Abstract
Machine learning (ML) is a branch of artificial intelligence that combines computer science, statistics, and decision theory to learn complex patterns from voluminous data. In the last decade, accumulating evidence has shown the utility of ML for prediction, diagnosis, and classification of hypertension and heart failure (HF). In addition, ML-enabled image analysis has potential value in assessing cardiac structure and function in an accurate, scalable, and efficient way. Considering the high burden of hypertension and HF in China and worldwide, ML may help address these challenges from different aspects. Indeed, prior studies have shown that ML can enhance each stage of patient care, from research and development, to daily clinical practice and population health. Through reviewing the published literature, the aims of the current systemic review are to summarize the utilities of ML for the care of those with hypertension and HF.
Key Words: algorithms, heart failure, hypertension machine learning
Abbreviations and Acronyms: ANN, artificial neural network; AUC, area under the curve; CNN, convolutional neural network; HFpEF, heart failure with preserved ejection fraction; LVDD, left ventricular diastolic dysfunction; LVH, left ventricular hypertrophy; LRM, linear or logistic regression model; ML, machine learning; RF, random forest; SVM, support vector machine
Central Illustration
Highlights
-
•
The use of ML for improving cardiovascular care has been extensively assessed in the last decade.
-
•
ML algorithms can help improve prediction, diagnosis, and classification of hypertension and heart failure.
-
•
ML-enabled image analysis has potential value in assessing cardiac structure and function in an accurate, scalable, and efficient way.
-
•
Studies are needed to investigate whether ML method can help improve the management of hypertension and heart failure.
The prevalence of hypertension continues to increase in China and worldwide due to the extended life expectancy in the general population (1, 2, 3, 4, 5). Hypertension is the single most important risk factor for heart failure (HF) (6, 7, 8). The China hypertension survey shows that approximately 1.3% of adults (estimated 13.7 million) have HF (9). Among HF patients with hypertension, only 57.7% receive antihypertensive therapy, and only 14.5% have their blood pressure under control (9). HF accounts for nearly one-fifth of overall cardiovascular hospitalizations and costs more than 5 billion US dollars annually in China (10,11). Improvement in screening, prevention, and management of hypertension is essential to reduce the burden of hypertension and associated HF (1, 2, 3, 4).
Machine learning (ML) is a branch of artificial intelligence that combines computer science, statistics, and decision theory to learn complex patterns from voluminous data (12, 13, 14). The clinical goals of ML include prediction, diagnosis, classification, automated image assessment, among others (12,15, 16, 17). In the last 5 years, more than 3,000 papers about ML in cardiovascular care have been published, with most studies focused on the areas of atherosclerosis, hypertension, and HF (15). Considering the high burden of hypertension and HF in China and worldwide, ML may help address these challenges from different aspects (1, 2, 3, 4). Indeed, prior studies show that ML could enhance each stage of patient care, from research and development to daily clinical practice and population health (18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39). By reviewing the published literature, the aim of the current systematic review is to summarize the use of ML for prediction, diagnosis, classification and automated image assessment of hypertension and HF (Central Illustration).
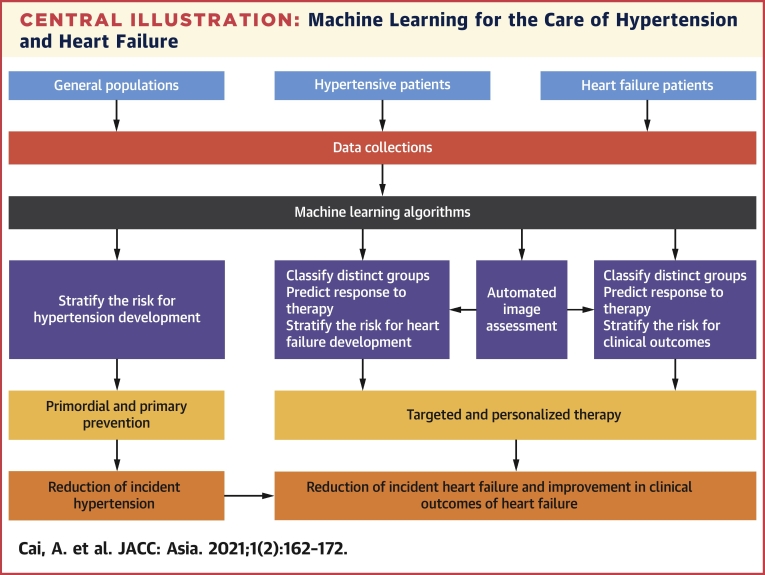
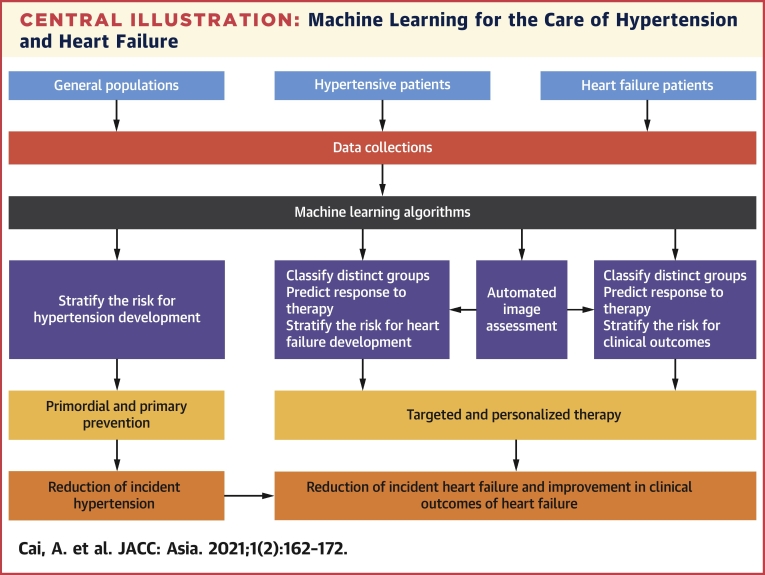
Central Illustration.
Machine Learning for the Care of Hypertension and Heart Failure
The potential utility of machine learning for the care of hypertension and heart failure is shown. Machine learning models can be used to stratify the risk of hypertension and heart failure development; classify hypertension and heart failure subgroups; predict response to specific therapy; and assist in automated image assessment.
From Hypertension to HF and Diagnostic Techniques
Exposure of the human heart to longstanding hypertension leads to hypertensive heart disease (7,8,40). Based on the clinical and pathophysiologic impacts of hypertension on the heart, Messerli et al (8) and Iriarte et al (41) proposed that hypertensive heart disease can be classified into four ascending categories. Degree I is characterized by the presence of left ventricular diastolic dysfunction (LVDD) without left ventricular hypertrophy (LVH); degree II is presence of LVDD with concentric LVH; degree III is symptomatic HF with preserved ejection fraction; and degree IV is symptomatic HF with reduced ejection fraction and left ventricular (LV) dilatation. Degrees I and II are considered subclinical stages of HF and degrees III and IV are considered the symptomatic clinical stage of HF. Several diagnostic modalities can be used to detect structural and functional alterations of the heart, and the advantages and limitations of each modality are summarized in Table 1. In the last decade, accumulating evidence has shown that combining ML techniques with each of these imaging modalities could introduce novel strategies for predicting, diagnosing, and classifying hypertension and HF, which will be discussed in the following section.
Table 1.
Techniques Used to Assess Cardiac Structure and Function
| 12-Lead ECG | Transthoracic Echocardiogram | Cardiac CT | Cardiac MR | |
|---|---|---|---|---|
| LA enlargement | Usually based on the duration of the negative phase of the P-wave in lead V1 >40 ms, with a sensitivity and specificity of 83% and 80%, respectively (83). | Usually based on LA volume index >40 ml/m2. Provides diagnostic and prognostic information. Accuracy is dependent on the image quality and operator experiences (84). | Better than 2D echo in estimating LA volume and anatomy. Radiate, time-consuming, more expensive, and less available than echo. | Gold standard for estimation of LA volume. Time-consuming, expensive, and limited accessible (85). |
| LV hypertrophy | Based on the Sokolow-Lyon criteria (SV1 or SV2 plus RV5 or RV6 >3.5 mV), or the Cornell criteria (RI plus SIII >2.5 mV). Low sensitivity (6.9%) but high specificity (98.8%) (86). | Based on LV mass index >115 g/m2 for men and >95 g/m2 for women. Higher sensitivity than ECG. More accessible, cheaper, and higher operator-dependent than cardiac CT/MR (87). | The utility of cardiac CT for assessment of LV hypertrophy is relatively limited. May be appropriate test for assessment of LV hypertrophy in individuals with known or suspected HF (88). | Gold standard for diagnosing LV hypertrophy. Time-consuming, expensive, and limited accessible (85). Appropriate test for assessment of LV hypertrophy in individuals with known or suspected HF (88). |
| LV enlargement | N/A | Available and reproducible. Apex foreshortened, endocardial dropout, and shape distortion compromise the accuracy of LV volume estimation. | May be appropriate test for assessment of LV enlargement in individuals with known or suspected HF (88). | Gold standard for evaluation of LV volume. Appropriate test for assessment of LV enlargement in individuals with known or suspected HF (88). |
| LV systolic dysfunction | N/A | LVEF is easily obtained but limited by high interobserver and intraobserver variability (89). GLS has reduced interobserver and intraobserver variability, and is more sensitive and specific in identifying subclinical LV systolic dysfunction (90). | May be appropriate test for assessment of LV systolic dysfunction in individuals with known or suspected HF (88). | Gold standard for evaluation of LV systolic function. Appropriate test for assessment of LV systolic dysfunction in individuals with known or suspected HF (88). |
| LV diastolic dysfunction | N/A | Diagnosed based on increased LA volume index, reduced septal or lateral e’ velocity, increased average E/e’ ratio and increased TR systolic jet velocity. | May be appropriate test for assessment of LV diastolic dysfunction in individuals with known or suspected HF (88). | Good agreement of cardiac MR and echo in assessment of LV diastolic dysfunction (91). Appropriate test for assessment of LV diastolic dysfunction in individuals with known or suspected HF (88). |
2D = 2-dimensional; CT = computed tomography; ECG = electrocardiogram; echo = echocardiogram; GLS = global longitudinal strain; HF = heart failure; LA = left atrial; LV = left ventricular; LVEF = left ventricular ejection fraction; MR = magnetic resonance; N/A = not applicable; TR = tricuspid regurgitation.
Computational Techniques for ML
Computational techniques for ML can be broadly classified to 2 categories: supervised and unsupervised learning. The advantages and limitations of some commonly used ML algorithms are summarized in Table 2. Supervised learning algorithms build models based on pairs of input features and labeled outcomes. They are characterized by the iterative analysis of data, with features selected, processed, and weighted so as to identify the best combination to fit the outcome of interest (42). The main goals of supervised learning include outcome prediction, disease classification, and parameter estimation. Linear or logistic regression models (LRMs), artificial neural network (ANN), random forest (RF), and support vector machine (SVM) algorithms are the most widely used. The details of applying the ML algorithm to build models for hypertension prediction are well documented (20,21,30, 31, 32, 33, 34, 35, 36, 37). For example, Huang et al (30) provide an example of using ANN to build a model for hypertension prediction. The investigators used a dataset to conduct an LRM to determine factors that predicted hypertension. Following the identification of these factors, they applied ANN to build models for hypertension prediction, and subsequently evaluated the performance of these ANN models in the testing dataset.
Table 2.
Computational Techniques for Machine Learning
| Advantages | Limitations | Application Example | |
|---|---|---|---|
| Supervised learning | |||
| Linear or logistic regression model | Easy to use, good for small dataset, easy to interpret and understand, and less tendency to be overfitting | Inappropriate for nonlinear modeling and large dataset, relatively low predictive accuracy, and unable to perform classification | Evaluate the risk of hypertension (30,31) and predict the incident hypertension (32) |
| Artificial neural network | Good for large dataset and nonlinear modeling, easy to identify potential interaction between variables | Time-consuming, difficult to interpret or understand (eg, black box effect), tendency to be overfitting, problem with generalizability, vulnerable to adversarial example, and requires hyperparameter tuning | Evaluate the risk of hypertension (30,33) |
| Random forest | Good for nonlinear modeling and variable importance assessment, well-suited for prediction and classification | Time-consuming, less useful for descriptive analysis, tendency to be overfitting, inappropriate for large dataset, and requires high computational power | Predict the incident hypertension (32), risk of hypertension (31), and predicting transitions in hypertension control status (34) |
| Support vector machines | High predictive accuracy, able to transform linear classifier to nonlinear classifier, good for small dataset, text classification, and image recognition | Inappropriate for large and noisy dataset, nonparametric inference (without P value), and not ideal for multiclass classification and high-dimensional space | Predict the incident hypertension (32) |
| Unsupervised learning | |||
| Cluster analysis | Easy to understand using dendrogram, insensitive to outliers (hierarchical clustering), and simple to use | Difficult to find a k value (number of clusters), sensitive to outliers (k-means clustering), does not work with missing data, arbitrary metric and linkage criteria, and nonparametric inference (without P value) | Classification of hypertension (35) |
| Principal component analysis | Less tendency to be overfitting, good for reducing noises and dimensionality of features, and minimum loss of information | Inappropriate for nonlinear modeling, difficult to understand or interpret, and possibility of losing information in some dimensions | Evaluation of medication adherence (39) |
| Combined tools | |||
| Ensemble | Less tendency to be overfitting, ideal for multiclass classification, easy to reduce biases, and can be used for a combination of results from different algorithms (supervised and unsupervised) | Sensitive to outlier | Prediction of incident hypertension (38) |
Different from supervised learning, unsupervised learning does not have an established relationship between inputs and outputs, and thus does not attempt to fit input features into an outcome. Instead, it builds a model using unlabeled data, with a goal of discovering the hidden/latent structure in the data and identifying the relationship between variables. Cluster analysis and principal component analysis are the 2 commonly used algorithms. Examples of cluster analysis for classifying hypertension, identifying patterns of cardiac remodeling, and classifying HF patients with distinct clinical characteristics and outcomes are well documented (21,35,43, 44, 45, 46, 47). For example, Lancaster et al (43) used cluster analysis for phenotyping echocardiographic variables in the assessment of LVDD. To do this, clustering models were constructed based on parameters of LV diastolic function, with the optimal number of clusters determined using the Calinski-Harabasz F criterion and item response theory. Agreement between the cluster designation and guideline-based diagnosis were measured using the kappa statistic. Through these processes, 2 distinct groups with unique LVDD patterns were identified.
Combined tools, using both supervised combined unsupervised learning techniques, can create an ensemble model. These algorithms have the ability to combine results from different algorithms, with 1 recent study showing that the ensemble model, built upon 3 different ML algorithms (SVM, RF, and ANN), had a better performance than routine echocardiographic parameters in differentiating physiological and pathological LVH (48).
The Utility of ML for Hypertension Care
The prediction and early diagnosis of hypertension is important to prevent the development of hypertension and associated health complications. Several models, which were based on conventional regression analysis, have been developed to predict the incidence of hypertension (49, 50, 51, 52, 53, 54). The Framingham hypertension risk score is 1 of the main scores, with a good discriminative ability (area under the curve [AUC] = 0.79) (49). Although the Framingham score was confirmed by a study of British individuals (55), the model is derived from the Caucasian populations and is based on single measurement of risk factors and blood pressure (49). Different from models derived from conventional regression analysis, models built upon ML algorithms are routinely validated with a separated dataset during model development. In addition, ML algorithms can differentiate which variable or set of variables is the most pragmatic in risk prediction. In the last decade, several studies have assessed the roles of ML for hypertension prediction. The important findings are summarized in Table 3. Ye et al (36) used electronic health record (EHR)–based data to predict the 1-year risk of incident hypertension through the development of the XGBoost model. XGBoost used features from 6 domains to build a predictive model. The results showed that the XGBoost model had good predictive value in both the training (AUC = 0.92) and validation (AUC = 0.87) cohorts. Another study using the EHR-based data showed that an RF model had a good performance (AUC = 0.84) in predicting the transition from control to hypertension (34).
Table 3.
Examples of ML for the Care of Hypertension
| First Author (Ref. #) | Disease Application |
Sample Size |
Variable Input | Output | Algorithms | Results |
|---|---|---|---|---|---|---|
| Huang et al (30) | Prediction of hypertension | 3,054 | Occupation, family history, educational level, alcohol intake, vegetable and fruit intake, salt, animal insides intake, physical exercise, body mass index, and blood pressure | Prevalent hypertension | LRM and ANN | ANN model was better than LRM in predicting the presence of hypertension |
| AlKaabi et al (31) | Prediction of hypertension | 987 | Age, sex, education, employment, tobacco use, physical activity, consumption of fruits and vegetables, mother history of hypertension, diabetes, cholesterol, and abdominal obesity | Prevalent hypertension | DT, RF and LRM | RF model had better prediction accuracy for screening the presence of hypertension |
| Kanegae et al (38) | Prediction of hypertension | 18,258 | Medical history, lifestyle factors, anthropometrics, and biochemical measurements | Incident hypertension | XGBoost, ensemble, and LRM | ML developed a highly precise model for predicting incident hypertension |
| Katz et al (35) | Classification of hypertension | 1,273 | Demographics, physical characteristics, laboratory, and echocardiographic indices | Hypertension phenotypes | Agglomerative hierarchical clustering | 2 distinct types of hypertension with different cardiac substrate |
| Wu et al (20) | Prediction of outcome | 508 | Left atrial diameter, HDL-C, big endothelin-1, right arm diastolic BP, right/left leg systolic BP, right leg diastolic BP, left arm systolic BP, mean nocturnal arterial oxygen saturation, past maximum systolic BP, and urea | Clinical outcomes | Recursive feature elimination and XGBoost | ML model was comparable with Cox proportional model for outcome prediction and better than recalibrated Framingham risk score model |
ANN = artificial neural network; BP = blood pressure; DT = decision tree; HDL-C = high-density lipoprotein–cholesterol; LRM = logistic regression model; ML = machine learning; RF = random forest.
The values of ML models in hypertension classification and outcome prediction are also well documented. For example, using cluster analysis, Katz et al (35) identified 2 distinct types of hypertension which were associated with the myocardial substrate for HF with preserved ejection fraction (HFpEF) (35). Park et al (37) compared the accuracy of an ML model and a conventional regression model for predicting cardio- and cerebrovascular outcomes in hypertensive populations, and the results showed that the ML model had a better performance. Wu et al (20) applied XGBoost to build a model for predicting clinical outcomes in young hypertensive patients. Compared to both the Cox proportion regression model and the recalibrated Framingham risk score, the XGBoost model had a better performance (20). These findings together suggest that ML can be a feasible and useful tool for classifying hypertension and predicting clinical outcomes in a more accurate way than conventional regression analysis.
The Use of ML for the Care of HF
Although prognosis has improved, the 5-year survival rate in patients with HF remains low, especially patients with HFpEF (56, 57, 58). In the last decade, ML has been applied for predicting, diagnosing, and classifying subclinical and clinical stages of HF (23,26,43,44,48,59, 60, 61, 62, 63). The preliminary results are promising (Table 4). Sabovčik et al (23) used ML algorithms to build models to screen for early stages of cardiac remodeling and dysfunction among general populations, showing that leveraging ML algorithms, routinely measured clinical, laboratory, and electrocardiographic (ECG) data can be used to predict LVDD and LVH with high accuracy. Based on ECG and clinical data, results from Kagiyama et al (26) showed that an ML model can help assess LV diastolic function with good performance in subjects referred for echocardiography. Convolutional neural network (CNN)–enabled ECG algorithm identified LV systolic dysfunction in dyspneic patients who presented to the emergency department, and its performance outperformed N-terminal pro-B type natriuretic peptide (AUC 0.85 vs 0.80, respectively) (60). Using EHR-based data, Choi et al (63) reported that a recurrent neural network model was better than conventional regression analysis for predicting early onset of incident HF. These results have important clinical implications. Current guidelines do not recommend routing use of transthoracic echocardiographic for screening and primary prevention of cardiac remodeling and dysfunction (3); however, combining ML algorithms with EHR-based data may provide a feasible and cost-effective way to detect early cardiac remodeling and dysfunction.
Table 4.
Example of ML for the Care of Subclinical and Clinical Stages of HF
| First Author (Ref. #) | Disease Application |
Sample Size |
Variable Input | Output | Algorithms | Results |
|---|---|---|---|---|---|---|
| Sabovcik et al (23) | Diagnosis of LVDD and LVH | 1,407 | 67 features and age, BMI, BP, history of hypertension, antihypertensive treatment, and electrocardiographic variables | Presence of LVDD and LVH | XGBoost, AdaBoost, RF, SVM, and LRM | The combination of ML and routinely measured data predicted LVDD and LVH with high accuracy. |
| Adedinsewo et al (60) | Diagnosis of LV systolic dysfunction | 1,606 | 12-lead ECG | Presence of reduced LVEF | CNN | CNN-enabled ECG algorithm effectively identified patients with LV systolic dysfunction, which outperformed NT-proBNP |
| Woolley et al (46) | Classification of HFpEF | 429 | 363 biomarkers | HFpEF subgroups | Cluster analysis | Cluster analysis identified four subgroups of HFpEF patients with distinct biomarker profiles, clinical features and outcomes |
| Narang et al (66) | Automated imaging acquisition | 240 | Echocardiographic indices | Cardiac view and echocardiographic indices | CNN | CNN-enabled automated image acquisition algorithm allowed novices in ultrasonography to obtain cardiac views for cardiac structure/function evaluation |
| Knackstedt et al (61) | Automated assessment of LVEF and LS | 255 | Apical 4- and 2-chamber echocardiographic imaging | Measures of LVEF and LS | ML-enabled software (AutoLV) | ML-enabled software provided rapid and reproducible assessment of LVEF and LS |
| Zhang et al (62) | Automated image interpretation | 14,035 Echocardiographic imaging data | Echocardiographic imaging | Cardiac view identification, chamber segmentation, and cardiac structure and function metrics | CNN-enabled fully automated assessment | CNN-enabled fully automated assessment identified cardiac view, segmented cardiac chamber, measured cardiac structure and function with high accuracy |
| Frizzell et al (92) | Prediction of readmission | 56,477 | Demographics, socioeconomics, medical history, HF characterization, medications used, vital signs, body weights, laboratories, and discharge interventions | 30-day HF readmission | Tree-augmented naïve Bayesian network, LRM with backward stepwise selection, LRM with LASSO, gradient boosted model, and RF | Prediction of 30-day HF readmissions was similar between ML models and traditional prediction models. |
| Ahmad et al (45) | Prediction of clinical outcome and HF subgroups classification | 44,886 | 86 variables for RF model; variables for cluster analysis include age, heart rate, creatinine, hemoglobin, weight, systolic BP, mean arterial pressure, and income | One-year survival; HF classification | RF and cluster analysis | ML models accurately predicted outcomes for HF patients. Cluster analysis identified 4 distinct HF phenotypes that differed significantly in outcomes and in response to therapy |
BMI = body mass index; CNN = convolutional neural network; HFpEF = heart failure with preserved ejection fraction; LASSO = Least Absolute Shrinkage and Selection Operator; LS = longitudinal strain; LVDD = left ventricular diastolic dysfunction; LVH = left ventricular hypertrophy; NT-proBNP = N-terminal pro-B type natriuretic peptide; SVM = support vector machine; other abbreviations as in Tables 1 and 3.
Accurate assessment of cardiac structure and function is essential for the diagnosis and management of subclinical and clinical stages of HF. Despite this, routine techniques used to assess cardiac structure and function are time-consuming, operator-dependent, and impacted by image quality, making visual assessment difficult and inaccurate. In the last decade, ML-enabled image analysis has become a widely used approach for automated image assessment. Preliminary results reveal good performance of the automated image assessment for the evaluation of cardiac structure and function (42,44,48,61,62,64, 65, 66). For example, Knackstedt et al (61) reported that a ML-enabled image analysis had a better performance than visual assessment for evaluating LV systolic function. In addition, fully automated image assessment had no interobserver and intraobserver variability and had high efficiency (61). Results from Zhang et al (62) showed that CNN-enabled automated image assessment could accurately identify specific cardiac views, segment cardiac chambers, quantify cardiac structure and function, and differentiate 3 different cardiac diseases. ML algorithm can also help guide novices without experience in echocardiography to obtain images for assessing cardiac structure and function (66). When using the ML algorithm, the novices obtained cardiac images, and results were comparable to those obtained by a trained sonographer (66). These results have important clinical implications. First, advanced diagnostic techniques can be less accessible in areas with limited resources. Automated image assessment may overcome these barriers and extend the reach of these techniques into remote areas. Second, automated image assessment is useful for standardizing cardiac image assessment and improving the quality of image interpretation. Third, fully automated image analysis can help assess cardiac structure and function in an efficient, scalable, and accurate way.
Another important use of ML for HF is in predicting clinical outcomes. Conventional LRM has poor discriminative ability in predicting HF or all-cause readmissions (67, 68, 69, 70, 71). Studies have suggested that an ML model might provide a better performance for predicting HF and all-cause readmissions (28,72). Compared to LRM, both RF and boosting models provided better performance for predicting 30- and 180-day HF readmissions (72). ML models were also better than conventional regression models for predicting mortality (27, 28, 29,45,73). Compared to LRM, an RF model had a better performance in predicting mortality in HFpEF patients (28). Through applying cluster analysis, 4 groups of HF patients with marked differences in 1-year survival and in response to therapy were identified (45). Similarly, 3 distinct HFpEF groups with markedly different clinical characteristics, cardiac structure and function, and clinical outcomes were identified using cluster analysis (47). These findings have important clinical implications. First, predictive models built upon ML algorithms can help physicians stratify and identify high-risk patients, which then allows for implementation of effective targeted therapy. Second, predictive models built upon ML algorithms can be used by physicians as decision-making tools to estimate the prognosis of HF patients, allowing for a more efficient use of health resources. Third, improvement in describing and classifying heterogenous clinical syndromes (such as HFpEF) can allow for development and deployment of targeted therapies for patients with similar phenotypes.
Illustration of ML for Care From Hypertension to HF
In the progression of hypertension to HF, the human heart undergoes complex pathophysiological alterations in structure and function, and ML may help explore the mechanisms underlying these alterations. For example, Wu et al (20) used recursive feature elimination and XGBoost to identify factors that contributed to incident HF and other clinical outcomes in young hypertensive patients. During features selection, recursive feature elimination ranked the variables by their contribution to the outcome of interest, regardless of whether the effect could be explained. Through these processes, potential key risk factors of HF were identified, including big endothelin-1, a precursor of endothelin-1. Mechanistically, the endothelin system is associated with oxidative stress and cardiac remodeling, all of which have been found related to HF development (74,75). These findings support the potential value of ML for exploring the mechanisms associated with the development of hypertensive heart disease and subsequent clinical HF.
In addition, ML can also assist in predicting and managing hypertensive patients who are at an increased risk of developing HF. As mentioned above, through applying unsupervised learning algorithm, distinct subgroups with unique pathophysiological features, which relate to HF development, can be identified. For example, Katz et al (35) used agglomerative hierarchical clustering to group hypertensive patients and 47 continuous variables. Two distinct groups with differences in clinical characteristics, cardiac structure and function, and indices of cardiac mechanics were identified. Compared to phenogroup 1, phenogroup 2 had a worse global longitudinal strain, an index of LV systolic function, and a worse early diastolic velocity (e’) and E/e’ ratio, indices of LV diastolic function, suggesting that these 2 hypertension groups might represent distinct subtypes that could benefit from specific targeted therapies for HF prevention. Indeed, accumulating evidence has shown the potential value of ML in identifying individuals who are at an increased risk of developing HF and who may have a good response to a specific therapy (29,46,76).
Limitations of ML
Although the results of ML are promising, several important limitations merit considerations. First is the black box effect. ML models are not easily explained and understood by physicians, and the processes by which ML models have achieved their performance are unclear, which is commonly referred to as the black box effect. This can result in physicians being less willing to engage in using this technology. In addition, the lack of easy interpretability means that it can be difficult to confirm whether the learned rules can be generalized into daily clinical practice. The second limitation is the tendency of some ML algorithms (such as ANN) to be overfitting. Overfitting is one of the most common problems encountered when using supervised ML algorithms, and it causes the failure of generalization of the learned rules into other clinical settings. Third is the concern over accuracy. The metric often used to evaluate the performance of ML models is AUC. However, the AUC is only a measure of discrimination, and it is unable to assess the calibration, an important metric used for evaluating whether the risk estimates are reliable (77). In addition, there is no single universally accepted AUC cut-off or range of acceptable AUC (78). Fourth is the vulnerability of ML algorithms to poor-quality or misinterpreted data. Biased or poor-quality data can lead to biased predictions or even facilitate manipulation of the results. For example, input data with slight modifications may cause a major change in the performance of ML models, which may be associated with serious medical sequelae. Accordingly, potential liability for physicians using artificial intelligence has recently been proposed (79). Fifth is the relatively limited data behind clinically applying results. The utility of ML for the management of hypertension and HF have not been well tested yet due to limitations of study design and physician’s lack of engagement in computer science (80). Studies are needed to assess whether clinical trials guided by ML could potentially improve the management of hypertension and HF in the future.
Future Directions
Future directions for ML include but are not limited to the following 3 important aspects. First is the application of ML to basic science. For example, a flow cytometer is routinely used to separate cells into different groups based on the similarities in light scattering and fluorescence. These processes are usually accomplished by manual partitioning cells through visual inspection. Notably, there are many problems with this approach, including that it is subjective, time-consuming, and there is difficulty in effectively analyzing high-dimensional data (81). ML-enabled image analysis may help automatically identify cells in an efficient, scalable, and accurate way (82). Clustering analysis can also help group cells with similar behavior and increase the accuracy in defining cell phenotypes. Through learning the morphology and motion patterns of progenitor cells, an ML method (such as ANN) can provide an alternative method to identify progenitor cells in the early stage of reprogramming process. Second is using ML for improvement in data collection and integration. Growing volumes of information from clinical notes, multiomics, biomedical analysis, wearable and sensor data, and imaging make it challenging for physicians to integrate and interpret these data in an efficient, straightforward, and accurate way. Moreover, these diverse, complex, and high-dimensional data exhibit nonlinear relationships and there are unknown interactions, which may lead to the failure of conforming to the assumptions of many classical statistical methods. The advantages in methodologies of ML algorithms as shown in Table 2 are useful to facilitate the integration of multiple variables and ideally suited to the task of learning pattern and gaining insight from complex and high-dimensional data. Third is developing a framework for incorporating ML into daily clinical practice. Cardiac structure and function assessment guided by ML can provide more reliable and accurate information, which increase the precision in identifying subclinical stage of HF. In addition, through rapidly integrating and analyzing complex and high-dimensional data with ML algorithms, physicians may promptly identify individuals who are at risk for hypertension or HF, or who are likely to become nonadherent or nonresponse to therapy, which can inform targeted therapy in a timely manner. Although ML is still in its earliest stage of development, we believe that with accumulating experience and knowledge, ML techniques will become part of a physician’s toolbox at the point of care for the diagnosis, prediction, classification, automated image assessment, and possibly management of hypertension and HF in the future.
Funding Support and Author Disclosures
Dr Feng has received funding from the National Key Research and Development Program of China (No.2017YFC1307603), the Natural Science Foundation of Guangdong Province (No.2020A1515010738), Science and Technology Plan Program of Guangzhou (No.201803040012), the Key Area R&D Program of Guangdong Province (No.2019B020227005), Guangdong Provincial People's Hospital Clinical Research Fund (Y012018085), and Climbing Plan of Guangdong Provincial People's Hospital (DFJH2020022). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.2018 Chinese guidelines for prevention and treatment of hypertension — a report of the Revision Committee of Chinese Guidelines for Prevention and Treatment of Hypertension. J Geriatric Cardiol. 2019;16:182–241. doi: 10.11909/j.issn.1671-5411.2019.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whelton P.K., Carey R.M., Aronow W.S., et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):2199–2269. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Williams B., Mancia G., Spiering W., et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 4.Unger T., Borghi C., Charchar F., et al. 2020 International Society of Hypertension Global Hypertension practice guidelines. Hypertension. 2020;38:982–1004. doi: 10.1097/HJH.0000000000002453. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z., Chen Z., Zhang L., et al. Status of hypertension in China: results from the China Hypertension Survey, 2012–2015. Circulation. 2018;137:2344–2356. doi: 10.1161/CIRCULATIONAHA.117.032380. [DOI] [PubMed] [Google Scholar]
- 6.Ma L.Y., Chen W.W., Gao R.L., et al. China cardiovascular diseases report 2018: an updated summary. J Geriatric Cardiol. 2020;17:1–8. doi: 10.11909/j.issn.1671-5411.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slivnick J., Lampert B.C. Hypertension and heart failure. Heart Fail Clin. 2019;15:531–541. doi: 10.1016/j.hfc.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Messerli F.H., Rimoldi S.F., Bangalore S. The transition from hypertension to heart failure: contemporary update. J Am Coll Cardiol HF. 2017;5:543–551. doi: 10.1016/j.jchf.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Hao G., Wang X., Chen Z., et al. Prevalence of heart failure and left ventricular dysfunction in China: the China Hypertension Survey, 2012–2015. Eur J Heart Fail. 2019;21:1329–1337. doi: 10.1002/ejhf.1629. [DOI] [PubMed] [Google Scholar]
- 10.Du X., Patel A., Anderson C.S., Dong J., Ma C. Epidemiology of cardiovascular disease in China and opportunities for improvement: JACC international. J Am Coll Cardiol. 2019;73:3135–3147. doi: 10.1016/j.jacc.2019.04.036. [DOI] [PubMed] [Google Scholar]
- 11.Cook C., Cole G., Asaria P., Jabbour R., Francis D.P. The annual global economic burden of heart failure. Int J Cardiol. 2014;171:368–376. doi: 10.1016/j.ijcard.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 12.Krittanawong C., Johnson K.W., Rosenson R.S., et al. Deep learning for cardiovascular medicine: a practical primer. Eur Heart J. 2019;40:2058–2073. doi: 10.1093/eurheartj/ehz056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghahramani Z. Probabilistic machine learning and artificial intelligence. Nature. 2015;521:452–459. doi: 10.1038/nature14541. [DOI] [PubMed] [Google Scholar]
- 14.Jordan M.I., Mitchell T.M. Machine learning: trends, perspectives, and prospects. Science. 2015;349:255–260. doi: 10.1126/science.aaa8415. [DOI] [PubMed] [Google Scholar]
- 15.Quer G., Arnaout R., Henne M., Arnaout R. Machine learning and the future of cardiovascular care: JACC state-of-the-art review. J Am Coll Cardiol. 2021;77:300–313. doi: 10.1016/j.jacc.2020.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson K.W., Torres Soto J., Glicksberg B.S., et al. Artificial intelligence in cardiology. J Am Coll Cardiol. 2018;71:2668–2679. doi: 10.1016/j.jacc.2018.03.521. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein B.A., Navar A.M., Carter R.E. Moving beyond regression techniques in cardiovascular risk prediction: applying machine learning to address analytic challenges. Eur Heart J. 2017;38:1805–1814. doi: 10.1093/eurheartj/ehw302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raghunath S., Pfeifer J.M., Ulloa-Cerna A.E., et al. Deep neural networks can predict new-onset atrial fibrillation from the 12-lead electrocardiogram and help identify those at risk of AF-related stroke. Circulation. 2021;143:1287–1298. doi: 10.1161/CIRCULATIONAHA.120.047829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L., Yuan M., An Z., et al. Prediction of hypertension, hyperglycemia and dyslipidemia from retinal fundus photographs via deep learning: a cross-sectional study of chronic diseases in central China. PloS One. 2020;15 doi: 10.1371/journal.pone.0233166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu X., Yuan X., Wang W., et al. Value of a machine learning approach for predicting clinical outcomes in young patients with hypertension. Hypertension. 2020;75:1271–1278. doi: 10.1161/HYPERTENSIONAHA.119.13404. [DOI] [PubMed] [Google Scholar]
- 21.Tsoi K.K.F., Chan N.B., Yiu K.K.L., Poon S.K.S., Lin B., Ho K. Machine learning clustering for blood pressure variability applied to Systolic Blood Pressure Intervention Trial (SPRINT) and the Hong Kong community cohort. Hypertension. 2020;76:569–576. doi: 10.1161/HYPERTENSIONAHA.119.14213. [DOI] [PubMed] [Google Scholar]
- 22.Sánchez-Cabo F., Rossello X., Fuster V., et al. Machine learning improves cardiovascular risk definition for young, asymptomatic individuals. J Am Coll Cardiol. 2020;76:1674–1685. doi: 10.1016/j.jacc.2020.08.017. [DOI] [PubMed] [Google Scholar]
- 23.Sabovčik F., Cauwenberghs N., Kouznetsov D., et al. Applying machine learning to detect early stages of cardiac remodelling and dysfunction. Eur Heart J Cardiovasc Imaging. Published online June 26, 2020 doi: 10.1093/ehjci/jeaa135. [DOI] [PubMed] [Google Scholar]
- 24.Ouyang D., He B., Ghorbani A., et al. Video-based AI for beat-to-beat assessment of cardiac function. Nature. 2020;580:252–256. doi: 10.1038/s41586-020-2145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kusunose K., Abe T., Haga A., et al. A deep learning approach for assessment of regional wall motion abnormality from echocardiographic images. J Am Coll Cardiol Img. 2020;13:374–381. doi: 10.1016/j.jcmg.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 26.Kagiyama N., Piccirilli M., Yanamala N., et al. Machine learning assessment of left ventricular diastolic function based on electrocardiographic features. J Am Coll Cardiol. 2020;76:930–941. doi: 10.1016/j.jacc.2020.06.061. [DOI] [PubMed] [Google Scholar]
- 27.Jing L., Ulloa Cerna A.E., Good C.W., et al. A machine learning approach to management of heart failure populations. J Am Coll Cardiol HF. 2020;8:578–587. doi: 10.1016/j.jchf.2020.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Angraal S., Mortazavi B.J., Gupta A., et al. Machine learning prediction of mortality and hospitalization in heart failure with preserved ejection fraction. J Am Coll Cardiol HF. 2020;8:12–21. doi: 10.1016/j.jchf.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 29.Adler E.D., Voors A.A., Klein L., et al. Improving risk prediction in heart failure using machine learning. Eur J Heart Fail. 2020;22:139–147. doi: 10.1002/ejhf.1628. [DOI] [PubMed] [Google Scholar]
- 30.Huang S., Xu Y., Yue L., et al. Evaluating the risk of hypertension using an artificial neural network method in rural residents over the age of 35 years in a Chinese area. Hypertension Res. 2010;33:722–726. doi: 10.1038/hr.2010.73. [DOI] [PubMed] [Google Scholar]
- 31.AlKaabi L.A., Ahmed L.S., Al Attiyah M.F., Abdel-Rahman M.E. Predicting hypertension using machine learning: findings from Qatar Biobank Study. PloS One. 2020;15 doi: 10.1371/journal.pone.0240370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakr S., Elshawi R., Ahmed A., et al. Using machine learning on cardiorespiratory fitness data for predicting hypertension: the Henry Ford ExercIse Testing (FIT) Project. PloS One. 2018;13 doi: 10.1371/journal.pone.0195344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.López-Martínez F., Núñez-Valdez E.R., Crespo R.G., García-Díaz V. An artificial neural network approach for predicting hypertension using NHANES data. Sci Rep. 2020;10:10620. doi: 10.1038/s41598-020-67640-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun J., McNaughton C.D., Zhang P., et al. Predicting changes in hypertension control using electronic health records from a chronic disease management program. J Am Med Informatics Assoc. 2014;21:337–344. doi: 10.1136/amiajnl-2013-002033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katz D.H., Deo R.C., Aguilar F.G., et al. Phenomapping for the identification of hypertensive patients with the myocardial substrate for heart failure with preserved ejection fraction. J Cardiovasc Transl Res. 2017;10:275–284. doi: 10.1007/s12265-017-9739-z. [DOI] [PubMed] [Google Scholar]
- 36.Ye C., Fu T., Hao S., et al. Prediction of incident hypertension within the next year: prospective study using statewide electronic health records and machine learning. J Med Internet Res. 2018;20:e22. doi: 10.2196/jmir.9268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park J., Kim J.W., Ryu B., Heo E., Jung S.Y., Yoo S. Patient-level prediction of cardio-cerebrovascular events in hypertension using nationwide claims data. J Med Internet Res. 2019;21:e11757. doi: 10.2196/11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanegae H., Suzuki K., Fukatani K., Ito T., Harada N., Kario K. Highly precise risk prediction model for new-onset hypertension using artificial intelligence techniques. J Clin Hypertens. 2020;22:445–450. doi: 10.1111/jch.13759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y., Li X., Mao L., et al. Factors affecting medication adherence in community-managed patients with hypertension based on the principal component analysis: evidence from Xinjiang, China. Patient Prefer Adherence. 2018;12:803–812. doi: 10.2147/PPA.S158662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalogeropoulos A.P., Goulbourne C., Butler J. Diagnosis and prevention of hypertensive heart failure. Heart Fail Clin. 2019;15:435–445. doi: 10.1016/j.hfc.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Iriarte M., Murga N., Sagastagoitia D., et al. Classification of hypertensive cardiomyopathy. Eur Heart J. 1993;14(suppl J):95–101. [PubMed] [Google Scholar]
- 42.Dey D., Slomka P.J., Leeson P., et al. Artificial intelligence in cardiovascular imaging: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:1317–1335. doi: 10.1016/j.jacc.2018.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lancaster M.C., Salem Omar A.M., Narula S., Kulkarni H., Narula J., Sengupta P.P. Phenotypic clustering of left ventricular diastolic function parameters: patterns and prognostic relevance. J Am Coll Cardiol Img. 2019;12:1149–1161. doi: 10.1016/j.jcmg.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 44.Omar A.M.S., Narula S., Abdel Rahman M.A., et al. Precision phenotyping in heart failure and pattern clustering of ultrasound data for the assessment of diastolic dysfunction. J Am Coll Cardiol Img. 2017;10:1291–1303. doi: 10.1016/j.jcmg.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 45.Ahmad T., Lund L.H., Rao P., et al. Machine learning methods improve prognostication, identify clinically distinct phenotypes, and detect heterogeneity in response to therapy in a large cohort of heart failure patients. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.008081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woolley R.J., Ceelen D., Ouwerkerk W., et al. Machine learning based on biomarker profiles identifies distinct subgroups of heart failure with preserved ejection fraction. Eur J Heart Fail. 2021;23:983–991. doi: 10.1002/ejhf.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shah S.J., Katz D.H., Selvaraj S., et al. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131:269–279. doi: 10.1161/CIRCULATIONAHA.114.010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Narula S., Shameer K., Salem Omar A.M., Dudley J.T., Sengupta P.P. Machine-learning algorithms to automate morphological and functional assessments in 2D echocardiography. J Am Coll Cardiol. 2016;68:2287–2295. doi: 10.1016/j.jacc.2016.08.062. [DOI] [PubMed] [Google Scholar]
- 49.Parikh N.I., Pencina M.J., Wang T.J., et al. A risk score for predicting near-term incidence of hypertension: the Framingham Heart Study. Ann Internal Med. 2008;148:102–110. doi: 10.7326/0003-4819-148-2-200801150-00005. [DOI] [PubMed] [Google Scholar]
- 50.Paynter N.P., Cook N.R., Everett B.M., Sesso H.D., Buring J.E., Ridker P.M. Prediction of incident hypertension risk in women with currently normal blood pressure. Am J Med. 2009;122:464–471. doi: 10.1016/j.amjmed.2008.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kshirsagar A.V., Chiu Y.L., Bomback A.S., et al. A hypertension risk score for middle-aged and older adults. J Clin Hypertens. 2010;12:800–808. doi: 10.1111/j.1751-7176.2010.00343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fava C., Sjögren M., Montagnana M., et al. Prediction of blood pressure changes over time and incidence of hypertension by a genetic risk score in Swedes. Hypertension. 2013;61:319–326. doi: 10.1161/HYPERTENSIONAHA.112.202655. [DOI] [PubMed] [Google Scholar]
- 53.Völzke H., Fung G., Ittermann T., et al. A new, accurate predictive model for incident hypertension. J Hypertens. 2013;31:2142–2150. doi: 10.1097/HJH.0b013e328364a16d. discussion 2150. [DOI] [PubMed] [Google Scholar]
- 54.Chen Y., Wang C., Liu Y., et al. Incident hypertension and its prediction model in a prospective northern urban Han Chinese cohort study. J Hypertens. 2016;30:794–800. doi: 10.1038/jhh.2016.23. [DOI] [PubMed] [Google Scholar]
- 55.Kivimäki M., Batty G.D., Singh-Manoux A., et al. Validating the Framingham Hypertension Risk Score: results from the Whitehall II study. Hypertension. 2009;54:496–501. doi: 10.1161/HYPERTENSIONAHA.109.132373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chinese guidelines for the diagnosis and treatment of heart failure 2018 [In Chinese] Zhonghua Xin Xue Guan Bing Za Zhi. 2018;46:760–789. doi: 10.3760/cma.j.issn.0253-3758.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 57.Yancy C.W., Jessup M., Bozkurt B., et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. doi: 10.1161/CIR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 58.Ponikowski P., Voors A.A., Anker S.D., et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 59.Huang M.S., Wang C.S., Chiang J.H., Liu P.Y., Tsai W.C. Automated recognition of regional wall motion abnormalities through deep neural network interpretation of transthoracic echocardiography. Circulation. 2020;142:1510–1520. doi: 10.1161/CIRCULATIONAHA.120.047530. [DOI] [PubMed] [Google Scholar]
- 60.Adedinsewo D., Carter R.E., Attia Z., et al. Artificial intelligence-enabled ECG algorithm to identify patients with left ventricular systolic dysfunction presenting to the emergency department with dyspnea. Circul Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.120.008437. [DOI] [PubMed] [Google Scholar]
- 61.Knackstedt C., Bekkers S.C., Schummers G., et al. Fully automated versus standard tracking of left ventricular ejection fraction and longitudinal strain: the FAST-EFs multicenter study. J Am Coll Cardiol. 2015;66:1456–1466. doi: 10.1016/j.jacc.2015.07.052. [DOI] [PubMed] [Google Scholar]
- 62.Zhang J., Gajjala S., Agrawal P., et al. Fully automated echocardiogram interpretation in clinical practice. Circulation. 2018;138:1623–1635. doi: 10.1161/CIRCULATIONAHA.118.034338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choi E., Schuetz A., Stewart W.F., Sun J. Using recurrent neural network models for early detection of heart failure onset. J Am Med Infromat Assoc. 2017;24:361–370. doi: 10.1093/jamia/ocw112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Litjens G., Ciompi F., Wolterink J.M., et al. State-of-the-art deep learning in cardiovascular image analysis. J Am Coll Cardiol Img. 2019;12:1549–1565. doi: 10.1016/j.jcmg.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 65.Chen C., Qin C., Qiu H., et al. Deep learning for cardiac image segmentation: a review. Front Cardiovasc Med. 2020;7:25. doi: 10.3389/fcvm.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Narang A., Bae R., Hong H., et al. Utility of a deep-learning algorithm to guide novices to acquire echocardiograms for limited diagnostic use. JAMA Cardiol. 2021;6:624–632. doi: 10.1001/jamacardio.2021.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kansagara D., Englander H., Salanitro A., et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306:1688–1698. doi: 10.1001/jama.2011.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ross J.S., Mulvey G.K., Stauffer B., et al. Statistical models and patient predictors of readmission for heart failure: a systematic review. Arch Intern Med. 2008;168:1371–1386. doi: 10.1001/archinte.168.13.1371. [DOI] [PubMed] [Google Scholar]
- 69.Rahimi K., Bennett D., Conrad N., et al. Risk prediction in patients with heart failure: a systematic review and analysis. J Am Coll Cardiol HF. 2014;2:440–446. doi: 10.1016/j.jchf.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 70.Hammill B.G., Curtis L.H., Fonarow G.C., et al. Incremental value of clinical data beyond claims data in predicting 30-day outcomes after heart failure hospitalization. Circ Cardiovasc Qual Outcomes. 2011;4:60–67. doi: 10.1161/CIRCOUTCOMES.110.954693. [DOI] [PubMed] [Google Scholar]
- 71.Eapen Z.J., Liang L., Fonarow G.C., et al. Validated, electronic health record deployable prediction models for assessing patient risk of 30-day rehospitalization and mortality in older heart failure patients. J Am Coll Cardiol HF. 2013;1:245–251. doi: 10.1016/j.jchf.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 72.Mortazavi B.J., Downing N.S., Bucholz E.M., et al. analysis of machine learning techniques for heart failure readmissions. Circ Cardiovasc Qual Outcomes. 2016;9:629–640. doi: 10.1161/CIRCOUTCOMES.116.003039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Subramanian D., Subramanian V., Deswal A., Mann D.L. New predictive models of heart failure mortality using time-series measurements and ensemble models. Circ Heart Fail. 2011;4:456–462. doi: 10.1161/CIRCHEARTFAILURE.110.958496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y., Tang Y., Zou Y., et al. Plasma level of big endothelin-1 predicts the prognosis in patients with hypertrophic cardiomyopathy. Int J Cardiol. 2017;243:283–289. doi: 10.1016/j.ijcard.2017.03.162. [DOI] [PubMed] [Google Scholar]
- 75.Schiffrin E.L. Vascular endothelin in hypertension. Vasc Pharmacol. 2005;43:19–29. doi: 10.1016/j.vph.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 76.Cohen J.B., Schrauben S.J., Zhao L., et al. Clinical phenogroups in heart failure with preserved ejection fraction: detailed phenotypes, prognosis, and response to spironolactone. J Am Coll Cardiol HF. 2020;8:172–184. doi: 10.1016/j.jchf.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Van Calster B., McLernon D.J., van Smeden M., Wynants L., Steyerberg E.W. Calibration: the Achilles heel of predictive analytics. BMC Med. 2019;17:230. doi: 10.1186/s12916-019-1466-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Steyerberg E.W., Harrell F.E, Jr, Borsboom G.J., Eijkemans M.J., Vergouwe Y., Habbema J.D. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–781. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 79.Price W.N., 2nd, Gerke S., Cohen I.G. Potential liability for physicians using artificial intelligence. JAMA. 2019;322:1765–1766. doi: 10.1001/jama.2019.15064. [DOI] [PubMed] [Google Scholar]
- 80.Krittanawong C., Bomback A.S., Baber U., Bangalore S., Messerli F.H., Wilson Tang W.H. Future direction for using artificial intelligence to predict and manage hypertension. Curr Hypertens Rep. 2018;20:75. doi: 10.1007/s11906-018-0875-x. [DOI] [PubMed] [Google Scholar]
- 81.Aghaeepour N., Finak G., Hoos H., et al. Critical assessment of automated flow cytometry data analysis techniques. Nat Methods. 2013;10:228–238. doi: 10.1038/nmeth.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brinkman R.R., Aghaeepour N., Finak G., Gottardo R., Mosmann T., Scheuermann R.H. Automated analysis of flow cytometry data comes of age. Cytometry. A. 2016;89:13–15. doi: 10.1002/cyto.a.22810. [DOI] [PubMed] [Google Scholar]
- 83.Munuswamy K., Alpert M.A., Martin R.H., Whiting R.B., Mechlin N.J. Sensitivity and specificity of commonly used electrocardiographic criteria for left atrial enlargement determined by M-mode echocardiography. Am J Cardiol. 1984;53:829–832. doi: 10.1016/0002-9149(84)90413-2. [DOI] [PubMed] [Google Scholar]
- 84.Nagueh S.F., Smiseth O.A., Appleton C.P., et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 85.Olsen F.J., Bertelsen L., de Knegt M.C., et al. Multimodality cardiac imaging for the assessment of left atrial function and the association with atrial arrhythmias. Circ Cardiovasc Imaging. 2016;9 doi: 10.1161/CIRCIMAGING.116.004947. [DOI] [PubMed] [Google Scholar]
- 86.Levy D., Labib S.B., Anderson K.M., Christiansen J.C., Kannel W.B., Castelli W.P. Determinants of sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy. Circulation. 1990;81:815–820. doi: 10.1161/01.cir.81.3.815. [DOI] [PubMed] [Google Scholar]
- 87.Lang R.M., Badano L.P., Mor-Avi V., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 88.Doherty J.U., Kort S., Mehran R., et al. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2019 appropriate use criteria for multimodality imaging in the assessment of cardiac structure and function in nonvalvular heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2019;73:488–516. doi: 10.1016/j.jacc.2018.10.038. [DOI] [PubMed] [Google Scholar]
- 89.Luis S.A., Chan J., Pellikka P.A. Echocardiographic assessment of left ventricular systolic function: an overview of contemporary techniques, including speckle-tracking echocardiography. Mayo Clinic Proc. 2019;94:125–138. doi: 10.1016/j.mayocp.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 90.Voigt J.U., Pedrizzetti G., Lysyansky P., et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Hear J Cardiovasc Imaging. 2015;16:1–11. doi: 10.1093/ehjci/jeu184. [DOI] [PubMed] [Google Scholar]
- 91.Ramos J.G., Fyrdahl A., Wieslander B., et al. Comprehensive cardiovascular magnetic resonance diastolic dysfunction grading shows very good agreement compared with echocardiography. J Am Coll Cardiol Img. 2020;13:2530–2542. doi: 10.1016/j.jcmg.2020.06.027. [DOI] [PubMed] [Google Scholar]
- 92.Frizzell J.D., Liang L., Schulte P.J., et al. Prediction of 30-day all-cause readmissions in patients hospitalized for heart failure: comparison of machine learning and other statistical approaches. JAMA Cardiol. 2017;2:204–209. doi: 10.1001/jamacardio.2016.3956. [DOI] [PubMed] [Google Scholar]