Abstract
The health of horseshoe crab (Limulus polyphemus) eggs is important not only to maintain horseshoe crab populations, but because they are a resource for higher trophic levels, such as fish and shorebirds. We examined the concentrations of arsenic, cadmium, chromium, lead, manganese, mercury and selenium in the eggs of horseshoe crabs from Delaware Bay (between New Jersey and Delaware, USA) in 1993, 1994, 1995, 1999, 2000, and 2012 to determine if there were significant temporal changes, and if levels appear to pose a health risk to the crabs themselves, or to predators that consume them. All metal levels declined in horseshoe crab eggs between 1994 and 2012, although the declines were much less consistent for mercury and chromium than for lead and chromium. Levels of contaminants found in these eggs are well below those known to cause adverse effects in the crabs themselves, or to organisms that consume them, such as migrating shorebirds.
Keywords: Delaware Bay, eggs, horseshoe crab, heavy metals, mercury
1. Introduction
Each spring horseshoe crabs migrate into Delaware Bay to spawn on the beaches and mudflats. Delaware Bay, bordered by Delaware and New Jersey, is the center of horseshoe crab (Limulus polyphemus) breeding in North America (Shuster 1982; Shuster and Botton 1985). In the late 1980s and 1990s, the numbers of horseshoe crabs taken for eel and conch bait greatly increased (Burger 1996a; ASMFC 1998, 1999). Concern about the apparent decline in horseshoe crabs along the Atlantic coast of the United States, particularly along Delaware Bay (Burger 1996a; ASMFC 1999; Botton 2000; Botton and Loveland 2001) resulted in the development of horseshoe crab management plan, with limits on take by the Atlantic States Marine Fisheries Council (ASMFC). This decrease in breeding horseshoe crabs, and thus in excess eggs, fueled additional concern for several species of shorebirds that relied on the eggs for a source of energy during their northward migration each spring (Botton et al. 1994; Burger, 1986), and the ASMFC considered shorebirds populations as one key aspect of the plan.
Amplexing pairs of horseshoe crabs deposit their eggs in the sand during high tide, and when they are abundant, subsequent pairs dig up the nests of previous females when they deposit their own eggs. Excess eggs in the surf, and those left on the sand by the receding tide are available for shorebirds to eat (Dunne 1982). Shorebirds concentrate along Delaware Bay beaches in directly proportion to the abundance of horseshoe crab eggs (Botton 1994). Thousands of shorebirds migrant through Delaware Bay each spring (Burger 1986, 1996b; Burger 1997; Botton 1994), and they rely on the horseshoe crab eggs (Castro and Myers 1993; Tsipoura and Burger 1999; Baker 2004; Niles 2008). There is evidence that several species of shorebirds have declined in the last 25 years (Morrison 2004, 2007; Andres 2013). Other species, such as laughing gulls (Larus atricilla) and herring gulls (Larus argentatus) also rely on the horseshoe crab eggs during the breeding season (Burger 1996c). During migration, shorebirds are faced with habitat loss, decreases in the prey base, predation, and human disturbance (Goss-Custard 2006).
Although the initial decline in spawning horseshoe crabs in Delaware Bay was attributed to overharvesting by fishermen that use them as bait (ASMFC 1999; Niles 2008; Kreamer and Michels 2009), the possibility exists that other factors, such as contaminants, may contribute to their decline. High contaminants in crab eggs might also pose a problem for shorebirds that feed extensively on them, as well as fish and other species that may eat eggs or young horseshoe crabs (Burger et al 2002, 2003).
In this paper we examine the levels of arsenic, cadmium, chromium, lead, manganese, mercury and selenium in the eggs of horseshoe crabs from Delaware Bay, New Jersey to determine whether there were temporal differences from 1993 to 2012, and whether metal levels are high enough to cause adverse health effects on the crabs themselves, or on predators that eat horseshoe crab eggs. If female crabs are sequestering metals in their eggs, then other species that eat them might be exposed. Examining heavy metal levels in horseshoe crabs eggs is critical because a series of experiments indicated that horseshoe crab eggs are vulnerable to heavy metals, with mercury, organotin, and cadmium being the most toxic (Botton 1998; Botton 2000) and Itow (1998a, 1998b). Impacts include mortality, lower limb regenerative abilities, segment defective embryos, and abnormal eyes (Itow, 1998a, 1998b), indicating clear toxic effects that differ by metal.
2. Materials and Methods
Eggs of horseshoe crabs were collected under appropriate permits from spawning beaches from Delaware Bay, between Delaware and New Jersey (Fig. 1). Eggs were collected either from recently-laid nests or from females prior to egg-laying (Burger 1997; Burger et al 2002, 2003), and were frozen for later digestion and analysis in the NIEHS Chemical Analysis Laboratory at the Environmental and Occupational Health Sciences Institute.
Figure 1.
Map of Delaware Bay, between the States of Delaware and New Jersey in the US, showing sampling sites.
Tissues were digested in ultrex ultrapure nitric acid in a microwave (MD 2000 CEM), using a digestion protocol of three stages of ten min each under 50, 100 and 150 pounds per square inch (3.5, 7.0 and 10.6 kg/cm2) at 70X power. Digested samples were subsequently diluted in deionized water.
Mercury was analyzed by cold vapor technique, and all other metals were analyzed by graphite furnace atomic absorption, including arsenic, cadmium, chromium, lead, manganese, and selenium. All concentrations in tissues are expressed in parts per billion (ng/g wet weight). Detection limits were 0.02 ppb for cadmium, 0.08 ppb for chromium, 0.15 ppb for lead, 0.09 ppb for manganese, 0.2 ppb for mercury, and 0.7 ppb for selenium. All specimens were run in batches that included blanks, a standard calibration curve and spiked specimens. The accepted recoveries for spikes ranged from 85 % to 105 % (batches with recoveries less than 85 % were rerun). The coefficient of variation (CV) on replicate, samples ranged from 3–7 %. Further quality control included periodic blind analysis of an aliquot from a large sample of known concentration, and blind runs of duplicate samples during the analysis for each metal (acceptable criterion = ± 15%).
Because metal concentrations approximated a log-normal distribution, the data were analyzed by non-parametric Kruskal-Wallis one way analysis of variance to compare concentrations among locations (SAS 2005). Both arithmetic and geometric means are given in tables to facilitate comparisons with other studies in the literature. Similarly, ppb levels are shown in tables and figures to provide maximum information, but health effects are discussed in ppm.
3. Results
There were significant location and temporal differences in levels of all metals for horseshoe crab eggs (Table 1). While this table presents all the information, it is useful to examine metals levels at individual beaches. Data were available for Cape Shore for all three time period; there were significant temporal differences, except for arsenic, which was not measured in the earlier years (Table 2). There were significant temporal differences for Villas, Baycove, and Reeds for 4 of 7 metals; there were no differences for arsenic, lead, and selenium (Table 3). There were significant temporal differences in all metals for horseshoe eggs collected from Forescue (the beach that was the greatest distance from the mouth of the bay, Table 4, Fig. 1).
Table 1.
Metal levels ppb (means and SE wet weight) in Horseshoe Crabs eggs from New Jersey collected 1993 through 2012. Analysis done on wet weight basis; results in ppb. Shown are the arithmetic means and standard error with the geometric means below.
| 1993 | 1993 | 1994 | 1995 | 1995 | 1999 | 1999 | 1999 | 1999 | 2000 | 2012 | 2012 | 2012 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Barnegat Bay | Cape Shore | Moore’s | Cape Shore | Fortescue | Cape Shore | Fortescue | Thompsons | Villas | Moore’s | Baycove | Fortescue | Reeds | Kruskal-Wallis X2 | |
| Number | 12 | 6 | 9 | 9 | 10 | 12 | 6 | 9 | 12 | 7 | 8 | 7 | 2 | |
| arsenic | 7545 ± 1028 | 8517± 1139 | 4352 ± 188 | 3668 ± 350 | 6629 ± 1384 | 4413 ± 375 | 3086 ± 198 | 3250 ± 1050 | 31 (<0.0001) | |||||
| 6789 | 8129 | 4318 | 3499 | 5875 | 4292 | 3048 | 3076 | |||||||
| cadmium | 7.3 ± 1.3 | 37.8 ± 10.3 | 311 ± 130 | 9.4 ± 1.9 | 38.5 ± 12.7 | 36.2 ± 7.8 | 31.4 ± 10.0 | 12.1 ± 3.1 | 99.1 ± 60.7 | 14.0 ± 4.0 | 1.0 ± 0.2 | 0.4 ± 0.2 | 8.2 ± 3.9 | 69 (< 0.0001) |
| 6.0 | 26.6 | 150 | 8.4 | 30.1 | 26.0 | 25.0 | 9.0 | 28.0 | 10.0 | 0.7 | 0.1 | 7.2 | ||
| chromium | 5059 ± 927 | 699 ± 306 | 178 ± 4 | 315 ± 29 | 91 ± 31 | 66 ± 24 | 65 ± 17 | 39 ± 6 | 40 ± 11 | 97.1 ± 19 | 40 ± 4.90 | 175 ± 35 | 70 (< 0.0001) | |
| 3934 | 466 | 177 | 295 | 61 | 46 | 48 | 35 | 34 | 86 | 38 | 171 | |||
| lead | 271 ± 41 | 1246 ± 412 | 206 ± 34 | 98 ± 7 | 78 ± 14 | 25 ± 9 | 64 ± 20 | 21 ± 7 | 48 ± 9 | 26 ± 9 | 37 ± 5.20 | 25 ± 5.04 | 86 ± 44 | 79 (< 0.0001) |
| 239 | 905 | 187 | 96 | 66 | 16 | 53 | 7 | 27 | 12 | 34.60 | 21 | 74 | ||
| manganese | 18371 ± 5560 | 4982 ± 518 | 7278 ± 130 | 6975 ± 306 | 2268 ± 201 | 2672 ± 933 | 2195 ± 383 | 2408 ± 281 | 2675 ± 365 | 7325 ± 754 | 4457 ± 257 | 6750 ± 2250 | 73 (< 0.0001) | |
| 14013 | 4801 | 7268 | 6918 | 2160 | 2077 | 1957 | 2246 | 2526 | 7052 | 4410 | 6364 | |||
| mercury | 27.9 ± 0.3 | 93 ± 8.0 | 5.8 ± 2.0 | 14.9 ± 0.3 | 22.5 ± 3.2 | 18.2 ± 6.0 | 12.6 ± 2.6 | 21.8 ± 3.9 | 16.8 ± 5.2 | 7.8 ± 2.0 | 1.0 ± 0.5 | 17.5 ± 5.4 | 56 (< 0.0001) | |
| 27.0 | 89.5 | 4.5 | 14.9 | 15.0 | 13.0 | 10.0 | 18.0 | 11.0 | 5.3 | 0.4 | 16.6 | |||
| selenium | 1965 ± 57 | 3468 ± 522 | 4040± 117 | 2962 ± 68 | 1141 ± 124 | 1616 ± 290 | 713 ± 112 | 969 ± 184 | 1112 ± 319 | 907 ± 169 | 996 ± 137 | 1300 ± 100 | 67 (< 0.0001) | |
| 1961 | 3113 | 4026 | 2955 | 1074 | 1483 | 651 | 748 | 788 | 789 | 935 | 1296 |
Table 2.
Metal levels ppb (means and SE wet weight) in Horseshoe Crabs eggs from New Jersey collected 1993, 1995, 1999. Analysis done on wet weight basis; results in ppb. Shown are the aritmetic means and standard error with the geometric means below.
| 1993 | 1995 | 1999 | ||
|---|---|---|---|---|
|
| ||||
| Cape Shore | Cape Shore | Cape Shore | Kruskal-Wallis X2 | |
|
| ||||
| Number | 6 | 9 | 12 | |
| arsenic | 7555 ± 1028 | |||
| 6789 | ||||
| cadmium | 37.8 ± 10.3 | 9.4 ± 1.9 | 36.2 ± 7.8 | 8.0 (0.02) |
| 27 | 8 | 26 | ||
| chromium | 5059 ± 927 | 178 ± 4.2 | 90.5 ± 30.7 | 17.7 (0.0001) |
| 3934 | 177 | 61 | ||
| lead | 1246 ± 412 | 98.2 ± 6.8 | 24.5 ± 8.7 | 19.0 (<0.0001) |
| 905 | 96 | 16 | ||
| manganese | 18371 ± 5560 | 7278 ± 130 | 2268 ± 201 | 19.6 (<0.0001) |
| 14013 | 7268 | 2160 | ||
| mercury | 5.8 ± 2.0 | 22.5 ± 3.2 | 6.4 (0.01) | |
| 5 | 15 | |||
| selenium | 1965 ± 57.0 | 4040 ± 117 | 1141 ± 124 | 22.2 (<0.0001) |
| 1961 | 4026 | 1074 | ||
Table 3.
Metal levels ppb (means and SE wet weight) in Horseshoe Crabs eggs from New Jersey collected 1999 and 2012. Analysis done on wet weight basis; results in ppb. Shown are the aritmetic means and standard error with the geometric means below.
| 1999 | 2012 | 2012 | ||
|---|---|---|---|---|
|
| ||||
| Villas | Reeds | Baycove | Kruskal-Wallis X2 | |
|
| ||||
| Number | 12 | 2 | 8 | |
| arsenic | 3668 ± 350 | 3250 ± 1050 | 4413 ± 375 | 3.4 NS |
| 3499 | 3076 | 4292 | ||
| cadmium | 99 ± 61 | 8.20 ± 3.90 | 0.98 ± 0.19 | 12.7 (0.002) |
| 28 | 7.18 | 0.69 | ||
| chromium | 39 ± 6 | 175 ± 35 | 97.1 ± 19 | 11.8 (0.003) |
| 35 | 171 | 86 | ||
| lead | 48 ± 9 | 86 ± 44 | 37 ± 5.20 | 1.6 NS |
| 27 | 74 | 34.60 | ||
| manganese | 2408 ± 281 | 6750 ± 2250 | 7325 ± 754 | 6.2 (0.04) |
| 2246 | 6364 | 7052 | ||
| mercury | 22 ± 4 | 18 ± 5 | 8 ± 2 | 15.8 (0.0004) |
| 18.0 | 17 | 5.30 | ||
| selenium | 969 ± 184 | 1300 ± 100 | 907 ± 169 | 2.1 NS |
| 748 | 1296 | 789 | ||
Table 4.
Metal levels ppb (means and SE wet weight) in Horseshoe Crabs eggs from New Jersey collected 1995, 1999 and 2012. Analysis done on wet weight basis; results in ppb. Shown are the aritmetic means and standard error with the geometric means below.
| 1995 | 1999 | 2012 | ||
|---|---|---|---|---|
|
| ||||
| Fortescue | Fortescue | Fortescue | Kruskal-Wallis X2 | |
|
| ||||
| Number | 10 | 6 | 7 | |
| arsenic | 8517 ± 1139 | 3086 ± 198 | 9.0 (0.003) | |
| 8129 | 3048 | |||
| cadmium | 39 ± 13 | 31 ± 10 | 0.38 ± 0.15 | 13.7 (0.001) |
| 30 | 25 | 0 | ||
| chromium | 315 ± 29 | 66 ± 24 | 40 ± 4.90 | 15.4 (0.0005) |
| 295 | 46 | 38 | ||
| lead | 78 ± 14 | 64 ± 20 | 25 ± 5.04 | 7.8 (0.02) |
| 65 | 53 | 21 | ||
| manganese | 6975 ± 306 | 2672 ± 933 | 4457 ± 257 | 15.1 (0.0005) |
| 6918 | 2077 | 4410 | ||
| mercury | 15 ± 0 | 18 ± 6 | 1.03 ± 0.50 | 13.4 (0.001) |
| 15 | 13 | 0.37 | ||
| selenium | 2962 ± 68 | 1616 ± 290 | 996 ± 137 | 17.1 (0.0002) |
| 2955 | 1483 | 935 | ||
Combining all the data on metal levels in horseshoe crab eggs, all from places where shorebirds forage, indicated significant temporal differences in all metals (Table 5). The overall patterns are shown in figure 2. While the decline was consistent for lead and chromium, it was less so for cadmium and mercury. This figure illustrates that the highest levels in cadmium and chromium were in 1994.
Table 5.
Metal levels ppb (means and SE wet weight) in Horseshoe Crabs eggs from New Jersey collected 1993 through 2012. Analysis done on wet weight basis; results in ppb. Shown are the aritmetic means and standard error with the geometric means below.
| 1993, 1994 & 1995 | 1999 & 2000 | 2012 | Kruskal-Wallis X2 | |
|---|---|---|---|---|
|
| ||||
| Number | 46 | 46 | 17 | |
| arsenic | 5899 ± 459 | 3729 ± 263 | 8.0 (0.005) | |
| 5235 | 3584 | |||
| cadmium | 78.7 ± 30.5 | 43.9 ± 16.3 | 1.58 ± 0.7 | 36.7 (<0.0001) |
| 21 | 18 | 0 | ||
| chromium | 1217 ± 355 | 61.4 ± 9.65 | 82.7 ± 14.3 | 60.1 (<0.0001) |
| 459 | 44 | 67 | ||
| lead | 289 ± 68 | 35.3 ± 4.96 | 37.6 ± 6.74 | 60.3 (<0.0001) |
| 165 | 17 | 31 | ||
| manganese | 8539 ± 1227 | 2405 ± 170 | 6076 ± 530 | 65.5 (<0.0001) |
| 7207 | 2181 | 5743 | ||
| mercury | 38.9 ± 6.84 | 18.9 ± 1.79 | 6.15 ± 1.67 | 23.0 (<0.0001) |
| 24 | 14 | 2 | ||
| selenium | 3206 ± 184 | 1070 ± 91.5 | 990 ± 98.9 | 59.0 (<0.0001) |
| 3025 | 882 | 897 | ||
Figure 2.
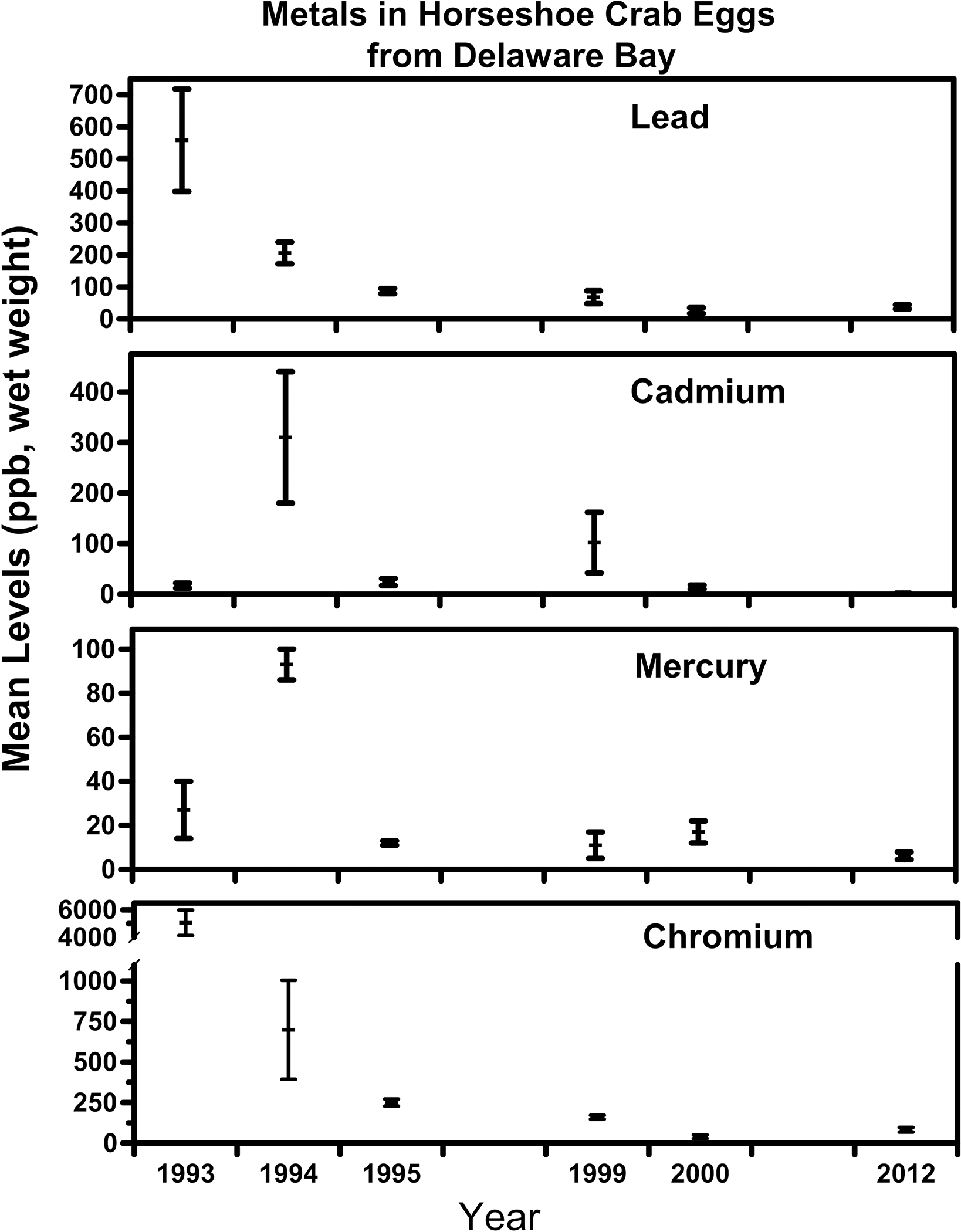
Temporal trends in levels of chromium, cadmium, lead, and mercury in ppb (wet weight).
The selenium:mercury molar ratios varied significantly by year (Table 6), but the pattern was not consistent. That is, the highest ratios occurred in 1995, and 2012. From 1995 until the present, the ratio was negatively correlated with mercury concentrations. The variation in selenium is interesting because selenium is regulated in the body (ATSDR 2003), and sequestering selenium in eggs may be a way of riding the body of selenium.
Table 6.
Total Mercury and Selenium levels (ppb, wet weight)(ug/g) in Horseshoe Crab eggs collected from New Jersey. Given are arithmetic means ± SE, standard deviation and Kendall Tau correlation coefficients.
| Year | n | Mercury Mean ± SE | Selenium Mean ± SE | Hg nmol/g wet wt. | Se nmol/g wet wt. | Se:Hg | Se:Hg Ratio Correlation with Hg tau (p) |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1993 | 18 | 27.1 ± 0.3 | 1965 ± 57 | 0.14 | 25 | 184 | a |
| 1994 | 9 | 93.2 ± 8.0 | 3468 ± 522 | 0.46 | 44 | 95 | NS |
| 1995 | 19 | 11.9 ± 1.3 | 3473 ± 142 | 0.06 | 44 | 743 | −0.7 (0.0003) |
| - | |||||||
| 1999 | 39 | 19.3 ± 1.9 | 1062 ± 94 | 0.10 | 13 | 140 | −0.60 (<0.0001) |
| 2000 | 7 | 16.8 ± 5.2 | 1112 ± 319 | 0.08 | 14 | 168 | −0.7 (0.02) |
| 2012 | 17 | 6.2 ± 1.7 | 990 ± 99 | 0.03 | 13 | 409 | −0.8 (<0.0001) |
|
| |||||||
| Kruskal Wallis X2 (p) | 46.6 (<0.0001) | 61.2 (<0.0001) | 32.8 (<0.0001) | ||||
Not available because too few samples overlapped.
4. Discussion
4.1. Temporal differences
Overall, there was a decline in the levels of cadmium, chromium, lead and mercury in the eggs of horseshoe crabs from 1993 to 2001 (Burger 1997; Burfer et al 2003, Fig. 2). The decline suggests that contaminants are not likely to be a problem for the crabs (see below). However, the declines were not similar for all metals, or for all locations. Lead and chromium clearly declined, while cadmium and mercury had high and low years, but did not exhibit a steady decline. Cadmium and mercury are contaminants that occur naturally in seawater, and are added to the environment by anthropogenic activities. While some legislation in the U.S. and elsewhere have resulted in declines in local exposure, atmospheric deposition continues to increase (Fitzgerald 2005)
Although mercury enters the environment from both natural and anthropogenic sources it is the anthropogenic sources that are of greatest concern because of both historic and recent changes, and the increasing demand for electric power generation releasing mercury to the atmosphere. Anthropogenic sources account for about 80% of the annual inputs of mercury to the environment; for many parts of the World, atmospheric deposition is the primary source of mercury (Driscoll et al 2006). The global contribution of mercury to the atmosphere is unevenly distributed. The Asian countries contribute about 54% of the mercury to total atmospheric sources. China alone contributes 28 % to the total emissions, followed by Africa (18%), and Europe (11%, Pacyna 2006). This seems likely to increase with further development in China. In the present study, although mercury declined, its decline was neither as consistent nor as dramatic as the declines in lead and chromium. The co-occurrence of highs in mercury and cadmium in 1994 may suggest a common cause, such as changes in currents that brought a different mix of seawater into the bay
4.2. Effects on the crabs
Interpreting levels of metals in invertebrates is difficult because both toxicity and susceptibility differ (Rainbow 1996). Understanding the effects of a given level of contaminant may depend upon the form. Further, the relationship between dose and tissue levels, and between tissue levels and toxicity is often not clear (Burger and Gochfeld 1997, 2001). Thus, reports often cite the level in tissues of vertebrates that suggest toxicity, but seldom relate these levels to those causing effects in higher-level consumers, except humans (Hiller and Barclay, 2011). In laboratory experiments, horseshoe crab eggs are vulnerable to heavy metals, with mercury, organotin, and cadmium being the most toxic (Itow 1998a,b; Botton et al; 1998; Botton 2000), Although Itow (1998a) did not find a high rate of abnormalities in the eggs or larvae of horseshoe crabs from Delaware Bay, tin, mercury, cadmium, chromium and zinc inhibited the regeneration of walking legs in adults.. Botton (1998) suggested that the relatively high tolerance of horseshoe crab eggs to heavy metals suggests that eggs might pose a problem for consumers (see below).
Arsenic levels in a number of marine crustaceans range up to 68 ppm, with muscle levels have been reported up to 50 ppm (Eisler 1994). In horseshoe crabs from Delaware Bay, levels were similar in muscle and eggs (Burger et al 2003). Levels in eggs have declined to means up to 9 ppm (Burger et al 2003, this study). Thus the arsenic levels in eggs from Delaware Bay are slightly lower than the range normally reported for other related arthropods.
Marine invertebrate populations are at increased risk because of their high sensitivity to cadmium (Eisler 1985a; Wren 1995). Cadmium concentrations of 0.0007 to 0.005 ppm are associated with sublethal effects such as decreased growth, lowered reproduction, and population alterations in invertebrates (Eisler 1985a). Cadmium levels are similar in muscle and eggs (Burger et al 2003). Cadmium levels in eggs of horseshoe crabs from Malaysia ranged from 0.52 to 3.64 ppm (mean of 2.28 ppm, Carcinoscorpius rotundicauda) and 0.02 to 4.11 ppm (mean of 3.59 ppm, Tachypleus gigas) (Hajeb, 2009). Eggs collected in Delaware Bay in 2012 averaged as high as 0.008 ppm, which might suggest some sensitivity to cadmium (Eisler 1985a). This bears further examination.
Average chromium levels in tissues of horseshoe crabs from previous work in Delaware Bay ranged up to 5.06 ppm (Burger 1997). Muscle and eggs levels are similar (Burger et al 2003). However, levels in eggs have declined from an average of 5 ppm to 0.17 ppm (this study).
Average lead levels in tissues of horseshoe crabs from previous work in Delaware Bay ranged up to 0.56 ppm (Burger 1997; Burger et al 2003); levels were similar among tissue types. In 2012, lead levels in eggs had means of up to 0.086 ppm for eggs in 2012 (this study). Lead levels in eggs of one species of horseshoe crabs (Carcinoscorpius rotundicauda) from Malaysia were non-detect, but eggs of another species, Tachypleus gigas, ranged from 10.0 to 25.8 ppm (mean of 18.26 ppm, Hajeb 2009).
Average manganese levels in tissues of horseshoe crabs from previous work in Delaware Bay ranged up to 7.12 ppm (Burger 1997), and then declined to 3.73 ppm (Burger et al 2002). In 2012, the highest mean level in eggs was 7.32 ppm. Average mercury levels in tissues of horseshoe crabs from previous work in Delaware Bay ranged up to 93 ppb (Burger 1997), compared to 130 ppb in 2000. However, in the present study we only measured mercury in eggs because of their overall importance in the food chain (see below). Average selenium levels in tissues of horseshoe crabs from previous work in Delaware Bay ranged up to 3.47 ppm (Burger 1997), and then declined to 1.62 ppm. In the present study, the highest average selenium level was 1.30 ppm.
Overall, for all metals where previous data were available (except mercury) on horseshoe crabs from Delaware Bay, the levels were similar to or lower than those previously reported. Given the low level of abnormalities in embryos of horseshoe crabs from Delaware Bay (Itow 1998a), it is unlikely that these levels are problematic for the horseshoe crabs.
4.3. Food chain effects
Horseshoe crabs are at the base of the food chain, particularly the eggs and young larvae stage. They are eaten by a wide range of invertebrates, fish, and birds. Understanding metals levels in important because vulnerable populations of young fish, breeding birds, and migrant shorebirds might receive a high dose of heavy metals during a critical migratory period when they must double their weight in two to three weeks (Castro et al 1989; Castro and Myers 1993; Tsipoura and Burger 1999). Horseshoe crab eggs are also important to laughing gulls that are breeding (Burger 1996c). Previous work indicated relatively low levels of heavy metals in the feathers of migrant shorebirds from this region (Burger 1993). However, feathers reflect circulation levels of metals in blood during feather formation (Monteiro 1996; Evers 2005), and thus those collected from shorebirds in Delaware Bay do not reflect local exposure. Local exposure would more likely be reflected in metal levels in blood.
Sensitive species of birds are adversely affected at diet arsenic concentrations of 120 ppm, and cadmium concentrations of 1 ppm (Eisler 1985a, 1994), which are well above the levels reported in this study (2012 arsenic levels averaged 4.41 ppm or less in eggs)..
Birds and fish can be adversely affected when cadmium levels exceed 1 ppm in the diet (Eisler 1986), which is well above the levels we found in the horseshoe crab eggs. Marine organisms, however, are less sensitive, perhaps because they live in the oceans that normally have higher cadmium levels than freshwater (Furness 1996). However, eggs and fry of fish are affected by chronic cadmium exposure, including growth deficits, hatching rates and deformities (Hansen 2002). There is great variation among fish species, suggestion the need for more studies with development in horseshoe crabs dosed with cadmium (Wren 1995).
Chromium levels of 10 ppm, and lead levels of 0.05 – 0.075 ppm in the diet are considered to cause adverse effects in birds (Eisler 1986, 1988). In the present study, mean chromium levels in eggs of up to 0.17 ppm suggest no cause for concern.
Lead levels in horseshoe eggs in 2012 averaged 0.08 ppm or less, also suggesting some cause for concern. Little similar data are available for manganese. Selenium levels of more than 5. 0 ppm are harmful, but lower levels may also be harmful (Eisler 1985b). In birds, levels of 10 ppm are harmful in eggs (Ohlendorf 1986), but harmful effects can occur at levels as low as 3 ppm (Heinz 1996). In this study, levels in eggs of horseshoe crabs averaged between 0.9–1.0 ppm, well below the effects level.
Mercury levels of 0.05 to 0.10 ppm in diet can cause developmental defects in young birds (Eisler 1987; Burger and Gochfeld 1997), and harmful effects in bird eggs occur at 3 ppm (Eisler 2000). The average levels in horseshoe crab eggs from all locations in 1999 and 2000 were well below 0.05 ppm (Burger 2003), suggesting no cause for concern even then. Mercury levels in horseshoe eggs in 2012 averaged up to 0.018 ppm, well below the effects levels, particularly given that marine organisms seem less sensitive to mercury than are terrestrial ones (Thompson and Furness 1989).
4.4. Selenium:mercury molar ratios.
Some researchers have suggested that mercury toxicity can be reduced by selenium (Lindh and Johansson 1987; Ralston et al 2008). Mercury binds to selenium with a high affinity, and research has focused on whether methylmercury toxicity is due to impaired synthesis of seleno-enzymes or inhibition of their activity (Ralston 2009; Ralston 2008). Mercury and methylmercury are irreversible selenoenzyme inhibitors that impair selenoprotein form and function. Selenoenzymes also play an important role in antioxidant defenses, explaining the oxidative damage attributable to methylmercury (Pinheiro 2009; Ralston and Raymond 2010). The toxicodynamics of the selenium and mercury interactions require extensive study as effects differ depending on the species and compounds of selenium and mercury (Khan and Wang, 2009; Dang and Wang 2011), as well as administration methods (Klimstra 2011). High maternal exposure to methylmercury in animals inhibits selenium-dependent enzyme activity in the brain while selenium supplementation is protective (Berry and Ralston, 2008). There is a limit to the protection of selenium on mercury toxicity, and selenium itself can be highly toxic (Klimstra 2012).
There is also evidence that selenium moderates mercury toxicity in free-ranging fish (Sormo 2011). Ralston and others have suggested that selenium:mercury molar ratios above 1 protect against mercury toxicity, and that these ratio should be an being an important consideration for risk assessment (Kaneko and Ralston 2007; Peterson 2009; Ralston and Raymond 2010).
Additionally, there is some evidence in humans that low levels of selenium are associated with increased coronary heart disease (Seppanen 2004), and high levels of selenium are associated with lower levels of nonfatal heart attacks (Mozaffarian 2009). Watanabe (2002) argued that the practical implications of the modifying effect of selenium on mercury toxicity are unclear because of the variability in toxicodynamics. Even so in places where mercury levels in eggs of horseshoe crabs are higher, and people consume them, the selenium:mercury molar ratios should be examined. The selenium:mercury molar ratios in horseshoe crab eggs from Delaware Bay ranged from a mean to 95 to a mean of 743. This suggests sufficient excess selenium to bind with mercury, reducing mercury toxicity.
5. Conclusions and management implications
Our overall conclusion is that the levels of contaminants found in horseshoe crab eggs are well below those known to cause adverse effects in the crabs themselves, or in organisms that consume their eggs. In Asia, local horseshoe crab eggs are eaten, and in some places, metal levels are in the toxic range (Hajeb 2009). From a risk perspective, it is clear that contaminant levels have generally declined in the eggs of horseshoe crabs over the last 20 years, suggesting that contaminants are not likely to be a problem for secondary consumers, such as young fish and migrating shorebirds.
In addition to the conservation issues related to preserving healthy populations of breeding horseshoe crabs, and to providing sufficient excess eggs for the food chain, including other invertebrates, fish, and migrating shorebirds, the region is dependent upon the tourists that come each spring to see the spectacle of the horseshoe crabs and shorebirds (Burger 1996a; Manion 2000; Niles 2012). The relatively low levels of metals in eggs of horseshoe crabs in 2012 suggests that because they provide no direct threat to the crabs themselves or to consumers, any populations shifts in either horseshoe crabs or shorebirds are not likely due to metals.
Acknowledgments
We thank L. Niles, M. Dey, K. Clark, R. Loveland and members of the ASMFC for discussions about horseshoe crabs. We especially thank M. Gochfeld and A. Spry for logistical help. This research was partially funded by the Nongame and Endangered Species Program of the NJ Department of Environmental Protection, Consortium for Risk Evaluation with Stakeholder Participation (CRESP) through the Department of Energy (AI # DE-FC01–95EW55084; DE-FG 26–00NT 40938), and NIESH (P30ES05022). The results, conclusions and interpretations reported herein are the sole responsibility of the authors, and should not in any way be interpreted as representing the views of the funding agencies.
References
- Andres BA, Smith PA, Morrison RG, Gratto-Trevor CL, Brown SC, & Friis CA (2013). Population estimates of North American shorebirds, 2012. Wader Study Group Bulletin, 119, 178–194. [Google Scholar]
- Atlantic States Marine Fisheries Commission (ASMFC ). (1998). Interstate fishery management plan for horseshoe crab. Washington, D.C. [Google Scholar]
- Atlantic States Marine Fisheries Commission (ASMFC ). (1999). Horseshoe Crab Stock Assessment for Peer Review. Stock assessment Report No. 98–01 (suppl.). Washington, D.C. [Google Scholar]
- Baker AJ, Gonzalez PM, Piersma T, & Niles LJ (2004). Rapid population decline in red knots: fitness consequences of refuelling rates and late arrival in Delaware Bay. Proceedings of the Royal Society of London, 271, 875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry MJ, & Ralston NVC (2008). Mercury toxicity and the mitigating role of selenium. EcoHealth, 5, 456–459. [DOI] [PubMed] [Google Scholar]
- Botton ML (2000). Toxicity of cadmium and mercury to horseshoe crab (Limulus polyphemus) embryos and larvae. Buletin of Environmental. Contamination and Toxicology, 64, 137–143. [DOI] [PubMed] [Google Scholar]
- Botton ML & Loveland RE (2001). Updating the life history of the Atlantic horseshoe crab, Limulus polyphemus. Jersey Shoreline, 20, 6–9. [Google Scholar]
- Botton ML, Loveland RE, & Jacobsen TR (1994). Site selection by migratory shorebirds in Delaware Bay, and its relationship to beach characteristics and abundance of horseshoe crab (Limulus polyphemus) eggs. Auk, 111, 605–616. [Google Scholar]
- Botton ML, Johnson K, & Helleby L (1998). Effects of copper and zinc on embryos and larvae of the horseshoe crab, Limulus polyphemus. Archives of Environmental Contamination and Toxicology, 64, 25–32 [DOI] [PubMed] [Google Scholar]
- Burger J (1986). The effect of human activities on shorebirds in two coastal bays in the Northeastern United States. Environmental Conservation, 13, 123–130. [Google Scholar]
- Burger J (1996a). A naturalist along the Jersey shore. New York, Rutgers University Press. [Google Scholar]
- Burger J (1996b). The effect of human activities on shorebirds in two coastal bays in the Northeastern United States. Environmental Conservation, 13, 123–130 [Google Scholar]
- Burger J (1996c). Laughing Gull. In Poole A and Gill F (Eds.), Birds of North America. No 225:1–28. [Google Scholar]
- Burger J (1997). Heavy metals in the eggs and muscle of horseshoe crabs (Limulus polyphemus) from Delaware Bay. Environmental Monitoring and Assessment 46, 279–287. [Google Scholar]
- Burger J & Gochfeld M (1997). Risk, mercury levels, and birds: relating adverse laboratory effects to field biomonitoring. Environmental Research 75, 160–172. [DOI] [PubMed] [Google Scholar]
- Burger J and Gochfeld M (2001). Effects of chemicals and pollution on seabirds. In Schreiber BA and Burger J (Eds.). Biology of marine birds (pp. 485–525). Florida, CRC Press. [Google Scholar]
- Burger J, Clark KL, & Niles L (1997). Importance of beach, mudflat and marsh for migrant shorebirds on Delaware Bay. Biological Conservation 79, 283–292. [Google Scholar]
- Burger J, Seyboldt S, Morganstein N, & Clark K (1993). Heavy metals and selenium in feathers of three shorebird species from Delaware Bay’, Environmental Monitoring and Assessment, 28, 189–198. [DOI] [PubMed] [Google Scholar]
- Burger J, Dixon C, Shukla T, Tsipoura N, & Gochfeld M (2002). Metal Levels in Horseshoe Crabs (Limulus polyphemus) from Maine to Florida. Environmental Research, 90, 227–236. [DOI] [PubMed] [Google Scholar]
- Burger J, Dixon C, Shukla T, Tsipoura N, Jensen H, Fitzgerald M, Ramon R, & Gochfeld M (2003). Metals in horseshoe crabs from Delaware Bay. Archives of Environmental Contamination and Toxicology, 44, 26–42. [DOI] [PubMed] [Google Scholar]
- Castro G, & Myers JP (1993). Shorebird predation on eggs of horseshoe crabs during spring stopover on Delaware Bay. Auk, 110, 927–930. [Google Scholar]
- Castro G, Myers JP, & Place AR (1989). Assimilation efficiency of sanderlings (Calidris alba) feeding on horseshoe crab eggs. Physiological Zoology, 62,716–731. [Google Scholar]
- Dang F, & Wang W (2011). Antagonistic interaction of mercury and selenium in a marine fish is dependent on their chemical species. Environmental Science and Technology, 45, 3116–3122. [DOI] [PubMed] [Google Scholar]
- Driscoll CT, Abbot M, Bullock R, Jansen J, Leonard D, Lindberg S, Munthe J, Pirrone N & Niles M (2006). Airsheds and watersheds. In: Harris R, Krabbenhoft DP, Mason R, Murray MW, Reash R, and Saltman T (Eds.), Ecosystem responses to mercury contamination (pp 12–46). Florida: CRC Press. [Google Scholar]
- Dunne P, Sibley D, Sutton C, & Wander W (1982). Aerial surveys in Delaware Bay: confirming an enormous spring staging area for shorebirds. Wader Study Group Buletin, 35, 32–33. [Google Scholar]
- Eisler R (1985a). Cadmium hazards to fish, wildlife, and invertebrates: a synoptic review. U.S. Fish & Wildlife Service, Biological Reports, 85 (1.2), Washington D.C. [Google Scholar]
- Eisler R (1985b). Selenium hazards to fish, wildlife, and invertebrates: a synoptic review. U.S. Fish & Wildlife Service, Biological Reports, 85 (1.5), Washington D.C. [Google Scholar]
- Eisler R (1986). Chromium hazards to fish, wildlife, and invertebrates: a synoptic review. U.S. Fish & Wildlife Service, Biological Reports 85, (1.6), Washington D.C. [Google Scholar]
- Eisler R (1987). Mercury hazards to fish, wildlife, and invertebrates: a synoptic review. U.S. Fish & Wildlife Service, Biological Reports, 85 (1.10), Washington D.C. [Google Scholar]
- Eisler R (1988). Lead hazards to fish, wildlife, and invertebrates: a synoptic review. U.S. Fish & Wildlife Service, Biological Reports, 85 (1.14), Washington D.C. [Google Scholar]
- Eisler R (1994). A review of arsenic hazards to plants and animals with emphasis on fishery and wildlife resources. In Nriagu JO (Ed.), Arsenic in the environment (pp 185–259). Florida: Lewis Publishing. [Google Scholar]
- Eisler R (2000). Handbook of chemical risk assessment: health hazards to humans, plants and animals. Florida: Lewis Publishing. [Google Scholar]
- Evers D, Burgess NM, Champoux L, Hoskins B, Major A, Goodale W. 2005. Patterns and interpretation of mercury exposure in freshwater avian communities in northeastern North America. Ecotoxicology, 14, 193–221 [DOI] [PubMed] [Google Scholar]
- Fitzgerald WF, Engstrom DR, Lamborg CH, Tseng CM, Balcom PH, Hammerschmidt CR 2005. Modern and historic atmospheric mercury fluxes in northern Alaska: global sources and Arctic depletion. Environmental Science and Technology, 39, 557–568. [DOI] [PubMed] [Google Scholar]
- Furness RW (1996). Cadmium in birds. In Beyer WM, Heinz GH, and Redmon AW (Eds.) Environmental contamination in wildlife: interpreting tissue concentrations. Boca Raton Fl. Pp 389–404 [Google Scholar]
- Goss-Custard JD, Triplet P, Sueur F, & West AD (2006). Critical thresholds of disturbance by people and raptors in foraging wading birds. Biological Conservation, 127, 88–97. [Google Scholar]
- Hansen JA, Welsh PG, Lipton J, & Suedkamp MJ (2002). The effects of long-term cadmium exposure on the growth and survival of juvenile bull trout (Salvelinus confluentus). Aquatic Toxicology, 58, 165–174. [DOI] [PubMed] [Google Scholar]
- Heinz GH (1996). Selenium in birds. In Beyer WM, Heinz GM, Redmon-Norwood E, and Norwood AW (Eds.), In Environmental contaminants in wildlife:interpreting tissue concentrations (pp. 447–458). Florida: Lewis Publishing. [Google Scholar]
- Hiller BJ, & Barclay JS (2011). Concentrations of heavy metals in American woodcock harvested in Connecticut. Archives of Environmental Contamination and Toxicology, 60, 156–164. [DOI] [PubMed] [Google Scholar]
- Itow T, Loveland RE, & Botton ML (1998a). Developmental abnormalities in horseshoe crab embryos caused by exposure to heavy metals. Archives of Environmental Contamination and Toxicology, 35, 33–40. [DOI] [PubMed] [Google Scholar]
- Itow T, Igarashi T, Botton ML, & Loveland RE (1998b). Heavy metals inhibit limb regeneration in horseshoe crab larvae. Archives of Environmental Contamination and Toxicology 35, 457–463 [DOI] [PubMed] [Google Scholar]
- Kaneko JJ, & Ralston NV (2007). Selenium and mercury in pelagic fish in the central north Pacific near Hawaii. Biological Trace Element Research, 119, 242–254. [DOI] [PubMed] [Google Scholar]
- Khan MAK, & Wang F (2009). Mercury-selenium compounds and their toxicological significance: toward a molecular understanding of the mercury-selenium antagonism. Environmental Toxicology and Chemistry, 28, 1567–1577. [DOI] [PubMed] [Google Scholar]
- Klimstra JD, Yee JL, Heinz GH, Hoffman DJ, & Stebbins KR (2012). Interactions between methylmercury and selenomethionine injected into Mallard eggs. Environmental Toxicology and Chemistry, 31, 579–584. [DOI] [PubMed] [Google Scholar]
- Kreamer G & Michels S. (2009). History of Horseshoe Crab Harvest in Delaware Bay. In Tanacredi JT, Botton ML & Smith DR (Eds),.Biology and Conservation of Horseshoe Crabs (pp. 299–313). New York: Springer. [Google Scholar]
- Lindh U, & Johansson E (1987). Protective effects of selenium against mercury toxicity as studied in the rat liver and kidney by nuclear analytical techniques. Biological Trace Element Research, 12, 109–120. [DOI] [PubMed] [Google Scholar]
- Manion MM, West RA, & Unsworth RE (2000). Economic assessment of the Atlantic Coast horseshoe crab fishery. U.S. Fish & Wildlife Service, Virginia. [Google Scholar]
- Monteiro LR (1996). Seabirds as monitors of mercury in the marine environment. Water, Air, Soil Pollution, 80, 851–870. [Google Scholar]
- Morrison RIG, Ross RK, &, Niles LJ (2004). Declines in wintering populations of Red Knots in southern South America, Condor, 106, 60–70. [Google Scholar]
- Morrison RIG, Davidson NC, & Wilson JR (2007). Survival of the fittest: body stores on migration and survival in Red Knots, Calidris canutus islandica. Journal of Field Ornithology, 38, 479–487. [Google Scholar]
- Mozaffarian D (2009) Fish, mercury, selenium and cardiovascular risk: current evidence and unanswered questions. International Journal of Environmental Research and Public Health, 6, 1894–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niles LJ, Sitters HP, Dey AD, Atkinson PW, Baker AJ, (2008). Status of the Red Knot, Calidris canutus rufa, in the Western Hemisphere. Studies of Avian Biology, 36, 1–185. [Google Scholar]
- Niles LJ, Burger J and Day A (2012). Life along the Delaware Bay: Gateway to a Million Shorebirds. New Jersey: Rutgers University Press. [Google Scholar]
- Statistical Analysis Systems (SAS). (1995), SAS Users’ Guide. Statistical Institute, Cary, North Carolina. [Google Scholar]
- Pacyna EG, Pacyna JM, Steenhuisen F and Wilson S (2006). Global anthropogenic mercury emissions inventory for 2000. Atmosphere and Environment 40, 4048–4063. [Google Scholar]
- Peterson SA, Ralston NVC, Whanger PD, Oldfield JE, and Mosher WD (2009). Selenium and mercury interactions with emphasis on fish tissue. Environmental Bioindicators, 4, 318–334. [Google Scholar]
- Pinheiro MCN, de Nascimento JLM, Silveira LCL, daRocha JBT, and Aschner M, (2009). Mercury and selenium – a review on aspects related to the health of human populations in the Amazon. Environmental Bioindicators, 4, 222–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainbow PS (1996). Heavy metals in aquatic vertebrates’, In Beyer WM, Heinz GH, Redmon-Norwood AW. eds. Environmental contaminants in wildlife: interpreting tissue concentrations. Lewis Pub. Boca Raton, FL. Pp. 405–425. [Google Scholar]
- Ralston NVC (2008). Selenium health benefit values as seafood safety criteria. Eco-Health, 5, 442–455. [DOI] [PubMed] [Google Scholar]
- Ralston NVC (2009). Introduction to 2nd issue on special topic: selenium and mercury as interactive environmental indicators. Environmental Bioindicators, 4, 286–290. [Google Scholar]
- Ralston NVC, and Raymond LJ (2010). Dietary selenium’s protective effects against methylmercury toxicity. Toxicology, 278, 112–123. [DOI] [PubMed] [Google Scholar]
- Ralston NVC, Ralston CR, Blackwell III JL, and Raymond LJ (2008). Dietary and tissue selenium in relation to methylmercury toxicity. Neurotoxicology, 29, 802–811. [DOI] [PubMed] [Google Scholar]
- Seppanen D, Soininen P, Salonen JT, Lotjonen S, and Laatikainen R (2004). Does mercury promote lipid peroxidation? An in vitro study concerning mercury, copper, and iron in peroxidation of low-density lipoprotein. Biological Trace Element Research, 101,117–132. [DOI] [PubMed] [Google Scholar]
- Shuster C,N Jr. (1982). A pictorial review of the natural history and ecology of the horseshoe crab (Limulus polyphemus), with references to other Limulidae. In Bonaventura J, Bonaventura C and Tesh S (Eds.), Physiology and Biology of Horseshoe Crabs (pp. 1–52), New York; Alan R. Liss. [PubMed] [Google Scholar]
- Shuster CN Jr. & Botton ML (1985). A contribution to the population biology of horseshoe crabs (Limulus polyphemus) in Delaware Bay. Estuaries, 8, 363–372 [Google Scholar]
- Thompson DR & Furness RW (1989). Comparison of the levels of total and organic mercury in seabird feathers. Marine Pollution Bulletin, 20, 577–579. [Google Scholar]
- Tsipoura N, and Burger J (1999). Shorebird diet during spring migration stop-over on Delaware Bay. Condor, 101, 635–644. [Google Scholar]
- Watanabe C (2002). Modification of mercury toxicity by selenium: practical importance. Tohoku Journal of Exposure Medicine, 196, 71–77. [DOI] [PubMed] [Google Scholar]
- Wren CD, Harris S, and Harttrup N (1995). Ecotoxicology of mercury and cadmium In, Beyer WM, Heinz GH, and Redmon AW (Eds.), Environmental contamination in wildlife: interpreting tissue concentrations (pp. 389–404). Florida: CRC Press. [Google Scholar]


