Abstract
For several decades, coronary artery bypass grafting has been regarded as the standard choice of revascularization for significant left main coronary artery (LMCA) disease. However, in conjunction with remarkable advancement of device technology and adjunctive pharmacology, percutaneous coronary intervention (PCI) offers a more expeditious approach with rapid recovery and is a safe and effective alternative in appropriately selected patients with LMCA disease. Several landmark randomized clinical trials showed that PCI with drug-eluting stents for LMCA disease is a safe option with similar long-term survival rates to coronary artery bypass grafting surgery, especially in those with low and intermediate anatomic risk. Although it is expected that the updated evidence from recent randomized clinical trials will determine the next guidelines for the foreseeable future, there are still unresolved and unmet issues of LMCA revascularization and PCI strategy. This paper provides a comprehensive review on the evolution and an update on the management of LMCA disease.
Key Words: coronary artery bypass grafting, left main coronary artery disease, percutaneous coronary intervention
Abbreviations and Acronyms: BMS, bare-metal stent(s); CABG, coronary artery bypass grafting; CAD, coronary artery disease; DAPT, dual antiplatelet therapy; DES, drug-eluting stent(s); DK, double-kissing; FFR, fractional flow reserve; iFR, instantaneous wave-free ratio; IVUS, intravascular ultrasound; LAD, left anterior descending artery; LCX, left circumflex artery; LMCA, left main coronary artery; LVEF, left ventricular ejection fraction; MACCE, major adverse cardiac or cerebrovascular events; MI, myocardial infarction; MLA, minimal lumen area; PCI, percutaneous coronary intervention; RCT, randomized clinical trial
Central Illustration
Highlights
-
•
With advancements in PCI, clinical outcomes after left main PCI have progressively improved.
-
•
Unmet needs still exist between clinical practice and the current evidence for left main PCI.
-
•
Better decision making of revascularization choice and PCI optimization should be emphasized to improve outcomes of LMCA disease.
-
•
Further research will provide further evidence to resolve conflicting issues on left main PCI.
Left main coronary artery (LMCA) disease represents the highest-risk lesion subset of coronary artery disease (CAD) because of the large amount (approximately 70%) of jeopardized myocardium, which is associated with significantly higher risks of cardiovascular morbidity and mortality as compared with other obstructive CAD.1 LMCA disease is not uncommon in patients with acute coronary syndrome and stable CAD, and it is frequently combined with concomitant multivessel disease.2 Thus, given that LMCA disease has an important prognostic value, current clinical practice guidelines strongly recommend revascularization in all patients with ≥50% stenosis of the LMCA,3 and the optimal revascularization strategy is crucial for the management of significant LMCA disease. Traditionally, coronary artery bypass grafting (CABG) has been the gold standard revascularization method for significant LMCA disease based on its established mortality benefit over medical therapy and documented long-term durability. In earlier periods, percutaneous coronary intervention (PCI) for LMCA disease was considered an alternative to CABG in highly selected patients or in those with hemodynamic instability or who are at high surgical risk.4
However, with remarkable advancements in the PCI field over the last 20 years, including device technology, PCI technique, adjunctive pharmacotherapy, and improved procedural expertise, PCI has become a reasonable alternative in a significant portion of patients with LMCA disease.5 Until recently, there have been several clinical registries and randomized clinical trials (RCTs) to evaluate the clinical effectiveness of PCI with stenting for LMCA disease relative to standard CABG.1,5 On the basis of these cumulative data, the optimal management of patients with LMCA disease is now guided by key clinical and anatomic factors, for which the heart team approach is increasingly emphasized. Furthermore, technological and conceptual advancements with functional and imaging concepts for left main PCI have been introduced in both techniques to alter optimal procedural decision making and approaches.6,7 In spite of these revolutions, unresolved issues on the treatment of LMCA disease in contemporary clinical practice still exist. In this review, we summarize the latest clinical evidence, practical applications, and special considerations as well as speculate on the future perspective of left main PCI.
Clinical Evidence Supporting Left Main PCI
Evolution of PCI for LMCA disease
Based on old data, the prognosis of patients with medically treated LMCA disease was very poor, with the 5-year cardiac mortality rate reaching >50%.8,9 Because earlier RCTs from 30 to 40 years ago demonstrated the superiority of surgical revascularization over medical treatment alone (which was limited by the lack of contemporary guideline-based medical therapy) for the 5- to 10-year survival in patients with LMCA disease,10, 11, 12, 13 CABG has been the gold standard of care for a long time. Meanwhile, the first PCI was performed by Dr Andreas Gruentzig with balloon angioplasty in 1977.14 However, plain balloon angioplasty was quickly abandoned after the first case series reported a poor long-term prognosis of patients with unprotected LMCA disease, although elective angioplasty was technically feasible.15 Thereafter, PCI with balloon angioplasty was performed on a limited basis, mostly in surgically ineligible conditions as a salvage procedure or in protected LMCA cases. With the introduction of metallic stents and dual antiplatelet therapy (DAPT), PCI for LMCA disease has been reconsidered as a revisited option since the mid-1990s, overcoming the shortcomings of balloon angioplasty (ie, acute recoil, abrupt closure, or coronary dissection). PCI with bare-metal stents (BMS) for LMCA disease was conducted in highly selective, elective, low-risk patients with technical feasibility and acceptable short-term and mid-term clinical outcomes.16, 17, 18, 19 However, the widespread use of BMS for complex LMCA disease was hampered by a high risk of restenosis and repeat revascularization.
In the early 2000s, drug-eluting stents (DES) with a remarkable reduction in angiographic and clinical restenosis were widely adopted, and PCI with DES implantation for LMCA disease has dramatically increased in daily clinical practice. At first, in several observational registries, PCI using early-generation DES compared with BMS demonstrated more favorable clinical outcomes after PCI of LMCA disease.20, 21, 22, 23, 24 With accumulating clinical evidence and shared experiences for such complex PCI, physicians’ threshold for performing PCI for LMCA disease has become less restrictive. Thus, PCI with DES has been increasingly performed for more complex clinical and anatomic situations.1 Also, given that newer-generation DES have demonstrated a lower risk of stent thrombosis and restenosis compared to first-generation DES,25,26 as well as that technological enhancements and procedural simplification have contributed to the increased use of PCI for such complex lesions, the unrestricted use of second-generation DES for LMCA disease has been more rapidly increasing, and better clinical outcomes were reported in diverse “real-world” registries.27, 28, 29
Latest evidence comparing PCI and CABG for LMCA disease
Several RCTs comparing PCI using early-generation DES with CABG for treating LMCA disease have suggested comparable clinical outcomes of PCI and CABG.30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42 Key elements and trial findings of RCTs comparing PCI with DES and CABG for LMCA revascularization are summarized in Table 1. During recent years, long-term follow-up up to 5 or 10 years has become available for a large number of patients who underwent percutaneous vs surgical revascularization of LMCA disease. Although revascularization is the established optimal management strategy for LMCA disease, recent long-term follow-up of landmark RCT data and several meta-analyses of contemporary RCTs have rekindled the debate over whether CABG or PCI is preferred and optimal.35,38,40,42, 43, 44, 45, 46, 47
Table 1.
Summary of Randomized Clinical Trials of PCI With DES Vs CABG for LMCA Disease
| LEMANS30,31 | Boudriot et al32 | SYNTAX-LM33, 34, 35 | PRECOMBAT36,38 | EXCEL39,40 | NOBLE41,42 | |
|---|---|---|---|---|---|---|
| Recruitment period | 2001-2004 | 2003-2009 | 2005-2007 | 2004-2009 | 2010-2014 | 2008-2015 |
| PCI/CABG, n/n | 52/53 | 100/101 | 357/348 | 300/300 | 948/957 | 592/592 |
| Follow-up, y | 10 | 1 | 5 10 (for mortality) |
10 | 5 | 5 |
| Diabetes, % | 18 | 36 | 25 | 32 | 29 | 15 |
| Bifurcation, % | 58 | 72 | 61 | 64 | 81 | 81 |
| SYNTAX score, mean | Not reported | 23 | 30 | 25 | 21 | 22 |
| Stent | BMS and DES (35%) | DP-SES | DP-PES | DP-SES | DP-EES | BP-BES and DP-SES (7.7%) |
| IVUS | Recommend | Infrequent | Infrequent | At discretion, 91% | Recommended, 77% | Recommended, 74% |
| FFR guidance | Not reported | Not reported | Infrequent | Not reported | Recommended, 9.0% | Recommended |
| LIMA, % | 72 | 99 | 97 | 94 | 99 | 96 |
| Off pump, % | 1.9 | 46 | Not reported | 64 | 29 | 16 |
| Primary trial endpoint | Change in LVEF | Cardiac death, MI, or TVR | Death, MI, stroke, or repeat revascularization 10-y all-cause death |
Death, MI, stroke, or TVR | Death, MI, or stroke | Death, nonprocedural MI, stroke, or repeat revascularization |
| Key finding | There was a trend toward higher LVEF at 10 y with PCI. | PCI was inferior to CABG at 1 y. | PCI was noninferior to CABG at 1 and 5 y in terms of death, MI, stroke, or repeat revascularization. No significant difference in 10-y all-cause death between PCI and CABG. | PCI was noninferior to CABG at 1, 5, and 10 y. | PCI was noninferior to CABG at 3 and 5 y. | PCI was inferior to CABG at 5 y. |
BMS = bare-metal stent; BP-BES = biodegradable polymer biolimus-eluting stent; CABG = coronary artery bypass grafting; DES = drug-eluting stent; DP-EES = durable polymer everolimus-eluting stent; DP-PES = durable polymer paclitaxel-eluting stent; DP-SES = durable-polymer sirolimus-eluting stent; EXCEL = Evaluation of Xience Everolimus Eluting Stent vs Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization; FFR = fractional flow reserve; IVUS = intravascular ultrasound; LEMANS = Left Main Stenting; LIMA = left internal mammary artery; LVEF = left ventricular ejection fraction; MI = myocardial infraction; NOBLE = Nordic-Baltic-British Left Main Revascularization; PCI = percutaneous coronary intervention; PRECOMBAT = Premier of Randomized Comparison of Bypass Surgery versus Angioplasty Using Sirolimus-Eluting Stent in Patients with Left Main Coronary Artery Disease; SYNTAX = Synergy Between Percutaneous Coronary Intervention With TAXUS and Cardiac Surgery; SYNTAX-LM = left main substudy of the SYNTAX (Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery); TVR = target vessel revascularization.
Currently, 2 RCTs, EXCEL (Evaluation of Xience Everolimus Eluting Stent vs Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization) and NOBLE (Nordic-Baltic-British Left Main Revascularization), were sufficiently powered to determine the clinical effectiveness of PCI with contemporary DES compared to standard CABG for LMCA disease.39, 40, 41, 42 However, these 2 landmark RCTs showed somewhat conflicting results; EXCEL found that PCI was noninferior to CABG,39,40 whereas NOBLE failed to show noninferiority for PCI compared with CABG.41,42 These opposing results might raise clinical uncertainty with regard to the relative very-long-term treatment effect of PCI vs CABG; especially, it is noteworthy that a relative treatment effect of PCI and CABG differed substantially over time. Given that a worrisome trend of late catch-up or event curves appeared to diverge toward favoring CABG over PCI during late periods of follow-up and that the benefits of CABG tend to become evident with longer follow-up,48 longer-term follow-up data of these trials are required to determine whether the relative long-term treatment effect of contemporary PCI and CABG is variable over time and to confirm any definitive conclusions.
To date, the most recent individual patient-level or study-level meta-analyses incorporating long-term follow-up results from key RCTs (eg, SYNTAX [Synergy Between Percutaneous Coronary Intervention With TAXUS and Cardiac Surgery], PRECOMBAT [Premier of Randomized Comparison of Bypass Surgery versus Angioplasty Using Sirolimus-Eluting Stent in Patients with Left Main Coronary Artery Disease], EXCEL, and NOBLE) are summarized in Table 2. These analyses showed similar long-term mortality and hard clinical endpoint of composite of death, myocardial infarction (MI), or stroke after PCI with DES as compared to CABG in patients with LMCA disease.43, 44, 45, 46,49, 50, 51 Based on the currently available totality of the evidence, CABG and PCI with DES for the treatment of LMCA disease have comparable risks for overall mortality and the composite of death, MI, or stroke up to 5 to 10 years of follow-up. The risks of procedural MI and stroke were greater with CABG, whereas the risk of spontaneous MI was greater with PCI, providing early benefit for PCI in terms of MI and stroke, which is subsequently offset by a higher risk of spontaneous MI and repeat revascularization during long-term follow-up.
Table 2.
Updated Meta-Analysis of RCTs of PCI With DES vs CABG for LMCA Disease
| Giacoppo et al43 | Palmerini et al44 | Head et al45 | Ahmad et al46 | Kuno et al49 | D’Ascenzo et al50 | Sabatine et al51 | |
|---|---|---|---|---|---|---|---|
| Publication year | 2017 | 2017 | 2018 | 2020 | 2020 | 2021 | 2021 |
| Number of RCTs | 4 | 6 | 11 | 5 | 4 | 4 | 4 |
| Number of participants, (PCI/CABG) | 4,394 (2,197/2,197) | 4,686 (2,347/2,339) | 4,478 of 11,518 (38.9%) had LMCA disease (2,233/2,245) |
4612 (2,303/2,309) | 4,394 (not reported specific numbers for PCI/CABG) | 4,394 (2,197/2,197) | 4,394 (2,197/2,197) |
| Mean follow-up duration | Not reported (1 RCT had a 3-y follow-up; 3 RCTs reported 5-y follow-up) | 39 months | 3.4 y | 67.1 months | Not reported (included trials with at least a 5-y follow-up) | Not reported (included trials with at least a 5-y follow-up) | Not reported (included trials with at least a 5-y follow-up) |
| Primary outcome | A composite of all-cause death, MI, or stroke | All-cause mortality | All-cause mortality | All-cause mortality | All-cause mortality | All-cause mortality | All-cause mortality |
| Pooled estimate for primary outcome | HR: 1.06; 95% CI: 0.85-1.32; P = 0.60 | HR: 0.99; 95% CI: 0.76-1.30; P = 0.74 | HR 1.07; 95% CI: 0.87-1.33; P = 0.52 | RR: 1.03; 95% CI: 0.81-1.32; P = 0.78 | HR: 1.11; 95% CI: 0.91–1.35; P = 0.30 | ORa: 0.93; 95% CI 0.71-1.21; P = 0.58 | HR: 1.10; 95% CI: 0.91-1.32; P = 0.33 |
| Additional analyses and secondary outcomes | Primary outcome in patients with low to intermediate SYNTAX score: HR: 1.02; 95% CI: 0.74-1.41; P = 0.89 Repeat revascularization: HR: 1.70; 95% CI 1.42-2.05; P < 0.001 All cause-death: HR: 1.04; 95% CI: 0.81-1.31; P = 0.77 Cardiac death: HR: 1.00; 95% CI: 0.72-1.31; P = 0.77 MI: HR: 1.48; 95% CI: 0.85-2.58; P = 0.17 Stroke: HR: 0.87; 95% CI: 0.39-1.92; P = 0.72 |
Cardiac mortality: HR: 1.01; 95% CI: 0.72-1.42; P = 0.83 Significant interaction for cardiac mortality between treatment and SYNTAX score: P for interaction = 0.03 MI: HR: 1.33; 95% CI: 0.84-2.11; P = 0.11 Stroke: HR: 0.71; 95% CI: 0.34-1.49; P = 0.31 Unplanned revascularization: HR: 1.74; 95% CI: 1.47-2.07; P < 0.001 All-cause death, MI, or stroke: HR: 1.06; 95% CI: 0.82-1.37; P = 0.39 |
In diabetic patients with LMCA disease: HR: 1.34; 95% CI 0.93-1.91; P = 0.11 For nondiabetic patients with LMCA disease: HR: 0.94; 95% CI: 0.72-1.23; P = 0.65; P for interaction = 0.13 LMCA disease with SYNTAX score 0-22: HR: 0.91; 95% CI: 0.60-1.36; P = 0.64 SYNTAX score 23-32: HR: 0.92; 95% CI: 0.65-1.30; P = 0.65 SYNTAX score ≥33: HR: 1.39; 95% CI: 0.94-2.06; P = 0.10; P for interaction = 0.38 |
Cardiac mortality: RR: 1.03; 95% CI: 0.79-1.34; P = 0.82 Stroke: RR: 0.74; 95% CI: 0.35-1.50; P = 0.40 MI: RR: 1.22; 95% CI: 0.96-1.56; P = 0.11 Unplanned revascularization: RR: 1.73; 95% CI: 1.49-2.02; P < 0.001 |
Cardiac mortality: HR: 1.13; 95% CI: 0.88-1.44; P = 0.34 MI: HR: 1.48; 95% CI: 0.88-2.48; P = 0.14 Stroke: HR: 0.81; 95% CI: 0.42–1.53; P = 0.53 Repeat revascularization: HR: 1.80; 95% CI: 1.52-2.13; P < 0.01 |
Cardiac mortality: ORa: 0.95; 95% CI: 0.68-1.32; P = 0.75 MACCEs (including repeat revascularization): ORa: 0.69; 95% CI: 0.60-0.79; P < 0.00001 MI: ORa: 0.48; 95% CI: 0.36-0.65; P < 0.0001 Repeat revascularization: ORa: 0.53; 95% CI: 0.45-0.64; P < 0.0001 Stroke: ORa: 1.17; 95% CI: 0.59-2.31; P = 0.66 |
Cardiac mortality: HR: 1.07; 95% CI: 0.83-1.37; P = 0.61 MI: HR: 2.35; 95% CI:1.71-3.23; P < 0.0001 Stroke: HR: 0.84; 95% CI: 0.59-1.21; P = 0.36 Repeat revascularization: HR: 1.78; 95% CI: 1.51-2.10; P < 0.0001 Bayesian analysis: 85.7% probability that death at 5 years was greater with PCI than with CABG (probably <1.0%; <0.2%/y) |
| Key findings | PCI and CABG showed comparable outcomes at 3-5 y of follow-up. | PCI had a similar risk of mortality compared with CABG, with an interaction effect suggesting relatively lower mortality with PCI in patients with low SYNTAX scores and relatively lower mortality with CABG in patients with high SYNTAX scores. | Patients with LMCA disease had similar survival with PCI and CABG, regardless of diabetes and SYNTAX score. | PCI with DES showed similar long-term mortality compared with CABG in patients with LMCA disease. | PCI with DES showed similar long-term mortality compared with CABG in patients with LMCA disease. | PCI with DES showed similar long-term mortality compared with CABG in patients with LMCA disease. | PCI with DES showed similar long-term mortality compared with CABG in patients with LMCA disease. |
LMCA = left main coronary artery; MACCE = major adverse cardiac or cerebrovascular event; MI = myocardial infraction; OR = odds ratio; RCT = randomized clinical trial; RR = relative risk; other abbreviations as in Table 1.
ORs for CABG as compared with PCI.
In the “real-world” clinical setting, patients and physicians are increasingly opting for less invasive PCI rather than CABG for LMCA disease.1,52 The worsening risk profile and increasing prevalence of complex comorbidities in patients undergoing PCI has been broadly observed in several nationwide and international registries.1,52, 53, 54 Enrollment in RCTs is frequently mandated by strict inclusion and exclusion criteria, which raises concerns about the generalizability of reported trial findings. Therefore, although PCI with contemporary DES is currently widely considered in “real-world” LMCA patients with a wide variety of clinical and anatomic complexities, it is quite challenging to directly apply the trial findings to unrestricted patients in routine PCI practice.
Current revascularization guidelines
Over the last decade, existing clinical practice guidelines continue to advocate CABG surgery as the Class I indication for myocardial revascularization. However, more recent RCTs and registry studies support PCI as a reasonable alternative in selective patients with less complex LMCA disease. As evidence has accumulated over time, guideline recommendations of PCI for LMCA disease have become less stringent, and key changes before and after the EXCEL and NOBLE trials are summarized in Table 3. Consistent with the 2014 European guidelines,55 the recent 2018 European guidelines kept a Class I Level of Evidence: A recommendation for CABG in all patients with LMCA disease regardless of anatomic complexity.56 After the publication of the landmark EXCEL and NOBLE trials and subsequent meta-analysis, this guideline still indicates that PCI is an appropriate alternative to CABG in LMCA disease with low to intermediate anatomic complexity, and all evidence levels have been upgraded from Level of Evidence: B to Level of Evidence: A. PCI is recommended as Class I, IIa, or III based on the SYNTAX score tertile.
Table 3.
Recent Changes of PCI Recommendation Guidelines for LMCA Disease
| Guidelines | Class of Recommendation | Level of Evidence |
|---|---|---|
| Pre-EXCEL and NOBLE | ||
| 2014 ESC/EACTS55 | I: LMCA disease with a SYNTAX score of ≤22 IIa: LMCA disease with a SYNTAX score of 23–32 III: LMCA disease with a SYNTAX score of ≥33 |
B |
| 2014 ACC/AHA57 | IIa: For SIHD patients when both of the following are present:
|
B B B |
| Post-EXCEL and NOBLE | ||
| 2018 ESC/EACTS56 | I: LMCA disease with a SYNTAX score of ≤22 IIa: LMCA disease with a SYNTAX score of 23–32 III: LMCA disease with a SYNTAX score of ≥33 |
A |
| 2021 ACC/AHA58 | IIa: In selected patients with SIHD and significant LMCA disease for whom PCI can provide equivalent revascularization to that possible with CABG, PCI is reasonable to improve survival. | B |
As compared to the European guidelines, U.S. practical guidelines proposed a more strict recommendation of PCI use for LMCA revascularization; the 2014 U.S. guidelines have provided a Class IIa Level of Evidence: B indication to PCI for patients with low anatomic risk (eg, a low SYNTAX score and ostial or shaft LMCA disease), a Class IIb Level of Evidence: B indication for those with intermediate anatomic risk (eg, low to intermediate SYNTAX score and bifurcation LMCA disease), and a Class III Level of Evidence: B indication for those with unfavorable anatomy (eg, highest tertile of the SYNTAX score, ≥33), also taking into account both anatomic condition and surgical risks.57 After the publication of the EXCEL, NOBLE, and ISCHEMIA (International Study of Comparative Health Effectiveness With Medical and Invasive Approaches) trials, the most updated 2021 American College of Cardiology/American Heart Association/Society for Cardiovascular Angiography and Interventions guideline for coronary revascularization provides a Class IIa indication (Level of Evidence: B nonrandomized) to PCI for patients in whom equivalent revascularization can be achieved with both PCI and CABG without subclassification according to clinical and anatomic risk profiles.58
Heart team approach
Current practical guidelines recommend multidisciplinary decision making (the heart team approach) in patients with complex CAD, including LMCA disease.56, 57, 58 As supporting evidence for PCI as a reasonable alternative in selected LMCA disease accumulated, physicians’ threshold for performing PCI at the LMCA has become lower. The rapid rise in the volume of left main PCI and the relative shrinkage of CABG was observed in the contemporary real-world multinational registry.1 Although the rise in PCI use is a widespread phenomenon observed across the world,52 there is marked variability in PCI-to-CABG ratios observed between countries or across regions under the same health care system59,60; it may raise concern on the underuse or inappropriate use of revascularization strategies. Furthermore, the available treatment options for patients in developing Asian countries would differ from those in the Western countries because of the less well-developed health care infrastructure (ie, physicians, medical services, and devices), and, also, many Asian patients would opt for PCI over CABG because of cultural, religious, or socioeconomic factors, such as the lack of social welfare support.61,62
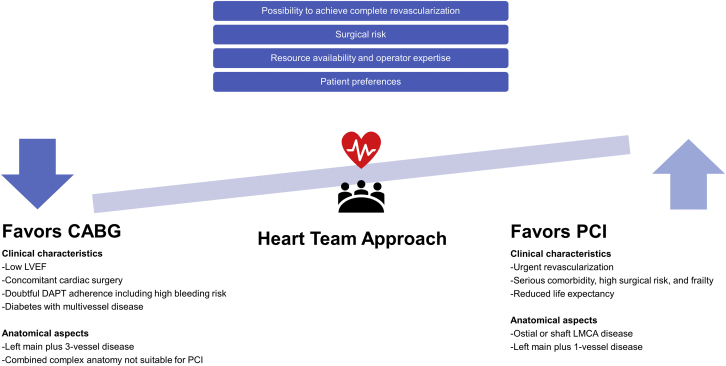
In this regard, the consistency and generality of the recommendations could be addressed by the heart team approach, which is becoming common practice in many Asian countries to encourage appropriate treatment. The long-term prognosis and decision making of optimal revascularization of patients with LMCA can be stratified by means of several key clinical, anatomic, and procedural factors (Figure 1). Over the last decade, there are several integrated and validated risk scores or models for anatomic and/or clinical variables that can be used to facilitate heart team discussion and informed discussion with patients (Table 4). The SYNTAX score is an anatomic scoring system reflecting the extent and complexity of CAD.63 Generally, patients with high anatomic complexity reflected by high SYNTAX scores are less likely to achieve complete revascularization with PCI; consequently, they have worse prognosis when treated with PCI. However, the prognosis of CABG is much less affected by anatomic complexity because CABG can presumably bypass all anatomic complex lesions.64 Rather, it is more related to clinical characteristics and comorbidities of patients, reflected in surgical risk scores (ie, EuroSCORE [European System for Cardiac Operative Risk Evaluation], STS [Society of Thoracic Surgeons] score, ACEF [Age, Creatinine, and Ejection Fraction] score). Like this, the complexity and extent of CAD should be adequately considered in the heart team discussion and patient counseling with the intention of achieving complete revascularization, because incomplete revascularization is associated with worse clinical outcomes regardless of the revascularization strategy.65 On the other hand, the SYNTAX II score was developed by combining anatomic and clinical factors for individualized guidance for decision making between PCI and CABG and, most recently, was refined to the SYNTAX Score II 2020 for predicting 10-year mortality and 5-year major adverse cardiac events.66 Additional factors that are not included in most risk models but also need to be considered include frailty, life expectancy, patient preference and patient-stated goals, expected quality-of-life improvement, doubtful DAPT adherence, operator skills, and local expertise. For patients who received the heart team decision of equipoise risk, the patient preference should be forefront. Depending on the combination of these factors mentioned, there are patients who are more likely to benefit from one approach instead of the other. In this clinical viewpoint, the heart team should deliver this knowledge effectively to the patient, discuss it with the patient, and reach an agreement on the optimal revascularization strategy.67
Figure 1.
Heart Team Approach to LMCA Revascularization
CABG = coronary artery bypass grafting; DAPT = dual antiplatelet therapy; LMCA = left main coronary artery; LVEF = left ventricular ejection fraction; PCI = percutaneous coronary intervention.
Table 4.
Contemporary Risk Scores for Decision Making or Risk Prediction for Left Main or Multivessel Coronary Artery Disease
| SYNTAX Score I | SYNTAX Score II | SYNTAX Score II 2020 | EuroSCORE | STS Score | ACEF Score | |
|---|---|---|---|---|---|---|
| Year of publication | 2005 | 2013 | 2020 | 1999: EuroSCORE I (additive) 2003: EuroSCORE I (logistic) 2012: EuroSCORE II |
2007 2018: entirely new risk model |
2009 |
| Clinical use | Angiographic tool for objectively grading the complexity of CAD and guiding decision making between CABG and PCI | To guide the optimum revascularization method in patients with complex CAD. | For predicting 10-y deaths and 5-y MACEs after CABG or PCI | The prediction of early mortality in cardiac surgical patients | Predicting the postoperative mortality in patients undergoing open heart surgery | Assessing operative mortality risk in elective cardiac operations |
| Development data set | Initially established itself as an anatomic-based tool to force the heart team to analyze the coronary angiogram and agree that equivalent revascularization (CABG and PCI) could be achieved | CABG and PCI cohorts of the SYNTAX trial (N = 1,800) | SYNTAXES (SYNTAX Extended Survival) study (N = 1,800) | EuroSCORE I: development data set (N = 13,302)/validation data set (N = 1,479) EuroSCORE II: development data set (N = 16,828)/validation data set (N = 5,553) |
Development data set: July 2011 to June 2014 STS Adult Cardiac Surgery Database data (isolated CABG [N = 439,092], isolated valve surgery [N = 150,150], combined procedure [N = 81,588]) Validation data set: July 2014 to December 2016 STS Adult Cardiac Surgery Database |
Development data set: 4,557 patients having surgery in the San Donato Hospital from 2001 to 2003 Validation data set: 4,091 patients having surgery in the same hospital from 2004 to 2007 |
| External validation set of initial publication | Not applicable | DELTA registry (N = 2,891) | 10-y death: only internally validated 5-y all-cause death and 5-y MACEs: externally validated by use of data from the FREEDOM, BEST, and PRECOMBAT trial cohort (N = 3,380) | Not applicable | Not applicable | Not applicable |
| Study population | Patients with complex CAD (3-vessel or LMCA disease) undergoing PCI or CABG | Same as SYNTAX score I | Same as SYNTAX score I | Cardiac surgical patients in Europe | Patients undergoing cardiac surgery | Patients undergoing an elective cardiac operation |
| Outcomes of interest | MACCEs (death, stroke, MI, and repeat revascularization) | 4-y mortality | All-cause death at 10 years 5-y all-cause death 5-y MACEs |
Postoperative mortality (death within 30 d of operation or within the same hospital admission) | Mortality and postoperative complications (a composite of morbidity and 30-d mortality, length of stay, neurologic injury, deep sternal wound infection, prolonged ventilation, renal failure, and reoperation) | Operative mortality (in-hospital mortality or mortality by 30 d after the operation for patients discharged from the hospital) |
| Calculator | http://www.syntaxscore.org/calculator/syntaxscore/frameset.htm | http://www.syntaxscore.org/calculator/syntaxscore/framesetss2.htm | https://syntaxscore2020.com/ | EuroSCORE I: http://www.euroscore.org/calcold.html EuroSCORE II: http://www.euroscore.org/calc.html (EuroSCORE II) |
https://riskcalc.sts.org/stswebriskcalc/calculate (periodically recalibrated) | Not applicable |
ACEF = Age, Creatinine, and Ejection Fraction; BEST = Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus Eluting Stent Implantation in the Treatment of Patients With Multivessel Coronary Artery Disease; CAD = coronary artery disease; DELTA = Drug- Eluting Stent for Left Main Coronary Artery Disease; EuroSCORE = European System for Cardiac Operative Risk Evaluation; FREEDOM = Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease; MACE = major adverse cardiovascular event; other abbreviations as in Table 1, Table 2, and Table 3.
Special Populations
Patients with diabetes
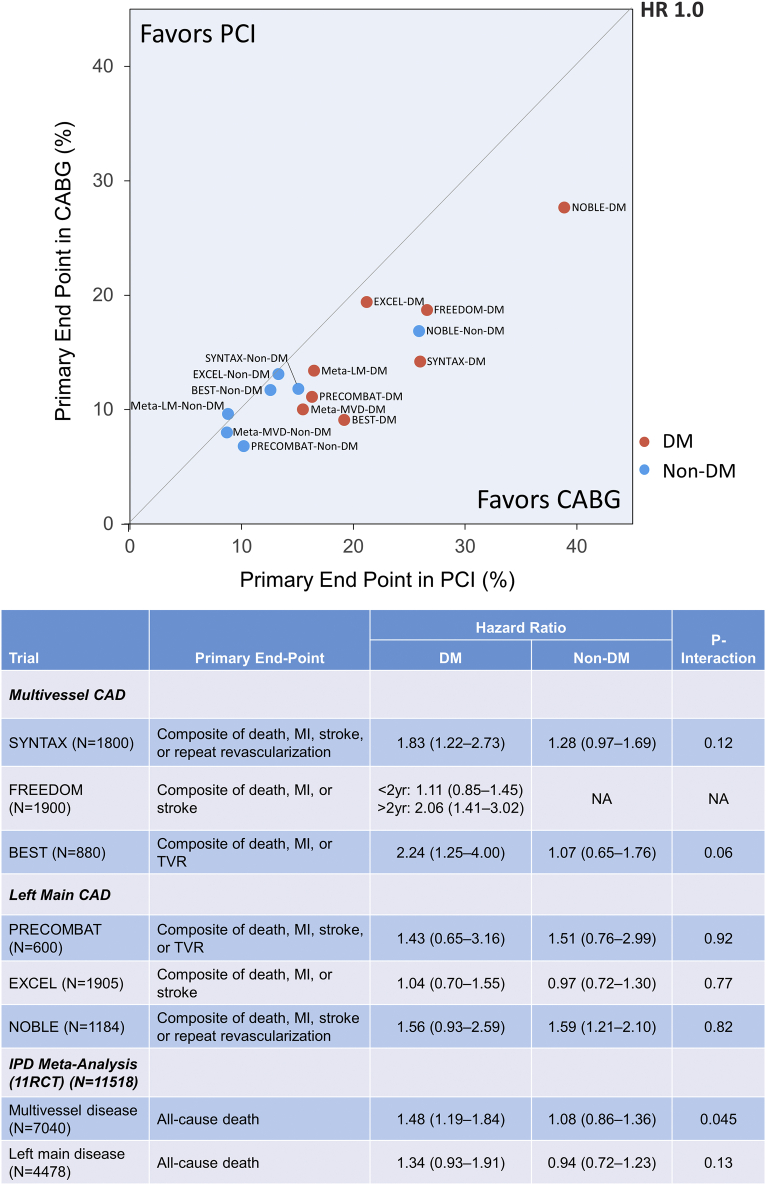
Patients with diabetes mellitus are more likely to have complex CAD, such as multivessel or LMCA disease, with a greater atherosclerotic burden.68 Diabetes is a well-known major determinant of the long-term outcome after both CABG and PCI.69 Although no specific recommendation was given regarding the optimal revascularization strategy in patients with diabetes and LMCA disease, current guidelines recommend CABG as the preferred revascularization strategy in diabetic patients with 3-vessel disease.56 However, the role of diabetes guiding the optimal revascularization strategy in patients with LMCA disease has recently been questioned. In fact, no dedicated RCT has compared the outcomes of PCI vs CABG for LMCA disease specifically in patients with diabetes. The relative treatment effect of PCI and CABG for multivessel and LMCA disease according to diabetic status are well summarized in Figure 2.70 In a pooled analysis of individual patient data from 11 RCTs, the presence of diabetes modified the treatment effect of PCI vs CABG on the 5-year mortality in patients with multivessel disease (without LMCA disease) (P for interaction = 0.045) but not in those with LMCA disease (P for interaction = 0.13).45 The subgroup analysis of EXCEL also showed similar results with comparable outcomes irrespective of diabetic status.71 In the 10-year report from the MAIN-COMPARE (Revascularization for Unprotected Left Main Coronary Artery Stenosis: Comparison of Percutaneous Coronary Angioplasty vs Surgical Revascularization) registry, the long-term risks of mortality and serious composite outcome were comparable after PCI and CABG in diabetic patients, demonstrating the diminishing impact of diabetes favoring CABG over PCI from the BMS to DES eras.72 These findings suggest the limited role of diabetes as a key factor for the decision making regarding LMCA revascularization strategy. Therefore, in contemporary practice, PCI can be a reasonable treatment option in diabetic patients with LMCA disease and relatively noncomplex CAD, whereas CABG should be considered for diabetic patients with more complex CAD.
Figure 2.
Treatment Effects of PCI and CABG According to Diabetic Status
BEST = Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus Eluting Stent Implantation in the Treatment of Patients With Multivessel Coronary Artery Disease; CABG = coronary artery bypass grafting; CAD = coronary artery disease; DM = diabetes mellitus; EXCEL = Evaluation of Xience Everolimus Eluting Stent vs Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization; FREEDOM = Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease; IPD = individual patient-level data; LM = left main; MVD = multivessel disease; NOBLE = Nordic-Baltic-British Left Main Revascularization; PCI = percutaneous coronary intervention; PRECOMBAT = Premier of Randomized Comparison of Bypass Surgery versus Angioplasty Using Sirolimus-Eluting Stent in Patients with Left Main Coronary Artery Disease; RCT = randomized clinical trial; SYNTAX = Synergy Between Percutaneous Coronary Intervention With TAXUS and Cardiac Surgery. Reproduced with permission from Park et al70 with permission. Copyright © 2019, Elsevier.
Ostial/shaft vs distal bifurcation lesions
The lesion location of LMCA disease is considered an important factor that affects the decision making on the revascularization strategy, and it is also reflected in current practice guidelines.56,57 For isolated ostial/shaft LMCA disease, PCI revealed comparable long-term outcomes compared to CABG.73,74 However, PCI for distal LMCA bifurcation lesions usually demands more complex procedural considerations and techniques and shows less favorable outcomes compared to PCI for ostial/shaft lesions.75,76 In the 10-year results of the MAIN-COMPARE registry, PCI with DES showed higher mortality and serious composite outcome rates than did CABG beyond 5 years among patients with LMCA bifurcation lesion, especially when treated with a 2-stent technique. In EXCEL, including 84.2% of patients with distal LMCA bifurcation disease, a trend toward late crossover in terms of hard endpoints over 3 years was noted in patients with LMCA bifurcation disease but not in patients with isolated ostial/shaft disease.77 Meanwhile, the higher risk of repeat revascularization in distal LMCA bifurcation lesions favoring CABG over PCI was consistent across trials. In a recent meta-analysis involving RCTs and adjusted observational studies and analyzing the different outcomes of PCI vs CABG according to lesion site, similar findings were also observed: CABG was associated with a lower risk of major adverse cardiac events compared to PCI in patients with distal LMCA disease, whereas both revascularization strategies showed comparable outcomes for ostial/shaft LMCA disease.78 Therefore, decision making on revascularization for LMCA bifurcation should be based on careful evaluation of the anatomic complexity and potential to achieve complete revascularization. Appropriate lesion selection followed by stent optimization with intravascular imaging would improve PCI outcomes, particularly when a complex 2-stent strategy is needed for the treatment of distal LMCA disease.
Patients with heart failure with reduced ejection fraction
The major long-term manifestations of significant LMCA disease could be associated with decompensated heart failure because of the large area of myocardium at risk.9,79 Patients with reduced left ventricular ejection fraction (LVEF) secondary to ischemic CAD have a high risk of mortality, reaching 60% over a 10-year follow-up with medical therapy alone. Until recently, there have been no dedicated RCTs to guide the optimal revascularization strategy in high-risk patients with LMCA disease and reduced heart function. In the SYNTAX trial, LVEF was an independent predictor of 4-year mortality and showed a moderate interaction effect on long-term mortality prediction with CABG and PCI.80 A meta-analysis including 16,191 patients with CAD and an LVEF of ≤40% (21 studies, mostly observational registries) reported lower mortality with CABG compared with PCI (HR: 0.82; 95% CI: 0.75-0.90; P < 0.001).81 In a recent report from the IRIS-MAIN (Interventional Research Incorporation Society-Left MAIN Revascularization) registry, CABG was associated with a lower risk of the composite outcome of death, MI, or stroke compared with PCI for patients with LMCA disease and moderately or severely reduced LVEF.82 It is worth noting that the differences in event rates between PCI and CABG in low-LVEF patients were diminished if complete revascularization was achieved with PCI. In summary, CABG would offer superior long-term outcomes for those with moderately or severely reduced LVEF if the surgical risk is acceptable, especially if complete revascularization cannot be achieved with PCI. Therefore, the severity of LV dysfunction should be considered in decision making on the optimal revascularization strategy in such high-risk patients, along with the potential to ensure complete revascularization.
Elderly patients
Although the average life expectancy is substantially increasing worldwide, the optimal revascularization strategy for elderly patients with complex CAD including multivessel and/or LMCA disease remains unclear. Elderly patients usually tend to have more complex and severe CAD and are frequently frailer than younger patients. Thus, treating physicians might be more reluctant to recommend an invasive surgical option for the treatment of complex CAD.83 In this context, currently available data or practical guidelines do not provide sufficient evidence-based recommendations for the management of elderly patients with complex CAD, including LMCA disease. In a recent subgroup analysis of the SYNTAX Extended study, elderly patients (>70 years old) with 3-vessel and/or LMCA disease had comparable 10-year all-cause mortality, life expectancy, 5-year major adverse cardiac or cerebrovascular events (MACCEs), and 5-year quality-of-life status after revascularization irrespective of CABG or PCI.84 In contrast, among nonelderly patients (<70 years of age), the 5-year risk of MACCEs was significantly higher with PCI than with CABG, which suggests that the beneficial effects of CABG over PCI on clinical outcomes observed in younger patients would not apply to elderly persons. In subgroup analyses of the IRIS-MAIN registry, significant interactions were absent for age or sex and revascularization methods of PCI and CABG for all-cause mortality, repeat revascularization, and MACCEs.85 From the clinical point of view, it might be less attractive in these elderly individuals to opt for a more invasive and complex surgical approach. Given the equivalent long-term survival risk and quality-of-life status between PCI and CABG, a less invasive strategy using PCI instead of CABG may be preferred for elderly patients, which should be finally discussed with both the patient and the heart team during the decision-making process.
Contemporary Intracoronary Imaging and Physiology for LMCA Disease
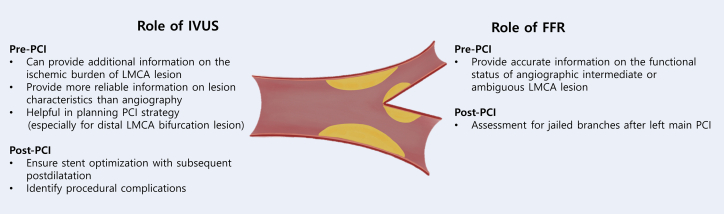
Evaluation of the LMCA lesion by coronary angiography is often hampered by the lack of a reference segment, lesion angulation, eccentricity, bifurcation anatomy, overlapping branches, and foreshortening.86 Invasively, a more detailed evaluation of the anatomic severity and hemodynamic significance of intermediate LMCA lesions can be obtained by intracoronary imaging with intravascular ultrasound (IVUS) imaging or physiologically with pressure wire assessment of the fractional flow reserve (FFR). In the contemporary clinical setting, information from intracoronary imaging functional assessment could improve the decision-making process of LMCA disease with the help of a heart team discussion. Also, these invasive imaging and physiology tools are increasingly being used to guide the appropriate PCI strategy and PCI optimization in daily left main PCI practice (Figure 3).
Figure 3.
IVUS and FFR Roles in Left Main PCI
FFR = fractional flow reserve; IVUS = intravascular ultrasound; LMCA = left main coronary artery; PCI = percutaneous coronary intervention;
IVUS guidance
In addition to coronary angiography, the use of IVUS is helpful in the determination of the vessel size, lumen area, and plaque extent and distribution within the LMCA and its main branches of the left anterior descending (LAD) artery and left circumflex (LCX) artery.87 The IVUS-derived minimal lumen area (MLA) can provide anatomic information on the ischemic burden of the LMCA lesion. A prior older U.S. study proposed an MLA cutoff value of 5.9 mm2 with a sensitivity and specificity of 93% and 94%, respectively, for an FFR of <0.75.88 Since then, the evaluation of IVUS in a multicenter prospective study of intermediate LMCA disease demonstrated that an MLA of ≥6 mm2 on IVUS is a safe value for deferring revascularization for intermediate LMCA disease.89 However, subsequent studies including Korean patients suggested that a smaller MLA of ≤4.5 mm2 would be associated with a functionally significant LMCA stenosis; thus, this value could be a potential criterion for deferral or revascularization treatment.90 One of the plausible explanations for different MLA cutoffs for the functional significance of LMCA lesions might be ethnic differences in the reference size of the coronary arteries; however, it remains unclear whether ethnicity per se predicts smaller coronary dimensions regardless of sex, dominance of the coronary system, or body size.91, 92, 93, 94 Currently, although there are several debates on optimal cutoff points, an MLA of ≥6 mm2 is generally accepted as the safe and optimal cutoff value for deferring treatment for LMCA disease. IVUS can also provide additional information on distal LMCA and bifurcation lesions by characterizing longitudinal plaque distribution. Ideally, pull backs from both the LAD and LCX should be used to obtain complete information on LMCA bifurcation and avoid overestimation of the MLA within LCX ostium by the noncoaxial orientation of the catheter from an LAD pull back.95
The other important value of IVUS is to ensure stent optimization of LMCA PCI. Intracoronary imaging can identify stent underexpansion or inapposition, edge dissection, or significant residual disease, which could not be detected on coronary angiography. Given that stent underexpansion after complex stenting is corrected by IVUS optimization, the use of IVUS guidance is associated with improved clinical outcomes after the procedure, particularly in patients with distal LMCA bifurcation lesions treated with 2 stents.96,97 The proposed best IVUS-derived minimal stent area criteria that predicted angiographic restenosis were 5.0 mm2 for the LCX ostium, 6.3 mm2 for the LAD ostium, 7.2 mm2 for the polygon of confluence, and 8.2 mm2 for the distal LMCA (the so-called 5-6-7-8 rule of criteria).98 Finally, IVUS is a valuable adjunctive tool for preinterventional lesion assessment and postinterventional stent optimization for significant LMCA disease. Given that the mechanistic benefit of IVUS for assessing plaque distribution, informing stenting strategy, and enhancing stent optimization is expected, the clinical impact of IVUS in reducing restenosis and stent thrombosis–related complications (especially for complex LMCA stenting) may be clinically relevant.6 The true prognostic effect of IVUS-guided PCI for LMCA disease should be confirmed through ongoing RCTs (ie, OPTIMAL [Optimization of Left Main Percutaneous Coronary Intervention With Intravascular Ultrasound; NCT04111770], INFINITE [Intravascular Ultrasound-Versus Angiography-Guided Percutaneous Coronary Intervention for Patients With Left Main Bifurcation Lesion; NCT04072003], and DKCRUSH VIII [IVUS-guided DK Crush Stenting Technique for Patients With Complex Bifurcation Lesions; NCT03770650]).
FFR assessment
In patients without angiographically significant LMCA stenosis or clinically relevant symptoms and/or ischemic evidence, the hemodynamic and functional significance of angiographically challenging LMCA lesions should be further evaluated. Given that angiography alone has inherent limitations in accurately evaluating functionally significant LMCA disease, there are significant interobserver variations and poor correlation between the severity of angiographic stenosis and its functional significance.99,100 With physiologic assessment by FFR, the visual-functional mismatch was identified in approximately 30% to 40% of intermediate LMCA disease,99,101 and such intermediate LMCA lesions with FFR values of >0.75 or 0.80 could be safely deferred.99,102 More recently, the instantaneous wave-free ratio (iFR) was introduced as a resting index of functional coronary stenosis. Although deferring the revascularization of non-LMCA obstructive CAD with an iFR of >0.89 was supported by compelling clinical data,103,104 the clinical application of iFR to LMCA disease may be still limited without solid outcome studies. In addition, the interpretation of FFR in LMCA lesions should be done carefully in cases with the presence of downstream stenoses in the LAD and/or LCX, which may underestimate the hemodynamic significance of LMCA disease.105
Furthermore, evidence for best practices with respect to technical details of LMCA PCI includes provisional stenting and an FFR-guided approach to jailed side branches. A recent observational study in Korea evaluated the long-term clinical impact of FFR in jailed LCX branch after simple crossover stenting in LMCA lesions.106 The patients with a high FFR in the jailed LCX had better 5-year target lesion failure (a composite of cardiac death, target vessel MI, or target lesion revascularization) outcomes than those with a low FFR. However, there was no significant difference in the clinical outcomes according to the angiographic percent diameter stenosis. This study suggests that FFR measurement in jailed LCX after left main stenting crossover can be helpful in selecting an adequate treatment strategy and may reduce unnecessary complex procedures. Also, among patients with LMCA disease and concomitant multivessel disease, the use of physiology-guided revascularization may significantly reduce the rate of MACCEs associated with non-LMCA multivessel PCI. In the FAME (Fractional Flow Reserve versus Angiography for Multivessel Evaluation) trial, physiology-guided PCI for multivessel CAD resulted in a significant reduction of a composite of death or MI compared to angiography-guided PCI.107
Contemporary PCI Techniques for LMCA Disease
PCI strategy and techniques
If the decision for a revascularization method is made to proceed with PCI after heart team discussion, several technical aspects of the PCI strategy should be considered to optimize PCI outcomes. As far as possible, left main PCI should be performed by experienced operators at catheterization laboratory facilities equipped with intracoronary imaging, invasive coronary physiology, and mechanical circulatory support.108 PCI of isolated ostium or shaft LMCA lesions is relatively straightforward; however, LMCA disease frequently involves distal bifurcation, which requires additional special considerations; LMCA bifurcation lesions should be approached systematically by evaluating the significance of the side branch lesion and its risk of being compromised based on known factors, and distal bifurcation PCI procedures should also be performed systematically. Operators also have to be fully familiar with their procedural steps and the associated challenges, with technical expertise.
Based on prior trials, an up-front 2-stent strategy for bifurcation lesion has been usually considered to be inferior to provisional stenting, mainly because of the higher rates of periprocedural MI, repeat revascularization, and stent thrombosis with multiple stents. However, the DEFINITION (Definitions and impact of complex bifurcation lesions on clinical outcomes after PCI using drug-eluting stents) II trial has potentially overcome such existing concerns and showed that the up-front 2-stent strategy could achieve better clinical outcomes compared with a provisional stenting strategy when complex bifurcation lesions are carefully selected.109 Until recently, there were 2 RCTs comparing the provisional stenting strategy and complex 2-stent technique for true distal LMCA bifurcation lesions, and the key findings are summarized in Table 5. The DKCRUSH-V trial demonstrated that PCI for true distal LMCA bifurcation lesions using a planned double-kissing (DK) crush 2-stent strategy resulted in a lower rate of target lesion failure (a composite of cardiac death, target vessel MI, or clinically driven target lesion revascularization) at 1 year than the provisional stenting strategy.110 In contrast, the EBC-MAIN (European Bifurcation Club Left Main Study) demonstrated that the stepwise layered provisional approach was associated with numerically (but not significantly) fewer major adverse cardiac events (MACEs) (a composite of death, MI, and target lesion revascularization) than planned dual stenting.111
Table 5.
Recent RCTs of Stenting Strategy for Distal Left Main Bifurcations
| DKCRUSH V Trial | EBC-MAIN Trial | |
|---|---|---|
| Year of publication | 2017 | 2021 |
| Design | Provisional strategy vs DK crush | Provisional strategy vs up-front 2-stent strategy |
| Number of patients | 482 | 467 |
| Mean age, y | 64.5 | 71.1 |
| Male sex, % | 80.2 | 76.9 |
| Diabetes, % | 27.2 | 27.4 |
| Sites | 26 sites (23 sites in Asia, 2 sites in United States, 1 site in Italy) | 31 sites in 11 European countries |
| Operator experience | ≥300 PCI/y and ≥20 left main PCI | ≥150 PCI/y |
| Lesion type | True unprotected left main bifurcation | True unprotected left main bifurcation |
| Anatomic complexity | ||
| Mean SYNTAX score | 30.6 | 22.9 |
| Distal bifurcation angle | 78° | 81.3° |
| Length of side branch lesion, mm | 16.4 | 6.9 |
| Complex bifurcation, % | 31.5 | Not classified |
| Use of IVUS guidance | Not mandated, 41.7% | Not mandated, 32.5% |
| Up-front 2-stent strategy | DK crush | Culotte (53%), T/TAP (33%), DK crush (5%) |
| Conversion rate to 2-stent in provisional strategy, % | 47 | 22 |
| Stents used in the study | Xience V (Abbott Vascular Inc), Endeavor Resolute (Medtronic Inc), Firebird 2 (MicroPort Medical) | Resolute Onyx (Medtronic Inc) |
| Primary endpoint | Target lesion failure, defined as a composite of cardiac death, target vessel MI, or target lesion revascularization | Death, MI, or target lesion revascularization |
| Key findings (provisional vs 2 stent) | 1 y: 10.7% vs 5.0%; P = 0.02 3 y: 16.9% vs 8.3%; P = 0.006 |
1 y: 14.7% vs 17.7%; P = 0.34 |
The initial single-stent crossover and provisional side branch approach is currently recommended for most cases of LMCA bifurcation lesions,112 which is technically less demanding compared to complex 2-stent techniques. Crossover stenting followed by the proximal optimization technique is the recommended way for the provisional single-stent strategy in LMCA bifurcation.113 Provisional stenting may cause angiographic side branch jailing by the mechanism of carina or plaque shift. The threshold for decision making on further complex procedures with side branch stenting may be lower in LMCA bifurcation because the discrepancy between the angiographic appearance and functional significance of the jailed side branch after provisional stenting can still exist.106,114 In this situation, the FFR measurement in the jailed side branch can be helpful in evaluating hemodynamic compromise in the LCX branch, which may reduce unnecessary additional complex procedures.106
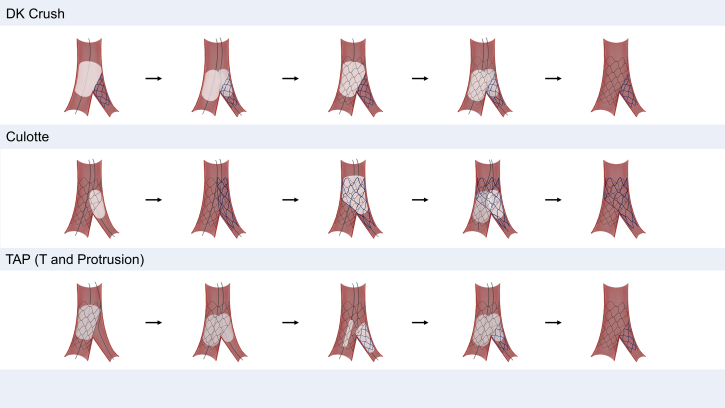
When a 2-stent strategy is necessary for the treatment of distal LMCA bifurcation lesions, which 2-stent technique should be preferred has been highly debated. Key steps of the 3 most commonly used contemporary 2-stent techniques (DK crush technique, culotte technique, and T-and-protrusion technique) are concisely illustrated in Figure 4. Like this, in contemporary PCI practice, an up-front 2-stent strategy is frequently required to secure the big side branch (ie, the LCX) in patients with complex true distal LMCA lesions. The 2-stent technique can be selected according to the bifurcation anatomy and the operator’s experience. Until recently, 2 dedicated RCTs comparing the DK crush technique with the culotte and provisional stenting for true LMCA bifurcation lesions demonstrated the superiority of the planned DK crush technique with respect to the primary composite ischemic endpoint over 3 years of long-term follow-up.115,116 In a recent network meta-analysis, including 5,711 patients treated using 5 different bifurcation PCI techniques (provisional, crush, culotte, T-stenting/T- stenting and protrusion, and DK crush), DK crush was associated with fewer MACEs compared with provisional stenting driven by lower target lesion revascularization, whereas no benefit of other 2-stent techniques over provisional stenting was observed.117 Although DK crush has emerged as a preferred strategy for true distal LMCA bifurcation lesions, it should be recognized that it is technically challenging and should be performed by experienced operators. Considering the difference in the preferred strategy of bifurcation PCI among Asian countries, performing PCI with the operator’s familiar technique with meticulous lesion selection may lead to more optimal procedures and better clinical outcomes. Meanwhile, when 2 stents are eventually required, this should be finalized with a kissing balloon inflation and repeat proximal optimization technique to optimize stent expansion and preserve bifurcation anatomy.112,118
Figure 4.
Contemporary Complex Bifurcation Stenting Techniques for Left Main Bifurcation Lesions
Each figure depicts the schematic representation of the key steps of complex 2-stent techniques. DK = double-kissing.
Clinical Unmet Need and Future Perspectives
Although several clinical studies have been conducted to determine the optimal revascularization strategy for LMCA disease, there are still unmet and debatable issues to be further resolved in the near future (Table 6). In addition, improving the terminology to describe “invasive treatment by PCI or CABG” is required, which has the potential to improve clinical decision making and guide future trial designs.119
Table 6.
Unmet Issues on Left Main Revascularization
| Topics | Controversial Points (Why This Issue Is Nonuniform Or Undefined? How Can We Resolve This Issue?) |
|---|---|
| MI definition | Because there is still no uniform definition of MI that does not penalize one of the revascularization approaches, different protocol definitions of MI were used in trials comparing PCI and CABG for LMCA disease. The interstudy heterogeneity for MI definitions can result in wide variability across trials and imprecision in estimating the overall treatment effect. Additional studies and efforts by trialists are warranted to improve standardization of the MI definition for future clinical trials comparing PCI and CABG. |
| Complete (CR) or incomplete revascularization (IR) | Reducing the burden of ischemia would improve clinical outcomes, and current evidence supports complete revascularization. Previous studies investigating the clinical impact of CR and IR have lacked standardized definitions of IR. Also, because of inherent selection bias on the results of previous studies, IR was more frequently associated with sicker patients and more anatomically complex CAD. There is a discrepancy in the long-term clinical outcomes of IR between PCI and CABG. In brief, clinical outcomes following IR seem more favorable after CABG than after PCI. Efforts are needed to standardize the definitions of CR and IR in future studies. Further study is required to validate the optimal degree of revascularization and a reasonable level of IR for acceptable long-term outcomes according to the revascularization strategy. Also, it is needed to identify some subsets of patients with LMCA disease who would benefit more from CR. |
| Role of IVUS or FFR | With regard to the clinical impact of IVUS guidance for left main PCI, there has been no large, multicenter, randomized clinical trial. Based on previous observation, IVUS was more frequently used in a substantially younger and less comorbid population, which might have influenced clinical outcomes. These studies rarely included a prespecified protocol for IVUS guidance and stent optimization. Although the potential role of IVUS in reducing LMCA restenosis and stent thrombosis–related complications may be clinically meaningful, a true clinical effect of IVUS guidance for LMCA PCI can be confirmed only through RCTs. Because it is highly unlikely that the efficacy of IVUS guidance in LMCA PCI is tested in RCTs, trials comparing IVUS-guided LMCA PCI with a prespecified optimization protocol vs CABG might provide further insight. CR based on the functional definition is the preferred strategy for PCI. However, the role of functional guidance for CABG is less clear. The clinical use of resting distal coronary pressure-to-aortic pressure ratio and iFR in guiding revascularization of LMCA disease is yet to be fully validated in RCTs. Further RCTs are needed to conclude these issues. |
| All-cause mortality or cardiac mortality | Controversy exists regarding whether all-cause mortality or cardiac mortality is preferred as a study endpoint in RCTs comparing PCI to CABG. There has been a debate over conflicting all-cause and cardiac mortality findings shown in the 5-y results of the EXCEL trial. The use of cardiac-specific mortality may exclude deaths related to the procedure, either through noncardiac mechanisms or because of misclassification. On the other hand, all-cause mortality is the most unbiased endpoint; however, it may lead to oversimplification by including death that is less attributable to the procedure. Efforts should also be made to find a better consensus and definition of cardiac mortality while discussing which mortality endpoint should be preferred. |
| Long-term follow-up data beyond 5 or 10 y | Until recently, long-term follow-up studies comparing contemporary PCI and CABG beyond 5 y were still limited. Limited follow-up could have penalized the CABG group because the long-term benefits of CABG over PCI have not typically been fully evident until 5 to 10 y after the procedure. Also, a substantial interaction between treatment effect and time for the risk of major adverse events was noted in EXCEL and NOBLE. Study participants in EXCEL and NOBLE will be followed up beyond 5 y, which will provide additional valuable information. |
| Optimal antithrombotic strategy and DAPT duration | The optimal strategy for DAPT following complex PCI, such as LMCA bifurcation PCI using the 2-stent technique, still remains unclear. Furthermore, it was suggested that the East Asian population tends to have a higher risk of bleeding events but a relatively lower risk of thrombotic events, namely, the East Asian paradox. A guideline and unique regimen specifically for Asian patients or the unique ischemic/bleeding risk score of Asian patients might be useful in tailoring DAPT for this population. |
| Role of SYNTAX score | The current guideline recommendation for LMCA revascularization is mainly based on the anatomic SYNTAX score. The SYNTAX score failed to clearly differentiate the comparative outcomes between CABG and PCI in EXCEL and NOBLE. The current role of the SYNTAX score as the key factor in decision making for optimal LMCA revascularization needs to be further debated in contemporary clinical practice settings. Also, the SYNTAX score should be interpreted with caution in the context of heart team discussion. |
Based on the totality of the clinical evidence available to date, CABG and PCI are equivalent in the treatment of patients with LMCA disease with low to intermediate anatomic complexity; however, the long-term equipoise between CABG and PCI in certain patient subsets is still conflicting. Also, a substantial interaction between time and the relative treatment effect of CABG and PCI with contemporary DES noted in recent RCTs—late catch-up in EXCEL or late divergence in NOBLE—should be further determined by longer follow-up of these landmark trials. Given that extended follow-up data of landmark RCTs will be available, further individual patient data meta-analyses would provide more comprehensive evidence to inform clinical decision making in patients with significant LMCA disease.
In addition, the current guideline recommendation for LMCA revascularization is mainly based on the anatomic SYNTAX score, which was based on a prespecified subgroup of patients with LMCA disease in the SYNTAX trial. Although the SYNTAX score remains a reasonable tool to evaluate the extent and complexity of CAD and to facilitate a shared decision-making process, the SYNTAX score failed to clearly differentiate the comparative outcomes between CABG and PCI in EXCEL, NOBLE, and an individual patient data meta-analysis, in contrast to the original result of the SYNTAX trial.40,42,45 As a nonphysiologic anatomic score, the SYNTAX score does not take into account clinical factors and physiologic significance. Therefore, the SYNTAX score should be interpreted with caution in the context of a heart team discussion. The current role of the SYNTAX score as the key factor in decision making for optimal LMCA revascularization needs to be further debated in the contemporary clinical practice setting.
For complex distal LMCA bifurcation PCI, the variability in strategic choice may result in heterogeneity in outcomes across different operators, institutions, and even countries. More convincing evidence is needed to appropriately define complex distal bifurcation lesions requiring the initial up-front 2-stent technique and the optimal side branch treatment. The optimal strategy for DAPT following complex PCI such as LMCA bifurcation PCI using the 2-stent technique also remains unclear. Furthermore, it was suggested that the East Asian population tends to have a higher risk of bleeding events but a relatively lower risk of thrombotic events, despite higher on-treatment platelet reactivity, namely, the East Asian paradox.”120 A guideline and unique regimen specifically for Asian patients, or a unique ischemic/bleeding risk score for Asian patients, might be useful in tailoring DAPT for this population.
Conclusions
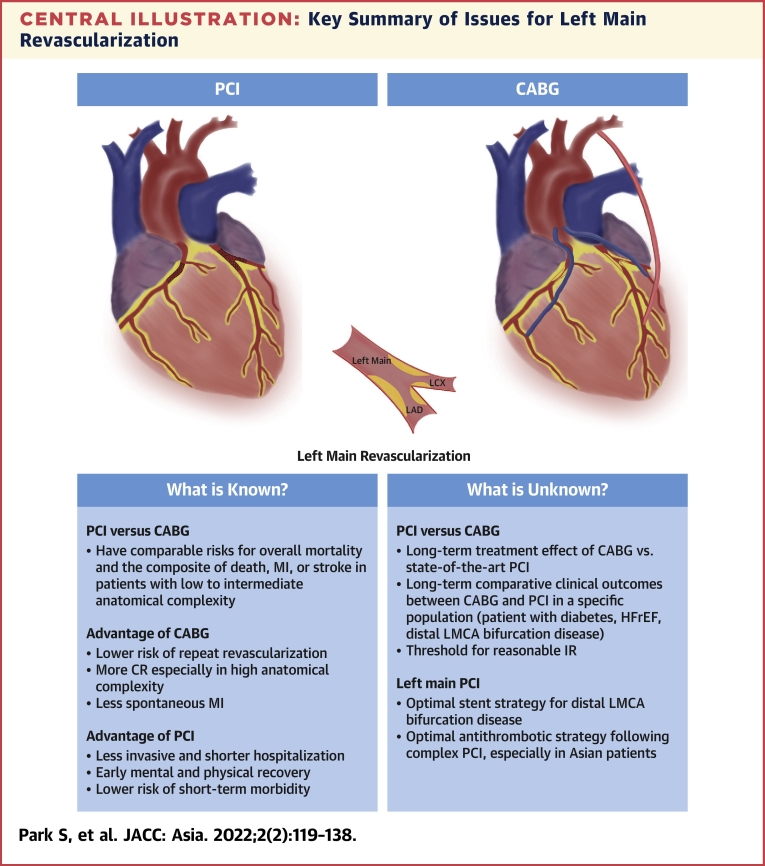
Over several decades, remarkable advancements in stent technology, PCI techniques, intracoronary imaging, physiologic guidance, and adjunctive pharmacotherapy have progressively improved clinical outcomes following PCI in patients with LMCA disease. In contemporary clinical practice, PCI for LMCA disease is widely considered in patients with a wide variety of clinical and anatomic complexities. However, a gap still exists between clinical practice and the current status of supporting evidence (Central Illustration). Given that large-sized RCTs for patients with LMCA disease are unlikely to be conducted in the near future, we hope that longer-term follow-up results of the prior landmark trials EXCEL and NOBLE will be available. In reality, there is no one-size-fits-all approach for LMCA revascularization. The heart team approach can play an important role in tailored decision making for individual patients, incorporating clinical factors, anatomic factors, and individual preferences. Also, further efforts should be made to optimize procedural outcomes and improve adjunctive medical management in both revascularization strategies.
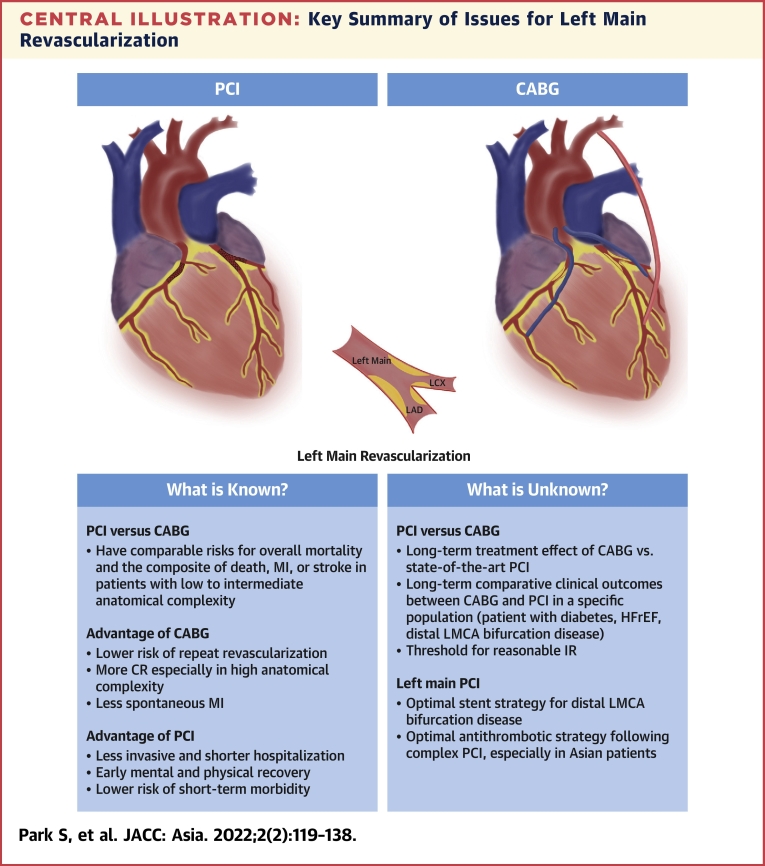
Central Illustration.
Key Summary of Issues for Left Main Revascularization
Key points are summarized for what is known and what is unknown for left main revascularization. CABG = coronary artery bypass grafting; CR = complete revascularization; IR = incomplete revascularization; PCI = percutaneous coronary intervention; LAD = left anterior descending artery; LCX = left circumflex artery; LMCA = left main coronary artery; MI = myocardial infarction.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Chang-Wook Nam, MD, served as the Guest Associate Editor for this paper. William F. Fearon, MD, served as the Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Lee P.H., Ahn J.M., Chang M., et al. Left main coronary artery disease: secular trends in patient characteristics, treatments, and outcomes. J Am Coll Cardiol. 2016;68:1233–1246. doi: 10.1016/j.jacc.2016.05.089. [DOI] [PubMed] [Google Scholar]
- 2.D’Ascenzo F., Presutti D.G., Picardi E., et al. Prevalence and non-invasive predictors of left main or three-vessel coronary disease: evidence from a collaborative international meta-analysis including 22 740 patients. Heart. 2012;98:914–919. doi: 10.1136/heartjnl-2011-301596. [DOI] [PubMed] [Google Scholar]
- 3.Levine G.N., Bates E.R., Blankenship J.C., et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58:2550–2583. doi: 10.1016/j.jacc.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Tan W.A., Tamai H., Park S.J., et al. Long-term clinical outcomes after unprotected left main trunk percutaneous revascularization in 279 patients. Circulation. 2001;104:1609–1614. doi: 10.1161/hc3901.096669. [DOI] [PubMed] [Google Scholar]
- 5.Park D.W., Park S.J. Percutaneous coronary intervention of left main disease: pre- and post-EXCEL (Evaluation of XIENCE Everolimus Eluting Stent Versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization) and NOBLE (Nordic-Baltic-British Left Main Revascularization Study) era. Circ Cardiovasc Interv. 2017;10(6) doi: 10.1161/CIRCINTERVENTIONS.117.004792. [DOI] [PubMed] [Google Scholar]
- 6.Park D.W., Park S.J. Intravascular ultrasound-guided percutaneous coronary intervention for left main disease: does procedural fine-tuning make a relevant clinical benefit? Circ Cardiovasc Interv. 2017;10(5) doi: 10.1161/CIRCINTERVENTIONS.117.005293. [DOI] [PubMed] [Google Scholar]
- 7.Park D.W., Park S.J. Contemporary state-of-the-art PCI with functional and imaging concepts: forethoughts on the FAME 3 trial. EuroIntervention. 2019;15:e219–e221. doi: 10.4244/EIJV15I3A40. [DOI] [PubMed] [Google Scholar]
- 8.Bruschke A.V., Proudfit W.L., Sones F.M., Jr. Progress study of 590 consecutive nonsurgical cases of coronary disease followed 5-9 years. II. Ventriculographic and other correlations. Circulation. 1973;47:1154–1163. doi: 10.1161/01.cir.47.6.1154. [DOI] [PubMed] [Google Scholar]
- 9.Conley M.J., Ely R.L., Kisslo J., Lee K.L., McNeer J.F., Rosati R.A. The prognostic spectrum of left main stenosis. Circulation. 1978;57:947–952. doi: 10.1161/01.cir.57.5.947. [DOI] [PubMed] [Google Scholar]
- 10.Takaro T., Hultgren H.N., Lipton M.J., Detre K.M. The VA cooperative randomized study of surgery for coronary arterial occlusive disease II. Subgroup with significant left main lesions. Circulation. 1976;54:III107–III117. [PubMed] [Google Scholar]
- 11.European Coronary Surgery Study Group Long-term results of prospective randomised study of coronary artery bypass surgery in stable angina pectoris. Lancet. 1982;2:1173–1180. [PubMed] [Google Scholar]
- 12.The Veterans Administration Coronary Artery Bypass Surgery Cooperative Study Group Eleven-year survival in the Veterans Administration randomized trial of coronary bypass surgery for stable angina. N Engl J Med. 1984;311:1333–1339. doi: 10.1056/NEJM198411223112102. [DOI] [PubMed] [Google Scholar]
- 13.Varnauskas E. Twelve-year follow-up of survival in the randomized European Coronary Surgery Study. N Engl J Med. 1988;319:332–337. doi: 10.1056/NEJM198808113190603. [DOI] [PubMed] [Google Scholar]
- 14.Rahimtoola S.H. First percutaneous catheter intervention for left main coronary artery disease: 30 years ago. J Am Coll Cardiol Intv. 2008;1:108. doi: 10.1016/j.jcin.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 15.O’Keefe J.H., Jr., Hartzler G.O., Rutherford B.D., et al. Left main coronary angioplasty: early and late results of 127 acute and elective procedures. Am J Cardiol. 1989;64:144–147. doi: 10.1016/0002-9149(89)90447-5. [DOI] [PubMed] [Google Scholar]
- 16.Park S.J., Park S.W., Hong M.K., et al. Stenting of unprotected left main coronary artery stenoses: immediate and late outcomes. J Am Coll Cardiol. 1998;31:37–42. doi: 10.1016/s0735-1097(97)00425-7. [DOI] [PubMed] [Google Scholar]
- 17.Silvestri M., Barragan P., Sainsous J., et al. Unprotected left main coronary artery stenting: immediate and medium-term outcomes of 140 elective procedures. J Am Coll Cardiol. 2000;35:1543–1550. doi: 10.1016/s0735-1097(00)00588-x. [DOI] [PubMed] [Google Scholar]
- 18.Black A., Cortina R., Bossi I., Choussat R., Fajadet J., Marco J. Unprotected left main coronary artery stenting: correlates of midterm survival and impact of patient selection. J Am Coll Cardiol. 2001;37:832–838. doi: 10.1016/s0735-1097(00)01176-1. [DOI] [PubMed] [Google Scholar]
- 19.Takagi T., Stankovic G., Finci L., et al. Results and long-term predictors of adverse clinical events after elective percutaneous interventions on unprotected left main coronary artery. Circulation. 2002;106:698–702. doi: 10.1161/01.cir.0000024983.34728.5d. [DOI] [PubMed] [Google Scholar]
- 20.Park S.J., Kim Y.H., Lee B.K., et al. Sirolimus-eluting stent implantation for unprotected left main coronary artery stenosis: comparison with bare metal stent implantation. J Am Coll Cardiol. 2005;45:351–356. doi: 10.1016/j.jacc.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 21.Valgimigli M., van Mieghem C.A., Ong A.T., et al. Short- and long-term clinical outcome after drug-eluting stent implantation for the percutaneous treatment of left main coronary artery disease: insights from the Rapamycin-Eluting and Taxus Stent Evaluated at Rotterdam Cardiology Hospital registries (RESEARCH and T-SEARCH) Circulation. 2005;111:1383–1389. doi: 10.1161/01.CIR.0000158486.20865.8B. [DOI] [PubMed] [Google Scholar]
- 22.Chieffo A., Stankovic G., Bonizzoni E., et al. Early and mid-term results of drug-eluting stent implantation in unprotected left main. Circulation. 2005;111:791–795. doi: 10.1161/01.CIR.0000155256.88940.F8. [DOI] [PubMed] [Google Scholar]
- 23.Kim Y.H., Park D.W., Lee S.W., et al. Long-term safety and effectiveness of unprotected left main coronary stenting with drug-eluting stents compared with bare-metal stents. Circulation. 2009;120:400–407. doi: 10.1161/CIRCULATIONAHA.108.800805. [DOI] [PubMed] [Google Scholar]
- 24.Pandya S.B., Kim Y.H., Meyers S.N., et al. Drug-eluting versus bare-metal stents in unprotected left main coronary artery stenosis a meta-analysis. J Am Coll Cardiol Intv. 2010;3:602–611. doi: 10.1016/j.jcin.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bangalore S., Kumar S., Fusaro M., et al. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed-treatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation. 2012;125:2873–2891. doi: 10.1161/CIRCULATIONAHA.112.097014. [DOI] [PubMed] [Google Scholar]
- 26.Palmerini T., Biondi-Zoccai G., Della Riva D., et al. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet. 2012;379:1393–1402. doi: 10.1016/S0140-6736(12)60324-9. [DOI] [PubMed] [Google Scholar]
- 27.Valenti R., Migliorini A., Parodi G., et al. Clinical and angiographic outcomes of patients treated with everolimus-eluting stents or first-generation Paclitaxel-eluting stents for unprotected left main disease. J Am Coll Cardiol. 2012;60:1217–1222. doi: 10.1016/j.jacc.2012.05.038. [DOI] [PubMed] [Google Scholar]
- 28.Kim Y.H., Park D.W., Ahn J.M., et al. Everolimus-eluting stent implantation for unprotected left main coronary artery stenosis. The PRECOMBAT-2 (Premier of Randomized Comparison of Bypass Surgery Versus Angioplasty Using Sirolimus-Eluting Stent in Patients With Left Main Coronary Artery Disease) study. J Am Coll Cardiol Intv. 2012;5:708–717. doi: 10.1016/j.jcin.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Buchanan G.L., Chieffo A., Bernelli C., et al. Two-year outcomes following unprotected left main stenting with first vs. new-generation drug-eluting stents: the FINE registry. EuroIntervention. 2013;9:809–816. doi: 10.4244/EIJV9I7A134. [DOI] [PubMed] [Google Scholar]
- 30.Buszman P.E., Kiesz S.R., Bochenek A., et al. Acute and late outcomes of unprotected left main stenting in comparison with surgical revascularization. J Am Coll Cardiol. 2008;51:538–545. doi: 10.1016/j.jacc.2007.09.054. [DOI] [PubMed] [Google Scholar]
- 31.Buszman P.E., Buszman P.P., Banasiewicz-Szkróbka I., et al. Left main stenting in comparison with surgical revascularization: 10-year outcomes of the (left main coronary artery stenting) LE MANS trial. J Am Coll Cardiol Intv. 2016;9:318–327. doi: 10.1016/j.jcin.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 32.Boudriot E., Thiele H., Walther T., et al. Randomized comparison of percutaneous coronary intervention with sirolimus-eluting stents versus coronary artery bypass grafting in unprotected left main stem stenosis. J Am Coll Cardiol. 2011;57:538–545. doi: 10.1016/j.jacc.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 33.Morice M.C., Serruys P.W., Kappetein A.P., et al. Outcomes in patients with de novo left main disease treated with either percutaneous coronary intervention using paclitaxel-eluting stents or coronary artery bypass graft treatment in the Synergy Between Percutaneous Coronary Intervention With TAXUS and Cardiac Surgery (SYNTAX) trial. Circulation. 2010;121:2645–2653. doi: 10.1161/CIRCULATIONAHA.109.899211. [DOI] [PubMed] [Google Scholar]
- 34.Morice M.C., Serruys P.W., Kappetein A.P., et al. Five-year outcomes in patients with left main disease treated with either percutaneous coronary intervention or coronary artery bypass grafting in the synergy between percutaneous coronary intervention with taxus and cardiac surgery trial. Circulation. 2014;129:2388–2394. doi: 10.1161/CIRCULATIONAHA.113.006689. [DOI] [PubMed] [Google Scholar]
- 35.Thuijs D., Kappetein A.P., Serruys P.W., et al. Percutaneous coronary intervention versus coronary artery bypass grafting in patients with three-vessel or left main coronary artery disease: 10-year follow-up of the multicentre randomised controlled SYNTAX trial. Lancet. 2019;394:1325–1334. doi: 10.1016/S0140-6736(19)31997-X. [DOI] [PubMed] [Google Scholar]
- 36.Park S.J., Kim Y.H., Park D.W., et al. Randomized trial of stents versus bypass surgery for left main coronary artery disease. N Engl J Med. 2011;364:1718–1727. doi: 10.1056/NEJMoa1100452. [DOI] [PubMed] [Google Scholar]
- 37.Ahn J.M., Roh J.H., Kim Y.H., et al. Randomized trial of stents versus bypass surgery for left main coronary artery disease: 5-year outcomes of the PRECOMBAT study. J Am Coll Cardiol. 2015;65:2198–2206. doi: 10.1016/j.jacc.2015.03.033. [DOI] [PubMed] [Google Scholar]
- 38.Park D.W., Ahn J.M., Park H., et al. Ten-year outcomes after drug-eluting stents versus coronary artery bypass grafting for left main coronary disease: extended follow-up of the PRECOMBAT trial. Circulation. 2020;141:1437–1446. doi: 10.1161/CIRCULATIONAHA.120.046039. [DOI] [PubMed] [Google Scholar]
- 39.Stone G.W., Sabik J.F., Serruys P.W., et al. Everolimus-eluting stents or bypass surgery for left main coronary artery disease. N Engl J Med. 2016;375:2223–2235. doi: 10.1056/NEJMoa1610227. [DOI] [PubMed] [Google Scholar]
- 40.Stone G.W., Kappetein A.P., Sabik J.F., et al. Five-year outcomes after PCI or CABG for left main coronary disease. N Engl J Med. 2019;381:1820–1830. doi: 10.1056/NEJMoa1909406. [DOI] [PubMed] [Google Scholar]
- 41.Mäkikallio T., Holm N.R., Lindsay M., et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis (NOBLE): a prospective, randomised, open-label, non-inferiority trial. Lancet. 2016;388:2743–2752. doi: 10.1016/S0140-6736(16)32052-9. [DOI] [PubMed] [Google Scholar]
- 42.Holm N.R., Mäkikallio T., Lindsay M.M., et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in the treatment of unprotected left main stenosis: updated 5-year outcomes from the randomised, non-inferiority NOBLE trial. Lancet. 2020;395:191–199. doi: 10.1016/S0140-6736(19)32972-1. [DOI] [PubMed] [Google Scholar]
- 43.Giacoppo D., Colleran R., Cassese S., et al. Percutaneous coronary intervention vs coronary artery bypass grafting in patients with left main coronary artery stenosis: a systematic review and meta-analysis. JAMA Cardiol. 2017;2:1079–1088. doi: 10.1001/jamacardio.2017.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palmerini T., Serruys P., Kappetein A.P., et al. Clinical outcomes with percutaneous coronary revascularization vs coronary artery bypass grafting surgery in patients with unprotected left main coronary artery disease: a meta-analysis of 6 randomized trials and 4,686 patients. Am Heart J. 2017;190:54–63. doi: 10.1016/j.ahj.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Head S.J., Milojevic M., Daemen J., et al. Mortality after coronary artery bypass grafting versus percutaneous coronary intervention with stenting for coronary artery disease: a pooled analysis of individual patient data. Lancet. 2018;391:939–948. doi: 10.1016/S0140-6736(18)30423-9. [DOI] [PubMed] [Google Scholar]
- 46.Ahmad Y., Howard J.P., Arnold A.D., et al. Mortality after drug-eluting stents vs. coronary artery bypass grafting for left main coronary artery disease: a meta-analysis of randomized controlled trials. Eur Heart J. 2020;41:3228–3235. doi: 10.1093/eurheartj/ehaa135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brophy J.M. Bayesian interpretation of the EXCEL trial and other randomized clinical trials of left main coronary artery revascularization. JAMA Int Med. 2020;180:986–992. doi: 10.1001/jamainternmed.2020.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Velazquez E.J., Lee K.L., Jones R.H., et al. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med. 2016;374:1511–1520. doi: 10.1056/NEJMoa1602001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuno T., Ueyama H., Rao S.V., et al. Percutaneous coronary intervention or coronary artery bypass graft surgery for left main coronary artery disease: a meta-analysis of randomized trials. Am Heart J. 2020;227:9–10. doi: 10.1016/j.ahj.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 50.D’Ascenzo F., De Filippo O., Elia E., et al. Percutaneous vs. surgical revascularization for patients with unprotected left main stenosis: a meta-analysis of 5-year follow-up randomized controlled trials. Eur Heart J Qual Care Clin Outcomes. 2021;7:476–485. doi: 10.1093/ehjqcco/qcaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sabatine M.S., Bergmark B.A., Murphy S.A., et al. Percutaneous coronary intervention with drug-eluting stents versus coronary artery bypass grafting in left main coronary artery disease: an individual patient data meta-analysis. Lancet. 2021;398(10318):2247–2257. doi: 10.1016/S0140-6736(21)02334-5. [DOI] [PubMed] [Google Scholar]
- 52.Kataruka A., Maynard C.C., Kearney K.E., et al. Temporal trends in percutaneous coronary intervention and coronary artery bypass grafting: insights from the Washington Cardiac Care Outcomes Assessment Program. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fokkema M.L., James S.K., Albertsson P., et al. Population trends in percutaneous coronary intervention: 20-year results from the SCAAR (Swedish Coronary Angiography and Angioplasty Registry) J Am Coll Cardiol. 2013;61:1222–1230. doi: 10.1016/j.jacc.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 54.Culler S.D., Kugelmass A.D., Brown P.P., Reynolds M.R., Simon A.W. Trends in coronary revascularization procedures among Medicare beneficiaries between 2008 and 2012. Circulation. 2015;131:362–370. doi: 10.1161/CIRCULATIONAHA.114.012485. [DOI] [PubMed] [Google Scholar]
- 55.Windecker S., Kolh P., Alfonso F., et al. 2014 ESC/EACTS guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur Heart J. 2014;35:2541–2619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 56.Neumann F.J., Sousa-Uva M., Ahlsson A., et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. doi: 10.1093/eurheartj/ehy855. [DOI] [PubMed] [Google Scholar]
- 57.Fihn S.D., Blankenship J.C., Alexander K.P., et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;64:1929–1949. doi: 10.1016/j.jacc.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 58.Lawton J.S., Tamis-Holland J.E., Bangalore S., et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(2):e21–e129. doi: 10.1016/j.jacc.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 59.Head S.J., Kaul S., Mack M.J., et al. The rationale for heart team decision-making for patients with stable, complex coronary artery disease. Eur Heart J. 2013;34:2510–2518. doi: 10.1093/eurheartj/eht059. [DOI] [PubMed] [Google Scholar]
- 60.Kim A.M., Park J.H., Cho S., Kang S., Yoon T.H., Kim Y. Factors associated with the rates of coronary artery bypass graft and percutaneous coronary intervention. BMC Cardiovasc Disord. 2019;19:275. doi: 10.1186/s12872-019-1264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Loh P.H., Lassen J.F., Jepson N., et al. Asia Pacific consensus document on coronary bifurcation interventions. EuroIntervention. 2020;16:e706–e714. doi: 10.4244/EIJ-D-19-00977. [DOI] [PubMed] [Google Scholar]
- 62.Kim C., Hong S.J., Ahn C.M., et al. Patient-centered decision-making of revascularization strategy for left main or multivessel coronary artery disease. Am J Cardiol. 2018;122:2005–2013. doi: 10.1016/j.amjcard.2018.08.064. [DOI] [PubMed] [Google Scholar]
- 63.Sianos G., Morel M.A., Kappetein A.P., et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1:219–227. [PubMed] [Google Scholar]
- 64.Yoon Y.H., Ahn J.M., Kang D.Y., et al. Impact of SYNTAX score on 10-year outcomes after revascularization for left main coronary artery disease. J Am Coll Cardiol Intv. 2020;13:361–371. doi: 10.1016/j.jcin.2019.10.020. [DOI] [PubMed] [Google Scholar]
- 65.Farooq V., Serruys P.W., Garcia-Garcia H.M., et al. The negative impact of incomplete angiographic revascularization on clinical outcomes and its association with total occlusions: the SYNTAX (Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery) trial. J Am Coll Cardiol. 2013;61:282–294. doi: 10.1016/j.jacc.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 66.Takahashi K., Serruys P.W., Fuster V., et al. Redevelopment and validation of the SYNTAX score II to individualise decision making between percutaneous and surgical revascularisation in patients with complex coronary artery disease: secondary analysis of the multicentre randomised controlled SYNTAXES trial with external cohort validation. Lancet. 2020;396:1399–1412. doi: 10.1016/S0140-6736(20)32114-0. [DOI] [PubMed] [Google Scholar]
- 67.Mesana T., Rodger N., Sherrard H. Heart teams: a new paradigm in health care. Can J Cardiol. 2018;34:815–818. doi: 10.1016/j.cjca.2018.02.028. [DOI] [PubMed] [Google Scholar]
- 68.Silvain J., Vignalou J.B., Barthélémy O., Kerneis M., Collet J.P., Montalescot G. Coronary revascularization in the diabetic patient. Circulation. 2014;130:918–922. doi: 10.1161/CIRCULATIONAHA.113.004382. [DOI] [PubMed] [Google Scholar]
- 69.Farkouh M.E., Domanski M., Sleeper L.A., et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–2384. doi: 10.1056/NEJMoa1211585. [DOI] [PubMed] [Google Scholar]
- 70.Park S.J., Park D.W. Diabetes in myocardial revascularization for left main coronary artery disease: predictor or decision maker? J Am Coll Cardiol. 2019;73:1629–1632. doi: 10.1016/j.jacc.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 71.Milojevic M., Serruys P.W., Sabik J.F., 3rd, et al. Bypass surgery or stenting for left main coronary artery disease in patients with diabetes. J Am Coll Cardiol. 2019;73:1616–1628. doi: 10.1016/j.jacc.2019.01.037. [DOI] [PubMed] [Google Scholar]
- 72.Lee K., Ahn J.M., Yoon Y.H., et al. Long-term (10-year) outcomes of stenting or bypass surgery for left main coronary artery disease in patients with and without diabetes mellitus. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.015372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Naganuma T., Chieffo A., Meliga E., et al. Long-term clinical outcomes after percutaneous coronary intervention versus coronary artery bypass grafting for ostial/midshaft lesions in unprotected left main coronary artery from the DELTA registry: a multicenter registry evaluating percutaneous coronary intervention versus coronary artery bypass grafting for left main treatment. J Am Coll Cardiol Intv. 2014;7:354–361. doi: 10.1016/j.jcin.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 74.Hyun J., Kim J.H., Jeong Y., et al. Long-term outcomes after PCI or CABG for left main coronary artery disease according to lesion location. J Am Coll Cardiol Intv. 2020;13:2825–2836. doi: 10.1016/j.jcin.2020.08.021. [DOI] [PubMed] [Google Scholar]
- 75.Naganuma T., Chieffo A., Meliga E., et al. Long-term clinical outcomes after percutaneous coronary intervention for ostial/mid-shaft lesions versus distal bifurcation lesions in unprotected left main coronary artery: the DELTA Registry (drug-eluting stent for left main coronary artery disease): a multicenter registry evaluating percutaneous coronary intervention versus coronary artery bypass grafting for left main treatment. J Am Coll Cardiol Intv. 2013;6:1242–1249. doi: 10.1016/j.jcin.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 76.Biondi-Zoccai G.G., Lotrionte M., Moretti C., et al. A collaborative systematic review and meta-analysis on 1278 patients undergoing percutaneous drug-eluting stenting for unprotected left main coronary artery disease. Am Heart J. 2008;155:274–283. doi: 10.1016/j.ahj.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 77.Gershlick A.H., Kandzari D.E., Banning A., et al. Outcomes after left main percutaneous coronary intervention versus coronary artery bypass grafting according to lesion site: results from the EXCEL trial. J Am Coll Cardiol Intv. 2018;11:1224–1233. doi: 10.1016/j.jcin.2018.03.040. [DOI] [PubMed] [Google Scholar]
- 78.De Filippo O., Di Franco A., Boretto P., et al. Percutaneous coronary intervention versus coronary artery surgery for left main disease according to lesion site: a meta-analysis. J Thorac Cardiovasc Surg. Published online August 21, 2021 doi: 10.1016/j.jtcvs.2021.08.040. [DOI] [PubMed] [Google Scholar]
- 79.Takaro T., Peduzzi P., Detre K.M., et al. Survival in subgroups of patients with left main coronary artery disease. Veterans Administration Cooperative Study of Surgery for Coronary Arterial Occlusive Disease. Circulation. 1982;66:14–22. doi: 10.1161/01.cir.66.1.14. [DOI] [PubMed] [Google Scholar]
- 80.Farooq V., van Klaveren D., Steyerberg E.W., et al. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: development and validation of SYNTAX score II. Lancet. 2013;381:639–650. doi: 10.1016/S0140-6736(13)60108-7. [DOI] [PubMed] [Google Scholar]
- 81.Wolff G., Dimitroulis D., Andreotti F., et al. Survival benefits of invasive versus conservative strategies in heart failure in patients with reduced ejection fraction and coronary artery disease: a meta-analysis. Circ Heart Fail. 2017;10(1) doi: 10.1161/CIRCHEARTFAILURE.116.003255. [DOI] [PubMed] [Google Scholar]
- 82.Park S., Ahn J.M., Kim T.O., et al. Revascularization in patients with left main coronary artery disease and left ventricular dysfunction. J Am Coll Cardiol. 2020;76:1395–1406. doi: 10.1016/j.jacc.2020.07.047. [DOI] [PubMed] [Google Scholar]
- 83.Tran D.T.T., Tu J.V., Dupuis J.Y., Bader Eddeen A., Sun L.Y. Association of frailty and long-term survival in patients undergoing coronary artery bypass grafting. J Am Heart Assoc. 2018;7(15) doi: 10.1161/JAHA.118.009882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ono M., Serruys P.W., Hara H., et al. 10-year follow-up after revascularization in elderly patients with complex coronary artery disease. J Am Coll Cardiol. 2021;77:2761–2773. doi: 10.1016/j.jacc.2021.04.016. [DOI] [PubMed] [Google Scholar]
- 85.Park H., Ahn J.M., Yoon Y.H., et al. Effect of age and sex on outcomes after stenting or bypass surgery in left main coronary artery disease. Am J Cardiol. 2019;124:678–687. doi: 10.1016/j.amjcard.2019.05.061. [DOI] [PubMed] [Google Scholar]
- 86.Lindstaedt M., Spiecker M., Perings C., et al. How good are experienced interventional cardiologists at predicting the functional significance of intermediate or equivocal left main coronary artery stenoses? Int J Cardiol. 2007;120:254–261. doi: 10.1016/j.ijcard.2006.11.220. [DOI] [PubMed] [Google Scholar]
- 87.Oviedo C., Maehara A., Mintz G.S., et al. Intravascular ultrasound classification of plaque distribution in left main coronary artery bifurcations: where is the plaque really located? Circ Cardiovasc Interv. 2010;3:105–112. doi: 10.1161/CIRCINTERVENTIONS.109.906016. [DOI] [PubMed] [Google Scholar]
- 88.Jasti V., Ivan E., Yalamanchili V., Wongpraparut N., Leesar M.A. Correlations between fractional flow reserve and intravascular ultrasound in patients with an ambiguous left main coronary artery stenosis. Circulation. 2004;110:2831–2836. doi: 10.1161/01.CIR.0000146338.62813.E7. [DOI] [PubMed] [Google Scholar]
- 89.de la Torre Hernandez J.M., Hernández Hernandez F., Alfonso F., et al. Prospective application of pre-defined intravascular ultrasound criteria for assessment of intermediate left main coronary artery lesions results from the multicenter LITRO study. J Am Coll Cardiol. 2011;58:351–358. doi: 10.1016/j.jacc.2011.02.064. [DOI] [PubMed] [Google Scholar]
- 90.Park S.J., Ahn J.M., Kang S.J., et al. Intravascular ultrasound-derived minimal lumen area criteria for functionally significant left main coronary artery stenosis. J Am Coll Cardiol Intv. 2014;7:868–874. doi: 10.1016/j.jcin.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 91.Rusinova R.P., Mintz G.S., Choi S.Y., et al. Intravascular ultrasound comparison of left main coronary artery disease between white and Asian patients. Am J Cardiol. 2013;111:979–984. doi: 10.1016/j.amjcard.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 92.Lip G.Y., Rathore V.S., Katira R., Watson R.D., Singh S.P. Do Indo-Asians have smaller coronary arteries? Postgrad Med J. 1999;75:463–466. doi: 10.1136/pgmj.75.886.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Skowronski J., Cho I., Mintz G.S., et al. Inter-ethnic differences in normal coronary anatomy between Caucasian (Polish) and Asian (Korean) populations. Eur J Radiol. 2020;130:109185. doi: 10.1016/j.ejrad.2020.109185. [DOI] [PubMed] [Google Scholar]
- 94.Dodge J.T., Jr., Brown B.G., Bolson E.L., Dodge H.T. Lumen diameter of normal human coronary arteries. Influence of age, sex, anatomic variation, and left ventricular hypertrophy or dilation. Circulation. 1992;86:232–246. doi: 10.1161/01.cir.86.1.232. [DOI] [PubMed] [Google Scholar]
- 95.Oviedo C., Maehara A., Mintz G.S., et al. Is accurate intravascular ultrasound evaluation of the left circumflex ostium from a left anterior descending to left main pullback possible? Am J Cardiol. 2010;105:948–954. doi: 10.1016/j.amjcard.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 96.de la Torre Hernandez J.M., Baz Alonso J.A., Gómez Hospital J.A., et al. Clinical impact of intravascular ultrasound guidance in drug-eluting stent implantation for unprotected left main coronary disease: pooled analysis at the patient-level of 4 registries. J Am Coll Cardiol Intv. 2014;7:244–254. doi: 10.1016/j.jcin.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 97.Park S.J., Kim Y.H., Park D.W., et al. Impact of intravascular ultrasound guidance on long-term mortality in stenting for unprotected left main coronary artery stenosis. Circ Cardiovasc Interv. 2009;2:167–177. doi: 10.1161/CIRCINTERVENTIONS.108.799494. [DOI] [PubMed] [Google Scholar]
- 98.Kang S.J., Ahn J.M., Song H., et al. Comprehensive intravascular ultrasound assessment of stent area and its impact on restenosis and adverse cardiac events in 403 patients with unprotected left main disease. Circ Cardiovasc Interv. 2011;4:562–569. doi: 10.1161/CIRCINTERVENTIONS.111.964643. [DOI] [PubMed] [Google Scholar]
- 99.Hamilos M., Muller O., Cuisset T., et al. Long-term clinical outcome after fractional flow reserve-guided treatment in patients with angiographically equivocal left main coronary artery stenosis. Circulation. 2009;120:1505–1512. doi: 10.1161/CIRCULATIONAHA.109.850073. [DOI] [PubMed] [Google Scholar]
- 100.Toth G., Hamilos M., Pyxaras S., et al. Evolving concepts of angiogram: fractional flow reserve discordances in 4000 coronary stenoses. Eur Heart J. 2014;35:2831–2838. doi: 10.1093/eurheartj/ehu094. [DOI] [PubMed] [Google Scholar]
- 101.Park S.J., Kang S.J., Ahn J.M., et al. Visual-functional mismatch between coronary angiography and fractional flow reserve. J Am Coll Cardiol Intv. 2012;5:1029–1036. doi: 10.1016/j.jcin.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 102.Puri R., Kapadia S.R., Nicholls S.J., Harvey J.E., Kataoka Y., Tuzcu E.M. Optimizing outcomes during left main percutaneous coronary intervention with intravascular ultrasound and fractional flow reserve: the current state of evidence. J Am Coll Cardiol Intv. 2012;5:697–707. doi: 10.1016/j.jcin.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 103.Götberg M., Christiansen E.H., Gudmundsdottir I.J., et al. Instantaneous wave-free ratio versus fractional flow reserve to guide PCI. N Engl J Med. 2017;376:1813–1823. doi: 10.1056/NEJMoa1616540. [DOI] [PubMed] [Google Scholar]
- 104.Davies J.E., Sen S., Dehbi H.M., et al. Use of the instantaneous wave-free ratio or fractional flow reserve in PCI. N Engl J Med. 2017;376:1824–1834. doi: 10.1056/NEJMoa1700445. [DOI] [PubMed] [Google Scholar]
- 105.Kim H.L., Koo B.K., Nam C.W., et al. Clinical and physiological outcomes of fractional flow reserve-guided percutaneous coronary intervention in patients with serial stenoses within one coronary artery. J Am Coll Cardiol Intv. 2012;5:1013–1018. doi: 10.1016/j.jcin.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 106.Lee C.H., Choi S.-W., Hwang J., et al. 5-year outcomes according to FFR of left circumflex coronary artery after left main crossover stenting. J Am Coll Cardiol Intv. 2019;12:847–855. doi: 10.1016/j.jcin.2019.02.037. [DOI] [PubMed] [Google Scholar]
- 107.Pijls N.H., Fearon W.F., Tonino P.A., et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. J Am Coll Cardiol. 2010;56:177–184. doi: 10.1016/j.jacc.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 108.Xu B., Redfors B., Yang Y., et al. Impact of operator experience and volume on outcomes after left main coronary artery percutaneous coronary intervention. J Am Coll Cardiol Intv. 2016;9:2086–2093. [Google Scholar]
- 109.Zhang J.J., Ye F., Xu K., et al. Multicentre, randomized comparison of two-stent and provisional stenting techniques in patients with complex coronary bifurcation lesions: the DEFINITION II trial. Eur Heart J. 2020;41:2523–2536. doi: 10.1093/eurheartj/ehaa543. [DOI] [PubMed] [Google Scholar]
- 110.Chen S.L., Zhang J.J., Han Y., et al. Double kissing crush versus provisional stenting for left main distal bifurcation lesions: DKCRUSH-V randomized trial. J Am Coll Cardiol. 2017;70:2605–2617. doi: 10.1016/j.jacc.2017.09.1066. [DOI] [PubMed] [Google Scholar]
- 111.Hildick-Smith D., Egred M., Banning A., et al. The European Bifurcation Club Left Main Coronary Stent study: a randomized comparison of stepwise provisional vs. systematic dual stenting strategies (EBC MAIN) Eur Heart J. 2021;42(37):3829–3839. doi: 10.1093/eurheartj/ehab283. [DOI] [PubMed] [Google Scholar]
- 112.Burzotta F., Lassen J.F., Lefèvre T., et al. Percutaneous coronary intervention for bifurcation coronary lesions: the 15(th) consensus document from the European Bifurcation Club. EuroIntervention. 2021;16:1307–1317. doi: 10.4244/EIJ-D-20-00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Finet G., Derimay F., Motreff P., et al. Comparative analysis of sequential proximal optimizing technique versus kissing balloon inflation technique in provisional bifurcation stenting: fractal coronary bifurcation bench test. J Am Coll Cardiol Intv. 2015;8:1308–1317. doi: 10.1016/j.jcin.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 114.Koo B.K., Kang H.J., Youn T.J., et al. Physiologic assessment of jailed side branch lesions using fractional flow reserve. J Am Coll Cardiol. 2005;46:633–637. doi: 10.1016/j.jacc.2005.04.054. [DOI] [PubMed] [Google Scholar]
- 115.Chen S.L., Xu B., Han Y.L., et al. Clinical outcome after DK crush versus culotte stenting of distal left main bifurcation lesions: the 3-year follow-up results of the DKCRUSH-III study. J Am Coll Cardiol Intv. 2015;8:1335–1342. doi: 10.1016/j.jcin.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 116.Chen X., Li X., Zhang J.J., et al. 3-year outcomes of the DKCRUSH-V trial comparing DK crush with provisional stenting for left main bifurcation lesions. J Am Coll Cardiol Intv. 2019;12:1927–1937. doi: 10.1016/j.jcin.2019.04.056. [DOI] [PubMed] [Google Scholar]
- 117.Di Gioia G., Sonck J., Ferenc M., et al. Clinical outcomes following coronary bifurcation PCI techniques: a systematic review and network meta-analysis comprising 5,711 patients. J Am Coll Cardiol Intv. 2020;13:1432–1444. doi: 10.1016/j.jcin.2020.03.054. [DOI] [PubMed] [Google Scholar]
- 118.Gaido L., D’Ascenzo F., Imori Y., et al. Impact of kissing balloon in patients treated with ultrathin stents for left main lesions and bifurcations: an analysis from the RAIN-CARDIOGROUP VII study. Circ Cardiovasc Interv. 2020;13 doi: 10.1161/CIRCINTERVENTIONS.119.008325. [DOI] [PubMed] [Google Scholar]
- 119.Doenst T., Bonow R.O., Bhatt D.L., Falk V., Gaudino M. Improving terminology to describe coronary artery procedures: JACC review topic of the week. J Am Coll Cardiol. 2021;78:180–188. doi: 10.1016/j.jacc.2021.05.010. [DOI] [PubMed] [Google Scholar]
- 120.Levine G.N., Jeong Y.H., Goto S., et al. Expert consensus document: World Heart Federation expert consensus statement on antiplatelet therapy in East Asian patients with ACS or undergoing PCI. Nat Rev Cardiol. 2014;11:597–606. doi: 10.1038/nrcardio.2014.104. [DOI] [PubMed] [Google Scholar]