajectory subtypes and results of premorbid/current IQ cluster analyses in 540 people with schizophrenia.
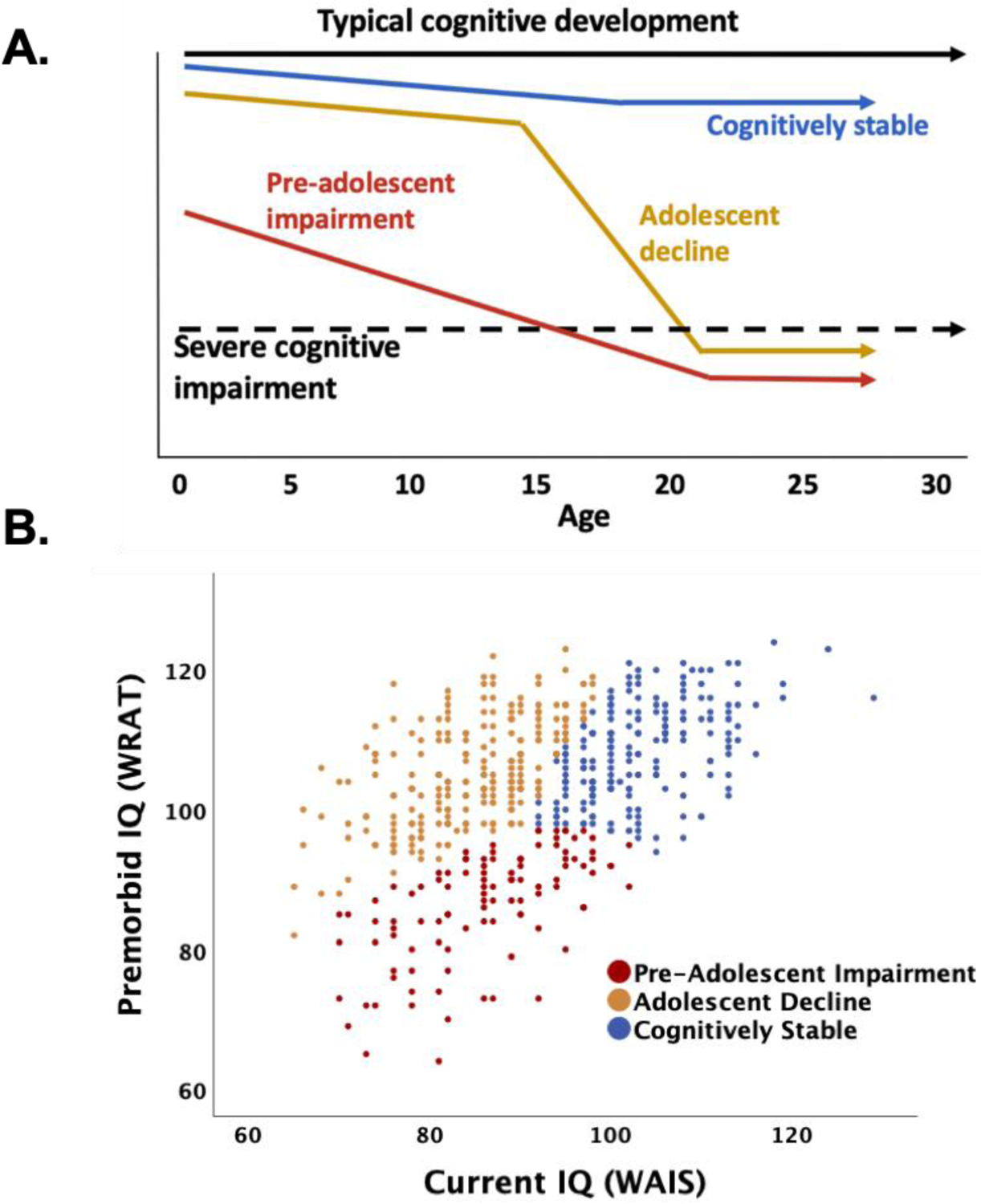
The schematic in Panel A depicts three commonly described trajectories of cognitive development up to and through schizophrenia diagnosis: one (red) with evidence of early cognitive impairment suggesting pre-adolescent developmental issues; one (blue) showing a more stable course of cognitive development through adolescence despite emerging psychosis; and one (gold) highlighting the adolescent time frame as a period of disrupted cognitive development. The scatterplot in Panel B shows the clustering of 540 schizophrenia cases on the basis of premorbid (WRAT, y-axis) and current IQ (WAIS, x-axis) into subgroups that align with the three cognitive trajectory subtypes depicted in Panel A and with results from earlier studies(17): one (red) with evidence of early life cognitive impairment (i.e., low WRAT or premorbid IQ), as well as evidence of ongoing cognitive impairment in adulthood (i.e., low WAIS or current IQ); one (blue) with relatively better early life cognitive performance (i.e., higher WRAT) and continued better performance in adulthood (i.e., higher WAIS); and one (gold) with evidence of relatively good premorbid cognitive performance (i.e., high WRAT), but accompanied in this subgroup by substantially impaired adult performance (i.e., low WAIS).
Our main goals were to derive and characterize IQ-based subgroups in a large schizophrenia sample, testing the proposition that these subgroups reflect three distinct trajectories of cognitive development, and to show contrasting profiles of genetic influence – represented here by four PGSs – across the subgroups. Different PGS can be derived simultaneously in a given sample using results from various genome-wide association studies (GWAS), thus allowing the construction of profiles of influence across multiple genetic dimensions(25). Given that our subgroups were defined by schizophrenia diagnosis and IQ performance patterns intended to reflect different trajectories of early cognitive development, we expected differences in profiles of PGSs for schizophrenia, cognition, educational attainment, and attention-deficit hyperactivity disorder (ADHD) that converged with our IQ-based subgrouping. We reasoned that symptom and outcome differences that have been reported in earlier schizophrenia cognitive subgroups studies(18–21) might reflect, in part, differences in underlying schizophrenia genetics. We reasoned further that PGS for ADHD, cognition, and education would covary with current and premorbid IQ.
METHODS
Participants
Our sample consisted of 746 individuals 18–60 years of age with DSM-IV schizophrenia disorders (540 genotyped), studied at the NIMH Clinical Center between 1996 and 2016. Each subject was diagnosed by consensus between clinician evaluators using the Structured Clinical Interview for DSM-IV Axis I Disorders(26) and available medical records. Schizophrenia participants were stably treated. Full siblings with no psychotic disorder history (N=370; limited to one per family; 247 genotyped) and community controls (N=1525; 844 genotyped) served as comparison samples. Participants from all samples were excluded if they had recent or extended past substance abuse history, serious medical, neurological, or neurodevelopmental conditions, or if there was a current learning disorder diagnosis (including dyslexia) or estimated WAIS IQ below 65. All participants gave written informed consent consistent with NIH IRB guidelines.
Assessment procedure
During a two-day assessment, participants provided demographic information, academic history and challenges (e.g., reading or attention difficulties), vocational history, and blood samples for genotyping. They completed a comprehensive neuropsychological battery that yielded a composite index of general cognitive ability(27)(details in Supplementary Methods). Main analyses focused on a four-subtest estimate of current WAIS IQ(28) and, to index premorbid IQ, the irregular word-reading test from the WRAT(12). The same clinicians who conducted SCID interviews rated participants on the Positive and Negative Syndrome Scale (PANSS)(29), from which composite scores for negative, positive and concrete/disorganized symptoms were derived(30).
Cluster analysis
Our hypothesis was that the three premorbid/current IQ schizophrenia subgroups identified in earlier studies would emerge from cluster analyses of WRAT and WAIS IQ. To test this, WRAT and WAIS performance data were analyzed for the full schizophrenia sample using the TwoStep Cluster Analysis procedure in SPSS, version 24 (SPSS, Armonk, NY: IBM Corp.; details in Supplementary Methods)(31,32). To reduce collinearity between the two indicators (r=.50 in schizophrenia sample), we used their average ([WRAT + WAIS]/2) and difference (WRAT – WAIS) (r=−.05) as input variables. Unsupervised clustering was performed 1000 times, with random re-orderings, to determine the optimal number of clusters. Results supported a three-cluster solution. Fifty additional analyses, each specifying three clusters, were used to determine the assignment of individuals to subgroups. General linear model (GLM), chi-square, logistic regression and Fisher’s LSD analyses were used to compare groups and subgroups on demographic and clinical variables, controlling for age, sex, and race.
Genotyping and PGS calculation and analysis
Genotypes were determined with Illumina Bead Chips (510K-2.5M SNP chips) (quality control and other genotyping details in Supplementary Methods). The first 10 principal components (PCs) of the whole genome data (PLINK version 1.90, https://www.cog-genomics.org/plink/1.9) were derived for use as population stratification covariates. To assess broad differences in subgroup genetics, we used GWAS summary statistics to construct four sets of PGSs in our sample. Schizophrenia PGS were based on statistics from the 2014 Psychiatric Genomics Consortium schizophrenia GWAS meta-analysis(33) (36,573 schizophrenia cases, after excluding the present sample). Three other PGS sets were based on more recent GWAS meta-analyses that did not include our sample (cognition based on 78,308 individuals (34), educational attainment based on 1.1 million individuals(35), and ADHD based on 20,183 individuals(36)). In order to match allele frequency variation in the discovery GWAS samples, PGS analyses in the current sample were limited to participants who clustered with HapMap3 CEU and TSI populations (i.e., Caucasians of European descent). After the ancestry restriction, we calculated schizophrenia, cognitive, educational attainment, and ADHD PGS for 540 people with schizophrenia, 247 of their unaffected full siblings, and 844 controls. We derived each of the four PGS at 10 p-value thresholds (ranging from PT<5×10E-08 to PT<1.0)(33). To concentrate the polygenic signal, the 10 scores were reduced to a single score through principal components analysis(37) (details in Supplementary Methods and Results).
The cognitive development trajectory subgroup assignment for each of the 247 unaffected siblings was carried over from his or her affected sibling, yielding parallel unaffected sibling subgroups. Controls were not assigned to subgroups. Pearson correlations characterized the bivariate associations between PGSs. All group-wise PGS analyses controlled for age, sex and 10 ancestry-based, genomic principal components. Multinomial logistic regression tested whether the four PGSs (entered together) predicted cognitive trajectory subgroup membership based on the chi-square difference between the full model and a covariates-only model without the PGSs. Effect size was estimated as the difference in Nagelkerke R2 estimates for the two models. Separate GLM analyses tested whether PGSs for schizophrenia, cognition, education, and ADHD differed by subgroup, with partial eta2 as the effect size metric. Fisher’s LSD analyses were used for pairwise comparisons of PGSs across diagnostic groups and across schizophrenia subgroups.
RESULTS
Overall Sample Characteristics
Table 1 (top) summarizes the characteristics of the 540 schizophrenia cases, 247 unaffected siblings, and 844 community controls included in the genetics analyses after ancestry restrictions. Mean age was in the lower 30’s for all groups. Relative to siblings and controls, schizophrenia cases were more likely to be male, showed cognitive impairments exceeding 1.0SD on average, and had markedly worse educational performance, employment, and global functioning. As a group, schizophrenia cases were chronic (mean illness duration 12.3 years, SD=9.5), with moderately severe symptomatology (e.g., PANSS Total mean=60.3, SD=20.9).
Table 1:
Descriptive statistics for PGS analysis samples, by diagnostic group (top) and schizophrenia subgroup (bottom).
| Diagnostic Group | Schizophrenia Cases (n=540) | Unaffected Siblings (n=247) | Community Controls (n=844) | Statistic | df | P-value | Effect Size | Pairwise | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean/N | SD/% | Mean/N | SD/% | Mean/N | SD/% | ||||||
| Demographics: | |||||||||||
| Age | 34.1 | 10.1 | 35.2 | 10.2 | 31.1 | 9.7 | F=25.0 | 2, 1627 | 2.09E-11 | 0.03 | SC=US>CC |
| Male | 407 | 75.4% | 117 | 47.4% | 390 | 46.2% | X2=114.4 | 2 | 7.11E-25 | 0.091 | SC>US=CC |
| Caucasian | 540 | 100.0% | 247 | 100.0% | 844 | 100.0% | na | na | na | - | - |
| Family SES | 52.9 | 11.8 | 53.4 | 12.2 | 51.6 | 11.9 | F=3.7 | 2, 1095 | 2.60E-02 | 0.007 | SC=US>CC |
| Functioning: | |||||||||||
| Education Years | 14.1 | 2.1 | 15.9 | 2.5 | 16.6 | 2.4 | F=211.6 | 2, 1616 | 2.44E-82 | 0.208 | SC<US<CC |
| Global Functioning | 45.2 | 14.1 | 85.3 | 6.4 | 87.8 | 3.9 | F=3519.7 | 2, 1572 | <.0001 | 0.817 | SC<US<CC |
| Learning Difficulties | 166 | 30.7% | 25 | 10.1% | 160 | 19.0% | X2=26.3 | 2 | 2.94E-07 | 0.028 | SC>CC>US |
| Current Employment | 165 | 30.5% | 209 | 84.5% | 533 | 79.5% | X2=305.5 | 2 | 2.05E-69 | 0.261 | SC<US=CC |
| Cognition: | |||||||||||
| WAIS Full Scale IQ | 92.0 | 11.4 | 106.4 | 10.8 | 109.3 | 9.2 | F=517.1 | 2, 1607 | 4.12E-174 | 0.392 | SC<US<CC |
| WRAT Reading | 102.9 | 10.9 | 106.2 | 10.5 | 109.4 | 8.4 | F=81.6 | 2, 1614 | 1.65E-34 | 0.092 | SC<US<CC |
| General Cognitiion* | −1.0 | 0.7 | −0.1 | 0.5 | 0.13 | 0.4 | F=676.4 | 2, 1565 | 1.96E-212 | 0.464 | SC<US<CC |
| Polygenic Scores: | |||||||||||
| Schizophrenia | 0.41 | 0.9 | −0.01 | 0.9 | −0.30 | 1.0 | F=89.6 | 2, 1616 | 1.23E-37 | 0.1 | SC>US>CC |
| Cognition | −0.04 | 1.0 | −0.15 | 1.0 | 0.13 | 1.0 | F=11.5 | 2, 1616 | 1.10E-05 | 0.014 | SC=US<CC |
| Education | 0.02 | 1.0 | −0.1 | 1.0 | 0.06 | 1.0 | F=4.2 | 2, 1616 | 0.016 | 0.005 | US<SC=CC |
| ADHD | 0.05 | 1.0 | 0.04 | 1.0 | −0.04 | 1.0 | F=2.1 | 2, 1616 | ns | - | - |
| Schizophrenia Subgroup | Pre-Adolescent Impairment (n=105) | Adolescent Decline (n=237) | Cognitively Stable (n=198) | Statistic | df | P-value | Effect Size | Pairwise | |||
| Mean/N | SD/% | Mean/N | SD/% | Mean/N | SD/% | ||||||
| Demographics: | |||||||||||
| Age | 32.4 | 8.4 | 32.3 | 9.9 | 37.1 | 10.6 | F=13.9 | 2, 536 | 1.00E-06 | 0.049 | PI=AD<CS |
| Male | 76 | 72.4% | 187 | 78.9% | 144 | 72.7% | X2=2.1 | 2 | ns | - | - |
| Caucasian | 105 | 100.0% | 237 | 100.0% | 198 | 100.0% | na | na | na | - | - |
| Family SES | 49.2 | 10.6 | 52.4 | 12.6 | 55.2 | 10.6 | F=7.4 | 2, 362 | 7.16E-04 | 0.039 | PI<AD<CS |
| Functioning: | |||||||||||
| Education Years | 13.3 | 1.9 | 13.8 | 2.0 | 14.8 | 2.2 | F=16.2 | 2, 533 | 1.48E-07 | 0.057 | PI<AD<CS |
| Global Functioning | 45.6 | 11.7 | 42.1 | 13.3 | 48.7 | 15.3 | F=9.8 | 2, 515 | 6.90E-05 | 0.046 | PI=CS>AD |
| Learning Difficulties | 50 | 47.6% | 71 | 30.0% | 44 | 22.2% | X2=15.5 | 2 | 4.36E-04 | 0.041 | PI>AD=CS |
| Currently Employed | 21 | 20.2% | 67 | 28.4% | 77 | 38.7% | X2=9.6 | 2 | 0.008 | 0.025 | PI=AD<CS |
| Cognition: | |||||||||||
| WAIS Full Scale IQ | 86.4 | 8.2 | 84.9 | 7.5 | 103.4 | 6.5 | F=355.5 | 2, 535 | 5.98E-99 | 0.571 | PI=AD<CS |
| WRAT Reading | 86.5 | 7.8 | 105.6 | 7.5 | 108.3 | 6.8 | F=324.3 | 2, 535 | 5.62E-93 | 0.548 | PI<AD<CS |
| General Cognition* | −1.5 | 0.6 | −1.4 | 0.6 | −0.5 | 0.5 | F=183.5 | 2, 518 | 5.81E-61 | 0.415 | PI=AD<CS |
| Clinical: | |||||||||||
| Duration of illness | 11.4 | 8 | 11.8 | 8.9 | 15 | 10.5 | F=0.7 | 2, 518 | ns | - | - |
| On antipsychotics | 103 | 98.1% | 203 | 92.6% | 180 | 90.7% | X2=5.58 | 2 | ns | - | - |
| CPZE | 651 | 427 | 611 | 413 | 531 | 357 | F=3.6 | 2, 474 | 0.029 | 0.015 | PI=AD>CS |
| PANSS Total (30–210) | 59.2 | 19.4 | 64.1 | 21.2 | 55.1 | 20.1 | F=7.1 | 2, 423 | 9.34E-04 | 0.032 | AD>PI=CS |
| Negative (6–42) | 15.6 | 8.5 | 17.4 | 8.9 | 14.4 | 8.5 | F=4.2 | 2, 451 | 0.016 | 0.018 | AD>CS |
| Positive (4–28) | 8.5 | 5.1 | 10.2 | 5.7 | 8.8 | 5.3 | F=3.4 | 2, 430 | 0.035 | 0.016 | PI=CS<AD |
| Disorganized (3–21) | 7.7 | 3.6 | 7.5 | 3.9 | 5.7 | 3.3 | F=14.9 | 2, 440 | 5.30E-07 | 0.064 | PI=AD>CS |
| Polygenic Scores: | |||||||||||
| Schizophrenia | 0.42 | 1.0 | 0.57 | 0.9 | 0.22 | 1.0 | F=4.5 | 2, 525 | 0.001 | 0.026 | AD>CS |
| Cognition | −0.28 | 0.9 | −0.07 | 1.0 | 0.12 | 1.0 | F=5.4 | 2, 525 | 0.005 | 0.02 | PI=AD<CS |
| Education | −0.37 | 0.9 | 0.03 | 1.1 | 0.23 | 0.9 | F=9.7 | 2, 525 | 7.20E-05 | 0.036 | PI<AD<CS |
| ADHD | 0.35 | 1.0 | −0.02 | 1.1 | −0.02 | 1.0 | F=5.1 | 2, 525 | 0.007 | 0.019 | PI>AD=CS |
Analyses control for age and sex and, in analyses of polygenic scores, for 10 ancestry principal components. For pairwise analyses, significance set at p<.05, after accounting for three comparisons. For continuous dependent variables, ‘effect size’ refers to partial eta2 from GLM analysis and, for categorical dependent variables, to the difference in Nagelkerke R2 estimates between a covariates-only logistic regression model and a model also including the independent variable of interest. ‘SC’, schizophrenia; ‘US’, unaffected sibling; ‘CC’, community control; ‘SES’, socio-economic status; ‘PI’, pre-adolescent impairment; ‘AD’, adolescent decline; ‘CS’ cognitively stable; ‘PANSS’, Positive and Negative Syndrome Scale; ‘CPZE’ chlorpromazine equivalents; ‘na’, not applicable; ‘ns’, not significant.
“General Cognition’ is a composite of 25 cognitive variables based on earlier work. Details are provided in the Supplementary Methods.
Clustering Based on WAIS and WRAT IQ Estimates
In 1000 unsupervised clustering runs, three-cluster solutions (56.1%) were the most frequent result, followed by four- and six-cluster solutions (23.2% and 11.3%, respectively). Subgroups emerging from the three-cluster solutions were consistent in size, individual subgroup assignments, and mean IQ indicator values. Across 50 further analyses, each constrained to yield three clusters, agreement in assignments of individuals to subgroups was high overall (kappa=.794) and by subgroup (kappas=.743–.857). Individuals were assigned to the same subgroup in all 50 runs 53.3% of the time and in at least 30 runs 88.6% of the time. Descriptive statistics for the 540 schizophrenia cases who met ancestry restrictions and had genotype information are shown in Table 1 (bottom). Descriptive statistics for all 746 cases, without the ancestry restriction (Table S1), demonstrate that, apart from race, the characteristics of the subgroups used in genetics analyses closely matched those for the unrestricted subgroups.
Across the cluster analyses, 86 individuals (11.4%) were less consistently assigned than others to a specific subgroup. Secondary, sensitivity analyses excluding these individuals from the subgroups revealed only minor effects on study results (Supplementary Results, Tables S4, S5).
Cognitive trajectory subgroup characteristics and comparisons
Figure 1A shows commonly described trajectories of cognitive development schematically. Figure 1B indicates how the subgroups derived through cluster analysis align with this cognitive development trajectories scheme and, along with Table 1, shows the expected patterns of premorbid (WRAT) and current (WAIS) IQ scores across the three cognitive trajectory subgroups. One subgroup of 198 cases (37%) had high mean scores on both of these indicators (“cognitively stable”); a second subgroup of 105 cases (19%) had both low premorbid and low current IQs (“pre-adolescent impairment”); and the third group included 193 cases (44%) with high premorbid but low current IQ (“adolescent decline”).
The subgroups also differed significantly on important cognitive, clinical and functional variables not used in the clustering (detailed in Table 1 and Figures 2A–F). Those in the cognitively stable subgroup showed markedly less general cognitive impairment than the other subgroups, relatively low symptoms, more education, and higher levels of employment. The adolescent decline subgroup had the highest levels of total and positive PANSS symptoms, the lowest ratings of global functioning, and generalized cognitive impairment. Members of the pre-adolescent impairment subgroup had the fewest years of education, the highest rates of childhood learning difficulties, generalized cognitive impairment, and low adult employment.
Figure 2: Behavioral characteristics across cognitive trajectory subgroups.
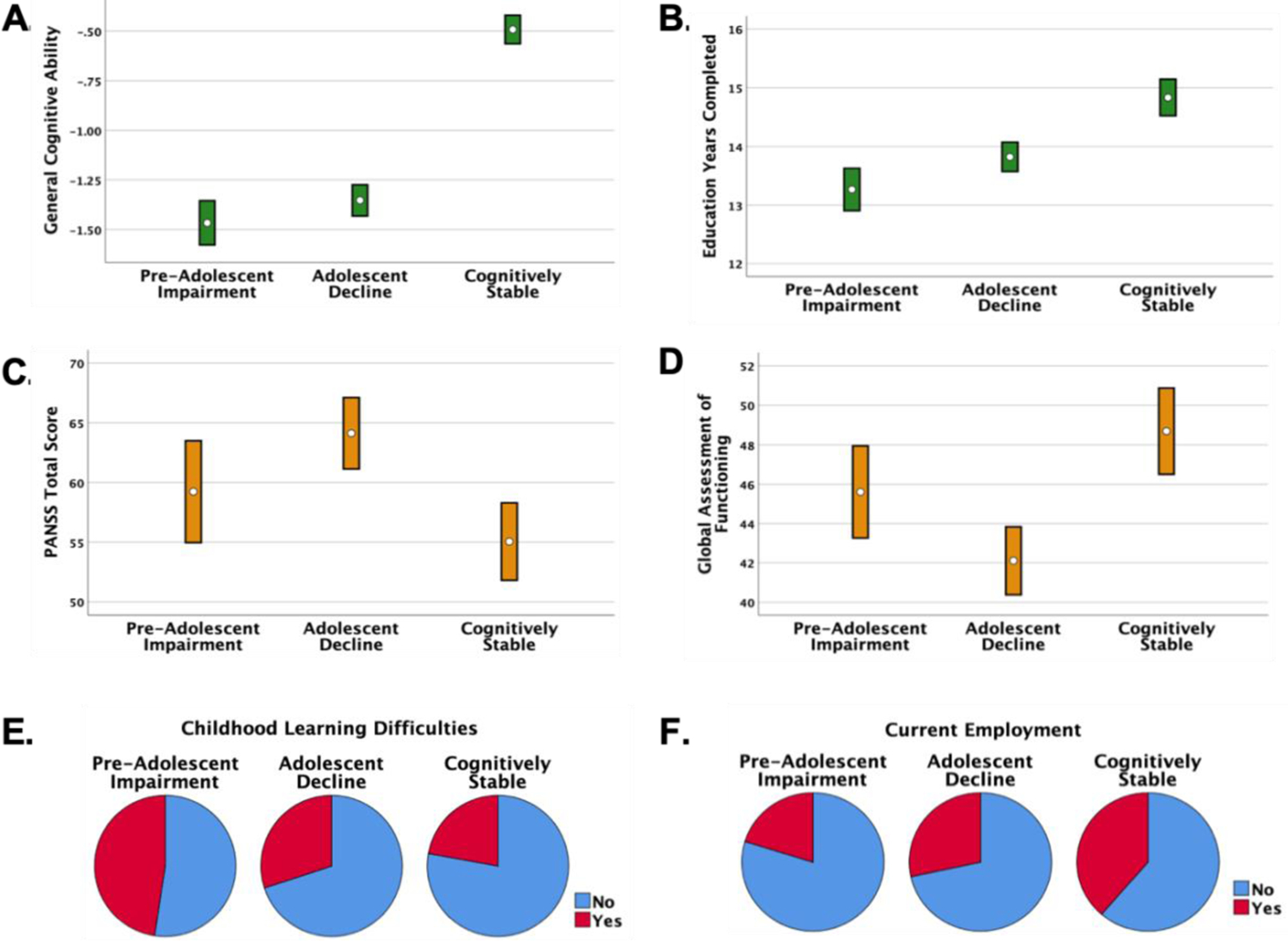
“PANSS”, Positive and Negative Syndrome Scale. Bars represent 95% confidence intervals. Statistical details are in Table 1. General cognitive ability (Panel A) is indexed by a composite score from a comprehensive neuropsychological battery(27) (see Supplementary Methods for additional information) – the cognitively stable subgroup shows relatively mild general cognitive impairment compared to the other subgroups. The cognitively stable group also completed the most education (Panel B), and the pre-adolescent impairment subgroup the least, with the adolescent decline subgroup intermediate. The adolescent decline subgroup was rated as having the highest levels of PANSS symptoms (Panel C) and the lowest level of overall functioning (Panel D). The pie charts illustrate that the pre-adolescent impairment subgroup included the highest proportion (47.6%) of individuals with learning difficulties (e.g., remedial classes, repeated grades) (Panel E), and that individuals in the cognitive stable subgroup were most likely to be employed (Panel F) at the time of study participation (38.7%).
When the cognitive trajectory subgroup assignments were carried over from schizophrenia cases to 247 of their unaffected siblings, the most prominent subgroup differences related to academic and cognitive performance (Supplementary Results and Table S6). Siblings of the cognitively stable schizophrenia cases had higher levels of education than siblings in the other subgroups. The siblings of pre-adolescent impairment cases performed relatively worse on WAIS, WRAT and general cognitive ability measures, while the cognitively stable siblings performed best. Adolescent decline siblings performed at an intermediate level relative to the other unaffected sibling subgroups. In general, the sibling findings conformed to the differences found across the schizophrenia subgroups, but with reduced effect sizes.
PGSs across diagnostic groups
Unsurprisingly, the schizophrenia cases had the highest schizophrenia genetic risk, as indexed by the schizophrenia PGS; the controls had the lowest, with unaffected siblings intermediate (Table 1, Figure 3A). PGSs for cognition and education varied within a narrower range. ADHD PGS did not differ across diagnostic groups. For all groups, PGSs for cognition and education were moderately positively correlated and other PGS correlations were quite modest (Supplementary Results, Table S2).
Figure 3. Polygenic scores (PGSs) by diagnostic group (total N=1631) and by schizophrenia cognitive trajectory subgroup (N=540).
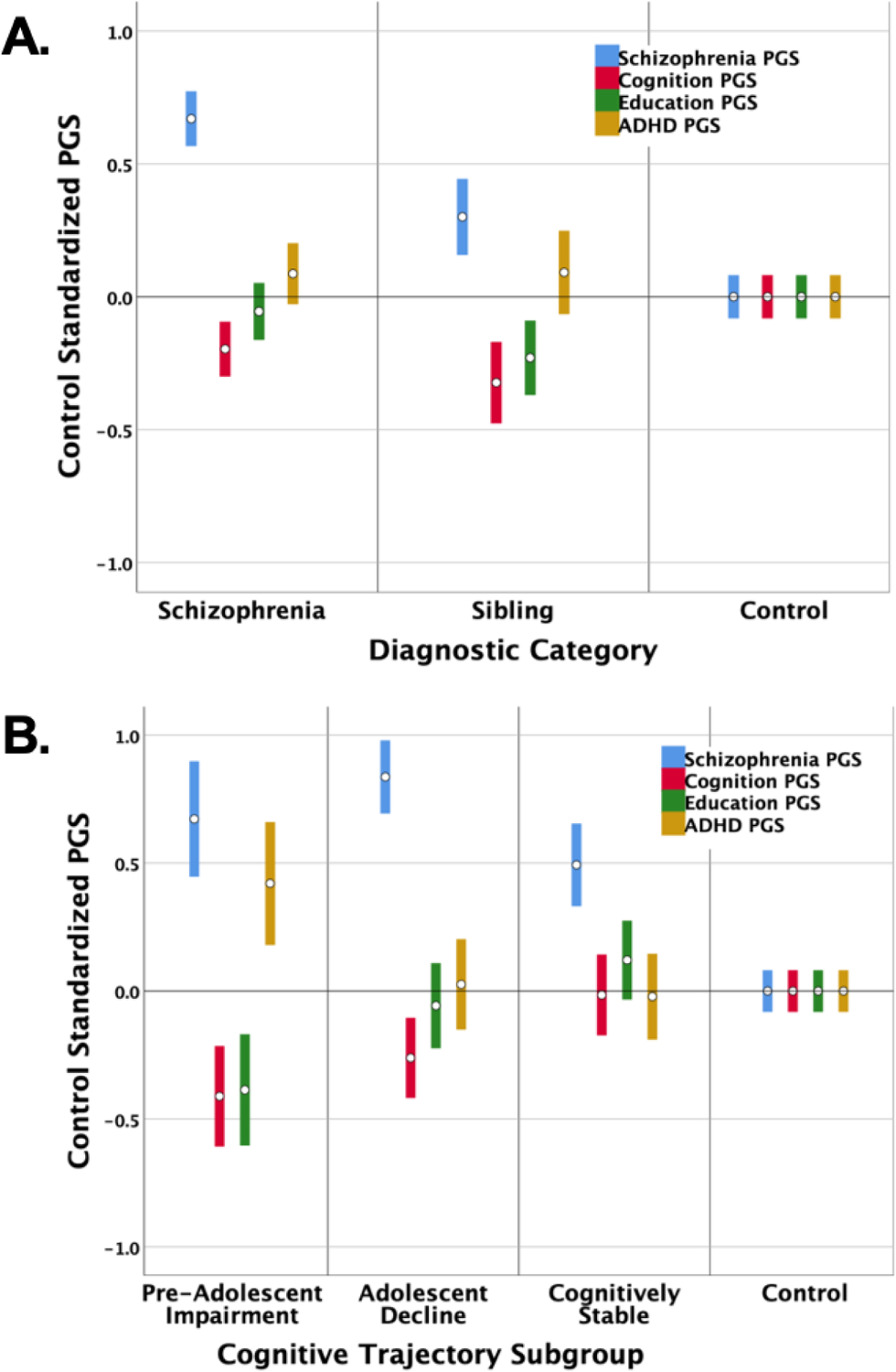
Bars represent 95% confidence intervals. Statistical details are in Tables 1 and 2. The figures depict the profiles of PGSs for the main diagnostic categories in our study (Panel A) and the schizophrenia cognitive trajectory subgroups (Panel B). PGSs were derived in our samples for schizophrenia (blue), cognition (red), educational attainment (green), and ADHD (gold). It warrants emphasis that for schizophrenia and ADHD PGS, higher standardized scores indicate higher disorder risk. For cognition and education PGSs, lower standardized scores predict worse cognitive and academic performance. All PGSs were adjusted to account for age, sex, and population stratification, and then standardized. We used control means and SD’s to standardize the PGSs so that controls serve as the reference for differences in PGSs across diagnostic categories and across cognitive trajectory subgroups.
PGSs in cognitive trajectory subgroups
All PGSs differed significantly across the three cognitive trajectory subgroups, with modest effect sizes (Table 1, Figure 3B) – and also differed in relation to controls (Table 2). Multinomial logistic regression confirmed that PGS patterns across the four polygenic scores significantly predicted cognitive trajectory subgroup membership (ΔX2[8]=43.83, p=6.10E-07, ES=.079). Within the multi-PGS model, all the PGSs remained individually significant except the cognition PGS, likely reflecting the previously reported association of cognition and education PGSs (35) (additional details in Supplementary Results, Table S2). Cognitively stable schizophrenia cases had somewhat elevated schizophrenia PGS but were similar to controls across other PGSs, even showing a nominally significant advantage in education PGS. They had advantageous cognitive PGS relative to the pre-adolescent impairment and adolescent decline subgroups and advantageous educational attainment PGS relative to the pre-adolescent impairment subgroup. The adolescent decline subgroup had elevated schizophrenia PGS, which were significantly higher than scores for the cognitively stable subgroup and controls, and unfavorable cognition PGS relative to the same comparison groups. The pre-adolescent impairment subgroup showed consistently disadvantageous PGSs relative to controls. Importantly, this was the only subgroup with significantly elevated ADHD PGS and significantly reduced educational attainment PGS.
Table 2:
GLM results for contrasts of each polygenic score (PGS) in each cognitive trajectory subgroup with control PGS
| Cognitively Stable (n=198) | Community Controls (n=844) | Statistic | df | P-value | Effect Size | |||
|---|---|---|---|---|---|---|---|---|
| Polygenic Scores: | Mean | SD | Mean | SD | ||||
| Schizophrenia | 0.22 | 1.0 | −0.30 | 1.0 | F=45.0 | 1, 1012 | 3.26E-11 | 0.042 |
| Cognition | 0.12 | 1.0 | 0.13 | 1.0 | F=0.1 | 1, 1012 | ns | - |
| Education | 0.23 | 0.9 | 0.06 | 1.0 | F=5.3 | 1, 1012 | 0.02 | 0.005 |
| ADHD | −0.02 | 1.0 | −0.04 | 1.0 | F=0.9 | 1, 1012 | ns | - |
| Adolescent Decline (n=237) | Community Controls (n=844) | Statistic | df | P-value | Effect Size | |||
| Polygenic Scores: | Mean | SD | Mean | SD | ||||
| Schizophrenia | 0.57 | 0.9 | −0.30 | 1.0 | F=168.3 | 1, 1067 | 7.66E-36 | 0.136 |
| Cognition | −0.08 | 1.0 | 0.13 | 1.0 | F=8.2 | 1, 1067 | 0.004 | 0.008 |
| Education | 0.02 | 1.1 | 0.06 | 1.0 | F=0.1 | 1, 1067 | ns | - |
| ADHD | −0.02 | 1.1 | −0.04 | 1.0 | F=0.5 | 1, 1067 | ns | - |
| Pre-Adolescent Impairment (n=105) | Community Controls (n=844) | Statistic | df | P-value | Effect Size | |||
| Polygenic Scores: | Mean | SD | Mean | SD | ||||
| Schizophrenia | 0.42 | 1.0 | −0.30 | 1.0 | F=52.6 | 1, 935 | 8.62E-13 | 0.053 |
| Cognition | −0.28 | 0.9 | 0.13 | 1.0 | F=18.6 | 1, 935 | 1.80E-05 | 0.019 |
| Education | −0.37 | 0.9 | 0.06 | 1.0 | F=17.8 | 1, 935 | 2.70E-05 | 0.019 |
| ADHD | 0.35 | 1.0 | −0.04 | 1.0 | F=16.6 | 1, 935 | 4.90E-05 | 0.017 |
All analyses control for age, sex, and 10 population stratification principal components. ‘ns’, not significant. ‘Effect size’ refers to partial eta2 from GLM analysis.
PGS profiles in 247 unaffected siblings were generally similar to profiles in corresponding subgroups of schizophrenia cases (compare Figures 3B and S2). As found in schizophrenia cases, PGS profile significantly predicted sibling cognitive trajectory subgroup assignment (ΔX2[8]=23.87, p=.002, ES=.093). While schizophrenia PGS and ADHD PGSs did not differ by subgroup in siblings, for both cognition and educational attainment, the siblings of the pre-adolescent impairment schizophrenia cases had significantly lower (i.e., more disadvantageous) PGSs than those in the other sibling subgroups (Table S6, S7, and Figure S2).
DISCUSSION
The goals of the current study were to use an IQ-based strategy in a large and extensively phenotyped schizophrenia sample to identify and characterize subgroups with different pre-diagnosis trajectories of cognitive development, and to test whether the profiles of four polygenic scores – separately summarizing the influence of common genetic variants associated with schizophrenia, general cognition, educational attainment, and ADHD – differed by subgroup. The resulting IQ patterns and PGS profiles were consistent with hypotheses and congruent in interesting ways. Cluster analysis based only on “premorbid” (WRAT) and current (WAIS) IQ strongly supported a three-subgroup model, similar to earlier studies(18,20,21), with “cognitively stable”, “adolescent decline,” and “pre-adolescent impairment” subgroups. Distinct cognitive, clinical and functional characteristics across subgroups helped validate the guiding cognitive development trajectories framework. Finally, profiles of the four PGSs showed a remarkable convergence with the developmental framework and with subgroup characteristics.
For 37% of the sample, relatively strong performance on both the WRAT and the WAIS suggested a stable cognitive development trajectory, with good early-life cognitive and educational functioning and a more limited impact of emerging psychosis in these areas. Subgroup clinical and functional characteristics in adulthood – beyond WRAT and WAIS results – indicated that individuals in this subgroup had a milder course of illness with low levels of schizophrenia symptomatology (particularly negative symptoms), a strong advantage in general cognitive performance, and higher levels of employment than their peers. The PGS profile for the cognitively stable subgroup was, likewise, relatively more benign than the profiles for the other schizophrenia subgroups. Individuals in this group were similar to controls on PGSs for cognition and ADHD. They showed a slight advantage on education PGS, which might relate to a previously-reported and counter-intuitive positive association between schizophrenia and education PGS.(35,38) These individuals were only disadvantaged relative to controls on schizophrenia PGS.
For the adolescent decline subgroup, which accounted for 44% of the sample, the matrix of findings was quite different. For these individuals, premorbid IQ in the normal range, combined with substantially impaired current IQ, suggested a disruption of cognitive development during adolescence, likely overlapping with psychosis prodrome and onset. Illness course and outcome for this subgroup was unfavorable. As adults, in addition to broadly impaired cognitive performance, adolescent decline subgroup members had the most severe schizophrenia symptoms among the subgroups, especially positive symptoms, low levels of employment, and the lowest Global Assessment of Functioning ratings. Those in the adolescent decline group also showed a distinct and unfavorable PGS profile, with a significant disadvantage in terms of schizophrenia and cognition PGSs relative to controls and those in the cognitively stable subgroup.
The pre-adolescent impairment subgroup, comprising 19% of the sample, was also distinctive. Substantial impairment in both WRAT and WAIS performance in this group suggested early-life divergence from typical cognitive development. Supporting this interpretation, the subgroup reported the highest rates of childhood learning problems and the fewest years of education completed. These individuals also had globally impaired cognition and low rates of employment in adulthood. Symptoms were intermediate relative to the cognitively stable and adolescent decline subgroups. Individuals in the pre-adolescent impairment subgroup showed a generalized profile of unfavorable PGSs across the four phenotypes. They were at a significant disadvantage in all PGSs relative to controls, and in all but schizophrenia PGS relative to the cognitively stable subgroup. It is particularly striking in light of evidence of early-life cognitive and academic abnormalities, that this was the only cognitive trajectory subgroup with robust disadvantages in both education and ADHD PGSs, perhaps suggesting a distinct genetic etiology for this schizophrenia subgroup.
The PGS profile for each unaffected sibling subgroup was consistent with the profile in the corresponding schizophrenia subgroup, although only the cognition and education PGSs differed significantly across the sibling subgroups. As with the schizophrenia subgroups, PGS profiles predicted sibling subgroup assignments, and sibling subgroup PGS differences converged with differences in observed academic and cognitive performance. This consistency of affected and unaffected sibling subgroup PGS profiles and associations is further evidence of the importance of inherited polygenic factors in distinguishing the cognitive trajectory subgroups.
Although cognitive impairment is common in schizophrenia, the extent of impairment – and the trajectory of development leading to impairment – vary considerably. A literature seeking to address this heterogeneity has proposed that developmental trajectory subgroups, with distinct patterns of course and outcome, can be formed using estimates of premorbid and current intellectual functioning.(17–21) Recent work has identified brain structure differences across subgroups,(22,23) but genetic differences have not been examined. With the completion in recent years of high quality GWAS for many common disorders and traits, PGSs are becoming accessible research tools. Combined with the increasing availability of large, comprehensively phenotyped and genotyped samples, PGSs have evolved rapidly as clinical tools as well, now providing actionable information in conditions such as coronary artery disease(39). Despite these promising developments, the clinical utility of PGSs in psychiatry is less clear. The variance in diagnostic status explained by any single PGS is not yet adequate to allow biologically informed diagnosis or helpful clinical stratification(2,3). Among other factors, co-morbidities and pleiotropy in psychiatric disorders, and genetic correlations between the disorders and traits such as cognition and education, greatly complicate the resolution of genetic influences and risk(38).
On the other hand, it is exactly these characteristics of psychiatric disorders that may make simultaneous analysis of multiple PGSs a potent strategy for resolving developmental and diagnostic heterogeneity(40). The present results offer support for this approach. The set of PGSs included diagnosis-based (schizophrenia and ADHD) and trait-based (cognition and education) PGSs, and both contributed to subgroup differentiation. Together, the four PGSs predicted 7.9% of the variance in cognitive trajectory subgroup membership. Although variance explained was relatively modest, employing a set of PGSs was useful as we shifted focus beyond simple case/control discrimination, toward the prediction of important within-diagnosis differences – and we draw encouragement from the fact that we accounted for within group variance at a level comparable to studies focused on between groups variance.(33,36) Thus, in ways that parallel recent findings for depression onset,(40) PGS profiles discriminated cognitive developmental trajectories in schizophrenia and were clinically informative to a degree, showing association with facets of illness course and outcome. To be clear, the present findings that four PGSs account for a modest proportion of variance across cognitive development trajectories in schizophrenia do not provide a basis for clinical stratification of new patients or individuals at risk of illness. However, this work illustrates how multiple PGSs might contribute in the future to stratification that has not been achievable in psychiatric disorders with single diagnosis PGSs.
Various limitations of the current work should be considered. Importantly, the samples are small by the standards of genetics analyses and the findings await replication(24). Although modest in size, the current sample offers a consistent ascertainment approach, comparison samples, and extensive clinical, cognitive, and functional data, as well as genotypes, and key statistical findings were robust. Another limitation involves the subgrouping approach. Lacking detailed information about developmental history in each case, we employed a proxy measure of “premorbid IQ” as one cornerstone of our subgrouping, as others have done(17), providing an estimate of cognitive performance in early adolescence, before the onset of psychosis. Contrasting characteristics of the subgroups provided some validation of the strategy but it would be preferable to analyze direct information about pre-diagnosis developmental history(40).
The use of current methods for creating PGSs also involves certain limitations. Although it is clear that psychiatric disorders involve both common and rare forms of genetic variation, PGSs reflect only common genetic variants, missing important elements of the genetic landscape(2). Further, PGS reflect small genetic effects across the genome and offer limited traction for the investigation of specific biological mechanisms. Importantly, the various GWAS on which current PGSs were based involved overwhelmingly Caucasian samples. We restricted our analyses accordingly. The strategies employed here may not be available for non-Caucasian samples until high quality GWAS in such samples are completed.
In conclusion, the findings of this study suggest that adult cognitive data can be used to generate schizophrenia subgroups with distinct trajectories of cognitive development, and that these subgroups are characterized by quite different profiles of psychiatric, cognitive and academic genetic influence.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported with funding from the Division of Intramural Research Programs, National Institute of Mental Health, National Institutes of Health, to programs within the NIMH Clinical and Translational Neuroscience Branch (KF Berman, PI): Clinical Study Number NCT 95-M-0150 and Annual Report Number MH002652-25.
Footnotes
The authors report no financial relationships with relevant commercial interests.
REFERENCES
- 1.Walker EF: Schizophrenia: A life-course developmental perspective. San Diego, CA, Academic Press, Inc.; 1991. [Google Scholar]
- 2.Smoller JW, Andreassen OA, Edenberg HJ, et al. Psychiatric genetics and the structure of psychopathology. Mol Psychiatry. 2019;24:409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinberger DR. Thinking About Schizophrenia in an Era of Genomic Medicine. Am J Psychiatry. 2019;176:12–20. [DOI] [PubMed] [Google Scholar]
- 4.Kendler KS, Ohlsson H, Keefe RSE, et al. The joint impact of cognitive performance in adolescence and familial cognitive aptitude on risk for major psychiatric disorders: a delineation of four potential pathways to illness. Mol Psychiatry. 2018;23:1076–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mollon J, Reichenberg A. Cognitive development prior to onset of psychosis. Psychological Medicine. 2018;48:392–403. [DOI] [PubMed] [Google Scholar]
- 6.Cole VT, Apud JA, Weinberger DR, et al. Using latent class growth analysis to form trajectories of premorbid adjustment in schizophrenia. J Abnorm Psychol. 2012;121:388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsen TK, Friis S, Haahr U, et al. Premorbid adjustment in first-episode non-affective psychosis: distinct patterns of pre-onset course. Br J Psychiatry. 2004;185:108–115. [DOI] [PubMed] [Google Scholar]
- 8.Kendler KS, Ohlsson H, Sundquist J, et al. IQ and schizophrenia in a Swedish national sample: their causal relationship and the interaction of IQ with genetic risk. Am J Psychiatry. 2015;172:259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacCabe JH. Population-based cohort studies on premorbid cognitive function in schizophrenia. Epidemiol Rev. 2008;30:77–83. [DOI] [PubMed] [Google Scholar]
- 10.Reichenberg A, Weiser M, Rapp MA, et al. Elaboration on premorbid intellectual performance in schizophrenia: premorbid intellectual decline and risk for schizophrenia. Arch Gen Psychiatry. 2005;62:1297–1304. [DOI] [PubMed] [Google Scholar]
- 11.Lewandowski KE, Cohen BM, Ongur D. Evolution of neuropsychological dysfunction during the course of schizophrenia and bipolar disorder. Psychol Med. 2011;41:225–241. [DOI] [PubMed] [Google Scholar]
- 12.Wilkinson G: Wide Range Achievement Test - Revision 3: Administration Manual. Wilmington, DE, Wide Range, Inc.; 1993. [Google Scholar]
- 13.Wechsler D: Wechsler Adult Intelligence Test - Fourth Edition. San Antonio, TX, PsychCorp; 2008. [Google Scholar]
- 14.O’Carroll R, Walker M, Dunan J, et al. Selecting controls for schizophrenia research studies: the use of the National Adult Reading Test (NART) is a measure of premorbid ability. Schizophr Res. 1992;8:137–141. [DOI] [PubMed] [Google Scholar]
- 15.Kremen WS, Pepple JR, Tsuang MT, et al. The “3 Rs” and neuropsychological function in schizophrenia: An empirical test of the matching fallacy. Neuropsychology. 1996;10:22–31. [DOI] [PubMed] [Google Scholar]
- 16.Horton LE, Tarbox SI, et al. Trajectories of premorbid childhood and adolescent functioning in schizophrenia-spectrum psychoses: A first-episode study. Psychiatry Res. 2015;227:339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weickert TW, Goldberg TE, Gold JM, et al. Cognitive impairments in patients with schizophrenia displaying preserved and compromised intellect. Arch Gen Psychiatry. 2000;57:907–913. [DOI] [PubMed] [Google Scholar]
- 18.Badcock JC, Dragovic M, Waters FA, et al. Dimensions of intelligence in schizophrenia: evidence from patients with preserved, deteriorated and compromised intellect. J Psychiatr Res. 2005;39:11–19. [DOI] [PubMed] [Google Scholar]
- 19.Joyce EM, Hutton SB, Mutsatsa SH, et al. Cognitive heterogeneity in first-episode schizophrenia. Br J Psychiatry. 2005;187:516–522. [DOI] [PubMed] [Google Scholar]
- 20.Leeson VC, Sharma P, Harrison M, et al. IQ trajectory, cognitive reserve, and clinical outcome following a first episode of psychosis: a 3-year longitudinal study. Schizophr Bull. 2011;37:768–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wells R, Swaminathan V, Sundram S, et al. The impact of premorbid and current intellect in schizophrenia: cognitive, symptom, and functional outcomes. NPJ Schizophr. 2015;1:15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Czepielewski LS, Wang L, Gama CS, et al. The Relationship of Intellectual Functioning and Cognitive Performance to Brain Structure in Schizophrenia. Schizophr Bull. 2017;43:355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woodward ND, Heckers S. Brain Structure in Neuropsychologically Defined Subgroups of Schizophrenia and Psychotic Bipolar Disorder. Schizophr Bull. 2015;41:1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dudbridge F. Power and predictive accuracy of polygenic risk scores. PLoS Genet. 2013;9:e1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krapohl E, Euesden J, Zabaneh D, et al. Phenome-wide analysis of genome-wide polygenic scores. Mol Psychiatry. 2016;21:1188–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.First M, Spitzer R, Gibbon M, et al. Structured Clinical Interview for Axis I DSM-IV. New York, Biometrics Research Dept, New State Psychiatric Institute; 1994. [Google Scholar]
- 27.Dickinson D, Goldberg TE, Gold JM, et al. Cognitive factor structure and invariance in people with schizophrenia, their unaffected siblings, and controls. Schizophr Bull. 2011;37:1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaufman A: Assessing adolescent and adult intelligence. Boston, MA, Allyn and Bacon, Inc.; 1990. [Google Scholar]
- 29.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 30.Wallwork RS, Fortgang R, Hashimoto R, et al. Searching for a consensus five-factor model of the Positive and Negative Syndrome Scale for schizophrenia. Schizophr Res. 2012;137:246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiu T, Fang D, Chen J, et al. A robust and scalable clustering algorithm for mixed type attributes in large database environment. in The 7th ACM SIGKDD International Conference on Knowledge Discovery and Data Mining 2001. pp. 263–268. [Google Scholar]
- 32.Norusis MJ: IBM SPSS Statistics 19 Statistical Procedures Companion. Upper Saddle River, NJ, Prentice Hall; 2011. [Google Scholar]
- 33.Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sniekers S, Stringer S, Watanabe K, et al. Genome-wide association meta-analysis of 78,308 individuals identifies new loci and genes influencing human intelligence. Nat Genet. 2017;49:1107–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JJ, Wedow R, Okbay A, et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet. 2018;50:1112–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demontis D, Walters RK, Martin J, et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bergen SE, Ploner A, Howrigan D, et al. Joint Contributions of Rare Copy Number Variants and Common SNPs to Risk for Schizophrenia. Am J Psychiatry. 2018:appiajp201817040467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brainstorm Consortium, Anttila V, Bulik-Sullivan B, et al. Analysis of shared heritability in common disorders of the brain. Science. 2018;360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khera AV, Chaffin M, Aragam KG, et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet. 2018;50:1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rice F, Riglin L, Thapar AK, et al. Characterizing Developmental Trajectories and the Role of Neuropsychiatric Genetic Risk Variants in Early-Onset Depression. JAMA Psychiatry. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


