Abstract
Background
The epidemiology and management of valvular heart disease (VHD) have changed with economic development and population aging in China in recent decades.
Objectives
This study sought to understand the distribution, etiology, and presentation and assess the current practice and outcomes of older patients with VHD in China.
Methods
The authors conducted the first nationwide survey of older patients with VHD between September and December 2016 from 69 hospitals in 28 provinces and municipalities throughout China. Hospitalized patients over 60 years of age with moderate-to-severe VHD, infective endocarditis, or previous valvular intervention were consecutively enrolled.
Results
Of 8,929 patients (median age of 69 years, 47.5% female), 8227 (92.1%) had native VHD. Mitral regurgitation was the most prevalent single VHD (26.9% of native VHD), followed by tricuspid regurgitation (16.5%), aortic regurgitation (10.6%), aortic stenosis (5.1%), and mitral stenosis (3.1%). Degenerative (37.2%), functional (21.8%), and rheumatic (15.0%) etiologies were the 3 most common causes. Among symptomatic patients with severe VHD, 37.3% underwent valvular intervention. The intervention rates decreased significantly with age across all types of VHD (Ptrend < 0.01). Valvular surgery covered 93.7% of interventions. The overall 1-year survival rate was 74.4% (95% CI: 63.4%-85.4%).
Conclusions
This study provides a unique national insight into the contemporary spectrum and management of older VHD patients in China. With the increase in the health care demand, more resources and efforts are required for early detection, effective intervention, and targeting innovation on advanced therapeutic techniques and devices to improve the outcomes.
Key Words: epidemiology, intervention, valvular heart disease
Abbreviations and Acronyms: AR, aortic regurgitation; AS, aortic stenosis; CT, computed tomography; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; MS, mitral stenosis; RHD, rheumatic heart disease; TAVR, transcatheter aortic valve replacement; TR, tricuspid regurgitation; VHD, valvular heart disease
Central Illustration
Valvular heart disease (VHD) represents a growing serious public health problem; early detection and intervention improve survival.1, 2, 3, 4, 5, 6 Disparities exist in the epidemiology and management of VHD across countries, especially between industrialized and developing countries.1, 2, 3, 4, 5,7 Moreover, the distribution, characteristics, and treatments have changed over time within nations.4,5,8,9 In China, the incidence of rheumatic VHD has decreased significantly, whereas degenerative and ischemic VHDs have increased in recent years due to rapid economic growth and population aging.4,5,10,11
Less invasive transcatheter techniques have emerged as alternatives to traditional valve surgery. Transcatheter aortic valve replacement (TAVR), as one of the representative techniques, was introduced to China in 2010 but was not commercially available until 2017. Therefore, it is essential to understand the contemporary characteristics and clinical practice of VHD before novel techniques change the treatment pattern so as to allocate health resources accordingly and target the innovation and translational research. However, to date, there are no nationwide data on VHD in China. The prevalence and complexity of VHD increase in older people, which affects decision making.4,6,7,12,13 Technical advances have increased the availability of interventions for them. This population is of particular interest for research and health care.1, 2, 3, 4 Therefore, we designed the first national survey of older patients with VHD to understand the contemporary spectrum, characteristics, treatments, and outcomes of VHD in China during a period of economic transition and population aging.
Methods
Study design
The China-DVD (China Elderly Valve Disease) study is a prospective, nationwide, multicenter cohort study of older inpatients with moderate or severe VHD (NCT02865798). The participating sites were required to consecutively enroll inpatients who met the inclusion criteria. Recruitment was performed from September to December 2016. Follow-up was done personally or telephonically at 6 and 12 months. This project was approved by the central and site Institutional Review Board or Ethics Committees. Written informed consent was obtained from eligible patients before registration.
Inclusion criteria and diagnostic definitions
All hospitalized patients ≥60 years of age who had clinically significant (moderate or severe) native valvular disease as defined by echocardiography, suspected or definite endocarditis based on the Duke criteria, or previous valvular intervention (percutaneous balloon dilatation, transcatheter intervention, valve repair, valve replacement) in the Departments of Cardiology or Cardiac Surgery were enrolled. Detailed criteria for clinically significant native VHD were the following: aortic stenosis (AS) with a valve area of ≤1.5 cm2 or a maximal jet velocity of ≥3 m/s or a mean pressure gradient of ≥20 mm Hg, aortic regurgitation (AR) with a grade of ≥2/4, mitral stenosis (MS) with a valve area of ≤2.0 cm2, mitral regurgitation (MR) with a grade of ≥2/4, pulmonary stenosis, pulmonary regurgitation, tricuspid stenosis, and tricuspid regurgitation (TR) with a moderate or severe grade. Severe VHD was defined as with a valve area of ≤1.0 cm2 or a maximal jet velocity of ≥4 m/s or mean pressure gradient of ≥40 mm Hg, AR with a regurgitation grade of ≥3/4, MS with a valve area of ≤1.5 cm2, MR with a regurgitation grade of ≥3/4, pulmonary stenosis with a maximal jet velocity of ≥4 m/s, PR with a severe grade, tricuspid stenosis with a valve area of ≤1.0 cm2 or pressure gradient of ≥5 mm Hg, and TR with severe grade.
Native VHD was characterized by no previous valvular intervention. Single native VHD was defined as only 1 affected valve without any other significant valve disease. Isolated native VHD was defined as a stenotic or regurgitant lesion on a single valve, and mixed VHD was defined as both significant stenosis and regurgitation on a single valve. Multiple native VHD was characterized by the combination of at least moderate stenotic or regurgitant lesions on ≥2 valves. Severe multiple VHD was defined by the presence of severe lesions on at least 1 valve.
Site selection, startup, and investigator training
The invited hospitals have both a Department of Cardiology and a Department of Cardiac Surgery with experienced staff for VHD diagnosis, assessment, and management. Eight hospitals withdrew due to the inability to consecutively enroll eligible patients. Finally, the study included 69 large academic hospitals from 28 provinces and municipalities throughout mainland China (Supplemental Methods, Supplemental Figure 1). This list of participating hospitals would ensure broad geographic coverage, accurate diagnoses, and representation of the current status of the management of VHD patients. The kick-off investigator meetings and training workshops were held on site. All local investigators received detailed training on the protocols and data collection. A standard echocardiography protocol was provided to operators and reporters at participating sites.
Study variables and data collection
Data variables with standard definitions included patient demographics, comorbidities, medical history, presentation, investigations, etiology, interventions, complications, medications, and outcomes. Vital status, rehospitalization, and intervention were collected during follow-up. The etiologies of VHD were classified based on clinical context, echocardiography, computed tomography (CT), and surgical findings (if available). Multiple etiologies were defined if multiple valve lesions had at least 2 causes.
Data collection was performed by trained cardiology fellows, junior cardiologists, or cardiothoracic surgeons at each site to ensure data accuracy and reliability. Site investigators were required to collect the data during the hospitalization and complete all the information in case report form upon the patient’s discharge or death. We also requested the sites to scan the medical records and echocardiography reports and send them to coordinator center of the study as resource documents for check. Randomly sampled sites received on-site audit. In addition, echocardiography videos of randomly sampled patients were reviewed and validated blindly at core echocardiography lab. Detailed data management and rigorous quality control are shown in the Supplemental Methods.
Statistical analysis
Patient characteristics, management, and outcomes were demonstrated and compared among different types of VHD. Intervention rates during the index hospitalization were calculated across different types of VHD among all patients, symptomatic patients with severe VHD, severe VHD patients with left ventricular ejection fraction (LVEF) <50%, and also patients with native degenerative MR and LVEF <60%. We also compared the etiologies and intervention rates according to age, as well as clinical characteristics between the patients with and without intervention. One-year survival after enrollment was assessed.
Continuous variables were described as mean ± SD or median (IQR) and compared using 1-way analysis of variance or the Kruskal-Wallis H test, as appropriate. Categorical variables were expressed as number (percentage), and comparisons were performed using the chi-square test. The Cochran-Armitage test was used in the trend analysis of intervention rates according to age. One-year survival rates were calculated using the Kaplan-Meier method with 95% CIs. The survival curves among moderate or severe patients without intervention were drawn. A 2-tailed P value <0.05 was considered statistically significant. Statistical analyses were performed using SAS software (version 9.4, SAS Institute).
Results
A total of 8,929 patients were included (Supplemental Figure 2): 70.5% were enrolled from cardiology wards and 29.5% from cardiac surgery wards. The primary reasons for hospital admissions included 3,491 (39.1%) patients for valve disease, 5,074 (56.8%) patients for other cardiac diseases, and 204 (2.3%) patients for noncardiac diseases (Supplemental Table 1).
Characteristics
The overall cohort had a median age of 69 years with 14.2% ≥80 years of age, and 47.5% were female. The majority of patients (89.4%) had symptoms, and New York Heart Association (NYHA) functional class ≥II covered 78.0% of patients. The proportions of comorbidities were 52.2% for hypertension (most common in AR), 19.3% for diabetes (most frequent in MR and AS), 32.5% for coronary artery disease (most common in MR), 11.8% for prior stroke, 5.5% for chronic obstructive pulmonary disease, and 7.2% for chronic renal insufficiency. Aortic disease was predominantly concurrent with aortic VHD (14.3% in AS and 11.1% in AR) (Table 1).
Table 1.
Characteristics of Older Patients With Valvular Heart Disease
| Total (N = 8,929) | Native Valvular Disease |
Previous Valve Intervention (n = 702) | P Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AS (n = 419) | AR (n = 875) | AS+AR (n = 186) | MS (n = 256) | MR (n = 2,209) | MS+MR (n = 109) | Right Sided (n = 1,384) | Multiple (n = 2,789) | ||||||
| Age, y | 69 (64-76) | 69 (65-76) | 69 (64-74) | 68 (64 -73) | 65 (60-69) | 69 (64-70) | 65 (62-70) | 73 (66-79) | 70 (65-77) | 67 (63-71) | <0.001 | ||
| Age ≥80 y | 1,270 (14.2) | 58 (13.8) | 86 (9.8) | 17 (9.1) | 13 (5.1) | 274 (12.4) | 2 (1.8) | 329 (23.8) | 468 (16.8) | 23 (3.3) | <0.001 | ||
| Female | 4,239 (47.5) | 154 (36.8) | 271 (71.0) | 68 (36.6) | 182 (71.1) | 1,029 (46.6) | 84 (77.1) | 689 (49.8) | 1365 (48.9) | 397 (56.6) | <0.001 | ||
| Symptom | 7,982 (89.4) | 387 (92.4) | 748 (85.5) | 173 (93.0) | 237 (92.6) | 1,996 (90.4) | 105 (96.3) | 1,178 (85.1) | 2,550 (91.4) | 608 (86.6) | <0.001 | ||
| NYHA functional class | 8,671 | 393 | 821 | 176 | 246 | 2,122 | 99 | 1,328 | 2,711 | 675 | <0.001 | ||
| I | 1,909 (22.0) | 54 (13.7) | 250 (30.5) | 17 (9.7) | 27 (11.0) | 466 (22.0) | 7 (7.1) | 478 (36.0) | 382 (14.1) | 128 (19.0) | |||
| II | 2,242 (25.8) | 126 (32.1) | 261 (31.8) | 48 (27.3) | 74 (30.1) | 583 (27.5) | 31 (31.3) | 368 (27.7) | 581 (21.4) | 170 (25.2) | |||
| III | 3,223 (37.2) | 168 (42.7) | 261 (31.8) | 85 (48.3) | 119 (48.4) | 748 (35.2) | 49 (49.5) | 346 (26.1) | 1,178 (43.5) | 269 (39.9) | |||
| IV | 1,297 (15.0) | 45 (11.5) | 49 (6.0) | 26 (14.8) | 26 (10.6) | 325 (15.3) | 12 (12.1) | 136 (10.2) | 570 (21.0) | 108 (16.0) | |||
| Angina | 2,486 (27.8) | 139 (33.2) | 317 (36.2) | 51 (27.4) | 34 (13.3) | 853 (38.6) | 14 (14.5) | 389 (28.1) | 606 (21.7) | 83 (11.8) | <0.001 | ||
| Syncope | 387 (4.3) | 37 (8.8) | 36 (4.1) | 15 (8.1) | 10 (3.9) | 78 (3.5) | 1 (0.9) | 73 (5.3) | 110 (3.9) | 27 (3.9) | <0.001 | ||
| Smoking | 2,627 (29.4) | 161(38.4) | 333 (38.1) | 65 (35.0) | 49 (19.1) | 686 (31.1) | 20 (18.4) | 386 (27.9) | 779 (27.9) | 148 (21.1) | <0.001 | ||
| Hypertension | 4,658 (52.2) | 207 (49.4) | 580 (66.3) | 91 (48.9) | 80 (31.3) | 1,319 (59.7) | 35 (32.1) | 766 (55.4) | 1315 (47.2) | 265 (37.8) | <0.001 | ||
| Dyslipidemia | 662 (7.4) | 61 (14.6) | 70 (8.0) | 29 (15.6) | 17 (6.6) | 205 (9.3) | 5 (4.6) | 88 (6.4) | 139 (5.0) | 48 (6.8) | <0.001 | ||
| Diabetes | 1,726 (19.3) | 88 (21.0) | 97 (11.1) | 23 (12.4) | 40 (15.6) | 599 (27.1) | 12 (11.0) | 255 (18.4) | 482 (17.3) | 130 (18.5) | <0.001 | ||
| CAD | 2,902 (32.5) | 132 (31.5) | 303 (34.6) | 34 (18.3) | 46 (18.0) | 989 (44.8) | 13 (11.9) | 440 (31.8) | 809 (29.0) | 136 (19.4) | <0.001 | ||
| Prior MI | 808 (9.0) | 16 (3.8) | 67 (7.7) | 5 (2.7) | 4 (1.6) | 369 (16.7) | 4 (3.7) | 107 (7.7) | 218 (7.8) | 18 (2.6) | <0.001 | ||
| Prior PCI | 989 (11.1) | 37 (8.8) | 98 (11.2) | 10 (5.4) | 6 (2.3) | 397 (18.0) | 4 (3.7) | 158 (11.4) | 247 (8.9) | 32 (4.6) | <0.001 | ||
| Prior CABG | 288 (3.2) | 12 (2.9) | 9 (1.0) | 3 (1.6) | 1 (0.4) | 79 (3.6) | 2 (1.8) | 32 (2.3) | 72 (2.6) | 78 (11.1) | <0.001 | ||
| Atrial fibrillation | 3,313 (37.1) | 35 (8.4) | 115 (13.1) | 19 (10.2) | 149 (58.2) | 515 (23.3) | 70 (64.2) | 612 (44.2) | 1377 (49.4) | 421 (60.0) | <0.001 | ||
| Aortic disease | 430 (4.8) | 60 (14.3) | 97 (11.1) | 26 (14.0) | 8 (3.1) | 47 (2.1) | 5 (4.6) | 19 (1.4) | 117 (4.2) | 51 (7.3) | <0.001 | ||
| PAD | 364 (4.1) | 25 (6.0) | 37 (4.2) | 9 (4.8) | 5 (2.0) | 95 (4.3) | 4 (3.7) | 74 (5.4) | 93 (3.3) | 22 (3.1) | 0.020 | ||
| Prior stroke | 1,050 (11.8) | 45 (10.7) | 97 (11.1) | 16 (8.6) | 33 (12.9) | 246 (11.1) | 16 (14.7) | 178 (12.9) | 333 (11.9) | 86 (12.3) | 0.471 | ||
| COPD | 487 (5.5) | 19 (4.5) | 48 (5.5) | 16 (8.6) | 13 (5.1) | 98 (4.4) | 3 (2.8) | 108 (7.8) | 156 (5.6) | 26 (3.7) | 0.001 | ||
| Renal insufficiency | 644 (7.2) | 28 (6.7) | 46 (5.3) | 12 (6.5) | 6 (2.3) | 166 (7.5) | 6 (5.5) | 83 (6.0) | 238 (8.5) | 59 (8.4) | 0.007 | ||
Values are median (IQR), n (%), or n. P values are tested for characteristics among patients with different types of native valvular diseases.
AR = aortic regurgitation = AS = aortic stenosis; CABG = coronary artery bypass grafting; CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease; MI = myocardial infarction; MR = mitral regurgitation; MS = mitral stenosis; NYHA = New York Heart Association; PAD = peripheral artery disease; PCI = percutaneous coronary intervention; PR = pulmonary regurgitation; PS = pulmonary stenosis; TR = tricuspid regurgitation; TS = tricuspid stenosis.
Distribution and etiology
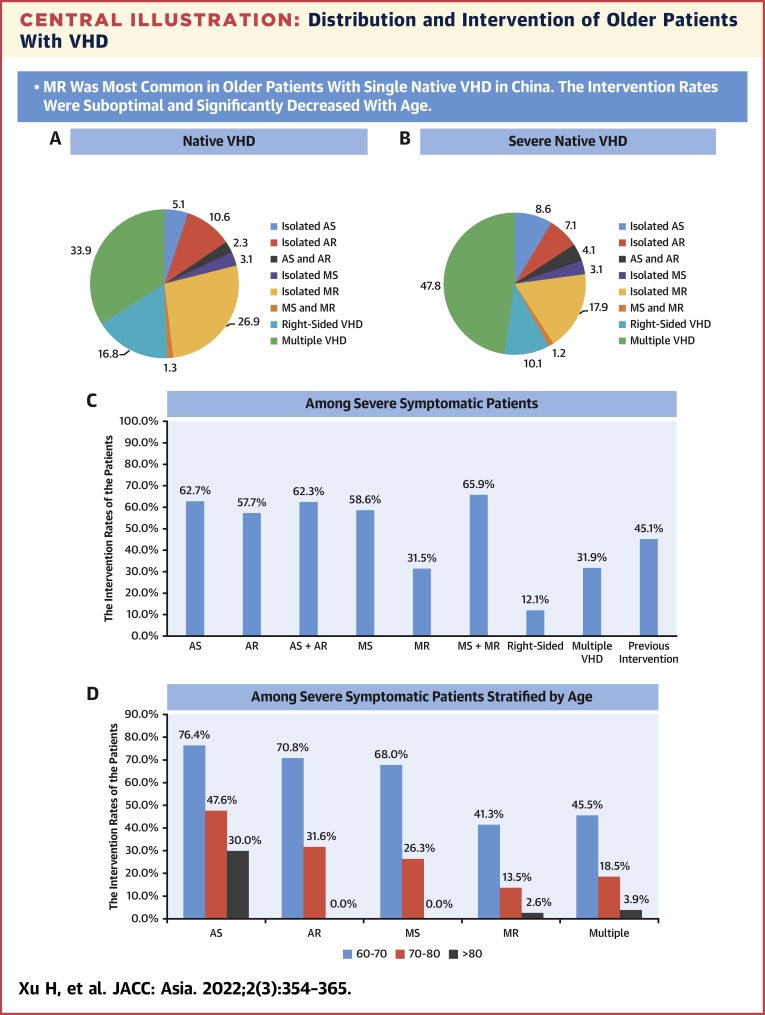
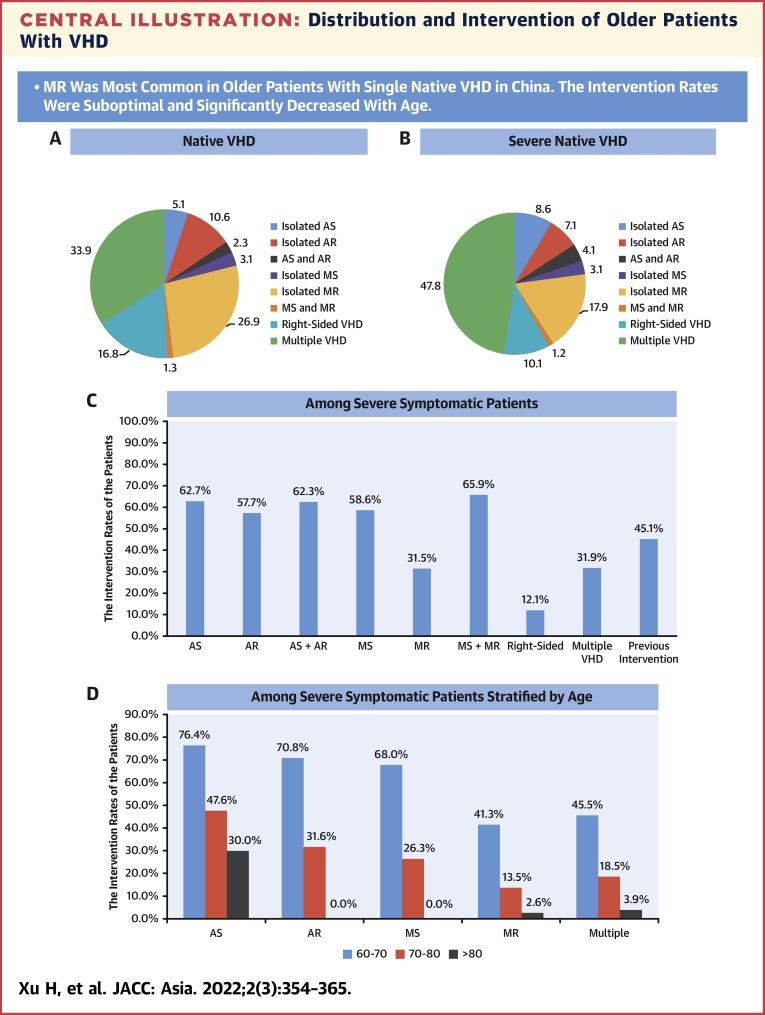
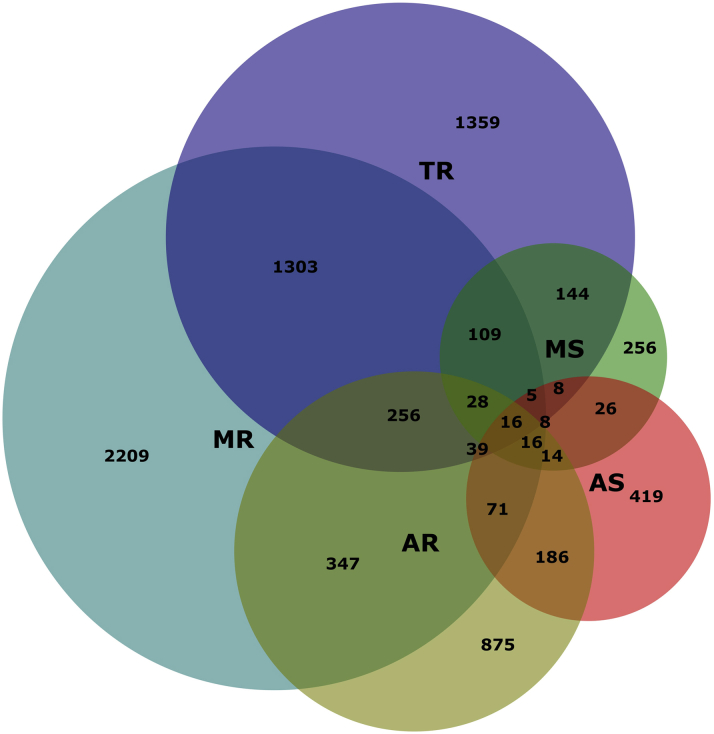
Among all patients, 8,227 (92.1%) had native VHD, and 702 (7.9%) had previous valvular intervention. Of patients with native VHD, 5,438 (66.1%) had single VHD: isolated MR was most prevalent (n = 2,209 [26.9%]), followed by TR (n = 1,359 [16.5%]), AR (n = 875 [10.6%]), AS (n = 419 [5.1%]), and MS (n = 256 [3.1%]). Severe single native VHD was identified in 7.5% of native VHD with MR, 4.1% with TR, 3.6% with AS, 3.0% with AR, and 1.3% with MS. Significant tricuspid stenosis, pulmonary stenosis, and pulmonary regurgitation were rare (<1%). Multiple VHD was present in 2,789 patients (33.9% of native VHD), of which the combination with MR and TR was most prevalent (n = 1,339 patients [16.3% of native VHD]) (Table 2, Central Illustration, Figure 1).
Table 2.
Distribution of Older Patients With Valvular Heart Disease
| Total (N = 8,929) | Native Valve Disease (n = 8,227) | Previous Intervention (n = 702) | |
|---|---|---|---|
| Isolated aortic stenosis | 430 (4.8) | 419 (5.1) | 11 (1.6) |
| Moderate | 128 (1.4) | 125 (1.5) | 3 (0.4) |
| Severe | 302 (3.4) | 294 (3.6) | 8 (1.1) |
| Isolated aortic regurgitation | 894 (10.0) | 875 (10.6) | 19 (2.7) |
| Moderate | 642 (7.2) | 631 (7.7) | 11 (1.6) |
| Severe | 252 (2.8) | 244 (3.0) | 8 (1.1) |
| Mixed aortic stenosis and regurgitation | 189 (2.1) | 186 (2.3) | 3 (0.4) |
| Moderate | 48 (0.5) | 47 (0.6) | 1 (0.1) |
| Severe | 141 (1.6) | 139 (1.7) | 2 (0.3) |
| Isolated mitral stenosis | 287 (3.2) | 256 (3.1) | 31 (4.4) |
| Moderate | 168 (1.9) | 149 (1.8) | 19 (2.7) |
| Severe | 119 (1.3) | 107 (1.3) | 12 (1.7) |
| Isolated mitral regurgitation | 2,248 (25.2) | 2,209 (26.9) | 39 (5.6) |
| Moderate | 1,616 (18.1) | 1,594 (19.4) | 22 (3.1) |
| Severe | 632 (7.1) | 615 (7.5) | 17 (2.4) |
| Mixed mitral stenosis and regurgitation | 121 (1.4) | 109 (1.3) | 12 (1.7) |
| Moderate | 75 (0.8) | 67 (0.8) | 8 (1.1) |
| Severe | 46 (0.5) | 42 (0.5) | 4 (0.6) |
| Isolated pulmonary stenosis | 4 (0.0) | 4 (0.1) | 0 |
| Moderate | 2 (0.0) | 2 (0.0) | 0 |
| Severe | 2 (0.0) | 2 (0.0) | 0 |
| Isolated pulmonary regurgitation | 15 (0.2) | 10 (0.1) | 5 (0.7) |
| Moderate | 10 (0.1) | 7 (0.1) | 3 (0.4) |
| Severe | 5 (0.1) | 3 (0.0) | 2 (0.3) |
| Mixed pulmonary stenosis and regurgitation | 0 | 0 | 0 |
| Isolated tricuspid stenosis | 13 (0.2) | 10 (0.1) | 3 (0.4) |
| Moderate | 7 (0.1) | 5 (0.1) | 2 (0.3) |
| Severe | 6 (0.1) | 5 (0.1) | 1 (0.1) |
| Isolated tricuspid regurgitation | 1,527 (17.1) | 1,359 (16.5) | 168 (23.9) |
| Moderate | 1,107 (12.4) | 1,022 (12.4) | 85 (12.1) |
| Severe | 420 (4.7) | 337 (4.1) | 83 (11.8) |
| Mixed tricuspid stenosis and regurgitation | 1 (0.0) | 1 (0.0) | 0 |
| Moderate | 1 (0.0) | 1 (0.0) | 0 |
| Severe | 0 | 0 | 0 |
| Multiple valvular disease | 3200 (35.8) | 2,789 (33.9) | 411 (58.6) |
| Moderate | 1469 (16.5) | 1150 (14.0) | 319 (45.4) |
| Severe | 1731 (19.4) | 1639 (19.9) | 92 (13.1) |
Values are n (%).
Central Illustration.
Distribution and Intervention of Older Patients With VHD
(A) Distribution of patients with native valvular heart disease (VHD). (B) Distribution of patients with severe native VHD. (C) The intervention rates among severe symptomatic patients according to types of VHD. (D) The intervention rates among severe symptomatic patients according to age groups. P values were <0.01 for all trends. AR = aortic regurgitation; AS = aortic stenosis; MR = mitral regurgitation; MS = mitral stenosis.
Figure 1.
Venn Diagram on Distribution of Single and Multiple Native Valve Disease
Venn diagram demonstrate the composition of isolated, mixed and multiple valve lesions and the numbers of these patients. AR = aortic regurgitation; AS = aortic stenosis; MR = mitral regurgitation; MS = mitral stenosis; TR = tricuspid regurgitation.
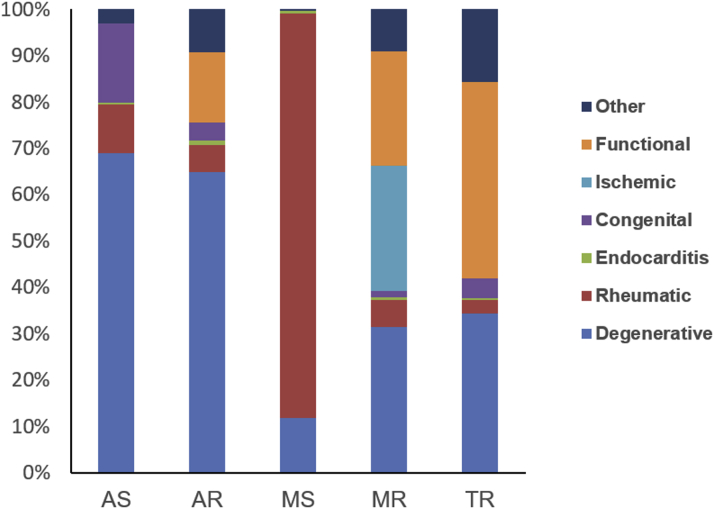
Degenerative (37.2%), functional (21.8%), and rheumatic (15.0%) etiologies were the 3 most common causes in patients with native VHD. A degenerative cause accounted for 69.1% of AS, followed by congenital cause (17.1%), and 64.8% of AR, followed by functional etiology related to aortic dilation or hypertension (15.2%). Degenerative etiology (31.4%) was the leading cause of MR, followed by ischemic (27.0%) and functional (24.8%) etiologies. A rheumatic cause remained most common in MS (87.4%). A functional cause was most frequent (42.5%) in TR, followed by degeneration (34.4%). In multiple VHD, 32.0% and 21.7% were as a result of degenerative and rheumatic causes, respectively (Figure 2, Supplemental Table 1). The etiologies of aortic and mitral VHD according to age are detailed in Supplemental Figure 3.
Figure 2.
Etiology of Native Valve Disease in Older Patients
The bar chart represents the proportions of different etiologies in patients with native valve diseases. Abbreviations as in Figure 1.
Investigations
Echocardiography demonstrated LVEF <50% in 31.1% (45.3% in MR) and pulmonary hypertension in 42.6% of patients. Transesophageal echocardiography was performed in 6.6% of all patients and in 18.8% of patients with intervention for baseline evaluation. Aortic CT was used in 4.6% of all patients (16.5% of AS patients and 8.2% of AR patients) and in 10.0% of patients with intervention. Coronary angiography was performed in 40.9% of all patients and in 66.9% of patients with intervention. Stress tests were performed in only 0.7% of all patients (Table 3, Supplemental Table 2).
Table 3.
Investigations of Older Patients With Valvular Heart Disease
| Total (N = 8,929) | Native Valve Disease |
Native Valve Disease |
Previous Valve Intervention (n = 702) | P Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolated AS (n = 419) | Isolated AR (n = 875) | AS+AR (n = 186) | Isolated MS (n = 256) | Isolated MR (n = 2,209) | MS+MR (n = 109) | Right Sided (n = 1,384) | Multiple (n = 2,789) | ||||
| NT-proBNP, pg/mL | 1,835.0 (630.4-4,614.0) | 973.7 (316.0-2,588.0) | 766.5 (211.0-2,370.0) | 1,679.5 (394.5-4,950.0) | 1,109.0 (621.4-1,944.0) | 1,923.0 (653.0-4,755.0) | 1,567.5 (545.0-4,357.0) | 1,660.0 (639.0-3,417.0) | 2,870.8 (1,275.0-7,031.0) | 1,094.0 (463.7-2,915.0) | <0.001 |
| Cr, μmol/L | 80.8 (67.0-101.0) | 771 (65.5-92.0) | 81.0 (67.0-100.0) | 78.8 (67.2-94.8) | 76.5 (63.0-89.3) | 81.0 (66.0-102.6) | 70.6 (63.0-86.1) | 79.2 (66.0-98.0) | 84.0 (68.6-106.0) | 79.0 (64.7-99.3) | <0.001 |
| TTE | |||||||||||
| LA | 45.0 (39.0-51.0) | 39.0 (35.0-44.0) | 39.0 (35.0-44.0) | 42.0 (38.0-47.0) | 49.0 (45.5-56.4) | 44.0 (40.0-49.0) | 51.0 (45.0-61.0) | 41.0 (36.0-47.0) | 48.0 (42.0-55.0) | 49.0 (42.0-57.0) | <0.001 |
| LVEDD | 52.0 (46.0-60.0) | 49.0 (45.0-54.0) | 57.0 (50.0-63.0) | 55.0 (51.0-61.0) | 47.0 (43.0-50.0) | 56.0 (50.0-63.0) | 48.0 (45.0-52.0) | 46.0 (43.0-51.0) | 54.0 (48.0-63.0) | 48.0 (44.0-54.5) | <0.001 |
| LVEF | <0.001 | ||||||||||
| <30% | 529 (5.9) | 9 (2.2) | 17 (1.9) | 5 (2.7) | 0 | 200 (9.1) | 0 | 43 (3.1) | 226 (8.1) | 29 (4.1) | |
| 30%-49% | 2,246 (25.2) | 56 (13.4) | 183 (20.9) | 38 (20.4) | 19 (7.4) | 800 (36.2) | 17 (15.6) | 185 (13.4) | 852 (30.6) | 96 (13.7) | |
| ≥50% | 6,154 (68.9) | 354 (84.5) | 675 (77.1) | 143 (76.9) | 237 (92.6) | 1,209 (54.7) | 92 (84.4) | 1,156 (83.5) | 1,711 (61.4) | 577 (82.2) | |
| Pulmonary hypertension | 3,799 (42.6) | 68 (16.2) | 99 (11.3) | 41 (22.0) | 112 (43.8) | 695 (31.5) | 39 (35.8) | 751 (54.3) | 1,718 (61.6) | 276 (39.3) | <0.001 |
| TEE | 591 (6.6) | 42 (10.0) | 55 (6.3) | 27 (14.5) | 31 (12.1) | 151 (6.8) | 13 (11.9) | 63 (4.6) | 157 (5.6) | 52 (7.4) | <0.001 |
| Stress test | 63 (0.7) | 7 (1.7) | 9 (1.0) | 0 | 3 (1.2) | 12 (0.6) | 2 (1.8) | 4 (0.3) | 19 (0.7) | 7 (1.0) | 0.034 |
| Aortic CT | 414 (4.6) | 69 (16.5) | 72 (8.2) | 41 (22.0) | 6 (2.3) | 41 (1.9) | 2 (1.8) | 35 (2.5) | 110 (3.9) | 38 (5.4) | <0.001 |
| Coronary angiography | 3,653 (40.9) | 231 (55.1) | 457 (52.2) | 93 (50.0) | 140 (54.7) | 1,072 (48.5) | 58 (53.2) | 437 (31.6) | 993 (35.6) | 172 (24.5) | <0.001 |
Values are median (IQR) or n (%). P values are tested for characteristics among the patients with different types of native valvular diseases.
Cr = creatinine; CT = computed tomography; LA = left atrium; LVEDD = left ventricular end-diastolic dimension; LVEF = left ventricular ejection fraction; NT-proBNP = N-terminal pro–B-type natriuretic peptide; TEE = transesophageal echocardiography; TTE = transthoracic echocardiography; other abbreviations as in Table 1.
Interventions
During index hospitalization, 2,190 (24.5%) patients underwent valve interventions (2,029 on native valve and 161 reoperated), and 207 patients were scheduled. Of patients undergoing intervention, 1,256 were severe symptomatic, 9 had severe VHD with LVEF <50%, 94 were severe but asymptomatic, and 831 had moderate VHD (277 of which had other cardiac procedures) (Supplemental Figure 4).
The intervention rate was 37.3% in symptomatic patients with severe VHD and 11.0% in patients with severe VHD and LVEF <50%. Specifically, interventions were performed in 62.7% of symptomatic patients with severe AS, 57.7% with severe AR, 58.6% with severe MS, 31.5% with severe MR, 45.1% with severe multiple VHD, and only 12.1% with severe right-sided VHD. The intervention rates decreased significantly with age across all types of VHD (all Ptrend < 0.01) (Central Illustration). The reasons for not performing an intervention in severe symptomatic patients included high operative risk in 33.0% and patients’ refusal owing to fear, culture, and affordability in 29.0% of patients. In the patients receiving intervention, 77.1% were ≤70 years of age, and 81.7% had LVEF >30%. Older age, more comorbidities and previous heart surgery, higher B-type natriuretic peptide and creatinine levels, and lower LVEF were observed in patients without intervention compared with those with intervention (Supplemental Table 3). The intervention rates in patients with severe VHD and LVEF <50% according to the types of VHD are detailed in Supplemental Figure 5. The intervention rate was 23.7% in symptomatic patients with degenerative severe MR and LVEF <60%.
The modes of intervention are shown in Table 4. Surgery covered 93.7% of interventions. TAVR was performed in only 7.5% of isolated AS patients and in 1 patient with AR. For MR, 44.3% received surgical valve repair, 55.0% underwent prosthetic valve replacement, and 2 patients received a MitraClip (Abbott Vascular). Bioprostheses were used in 57.0% of surgical patients. Simultaneously, 12.7% of patients underwent coronary artery bypass grafting, 7.3% underwent aortic surgery, and 12.9% underwent antiarrhythmic surgery during the valvular intervention. Perioperative complications occurred in 10.7% of patients, and the in-hospital mortality rate was 1.7%, with the highest rate (3.3%) in AS patients (Table 4, Supplemental Table 4).
Table 4.
Interventions of Older Patients With Valvular Heart Disease
| Total (N = 8,929) | Native Valve Disease |
Previous Valve Intervention (n = 702) | P Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AS (n = 419) | AR (n = 875) | AS+AR (n = 186) | MS (n = 256) | MR (n = 2,209) | MS+MR (n = 109) | Right Sided (n = 1,384) | Multiple (n = 2,789) | ||||
| Intervention | 2,190 (24.5) | 244 (58.2) | 268 (30.6) | 116 (62.4) | 151 (59.0) | 375 (17.0) | 66 (60.6) | 93 (6.7) | 716 (25.7) | 161 (22.9) | <0.001 |
| Surgical valve repair | 292 (13.9) | 0 | 12 (4.7) | 0 | 0 | 141 (44.3) | 0 | 50 (79.4) | 69 (9.8) | 8 (4.8) | |
| Surgical valve replacement | 1,211 (57.7) | 219 (91.6) | 241 (94.9) | 96 (92.3) | 134 (92.4) | 175 (55.0) | 64 (100) | 0 | 184 (26.2) | 87 (52.7) | |
| Balloon dilation | 91 (4.3) | 2 (0.8) | 0 | 0 | 11 (7.6) | 0 | 0 | 13 (20.6) | 24 (3.4) | 8 (4.8) | |
| TAVR | 39 (1.9) | 18 (7.5) | 1 (0.4) | 8 (7.7) | 0 | 0 | 0 | 0 | 11 (1.6) | 1 (0.6) | |
| Mitral clip | 2 (0.1) | 0 | 0 | 0 | 0 | 2 (0.6) | 0 | 0 | 0 | 0 | |
| Multiple mode | 465 (22.1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 414 (59.0) | 51 (30.9) | |
| Bioprosthesis | 981 (56.6) | 145 (62.5) | 165 (69.6) | 63 (58.9) | 74 (54.4) | 109 (61.2) | 32 (49.2) | 0 | 306 (50.2) | 70 (50.0) | <0.001 |
| CABG | 279 (12.7) | 40 (16.4) | 36 (13.4) | 10 (8.6) | 21 (13.9) | 80 (21.3) | 3 (4.5) | 13 (14.0) | 72 (10.1) | 4 (2.5) | <0.001 |
| Aortic surgery | 160 (7.3) | 41 (16.8) | 59 (22.0) | 16 (13.8) | 0 | 1 (0.3) | 1 (1.5) | 2 (2.2) | 34 (4.7) | 6 (7.5) | <0.001 |
| Antiarrhythmic surgery | 283 (12.9) | 13 (5.3) | 8 (3.0) | 5 (4.3) | 28 (18.5) | 41 (10.9) | 14 (21.2) | 14 (15.1) | 138 (19.3) | 22 (13.7) | <0.001 |
| Complications | 233 (10.7) | 37 (15.2) | 28 (10.5) | 10 (8.6) | 15 (9.9) | 31 (8.3) | 3 (4.6) | 6 (6.5) | 83 (11.6) | 20 (12.4) | <0.001 |
| In-hospital death | 37 (1.7) | 8 (3.3) | 5 (1.9) | 3 (2.6) | 1 (0.7) | 3 (0.8) | 1 (1.5) | 0 | 14 (2.0) | 2 (1.2) | 0.268 |
Values are n (%). P values are tested for characteristics among patients with different types of native valvular diseases.
TAVR = transcatheter aortic valve replacement; other abbreviations as in Table 1.
One-year follow-up
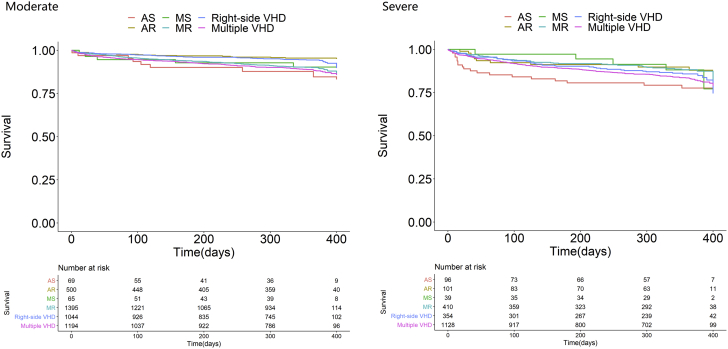
Overall, 131 patients died during the index hospitalization. Of the 8,798 patients discharged alive, 6-month follow-up was available in 7,363 (84.0%) patients, including 456 deaths, and 1-year follow-up was available in 6,259 patients. During follow-up, 2,359 patients were rehospitalized. In patients without a valvular intervention during the index hospitalization, 238 underwent valvular intervention (Supplemental Table 5). The 1-year Kaplan-Meier survival rate was 74.4% (95% CI: 63.4%-85.4%) in all patients. The survival curves among moderate or severe patients without intervention are shown in Figure 3. Among severe symptomatic patients without any intervention, the survival rate was 78.0% for AS, 90.9% for AR, 77.5% for MS, 71.5% for MR, 69.6% for right-sided VHD, and 77.8% for multiple VHD (Supplemental Table 5).
Figure 3.
1-Year Survival Rates of Older Patients With Native Moderate or Severe Valve Disease
The Kaplan-Meier curves of one-year survival are shown according to different VHD in patients with moderate or severe valve diseases. VHD = valvular heart disease; other abbreviations as in Figure 1.
Discussion
This study provided comprehensive insights into the contemporary spectrum, etiologies, characteristics, assessments, management, and outcomes of VHD in China, a large developing country, during a period of economic transition and population aging. It demonstrated that MR was the most common VHD, and AR was more prevalent than AS in older patients. A degenerative cause was most frequent, followed by functional and rheumatic etiologies. The majority of inpatients were at an advanced stage of disease with cardiac insufficiency. The intervention rate was as low as 37.3% in symptomatic patients with severe VHD and significantly decreased with age. A proportion of patients refused surgery owing to high operative risk, fear, and culture. Surgical valve intervention remained the main modality. The overall 1-year survival rate was 74.4%, with a lower rate in patients without intervention.
Distribution and etiology
Variations exist in the prevalence of VHD across regions and populations.1, 2, 3, 4, 5 The differences depend on numerous factors, including not only demographic details, but also the nature of patient group and methodologies such as definition of valve disease and standardization of diagnosis in the different studies as potential reasons.14 Our data showed that MR was most common, followed by TR, AR, AS, and MS in older inpatients with native VHD in China. In a population-based study in the United States, MR was also most frequent in left-sided VHD. However, the 2001 Euro Heart Survey on VHD and 2017 European VHD II Survey showed that AS was the most prevalent native left-sided VHD, followed by MR, AR, and MS.15,16 The finding on MR, TR, and AR prevalence in older Chinese patients warrants the need for design and innovation directed toward these VHDs in less invasive technology and devices. Additionally, AS represents a late manifestation of age-related progressive valve degeneration and is associated with reduced life expectancy.8,17,18 The frequency of severe AS was higher than severe AR in our cohort. The prevalence of AS is anticipated to increase in the future in China with population aging and economic development. Therefore, more health care resources and research should focus on the increasingly prevalent or poor-prognostic types of VHD.
Valve diseases are mainly related to aging in developed countries while rheumatic heart disease (RHD) predominates in developing regions.1, 2, 3, 4, 5,19 Our study showed that degenerative, functional, and rheumatic etiologies were the 3 most common causes in older patients with VHD. Rheumatic valvular disease still accounted for 15.0% of VHD in older people, suggesting that these patients had suffered from RHD from a young age, or they experienced original rheumatic valvular lesion and later degenerative calcification on the valve with increasing age. This concomitance of rheumatic and degenerative etiologies is a unique phenomenon during the transition period of economic development and population aging, which may lead to special structure characteristics and affect the intervention technique. The global burden of nonrheumatic VHD increased between 1990 and 2017.4 However, a high prevalence of RHD persists in Oceania, central sub-Saharan Africa, and South Asia.5 Gross domestic product and education level affect disease-related severity and left ventricular dysfunction.20 Additionally, a congenital cause (17%) in older Chinese patients with AS was higher than that (<5%) in European patients.21 Functional AR covered a proportion of patients (15%). The higher prevalence of congenital AS and functional AR warrants research into TAVR technology and devices tailored for the Chinese population.
Regarding MR, predominant degenerative etiology (31%) was still lower than that (>60%) in European patients in the Euro Heart Survey.15 Half of MR is secondary to ischemic heart disease or left ventricular dilation, suggesting the high burden of secondary MR in China. Considerable effort should be made regarding the treatment of original diseases leading to secondary valve lesions and the expansion of preventive strategies to reduce the incidence and halt the progression of VHD.
Patient characteristics and investigations
Older patients had high frequencies of cardiovascular risk factors, comorbidities, and complex multiorgan diseases in our registry. Hypertension, diabetes, atrial fibrillation, NYHA functional class, chronic obstructive pulmonary disease, and chronic kidney disease were identified as independent predictors of major cardiovascular events in geriatric patients with VHD. These problems not only affect prognosis and life expectancy, but also increase the operative risk as a barrier to and challenge of intervention.22, 23, 24 Effective control of risk factors and treatment for comorbidities potentially slow the progression of VHD, improve the outcomes, make it easier to receive the intervention, and decrease the operative risk.
Most of the older patients admitted to hospital had symptoms (89.4%) and NYHA functional class ≥II (78.0%). Left ventricular dysfunction was present in 31.1% of patients. Transesophageal echocardiography was less frequently performed in our study than in the Euro Heart Survey and VHD II Survey (6.6% vs 18.6% and 18.5%).15,16 CT in patients with aortic VHD is also less performed, probably owing to fewer TAVRs.16 Early detection, appropriate evaluation, and referral are key to improving the prognosis of VHD and need to be emphasized in China.
Interventions
Severe VHD with symptoms or left ventricular dysfunction carries a poor prognosis, while intervention improves the outcomes and quality of life even in the high-aged individuals.2,22, 23, 24 Guidelines on VHD recommend intervention therapy for these patients.25,26 In our study, however, the intervention rates were only 37.0% in severe symptomatic patients and 11.0% in patients with severe VHD and LVEF <50%. Interventions were performed in about 60% of symptomatic patients with severe aortic VHD. Several clinical trials showed that intervention improved survival and functional capacity of MR patients.27,28 However, the intervention rate for MR is consistently suboptimal worldwide29; in our study, it was 32% in symptomatic patients with severe MR. The number of potential candidates for MR intervention may be higher in China due to the high prevalence, which should be considered for health care resources planning.
In our study, 33.0% of patients were denied an intervention due to high operative risk. These patients had older age, more comorbidities, previous heart surgery, and low LVEF. The intervention rates decreased significantly with age across all types of VHD. Previous studies demonstrated that advanced age, comorbidities with chronic renal insufficiency, increased comorbidity index, and left ventricular dysfunction are independent or most striking factors for treatment decision making.29, 30, 31 In addition, 29.0% of patients refused surgery owing to fear, culture, or affordability. Despite advances in techniques, these patients were concerned about the complications of thoracotomy and had cultural reservations about open-heart surgery. Therefore, less invasive procedures are attractive for these patients and will increase the intervention rate. Enhancing their awareness of the therapeutic benefit of intervention independent of age is required. Moreover, economic factor affects the intervention rate with variations in intervention strategies according to country income level.32
Valvular surgery remains the main modality of intervention in China. TAVR was performed in only 7.5% of cases with AS, which is substantially lower than in Europe (39% in 2017), the United States (43% in 2016), and Germany (59% in 2015).16,33,34 With advances in TAVR, this proportion is expected to increase. The operative mortality for mitral valve replacement is higher than that for repair in MR patients.35 In our data, 44.0% of patients underwent valve repair.
1-year survival
The 1-year survival was relatively low. Among symptomatic patients with native left-sided severe VHD without any intervention, the lowest survival rate was observed in patients with MR, which may be related to increased cardiac insufficiency. An increasing intervention rate may improve survival and benefit the older patients.
Study limitations
This inpatient registry was not a community-based epidemiological study. Undiagnosed and mild cases were not included, and it was not possible to determine the prevalence of VHD in the general population of China. Nonetheless, we provided a national overview of the distribution pattern, presentation, and management of clinically significant valvular diseases in hospitalized patients. The study may not have captured all the characteristics of patients with VHD and the bias of hospital selection may exist. However, it included 69 large hospitals from 28 provinces and municipalities throughout China and represents the national contemporary status using accurate and reliable comprehensive data. Even considering the detailed 1-year follow-up, this was not a longitudinal study. Moreover, survival bias cannot be excluded in the analysis of follow-up owing to missing data.
Conclusions
The China-DVD study illustrates the unique distribution, characteristics, and management of older hospitalized patients with VHD in China. More efforts should be made regarding the treatment of original diseases and development of preventive strategies to lower the incidence and progression of VHD. Moreover, early identification and specialist referral for evaluation, timely intervention, enhanced innovation and translational research directed toward advanced techniques and devices, increased application of less invasive interventions, and efficient organization and allocation of health care infrastructure and resources to meet the needs should be highlighted.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Among older patients with single VHD in China, MR was most prevalent. Severe symptomatic patients were markedly undertreated with surgical valve intervention as the main modality, and the intervention rates decreased significantly with age across all types of VHD.
TRANSLATIONAL OUTLOOK: More translational research should be directed toward the increasingly prevalent or poor prognostic types of VHD. Early identification, effective intervention, and widespread application and innovation of less invasive procedures should be highlighted to increase the intervention rate and improve the prognosis among older patients.
Funding Support and Author Disclosures
This work was supported by National Twelfth Five-year Science and Technology Support Projects by Ministry of Science and Technology of China (Grant No. 2015BAI12B02). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank all investigators, coordinators, project managers, and data managers at the China-DVD study group.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For an expanded Methods section and supplemental tables and figures, please see the online version of this paper.
Appendix
References
- 1.Iung B., Vahanian A. Epidemiology of valvular heart disease in the adult. Nat Rev Cardiol. 2011;8(3):162–172. doi: 10.1038/nrcardio.2010.202. [DOI] [PubMed] [Google Scholar]
- 2.Iung B., Vahanian A. Epidemiology of acquired valvular heart disease. Can J Cardiol. 2014;30(9):962–970. doi: 10.1016/j.cjca.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 3.Coffey S., Cairns B.J., Iung B. The modern epidemiology of heart valve disease. Heart. 2016;102(1):75–85. doi: 10.1136/heartjnl-2014-307020. [DOI] [PubMed] [Google Scholar]
- 4.Yadgir S., Johnson C.O., Aboyans V., et al. Global, regional, and national burden of calcific aortic valve and degenerative mitral valve diseases, 1990-2017. Circulation. 2020;141(21):1670–1680. doi: 10.1161/CIRCULATIONAHA.119.043391. [DOI] [PubMed] [Google Scholar]
- 5.Watkins D.A., Johnson C.O., Colquhoun S.M., et al. Global, regional, and national burden of rheumatic heart disease, 1990-2015. N Engl J Med. 2017;377:713–722. doi: 10.1056/NEJMoa1603693. [DOI] [PubMed] [Google Scholar]
- 6.Nkomo V.T. Burden of valvular heart disease: a population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 7.Iung B., Vahanian A. Valvular heart diseases in older people. Lancet. 2006;368(9540):969–971. doi: 10.1016/S0140-6736(06)69216-7. [DOI] [PubMed] [Google Scholar]
- 8.Martinsson A., Li X., Anderson C., Nilsson J., Smith J.G., Sundquist K. Temporal trends in the incidence and prognosis of aortic stenosis a nationwide study of the Swedish population. Circulation. 2015;131:988–994. doi: 10.1161/CIRCULATIONAHA.114.012906. [DOI] [PubMed] [Google Scholar]
- 9.Jang S.Y., Ju E.Y., Seo S.R., et al. Changes in the etiology of valvular heart disease in the rapidly aging Korean population. Int J Cardiol. 2014;174(2):355–359. doi: 10.1016/j.ijcard.2014.04.112. [DOI] [PubMed] [Google Scholar]
- 10.Zhimin W., Yubao Z., Lei S., et al. Prevalence of chronic rheumatic heart disease in Chinese adults. Int J Cardiol. 2006;107(3):356–359. doi: 10.1016/j.ijcard.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 11.Liu F.Z., Xue Y.M., Liao H.T., et al. Five-year epidemiological survey of valvular heart disease: changes in morbidity, etiological spectrum and management in a cardiovascular center of Southern China. J Throrac Dis. 2014;6:1724–1730. doi: 10.3978/j.issn.2072-1439.2014.11.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.d'Arcy J.L., Coffey S., Loudon M.A., et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: the OxVALVE Population Cohort Study. Eur Heart J. 2016;37:3515–3522. doi: 10.1093/eurheartj/ehw229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andell P., Li X., Martinsson A., et al. Epidemiology of valvular heart disease in a Swedish nationwide hospital-based register study. Heart. 2017;103:1696–1703. doi: 10.1136/heartjnl-2016-310894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otto C.M., Bonow R.O. Valvular Heart Disease: A Companion to Braunwald’s Heart Disease. 4th. ed. Elsevier Saunders; 2014. Epidemiology of valvular heart disease; pp. 1–13. [Google Scholar]
- 15.Iung B., Baron G., Butchart E.G., et al. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24(13):1231–1243. doi: 10.1016/s0195-668x(03)00201-x. [DOI] [PubMed] [Google Scholar]
- 16.Iung B., Delgado V., Rosenhek R., et al. EORP VHD II Investigators Contemporary presentation and management of valvular heart disease: the EURObservationalResearch Programme Valvular Heart Disease II Survey. Circulation. 2019;140(14):1156–1169. doi: 10.1161/CIRCULATIONAHA.119.041080. [DOI] [PubMed] [Google Scholar]
- 17.Smith J.G., Luk K., Schulz C.A., et al. Cohorts for Heart and Aging Research in Genetic Epidemiology (CHARGE) Extracoronary Calcium Working Group Association of low-density lipoprotein cholesterol-related genetic variants with aortic valve calcium and incident aortic stenosis. JAMA. 2014;312(17):1764–1771. doi: 10.1001/jama.2014.13959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonow R.O., Greenland P. Population-wide trends in aortic stenosis incidence and outcomes. Circulation. 2015;131(11):969–971. doi: 10.1161/CIRCULATIONAHA.115.014846. [DOI] [PubMed] [Google Scholar]
- 19.Nkomo V.T. Epidemiology and prevention of valvular disease and infective endocarditis in Africa. Heart. 2007;93:1510–1519. doi: 10.1136/hrt.2007.118810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kingué S., Ba S.A., Balde D., et al. Working Group on Tropical Cardiology of the Société française de cardiologie The VALVAFRIC study: a registry of rheumatic heart disease in Western and Central Africa. Arch Cardiovasc Dis. 2016;109(5):321–329. doi: 10.1016/j.acvd.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Iung B., Baron G., Tornos P., Gohlke-Bärwolf C., Butchart E.G., Vahanian A. Valvular heart disease in the community: a European experience. Curr Probl Cardiol. 2007;32(11):609–661. doi: 10.1016/j.cpcardiol.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Kodali S.K., Velagapudi P., Hahn R.T., Abbott D., Leon M.B. Valvular heart disease in patients ≥80 years of age. J Am Coll Cardiol. 2018;71(18):2058–2072. doi: 10.1016/j.jacc.2018.03.459. [DOI] [PubMed] [Google Scholar]
- 23.Barreto-Filho J.A., Wang Y., Dodson J.A., et al. Trends in aortic valve replacement for older patients in the United States, 1999-2011. JAMA. 2013;310:2078–2085. doi: 10.1001/jama.2013.282437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song F., Liu F.Z., Liang Y.F., et al. Clinical, sonographic characteristics and longterm prognosis of valvular heart disease in older patients. J Geriatr Cardiol. 2019;16(1):33–41. doi: 10.11909/j.issn.1671-5411.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baumgartner H., Falk V., Bax J.J., et al. ESC Scientific Document Group 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38(36):2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 26.Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2020;77(4):e25–e197. doi: 10.1016/j.jacc.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 27.Feldman T., Foster E., Glower D.D., et al. EVEREST II Investigators Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395–1406. doi: 10.1056/NEJMoa1009355. [DOI] [PubMed] [Google Scholar]
- 28.Feldman T., Kar S., Elmariah S., et al. EVEREST II Investigators Randomized comparison of percutaneous repair and surgery for mitral regurgitation: 5-year results of EVEREST II. J Am Coll Cardiol. 2015;66:2844–2854. doi: 10.1016/j.jacc.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 29.Dziadzko V., Clavel M.A., Dziadzko M., et al. Outcome and undertreatment of mitral regurgitation: a community cohort study. Lancet. 2018;391(10124):960–969. doi: 10.1016/S0140-6736(18)30473-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu K., Li J., Wan Y., et al. Heart valve disease in older Chinese population: effect of advanced age and comorbidities on treatment decision-making and outcomes. J Geriatr Cardiol. 2016;13:593–601. doi: 10.11909/j.issn.1671-5411.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iung B., Cachier A., Baron G., et al. Decision-making in older patients with severe aortic stenosis: why are so many denied surgery? Euro Heart J. 2005;26(24):2714–2720. doi: 10.1093/eurheartj/ehi471. [DOI] [PubMed] [Google Scholar]
- 32.Zühlke L., Engel M.E., Karthikeyan G., et al. Characteristics, complications, and gaps in evidence-based interventions in rheumatic heart disease: the Global Rheumatic Heart Disease Registry (the REMEDY study) Eur Heart J. 2015;36(18):1115–11522a. doi: 10.1093/eurheartj/ehu449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stachon P., Zehender M., Bode C., von Zur Mühlen C., Kaier K. Development and in-hospital mortality of transcatheter and surgical aortic valve replacement in 2015 in Germany. J Am Coll Cardiol. 2018;72:475–476. doi: 10.1016/j.jacc.2018.04.077. [DOI] [PubMed] [Google Scholar]
- 34.Alkhouli M., Alqahtani F., Ziada K.M., Aljohani S., Holmes D.R., Mathew V. Contemporary trends in the management of aortic stenosis in the USA. Eur Heart J. 2020;41(8):921–928. doi: 10.1093/eurheartj/ehz568. [DOI] [PubMed] [Google Scholar]
- 35.Gammie J.S., Sheng S., Griffith B.P., et al. Trends in mitral valve surgery in the United States: results from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg. 2009;87(5):1431–1437. doi: 10.1016/j.athoracsur.2009.01.064. [discussion: 1437-1439] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.