Abstract
Brugada syndrome (BrS) is one of the main inherited arrhythmia syndromes causing ventricular fibrillation (VF) and sudden cardiac death in young to middle-aged men, especially in Asians. The diagnosis of BrS is based on spontaneous or drug-provoked type 1 Brugada electrocardiogram. The current reliable therapy for BrS patients with VF history is the implantation of an implantable cardioverter-defibrillator. As for BrS patients without VF history, how asymptomatic BrS patients should effectively be treated is still uncertain because risk stratification of the BrS is still inadequate. Various parameters and combinations of several parameters have been reported for risk stratification of BrS. The SCN5A gene is believed to be the only gene that is responsible for BrS, and it has been reported to be useful for risk stratification. This review focuses on risk stratification of BrS patients, and focuses specifically on BrS patients of Asian descent.
Key Words: Asians, Brugada syndrome, risk stratification, SCN5A, ventricular fibrillation
Abbreviations and Acronyms: APHRS, Asia Pacific Heart Rhythm Society; BrS, Brugada syndrome; ECG, electrocardiogram; HRS, Heart Rhythm Society; ICD, implantable cardioverter-defibrillator; SAD, sudden arrhythmic death; SCD, sudden cardiac death; VF, ventricular fibrillation
Central Illustration
Highlights
-
•
SCD is a leading problem worldwide and early detection of diseases causing SCD is important.
-
•
The rate of sudden arrhythmic death is highest in Asians and BrS is one of the main SCD causes in Asians.
-
•
This review focuses on the epidemiology, diagnosis, prognosis, risk stratification, genetics, and therapy of BrS based on currently available evidence including ethnic differences.
-
•
Clarification of the pathophysiology and risk stratification of BrS is still insufficient. With the current guidelines alone, handling BrS cases in clinical practice is difficult, and further clinical and basic explanation is required in the future.
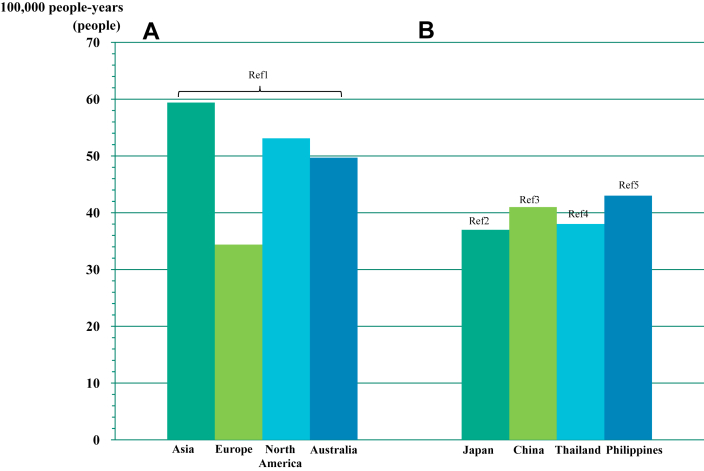
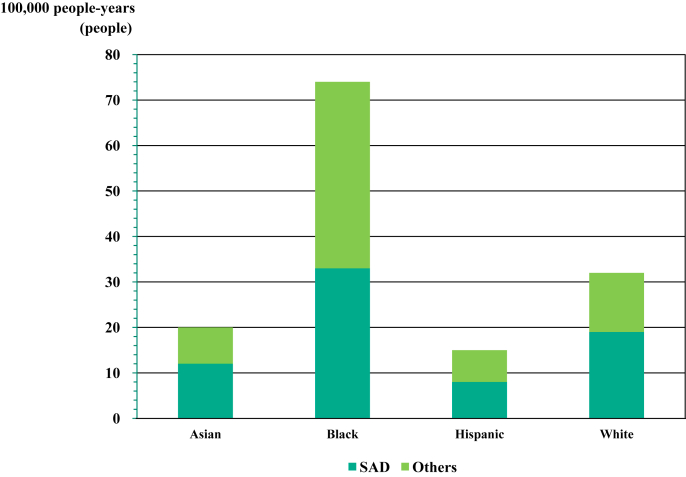
Sudden cardiac death (SCD) is a leading problem worldwide. Globally, the incidence of SCD per 100,000 people per year was estimated to be 34.4 in Europe, 53.1 people in North America, 59.4 people in Asia, and 49.7 people in Australia (Figure 1A).1 In other reports, the incidence of SCD per 100,000 people per year in Asia has been reported as 37 in Japan,2 41 in China,3 38 in Thailand,4 and 43 in the Philippines5 (Figure 1B). Moreover, the POST SCD (Postmortem Systematic Investigation of SCD) Study in 2018 reported that the incidence ratios for the World Health Organization SCD including sudden arrhythmic death (SAD) were more than 2- and 3-fold higher in men vs women, and highest in black people, lowest in Hispanics, and intermediate in Asians and white people (Figure 2).6
Figure 1.
Ethnic Difference of Incidence Rate of SCD
(A) The incidence of sudden cardiac death (SCD) per 100,000 people-years was estimated to be 34.4 in Europe, 53.1 in North America, 59.4 in Asia, and 49.7 in Australia, respectively.1(B) In the other reports, the SCD per 100,000 people-years has been reported as 37 in Japan,2 41 in China,3 38 in Thailand,4 and 43 in Philippines,5 respectively.
Figure 2.
Ethnic Difference of Incidence Rate of SAD
The POST SCD (Postmortem Systematic Investigation of SCD) Study in 2018 reported that the incidence rate ratios for the World Health Organization sudden cardiac death (SCD) were more than 2- and 3-fold higher in men vs women, and highest, lowest, and intermediate in blacks, Hispanics, and Asians and whites, respectively. In addition, the rate of sudden arrhythmic death (SAD) among all the SCDs was highest in Asians (61.9%) and lowest in blacks (44.6%).
As for SCD survival rate, Berdowski et al1 reported that the survival rate to discharge was reported to be 7.6% in Europe, 6.8% in North America, 3.0% in Asia, and 9.7% in Australia. According to a report by the Fire and Disaster Management Agency of Japan’s Ministry of Internal Affairs and Communications in 2020, the 1-month survival rate was 55.9% and the rehabilitation rate was 48.2% in SCD patients using bystander cardiopulmonary resuscitation and automated external defibrillator, whereas the 1-month survival rate was 9.0% and the rehabilitation rate was 4.5% in those without. Bystander cardiopulmonary resuscitation and automated external defibrillator use are effective but currently insufficient worldwide.7 According to data from the Pan-Asian Resuscitation Outcomes Study between 2011 and 2016, the national Utstein (bystander witnessed, shockable rhythm) 30-day survival-to-discharge rate was 11.6%-23.1% in Singapore.8 This was related to the implementation of a 5-year national plan for prehospital emergency care, which consisted of both community, prehospital community policies, and implementation measures.8
Detecting and intervening in the diseases that cause SCD at an early stage are also important. As for causes of SCD listed in the 2020 Asia Pacific Heart Rhythm Society/Heart Rhythm Society (APHRS/HRS) expert consensus statement on the investigation of SCD,7 the most common SCD cause in young people younger than 35 years are inherited heart diseases, including inherited arrhythmia syndromes. Coronary artery disease is the most common SCD cause from 35 years of age, but inherited arrhythmia syndromes also continue to be a common SCD cause up until 50 years of age7. According to the POST SCD Study,6 the rate of SAD among all the SCD was highest in Asians (61.9%) and lowest in black people (44.6%) (Figure 2). In Japan, the Hisayama study (1962-2009) was reported in 2013 as only the autopsy data. This study demonstrated that the most common cause of SCD was coronary artery diseases, but the rate was <30%.9 In the autopsy data of Chinese adults, unexplained sudden death was a major cause and accounted for 22.5% of victims younger than 35 years of age.10 According to data of 289 SCD victims in Hong Kong, 35% of the deaths were caused by coronary artery disease, 40% of the deaths were caused by structural heart diseases, and 25% of the deaths were unexplained. Among the unexplained cases, 85% had negative autopsy, suggesting SAD.11
According to data reported by Nowbar et al12 in 2019, based on an analysis of data from health organizations, the mortality rate from ischemic heart disease worldwide is 57.4 per 100,000 population in Japan, 140 in Turkey, 80.2 in France, 184.9 in the United States, and 260.9 in the United Kingdom Lower mortality rates associated with ischemic heart disease were noted in Asia compared with Europe and the United States.12 Therefore, the role of inherited arrhythmia syndromes in SCD is particularly important in Asia.13 The main inherited arrhythmia syndromes are Brugada syndrome (BrS), long-QT syndrome, short-QT syndrome, and catecholaminergic polymorphic ventricular tachycardia. Among them, BrS is one of the major cause of SCD in young to middle-aged men and it is the most common cause in some Asian countries.14,15 According to the 2005-2015 All-Japan Utstein Registry data, the 18- to 39-year-old male group showed a higher rate of SCD events at midnight to early morning compared with that of other age groups.16 This may reflect a BrS characteristic, which is that BrS is more common in men and is more likely to cause sudden death at night or at rest. Thus, this review article specifically focuses on BrS under the theme of inherited arrhythmia syndromes in Asia.
BrS Epidemiology and Diagnosis
BrS is one of the inherited arrhythmia syndromes, is characterized by a type 1 Brugada electrocardiogram (ECG) pattern of ST-segment elevation in the right precordial leads, and has a high SCD risk caused by ventricular fibrillation (VF).17 BrS is 8-10 times more prevalent in males than in females18 and typically manifests during adulthood at a mean age of 45 years.19
BrS can be diagnosed in patients with ST-segment elevation with type 1 morphology, characterized by ST-segment elevation ≥2 mm in at least one lead in the right precordial leads V1, V2, positioned in the second, third, or fourth intercostal space, occurring either spontaneously or after administration of sodium channel blockers after HRS/European Heart Rhythm Association/APHRS expert consensus statement (Figure 3).15,19,20 Prevalence of BrS is much higher in Asian countries, especially Thailand, Philippines, and Japan.21 The estimated prevalence of BrS ranges from 0.02%-0.1% in Europe and from 0.1%-0.25% in Asia.22,23 According to a meta-analysis in 2018, the global BrS pool prevalence is 0.5 per 1,000. Southeast Asians show the highest prevalence (3.7 per 1,000), and North Africans the lowest (0 per 1,000). The prevalence of Asians is 9 times more often than in Caucasians, and 36 times more common than Hispanics.24 Thus, BrS is a disease of much concern, especially in Asia.
Figure 3.
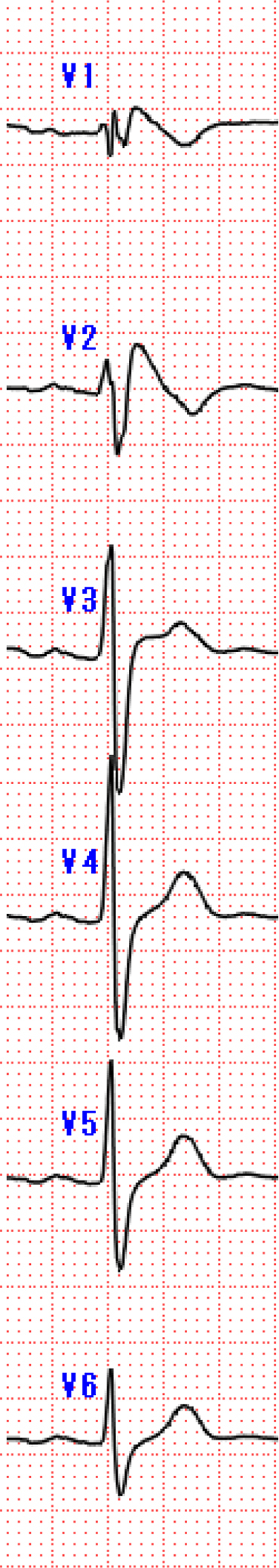
Type1 BrS ECG
Type 1 Brugada type electrocardiograms (ECGs), whether spontaneous or after sodium channel blockers provocation, are characterized by a coved type ST-segment elevation ≥2 mm in at least one lead located in the right precordial leads V1, V2, positioned in the second, third, or fourth intercostal space. BrS = Brugada syndrome.
BrS Prognosis and Risk Stratification
Kamakura et al25 reported that family history of SCD at younger than 45 years of age and coexistence of early repolarization pattern in inferolateral leads, in addition to Brugada ECG, were independent predictors of fatal arrhythmic events in a Japanese cohort, although spontaneous type 1 ECG and inducibility of VF by the electrophysiological study were not reliable parameters. Another Japanese cohort, including 460 BrS patients with type 1 ECG, reported that VF events were observed in 27 of 84 patients (32%, 8.4%/y) with a history of VF, 8 of 109 patients with a history of syncope alone (7%, 1.7%/y), and 3 of 267 asymptomatic patients (1%, 0.3%/y).26 Similarly, in the European registry (FINGER [France, Italy, Netherlands, Germany] Brugada syndrome registry), VF events were reported to be 7.7%/y in those with a history of VF, 1.9%/y in those with only syncope, and 0.5%/y in asymptomatic BrS patients.27 A recent meta-analysis from Asia reported that a SCD history in family members younger than 40 years of age held a significant increase in the risk of major arrhythmic events.28 Moreover, SCD risk stratification in BrS has not been completely explained and is still controversial (Table 1). Many previous studies, including 2 large European BrS registries (FINGER and PRELUDE [PRogrammed ELectrical stimUlation preDictive Value]), reported that a syncope history was significantly associated with VF events.25,27,29, 30, 31 Predicting the risk by a single parameter is difficult, although various parameters for risk stratification have been reported. We reported a novel logistic model using previously reported noninvasive risk factors for VF in patients with BrS (a combination of the history of syncope, r-J interval in V1, QRS duration in V6, and Tpeak to Tend dispersion), and it was useful for assessing risk stratification in routine clinical practice.32 The Shanghai score33,34 and Sciera score,35 which are both diagnostic scores consisting of a combination of multiple factors, have been devised for VF risk stratification in BrS.
Table 1.
Parameters for Risk Stratification of VF Events in BrS
| Brugada et al30 | Eckardt et al31 | Kamakura et al25 | FINGER27 | PRELUDE29 | Takagi et al26 | Kawazoe et al32 | Sieira et al35 | Yamagata and Shimizu39 | Kawada and Morita33,34 | BRUGADA-RISK33 | Rattanawong et al28 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | 2002 | 2005 | 2009 | 2010 | 2012 | 2007 | 2016 | 2017 | 2017 | 2018 | 2021 | 2021 |
| N | 334 | 212 | 330 | 1,029 | 308 | 430 | 143 | 400 | 415 | 393 | 1,110 | 3,386 |
| Prior VF | a | a | a | a | a | a | a | a | a | a | ||
| Syncope | a | a | b | a | a | a | a | a | a | a | ||
| Spontaneous type1 | a | a | b | a | a | b | b | a | a | a | ||
| FH of SCD | b | b | a | b | b | b | a | a | aOnly age <40 y | |||
| VF inducibility | a | b | b | b | b | b | b | a | ||||
| Male | b | b | a | b | ||||||||
| SCN5A mutation | a | a | ||||||||||
| Others | Logistic model | Sieira score | Shanghai score | Meta-analysis | ||||||||
| QRS duration in lead V2 >90 ms | Syncope | +Atrial fibrillation | +Early repolarization | |||||||||
| Inferolateral J-wave | +r–J interval in V1 | +Type 1 Brugada ECG pattern | ||||||||||
| Horizontal ST-segment morphology | +QRS duration in V6 | In peripheral leads | ||||||||||
| After J-wave | +Tp-e dispersion |
BRS = Brugada syndrome; BRUGADA RISK =A Primary Prevention Clinical Risk Score Model for Patients With Brugada Syndrome; ECG = electrocardiogram; FH = family history; FINGER = France, Italy, Netherlands Germany registry; PRELUDE = PRogrammed ELectrical stimUlation preDictive Value); SSCD = sudden cardiac death; VF = ventricular fibrillation.
Risk factors associated to events.
Risk factors not associated to events.
Shanghai Score is a risk stratification scoring system that integrates ECG, clinical history, family history, and genetic test, with a score of 3.5 or higher as probable/definite BrS. A notable point of the Shanghai score is that the spontaneous type 1 ECG is assigned the highest point (3.5 points) among other parameters, making spontaneous type 1 ECG the main focus of the scoring system, whereas a drug-induced type 1 Brugada ECG (type 2 or 3 Brugada ECG at baseline) is assigned 2 points.33 Kawada et al33 validated the appropriateness of the Shanghai Score System for diagnosis of 393 Japanese BrS patients. They reported that an increase in score was associated with an increase in the frequency of ventricular tachycardia/VF events, and that VF events did not occur in any BrS patients with a score of <3.5.34 The results of this study suggested the credibility of the Shanghai score and showed that a rigorous diagnosis of BrS was important.
However, Probst et al36 recently reported that these risk scores were inadequate for stratifying the risk of arrhythmic events in intermediate-risk patients. According to Japanese guidelines,37 BrS patients with a VF history or aborted cardiac arrest, in addition to type 1 ECG, have been indicated for implantable cardioverter-defibrillator (ICD) implantation as Class I indication. An ICD is recommended as Class IIa indication if arrhythmogenic syncope is found in patients with type 1 ECG. In addition, an unexplained syncope, induction of VF by a single or 2 consecutive extraventricular stimulations,38 family history of sudden death,25,28 and SCN5A mutation39 are all considered mild to moderate risk.
A recent multicenter study (BRUGADA-RISK [A Primary Prevention Clinical Risk Score Model for Patients With Brugada Syndrome]), including 1,110 patients with BrS, identified that arrhythmia-related syncope, spontaneous type 1 Brugada ECG, early repolarization pattern, and type 1 Brugada ECG pattern in peripheral leads were associated with a higher risk of SCD.40 BRUGADA-RISK estimated the risk of VF or SCD at 5 years in patients with BrS and the Brugada risk calculator was invented using these 4 parameters. 41 Given the high prevalence of BrS in Asians, this simple Brugada risk calculator may contribute significantly to the prevention of sudden death in family members of BrS patients. Milman et al42 investigated the ethnic differences of clinical characteristics and arrhythmic events between Western and Asian patients with BrS. They suggested that Asian BrS patients present almost exclusively as male adults and more often have aborted cardiac arrest and spontaneous type 1 ECG than white BrS patients. On the other hand, white BrS patients have a higher rate of family history of SCD and SCN5A mutation than Asian BrS patients. Moreover, the SABRUS (Survey on Arrhythmic Events in Brugada Syndrome) revealed that a shorter time-to-first appropriate ICD therapy was observed in Asian BrS patients.43 In consideration of the racial differences in the characteristics of BrS, the validation of the Brugada risk calculator in Asian BrS patients would be required as a multicenter study.
Genetics
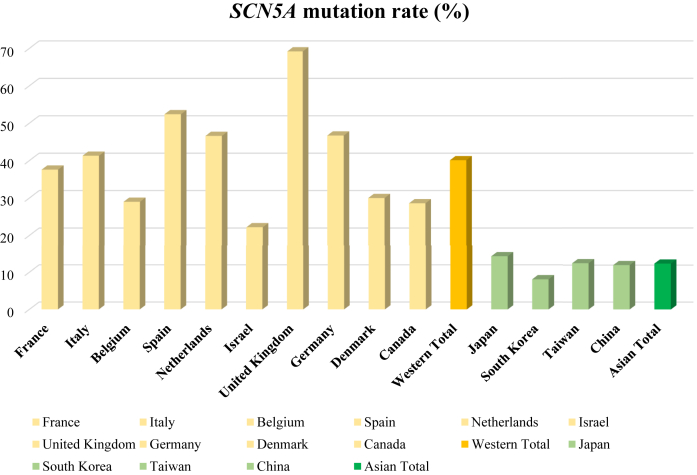
The SCN5A gene, which codes for cardiac voltage-gated sodium channels, recently has been agreed on as the only reliable gene causing BrS.44 The SCN5A gene accounts for the vast majority of patients with BrS in whom the mutation was identified. The Survey on Arrhythmic Events in BrS including 678 BrS patients reported that the SCN5A mutation was more frequently identified in whites than in Asians (40.1% vs 13.2%) (Figure 4).42 Many other susceptibility genes have been reported, but their frequencies were rare and their association with the Brugada phenotype is currently limited.45,46 A recent evidence-based review of genes also concluded that only the SCN5A gene is classified as having definitive evidence as a cause for BrS (Table 2).47
Figure 4.
Mean SCN5A Mutation Rates in Western and Asian Countries (SABRUS)
SABRUS (Survey on Arrhythmic Events in BrS), including 678 Brugada syndrome patients, reported that the SCN5A mutation was more frequently identified in whites than in Asians (40.1% vs 13.2%).
Table 2.
Genes Associated With BrS
| Name | Gene | Protein | Prevalence |
|---|---|---|---|
| BrS1 | SCN5A | α-Subunit Nav1.5 sodium channel | 20%-25% |
| BrS2 | GPD1L | Glycerol-3-phosphate dehydrogenase 1-like | Rare |
| BrS3 | CACNA1C | α-Subunit α1C Cav1.2 calcium channel | 1%-2% |
| BrS4 | CACNB2b | β-Subunit Cavβ2b calcium channel | 1%-2% |
| BrS5 | SCN1b | β-Subunit Navβ1 sodium channel | Rare |
| BrS6 | KCNE3 | β-Subunit MiRP2 potassium channel | Rare |
| BrS7 | SCN3b | β-Subunit Navβ3 sodium channel | Rare |
| BrS8 | HCN4 | Hyperpolarization-activated cyclic nucleotide-gated channel 4 | Rare |
| BrS9 | KCND3 | α-Subunit KV4.3 potassium channel | Rare |
| BrS10 | KCNJ8 | α-Subunit KIR6.1 potassium channel | Rare |
| BrS11 | CACNA2D1 | δ-Subunit Cavα2δ1 calcium channel | Rare |
| BrS12 | KCNE5 | β-Subunit potassium channel | Rare |
| BrS13 | RANGRF | RAN guanine nucleotide release factor | Rare |
| BrS14 | KCND2 | α-Subunit KV4.2 potassium channel | Rare |
| BrS15 | TRPM4 | Calcium-activated nonselective ion channel | Rare |
| BrS16 | SCN2B | β-subunit Navβ2 sodium channel | Rare |
| BrS17 | PKP2 | Plakophilin 2 | Rare |
| BrS18 | ABCC9 | ATP-sensitive potassium channels | Rare |
| BrS19 | SLMAP | Sarcolemma-associated protein | Rare |
| BrS20 | KCNH2 | α-Subunit of HERG potassium channel | Rare |
| BrS21 | SCN10A | α-Subunit Nav1.8 sodium channel | 1%-16% |
| BrS22 | FGF12 | Fibroblast growth factor 12 | Rare |
| BrS23 | SEMA3A | Semaphorin family protein | Rare |
ATP = adenosine triphosphate; other abbreviation as in Table 1.
Predictive values of SCN5A mutations for VF events have been controversial for a long time. SCN5A mutations were reported not to be associated with VF events in European BrS cohorts.27 However, a recent study in Europe reported contrary evidence that patients harboring SCN5A mutations exhibit more pronounced epicardial electrical abnormalities as an arrhythmogenic substrate and have a more aggressive clinical presentation.48 The current Japanese multicenter prospective registry, including 415 probands with BrS, demonstrated that probands carrying SCN5A mutations had more conduction abnormalities on ECG and have a higher risk for cardiac events than probands without SCN5A mutations.40 A large number of SCN5A variants have been reported to underlie BrS, but only a few missense variants had available functional data.49 The in silico assessment of channel function does not always reflect BrS phenotype and risk stratification.50 Most recently, Ishikawa51 reported that functionally proved loss-of-function SCN5A mutations were a significant predictor for subsequent VF events in a similar Japanese BrS registry. In a genome-wide association study, 3 single-nucleotide variation (SNV) (formerly SNP) were reported to be associated with BrS.52 The result in replication was confirmed and the HEY2 SNV was reported to be an useful prognostic marker for Japanese BrS.53 Genetic diagnosis is currently difficult because genes responsible for BrS have not been completely elucidated. However, the accumulation of many studies has made it possible to use SCN5A mutations as one of the risk stratifications. BrS biomarkers have also been investigated and α-cardiac actin, α-skeletal actin, keratin, and connexin-43 were reported to be useful for BrS risk stratification, although not enough consensus was noted.54 Overlapping between BrS and arrhythmogenic right ventricular cardiomyopathy has long been debated. In 2020, Scheirlynck et al55 reported that dilation of the right ventricular outflow, in addition to type 1 ECG, exacerbated the occurrence of arrhythmic events in patients with BrS. They suggested that BrS is not just a primary electrical disease but also overlapping phenotypes of ion channelopathy and structural abnormalities through the disruption of the voltage-gated sodium channel cytoskeleton/desmosome pathway.55
Therapy
The only proven and effective treatment strategy to prevent sudden death in cases of BrS is ICD. Moreover, ICD implantation is a Class I indication for BrS patients with spontaneous type 1 BrS ECG and a history of VF. ICD implantation is indicated for Class IIa in BrS patients with type 1 ECG who have a history of arrhythmogenic syncope.15 According to Japanese guidelines,37,56 ICD implantation is indicated for Class IIb if VF is induced by a single or 2 consecutive extraventricular stimulations in asymptomatic BrS patients combined with other clinical findings, other abnormal ECG findings, or SCN5A gene mutations. The HRS/European Heart Rhythm Association/APHRS expert consensus statement also indicates that VF induction by electrophysiological examination is Class IIb.15
The most effective drug to prevent VF development in BrS patients is quinidine, which suppresses Ito.57 In Japan, the multichannel blocker, beprizil, is used to prevent VF in BrS. It has been reported that beprizil prevented VF in BrS patients by suppressing multiple K channels including Ito and up-regulating Na channels.58 Nademanee et al59 reported that electrical epicardial substrate ablation in the right ventricular outflow tract in BrS patients can prevent VF inducibility in a high-risk population. Epicardial ablation was effective because an arrhythmogenic substrate in a wide area on the epicardial side of the right ventricle can be observed in most patients. Ablation therapy for BrS patients is currently indicated as a Class IIb indication by Japanese guidelines and is performed to save lives in high-risk BrS patients with repeated VF and VF storm cases.15,38 The level of evidence for the guideline may increase in the future as more information on BrS ablation cases in Asia is accumulated.
Conclusions
We reviewed the racial differences in the prevalence and the causes of SCD. Asians have a lower frequency of SCD than blacks, however, Asians have a higher mortality rate from sudden lethal arrhythmias. Among them, BrS is one of the most important causes of SCD in Asians. We show the flow from diagnosis to treatment of BrS in the Central Illustration. The risk stratification of BrS has long been investigated, and multifactorial combinations are becoming mainstream. Because of the racial differences in the characteristics of BrS, the validation of various risk factors for stratification will be necessary in Asian BrS patients. Although genetic background of BrS has not been fully understood, SCN5A mutations may help stratify the risk of BrS. Because BrS may not be a monogenic disease, further research including multigene risk assessments and omics will be required in the future to reduce SCD in Asians.
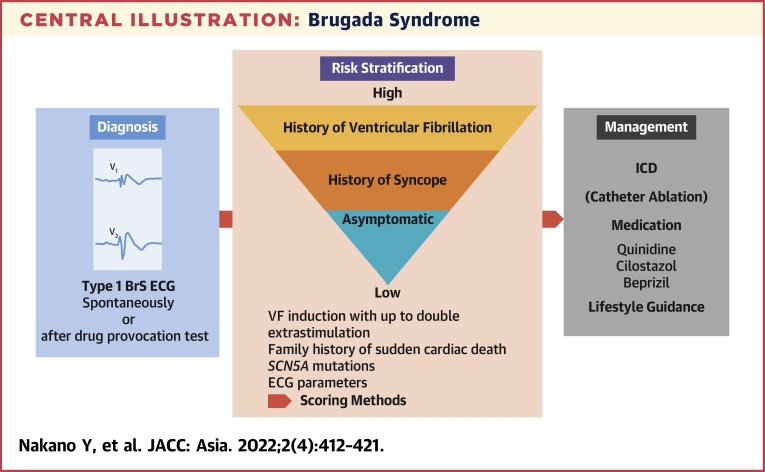
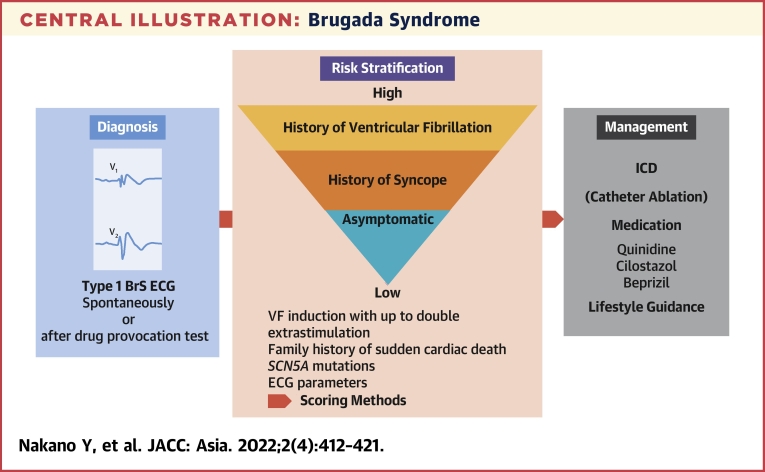
Central Illustration.
Brugada Syndrome
This illustration shows the flow from diagnosis to treatment of Brugada Syndrome (BrS). BrS is diagnosed with a spontaneously type 1 Brugada electrocardiogram (ECG) or type 1 Brugada ECG after provocative drug test. Ventricular fibrillation (VF) induction with up to double extra stimulation, family history of sudden cardiac death, SCN5A mutations, and ECG parameters are helpful for risk stratification. Recently, the scoring method, which combines several risks, is also used. In cases of secondary prevention, implantable cardioverter-defibrillator (ICD) is indicated, and in asymptomatic cases, oral administration of quinidine is performed depending on risk stratification in addition to lifestyle guidance.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank the members of the clerical and medical staff at Hiroshima University Hospital for their assistance. They thank Laverte School of English and their staff for editing a draft of the manuscript.
Footnotes
Ali J. Marian, MD, served as Guest Associate Editor for this paper. Yibin Wang, PHD, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Berdowski J., Berg R.A., Tijssen J.G., Koster R.W. Global incidences of out-of-hospital cardiac arrest and survival rates: systematic review of 67 prospective studies. Resuscitation. 2010;81:1479–1487. doi: 10.1016/j.resuscitation.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Tokashiki T., Muratani A., Kimura Y., Muratani H., Fukiyama K. Sudden death in the general population in Okinawa: incidence and causes of death. Jpn Circ J. 1999;63:37–42. doi: 10.1253/jcj.63.37. [DOI] [PubMed] [Google Scholar]
- 3.Hua W., Zhang L.F., Wu Y.F., et al. Incidence of sudden cardiac death in China: analysis of 4 regional populations. J Am Coll Cardiol. 2009;54:1110–1118. doi: 10.1016/j.jacc.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 4.Tungsanga K., Sriboonlue P. Sudden unexplained death syndrome in Northeast Thailand. Int J Epidemiol. 1993;22:81–87. doi: 10.1093/ije/22.1.81. [DOI] [PubMed] [Google Scholar]
- 5.Gervacio-Domingo G., Punzalan F.E., Amarillo M.L., Dans A. Sudden unexplained death during sleep occurred commonly in the general population in the Philippines: a sub study of the national nutrition and health survey. J Clin Epidemiol. 2007;60:567–571. doi: 10.1016/j.jclinepi.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Tseng Z.H., Olgin J.E., Vittinghoff E., et al. Prospective countywide surveillance and autopsy characterization of sudden cardiac death: POST SCD study. Circulation. 2018;137:2689–2700. doi: 10.1161/CIRCULATIONAHA.117.033427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stiles M.K., Wilde A.A.M., Abrams D.J., et al. 2020 APHRS/HRS expert consensus statement on the investigation of decedents with sudden unexplained death and patients with sudden cardiac arrest, and of their families. J Arrhythm. 2021;37:481–534. doi: 10.1002/joa3.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho A.F.W., De Souza N.N.A., Blewer A.L., et al. Singapore Pan-Asian Resuscitation Outcomes Study (PAROS) investigators. Implementation of a National 5-year Plan for Prehospital Emergency Care in Singapore and Impact on Out-of-Hospital Cardiac Arrest Outcomes from 2011 to 2016. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.015368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagata M., Ninomiya T., Doi Y., et al. Temporal trends in sudden unexpected death in a general population: the Hisayama study. Am Heart J. 2013;165:932–938. doi: 10.1016/j.ahj.2013.02.028. [DOI] [PubMed] [Google Scholar]
- 10.Wang H., Yao Q., Zhu S., et al. The autopsy study of 553 cases of sudden cardiac death in Chinese adults. Heart Vessels. 2014;29:486–495. doi: 10.1007/s00380-013-0388-0. [DOI] [PubMed] [Google Scholar]
- 11.Mak C.M., Mok N.S., Shum H.C., et al. Sudden arrhythmia death syndrome in young victims: a five-year retrospective review and two-year prospective molecular autopsy study by next-generation sequencing and clinical evaluation of their first-degree relatives. Hong Kong Med J. 2019;25:21–29. doi: 10.12809/hkmj187256. [DOI] [PubMed] [Google Scholar]
- 12.Nowbar A.N., Gitto M., Howard J.P., Francis D.P., Al-Lamee R. Mortality from ischemic heart disease. Circ Cardiovasc Qual Outcomes. 2019;12 doi: 10.1161/CIRCOUTCOMES.118.005375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi M., Shimizu W., Albert C.M. The spectrum of epidemiology underlying sudden cardiac death. Circ Res. 2015;116:1887–1906. doi: 10.1161/CIRCRESAHA.116.304521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hermida J.S., Lemoine J.L., Aoun F.B., Jarry G., Rey J.L., Quiret J.C. Prevalence of the Brugada syndrome in an apparently healthy population. Am J Cardiol. 2000;86:91–94. doi: 10.1016/s0002-9149(00)00835-3. [DOI] [PubMed] [Google Scholar]
- 15.Priori S.G., Wilde A.A., Horie M., et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–1963. doi: 10.1016/j.hrthm.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Otsuki S., Aiba T., Tahara Y., et al. Intra-day change in occurrence of out-of-hospital ventricular fibrillation in Japan: the JCS-ReSS study. Int J Cardiol. 2020;318:54–60. doi: 10.1016/j.ijcard.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu W., Matsuo K., Kokubo Y., et al. Sex hormone and gender difference - role of testosterone on male predominance in Brugada syndrome. J Cardiovasc Electrophysiol. 2007;18:415–421. doi: 10.1111/j.1540-8167.2006.00743.x. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu W. Clinical features of Brugada syndrome. J Arrhythm. 2013;29:65–70. [Google Scholar]
- 19.Brugada P., Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20:1391–1396. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 20.Antzelevitch C., Brugada P., Borggrefe M., et al. Brugada syndrome. Report of the second consensus conference. Endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111:659–670. doi: 10.1161/01.CIR.0000152479.54298.51. [DOI] [PubMed] [Google Scholar]
- 21.Wilde A.A., Antzelevitch C., Borggrefe M., et al. Proposed diagnostic criteria for the Brugada syndrome: consensus report. Circulation. 2002;106:2514–2519. doi: 10.1161/01.cir.0000034169.45752.4a. [DOI] [PubMed] [Google Scholar]
- 22.Miyasaka Y., Tsuji H., Yamada K., et al. Prevalence and mortality of the Brugada-type electrocardiogram in one city in Japan. J Am Coll Cardiol. 2001;38:771–774. doi: 10.1016/s0735-1097(01)01419-x. [DOI] [PubMed] [Google Scholar]
- 23.Matsuo K., Akahoshi M., Nakashima E., et al. The prevalence, incidence and prognostic value of the Brugada-type electrocardiogram: a population-based study of four decades. J Am Coll Cardiol. 2001;38:765–770. doi: 10.1016/s0735-1097(01)01421-8. [DOI] [PubMed] [Google Scholar]
- 24.Vutthikraivit W., Rattanawong P., Putthapiban P., et al. Worldwide prevalence of Brugada syndrome: a systematic review and meta-analysis. Acta Cardiol Sin. 2018;34:267–277. doi: 10.6515/ACS.201805_34(3).20180302B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamakura S., Ohe T., Nakazawa K., et al. Long-term prognosis of probands with Brugada-pattern ST-elevation in leads V1-V3. Circ Arrhythm Electrophysiol. 2009;2:495–503. doi: 10.1161/CIRCEP.108.816892. [DOI] [PubMed] [Google Scholar]
- 26.Takagi M., Aonuma K., Sekiguchi Y., et al. Japan idiopathic ventricular fibrillation study (J-IVFS) investigators. The prognostic value of early repolarization (J wave) and ST segment morphology after J wave in Brugada syndrome: multicenter study in Japan. Heart Rhythm. 2013;10:533–539. doi: 10.1016/j.hrthm.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 27.Probst V., Veltmann C., Eckardt L., et al. Long-term prognosis of patients diagnosed with Brugada syndrome: results from the FINGER Brugada syndrome registry. Circulation. 2010;121:635–643. doi: 10.1161/CIRCULATIONAHA.109.887026. [DOI] [PubMed] [Google Scholar]
- 28.Rattanawong P., Kewcharoen J., Kanitsoraphan C., et al. Does the age of sudden cardiac death in family members matter in Brugada syndrome? J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.019788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Priori S.G., Gasparini M., Napolitano C., et al. Risk stratification in Brugada syndrome: results of the Prelude registry. J Am Coll Cardiol. 2012;59:37–45. doi: 10.1016/j.jacc.2011.08.064. [DOI] [PubMed] [Google Scholar]
- 30.Brugada J., Brugada R., Antzelevitch C., Towbin J., Nademanee K., Brugada P. Long-term follow-up of individuals with the electrocardiographic pattern of right bundle-branch block and ST-segment elevation in precordial leads V1 to V3. Circulation. 2002;105:73–78. doi: 10.1161/hc0102.101354. [DOI] [PubMed] [Google Scholar]
- 31.Eckardt L., Probst V., Smits J.P., et al. Long-term prognosis of individuals with right precordial ST-segment-elevation Brugada syndrome. Circulation. 2005;111:257–263. doi: 10.1161/01.CIR.0000153267.21278.8D. [DOI] [PubMed] [Google Scholar]
- 32.Kawazoe H., Nakano Y., Ochi H., et al. Risk stratification of ventricular fibrillation in Brugada syndrome using noninvasive scoring methods. Heart Rhythm. 2016;13:1947–1954. doi: 10.1016/j.hrthm.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Kawada S., Morita H., Antzelevitch C., et al. Shanghai score system for diagnosis of Brugada syndrome: validation of the score system and system and reclassification of the patients. J Am Clin Cardiol EP. 2018;4:724–730. doi: 10.1016/j.jacep.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Antzelevitch C., Yan G.X., Ackerman M.J., et al. J-Wave syndromes expert consensus conference report: emerging concepts and gaps in knowledge. J Arrhythm. 2016;32:315–339. doi: 10.1016/j.joa.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sieira J., Conte G., Ciconte G., et al. A score model to predict risk of events in patients with Brugada syndrome. Eur Heart J. 2017;38:1756–1763. doi: 10.1093/eurheartj/ehx119. [DOI] [PubMed] [Google Scholar]
- 36.Probst V., Goronflot T., Anys S., et al. Robustness and relevance of predictive score in sudden cardiac death for patients with Brugada syndrome. Eur Heart J. 2021;42:1687–1695. doi: 10.1093/eurheartj/ehaa763. [DOI] [PubMed] [Google Scholar]
- 37.Nogami A., Kurita T., Abe H., et al. JCS/JHRS 2019 guideline on non-pharmacotherapy of cardiac arrhythmias. J Arrhythm. 2021;37:709–870. doi: 10.1002/joa3.12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sroubek J., Probst V., Mazzanti A., et al. Programmed ventricular stimulation for risk stratification in the Brugada syndrome: a pooled analysis. Circulation. 2016;133:622–630. doi: 10.1161/CIRCULATIONAHA.115.017885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamagata K., Horie M., Aiba T., et al. Genotype-phenotype correlation of SCN5A mutation for the clinical and electrocardiographic characteristics of probands with Brugada syndrome: a Japanese multicenter registry. Circulation. 2017;135:2255–2270. doi: 10.1161/CIRCULATIONAHA.117.027983. [DOI] [PubMed] [Google Scholar]
- 40.Honarbakhsh S., Providencia R., Garcia-Hernandez J., et al. A primary prevention clinical risk score model for patients with Brugada syndrome (BRUGADA-RISK) J Am Coll Cardiol EP. 2021;7:210–222. doi: 10.1016/j.jacep.2020.08.032. [DOI] [PubMed] [Google Scholar]
- 41.Brugada Syndrome Risk Stratification (Desktop) http://brugadariskscore.com Available at:
- 42.Milman A., Andorin A., Postema P.G., et al. Ethnic differences in patients with Brugada syndrome and arrhythmic events: new insights from survey on arrhythmic events in Brugada syndrome. Heart Rhythm. 2019;16:1468–1474. doi: 10.1016/j.hrthm.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Milman A., Hochstadt A., Andorin A., et al. Time-to-first appropriate shock in patients implanted prophylactically with an implantable cardioverter-defibrillator: data from the survey on arrhythmic events in Brugada syndrome (SABRUS) Europace. 2019;21:796–802. doi: 10.1093/europace/euy301. [DOI] [PubMed] [Google Scholar]
- 44.Chen Q., Kirsch G.E., Zhang D., et al. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature. 1998;392:293–296. doi: 10.1038/32675. [DOI] [PubMed] [Google Scholar]
- 45.Ingles J., Macciocca I., Morales A., Thomson K. Genetic testing in inherited heart diseases. Heart Lung Circ. 2020;29:505–511. doi: 10.1016/j.hlc.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 46.Gray B., Behr E.R. New insights into the genetic basis of inherited arrhythmia syndromes. Circ Cardiovasc Genet. 2016;9:569–577. doi: 10.1161/CIRCGENETICS.116.001571. [DOI] [PubMed] [Google Scholar]
- 47.Hosseini S.M., Kim R., Udupa S., et al. Reappraisal of reported genes for sudden arrhythmic death: evidence-based evaluation of gene validity for Brugada syndrome. National Institutes of Health Clinical Genome Resource Consortium. Circulation. 2018;138:1195–1205. doi: 10.1161/CIRCULATIONAHA.118.035070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ciconte G., Monasky M.M., Santinelli V., et al. Brugada syndrome genetics is associated with phenotype severity. Eur Heart J. 2021;42:1082–1090. doi: 10.1093/eurheartj/ehaa942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Denham N.C., Pearman C.M., Ding W.Y., et al. Wern yew. J Cardiovasc Electrophysiol. 2019;30:118–127. doi: 10.1111/jce.13740. [DOI] [PubMed] [Google Scholar]
- 50.Pearman C.M., Denham N.C., Mills R.W., et al. Relationship between sodium channel function and clinical phenotype in SCN5A variants associated with Brugada syndrome. Hum Mutat. 2020;41:2195–2204. doi: 10.1002/humu.24128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ishikawa T. Functionaly validated SCN5A variants allow interpretation of pathogenicity and the 1 prediction of lethal events in Brugada syndrome. Eur Heart J. 2021;42(29):2854–2863. doi: 10.1093/eurheartj/ehab254. [DOI] [PubMed] [Google Scholar]
- 52.Bezzina C.R., Barc J., Mizusawa Y., et al. Common variants at SCN5A-SCN10A and HEY2 are associated with Brugada syndrome, a rare disease with high risk of sudden cardiac death. Nat Genet. 2013;45:1044–1049. doi: 10.1038/ng.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakano Y., Ochi H., Onohara Y., et al. Common variant near HEY2 has a protective effect on ventricular fibrillation occurrence in Brugada syndrome by regulating the repolarization current. Circ Arrhythm Electrophysiol. 2016;9 doi: 10.1161/CIRCEP.115.003436. [DOI] [PubMed] [Google Scholar]
- 54.Chatterjee D., Pieroni M., Fatah M., et al. An autoantibody profile detects Brugada syndrome and identifies abnormally expressed myocardial proteins. Eur Heart J. 2020;41:2878–2890. doi: 10.1093/eurheartj/ehaa383. [DOI] [PubMed] [Google Scholar]
- 55.Scheirlynck E., Chivulescu M., Lie Ø.H., et al. Worse prognosis in Brugada syndrome patients with arrhythmogenic cardiomyopathy features. J Am Coll Cardiol EP. 2020;6:1353–1363. doi: 10.1016/j.jacep.2020.05.026. [DOI] [PubMed] [Google Scholar]
- 56.Ono K., Iwasaki Y.K., Akao M., et al. JCS/JHRS 2020 guideline on pharmacotherapy of cardiac arrhythmias. Circ J. Published online on March 11, 2022 doi: 10.1253/circj.CJ-20-1212. [DOI] [PubMed] [Google Scholar]
- 57.Belhassen B., Rahkovich M., Michowitz Y., Glick A., Viskin S. Management of Brugada syndrome: thirty-three-year experience using electrophysiologically guided therapy with class 1A antiarrhythmic drugs. Circ Arrhythm Electrophysiol. 2015;8:1393–1402. doi: 10.1161/CIRCEP.115.003109. [DOI] [PubMed] [Google Scholar]
- 58.Kang L., Zheng M.Q., Morishima M., Wang Y., Kaku T., Ono K. Bepridil up-regulates cardiac Na+ channels as a long-term effect by blunting proteasome signals through inhibition of calmodulin activity. Br J Pharmacol. 2009;157:404–414. doi: 10.1111/j.1476-5381.2009.00174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nademanee K., Veerakul G., Chandanamattha P., et al. Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation. 2011;123:1270–1279. doi: 10.1161/CIRCULATIONAHA.110.972612. [DOI] [PubMed] [Google Scholar]