Abstract
Background
Diagnosis of cardiac sarcoidosis (CS) is sometimes difficult due to a low positive rate of epithelioid granulomas by endomyocardial biopsy (EMB). Accordingly, Japanese guidelines can allow the CS diagnosis using clinical data alone without EMB results (clinical CS) since 2006. However, little is known about prognosis and outcome of clinical CS.
Objectives
Purpose of this study was to analyze the prognosis, outcomes, and response to corticosteroid of clinical CS using large-scale cohort survey.
Methods
Overall, 422 CS patients (mean age 60 ± 13 years, 68% female, median follow-up period of 5 years), including 345 clinical CS and 77 EMB-positive patients, histologically diagnosed CS (histological CS) by Japanese guidelines, were enrolled and examined.
Results
Clinical profile (age, sex, initial cardiac arrhythmias, and abnormal uptake of gallium-67 scintigraphy or 18F-fluorodeoxyglucose positron emission tomography in heart) was similar in both groups. Although clinical CS had better prognosis (P = 0.018) and outcome (all-cause death, appropriate defibrillator therapy, and heart transplantation; P = 0.008), multivariate Cox hazard analysis revealed that left ventricular ejection fraction (LVEF) and sustained ventricular tachycardia history were independently associated with outcome (P < 0.001 and P = 0.002, respectively), but not with the diagnosed CS category. Moreover, similar LVEF recovery after corticosteroid was observed in both groups with low LVEF (≤35%) at the 1-year follow-up period (P < 0.001).
Conclusions
In clinical CS according to the Japanese guideline, prophylactic implantable-cardioverter-defibrillator and immunosuppressive therapy are important in patients with low LVEF or ventricular tachycardia history, similar to histological CS.
Key Words: cardiac sarcoidosis, clinical diagnosis, histological diagnosis, prognosis
Abbreviations and Acronyms: 18F-FDG, 18F-fluorodeoxyglucose; 67Ga, gallium-67; AVB, atrioventricular block; BAL, bronchoalveolar lavage; CMR, cardiac magnetic resonance; CRT, cardiac resynchronization therapy; CS, cardiac sarcoidosis; EMB, endomyocardial biopsy; HRS, Heart Rhythm Society; ICD, implantable cardioverter-defibrillator; JCS, Japanese Circulation Society; JSSOG, Japanese Society of Sarcoidosis and Other Granulomatous diseases; LV, left ventricular; LVEF, left ventricular ejection fraction; PET, positron emission tomography; RFCA, radiofrequency catheter ablation; RV, right ventricle/ventricular; VT, ventricular tachycardia
Central Illustration
Sarcoidosis is a systemic inflammatory granulomatous disease that affects various organs (1). Although sarcoidosis is believed to be a relatively benign disease, cardiac sarcoidosis (CS), which affects the cardiovascular system, is an important predictor of poor prognosis in cases of systemic sarcoidosis due to heart failure and various serious types of fatal arrhythmias, such as atrioventricular block (AVB) and ventricular tachyarrhythmias (2, 3, 4, 5, 6, 7). Currently, immunosuppressive drugs comprise the first-line treatment (8,9), and nonpharmacological therapies, including pacemakers, implantable cardioverter-defibrillators (ICDs), cardiac resynchronization therapy (CRT), and radiofrequency catheter ablation (RFCA), are sometimes required for the management of CS.
Although different management strategies for CS have been identified, critical problems about the CS diagnosis have been still remained. CS is sometimes misdiagnosed as dilated cardiomyopathy, arrhythmogenic cardiomyopathy, or idiopathic ventricular aneurysm by echocardiography or electrocardiogram alone. Therefore, performing an endomyocardial biopsy (EMB) is important, and the current guidelines encourage the use of an electrophysiological or image-guided EMB to increase the sensitivity to diagnose CS (10,11), but making a definite diagnosis of CS is still difficult due to the low positive rate of EMB results (12). Accordingly, Japanese guidelines can allow the CS diagnosis using the clinical data alone without EMB results (clinical CS) since 2006 (13) and these diagnoses are considered definite diagnoses of CS, which are equivalent to the histologically diagnosed CS (histological CS). This guideline has been updated recently (14). The latest Japanese guideline (Japanese Circulation Society [JCS] 2016) enables making a diagnosis of clinical CS using the following criteria: clinical CS was diagnosed by: 1) identifying epithelioid granulomas in organs other than the heart, and clinical findings that strongly suggest cardiac involvement; or 2) clinical findings that are strongly suggestive of pulmonary or ophthalmic sarcoidosis, and confirmation of at least 2 of the 5 characteristic laboratory findings of sarcoidosis (bilateral hilar lymphadenopathy, high serum angiotensin-converting enzyme activity or elevated serum lysozyme levels, high serum soluble interleukin-2 receptor levels, significant tracer accumulation in gallium-67 [67Ga] citrate scintigraphy or 18F-fluorodeoxyglucose positron emission tomography [18F-FDG PET], and a high percentage of lymphocytes with a CD4/CD8 ratio of >3.5 in bronchoalveolar lavage [BAL] fluid).
These “clinical” CS diagnoses were the Japanese original diagnosis, and they were previously diagnosed as “probable” or “undefined” CS using the expert consensus statement from the Heart Rhythm Society (HRS) in 2014 (10). However, data on prognosis of those clinical CS were still lacking in the large-scale study.
Therefore, we examined the long-term prognosis and clinical outcome of “histological” and “clinical” CS, diagnosed by JCS 2016, and try to examine the feasibility of applying this guideline.
Methods
Study population
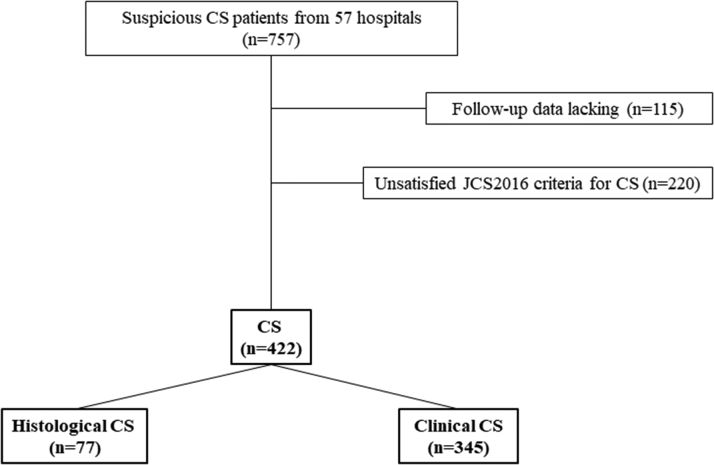
This was a multicenter retrospective cohort study based on a Japanese nationwide cohort survey on CS from 2014 to 2016 (UMIN000021299). Patients suspected of having CS were included and patients who had not satisfied the criteria for CS proposed by the JCS 2016 were excluded. Finally, data from 422 CS patients were analyzed (Table 1, Figure 1). The median follow-up period was 5 (interquartile range: 2-8) years. This study was approved by the institutional review committee of the National Cerebral and Cardiovascular Center (M26-016-5, June 4, 2014) and the individual hospitals. In this study, we analyzed anonymized data after patients’ agreement to treatment, and we applied the opt-out method to obtain informed consent by poster or leaflet that were approved by each institutional review committee. The study complied with the principles of the Declaration of Helsinki.
Table 1.
Difference Between Cardiac Sarcoidosis Diagnosis Categories According to JCS 2016 and HRS 2014
| Involved Organ of Sarcoidosis |
CS Diagnosis |
This Study |
||||
|---|---|---|---|---|---|---|
| Heart |
Other Organs |
JCS 2016 Guideline | HRS 2014 Statement | Total (N = 422) | ||
| Positive EMB Findings | Clinical Sign Manifestations | Positive Biopsy Findings | Clinical Sign Manifestations | |||
| 〇 | 〇 | |||||
| 〇 | 〇 | Histological CS | CS | 77 (18) | ||
| 〇 | ||||||
| 〇 | 〇 | Probable CS | 165 (39) | |||
| 〇 | 〇 | Clinical CS | ||||
| 〇 | Undefined | 180 (43) | ||||
Values are n (%).
CS = cardiac sarcoidosis; EMB = endomyocardial biopsy; HRS = Heart Rhythm Society; JCS = Japanese Circulation Society.
Figure 1.
Study Population
CS = cardiac sarcoidosis; JCS = Japanese Circulation Society.
Definition of histological and clinical CS in HRS 2014 and JCS 2016
Table 1 shows the details of the CS diagnosis category according to the current HRS 2014 and JCS 2016 guideline. If positive EMB findings were obtained, a histological diagnosis was easily made according to both the HRS 2014 and JCS 2016 guidelines. However, if a positive EMB finding was not obtained, a diagnosis of clinical CS was made based on biopsy results of samples obtained from other organs according to the HRS 2014 statement. The HRS 2014 criteria required positive biopsy findings in any organ; therefore, a diagnosis of clinical CS was made based on the combination of histological findings in other organs and cardiac manifestations that suggested “probable” CS. If a positive biopsy result was not obtained in other organs, patients were diagnosed with “undefined” CS based on the recommendations of the HRS 2014 statement. Conversely, the JCS 2016 guideline enabled a clinical diagnosis of CS to be made based on the clinical manifestation alone.
Components of the nationwide cohort survey
The components of the nationwide cohort survey consisted of 2 questionnaires that were used to determine the diagnosis, treatment, and clinical outcome of CS. All questions were directed to the hospitals and not to the patients. A total of 57 Japanese hospitals responded to this survey (see list of contributors in the Supplemental Appendix).
Components of the questionnaire used for making a diagnosis of CS
The survey included the examination findings from the CS diagnostic criteria of the Japanese Society of Sarcoidosis and Other Granulomatous diseases (JSSOG) in 2015 and JCS 2016. A detailed list of these items has been previously published (14). They include the following findings: histological biopsy of the heart or another organ, history of arrhythmia, electrocardiogram, echocardiogram), 67Ga scintigraphy, 18F-FDG PET of the heart and other organs, myocardial perfusion scintigraphy, gadolinium-enhanced cardiac magnetic resonance (CMR) results, chest radiograph, blood laboratory testing, and the BAL fluid test. Left ventricular ejection fraction (LVEF) and left ventricular (LV) septal wall thinning were evaluated by echocardiogram in this survey.
Clinical profile at the time of diagnosis and outcomes during the follow-up period
This survey also included components of treatment and follow-up data. The treatment data included the following items: corticosteroid therapy, drug therapy for arrhythmias or heart failure, cardiac implantable electronic device implantations (pacemakers, ICDs, CRT with pacemaker, CRT with defibrillator), and RFCA. Follow-up data included all-cause death and appropriate and inappropriate ICD therapies.
Study protocol
We compared the clinical characteristics at the time of CS diagnosis in the different diagnosis categories (histological CS and clinical CS according to the JCS 2016) and examined the prognosis and clinical factors associated with all-cause death and adverse events. Adverse events were defined as all-cause death, the use of appropriate ICD therapies, and heart transplantation. The effect of corticosteroids on LVEF was also examined in both groups according to the baseline LVEF. All-cause death and adverse events were also examined in patients with histological CS and clinical CS during each cardiac function (LVEF ≤35%, 35% < LVEF < 50%, LVEF ≥50%).
Statistical analysis
Statistical analyses were performed using JMP version 13 software (SAS Institute). Continuous variables are presented as the mean ± SD for variables with normal distribution and as median (interquartile range) for variables with skewed distribution. Categorical data are expressed as count and percentage. Differences among the groups were analyzed using the Mann-Whitney U test, t test for paired data, and chi-square test and Fisher’s exact test for unpaired data, as appropriate. Survival curves for all-cause death and adverse events were plotted using the Kaplan-Meier method, and all-cause death–free and adverse event–free survival was compared with the log-rank test. In this study, the follow-up start date for patients was the data of CS diagnosis and the follow-up end date was the date of all-cause death, appropriate ICD therapy uses, heart transplantation, or final visit to each institution. A univariate Cox proportional hazards model analysis was performed to assess the significant variables associated with the prediction of all-cause death and adverse events in CS patients. Variables with P values of <0.05 were entered into the final multivariate Cox proportional hazards model to identify the independent predictors of all-cause death and those of adverse events in CS patients. The Cox proportional model included a hazard ratio, P value, and 95% confidence intervals. All tests were 2-sided, and P values of <0.05 were considered statistically significant.
Results
Diagnosis of CS based on JCS 2016 and HRS 2014
Table 1 shows the difference in the diagnosed CS category between JCS 2016 and HRS 2014. Among the definite CS patients (n = 422), 77 (18%) patients had histological CS and 345 (82%) patients had clinical CS according to the JCS 2016 guideline. According to the HRS 2014 guideline, 77 (18%) patients had CS, 165 (39%) had probable CS, and 180 (43%) had undefined CS.
Baseline clinical characteristics of CS patients
The baseline clinical characteristics are shown in Table 2 and Figure 2. The mean age was 60 ± 13 years, and 68% of the patients were women. Regarding the initial cardiac arrhythmias at the time of CS diagnosis, the presence of AVB and VT was 41% and 18%, respectively. The mean LVEF was 49%, and the frequency of LVEF <50% and LVEF ≤35% was 48% and 23%, respectively. The frequency of LV septal wall thinning was 47%. 67Ga scintigraphy of the heart was performed in most patients to make a diagnosis of CS, and the positive rate of 67Ga scintigraphy was 67%. Conversely, in patients who underwent perfusion scintigraphy, CMR, and the BAL fluid test, the positive rates were considerably high (81%, 85%, and 76%, respectively).
Table 2.
Baseline Clinical Characteristics of Patients With Cardiac Sarcoidosis
| All Patients (N = 422) | Histological CS (n = 77) | Clinical CS (n = 345) | P Value | |
|---|---|---|---|---|
| Age, y | 60 ± 13 | 59 ± 11 | 61 ± 13 | 0.288 |
| Female | 288 (68) | 53 (69) | 235 (68) | 0.903 |
| UCG findings at diagnosis | ||||
| LVDd, mm | 53.8 ± 9.0 | 56.5 ± 9.7 | 53.2 ± 8.8 | 0.004 |
| LVDs, mm | 40.1 ± 11.6 | 44.9 ± 11.4 | 39.0 ± 11.4 | <0.001 |
| LVEF, % | 48.6 ± 15.9 | 40.0 ± 15.8 | 50.5 ± 15.3 | <0.001 |
| LVSWT | 197 (47) | 35 (46) | 162 (47) | 0.811 |
| UCG abnormality (including LVSWT) | 205 (49) | 34 (44) | 171 (50) | 0.391 |
| Abnormal uptake of 67Ga scintigraphy or 18FDG PET in the heart | 273/406 (67) | 49/74 (66) | 224/332 (67) | 0.835 |
| Blood flow defect on perfusion scintigraphy | 225/278 (81) | 44/51 (86) | 181/227 (80) | 0.283 |
| LGE in CMR | 184/216 (85) | 31/36 (86) | 153/180 (85) | 0.864 |
| Myocardial abnormality without epithelioid granulomas on endomyocardial biopsya | 106/245 (43) | 64/73 (88) | 42/172 (24) | <0.001 |
| Laboratory findings of sarcoidosis | ||||
| Bilateral hilar lymphadenopathy | 322/419 (77) | 34/76 (45) | 288/343 (84) | <0.001 |
| Serum ACE activity levels, IU/L | 18.6 ± 9.9 | 17.5 ± 11.3 | 18.8 ± 9.6 | 0.325 |
| Serum lysozyme levels, μg/mL | 11.9 ± 7.7 | 11.5 ± 9.4 | 12.0 ± 7.3 | 0.694 |
| Serum sIL-2R levels, U/mL | 821 ± 794 | 783 ± 867 | 828 ± 785 | 0.820 |
| Abnormal uptake of 67Ga citrate scintigraphy or 18FDG PET in any organ | 323/412 (78) | 41/74 (55) | 282/338 (83) | <0.001 |
| Abnormality in BAL fluid | 110/144 (76) | 4/8 (50) | 106/136 (78) | 0.071 |
| Therapy | ||||
| Corticosteroid therapy | 355 (84) | 67 (87) | 288 (83) | 0.443 |
| Maintenance dose of corticosteroids, mg/d | 7.5 ± 6.9 | 6.9 ± 3.9 | 7.6 ± 7.4 | 0.486 |
| β-blocker | 294 (70) | 67 (87) | 227 (66) | <0.001 |
| ACE inhibitor/ARB | 232 (55) | 45 (58) | 187 (54) | 0.499 |
| PM | 113 (27) | 13 (17) | 100 (29) | 0.030 |
| ICD | 69 (16) | 13 (17) | 56 (16) | 0.889 |
| CRT-P | 9 (2) | 3 (4) | 6 (2) | 0.236 |
| CRT-D | 69 (16) | 21 (27) | 48 (14) | 0.004 |
| RFCA | 37 (9) | 11 (14) | 26 (8) | 0.058 |
Values are mean ± SD, n (%), or n/N (%).
18F-FDG = 18F-fluorodeoxyglucose; 67Ga = gallium-67; ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; BAL = bronchoalveolar lavage; CMR = cardiac magnetic resonance imaging; CRT-D = cardiac resynchronization therapy with defibrillator; CRT-P = cardiac resynchronization therapy with pacemaker; CS = cardiac sarcoidosis; ICD = implantable cardioverter-defibrillator; LGE = late gadolinium enhancement; LVDd = left ventricular diastolic diameter; LVDs = left ventricular systolic diameter; LVEF = left ventricular ejection fraction; LVSWT = left ventricular septal wall thinning; PET = positron emission tomography; PM = pacemaker; RFCA = radiofrequency catheter ablation; sIL-2R = soluble interleukin-2 receptor; UCG = echocardiogram.
Myocardial abnormality without epithelioid granulomas on endomyocardial biopsy was defined as monocyte infiltration and moderate or severe myocardial interstitial fibrosis.
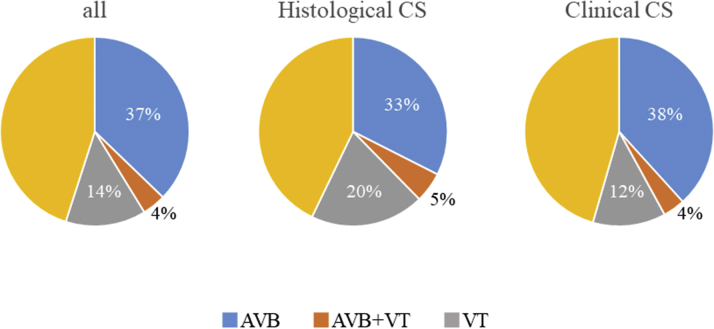
Figure 2.
Initial Cardiac Arrhythmias at the Time of CS Diagnosis
AVB = atrioventricular block; CS = cardiac sarcoidosis; VT = ventricular tachycardia.
Corticosteroids were used in 84% of the patients in this study, and the mean maintenance dose was 7.5 mg/d. A total of 138 patients underwent ICD or CRT with defibrillator implantation and 37 patients underwent RFCA for VT management during the follow-up period.
Clinical features, prognosis, and effect of corticosteroids on cardiac function in histological and clinical CS
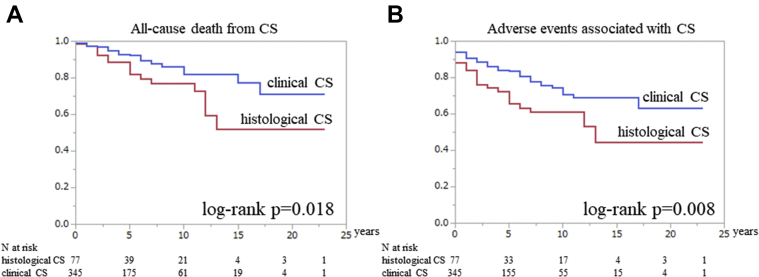
Table 2 and Figure 2 highlights the clinical features of histological and clinical CS. AVB or VT history at the time of making a diagnosis of CS was similar in the 2 groups (38% vs 42% [P = 0.482] and 25% vs 16% [P = 0.080], respectively). LVEF was significantly higher in the clinical CS group than in the histological CS group (P < 0.001). Kaplan-Meier analysis of all CS patients showed that all-cause death–free survival was 90% at 5 years and 81% at 10 years. Adverse event-free survival was 80% at 5 years and 69% at 10 years. The Kaplan-Meier curve revealed that clinical CS had a better prognosis than histological CS; however, the prognosis of clinical CS was still considerably poor (free from all-cause death: 92% vs 82% at 5 years and 82% vs 77% at 10 years [log-rank P = 0.018]; free from adverse events: 84% vs 66% at 5 years and 70% vs 61% at 10 years [log-rank P = 0.008]) (Figures 3A and 3B).
Figure 3.
Comparison of Prognosis Between Histological and Clinical CS Patients
The Kaplan-Meier curves reveal the frequency of (A) all-cause death–free and (B) adverse event–free survival between histological and clinical cardiac sarcoidosis (CS). The red line indicates histological CS, blue line indicates clinical CS.
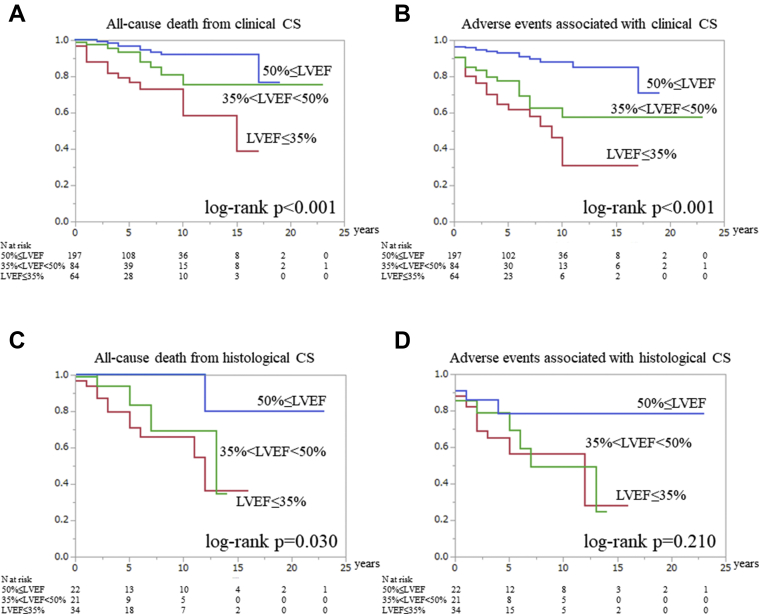
We also analyzed all-cause death and adverse events in the clinical CS patients according to the baseline LVEF. The Kaplan-Meier curves significantly differed (log-rank P < 0.001 and P < 0.001, respectively) (Figures 4A and 4B). In the Kaplan-Meier curve for all-cause death, an LVEF ≤35% had worse prognosis compared with an LVEF ≥50% (LVEF ≥50% vs 35% < LVEF <50%: log-rank P = 0.044, 35% < LVEF <50% vs LVEF ≤35%: log-rank P = 0.018; LVEF ≥50% vs LVEF ≤35%: log-rank P < 0.001) (Figure 4A). The Kaplan-Meier estimates for adverse events revealed that a 35% < LVEF <50% and LVEF ≤35% had a similar and poor prognosis compared with a LVEF ≥50% (LVEF ≥50% vs 35% < LVEF <50%: log-rank P < 0.001; 35% < LVEF ≤50% vs LVEF ≤35%: log-rank P = 0.112, LVEF ≥50% vs LVEF ≤35%: log-rank P < 0.001) (Figure 4B).
Figure 4.
The Prognosis of Clinical CS During Each Cardiac Function
The Kaplan-Meier curves reveal the frequency of (A) all-cause death–free and (B) adverse event–free survival in clinical cardiac sarcoidosis (CS) patients during each cardiac function. The Kaplan-Meier curves reveal the frequency of (C) all-cause death–free and (D) adverse event–free survival in histological CS patients during each cardiac function. The blue line indicates left ventricular ejection fraction (LVEF) ≥50%, the green line indicates 35% < LVEF <50%, and the red line indicates LVEF ≤35%).
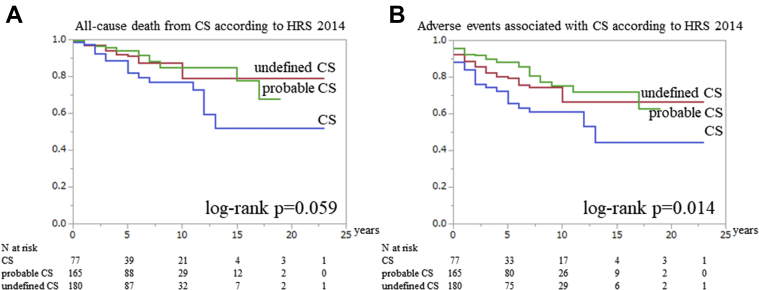
We analyzed all-cause death and adverse events in patients with histological, probable, and undefined CS who were diagnosed according to the HRS 2014 statement (Supplemental Table 1 highlights the clinical features of probable and undefined CS). The Kaplan-Meier curve showed that differences in all-cause death–free survival were not statistically significant (log-rank P = 0.059); however, an adverse event was observed more in histological CS than in the other categories (log-rank P = 0.014) (Figures 5A and 5B).
Figure 5.
The Prognosis Among the 3 Types of CS Classified According to HRS 2014
The Kaplan-Meier curves reveal the frequency of (A) all-cause death–free and (B) adverse event–free survival for the 3 types of cardiac sarcoidosis (CS) classified according to the Heart Rhythm Society (HRS) 2014 guideline. The blue line indicates CS, the green line indicates probable CS, and the red line indicates undefined CS.
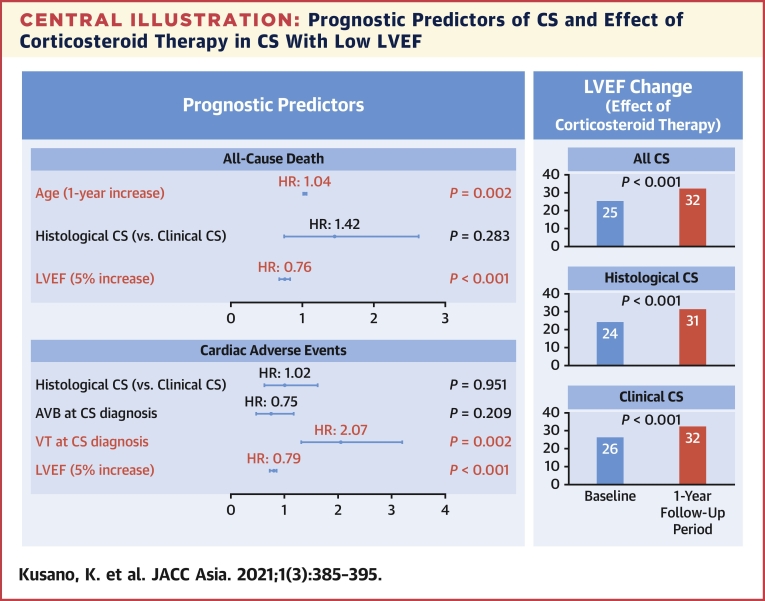
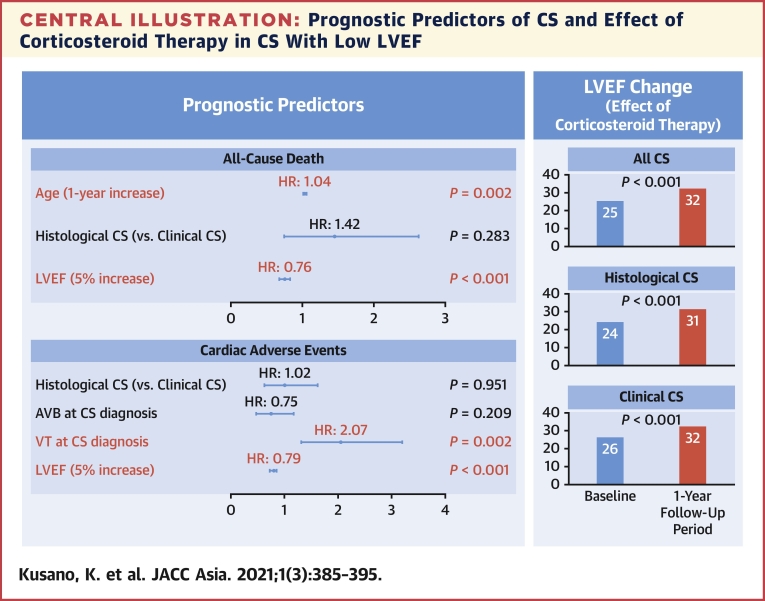
LV function at baseline and after 1 year of follow-up in CS patients who underwent corticosteroid therapy was as follows: LVEF in CS patients with normal or moderately impaired cardiac function (LVEF >35%) did not improve; however, the LVEF in CS patients (both histological and clinical diagnosis categories) with low cardiac function (LVEF ≤35%) improved significantly at the 1-year follow-up (histological CS: P = 0.001; clinical CS: P < 0.001) (Table 3).
Table 3.
LV Function at Baseline and After 1 Year of Follow-Up in CS Patients Who Underwent Corticosteroid Therapy
| Initial LV Function | Baseline (%) | 1-Year Follow-Up Period (%) | P Value |
|---|---|---|---|
| All CS | |||
| Normal (LVEF ≥50%), n = 106 | 60.9 ± 6.8 | 59.7 ± 12.5 | 0.865 |
| Moderate (50% > LVEF >35%), n = 63 | 43.0 ± 3.9 | 41.2 ± 10.4 | 0.910 |
| Low (LVEF ≤35%), n = 48 | 25.3 ± 6.5 | 32.0 ± 10.0 | <0.001 |
| Histological CS | |||
| Normal (LVEF ≥50%), n = 9 | 60.9 ± 6.7 | 51.7 ± 18.8 | 0.929 |
| Moderate (50% > LVEF >35%), n = 11 | 41.6 ± 4.1 | 33.7 ± 9.1 | 0.983 |
| Low (LVEF ≤35%), n = 19 | 24.1 ± 7.0 | 31.2 ± 11.1 | 0.001 |
| Clinical CS | |||
| Normal (LVEF ≥50%), n = 97 | 60.9 ± 6.9 | 60.4 ± 11.6 | 0.677 |
| Moderate (50% > LVEF >35%), n = 52 | 43.3 ± 3.8 | 42.8 ± 10.0 | 0.649 |
| Low (LVEF ≤35%), n = 29 | 26.1 ± 6.1 | 32.4 ± 9.3 | <0.001 |
Values are mean ± SD.
LV = left ventricle; other abbreviations as in Table 2.
Prognostic factors of histological and clinical CS
The prognostic factors for all-cause death and adverse events in the CS patients are shown in Tables 4 and 5, respectively. Increasing age and low LVEF values were independent predictors of all-cause death (P = 0.002 and P < 0.001, respectively), and VT presence and low LVEF were independent predictors of adverse events (P = 0.002 and P < 0.001, respectively). A low EF at the time of CS diagnosis was the most important prognostic factor in CS patients.
Table 4.
The Predictors of All-Cause Death in CS Patients by a Cox Proportional Hazards Model
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| HR | P Value | 95% CI | HR | P Value | 95% CI | |
| Age (1-y increase) | 1.04 | <0.001 | 1.02-1.06 | 1.04 | 0.002 | 1.01-1.06 |
| Female (vs male) | 0.80 | 0.459 | 0.45-1.46 | |||
| Histological CS (vs clinical CS) | 2.01 | 0.029 | 1.08-3.59 | 1.42 | 0.283 | 0.74-2.62 |
| AVB at CS diagnosis | 0.62 | 0.106 | 0.33-1.10 | |||
| VT at CS diagnosis | 1.10 | 0.789 | 0.53-2.07 | |||
| LVEF (5% increase) | 0.73 | <0.001 | 0.66-0.80 | 0.76 | <0.001 | 0.68-0.83 |
| Abnormal uptake of 67Ga scintigraphy or 18F-FDG PET in the heart | 0.85 | 0.603 | 0.47-1.58 | |||
AVB = atrioventricular block; CI = confidence interval; VT = ventricular tachycardia; other abbreviations as in Table 2.
Table 5.
The Predictors of Cardiac Adverse Events in CS Patients by a Cox Proportional Hazards Model
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| HR | P Value | 95% CI | HR | P Value | 95% CI | |
| Age (1-y increase) | 1.01 | 0.103 | 1.00-1.03 | |||
| Female (vs male) | 0.81 | 0.329 | 0.53-1.25 | |||
| Histological CS (vs clinical CS) | 1.81 | 0.015 | 1.13-2.82 | 1.02 | 0.951 | 0.62-1.62 |
| AVB at CS diagnosis | 0.58 | 0.014 | 0.37-0.90 | 0.75 | 0.209 | 0.47-1.17 |
| VT at CS diagnosis | 2.76 | <0.001 | 1.77-4.21 | 2.07 | 0.002 | 1.31-3.20 |
| LVEF (5% increase) | 0.78 | <0.001 | 0.73-0.84 | 0.79 | <0.001 | 0.73-0.85 |
| Abnormal uptake of 67Ga scintigraphy or 18F-FDG PET in the heart | 1.39 | 0.160 | 0.88-2.26 | |||
Discussion
Major findings
Our findings demonstrated that clinical CS had a better prognosis than EMB-positive histological CS, but a multivariate analysis revealed that the baseline cardiac function and a history of ventricular arrhythmias were related to their prognosis and clinical outcomes, regardless of CS diagnosis category (histological or clinical). In addition, the response of corticosteroid was similar in both histological and clinical CS patients. These data indicated that prophylactic ICD implantation with immunosuppressive therapy for clinical CS was important in CS patients with reduced LV function, and the diagnosis of “clinical CS” according to the JCS 2016 guideline could be applied in the clinical practice (Central Illustration).
Central Illustration.
Prognostic Predictors of CS and Effect of Corticosteroid Therapy in CS With Low LVEF
AVB = atrioventricular block; CI = confidence interval; CS = cardiac sarcoidosis; HR = hazard ratio; LVEF = left ventricular ejection fraction; VT = ventricular tachycardia.
CS diagnosis: Trends over time
There are currently 4 international guidelines or expert consensus documents for the diagnosis and treatment of CS. The first guideline was issued in 1992 from Japan and was revised in 2006 and 2015 (JSSOG 2015) (14, 15, 16). The second one was published by the World Association of Sarcoidosis and Other Granulomatous Disorders in 1999 as a sarcoidosis assessment instrument developed by the steering committee of the ACCESS (A Case Controlled Etiologic Study of Sarcoidosis) trial and revised in 2014 (11). The third one was the HRS expert consensus document from the United States published in 2014 (10). The last one was the Japanese guideline on the diagnosis and treatment of CS (JCS 2016) (14). The critical difference in the diagnosis of CS between the Japanese guidelines (JSSOG 2015/JCS 2016) and others (World Association of Sarcoidosis and Other Granulomatous Disorders and HRS 2014) is that the JSSOG 2015 and JCS 2016 guidelines do not require any histological proof of EMB because of the low rate of positive biopsy results in the heart (12). This concept is a Japan-original one and is quite unique, but the feasibility of this Japanese guideline was not examined previously. There is one study showing the diagnosis of CS based on JCS 2016 in patients treated with corticosteroid as CS (17). According to this study, the diagnostic accuracy of JCS 2016 is as follows: sensitivity 81%, specificity 87%, positive predictive value 76%, and negative predictive value 90%. Because there are no accurate data using autopsy cases still now and the frequency of positive endomyocardial biopsies findings is low (12), further study is needed to verify the accuracy of JCS 2016.
Prognosis of CS
In this cohort, the 5- and 10-year mortality rates were approximately 10% and 20%, respectively. A previous report of Smedema et al in 2005 (18) revealed very poor prognosis (the mortality of CS was 25% at 15 months). In contrast, recent studies demonstrated improving prognoses. Kandolin et al (19) reported that the 5- and 10-year mortality rates were approximately 20% and 30%, respectively, in 2015. Kaj et al (20) reported that the 5- and 10-year mortality rates were approximately 15% and 25%, respectively. Kandolin et al (19) included a considerable number of scheduled transplantations in the outcome of their analysis, and Kaj et al (20) included cases detected at autopsy without corticosteroid therapy; thus, the prognosis of CS in their reports might have also been overestimated. Thus, our mortality results were consistent with those of previous reports. The significant improvement in mortality may be related to the various recent therapeutic advances for the management of CS, such as drugs, pacemakers, ICDs, CRT, and RFCA.
Clinical profile and prognosis of histological and clinical CS
In this cohort, clinical profile including age, sex, and abnormal uptake of 67Ga scintigraphy or 18FDG PET in the heart was similar in histological and clinical CS groups. Furthermore, AVB or VT history was similar in the 2 groups. In contrast, LVEF of clinical CS was significantly higher than that of histological CS and clinical CS had a better prognosis than histological CS. Dilated cardiomyopathy has a better prognosis (21) and lower frequency of appropriate ICD therapy (7) compared to CS. If dilated cardiomyopathy was included in clinical CS, there should be a significant difference in the diagnostic category. However, multivariate analysis revealed that baseline cardiac function was the most important determinant of prognosis but not of the diagnostic category (histological or clinical CS). This finding suggests that the clinical CS diagnosis made at an earlier stage and early commencement of treatment may lead to better results. The frequency of positive findings from endomyocardial biopsies is usually about 20% in CS patients (12) and is very low in CS patients with preserved LVEF (22,23). In Japan, EMBs are commonly performed in the right ventricle (RV); thus, negative biopsy results in patients in the clinical CS category indicate that less disease extension occurs in clinical CS patients. One report using PET evaluation revealed that all patients with abnormal cardiac PET findings had LV abnormalities and that some patients with RV involvement had a higher event rate; thus, the presence of RV involvement may be a marker for disease severity (24). These findings suggest that histological CS may have LV and RV involvement at an advanced stage of CS, and that clinical CS may only have LV involvement at a relatively early stage. Accordingly, we conclude that the clinical diagnosis of CS according to the JCS 2016 guideline can be applied in the clinical setting and may be valuable in selecting the appropriate CS therapy.
Effect of corticosteroid therapy
In this cohort, a significant improvement in LVEF during the 1-year follow-up period after corticosteroid therapy was observed in CS patients with the lowest cardiac function (LVEF ≤35%). Several previous studies have reported that corticosteroid therapy prevented a worsening of LVEF in CS patients with normal cardiac function (25, 26, 27) or improved LVEF in patients with an LVEF <35% (19); these findings are similar to those of our study. Furthermore, these findings suggest that corticosteroid therapy is a definitively mainstream therapy for CS, even in the advanced stages of CS.
The maintenance dose of corticosteroid was 7.5 mg/d in this study. Although there is no established protocol for corticosteroid therapy in CS (28), the JCS 2016 guideline recommends lifelong corticosteroid use at a dose of 5 to 10 mg/d for the management of CS. However, further studies are required to establish a standardized maintenance dose of corticosteroid.
Study Limitations
This multicenter retrospective cohort study was based on a nationwide cohort survey; thus, there were several limitations. First, EMB was not performed in all cases. Consequently, several histological CS patients might have been erroneously included as having clinical CS in this survey. Further studies on clinical CS patients who undergo EMB are required. Second, we did not examine the clinical outcome of corticosteroid therapy in detail. There was no definition that could be used to estimate the effect of the corticosteroid therapy and management protocol, including the maintenance dose of corticosteroids. Therefore, evaluation of the clinical outcomes of corticosteroid therapy depended on the opinion of each treating physician. Third, the good prognosis and LVEF improvement may be related to the various recent therapeutic advances, such as drugs and nonpharmacological therapies, but we could not evaluate the effect of individual treatments in this study. This topic is important and should be estimated in future. Finally, the frequency of performing CMR was low (51%), and CMR was excluded from the Cox proportional hazards model in this study. CMR is an important examination in the diagnosis of CS and an evaluation of this topic is needed in future studies.
Conclusions
In clinical CS according to Japanese guideline, prophylactic implantable cardioverter-defibrillator and immunosuppressive therapy are important in patients with low LVEF or VT history, similar to histological CS.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Clinical CS according to Japanese guidelines could be applied in the clinical practice. Corticosteroid therapy for clinical CS with low LVEF is effective and important. Corticosteroid therapy could be recommended not only for clinical CS, but also for histological CS.
TRANSLATIONAL OUTLOOK: Corticosteroid therapy should be considered for CS patients at all stages. However, there was no definition that could be used to estimate the effect of the corticosteroid therapy and management protocol, including the maintenance dose of corticosteroids. The Japanese guideline recommends lifelong corticosteroid use at a dose of 5 to 10 mg/d for the management of CS. However, further studies are required to establish a standardized maintenance dose of corticosteroid.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank all of their colleagues in the 56 hospitals that participated in this study. They also thank Mrs. Yoshiko Takenobu for the data collection.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a list of contributing institutions and a supplemental table, please see the online version of this paper.
Appendix
References
- 1.Kusano K.F., Satomi K. Diagnosis and treatment of cardiac sarcoidosis. Heart. 2016;102:184–190. doi: 10.1136/heartjnl-2015-307877. [DOI] [PubMed] [Google Scholar]
- 2.Roberts W.C., McAllister H.A., Jr., Ferrans V.J. Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11) Am J Med. 1977;63:86–108. doi: 10.1016/0002-9343(77)90121-8. [DOI] [PubMed] [Google Scholar]
- 3.Yazaki Y., Isobe M., Hiroe M., et al. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol. 2001;88:1006–1010. doi: 10.1016/s0002-9149(01)01978-6. [DOI] [PubMed] [Google Scholar]
- 4.Sekiguchi M., Hiroe M., Take M., Hirosawa K. Clinical and histopathological profile of sarcoidosis of the heart and acute idiopathic myocarditis. Concepts through a study employing endomyocardial biopsy. II. Myocarditis. Jpn Circ J. 1980;44:264–273. doi: 10.1253/jcj.44.264. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi K., Okumura Y., Watanabe I., et al. A case of cardiac sarcoidosis presenting with double tachycardia. J Arrhythm. 2015;31:58–59. doi: 10.1016/j.joa.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banba K., Kusano K.F., Nakamura K., et al. Relationship between arrhythmogenesis and disease activity in cardiac sarcoidosis. Heart Rhythm. 2007;4:1292–1299. doi: 10.1016/j.hrthm.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Takaya Y., Kusano K., Nishii N., Nakamura K., Ito H. Early and frequent defibrillator discharge in patients with cardiac sarcoidosis compared with patients with idiopathic dilated cardiomyopathy. Int J Cardiol. 2017;240:302–306. doi: 10.1016/j.ijcard.2017.04.044. [DOI] [PubMed] [Google Scholar]
- 8.Takaya Y., Kusano K.F., Nakamura K., et al. Reduction of myocardial inflammation with steroid is not necessarily associated with improvement in left ventricular function in patients with cardiac sarcoidosis: predictors of functional improvement. Int J Cardiol. 2014;176:522–525. doi: 10.1016/j.ijcard.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 9.Grutters J.C., van den Bosch J.M. Corticosteroid treatment in sarcoidosis. Eur Respir J. 2006;28:627–636. doi: 10.1183/09031936.06.00105805. [DOI] [PubMed] [Google Scholar]
- 10.Birnie D.H., Sauer W.H., Bogun F., et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–1323. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 11.Judson M.A., Costabel U., Drent M., et al. The WASOG Sarcoidosis Organ Assessment Instrument: an update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31:19–27. [PubMed] [Google Scholar]
- 12.Uemura A., Morimoto S., Hiramitsu S., Kato Y., Ito T., Hishida H. Histologic diagnostic rate of cardiac sarcoidosis: evaluation of endomyocardial biopsies. Am Heart J. 1999;138:299–302. doi: 10.1016/s0002-8703(99)70115-8. [DOI] [PubMed] [Google Scholar]
- 13.JCS Joint Working Group Guidelines for diagnosis and treatment of myocarditis (JCS 2009): digest version. Circ J. 2011;75:734–743. doi: 10.1253/circj.cj-88-0008. [DOI] [PubMed] [Google Scholar]
- 14.Terasaki F., Azuma A., Anzai T., et al. JCS 2016 Guideline on Diagnosis and Treatment of Cardiac Sarcoidosis-Digest Version. Circ J. 2019;83:2329–2388. doi: 10.1253/circj.CJ-19-0508. [DOI] [PubMed] [Google Scholar]
- 15.The Japan Society of Sarcoidosis and Other Granulomatous Disorders, the Diffuse Pulmonary Study Group of the Health and Labor Sciences Research Grant-supported Rare/Intractable Disease Project, et al. [Committee for revision of the diagnostic standard for sarcoidosis. Diagnostic standard and guideline for sarcoidosis - 2006] Jpn J Sarcoidosis Other Granulomat Disord. 2007;27:89–102. [Google Scholar]
- 16.Terasaki F., Yoshinaga K. New guidelines for diagnosis of cardiac sarcoidosis in Japan. Ann Nucl Cardiol. 2017;3:42–45. [Google Scholar]
- 17.Kawai H., Sarai M., Kato Y., et al. Diagnosis of isolated cardiac sarcoidosis based on new guidelines. ESC Heart Fail. 2020;7:2662–2671. doi: 10.1002/ehf2.12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smedema J.P., Snoep G., van Kroonenburgh M.P., et al. Cardiac involvement in patients with pulmonary sarcoidosis assessed at two university medical centers in the Netherlands. Chest. 2005;128:30–35. doi: 10.1378/chest.128.1.30. [DOI] [PubMed] [Google Scholar]
- 19.Kandolin R., Lehtonen J., Airaksinen J., et al. Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation. 2015;131:624–632. doi: 10.1161/CIRCULATIONAHA.114.011522. [DOI] [PubMed] [Google Scholar]
- 20.Ekstrom K., Lehtonen J., Nordenswan H.K., et al. Sudden death in cardiac sarcoidosis: an analysis of nationwide clinical and cause-of-death registries. Eur Heart J. 2019;40:3121–3128. doi: 10.1093/eurheartj/ehz428. [DOI] [PubMed] [Google Scholar]
- 21.Yazaki Y., Isobe M., Hiramitsu S., et al. Comparison of clinical features and prognosis of cardiac sarcoidosis and idiopathic dilated cardiomyopathy. Am J Cardiol. 1998;82:537–540. doi: 10.1016/s0002-9149(98)00377-4. [DOI] [PubMed] [Google Scholar]
- 22.Ardehali H., Howard D.L., Hariri A., et al. A positive endomyocardial biopsy result for sarcoid is associated with poor prognosis in patients with initially unexplained cardiomyopathy. Am Heart J. 2005;150:459–463. doi: 10.1016/j.ahj.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Morimoto S., Kato S., Hiramitsu S., et al. Narrowing of the left ventricular cavity associated with transient ventricular wall thickening reduces stroke volume in patients with acute myocarditis. Circ J. 2003;67:490–494. doi: 10.1253/circj.67.490. [DOI] [PubMed] [Google Scholar]
- 24.Blankstein R., Osborne M., Naya M., et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63:329–336. doi: 10.1016/j.jacc.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiu C.Z., Nakatani S., Zhang G., et al. Prevention of left ventricular remodeling by long-term corticosteroid therapy in patients with cardiac sarcoidosis. Am J Cardiol. 2005;95:143–146. doi: 10.1016/j.amjcard.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 26.Kato Y., Morimoto S., Uemura A., Hiramitsu S., Ito T., Hishida H. Efficacy of corticosteroids in sarcoidosis presenting with atrioventricular block. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20:133–137. [PubMed] [Google Scholar]
- 27.Yodogawa K., Seino Y., Ohara T., Takayama H., Katoh T., Mizuno K. Effect of corticosteroid therapy on ventricular arrhythmias in patients with cardiac sarcoidosis. Ann Noninvasive Electrocardiol. 2011;16:140–147. doi: 10.1111/j.1542-474X.2011.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bussinguer M., Danielian A., Sharma O.P. Cardiac sarcoidosis: diagnosis and management. Curr Treat Options Cardiovasc Med. 2012;14:652–664. doi: 10.1007/s11936-012-0208-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.