Abstract
Background
Aortic regurgitation (AR) is a common comorbidity in patients with aortic stenosis (AS), but coexisting AR has often been excluded from major clinical studies on AS. The impact of coexisting AR on the natural history of AS has not been well-described.
Objectives
The authors compared clinical outcomes in medically managed patients with moderate-to-severe AS with or without coexisting AR.
Methods
Consecutive patients (N = 1,188) with index echocardiographic diagnosis of moderate-to-severe AS (aortic valve area <1.5 cm2) were studied. All patients were medically managed and were divided into those with coexisting AR (at least moderate severity) and those without. Adverse composite clinical outcomes were defined as mortality or admissions for congestive cardiac failure on subsequent follow-up. The authors compared differences in clinical profile and outcomes between the groups.
Results
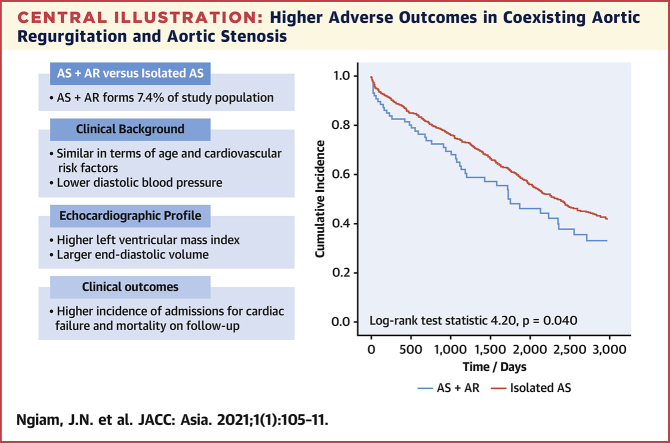
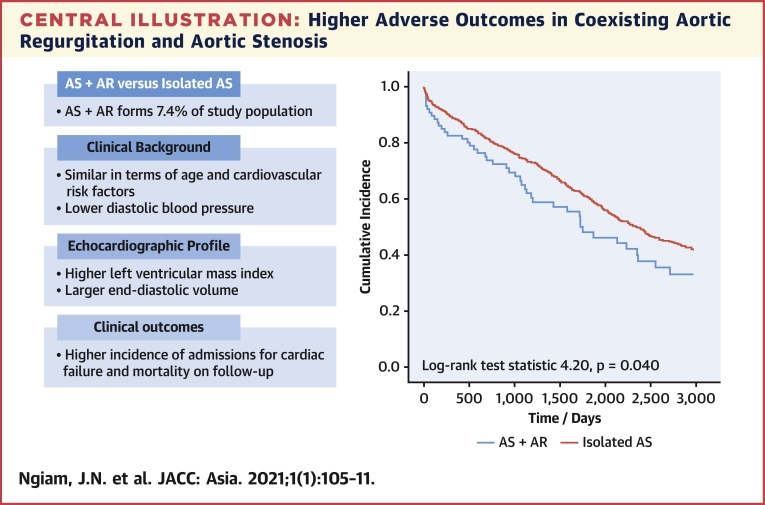
There were 88 patients (7.4%) with coexisting AR and AS. These patients did not differ significantly in age, but had lower body mass index (22.9 ± 3.8 vs 25.3 ± 5.1 kg/m2), lower diastolic blood pressure (68.7 ± 10.7 vs 72.2 ± 12.3 mm Hg), larger end-diastolic volume index (68.8 ± 18.8 vs 60.4 ± 17.8 mL/m2) and larger left ventricular mass index (118.6 ± 36.4 vs 108.9 ± 33.1 g/m2). The prevalence of cardiovascular risk factors did not differ significantly. Coexisting AR was associated with increased incidence of adverse outcomes (log-rank 4.20; P = 0.040). On multivariable Cox regression, coexisting AR remained independently associated with adverse outcomes (HR: 1.36; 95% CI: 1.02-1.82) after adjusting for age, AS severity, left ventricular ejection fraction, and year of study.
Conclusions
In patients with AS, coexisting AR was associated with changes in echocardiographic profile and adverse outcomes.
Key Words: aortic regurgitation, aortic stenosis, clinical outcomes
Abbreviations and Acronyms: AR, aortic regurgitation; AS, aortic stenosis; AVA, aortic valve area; AVR, aortic valve replacement; LV, left ventricle/ventricular; LVEF, left ventricular ejection fraction; TAVR, transcatheter aortic valve replacement
Central Illustration
In the context of patients with moderate-to-severe aortic stenosis (AS), the presence of coexisting aortic regurgitation (AR) may be an important, but under-recognized, comorbidity (1). The underlying etiology for the development of AS, such as congenital bicuspid aortic valves or calcific degenerative aortic valves, may also predispose patients to the development of AR (2). In patients with mixed aortic valve disease with associated symptoms, aortic valve replacement (AVR) has conventionally been the mainstay of therapy (2). Many studies have begun to evaluate the predictors of long-term surgical and functional outcomes in patients after AVR. Preoperatively, clinical profile such as age, presence of heart failure, coronary artery disease, as well as echocardiographic parameters including the valve gradient, left ventricular (LV) ejection fraction (LVEF), and presence of LV hypertrophy may influence surgical outcomes (3,4).
More recently, it has been demonstrated that the preoperative presence of AR was associated with adverse postoperative outcomes in patients undergoing surgical AVR for severe AS, with less improvement in LV function and functional capacity (1,5,6). Transcatheter AVR (TAVR) has been gaining popularity for management of significant AS (7). It is possible that significant AR would not have an impact on the prognosis of patients who had TAVR for severe AS (8,9).
In many large clinical trials on AS, the presence of coexisting AR was an exclusion criterion (10,11). Therefore, the prevalence, natural history, and clinical outcomes of patients with AS and coexisting AR remained poorly understood. A retrospective analysis of 306 patients with medically managed severe AS found 24% of patients (n = 74) had coexisting AR (12). The presence of AR was associated with adverse clinical outcomes on subsequent follow-up of these subjects. We aimed to further investigate the impact of coexisting AR on the echocardiographic profile and clinical outcomes in patients with medically managed moderate-to-severe AS.
Methods
A total of 1,188 consecutive patients with an index echocardiographic diagnosis of moderate-to-severe AS (aortic valve area [AVA] <1.5 cm2) and preserved LVEF (>50%) from a single tertiary center from years 2001-2015 were examined. They were divided into patients with or without coexisting AR (of at least moderate severity). The diagnosis and classification of AS and AR, as well as other echocardiographic parameters studied, were made in accordance to guidelines of the American Society of Echocardiography/European Association of Cardiovascular Imaging (13, 14, 15).
Only the index echocardiographic study was considered for patients who had multiple echocardiographic studies during the study period. Patients with polyvalvular disease of at least moderate severity (including mitral regurgitation) were excluded from the study. We exclusively examined patients that were medically managed, and those patients who underwent valve replacement (transcatheter or surgical) were excluded from analysis. For patients in whom valve replacement had been indicated, the patients had either declined the procedure or had been deemed to be medically unfit for the procedure.
For each patient examined, clinical and echocardiographic parameters at baseline were collected. Patients were subsequently followed up prospectively for at least 3 years from the index echocardiographic study for clinical outcomes in the form of all-cause mortality or admissions for congestive cardiac failure. The adverse composite clinical outcome was defined as either admission for congestive cardiac failure or all-cause mortality at follow-up.
We divided the study population into 2 groups: those with and those without coexisting AR. The baseline characteristics and echocardiographic profile were then compared. Univariate analyses were employed, including Student’s t-tests to compare continuous variables and Pearson’s chi-square test (or Fisher’s exact test where appropriate) for categorical variables. We examined the association of each parameter (including clinical parameters [age, body mass index, blood pressure, sex, past medical history] and echocardiographic parameters [left atrial and ventricular dimensions, and transaortic mean pressure gradient, velocity, and valve area]) with adverse clinical outcomes on univariate Cox regression. Important parameters that had significant association on univariate analyses (P < 0.05) and were not collinear were subsequently fit into a multivariable model. A multivariable Cox regression model was subsequently constructed to evaluate parameters independently associated with adverse clinical outcomes. We adjusted for age, AS severity, and LVEF and year of echocardiographic study in the multivariable model. Kaplan-Meier survival curves (time-to-event analysis) were also constructed to compare adverse composite clinical outcomes between the 2 groups, with the log-rank test statistic being calculated.
Subsequently, a subgroup cohort matched in a 1:1 ratio of patients with coexisting AR to non-AR was derived, and its characteristics were examined. Multivariable Cox regression was employed to examine parameters associated with adverse clinical outcomes. A P value of <0.05 was considered significant. All data analysis was performed on SPSS software version 20.0 (SPSS). This study was approved by the National Healthcare Group Domain Specific Review Board (Singapore) before the conduct of the study.
Results
Of the 1,188 consecutive patients studied with moderate-to-severe AS on medical therapy, 7.4% (n = 88) had coexisting moderate AR. The patients with coexisting AR were similar in age (70.7 ± 14.8 vs 72.7 ± 12.5 years; P = 0.167), sex (45.4% vs 37.9% male; P = 0.303) but had lower body mass index (22.9 ± 3.8 vs 25.3 ± 5.1 kg/m2; P < 0.001) compared with those with isolated AS. Although systolic blood pressure was not significantly different (141.5 ± 25.5 vs 140.5 ± 24.4 mm Hg; P = 0.704), the diastolic blood pressure was slightly lower in the group with coexisting AR (68.7 ± 10.7 vs 72.2 ± 12.3 mm Hg; P = 0.009). There were no significant differences in the prevalence of hypertension (61.4% vs 66.0%), diabetes mellitus (31.8% vs 35.2%), and hyperlipidemia (11.4% vs 12.1%) in the group with coexisting AR compared with isolated AS (Table 1).
Table 1.
Baseline Clinical and Echocardiographic Profiles of Moderate-to-Severe AS in Patients With or Without AR
| Both AS and AR (n = 88) | Isolated AS (n = 1,100) | Mean Difference OR (95% CI) | P Value | |
|---|---|---|---|---|
| Age, y | 70.7 ± 14.8 | 72.7 ± 12.5 | −2.0 (−4.7 to 0.8) | 0.167 |
| Body mass index, g/m2 | 22.9 ± 3.8 | 25.3 ± 5.1 | −2.4 (−3.5 to −1.3) | <0.001 |
| Systolic blood pressure, mm Hg | 141.5 ± 25.5 | 140.5 ± 24.4 | 1.0 (−4.3 to 6.4) | 0.704 |
| Diastolic blood pressure, mm Hg | 68.7 ± 10.7 | 72.2 ± 12.3 | −3.5 (−6.1 to −0.9) | 0.009 |
| Male | 45.4 | 37.9 | 1.34 (0.76 to 2.36) | 0.303 |
| Hypertension | 61.4 | 66.0 | 0.82 (0.43 to 1.55) | 0.538 |
| Diabetes mellitus | 31.8 | 35.2 | 0.86 (0.44 to 1.67) | 0.653 |
| Hyperlipidemia | 50.0 | 50.4 | 0.99 (0.53 to 1.84) | 0.963 |
| Atrial fibrillation | 11.4 | 12.1 | 0.93 (0.35 to 2.48) | 0.891 |
| Ischemic heart disease | 29.5 | 28.1 | 1.07 (0.54 to 2.13) | 0.837 |
| Left ventricular internal diameter in diastole, mm | 47.7 ± 6.2 | 45.8 ± 5.8 | 2.0 (0.7 to 3.2) | 0.003 |
| End-diastolic volume index, mL/m2 | 68.8 ± 18.8 | 60.4 ± 17.8 | 8.3 (4.5 to 12.2) | <0.001 |
| Stroke volume index, mL/m2 | 46.3 ± 12.1 | 40.4 ± 11.6 | 5.9 (3.4 to 8.4) | <0.001 |
| Left ventricular ejection fraction, % | 68.0 ± 6.8 | 67.4 ± 7.4 | 0.6 (−1.1 to 2.2) | 0.508 |
| Left ventricular mass index, g/m2 | 118.6 ± 36.4 | 108.9 ± 33.1 | 9.8 (2.3 to 17.2) | 0.010 |
| Left atrial diameter, mm | 41.6 ± 8.2 | 40.8 ± 8.4 | 0.8 (−1.0 to 2.6) | 0.380 |
| Peak transaortic velocity, cm/s | 348.2 ± 88.5 | 302.6 ± 88.2 | 45.6 (26.5 to 64.8) | <0.001 |
| Transaortic mean pressure gradient, mm Hg | 29.2 ± 16.7 | 23.5 ± 19.6 | 5.7 (2.2 to 9.9) | 0.009 |
| Aortic valve area, cm2 | 1.03 ± 0.28 | 1.09 ± 0.28 | −0.1 (−0.11 to 0.01) | 0.060 |
Values are mean ± SD or % unless otherwise indicated. Moderate-to-severe aortic stenosis (AS) is defined as aortic valve area <1.5 cm2.
AR = aortic regurgitation.
In terms of echocardiographic profile, the patients with coexisting AR had larger LV cavities, as evidenced by a larger LV internal diameter in diastole (47.7 ± 6.2 vs 45.8 ± 5.8 mm; P = 0.003) and higher end-diastolic volume index (68.8 ± 18.8 vs 60.4 ± 17.8 mL/m2; P < 0.001). Stroke volume index was higher in the group with coexisting AR (46.3 ± 12.1 vs 40.4 ± 11.6 mL/m2; P < 0.001), although the LVEF was comparable (68.0% ± 36.4% vs 67.4% ± 7.4%). LV mass was higher in patients with coexisting AR (118.6 ± 36.4 vs 108.9 ± 33.1 g/m2; P = 0.010), although there was no significant difference in the left atrial diameter or prevalence of atrial fibrillation (Table 1). In terms of aortic valve stenosis severity, there was a higher mean pressure gradient in the group with coexisting AR (29.2 ± 16.7 vs 23.5 ± 19.6 mm Hg; P = 0.009), but the AVA did not differ significantly (1.03 ± 0.28 vs 1.09 ± 0.28 cm2; P = 0.060) between the groups.
Composite adverse clinical outcomes comprised of either admission for congestive cardiac failure or all-cause mortality. On subsequent follow-up for at least 3 years after the index echocardiographic study, patients with coexisting AR had significantly poorer clinical outcomes compared with those with isolated AS (Central Illustration) (Kaplan-Meier log-rank statistic 4.20; P = 0.040). Each parameter was examined for its association with adverse outcomes on univariate Cox regression. Older age (unadjusted HR: 1.03; 95% CI: 1.02-1.04; P < 0.001), smaller AVA (unadjusted HR: 0.48; 95% CI: 0.37-0.63; P < 0.001), and reduced LVEF (unadjusted HR: 0.98; 95% CI: 0.97-0.99; P = 0.002) were associated with adverse outcomes (Table 2). These parameters (age, AVA, LVEF, and presence of coexisting AR) and year of echocardiographic study were fit into a multivariable Cox regression model. The multivariable model demonstrated that coexisting AR remained independently associated with an increased incidence of adverse clinical outcomes (adjusted HR: 1.36; 95% CI: 1.02-1.82; P = 0.036) (Table 3).
Central Illustration.
Higher Adverse Outcomes in Coexisting Aortic Regurgitation and Aortic Stenosis
In retrospective cohort of patients with index echocardiographic diagnosis of moderate-to-severe aortic stenosis (AS; aortic valve area <1.5 cm2), patients with coexisting aortic regurgitation (AR) demonstrated increased incidence of adverse composite clinical outcomes (admissions for congestive cardiac failure or mortality) compared with patients with isolated aortic stenosis (Kaplan-Meier log-rank test statistic 4.20; P = 0.040) on subsequent follow-up.
Table 2.
Univariate Cox Regression of Adverse Clinical Outcomes at Follow-Up
| Unadjusted HR (95% CI) | P Value | |
|---|---|---|
| Age, y | 1.03 (1.02–1.04) | <0.001 |
| Body mass index, g/m2 | 0.98 (0.96–0.99) | 0.009 |
| Systolic blood pressure, mm Hg | 0.99 (0.99–1.01) | 0.158 |
| Diastolic blood pressure, mm Hg | 0.98 (0.97–0.99) | <0.001 |
| Male | 1.22 (1.04–1.42) | 0.016 |
| Hypertension | 1.05 (0.88–1.26) | 0.560 |
| Diabetes mellitus | 1.08 (0.92–1.27) | 0.925 |
| Hyperlipidemia | 1.02 (0.87–1.19) | 0.847 |
| Atrial fibrillation | 1.28 (1.03–1.59) | 0.027 |
| Ischemic heart disease | 1.29 (1.10–1.52) | 0.002 |
| Left ventricular internal diameter in diastole, mm | 1.01 (0.99–1.01) | 0.897 |
| End-diastolic volume index, mL/m2 | 1.01 (0.99–1.01) | 0.236 |
| Stroke volume index, mL/m2 | 0.99 (0.99–1.01) | 0.865 |
| Left ventricular ejection fraction, % | 0.98 (0.97–0.99) | 0.002 |
| Left ventricular mass index, g/m2 | 1.01 (1.00–1.01) | <0.001 |
| Left atrial diameter, mm | 1.03 (1.02–1.04) | <0.001 |
| Peak transaortic velocity, cm/s | 1.01 (1.00–1.01) | <0.001 |
| Transaortic mean pressure gradient, mm Hg | 1.01 (1.01–1.02) | <0.001 |
| Aortic valve area, cm2 | 0.48 (0.37–0.63) | <0.001 |
Table 3.
Multivariable Cox Regression Showing the Association of Coexisting AR With Adverse Outcomes in Patients With Moderate-to-Severe AS
| Adjusted HR (95% CI) | P Value | |
|---|---|---|
| Age, y | 1.03 (1.02–1.04) | <0.001 |
| Aortic valve area, cm2 | 0.42 (0.32–0.56) | <0.001 |
| Left ventricular ejection fraction, % | 0.99 (0.97–0.99) | 0.008 |
| Year of index echocardiographic study | 1.16 (1.13–1.19) | <0.001 |
| Coexisting aortic regurgitation | 1.36 (1.02–1.82) | 0.039 |
Moderate-to-severe aortic stenosis is defined as an aortic valve area <1.5 cm2.
Abbreviations as in Table 1.
A subgroup cohort matched by AVA (1:1 coexisting AR to non-AR) of patients was subsequently examined, and the characteristics of this cohort are presented in Supplemental Table 1. Multivariable Cox regression analyses adjusting for age, LVEF, and AVA demonstrated that the presence of coexisting AR still remained independently associated with an increased incidence of adverse clinical outcomes in this subgroup (adjusted HR: 1.57; 95% CI: 1.02-2.43; P = 0.043) (Table 4).
Table 4.
Multivariable Cox Regression Showing the Association of Coexisting AR With Increased Incidence of Adverse Clinical Outcomes in a Subgroup Cohort Matched 1:1 With Non-AR
| Adjusted HR (95% CI) | P Value | |
|---|---|---|
| Age, y | 1.04 (1.02–1.06) | <0.001 |
| Aortic valve area, cm2 | 0.32 (0.18–0.57) | <0.001 |
| Left ventricular ejection fraction, % | 1.00 (0.98–1.04) | 0.489 |
| Coexisting aortic regurgitation | 1.57 (1.02–2.43) | 0.043 |
The aortic regurgitation (AR) and non-AR patients are matched by aortic valve area.
Discussion
Our findings demonstrated significant differences in the echocardiographic profile and clinical outcomes in patients with or without coexisting AR. Of note, the presence of coexisting AR was associated with a greater degree of LV hypertrophy and remodeling, as evidenced by larger LV volumes and greater LV mass index. These findings were also similar in previous studies on surgical AVR in AS patients with or without coexisting AR (16, 17, 18) Indeed, the presence of significant volume overload on the LV due to the presence of AR may add to the degree of hypertrophy and remodeling of the LV in these patients (1).
In surgical patients, there has been significant controversy as to whether the presence of significant AR preoperatively would be associated with adverse postoperative outcomes. The differences in findings had been attributed to the heterogeneity in the study cohorts, where the underlying etiology of AS may vary considerably, along with differing degree of severity of AR that had been included in each study (3,19). More recent large studies have concluded that there has been no significant difference in operative outcomes in patients with AS compared with those with AS and coexisting AR (1,20). Instead, conventional parameters such as sex, mean transaortic pressure gradient, prosthesis size and type, as well as associated coronary artery bypass grafting had been consistently identified as important predictors of outcomes postsurgery (4,21,22).
Nevertheless, the natural history of AS with coexisting AR had not been comprehensively studied in patients who were medically managed. Several large AS trials had excluded patients with concomitant AR. Our study found that the presence of concomitant AR in patients portended adverse clinical outcomes in patients that did not undergo surgical AVR or TAVR. Our findings expand on those that were reported by a similar, smaller retrospective study on a cohort of medically managed patients with AS from Japan, where the investigators reported that concomitant AR had been associated with a higher incidence of adverse events (12).
Several pathophysiological mechanisms may contribute to poorer outcomes in patients with coexisting AR and AS (23). Significant AS and AR affect both pressure as well as volume overload on the LV. The stenotic valve leads to LV hypertrophy, which increases LV stiffness and reduces compliance, which correspondingly limits the degree of LV dilation possible as a result of the volume overload from AR (5,24). Prior studies had reported that patients would develop symptoms earlier at a lower cutoff of LV end-systolic dimension, because the presence of significant LV hypertrophy does not allow for as much LV dilation to accommodate the volume overload (6).
Similar to previous studies, we also observed an increase in stroke volume with the presence of coexisting AR. This meant that at the same degree of AS, the additional volume overload due to the AR would add further to the pressure overload, resulting in higher peak velocities and pressure gradients (25). Indeed, we also found that for similar AVA, the patients with coexisting AR had higher transaortic peak velocities and transaortic mean pressure gradients. Although the peak velocities and the pressure gradients are expected to be augmented by the coexisting AR and consequently “overestimate” the severity of the underlying AS, these parameters had still been shown to correlate well with the development of symptoms (21,22).
Patients with AS and coexisting AR are more likely to be younger and have congenital aortic valve disease. The younger age may be associated with fewer medical comorbidities and thus better clinical outcomes on follow-up. However, we demonstrated that patients with AS and AR were only slightly younger and did not differ significantly in terms of the prevalence of cardiovascular risk factors such as diabetes mellitus, hypertension, or hyperlipidemia. Indeed, although congenital bicuspid aortic valves often predispose patients to the development of AR, there may be other contributory mechanisms that are less recognized. Although we did not specifically study the etiology for the underlying AS, we postulate that other pathophysiological mechanisms such as rheumatic aortic valve disease and calcific degenerative aortic valve disease may also result in AS with coexisting AR, which can explain the presence of this phenomenon even in older patients with AS (26, 27, 28).
Study limitations
We retrospectively examined a relatively large, but single-center, predominantly Asian cohort of patients with moderate-to-severe AS. The number of patients with coexisting AR (n = 88, 7.4%) had been relatively few. Although we controlled for some confounders with multivariable Cox regression, propensity-matched analyses may be possible with larger prospective cohorts to better account for bias. Nevertheless, we were able to demonstrate significant differences in the echocardiographic profile and clinical outcomes between the groups on subsequent follow-up. The cross-sectional nature of the study meant that it was subject to lead-time bias, where the patients studied may have been at different time points along the progression of AS. We studied only the index echocardiographic study, which may skew the study population to those earlier in the course of the natural history of AS. Examining serial echocardiographic studies over time in the future would also be helpful to compare the progression of severity of AS and AR between the groups.
The patients studied were also a heterogeneous group with moderate-to-severe AS. We did not examine whether these patients had been symptomatic from their AS. Furthermore, all patients were medically managed, and those who had undergone valve replacement were excluded. However, the patients who were medically managed may have been either unfit for valve replacement or had declined to undergo the procedure. These limitations result in heterogeneity of the cohort that may confound the findings described because they had not been adjusted for in the statistical analyses. Nevertheless, findings remain exploratory and demonstrated coexisting AR to be associated with adverse clinical outcomes in patients with AS. Further prospective studies would be important to evaluate its impact on clinical management of these patients.
Conclusions
Medically managed patients with moderate-to-severe AS and coexisting AR have often been excluded from large clinical studies, therefore, the impact of coexisting AR on the natural history of AS has not been well described. Our study shows that the presence of coexisting AR in these patients was associated with changes in echocardiographic profile and adverse composite clinical outcomes.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: This study adds to medical knowledge and patient care in that it demonstrates that coexisting aortic regurgitation portended a poorer prognosis in patients with moderate-to-severe aortic stenosis and had a distinct echocardiographic profile. The study examined a retrospective observational cohort of 1,188 patients with mild-to-moderate aortic stenosis; we found that 7.4% of patients had coexisting aortic regurgitation, which was associated with lower diastolic blood pressure, larger end-diastolic volume, and higher left ventricular mass index. This was also associated with adverse clinical outcomes, including admissions for congestive cardiac failure and mortality.
TRANSLATIONAL OUTLOOK: This may help guide clinicians in the prognostication of patients with coexisting aortic stenosis and regurgitation. Our findings may also have clinical implications for earlier valvular interventions but will require future prospective study.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental table, please see the online version of this paper.
Appendix
References
- 1.Catovic S., Popovic Z.B., Tasic B., et al. Impact of concomitant aortic regurgitation on long-term outcome after surgical aortic valve replacement in patients with severe aortic stenosis. J Cardiothorac Surg. 2011;6:51. doi: 10.1186/1749-8090-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonow R.O., Carabello B., Chatterjee K., et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease) J Am Coll Cardiol. 2006;48:e1–e148. doi: 10.1016/j.jacc.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Logeais Y., Langanay T., Roussin R., et al. Surgery for aortic stenosis in elderly patients. A study of surgical risk and predictive factors. Circulation. 1994;90:2891–2898. doi: 10.1161/01.cir.90.6.2891. [DOI] [PubMed] [Google Scholar]
- 4.Lund O. Preoperative risk evaluation and stratification of long-term survival after valve replacement for aortic stenosis. Circulation. 1990;82:124–139. doi: 10.1161/01.cir.82.1.124. [DOI] [PubMed] [Google Scholar]
- 5.Egbe A.C., Poterucha J.T., Warnes C.A. Mixed aortic valve disease: midterm outcome and predictors of adverse events. Eur Heart J. 2016;37:2671–2678. doi: 10.1093/eurheartj/ehw079. [DOI] [PubMed] [Google Scholar]
- 6.Rashedi N., Popović Z.B., Stewart W.J., et al. Outcomes of asymptomatic adults with combined aortic stenosis and regurgitation. J Am Soc Echocardiogr. 2014;27:829–837. doi: 10.1016/j.echo.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Tay E.L.W., Ngiam J.N., Kong W.K.F., et al. Management of severe aortic stenosis: the Singapore and Asian perspective. Singapore Med J. 2018;59:452–454. doi: 10.11622/smedj.2018103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chieffo A., Van Mieghem N.M., Tchetche D., et al. Impact of mixed aortic valve stenosis on VARC-2 outcomes and postprocedural aortic regurgitation in patients undergoing transcatheter aortic valve implantation: results from the International Multicentric Study PRAGMATIC (Pooled Rotterdam-Milan-Toulouse in Collaboration) Catheter Cardiovasc Interv. 2015;86:875–885. doi: 10.1002/ccd.25975. [DOI] [PubMed] [Google Scholar]
- 9.Abdelghani M., Cavalcante R., Miyazaki Y., et al. Transcatheter aortic valve implantation for mixed versus pure stenotic aortic valve disease. EuroIntervention. 2017;13:1157–1165. doi: 10.4244/EIJ-D-17-00328. [DOI] [PubMed] [Google Scholar]
- 10.Smith C.R., Leon M.B., Mack M.J., et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 11.Leon M.B., Smith C.R., Mack M., et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 12.Honda S., Kitai T., Okada Y., et al. Impact of aortic regurgitation on the prognosis of severe aortic stenosis. Heart. 2012;98:1591–1594. doi: 10.1136/heartjnl-2012-302089. [DOI] [PubMed] [Google Scholar]
- 13.Baumgartner H., Hung J., Bermejo J., et al. Recommendations of the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2017;18:254–275. doi: 10.1093/ehjci/jew335. [DOI] [PubMed] [Google Scholar]
- 14.Lang R.M., Badano L.P., Mor-Avi V., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Baumgartner H., Hung J., Bermejo J., et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1–23. doi: 10.1016/j.echo.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 16.Stephan P.J., Henry A.C., Hebeler R.F., Jr., et al. Comparison of age, gender, number of aortic valve cusps, concomitant coronary artery bypass grafting, and magnitude of left ventricular systemic arterial peak systolic gradient in adults having aortic valve replacement for isolated aortic stenosis. Am J Cardiol. 1997;79:166–172. doi: 10.1016/s0002-9149(96)00705-9. [DOI] [PubMed] [Google Scholar]
- 17.Krayenbuehel H.P., Turina M., Hess O.M., et al. Pre- and postoperative left ventricular contractile function in patients with aortic valve disease. Br Heart J. 1979;41:204–213. doi: 10.1136/hrt.41.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang M.H., Hammermeister K.E., Oprian C., et al. Preoperative identification of patients likely to have left ventricular dysfunction after aortic valve replacement. Participants in the Veterans Administration cooperative study on valvular heart disease. Circulation. 1989;80:I65–I76. [PubMed] [Google Scholar]
- 19.Craver J.M., Weintraub W.S., Jones E.L., et al. Predictors of mortality, complications, and length of stay in aortic valve replacement for aortic stenosis. Circulation. 1988;78:I85–I90. [PubMed] [Google Scholar]
- 20.Sharony R., Grossi E.A., Saunders P.C., et al. Aortic valve replacement in patients with impaired ventricular function. Ann Thorac Surg. 2003;75:1808–1814. doi: 10.1016/s0003-4975(03)00117-6. [DOI] [PubMed] [Google Scholar]
- 21.Pibarot P., Dumesnil J.G. Hemodynamic and clinical impact of prosthesis-patient mismatch in the aortic valve position and its prevention. J Am Coll Cardiol. 2000;36:1131–1141. doi: 10.1016/s0735-1097(00)00859-7. [DOI] [PubMed] [Google Scholar]
- 22.Medalion B., Blackstone E.H., Lytle B.W., et al. Aortic valve replacement: is valve size important? J Thorac Cardiovasc Surg. 2000;119:963–974. doi: 10.1016/S0022-5223(00)70091-2. [DOI] [PubMed] [Google Scholar]
- 23.Unger P., Pibarot P., Tribouilloy C., et al. Multiple and mixed valvular heart diseases: pathophysiology, imaging, and management. Circ Cardiovasc Imaging. 2018;11 doi: 10.1161/CIRCIMAGING.118.007862. [DOI] [PubMed] [Google Scholar]
- 24.Egbe A.C., Luis S.A., Padang R., et al. Outcomes in moderate mixed aortic valve disease: is it time for a paradigm shift? J Am Coll Cardiol. 2016;67:2321–2329. doi: 10.1016/j.jacc.2016.03.509. [DOI] [PubMed] [Google Scholar]
- 25.Zilberszac R., Gabriel H., Schemper M., et al. Outcome of combined stenotic and regurgitant aortic valve disease. J Am Coll Cardiol. 2013;61:1489–1495. doi: 10.1016/j.jacc.2012.11.070. [DOI] [PubMed] [Google Scholar]
- 26.Rajamannan N.M., Evans F.J., Aikawa E., et al. Calcific aortic valve disease: not simply a degenerative process: a review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: calcific aortic valve disease-2011 update. Circulation. 2011;124:1783–1791. doi: 10.1161/CIRCULATIONAHA.110.006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michelena H.I., Khanna A.D., Mahoney D., et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. 2011;306:1104–1112. doi: 10.1001/jama.2011.1286. [DOI] [PubMed] [Google Scholar]
- 28.Coffey S., Cox B., Williams M.J. The prevalence, incidence, progression, and risks of aortic valve sclerosis: a systematic review and meta-analysis. J Am Coll Cardiol. 2014;63:2852–2861. doi: 10.1016/j.jacc.2014.04.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.