Abstract
Background
Atrial fibrillation (AF) is common in heart failure with preserved ejection fraction (HFpEF).
Objectives
This study aimed to investigate the prognostic value of echocardiographic markers of congestion that can be applied to both AF and patients without AF with HFpEF.
Methods
We conducted a multicenter study of 505 patients with HFpEF admitted to hospitals for acute decompensated heart failure. The ratio of early diastolic transmitral flow velocity to mitral annulus velocity (E/e′), the tricuspid regurgitation peak velocity, and the collapsibility of the inferior vena cava were obtained at discharge. Congestion was determined by echocardiography if any one of E/e′ ≥14 (E/e′ ≥11 for AF), tricuspid regurgitation peak velocity ≥2.8 m/s, or inferior vena cava collapsibility <50% was positive. We classified patients into grade A, grade B, and grade C according to the number of positive congestion indices. The primary endpoint was the composite of cardiovascular death and heart failure hospitalization.
Results
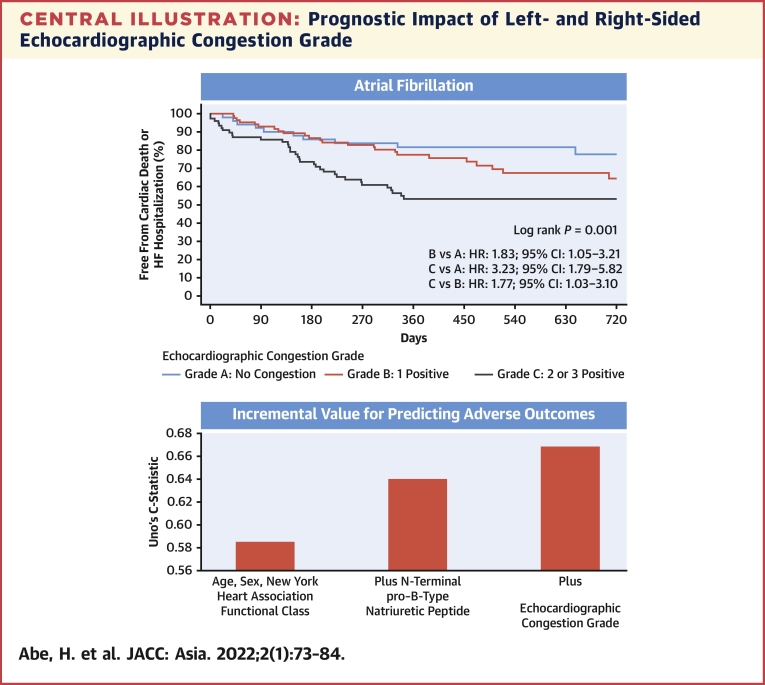
During the follow-up period (median: 373 days), 162 (32%) patients experienced the primary endpoint. Grade C patients had a higher risk for the primary endpoint than grade A (HR: 2.98; 95% CI: 1.97-4.52) and grade B patients (HR: 1.92; 95% CI: 1.29-2.86) (log-rank P < 0.0001). Echocardiographic congestion grade improved the predictive value when added to the age, sex, New York Heart Association functional class, and N-terminal pro–B-type natriuretic peptide, not only in sinus rhythm (Uno C-statistic: 0.670 vs 0.655) but in AF (Uno C-statistic: 0.667 vs 0.639).
Conclusions
Echocardiographic congestion grade has prognostic value in patients with HFpEF with and without AF.
Key Words: congestion, echocardiography, heart failure with preserved ejection fraction, prognosis
Abbreviations and Acronyms: AF, atrial fibrillation; ASE, American Society of Echocardiography; E/e′, ratio of early diastolic transmitral flow velocity to mitral annulus velocity; EACVI, European Association of Cardiovascular Imaging; HFpEF, heart failure with preserved ejection fraction; IVC, inferior vena cava; IVCC, inferior vena cava collapsibility; NT-proBNP, N-terminal pro–B-type natriuretic peptide; NYHA, New York Heart Association; TRV, tricuspid regurgitation peak velocity
Central Illustration
With the increasing incidence of heart failure with preserved ejection fraction (HFpEF) without accompanying improvements in survival, risk stratification is important for optimizing the treatment of HFpEF.1 Recently, objective evidence of cardiogenic pulmonary or systemic congestion was proposed as an important part of the universal definition of heart failure.2 Pulmonary congestion characterized by elevated left atrial pressure and subsequent pulmonary hypertension can be estimated by an echocardiographic marker of left-sided congestion. Systemic congestion characterized by elevated right atrial pressure can be estimated by an echocardiographic marker of right-sided congestion. Right-sided congestion as well as left-sided congestion have negative impacts on a heart failure prognosis.3,4 Although echocardiography is a useful noninvasive means with which to evaluate left- and right-sided congestion, the prognostic impact of the measurement of the inferior vena cava (IVC) as a measure of right-sided congestion has received little attention.5,6 In contrast, echocardiographic assessment of left-sided congestion has received much attention.7 The American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI) guidelines for the evaluation of left ventricular diastolic function proposed an algorithm for estimating left ventricular filling pressure.8 A major limitation with this algorithm is that the ratio of early and late transmitral flow peak velocity cannot be available in patients with atrial fibrillation (AF) despite the high prevalence of AF in HFpEF. In addition, the left atrial volume index in patients with AF is not simply a measure of left atrial pressure but reflects left atrial remodeling due to AF. On the other hand, the ratio of early diastolic transmitral flow velocity to mitral annulus velocity (E/e′), tricuspid regurgitation peak velocity (TRV), and the IVC diameter, which together assess left- and right-sided congestion, are all available in patients with AF with HFpEF. Nonetheless, the prognostic impact of integrated echocardiographic assessment of left- and right-sided congestion has not been thoroughly investigated in HFpEF.
In this study, we determined congestion to be present if any one of the averaged E/e′ ≥14 (≥11 for AF), TRV ≥2.8 m/s, or IVCC < 50% was positive using echocardiography. We further classified patients into grade A (no congestion), grade B (1 index was positive), and grade C (2 or 3 indices were positive) at discharge. The purpose of this observational multicenter study was to assess the prognostic impact of echocardiographically evaluated left- and right-sided congestion in patients with HFpEF with AF as well as in those in sinus rhythm.
Methods
Study subjects
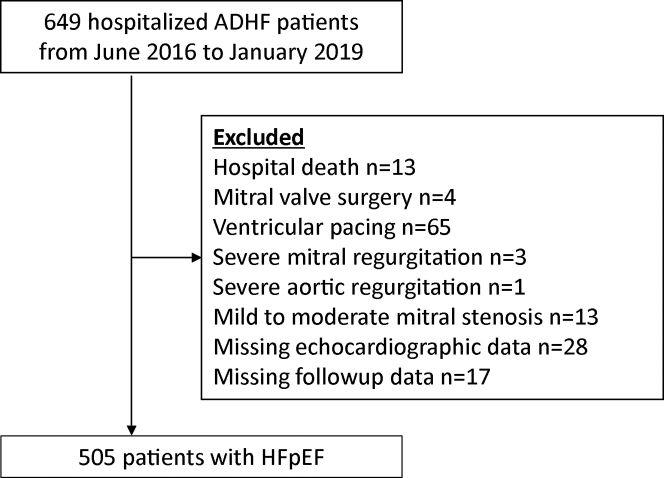
This study is a post hoc analysis of the PURSUIT-HFpEF (Prospective Multicenter Observational Study of Heart Failure With Preserved Ejection Fraction) registry. The rationale and design of the PURSUIT-HFpEF registry have been previously described.9 Collaborating hospitals in the Osaka urban area recorded clinical, echocardiographic, and outcome data of patients with acute decompensated heart failure and preserved left ventricular ejection fraction (≥50%) (UMIN000021831). The anonymized data were transferred to the data center of Osaka University Hospital for analysis. Acute decompensated heart failure was diagnosed based on the following inclusion criteria: 1) clinical symptoms and signs according to the Framingham Heart Study criteria; and 2) serum N-terminal pro–B-type natriuretic peptide (NT-proBNP) level of ≥400 pg/mL or brain natriuretic peptide of ≥100 pg/mL on admission. Of 649 hospitalized patients with acute decompensated heart failure, 505 patients were analyzed in this study (Figure 1). The study complies with the Declaration of Helsinki, and the protocol was approved by the local ethics committee of each participating hospital, including the National Hospital Organization Osaka National Hospital Institutional Review Board No. 2 (approval no. 16024) and Osaka University Graduate School of Medicine. All patients provided written informed consent for the use of their data.
Figure 1.
Flowchart of the Study Population
ADHF = acute decompensated heart failure; HFpEF = heart failure with preserved ejection fraction.
Echocardiography
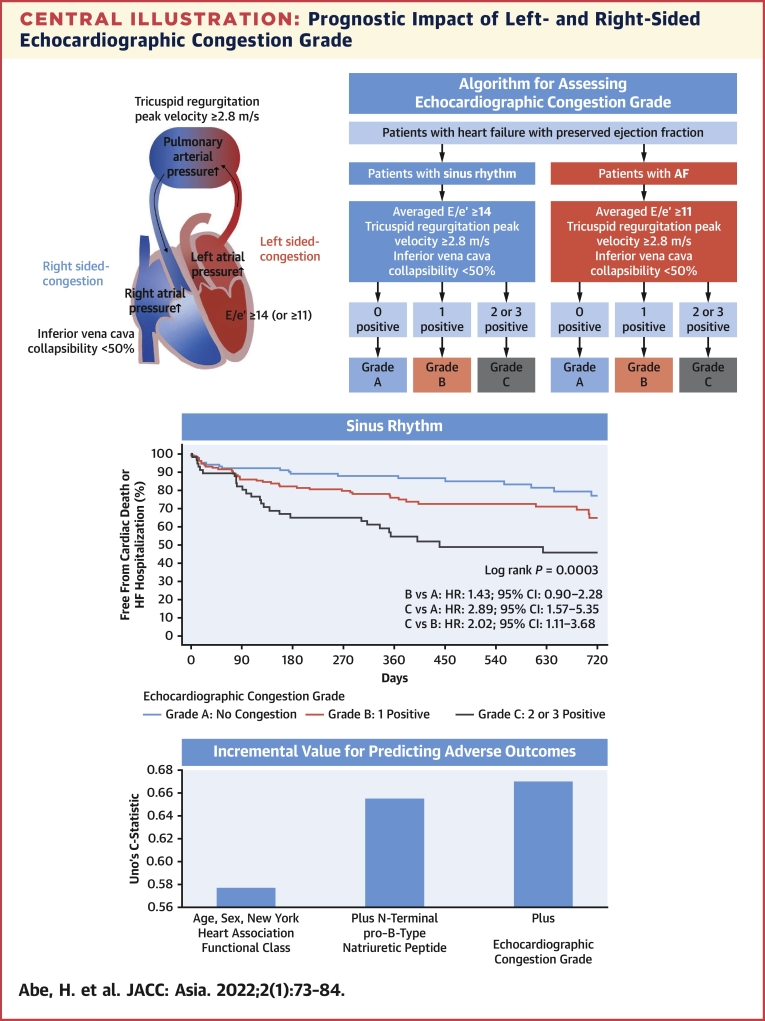
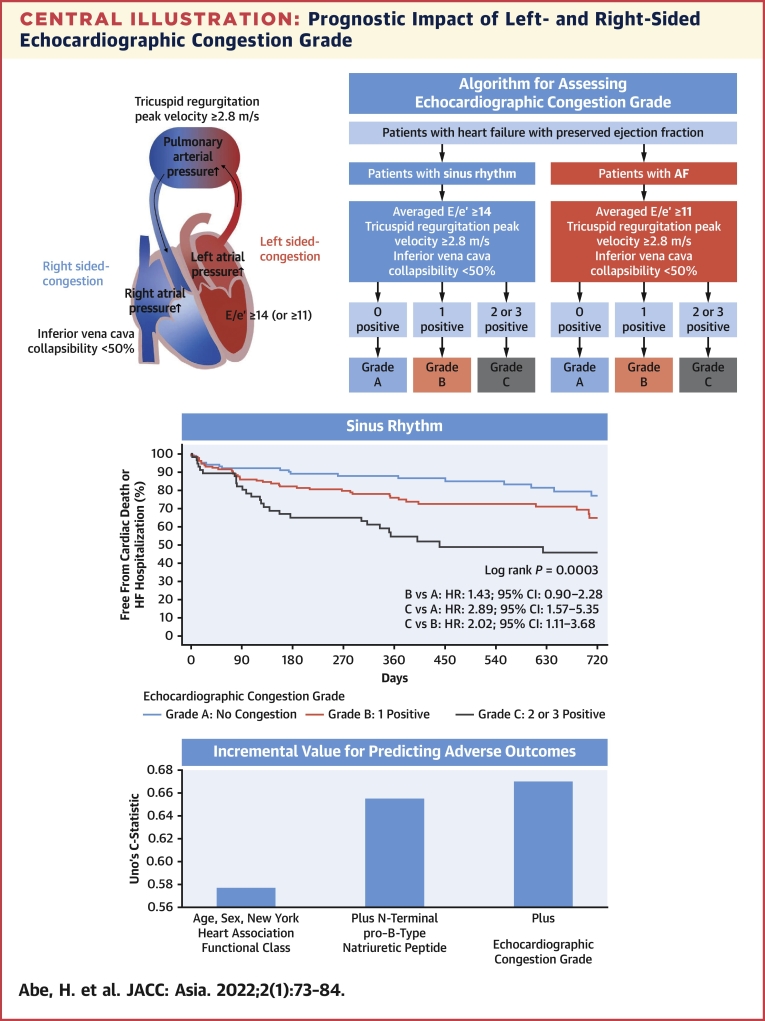
Comprehensive transthoracic echocardiography was performed after the treatment and resolution of heart failure symptoms. Conventional echocardiographic variables were recorded and analyzed by an experienced sonographer in each hospital according to the current ASE/EACVI recommendations.8,10,11 Left ventricular volumes and left ventricular ejection fraction were calculated using the biplane Simpson method. Peak velocity of early mitral inflow (E) was recorded. Tissue Doppler imaging was used to measure the early peak diastolic velocity (e′) of the septal and lateral mitral annulus. The averaged E/e′ ratio was calculated as E divided by the mean of the septal and lateral e′ velocities. TRV was also recorded. In patients with AF, the E/e′ ratio and TRV were measured by the method used by each hospital in daily echocardiography. At most hospitals, the E/e′ ratio and TRV were measured by a single representative beat assessment or index beat assessment (Supplemental Figure 1). The single representative beat assessment measured E, e′, and TRV that appeared to have an averaged value, judged by an experienced cardiac sonographer. Index beat assessment measured the E/e′ ratio and TRV, whose preceding R-R interval and prepreceding R-R interval were similar to those used for calculating E/e′ and TRV. With the patient supine, the diameters of the IVC were measured approximately 3 cm before the merger with the right atrium at end expiration (IVC max) and at inspiration with sniffing (IVC min).11 The collapsibility of the IVC (IVCC) was calculated as IVC max minus IVC min divided by IVC max. We used IVCC as a surrogate marker for right-sided congestion. Although the IVC dimensions are influenced by the body size along with elevated right atrial pressures, the cutoff point of IVCC for estimating right atrial pressure has been known to be unaffected by the difference in body surface area.12 Left atrial volume index was measured by the biplane method and indexed by the body surface area. Stroke volume was calculated as the product of the diameter of the left ventricular outflow tract and the velocity-time integral measured by a pulsed-wave Doppler method. Cardiac index was calculated by dividing the product of stroke volume and heart rate by body surface area. Echocardiographic congestion was determined if any one of those was present: averaged values of E/e′ were ≥14 for sinus rhythm or ≥11 for AF, TRV was ≥2.8 m/s, or IVCC was <50% at discharge. Echocardiographic cutoff values of averaged E/e′, TRV, and IVCC were adopted from the ASE/EACVI guidelines.8,11 Echocardiographic congestion grade was classified into grade A (no congestion), grade B (1 index was positive), and grade C (2 or 3 indices were positive) at discharge. If the indices were not available, they were considered negative (Central Illustration).
Central Illustration.
Prognostic Impact of Left- and Right-Sided Echocardiographic Congestion Grade
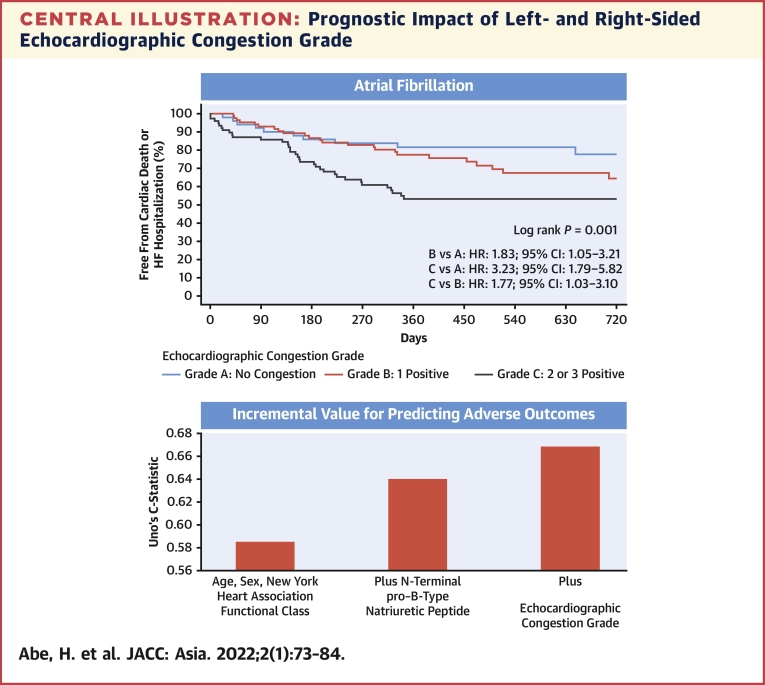
As shown in the flow chart, echocardiographic congestion grade was determined by the number of positive findings using different criteria for averaged E/e′ in sinus rhythm and AF. Echocardiographic congestion grade was determined for patients for whom at least 1 of the 3 indices was obtained. Only positive indices were used in the flowchart, and indices that could not be obtained were considered negative. Echocardiographic congestion grade was classified into grade A (no congestion), grade B (1 index was positive), and grade C (2 or 3 indices were positive) at discharge. The Kaplan-Meier curves for cardiovascular mortality and heart failure hospitalization among the 3 echocardiographic congestion grades were shown. Echocardiographic congestion grade improved the predictive value when added to age, sex, New York Heart Association functional class, and N-terminal pro–B-type natriuretic peptide, not only in sinus rhythm but also in AF. AF = atrial fibrillation; E/e′ = the ratio of early transmitral flow peak velocity to early diastolic peak velocity of the mitral annular plane; HF = heart failure.
Clinical outcomes
All patients were followed up by each admitting hospital. Survival data were obtained by dedicated coordinators and investigators via direct contact with patients and their physicians at the hospital or in outpatient settings, via a telephone interview with their families, or by mail. The primary endpoint was the composite of cardiovascular death or hospitalization for worsening heart failure.
Statistical analyses
All continuous variables are expressed as mean ± SD or median and interquartile range (25th to 75th percentile) as appropriate, and categorical variables are expressed as percentages. Student’s t-test or 1-way analysis of variance was used to compare differences in normally distributed continuous variables. The Kruskal-Wallis rank sum test was used to compare differences in nonnormally distributed data. The chi-square test was used to compare between-group differences in categorical variables. Cox hazard analyses were used to determine independent predictors for cardiovascular death or hospitalization for worsening heart failure. The multivariable Cox hazard model included well-established major confounders for heart failure, such as age, sex, New York Heart Association (NYHA) functional class, hemoglobin, serum sodium, albumin, total bilirubin, and NT-proBNP at discharge. For the Cox hazard analysis of echocardiographic congestion grade, grades A, B, and C were set as continuous variables 0, 1, and 2. Uno’s C-statistic of each model was used as a measure of discrimination to predict survival time.13 The initial clinical model included age, sex, and NYHA functional class. The second model included age, sex, and NYHA functional class plus NT-proBNP. The third model included age, sex, NYHA functional class, and NT-proBNP plus echocardiographic congestion grade. We also performed 10-fold cross validation. Patients were split randomly into 10 groups. For each group, step 1 removed that group from analysis, step 2 fitted the model on the remaining 9 groups, step 3 used the model to predict the outcome in the group that was removed, and step 4 stored predictive measures. Finally, we averaged 10 predictive values of Uno’s C-statistic. Additional statistical analyses are described in the supplemental tables and figures. Event-free survival after discharge was estimated using the Kaplan-Meier analysis and compared using the log-rank test. MedCalc Statistical Software, version 20.008 (MedCalc Software Ltd); SPSS for Windows, version 23.0 (IBM Corp); and EZR, version 1.54 (Saitama Medical Center, Jichi Medical University) were used to perform all statistical analyses. A P value of <0.05 was considered statistically significant.
Results
Patient characteristics
Patient characteristics are summarized in Table 1. Age and comorbidities are given on admission, and all the others are at discharge. The median age was 82 years. Of the 505 patients, 289 (57%) were in sinus rhythm, and 216 (43%) were in AF. Female sex and NYHA functional class II were more common in grade B/C than in grade A. Hemoglobin was significantly lower, and NT-proBNP was significantly higher, in grade C than in grades A or B.
Table 1.
Baseline Characteristics
| Total (N = 505) | Grade A (n = 153) | Grade B (n = 217) | Grade C (n = 135) | P Value | |
|---|---|---|---|---|---|
| Age, y | 82 (76-87) | 80 (74-84) | 83 (76-87)a | 84 (78-89)b,c | <0.0001 |
| Female | 273 (54) | 63 (41) | 126 (58) | 84 (62) | 0.0005 |
| BMI, kg/m2 | 21.4 (18.7-24.3) | 20.8 (18.5-23.8) | 21.7 (18.8-24.6) | 21.6 (19.3-24.7) | 0.096 |
| SBP, mm Hg | 118 (18) | 118 (18) | 119 (18) | 120 (18) | 0.78 |
| Heart rate, beats/min | 71 (13) | 72 (13) | 71 (13) | 71 (14) | 0.94 |
| AF | 216 (43) | 51 (33) | 87 (40) | 78 (58) | 0.0001 |
| NYHA functional class | |||||
| I | 188 (37) | 74 (48) | 75 (35) | 39 (29) | |
| II | 288 (57) | 73 (48) | 127 (59) | 88 (65) | |
| III or IV | 24 (5) | 5 (3) | 11 (5) | 8 (6) | 0.013 |
| Comorbidities | |||||
| Ischemic | 135 (27) | 49 (32) | 51 (24) | 35 (26) | 0.18 |
| Hypertension | 435 (86) | 128 (83) | 189 (87) | 118 (87) | 0.50 |
| DM | 176 (35) | 45 (29) | 82 (38) | 49 (36) | 0.20 |
| COPD | 36 (7) | 11 (7) | 14 (6) | 11 (8) | 0.78 |
| Medications | |||||
| Loop diuretics | 406 (80) | 120 (78) | 168 (77) | 118 (87) | 0.055 |
| Other diuretics | 103 (20) | 23 (15) | 47 (22) | 33 (24) | 0.11 |
| ACE inhibitor or ARB | 276 (55) | 78 (51) | 126 (58) | 72 (53) | 0.38 |
| Beta blocker | 292 (58) | 97 (63) | 116 (53) | 79 (59) | 0.18 |
| Aldosterone antagonist | 202 (40) | 61 (39) | 83 (38) | 58 (43) | 0.68 |
| Laboratory data | |||||
| Hemoglobin, g/dL | 11.5 ± 2.0 | 11.7 ± 1.9 | 11.6 ± 2.2 | 11.1 ± 1.9b,c | 0.036 |
| Serum sodium, mEq/L | 139 (137-141) | 139 (137-141) | 140 (137-141) | 139 (137-141) | 0.24 |
| Albumin, g/dL | 3.4 (3.1-3.7) | 3.4 (3.2-3.7) | 3.4 (3.1-3.7) | 3.3 (3.0-3.7) | 0.27 |
| Total bilirubin, mg/dL | 0.6 (0.4-0.8) | 0.6 (0.4-0.8) | 0.6 (0.4-0.7) | 0.6 (0.4-0.9) | 0.22 |
| BUN, mg/dL | 24 (18-34) | 24 (18-33) | 23 (18-33) | 25 (19-36) | 0.48 |
| eGFR, mL/min/1.73 m2 | 43 ± 19 | 44 ± 21 | 44 ± 19 | 41 ± 17 | 0.18 |
| NT-proBNP, pg/mL | 1,100 (531-2,600) | 887 (426-1,697) | 929 (485-2,556) | 1,782 (810-4,058)b,c | <0.0001 |
| CRP, mg/L | 0.29 (0.10-0.80) | 0.28 (0.10-0.88) | 0.29 (0.10-0.62) | 0.30 (0.12-1.21) | 0.32 |
Values are median (interquartile range), n (%), or mean ± SD. Age and comorbidities are given on admission, and all the others are at discharge. The statistical difference between variables is given for the comparison between echocardiographic congestion grades.
ACE = angiotensin converting enzyme; AF = atrial fibrillation; ARB = angiotensin II receptor blocker; BMI = body mass index; BUN = blood urea nitrogen; COPD = chronic obstructive pulmonary disease; CRP = C-reactive protein; DM = diabetes mellitus; eGFR = estimated glomerular filtration ratio; NT-proBNP = N-terminal pro–B-type natriuretic peptide; NYHA = New York Heart Association; SBP = systolic blood pressure.
P < 0.05, grade B versus grade A.
P < 0.05, grade C versus grade A.
P < 0.05, grade C versus grade B.
Echocardiographic characteristics
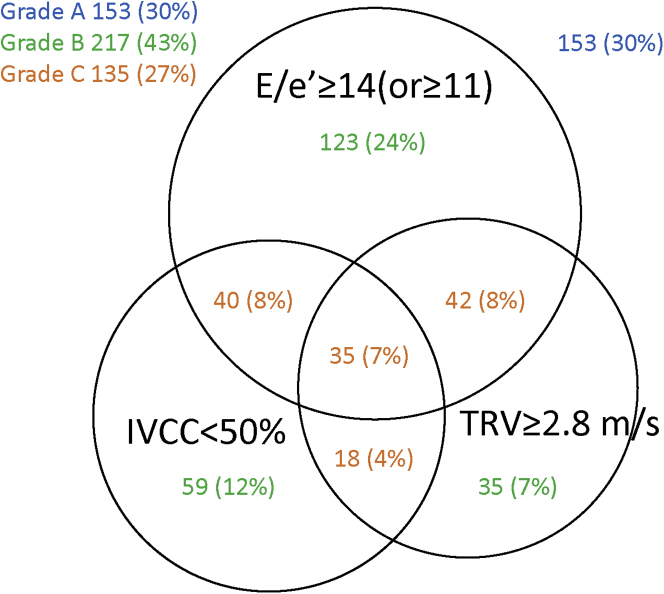
Echocardiographic characteristics are summarized in Table 2. E/e′, TRV, and IVCC showed significant differences among the groups at discharge. TRV was measurable without enhancing the signal by administration of intravenous saline or echocardiography contrast agents in 90% of patients with sinus rhythm and in 92% of patients with AF. E/e′ improved significantly from 16.9 ± 7.5 on admission to 15.9 ± 6.4 at discharge (P = 0.001). TRV improved significantly from 3.0 ± 0.5 m/s on admission to 2.6 ± 0.4 m/s at discharge (P < 0.0001). IVCC improved significantly from 42% ± 21% on admission to 56% ± 19% at discharge (P < 0.0001). Nevertheless, 352 (70%) of the 505 patients had at least 1 sign of congestion, and 135 (27%) had 2 or 3 signs of congestion even after the decongestion therapy (Figure 2).
Table 2.
Echocardiographic Characteristics
| Total (N = 505) | Grade A (n = 153) | Grade B (n = 217) | Grade C (n = 135) | P Value | |
|---|---|---|---|---|---|
| EDVI, mL/m2 | 51 (39-65) | 40 (21-53) | 48 (38-65) | 52 (40-65) | 0.49 |
| ESVI, mL/m2 | 19 (14-26) | 21 (15-26) | 18 (13-25) | 20 (14-26) | 0.26 |
| LVEF, % | 61 ± 8 | 60 ± 8 | 61 ± 7 | 60 ± 9 | 0.73 |
| E, m/s | 0.83 ± 0.29 | 0.67 ± 0.21 | 0.83 ± 0.26a | 1.01 ± 0.30b,c | <0.001 |
| Septal e′, m/s | 0.056 ± 0.019 | 0.058 ± 0.020 | 0.055 ± 0.019 | 0.055 ± 0.019 | 0.17 |
| Lateral e′, m/s | 0.076 ± 0.027 | 0.081 ± 0.026 | 0.074 ± 0.028a | 0.073 ± 0.024b | 0.020 |
| Septal E/e′ | 16.1 ± 6.5 | 11.8 ± 3.0 | 16.5 ± 6.4a | 20.0 ± 6.9b,c | <0.001 |
| Lateral E/e′ | 12.1 ± 5.3 | 8.5 ± 1.9 | 12.6 ± 5.5a | 14.9 ± 5.3b,c | <0.001 |
| Average E/e′ | 13.7 ± 5.5 | 9.7 ± 1.9 | 14.2 ± 5.6a | 16.9 ± 5.4b,c | <0.001 |
| TRV, m/s | 2.6 ± 0.4 | 2.4 ± 0.2 | 2.5 ± 0.4a | 2.9 ± 0.5b,c | <0.001 |
| IVCmax, mm | 13 (11-17) | 12 (10-15) | 13 (10-16)a | 16 (13-19)b,c | <0.0001 |
| IVCmin, mm | 7 (4-8) | 4 (3-6) | 6 (4-7)a | 9 (7-12)b,c | <0.0001 |
| IVCC, % | 55 ± 19 | 65 ± 14 | 56 ± 18a | 44 ± 18b,c | <0.001 |
| LAVI, mL/m2 | 51 (36-64) | 46 (32-62) | 47 (36-61) | 58 (45-78)b,c | <0.0001 |
| LV mass index, g/m2 | 101 (83-120) | 100 (83-119) | 101 (81-120) | 104 (89-123) | 0.34 |
| Cardiac index, L/min/m2 | 2.57 (2.03-3.22) | 2.52 (1.98-3.18) | 2.61 (2.12-3.23) | 2.57 (2.01-3.22) | 0.78 |
Values are median (interquartile range) or mean ± SD. The statistical difference between variables is given for the comparison between echocardiographic congestion grades.
E = early transmitral flow peak velocity; e′ = early diastolic peak velocity of the mitral annular plane; E/e′ = the ratio of early transmitral flow peak velocity to early diastolic peak velocity of the mitral annular plane; EDVI = end-diastolic left ventricular volume index; ESVI = end-systolic left ventricular volume index; IVCC = inferior vena cava collapsibility; IVCmax = maximal diameter of inferior vena cava at expiration; IVCmin = minimal diameter of inferior vena cava with sniffing; LAVI = left atrial volume index; LV = left ventricular; LVEF = left ventricular ejection fraction; TRV = tricuspid regurgitation peak velocity.
P < 0.05, grade B versus grade A.
P < 0.05, grade C versus grade A.
P < 0.05, grade C versus grade B.
Figure 2.
Interrelationship Between E/e′, TRV, and IVCC Among Patients With Heart Failure With Preserved Ejection Fraction
Even after decongestion therapy, 27% of patients showed grade C echocardiographic congestion. E/e′ = the ratio of early transmitral flow peak velocity to early diastolic peak velocity of the mitral annular plane; IVCC = inferior vena cava collapsibility; TRV = tricuspid regurgitation peak velocity.
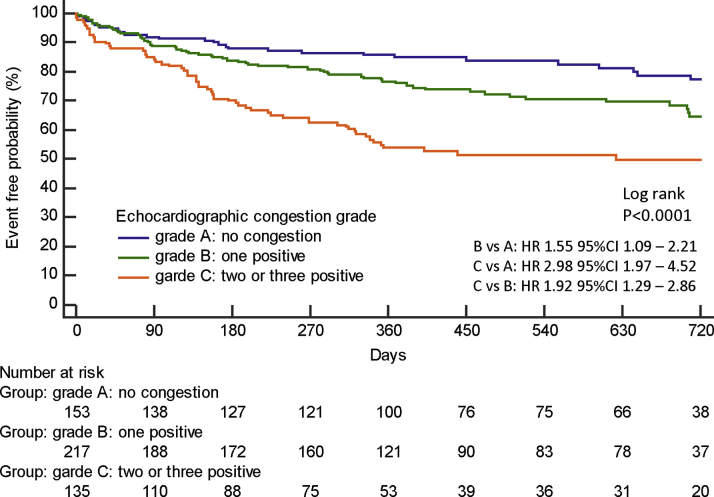
Cardiovascular death and heart failure hospitalization
During a median follow-up of 373 days (range: 198-706 days), all-cause death occurred in 77 (15%) patients, which included 22 (4%) cardiovascular deaths and 55 (11%) noncardiovascular deaths. Hospitalization for worsening heart failure occurred in 140 (28%) patients.
Table 3 shows univariable and multivariable Cox hazard analyses for cardiovascular death and heart failure hospitalization. Log NT-proBNP and echocardiographic congestion grade were independent predictors for the primary endpoint (HR: 2.31; 95% CI: 1.66-3.23; P < 0.0001; and HR: 1.51; 95% CI: 1.19-1.92; P = 0.0006, respectively). The HR of echocardiographic congestion grade B and grade C increased in the prediction of the primary endpoint (HR: 1.59; 95% CI: 1.00-2.51; P = 0.049; and HR: 2.37; 95% CI: 1.48-3.81; P = 0.003, respectively) when a categorical value with grade A was considered as the reference. Table 4 shows the multivariable Cox hazard analysis for the primary endpoint in patients with HFpEF in both sinus rhythm and AF. NT-proBNP and echocardiographic congestion grade were associated with the primary endpoint both in sinus rhythm and AF. Additional multivariable Cox hazard analyses were performed in the subgroup of patients with AF by including the grade of mitral regurgitation and tricuspid regurgitation. Even if the grade of mitral regurgitation and tricuspid regurgitation was included, E/e′, TRV, and IVCC was still the best combination in the prediction of the primary endpoint (HR: 1.70; 95% CI: 1.22-2.36; P = 0.002) in patients with AF (Supplemental Table 1).
Table 3.
Univariable and Multivariable Cox Hazard Analysis for the Primary Endpoint
| Univariable Analysis |
Multivariable Analysis |
|||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age | 1.02 (1.01-1.04) | 0.011 | 1.01 (0.99-1.03) | 0.28 |
| Female | 1.21 (0.88-1.65) | 0.23 | 0.93 (0.65-1.32) | 0.68 |
| NYHA functional class | 1.30 (0.99-1.69) | 0.060 | 1.03 (0.76-1.41) | 0.83 |
| Hemoglobin | 0.87 (0.80-0.94) | 0.0009 | 0.94 (0.85-1.04) | 0.21 |
| Serum sodium | 1.02 (0.97-1.07) | 0.39 | 1.02 (0.98-1.08) | 0.28 |
| Albumin | 0.56 (0.40-0.78) | 0.0007 | 0.73 (0.48-1.10) | 0.13 |
| Total bilirubin | 1.03 (0.64-1.66) | 0.89 | 1.28 (0.76-2.14) | 0.35 |
| Log NT-proBNP | 2.56 (1.91-3.44) | <0.0001 | 2.31 (1.66-3.23) | <0.0001 |
| Average E/e′ ≥14 or 11 | 1.64 (1.17-2.28) | 0.004 | ||
| TRV ≥2.8 | 1.91 (1.38-2.64) | 0.0001 | ||
| IVCC <50% | 1.65 (1.20-2.27) | 0.002 | ||
| Echocardiographic congestion grade | 1.76 (1.43-2.18) | <0.0001 | 1.51 (1.19-1.92) | 0.0006 |
Multivariable Cox hazard model included clinical variables (age, sex, and NYHA functional class), biomarkers (hemoglobin, serum sodium, albumin, total bilirubin, and NT-proBNP), and echocardiographic congestion grade determined by average E/e′, TRV, and IVCC at discharge.
Table 4.
Multivariable Cox Hazard Analysis for the Primary Endpoint in Patients With Sinus Rhythm and AF
| Sinus Rhythm |
Atrial Fibrillation |
|||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age | 1.02 (0.99-1.04) | 0.13 | 1.01 (0.97-1.04) | 0.73 |
| Female | 0.94 (0.59-1.50) | 0.80 | 0.90 (0.54-1.50) | 0.66 |
| NYHA functional class | 0.81 (0.53-1.23) | 0.33 | 1.50 (0.95-2.36) | 0.079 |
| Hemoglobin | 0.94 (0.83-1.07) | 0.35 | 0.98 (0.86-1.11) | 0.72 |
| Log NT-proBNP | 2.18 (1.50-3.16) | <0.0001 | 2.50 (1.21-5.16) | 0.013 |
| Echocardiographic congestion grade | 1.48 (1.09-2.01) | 0.012 | 1.61 (1.12-2.31) | 0.011 |
Patients with grade C echocardiographic abnormalities had a higher risk for the primary endpoint than patients with grade A or grade B (Figure 3). These results were similarly observed in the subgroup of sinus rhythm and AF (Central Illustration).
Figure 3.
Event-Free Probability in Patients With Heart Failure With Preserved Ejection Fraction
The Kaplan-Meier curves for cardiovascular mortality and heart failure hospitalization among the 3 echocardiographic congestion grades are shown. Grade C echocardiographic congestion showed poor prognosis in patients with heart failure with preserved ejection fraction.
Predictive value of echocardiographic congestion grade
Table 5 shows the discrimination abilities of the Cox models. Echocardiographic congestion grade improved the predictive value when added to a Cox model that includes clinical factors (age, sex, and NYHA functional class) and NT-proBNP in patients with HFpEF in both sinus rhythm and AF.
Table 5.
Discrimination Abilities of the Cox Models
| Uno’s C-Statistic (95% CI) | Uno’s C-Statistic, Averaged Value of 10-Fold Cross Validation | |
|---|---|---|
| All | ||
| Clinical | 0.561 (0.501-0.622) | 0.576 |
| Clinical + NT-proBNP | 0.658 (0.604-0.711) | 0.645 |
| Clinical + NT-proBNP + echocardiographic congestion grade | 0.671 (0.619-0.722) | 0.666 |
| Sinus rhythm | ||
| Clinical | 0.566 (0.475-0.658) | 0.577 |
| Clinical + NT-proBNP | 0.672 (0.601-0.744) | 0.655 |
| Clinical + NT-proBNP + echocardiographic congestion grade | 0.673 (0.601-0.745) | 0.670 |
| Atrial fibrillation | ||
| Clinical | 0.560 (0.465-0.655) | 0.585 |
| Clinical + NT-proBNP | 0.649 (0.562-0.737) | 0.639 |
| Clinical + NT-proBNP + echocardiographic congestion grade | 0.686 (0.604-0.769) | 0.667 |
Clinical model included age, sex, and New York Heart Association functional class.
NT-proBNP = N-terminal pro B-type natriuretic peptide.
The addition of echocardiographic congestion grade to a logistic regression model that includes clinical factors (age, sex, and NYHA functional class) and NT-proBNP significantly improved the area under the curve in the prediction of the 1-year primary endpoint (Supplemental Figure 2). The predictive ability of this logistic regression model is shown in Supplemental Table 2.
Discussion
The present study demonstrates that echocardiographic congestion grade consisting of averaged E/e′, TRV, and IVCC at discharge predicted adverse outcomes not only in sinus rhythm but also in patients with AF with HFpEF. Second, echocardiographic congestion grade may add an incremental value for predicting adverse outcomes over the clinical factors (age, sex, and NYHA functional class) and NT-proBNP.
Potential role of IVCC evaluation in HFpEF
We demonstrated the prognostic impact of echocardiographic congestion grading that includes IVCC for right-sided congestion in addition to E/e′ and TRV for left-sided congestion. Physical findings of right-sided congestion such as jugular venous distention, bilateral peripheral edema, and ascites provide us with not only diagnostic information but also prognosis.3,14,15 Earlier studies of echocardiography showed that increased IVC diameter as a sign of right-sided congestion was associated with adverse outcomes in chronic and acute heart failure.5,6 Nonetheless, the prognostic impact of the measurement of IVC has received little attention in HFpEF.
Right-sided congestion, which can be measured as IVCC, is considered to reflect various pathophysiologic features of HFpEF. Although the pathophysiology of HFpEF was initially thought to be caused by left ventricular diastolic dysfunction, recent studies have suggested more complex involvement of multiple abnormalities.16 An increase in left and right atrial pressures occurs in many patients in the absence of weight gain or total body volume increase.17 Values of right atrial pressure are affected by many variables such as venous return and stressed volume regulated by the autonomic nervous system, abdominal pressure inducing an intercompartmental fluid shift from the splanchnic vessels to the IVC, intrathoracic pressure, and pulmonary arterial resistance.18 These mechanisms can predispose patients to heart failure exacerbations regardless of total body volume status.19 From the results of these earlier studies, IVCC as right-sided congestion is considered to be important in predicting prognosis in HFpEF.
Echocardiographic congestion grade in AF
In our study, echocardiographic congestion was evaluated by 3 variables consisting of averaged E/e′, TRV, and IVCC, all of which can be applied to AF. AF existed in 43% of patients at discharge, which cannot be ignored in our HFpEF study. Elevated left ventricular filling pressure leads to left atrial stretching and remodeling and to increased pulmonary pressures and right ventricular afterload.20,21 Patients with HFpEF with AF had higher pulmonary capillary wedge pressure and mean pulmonary artery pressure compared to patients with HFpEF in sinus rhythm.22 The presence of pulmonary hypertension in AF is associated with more right atrial dilatation and higher right atrial pressures compared to pulmonary hypertension in patients without AF.23 These earlier studies suggested the sequential hemodynamic link from left to right and the critical role of E/e′, TRV, and IVCC when evaluating congestion in patients with HFpEF with AF.
Technical aspects of echocardiographic congestion grade
We included IVCC in echocardiographic congestion grade. Major studies evaluating the correlation between right atrial pressure and IVC are well summarized in a review article.24 Accuracy is a major concern in applying IVC dimension and collapsibility for estimating right atrial pressure. Additional 2-dimensional methods besides IVC evaluation or 3-dimensional methods of IVC evaluation for estimating right atrial pressure may aid in improving right atrial pressure estimation.24,25 Although there are controversies over the accurate estimation of right atrial pressure, we adopted IVCC, which is a simple and feasible index for right-sided congestion independent of body surface area.
In this study, 39% of hospitals used the index beat assessment to measure E and e′ velocities whose preceding R-R interval and prepreceding R-R interval were similar to those used for calculating E/e′ in patients with AF (Supplemental Figure 1). R-R interval irregularity is an issue in E/e′ measurement in AF. Simultaneous assessment of early transmitral flow and e′ velocities for estimating elevated left ventricular filling pressure has overcome the R-R irregularity in patients with AF. However, this simultaneous assessment is only available with a specific ultrasound apparatus.26, 27, 28 Index beat assessment of left ventricular function is a promising method to overcome R-R irregularity in patients with AF.29
Five studies of E/e′ showed significant association with pulmonary capillary wedge pressure or left ventricular pressure at end diastole in patients with AF. The cutoff value of E/e′ differs depending on whether septal E/e′,30,31 lateral E/e′,26,27 or averaged E/e′28 is measured and whether it predicts pulmonary capillary wedge pressure or left ventricular pressure at end diastole, ranging from 9 to 16. We determined a cutoff criterion of averaged E/e′ of ≥11 in patients with AF with HFpEF, which is similar to the value of septal E/e′ of ≥11 recommended by the ASE/EACVI guidelines.
Possible explanations and implications
We showed that echocardiographic congestion grade may add an incremental value for predicting adverse outcomes over the clinical factors (age, sex, and NYHA functional class) and NT-proBNP in HFpEF. Echocardiography may have detected insufficient decongestion, which may partly be due to a poor response to diuretic therapy.
Elevated NT-proBNP value may also reflect the residual congestion. The prognostic role of NT-proBNP in patients with HFpEF has been established in large cohort studies.32,33 The results of our study also showed the strong prognostic value of NT-proBNP as described in Tables 3 and 4. However, NT-proBNP in HFpEF is affected by AF, renal dysfunction, and obesity, making it difficult to interpret the cutoff value of NT-proBNP. NT-proBNP may increase in the presence of AF regardless of the left ventricular filling pressure.34,35
Echocardiographic congestion grade can be used in both sinus rhythm and AF, which is not affected by renal dysfunction and body size. The 3 indices of E/e′, TRV, and IVCC are key features in the hemodynamic link from left to right of HFpEF.16, 17, 18 Hence, echocardiographic congestion grade may be a prognostic marker that covers the weak points of NT-proBNP by reflecting the key hemodynamics of HFpEF. Echocardiographic congestion grade is thereby potentially useful in the evaluation of treatment efficacy and may help clinicians plan the safe discharge of patients with HFpEF.
Study limitations
The patient population needs attention for interpreting the results of our study. First, many patients were older than 80 years and had renal dysfunction, which may increase E/e′ and NT-proBNP values. However, the results of clinical studies in elderly patients with HFpEF will become more important because heart failure prevalence increases in the elderly population not only in Japan but also in the United States and European countries.36, 37, 38 Second, the entry requirement of admission NT-proBNP of ≥400 pg/mL or brain natriuretic peptide of ≥100 pg/mL is based on the recommendation of the Japanese Heart Failure Society, which is different from European Society of Cardiology guidelines. Third, we excluded patients without echocardiography data and follow-up data.
Cardiac sonographers were not blinded to clinical information, which may have caused a measurement bias. We did not perform a validation study to justify the incremental value of echocardiographic congestion grade over the clinical factors and NT-proBNP. This may limit the generalizability of our results. Because this was an observational study, the question of whether therapeutic strategies aimed to improve echocardiographic congestion grade alter the composite endpoint warrants future investigation.
Conclusions
Echocardiographic left- and right-sided congestion grade may add an incremental value for predicting adverse outcomes over the clinical factors (age, sex, and NYHA functional class) and NT-proBNP, not only in sinus rhythm but in patients with AF with HFpEF. The prognostic performance of this simplified and integrated echocardiographic congestion grade should be evaluated on a large scale. Whether echocardiographic congestion grade–guided heart failure treatment is efficacious deserves further investigation.
Perspectives.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS: Echocardiographic congestion grade provides prognostic value not only in sinus rhythm but also in atrial fibrillation patients with HFpEF. Confirmation of congestion by echocardiography may allow clinicians to guide further medical therapies for HFpEF.
TRANSLATIONAL OUTLOOK: The prognostic performance of echocardiographic congestion grade should be widely evaluated. Echocardiographic congestion grade–guided heart failure treatment warrants further investigation.
Funding Support and Author Disclosures
This work was funded by Roche Diagnostics K.K. and Fuji Film Toyama Chemical Co. Ltd. Dr Abe has received grants from Boehringer Ingelheim Japan. Dr Hikoso has received personal fees from Daiichi Sankyo Company, Bayer, Astellas Pharma, Pfizer Pharmaceuticals, and Boehringer Ingelheim Japan and has received grants from Roche Diagnostics, Fujifilm Toyama Chemical, and Actelion Pharmaceuticals. Dr Nakatani has received honoraria from Roche Diagnostics. Dr Koretsune has received personal fees from Daiichi Sankyo Company. Dr Sakata has received personal fees from Otsuka Pharmaceutical, Ono Pharmaceutical, Daiichi Sankyo Company, Mitsubishi Tanabe Pharma Corporation, and Actelion Pharmaceuticals and has received grants from Roche Diagnostic, Fujifilm Toyama Chemical, Abbott Medical Japan, Otsuka Pharmaceutical, Daiichi Sankyo Company, Mitsubishi Tanabe Pharma Corporation, and Biotronik. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgements
The authors thank Nagisa Yoshioka, Kyoko Tatsumi, Satomi Kishimoto, Noriko Murakami, and Sugako Mitsuoka for their excellent assistance with data collection.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a list of the Osaka Cardiovascular Conference-Heart Failure Investigators as well as supplemental tables and figures, please see the online version of this paper.
Appendix
References
- 1.Owan T.E., Hodge D.O., Herges R.M., Jacobsen S.J., Roger V.L., Redfield M.M. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Bozkurt B., Coats A.J.S., Tsutsui H., et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail. 2021;23:352–380. doi: 10.1002/ejhf.2115. [DOI] [PubMed] [Google Scholar]
- 3.Drazner M.H., Rame J.E., Stevenson L.W., Dries D.L. Prognostic importance of elevated jugular venous pressure and a third heart sound in patients with heart failure. N Engl J Med. 2001;345:574–581. doi: 10.1056/NEJMoa010641. [DOI] [PubMed] [Google Scholar]
- 4.Mullens W., Abrahams Z., Francis G.S., et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–596. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pellicori P., Graubelli V., Zhang J., et al. IVC diameter in patients with chronic heart failure: relationship and prognostic significance. J Am Coll Cardiol Img. 2013;6:16–28. doi: 10.1016/j.jcmg.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Goonewardena S.N., Gemignani A., Ronan A., et al. Comparison of hand-carried ultrasound assessment of the inferior vena cava and N-terminal pro-brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure. J Am Coll Cardiol Img. 2008;1:595–601. doi: 10.1016/j.jcmg.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Sharifov O.F., Schiros C., Aban I., Denney T.S., Gupta H. Diagnostic accuracy of tissue Doppler index E/e’ for evaluating left ventricular filling pressure and diastolic dysfunction/heart failure with preserved ejection fraction: a systematic review and meta-analysis. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.002530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagueh S.F., Smiseth O.A., Appleton C.P., et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Suna S., Hikoso S., Yamada T., et al. Study protocol for the PURSUIT-HFpEF study: a prospective, multicenter, observational study of patients with heart failure with preserved ejection fraction. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-038294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang R.M., Badano L.P., Mor-Avi V., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Rudski L.G., Lai W.W., Afilao J., et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Taniguchi T., Ohtani T., Nakatani S., et al. Impact of body size on inferior vena cava parameters for estimating right atrial pressure: a need for standardization? J Am Soc Echocardiogr. 2015;28:1420–1427. doi: 10.1016/j.echo.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Uno H., Cai T., Pencina M.J., D’Agostino R.B., Wei L.J. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med. 2011;30:1105–1117. doi: 10.1002/sim.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKee P.A., Castelli W.P., McNamara P.M., Kannel W.B. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 15.Thibodeau J.T., Drazner M.H. The role of the clinical examination in patients with heart failure. J Am Coll Cardiol HF. 2018;6:543–551. doi: 10.1016/j.jchf.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Burkhoff D., Maurer M.S., Packer M. Heart failure with a normal ejection fraction: is it really a disorder of diastolic function? Circulation. 2003;107:656–658. doi: 10.1161/01.cir.0000053947.82595.03. [DOI] [PubMed] [Google Scholar]
- 17.Miller W.L. Fluid volume overload and congestion in heart failure: time to reconsider pathophysiology and how volume is assessed. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.002922. [DOI] [PubMed] [Google Scholar]
- 18.Gelman S. Venous function and central venous pressure: a physiologic story. Anesthesiology. 2008;108:735–748. doi: 10.1097/ALN.0b013e3181672607. [DOI] [PubMed] [Google Scholar]
- 19.Fudim M., Hernandez A.F., Felker G.M. Role of volume redistribution in the congestion of heart failure. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotecha D., Lam C.S.P., van Veldhuisen D.J., van Gelder I.C., Voors A.A., Rienstra M. Heart failure with preserved ejection fraction and atrial fibrillation: vicious twins. J Am Coll Cardiol. 2016;68:2217–2228. doi: 10.1016/j.jacc.2016.08.048. [DOI] [PubMed] [Google Scholar]
- 21.Vachiery J.L., Adir Y., Barbera J.A., et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62(25 suppl):D100–D108. doi: 10.1016/j.jacc.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 22.Lam C.S., Rienstra M., Tay W.T., et al. Atrial fibrillation in heart failure with preserved ejection fraction: association with exercise capacity, left ventricular filling pressures, natriuretic peptides, and left atrial volume. J Am Coll Cardiol HF. 2017;5:92–98. doi: 10.1016/j.jchf.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Rottlaender D., Motloch L.J., Schmidt D., et al. Clinical impact of atrial fibrillation in patients with pulmonary hypertension. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beigel R., Cercek B., Luo H., Siegel R.J. Noninvasive evaluation of right atrial pressure. J Am Soc Echocardiogr. 2013;26:1033–1042. doi: 10.1016/j.echo.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Seo Y., Iida N., Yamamoto M., Machino-Ohtsuka T., Ishizu T., Aonuma K. Estimation of central venous pressure using the ratio of short to long diameter from cross-sectional images of the inferior vena cava. J Am Soc Echocardiogr. 2017;30:461–467. doi: 10.1016/j.echo.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Kusunose K., Yamada H., Nishio S., et al. Clinical utility of single-beat E/e′ obtained by simultaneous recording of flow and tissue Doppler velocities in atrial fibrillation with preserved systolic function. J Am Coll Cardiol Img. 2009;2:1147–1156. doi: 10.1016/j.jcmg.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Li C., Zhang J., Zhou C., Huang L., Tang H., Rao L. Will simultaneous measurement of E/e′ index facilitate the non-invasive assessment of left ventricular filling pressure in patients with non-valvular atrial fibrillation? Eur J Echocardiogr. 2010;11:296–301. doi: 10.1093/ejechocard/jep218. [DOI] [PubMed] [Google Scholar]
- 28.Wada Y., Murata K., Tanaka T., et al. Simultaneous Doppler tracing of transmitral inflow and mitral annular velocity as an estimate of elevated left ventricular filling pressure in patients with atrial fibrillation. Circ J. 2012;76:675–681. doi: 10.1253/circj.cj-11-0703. [DOI] [PubMed] [Google Scholar]
- 29.Kusunose K., Yamada H., Nishio S., et al. Index-beat assessment of left ventricular systolic and diastolic function during atrial fibrillation using myocardial strain and strain rate. J Am Soc Echocardiogr. 2012;25:953–959. doi: 10.1016/j.echo.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Sohn D.W., Song J.M., Zo J.H., et al. Mitral annulus velocity in the evaluation of left ventricular diastolic function in atrial fibrillation. J Am Soc Echocardiogr. 1999;12:927–931. doi: 10.1016/s0894-7317(99)70145-8. [DOI] [PubMed] [Google Scholar]
- 31.Senechal M., O’Connor K., Deblois J., et al. A simple Doppler echocardiography method to evaluate pulmonary capillary wedge pressure in patients with atrial fibrillation. Echocardiography. 2008;25:57–63. doi: 10.1111/j.1540-8175.2007.00555.x. [DOI] [PubMed] [Google Scholar]
- 32.Anand I.S., Rector T.S., Cleland J.G., et al. Prognostic value of baseline plasma amino-terminal pro-brain natriuretic peptide and its interactions with irbesartan treatment effects in patients with heart failure and preserved ejection fraction: findings from the I-PRESERVE trial. Circ Heart Fail. 2011;4:569–577. doi: 10.1161/CIRCHEARTFAILURE.111.962654. [DOI] [PubMed] [Google Scholar]
- 33.Cunningham J.W., Vaduganathan M., Claggett B.L., et al. Effects of sacubitril/valsartan on N-terminal pro-B-type natriuretic peptide in heart failure with preserved ejection fraction. J Am Coll Cardiol HF. 2020;8:372–381. doi: 10.1016/j.jchf.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Patton K.K., Ellinor R.T., Heckbert S.R., et al. N-terminal pro-B-type natriuretic peptide is a major predictor of the development of atrial fibrillation: the Cardiovascular Health Study. Circulation. 2009;120:1768–1774. doi: 10.1161/CIRCULATIONAHA.109.873265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellinor P.T., Low A.F., Patton K.K., Shea M.A., MacRae C.A. Discordant atrial natriuretic peptide and brain natriuretic peptide levels in lone atrial fibrillation. J Am Coll Cardiol. 2005;45:82–86. doi: 10.1016/j.jacc.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 36.Shiraishi Y., Kohsaka S., Sato N., et al. 9-Year trend in the management of acute heart failure in Japan: a report from the national consortium of acute heart failure registries. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.008687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Redfield M.M., Jacobsen S.J., Burnett J.C., Jr., Mahoney D.W., Bailey K.R., Rodeheffer R.J. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 38.Magnussen C., Niiranen T.J., Ojeda F.M., et al. Sex-specific epidemiology of heart failure risk and mortality in Europe: results from the BiomarCaRE consortium. J Am Coll Cardiol HF. 2019;7:204–213. doi: 10.1016/j.jchf.2018.08.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.