Abstract
Background
Patients with heart failure with reduced ejection fraction (HFrEF) in Asia exhibit many differences from those in other parts of the world.
Objectives
This study sought to investigate the efficacy and safety of dapagliflozin, compared with placebo, in HFrEF patients in Asia, compared with those elsewhere, enrolled in the DAPA-HF (Dapagliflozin and Prevention of Adverse-outcomes in Heart Failure) trial.
Methods
Patients in New York Heart Association functional class II to IV with a left ventricular ejection fraction ≤40% and elevated N-terminal pro–B-type natriuretic peptide were eligible for the DAPA-HF trial. The primary outcome in the DAPA-HF trial was the composite of an episode of worsening HF (HF hospitalization or urgent HF visit requiring intravenous therapy) or cardiovascular death.
Results
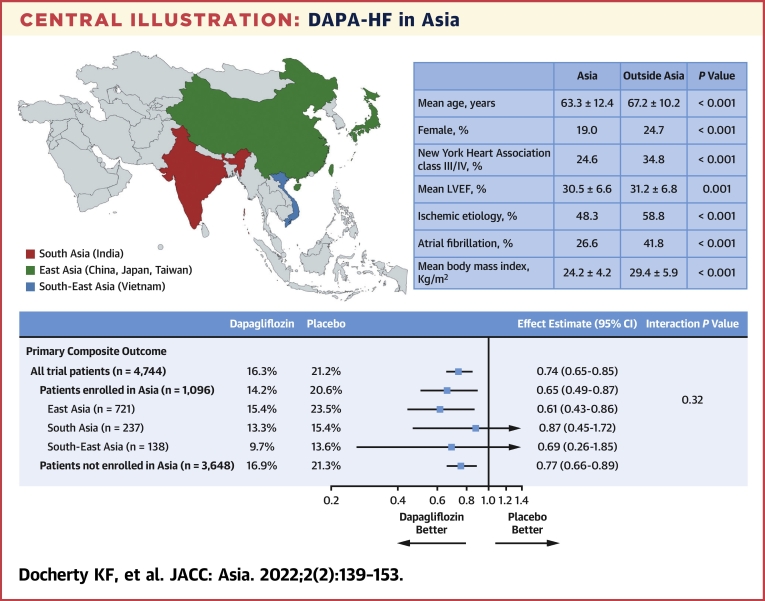
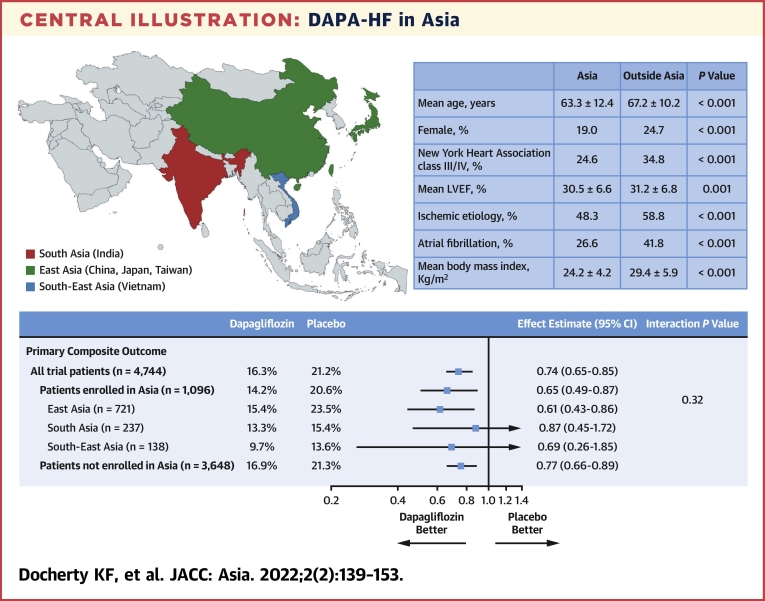
Of the 4,744 patients in the DAPA-HF trial, 1,096 (23.1%) were enrolled in Asia; 721 (15.2% overall, 65.8% of patients in Asia) were enrolled in East Asia (237 in China, 343 in Japan, and 141 in Taiwan), 138 (2.9% overall, 12.6% in Asia) in South-East Asia (Vietnam), and 237 (5.0% overall, 21.6% in Asia) in South Asia (India). Patients from Asia had similar rates of worsening HF events and mortality compared with patients elsewhere. Compared with placebo, dapagliflozin reduced the risk of the primary endpoint to the same extent in patients from Asia (HR: 0.65; 95% CI: 0.49 to 0.87) as elsewhere (HR: 0.77; 95% CI: 0.66 to 0.89) (P for interaction = 0.32). Consistent benefits were observed for the other prespecified outcomes and among the regions of Asia. Study drug discontinuation and prespecified adverse events did not differ between regions.
Conclusions
Dapagliflozin, compared with placebo, reduced the risk of worsening HF events and cardiovascular death to the same extent in Asian patients as elsewhere. (Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients With Chronic Heart Failure [DAPA-HF]; NCT03036124)
Key Words: Asia, ejection fraction, heart failure, SGLT2 inhibitor
Abbreviations and Acronyms: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; eGFR, estimated glomerular filtration rate; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; KCCQ-TSS, Kansas City Cardiomyopathy Questionnaire total symptom score; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro–B-type natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure; SGLT2, sodium-glucose cotransporter 2
Central Illustration
Sodium-glucose cotransporter 2 (SGLT2) inhibitors have recently been shown to be of therapeutic value in patients with type 2 diabetes, chronic kidney disease, and heart failure with reduced ejection fraction (HFrEF), conditions that commonly coexist and are interrelated pathophysiologically.1, 2, 3, 4, 5, 6, 7, 8, 9 However, differences between patients in Asia and elsewhere, and among patients in different regions of Asia, have been reported for each of these conditions.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35 Because of this, a full understanding of the efficacy and tolerability of SGLT2 inhibitors in Asian patients is clinically important, especially as nearly 60% of people in the world live in Asia, and China and India together constitute about 37% of the global population.
Patients in Asia develop type 2 diabetes at an earlier age, possibly reflecting different genetic susceptibility, and with a lower body mass index (BMI), compared with Western patients.10 Asian patients may have higher visceral adiposity, despite lower BMI, and greater pancreatic beta cell dysfunction.10, 11, 12,19 East Asian patients with type 2 diabetes have a higher risk of developing kidney disease than in any other region of the world but may respond more favorably to renin-angiotensin system blockade.29, 30, 31, 32 Patients with HFrEF in Asia also differ markedly from elsewhere, and there is also considerable heterogeneity within Asia.20, 21, 22, 23, 24, 25, 26, 27 In most Asian countries, HF develops at a much younger age than in the West and is less likely to be caused by coronary artery disease, particularly in East Asia. Asian patients with HFrEF have a lower BMI and blood pressure than those in Europe and North America.20, 21, 22, 23, 24, 25, 26, 27 Concomitant atrial fibrillation is less frequent in Asian patients, and natriuretic peptide concentrations lower, than among patients in the West.20, 21, 22, 23, 24, 25, 26, 27,33 The use of HFrEF treatment varies markedly between Asia and Europe and North America, with much lower use of diuretics and devices but greater use of digoxin (although considerable heterogeneity exists within Asia).20, 21, 22, 23, 24, 25, 26, 27
Also relevant is the salt-sensitive hypertension phenotype reported in Asia, possibly reflecting reduced ability to excrete a sodium load. Coupled with possible differences in pharmacogenetic background, these considerations make a detailed analysis of the efficacy and safety of SGLT2 inhibitors in Asian patients of interest.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18,34,35 Therefore, we have examined these outcomes in patients randomized in Asia in the DAPA-HF (Dapagliflozin and Prevention of Adverse-outcomes in Heart Failure) trial.6,36,37
Methods
The DAPA-HF trial was a randomized, double-blind trial that evaluated the efficacy and safety of dapagliflozin 10 mg once daily compared with matching placebo in patients with HFrEF. Randomized therapy was added to standard background treatment. All patients provided written informed consent and the trial protocol was approved by Ethics Committees at the 410 participating investigative centers.
Patients randomized
People in New York Heart Association (NYHA) functional class II to IV with a diagnosis of HF for ≥2 months were eligible if they had an left ventricular ejection fraction (LVEF) ≤40%, modest elevation of N-terminal pro–B-type natriuretic peptide (NT-proBNP), and were optimally treated with pharmacological and device therapy for HFrEF. Key exclusion criteria included type 1 diabetes, symptoms of hypotension or systolic blood pressure <95 mm Hg, and estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2.
Asian countries and regions
Patients were recruited in 20 countries, including 5 in Asia.6,37 Three of these 5 were in East Asia (China, Japan, and Taiwan), 1 in South-East Asia (Vietnam), and 1 in South Asia (India).
Clinical outcomes
The primary outcome was the composite of an episode of worsening HF (HF hospitalization or an urgent visit for worsening HF involving administration of intravenous therapy) or cardiovascular death, whichever occurred first. The secondary outcomes were the occurrence of HF hospitalization or cardiovascular death (we also examined the components of this composite); total HF hospitalizations (first and repeat) or cardiovascular death; change from baseline to 8 months in Kansas City Cardiomyopathy Questionnaire total symptom score (KCCQ-TSS); a composite worsening renal function endpoint; and all-cause mortality. Because there were few renal events overall, this endpoint was not analyzed in the present subgroup analysis. Several exploratory endpoints were prespecified, including change from baseline in systolic blood pressure (SBP), weight, hematocrit, and eGFR.
Prespecified safety analyses included serious adverse events, adverse events leading to discontinuation of randomized treatment, and “adverse events of interest.”
Statistical analyses
Baseline characteristics were summarized as frequency and percentage, mean ± SD, or median (IQR).
Time-to-event data for the primary outcome and secondary clinical outcomes, regardless of treatment allocation, were estimated using the Kaplan-Meier method and Cox proportional hazards models, stratified according to diabetes mellitus status, with a history of HF hospitalization and treatment group assignment as fixed-effect factors to calculate HRs, 95% CIs, and 2-sided P values. Total, including recurrent, events were evaluated with semiparametric proportional rates models, as described previously.6 The models for all-cause death did not include an adjustment for history of HF hospitalization. In addition, adjusted HRs were calculated from models including age, sex, heart rate, SBP, BMI, HF etiology, LVEF, NYHA functional class, NT-proBNP, atrial fibrillation, and eGFR.
The efficacy of dapagliflozin in Asia as a whole, and according to Asian country or region, was also examined using Cox proportional hazards models stratified according to diabetes status, with a history of HF hospitalization and treatment group assignment as explanatory variables (for all-cause death, history of HF hospitalization was not included in the model). The presence of a treatment effect interaction with geographical region (Asia vs non-Asia) was examined by means of inclusion of a region-by-treatment interaction term in these models.
The difference between groups in the change in KCCQ-TSS from baseline to 8 months in surviving patients was analyzed using a linear regression model adjusting for baseline value. Responder analyses were also carried out, calculating the proportions of patients with a clinically meaningful change (≥5 points) in KCCQ-TSS by 8 months, with the treatment effect expressed as an OR. Multiple imputation was used to account for missing KCCQ-TSS values using methods described previously.6,38
Longitudinal measures, including SBP, weight, hematocrit, and eGFR, were analyzed using a mixed-effect model for repeated measurements with unstructured covariance (adjusted for baseline values, visit, randomized treatment, and interaction of treatment and visit with a random intercept and slope per patient). The least-squares mean differences between treatment groups were estimated with 95% CIs and plotted for each group.
The effect of dapagliflozin, compared with placebo, on the incidence of new onset diabetes in patients without diabetes at baseline was examined using logistic regression as previously described.39
For prespecified safety outcomes, interaction P values were derived from logistic regression models including an interaction term between region (Asia/outside Asia) and randomized treatment.
All analyses were conducted using STATA version 16.1 (StataCorp) and SAS version 9.4 (SAS Institute). All analyses contained in this manuscript are post hoc and should be considered as exploratory. A P value of 0.05 was considered statistically significant.
Results
Of the 4,744 patients randomized in the DAPA-HF trial, 1,096 (23.1%) were enrolled in Asia. Of these, 721 (15.2% overall, 65.8% of patients in Asia) were enrolled in East Asia (237 in China, 343 in Japan, and 141 in Taiwan). Another 138 (2.9% overall, 12.6% in Asia) patients were enrolled in South-East Asia (Vietnam) and 237 (5.0% overall, 21.6% in Asia) were in South Asia (India). Of the 1,096 patients enrolled in Asia, 1,094 (99.8%) were reported to be of Asian race, and hereafter the adjective “Asian” is used to describe patients enrolled in an Asian country.
Patient characteristics: Asia versus overall population
Patients in Asia were considerably younger (mean 63.3 years of age) than those enrolled outside Asia (67.2 years of age) and in the overall trial population (66.3 years of age) (Table 1). More patients in Asia (81.0%) were male, compared with participants enrolled outside Asia (75.3%) and in the trial overall (76.6%). Asian patients had a much lower BMI and less evidence of coronary heart disease than in the trial overall. The proportion of patients enrolled in Asia with a BMI ≥30 kg/m2 was 9.9% versus 42.9% among those enrolled outside Asia. The prevalence of hypertension and chronic kidney disease (eGFR <60 mL/min/1.73 m2) was lower than in Asian patients than in the trial overall, whereas the prevalence of diabetes was similar. The prevalence of atrial fibrillation was considerably lower in Asia (26.6%) than in participants enrolled outside Asia (41.8%) and in the trial overall (38.3%). Patients in Asia had a higher heart rate and lower SBP than those from outside Asia.
Table 1.
Baseline Characteristics Overall and According to Geographic Region (Asia, Outside Asia, and Among Regions of Asia)
| Overall (N = 4,744) | Outside Asia (n = 3,648) | Asia (n = 1,096) | Asia vs. Outside Asia (P Value) | East Asia (n = 721) | South Asia (n = 237) | South-East Asia (n = 138) | Within Asia (P Value) | |
|---|---|---|---|---|---|---|---|---|
| Age, y | 66.3 ± 10.9 | 67.2 ± 10.2 | 63.3 ± 12.4 | <0.001 | 66.1 ± 11.8 | 56.6 ± 11.1 | 60.7 ± 12.5 | <0.001 |
| Sex | <0.001 | <0.001 | ||||||
| Female | 1,109 (23.4) | 901 (24.7) | 208 (19.0) | 112 (15.5) | 54 (22.8) | 42 (30.4) | ||
| Male | 3,635 (76.6) | 2,747 (75.3) | 888 (81.0) | 609 (84.5) | 183 (77.2) | 96 (69.6) | ||
| Race | <0.001 | 0.11 | ||||||
| White | 3,333 (70.3) | 3,332 (91.3) | 1 (0.1) | 0 (0) | 0 (0) | 1 (0.7) | ||
| Black | 226 (4.8) | 226 (6.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Asian | 1,116 (23.5) | 22 (0.6) | 1,094 (99.8) | 720 (99.9) | 237 (100) | 137 (99.3) | ||
| Other | 69 (1.5) | 68 (1.9) | 1 (0.1) | 1 (0.1) | 0 (0) | 0 (0) | ||
| NYHA functional class | <0.001 | <0.001 | ||||||
| II | 3,203 (67.5) | 2,377 (65.2) | 826 (75.4) | 520 (72.1) | 191 (80.6) | 115 (83.3) | ||
| III | 1,498 (31.6) | 1,252 (34.3) | 246 (22.4) | 177 (24.5) | 46 (19.4) | 23 (16.7) | ||
| IV | 43 (0.9) | 19 (0.5) | 24 (2.2) | 24 (3.3) | 0 (0.0) | 0 (0.0) | ||
| Heart rate, beats/min | 71.5 ± 11.7 | 70.6 ± 11.5 | 74.3 ± 12.0 | <0.001 | 73.2 ± 12.0 | 78.6 ± 10.9 | 73.1 ± 11.6 | <0.001 |
| Systolic blood pressure, mm Hg | 121.8 ± 16.3 | 122.9 ± 16.2 | 118.3 ± 16.3 | <0.001 | 118.9 ± 16.7 | 117.0 ± 14.5 | 117.0 ± 16.9 | 0.19 |
| Left ventricular ejection fraction, % | 31.1 ± 6.8 | 31.2 ± 6.8 | 30.5 ± 6.6 | 0.001 | 31.4 ± 6.3 | 27.8 ± 6.3 | 30.3 ± 7.2 | <0.001 |
| ≤30% | 2,161 (45.6) | 1,616 (44.3) | 545 (49.7) | 0.002 | 305 (42.3) | 175 (73.8) | 65 (47.1) | <0.001 |
| NT-proBNP, pg/mL | 1,437(857-2,650) | 1,464 (874-2721) | 1,368(812-2,436) | 0.022 | 1,420 (872-2,485) | 1,416 (759-2,665) | 996 (606-1,993) | <0.001 |
| AF on baseline ECG | 1,948 (1,265-3,204) | 1,981 (1,275-3,259) | 1,878 (1,236-2,985) | 0.41 | 1,898 (1,239-3,049) | 2,116 (1,846-3110) | 1,563 (1,084-2,286) | 0.10 |
| No AF on baseline ECG | 1,290 (772-2,415) | 1,302 (778-2,471) | 1,249 (746-2267) | 0.30 | 1,271 (800-2,242) | 1,383 (746-2634) | 848 (567-1,887) | <0.001 |
| KCCQ-TSSa | 77.1 (58.3-91.7) | 75.0 (56.2-89.6) | 86.5 (71.9-96.9) | <0.001 | 88.5 (75.0-97.9) | 79.2 (65.6-90.1) | 86.5 (76.0-100.0) | <0.001 |
| KCCQ-CSS | 74.3 (56.9-88.2) | 70.8 (54.3-86.1) | 81.9 (69.4-93.1) | <0.001 | 83.3 (72.2-94.4) | 73.6 (61.1-85.3) | 81.9 (68.1-95.6) | <0.001 |
| KCCQ-OSS | 70.8 (53.6-85.0) | 68.8 (51.2-84.6) | 77.2 (63.3-87.5) | <0.001 | 79.3 (67.2-89.2) | 67.7 (53.5-81.2) | 77.1 (57.9-89.2) | <0.001 |
| Body mass index, kg/m2 | 28.2 ± 6.0 | 29.4 ± 5.9 | 24.2 ± 4.2 | <0.001 | 24.5 ± 4.4 | 23.9 ± 4.1 | 22.9 ± 3.4 | <0.001 |
| ≥30 kg/m2 | 1672 (35.3) | 1,564 (42.9) | 108 (9.9) | <0.001 | 83 (11.5) | 21 (8.9) | 4 (2.9) | 0.007 |
| Principal cause of heart failure | <0.001 | 0.002 | ||||||
| Ischemic | 2,674 (56.4) | 2,145 (58.8) | 529 (48.3) | 328 (45.5) | 119 (50.2) | 82 (59.4) | ||
| Nonischemic | 1,687 (35.6) | 1,224 (33.6) | 463 (42.2) | 329 (45.6) | 86 (36.3) | 48 (34.8) | ||
| Unknown | 383 (8.1) | 279 (7.6) | 104 (9.5) | 64 (8.9) | 32 (13.5) | 8 (5.8) | ||
| Medical history | ||||||||
| Hospitalization for heart failure | 2,251 (47.4) | 1,657 (45.4) | 594 (54.2) | <0.001 | 477 (66.2) | 78 (32.9) | 39 (28.3) | <0.001 |
| Atrial fibrillation | 1,818 (38.3) | 1,526 (41.8) | 292 (26.6) | <0.001 | 252 (35.0) | 11 (4.6) | 29 (21.0) | <0.001 |
| Type 2 diabetes | 1,983 (41.8) | 1,536 (42.1) | 447 (40.8) | 0.44 | 326 (45.2) | 80 (33.8) | 41 (29.7) | <0.001 |
| Hypertension | 3,523 (74.3) | 2,905 (79.6) | 618 (56.4) | <0.001 | 454 (63.0) | 78 (32.9) | 86 (62.3) | <0.001 |
| PCI | 1,624 (34.2) | 1,290 (35.4) | 334 (30.5) | 0.003 | 251 (34.8) | 50 (21.1) | 33 (23.9) | <0.001 |
| CABG | 799 (16.8) | 696 (19.1) | 103 (9.4) | <0.001 | 78 (10.8) | 16 (6.8) | 9 (6.5) | 0.082 |
| eGFR of body surface area, mL/min/1.73 m2 | 65.8 ± 19.4 | 64.5 ± 18.7 | 70.2 ± 21.0 | <0.001 | 67.7 ± 20.4 | 76.9 ± 22.1 | 71.4 ± 20.3 | <0.001 |
| eGFR rate <60 mL/min/1.73 m2 | 1,926 (40.6) | 1,561 (42.8) | 365 (33.3) | <0.001 | 271 (37.6) | 56 (23.6) | 38 (27.5) | <0.001 |
| Device therapy | ||||||||
| Implantable cardioverter-defibrillatorb | 1,242 (26.2) | 1,148 (31.5) | 94 (8.6) | <0.001 | 80 (11.1) | 13 (5.5) | 1 (0.7) | <0.001 |
| Cardiac resynchronization therapyc | 354 (7.5) | 298 (8.2) | 56 (5.1) | <0.001 | 52 (7.2) | 3 (1.3) | 1 (0.7) | <0.001 |
| Heart failure medication at randomization visit | ||||||||
| Diuretic | 4,008 (84.5) | 3,130 (85.8) | 878 (80.1) | <0.001 | 535 (74.2) | 222 (93.7) | 121 (87.7) | <0.001 |
| ACE inhibitor | 2,661 (56.1) | 2,146 (58.8) | 515 (47.0) | <0.001 | 303 (42.0) | 154 (65.0) | 58 (42.0) | <0.001 |
| ARB | 1,307 (27.6) | 896 (24.6) | 411 (37.5) | <0.001 | 293 (40.6) | 42 (17.7) | 76 (55.1) | <0.001 |
| ≥50% of target dose of ACE inhibitor/ARBd | 1,517 (38.4) | 1,370 (45.2) | 147 (15.9) | <0.001 | 71 (12.0) | 50 (25.5) | 26 (19.4) | <0.001 |
| Sacubitril-valsartan | 508 (10.7) | 451 (12.4) | 57 (5.2) | <0.001 | 36 (5.0) | 20 (8.4) | 1 (0.7) | 0.005 |
| Beta-blocker | 4,558 (96.1) | 3,556 (97.5) | 1002 (91.4) | <0.001 | 657 (91.1) | 224 (94.5) | 121 (87.7) | 0.066 |
| ≥50% of target dose of beta-blockerd | 2,349 (51.5) | 2,183 (61.4) | 166 (16.6) | <0.001 | 117 (17.8) | 14 (6.2) | 35 (28.9) | <0.001 |
| MRA | 3,370 (71.0) | 2,600 (71.3) | 770 (70.3) | 0.52 | 456 (63.2) | 180 (75.9) | 134 (97.1) | <0.001 |
| ≥50% of target dose of MRAd | 2,953 (87.6) | 2,402 (92.4) | 551 (71.6) | <0.001 | 248 (54.4) | 177 (98.3) | 126 (94.0) | <0.001 |
| Digitalis | 887 (18.7) | 649 (17.8) | 238 (21.7) | 0.003 | 144 (20.0) | 54 (22.8) | 40 (29.0) | 0.057 |
| Anticoagulant | 1,969 (41.5) | 1,666 (45.7) | 303 (27.6) | <0.001 | 261 (36.2) | 15 (6.3) | 27 (19.6) | <0.001 |
| History of AF | 1,529/1,818 (84.1) | 1,311/1,526 (85.9) | 218/292 (74.7) | <0.001 | 192/252 (76.2) | 3/11 (27.3) | 23/29 (79.3) | 0.001 |
| No history of AF | 440/2,926 (15.0) | 355/2,122 (16.7) | 85/804 (10.6) | <0.001 | 69/469 (14.7) | 12/226 (5.3) | 4/109 (3.7) | <0.001 |
Values are mean ± SD, n (%), median (IQR), or n/n (%). Percentages may not total 100 because of rounding. The countries included in the Asia regions were from East Asia—China (n = 237), Japan (n = 343), Taiwan (n = 141); South Asia—India (n = 237); and South-East Asia—Vietnam (n = 138).
ACE = angiotensin-converting enzyme; AF = atrial fibrillation; ARB = angiotensin-receptor blocker; ARNI = angiotensin receptor-neprilysin inhibitor; CABG = coronary artery bypass grafting; ECG = electrocardiogram; eGFR = estimated glomerular filtration rate; KCCQ-CSS = Kansas City Cardiomyopathy Questionnaire clinical summary score; KCCQ-OSS = Kansas City Cardiomyopathy Questionnaire overall summary score; KCCQ-TSS = Kansas City Cardiomyopathy Questionnaire total symptom score; LVEF = left ventricular ejection fraction; MRA = mineralocorticoid receptor antagonist; NT-proBNP = N-terminal pro–B-type natriuretic peptide; NYHA = New York Heart association; PCI = percutaneous coronary intervention.
Range from 0 to 100, with higher scores indicating fewer symptoms and physical limitations associated with heart failure. A score of 75 or above is considered to reflect satisfactory health status.
Either implantable cardioverter-defibrillator or cardiac resynchronization therapy with a defibrillator.
Cardiac resynchronization therapy with or without a defibrillator.
Percentages are of those taking the medication at baseline and with available dose information.
Patients in Asia had a higher (better) median KCCQ-TSS, and a more favorable NYHA functional class distribution, than in the trial overall cohort, and a slightly lower NT-proBNP. Examination of patients according to presence or absence of atrial fibrillation or flutter on the baseline electrocardiogram showed that NT-proBNP was still lower in Asian patients, irrespective of rhythm. Mean LVEF was similar in participants enrolled in Asia and the trial overall, but a history of HF hospitalization was more common among patients from Asia.
Compared with the trial overall, patients in Asia were more likely to be treated with an angiotensin receptor blocker (ARB) (37.5% in Asia and 27.6% overall) and digoxin (21.7% vs 18.7%) but were less likely to be treated with a diuretic (80.1% vs 84.5%), sacubitril/valsartan (5.2% vs 10.7%), a defibrillating device (8.6% vs 26.2%), or cardiac resynchronization therapy (5.1% vs 7.5%). Mineralocorticoid receptor antagonist use was similar (70.3% vs 71.0%). Use of anticoagulation was particularly low in Asia (27.6%) compared with overall (41.5%), even accounting for the difference in the prevalence of atrial fibrillation. Of those taking the medications at baseline and with available dose information, a lower proportion of patients in Asia, as compared with the overall population, were taking ≥50% of guideline-recommended target doses of an angiotensin-converting enzyme (ACE) inhibitor or ARB (15.9% vs 38.4%), beta-blocker (16.6% vs 51.5%), and mineralocorticoid receptor antagonist (71.6% vs 87.6%).
Patient characteristics: All Asia versus Asian regions/countries
There was as much variation within Asia as between Asia and the rest of the world (Table 1). Age varied greatly across Asia, with the youngest average age in South Asia (India) (56.6 years) and the oldest East Asia (66.1 years). Other notable differences were the higher mean heart rate (78.6 beats/min) and much lower mean LVEF (27.8%), the prevalence of atrial fibrillation (4.6%) and chronic kidney disease (23.6%), and history of hypertension (32.9%) in South Asia, compared with Asia overall and the trial overall. Device use was also particularly low in South Asia. South-East Asia (Vietnam) also differed markedly from Asia overall, with a lower median NT-proBNP, BMI, prior HF hospitalization, and use of sacubitril/valsartan, anticoagulants, and devices.
Outcomes in patients enrolled in Asia
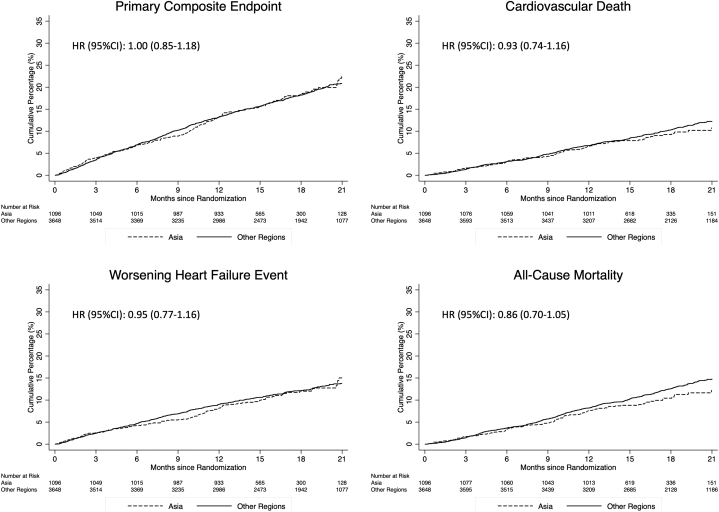
Compared with participants enrolled outside Asia, those enrolled in Asia had a similar risk of the primary composite outcome of a worsening HF event or cardiovascular death (Table 2, Figure 1). The risk of each component of this composite was also similar in the 2 different geographic regions. The risk of all-cause death was also similar in patients enrolled in Asia and outside Asia. These findings remained similar after adjustment for other prognostic variables (Table 2).
Table 2.
Cardiovascular Outcomes in Patients Enrolled in Asia and Outside Asia
| Patients Enrolled in Asia (n = 1,096) | Patients Not Enrolled in Asia (n = 3,648) | |
|---|---|---|
| Primary composite endpointa | ||
| Event rate per 100 person-y | 13.9 (12.0-16.0) | 13.4 (12.5-14.5) |
| Unadjusted HR (95% CI) | 1.00 (0.85-1.18), P = 0.98 | 1.00 (referent) |
| Adjusted HR (95% CI) | 1.19 (0.99-1.42), P = 0.06 | 1.00 (referent) |
| Cardiovascular death | ||
| Event rate per 100 person-y | 6.7 (5.5-8.2) | 7.3 (6.6-8.1) |
| Unadjusted HR (95% CI) | 0.93 (0.74-1.16), P = 0.50 | 1.00 (referent) |
| Adjusted HR (95% CI) | 0.98 (0.77-1.26), P = 0.90 | 1.00 (referent) |
| Worsening HF event | ||
| Event rate per 100 person-y | 8.6 (7.2-10.3) | 8.6 (7.8-9.4) |
| Unadjusted HR (95% CI) | 0.95 (0.77-1.16), P = 0.60 | 1.00 (referent) |
| Adjusted HR (95% CI) | 1.22 (0.98-1.53), P = 0.08 | 1.00 (referent) |
| All-cause mortality | ||
| Event rate per 100 person-y | 7.6 (6.3-9.2) | 9.0 (8.2-9.8) |
| Unadjusted HR (95% CI) | 0.86 (0.70-1.05), P = 0.15 | 1.00 (referent) |
| Adjusted HR (95% CI) | 0.93 (0.74-1.17), P = 0.56 | 1.00 (referent) |
| Total heart failure hospitalizations and cardiovascular deathb | ||
| Event rate per 100 person-y | 18.5 (16.4-20.8) | 19.0 (17.9-20.2) |
| Unadjusted RR (95% CI) | 0.96 (0.80-1.15), P = 0.62 | 1.00 (referent) |
| Adjusted RR (95% CI) | 1.13 (0.93-1.37), P = 0.23 | 1.00 (referent) |
Unadjusted analysis includes factors for Asia/not Asia, randomized treatment, and history of HF hospitalization and is stratified by diabetes status. Adjusted analysis includes factors for Asia/not Asia, randomized treatment, history of heart failure hospitalization, age, sex, heart rate, systolic blood pressure, BMI, ischemic etiology of heart failure, LVEF, NYHA functional class, NT-proBNP, atrial fibrillation, and eGFR (and stratified by type 2 diabetes status).
Abbreviations as in Table 1.
The primary outcome was a composite of worsening heart failure (hospitalization or an urgent visit resulting in intravenous therapy for heart failure) or death from cardiovascular causes.
Risk estimate presented is a rate ratio (RR).
Figure 1.
Cumulative Incidence of Cardiovascular Outcomes According to Geographic Region
The primary outcome was a composite of death from cardiovascular causes, hospitalization for heart failure, or an urgent visit resulting in intravenous therapy for heart failure. The cumulative incidences of the primary outcome, hospitalization for heart failure, death from cardiovascular causes, and death from any cause were estimated with the use of the Kaplan-Meier method.
Effects of dapagliflozin in patients enrolled in Asia
Primary composite outcome
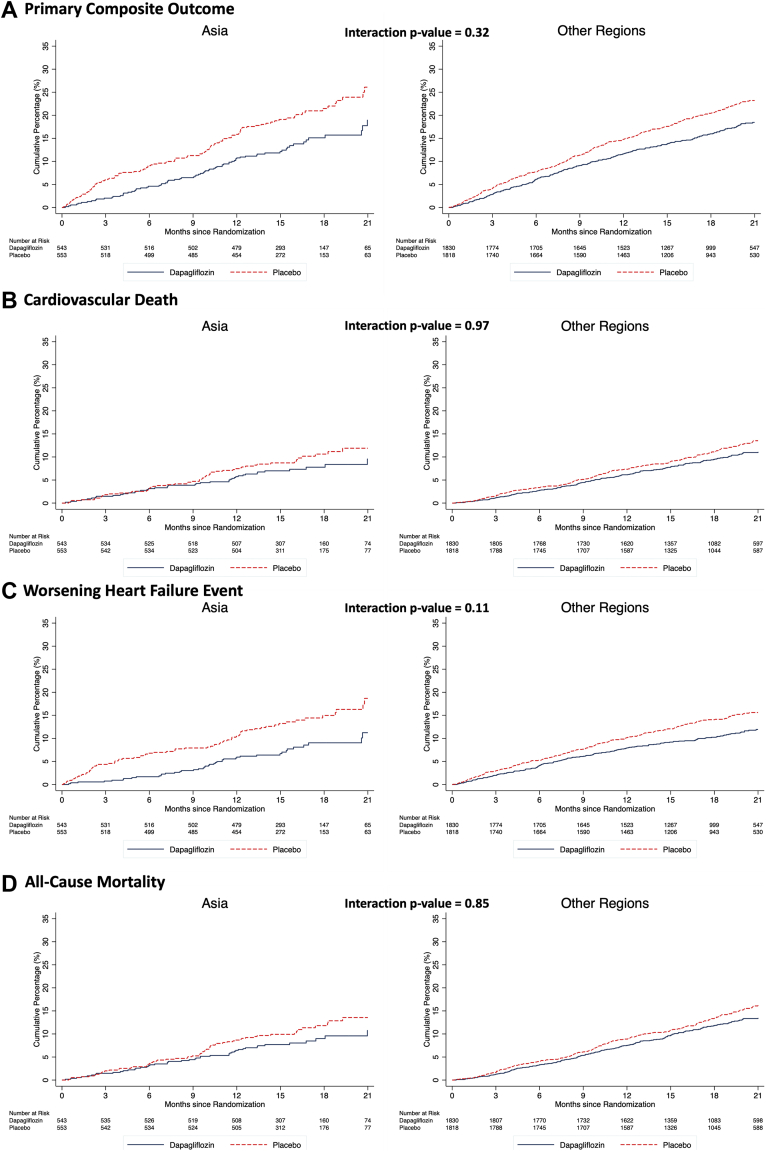
Dapagliflozin reduced the risk of worsening HF or cardiovascular death to the same extent in patients enrolled in Asia (HR: 0.65; 95% CI: 0.49 to 0.87) as in participants enrolled outside Asia (HR: 0.77; 95% CI: 0.66 to 0.89), with no interaction between geographical region and effect of treatment (P-interaction = 0.32) (Figure 2 and Table 3). Both components of the primary composite endpoint were reduced to the same extent in Asian patients as among patients enrolled outside Asia: the HR was 0.53 (95% CI: 0.37 to 0.78) for worsening HF events in Asian patients and 0.75 (95% CI: 0.62 to 0.91) in those from outside Asia (P for interaction = 0.11) and the HR for cardiovascular death was 0.83 (95% CI: 0.56 to 1.23) in Asian patients and 0.82 (95% CI: 0.67 to 1.00) elsewhere (P for interaction = 0.97).
Figure 2.
Effect of Dapagliflozin on Cardiovascular Outcomes According to Geographic Region
The primary outcome was a composite of death from cardiovascular causes, hospitalization for heart failure, or an urgent visit resulting in intravenous therapy for heart failure. The cumulative incidences of (A) the primary outcome, (B) hospitalization for heart failure, (C) death from cardiovascular causes, and (D) death from any cause were estimated with the use of the Kaplan-Meier method.
Table 3.
Effect of Dapagliflozin on Clinical Outcomes in Patients Enrolled in Asia and Outside Asia
| Patients Enrolled in Asia (n = 1,096) |
Patients Not Enrolled in Asia (n = 3,648) |
Interaction P Value | |||
|---|---|---|---|---|---|
| Dapagliflozin | Placebo | Dapagliflozin | Placebo | ||
| Primary composite outcomea | |||||
| Number of events | 77/543 (14.2) | 114/553 (20.6) | 309/1830 (16.9) | 388/1818 (21.3) | |
| Rate (95% CI) | 11.0 (8.8-13.7) | 16.8 (14.0-20.2) | 11.7 (10.5-13.1) | 15.2 (13.8-16.8) | |
| HR (95% CI) | 0.65 (0.49-0.87) | 0.77 (0.66-0.89) | 0.32 | ||
| Cardiovascular death | |||||
| Number of events | 44/543 (8.1) | 54/553 (9.8) | 183/1830 (10.0) | 219/1818 (12.0) | |
| Rate (95% CI) | 6.1 (4.5-8.2) | 7.4 (5.6-9.6) | 6.6 (5.7-7.7) | 8.1 (7.1-9.2) | |
| HR (95% CI) | 0.83 (0.56-1.23) | 0.82 (0.67-1.00) | 0.97 | ||
| Worsening heart failure eventa | |||||
| Number of events | 42/543 (7.7) | 76/553 (13.7) | 195/1830 (10.7) | 250/1818 (13.8) | |
| Rate (95% CI) | 6.0 (4.4-8.1) | 11.2 (9.0-14.1) | 7.4 (6.4-8.5) | 9.8 (8.7-11.1) | |
| HR (95% CI) | 0.53 (0.37-0.78) | 0.75 (0.62-0.91) | 0.11 | ||
| All-cause death | |||||
| Number of events | 49/543 (9.0) | 62/553 (11.2) | 227/1830 (12.4) | 267/1818 (14.7) | |
| Rate (95% CI) | 6.8 (5.1-8.9) | 8.4 (6.6-10.8) | 8.2 (7.2-9.3) | 9.8 (8.7-11.1) | |
| HR (95% CI) | 0.80 (0.55-1.17) | 0.83 (0.70-1.00) | 0.85 | ||
| First and recurrent heart failure hospitalization and cardiovascular death | |||||
| Number of events | 105 | 162 | 462 | 580 | |
| Rate (95% CI) | 15.6 (12.0-17.6) | 22.4 (19.2-26.1) | 16.8 (15.3-18.4) | 21.4 (19.7-23.2) | |
| Rate ratio (95% CI) | 0.65 (0.47-0.90) | 0.78 (0.66-0.93) | 0.32 | ||
| KCCQ-TSS | |||||
| Mean change in score at 8 mo (95% CI) | 4.7 (3.1-6.4) | 1.1 (-0.6-2.7) | 6.5 (5.5-7.4) | 4.0 (3.0-5.0) | 0.36 |
| Patients with ≥5-point improvement at 8 mo | 59.7 (55.3-64.2) | 50.4 (45.9-54.8) | 57.8 (55.4-60.3) | 51.1 (48.7-53.4) | |
| OR (95% CI) | 1.20 (1.05-1.37) | 1.14 (1.06-1.22) | 0.47 | ||
| Patients with ≥5-point deterioration at 8 mo | 27.5 (23.4-31.6) | 34.8 (30.5-39.1) | 24.7 (22.6-26.7) | 32.3 (30.0-34.6) | |
| OR (95% CI) | 0.85 (0.74-0.98) | 0.83 (0.77-0.90) | 0.81 | ||
Values are n/N (%), unless otherwise indicated. Event rates presented per 100 patient-years. HRs and 95% CIs were estimated with the use of Cox regression models, stratified according to diabetes status, with a history of hospitalization for heart failure and treatment group assignment as explanatory variables (for all-cause mortality history of hospitalization for heart failure was not included in the model).
KCCQ-TSS = Kansas City Cardiomyopathy Questionnaire total symptom score
The primary outcome was a composite of worsening heart failure (hospitalization or an urgent visit resulting in intravenous therapy for heart failure) or death from cardiovascular causes.
Secondary outcomes
HRs, rate ratios, and ORs for the effect of dapagliflozin compared with placebo on the secondary clinical endpoints are displayed in Table 3 and Figure 2. The effect of dapagliflozin in Asian patients was consistent with that in patients enrolled elsewhere for each secondary endpoint (Table 3). In particular, the rate ratio for the composite outcome of total (first and repeat) HF hospitalizations and cardiovascular death was 0.65 (95% CI: 0.47 to 0.90) in Asian patients compared with 0.78 (95% CI: 0.66 to 0.93) in patients enrolled outside Asia (P for interaction = 0.32). For all-cause mortality, the HR was 0.80 (95% CI: 0.55 to 1.17) in Asian patients and 0.83 (95% CI: 0.70 to 1.00) in other patients (P for interaction = 0.85).
The proportion of patients with an improvement of KCCQ-TSS of ≥5 points was greater with dapagliflozin, compared with placebo, with an effect in Asian patients (OR: 1.20; 95% CI: 1.05 to 1.37) that was consistent with that in patients enrolled outside Asia (OR: 1.14; 95% CI: 1.06 to 1.22) (P-interaction = 0.47). Conversely, the proportion of patients with a decrease in KCCQ-TSS of ≥5 points was smaller in those treated with dapagliflozin, compared with placebo, in Asian patients (OR: 0.85; 95% CI: 0.74 to 0.98), consistent with that in patients enrolled outside Asia (OR: 0.83; 95% CI: 0.77 to 0.90) (P for interaction = 0.81).
Effect of dapagliflozin in regions of Asia
The benefits of dapagliflozin on all outcomes were consistent across the regions of Asia: East Asia (China, Japan and Taiwan), South-East Asia (Vietnam), and South Asia (India) (Central Illustration).
Central Illustration.
DAPA-HF in Asia
Effect estimates are displayed as HRs and 95% CIs were estimated with the use of Cox regression models, stratified according to diabetes status, with a history of hospitalization for heart failure and treatment group assignment as explanatory variables. “All trial patients” refers to all 4,744 patients randomized in 20 countries worldwide. The interaction P value is from a test for interaction between region (Asia/non-Asia) and treatment effect. BMI = body mass index; DAPA-HF = Dapagliflozin and Prevention of Adverse-outcomes in Heart Failure; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association.
Changes in weight, SBP, hematocrit, and eGFR
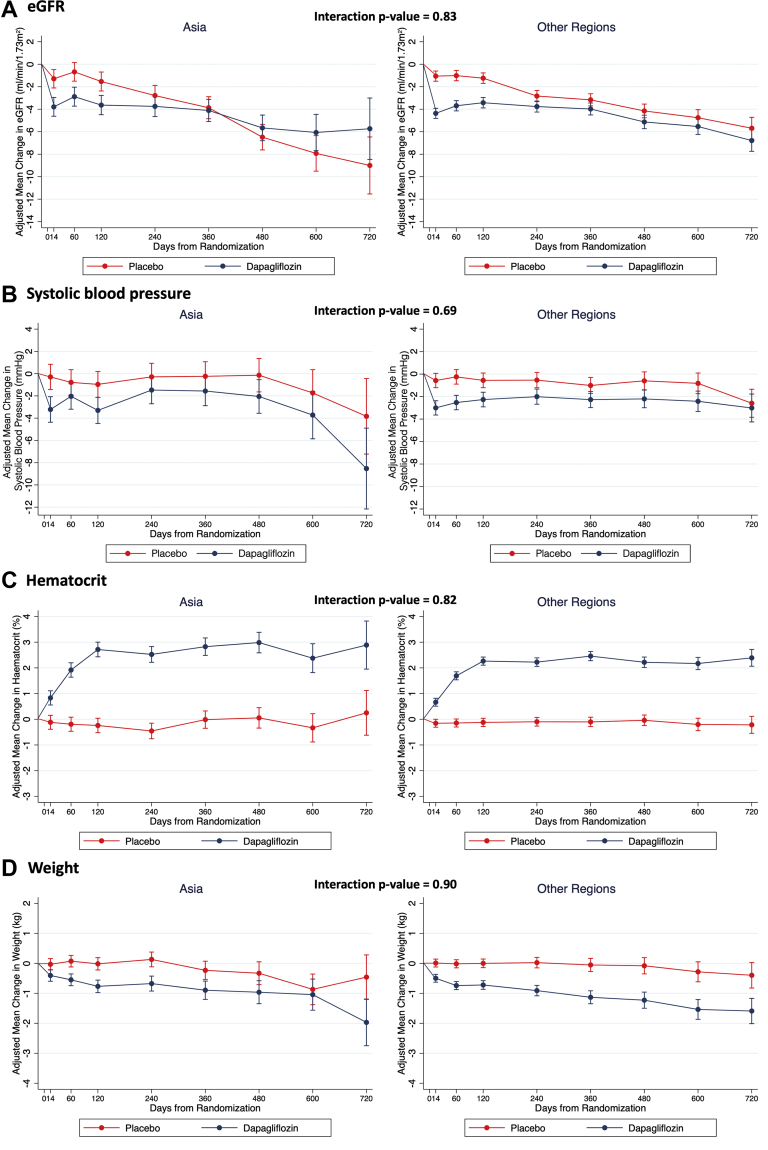
Changes in weight, SBP, hematocrit, and eGFR, adjusting for baseline value, are shown in Figure 3. There was an initial decrease in eGFR at 14 days following randomization with dapagliflozin in Asian patients (placebo-corrected change, -2.50 [95% CI: -3.67 to -1.33] mL/min/1.73 m2) that was consistent with the decline in other patients (-3.31 [95% CI: -3.95 to -2.67] mL/min/1.73 m2) (P for interaction = 0.83) (Figure 3A). The difference in eGFR slope over time in patients enrolled in Asia was 2.62 (95% CI: 1.63-3.61) mL/min/1.73 m2/y (-1.46 [95% CI: -2.16 to -0.75] mL/min/1.73 m2/y with dapagliflozin vs -4.08 [95% CI: -4.78 to -3.38] mL/min/1.73 m2/y with placebo), compared with a difference of 1.51 (95% CI: 1.01 to 2.01) mL/min/1.73 m2 among patients from outside Asia (-1.00 [95% CI: -1.35 to -0.65] in the dapagliflozin group, -2.51 [95% CI: -2.87 to -2.16] in the placebo group).
Figure 3.
Effect of Dapagliflozin on Vital Signs and Laboratory Measures by Geographic Region
(A) Change in estimated glomerular filtration rate (eGFR) from baseline; (B) change in systolic blood pressure from baseline; (C) change in hematocrit from baseline; (D) change in weight from baseline. Means and 95% CIs were derived from a mixed-effect model adjusted for baseline values, visit, randomized treatment, and interaction of treatment and visit with a random intercept and slope per patient. Least-square mean changes along with 95% CI are shown.
SBP declined to a similar extent in Asian patients (placebo-corrected change at 2 months, -1.25 [95% CI: -2.88 to 0.37] mm Hg) and those enrolled elsewhere (-2.29 [95% CI: -3.20 to -1.38] mm Hg), with no interaction between region and the effect of dapagliflozin (P-interaction = 0.69) (Figure 3B). The decrease in weight with dapagliflozin in Asian patients at 4 months (placebo-corrected change, -0.75 [95% CI: -1.04 to -0.46] kg) was consistent with the decrease in patients enrolled elsewhere (-0.72 [95% CI: -0.92 to -0.52] kg), and the placebo-corrected difference in weight with dapagliflozin was similar in Asians and non-Asians throughout the trial (P for interaction = 0.90) (Figure 3D).
Hematocrit increased with dapagliflozin in Asian and non-Asian patients, with a plateau reached after approximately 4 months (placebo-corrected increase, 2.96% [95% CI: 2.56%-3.36%] in Asian patients vs 2.38% [95% CI: 2.16%-2.61%] in others), with no interaction between region and the effect of dapagliflozin (P for interaction = 0.82).
In Asian patients, NT-proBNP decreased by 260 (95% CI: 89-433) pg/mL in the dapagliflozin group and increased by 258 (95% CI: -20 to 535) pg/mL in the placebo group (placebo-corrected difference -535 [95% CI: -851 to -220] pg/mL). The corresponding results in non-Asian patients were a decrease of 176 (95% CI: 55-298) pg/mL in the dapagliflozin group and an increase of 52 (95% CI: -89 to 194) pg/mL in the placebo group (placebo-corrected difference, -233 [95% CI: -409 to -58] pg/mL) (P for interaction = 0.10).
New onset diabetes
In patients without diabetes at baseline, the incidence of new onset diabetes was lower in patients randomized to dapagliflozin as compared with placebo in both Asian patients (OR: 0.47; 95% CI: 0.22 to 1.01) and non-Asian patients (OR: 0.74; 95% CI: 0.51 to 1.06) (P for interaction = 0.29).
Safety analyses
In general, the proportions of Asian patients who discontinued trial treatment or experienced adverse events according to randomized treatment assignment were similar to those among patients enrolled elsewhere (Table 4).
Table 4.
Occurrence of Prespecified Adverse Events in Patients Enrolled in Asia and Outside Asia
| Patients Enrolled in Asia (n = 1,092) |
Patients Not Enrolled in Asia (n = 3,644) |
Interaction P Value | |||
|---|---|---|---|---|---|
| Dapagliflozin (n = 540) | Placebo (n = 552) | Dapagliflozin (n = 1,828) | Placebo (n = 1,816) | ||
| Discontinuation of trial treatment for any reason | 52 (9.6) | 61 (11.1) | 197 (10.8) | 197 (10.8) | 0.52 |
| Discontinuation of trial treatment due to adverse event | 20 (3.7) | 26 (4.7) | 91 (5.0) | 90 (5.0) | 0.45 |
| Volume depletion | 37 (6.9) | 30 (5.4) | 141 (7.7) | 132 (7.3) | 0.52 |
| Renal adverse event | 24 (4.4) | 40 (7.2) | 129 (7.1) | 130 (7.2) | 0.09 |
| Fracture | 9 (1.7) | 12 (2.2) | 40 (2.2) | 38 (2.1) | 0.53 |
| Amputation | 0 (0.0) | 1 (0.2) | 13 (0.7) | 11 (0.6) | — |
| Major hypoglycemia | 0 (0.0) | 1 (0.2) | 4 (0.2) | 3 (0.2) | — |
| Diabetic ketoacidosis | 0 (0.0) | 0 (0.0) | 3 (0.2) | 0 (0.0) | — |
Values are n (%). The safety population included all the patients who had undergone randomization and received at least 1 dose of dapagliflozin or placebo (n = 2,368 in both treatment groups).
Discussion
With the globalization of clinical trials, the proportion of patients enrolled in major HFrEF trials from Asian countries has increased over the past 10 years from essentially zero to a weighted average of 20%.20, 21, 22, 23, 24, 25, 26, 27,40 Although 23.1% of patients in the DAPA-HF trial were randomized in an Asian country, this proportion still falls far short of the proportion of the world’s population (approximately 60%) that lives in that region and the burden of HF is anticipated to increase substantially there shortly, particularly in South Asia.40
Despite the modest number of Asian patients in the DAPA-HF trial, clear differences in baseline characteristics and outcomes were demonstrable between Asian patients and the overall trial population (and among Asian countries/regions). However, the efficacy and tolerability of dapagliflozin were the same in Asian patients as in the trial overall as well as among patients in different Asian countries/regions.
Baseline characteristics and outcomes in asian patients
We were able to confirm the differences in the clinical profile between Asian and other patients described in prior studies.20, 21, 22, 23, 24, 25, 26, 27 As expected, Asian patients were younger, had a lower BMI, and were less likely to have an ischemic etiology and atrial fibrillation. Asian patients had a lower NT-proBNP despite a lower prevalence of atrial fibrillation and a lower mean LVEF than other patients.20, 21, 22, 23, 24, 25, 26, 27 Both physician-assessed functional limitation (ie, assessed using NYHA functional class) and patient-reported symptoms (ie, KCCQ-TSS) were better in Asian patients than in the trial overall. This puzzling finding is consistent with other recent data.27 There were also notable differences in the treatment of patients enrolled in Asia, compared with the overall trial population. The lower use of diuretics, in East Asia in particular, may reflect the better symptom profile just described or other issues such as climatic conditions and resulting insensible fluid loss. Some, such as the use of implantable cardioverter-defibrillators and cardiac resynchronization therapy, probably reflect economic circumstances, supported by the gradient observed in the use of these devices across Asia.20, 21, 22, 23, 24, 25, 26, 27 Economic circumstances are probably also a consideration concerning the use of sacubitril/valsartan, although slow approval in countries such as Japan is also relevant. Greater use of ARBs in East Asia is believed to reflect a higher incidence of cough with ACE inhibitors among patients in that region. Consistent with previous reports, the proportions of patients taking ≥50% of guideline-directed doses of ACE inhibitors or ARBs, beta-blockers, and mineralocorticoid receptor antagonists were lower in Asia compared with the overall trial population.40 The low use of anticoagulants in Asia is also well recognized, possibly reflecting concern about greater bleeding risk in Asian patients, although there is no convincing evidence that this is true for direct oral anticoagulants.20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37
Despite the numerous differences in demographics, medical history, HF characteristics, and background therapy described, the rates of each of the prespecified cardiovascular outcomes were similar in patients in Asia compared with those from the rest of the world, even after adjustment for differences in key predictors of these outcomes.
Efficacy and safety of dapagliflozin in asian patients
The magnitude of the effect of dapagliflozin on the primary composite outcome of a worsening HF event or cardiovascular death was similar in Asian patients to that observed in the trial overall and among the different Asian countries and regions participating in the DAPA-HF trial. The effect of dapagliflozin on the prespecified secondary outcomes, including mortality, was also similar in Asian patients.
Improvement in patient health status by reducing the symptom burden and improving physical function and quality of life is a key aim of HF treatment.38 Dapagliflozin increased the proportion of patients with a clinically significant improvement in symptoms at 8 months and reduced the proportion with a clinically meaningful deterioration, to the same extent in Asian patients as in the trial overall, even though patients in Asia had a better health-related quality of life to begin with.38
The effects of dapagliflozin on weight, SBP, hematocrit, and eGFR in Asian patients were directionally similar to those observed in the trial overall. These observations are relevant to the tolerability of dapagliflozin, which was similar in Asian patients to that in the trial overall. Indeed, the attenuation in the rate of decline in eGFR over time (“eGFR slope”) by dapagliflozin was numerically greater in patients from Asia than from elsewhere. These data are reassuring given concerns that have been raised about weight loss in patients with a low BMI, and diuresis and SBP reduction in patients with a relatively low starting SBP.13, 14, 15, 16, 17, 18,28,29,34 We did not collect all adverse events in the DAPA-HF trial (only serious adverse events were collected). In other trials that did collect all adverse events, the rate of genital skin fungal infection has been reported at a lower rate in patients in Asia than in studies conducted in other geographic regions.13, 14, 15, 16, 17, 18,28,29,34
Study limitations
While Geographic region and race were predefined subgroup analyses, the analyses reported here were done post hoc for the reasons outlined previously. Although, the proportion of patients enrolled from Asian countries in the DAPA-HF trial was larger than in most prior trials, the number was relatively modest, and Asian patients continue to be underrepresented in clinical trials, relative to the proportion of the global population.24,35,41,42 Initiatives are underway to increase representativeness in trials, and increasing data sharing should also allow for meta-analyses of individual patient data giving more robust estimates of the benefits and harms of new therapies in subgroups of patients. A useful discussion of approaches to enhancing recruitment in trials from Asian countries is published elsewhere.26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43
The heterogeneity in patient characteristics across Asia seen in the DAPA-HF trial means that these results may not be generalizable to populations or regions not included (eg, Middle Eastern countries). The inclusion and exclusion criteria precluded the enrollment of hospitalized and other very high-risk patients. These limitations might affect the generalizability of our results. We were unable to fully examine the geographic, climatic, and other environmental factors; diet and lifestyle; cultural influences; type of health care system; economic considerations; race or ethnicity; and genetic variability that might underlie the differences between patients in Asia and elsewhere and among Asian countries and regions.
Conclusions
In the DAPA-HF trial, dapagliflozin, compared with placebo, reduced the risk of worsening HF events, cardiovascular death, and all-cause death, and improved symptoms, to the same extent in Asian patients as patients from other geographic regions. These findings provide further support for the use of dapagliflozin as a new treatment option for Asian patients with HFrEF.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: There are significant inter- and intraregional differences across the world in patients with HFrEF.
COMPETENCY IN PATIENT CARE: Evidence of a consistent benefit and safety of the SGLT2 inhibitor dapagliflozin in Asian patients, as compared with patients from outside Asia, means that dapagliflozin should be considered as a foundational treatment for HFrEF in Asian patients.
TRANSLATIONAL OUTLOOK: In the DAPA-HF trial, as reported previously, a smaller proportion of Asian patients, as compared with the overall trial population, were taking guideline-recommended target doses of disease-modifying treatments. Further research is required to understand the reasons underlying this difference.
Funding Support and Author Disclosures
This study was funded by AstraZeneca. Dr McMurray was supported by a British Heart Foundation Centre of Research Excellence Grant RE/18/6/34217. Dr Docherty has received grant support from Novartis and speaker fees from AstraZeneca; and his employer, the University of Glasgow, has been remunerated for his time spent working on the DAPA-HF trial. Dr Anand has received fees for serving on a steering committee from AstraZeneca, ARCA biopharma, Amgen, and LivaNova; for serving as chair of a data and safety monitoring board from Boston Scientific; for serving on an endpoint committee from Boehringer Ingelheim; and for serving on an advisory board from Zensun. Dr Chiang has received honorarium for lectures from AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Merck Sharp & Dohme, Novartis, Pfizer, and Sanofi. Dr Chopra has received consulting fees from Novartis. Dr Desai has received consulting fees from Abbott, Biofourmis, Boston Scientific, Boehringer Ingelheim, DalCor Pharmaceuticals, and Regeneron; grant support (paid to Brigham and Women’s Hospital) and consulting fees from Alnylam Pharmaceuticals and Novartis; and advisory board fees from Corvidia and Relypsa. Dr Kitakaze has received grant support and lecture fees from Astellas Pharma, Sanofi, Pfizer, Ono Pharmaceutical, Novartis, and Mitsubishi Tanabe Pharma; lecture fees from Daiichi Sankyo, Bayer, Boehringer Ingelheim, Kowa Pharmaceutical, Sawai Pharmaceutical, MSD, Shionogi, Kureha, Taisho Toyama Pharmaceutical, Takeda Pharmaceutical, and Toa Eiyo; and manuscript fees from Japan Medical Data Center. Dr Verma has received grant support, lecture fees, and advisory board fees from AstraZeneca, Boehringer Ingelheim, Bayer, Janssen, and Merck; lecture fees from Sun Pharmaceutical Industries and EOCI Pharmacomm; grant support and advisory board fees from Amgen; and lecture fees and advisory board fees from Sanofi and Eli Lilly. Dr Vinh has reported that he has no relationships relevant to the contents of this paper to disclose. Dr Inzucchi has received personal fees and nonfinancial support from AstraZeneca, Boehringer Ingelheim, Sanofi/Lexicon, Merck, VTV Therapeutics, and Abbott/Alere; and personal fees from AstraZeneca and Zafgen. Dr Køber has received other support from AstraZeneca and personal fees from Novartis and Bristol Myers Squibb as a speaker. Dr Kosiborod has received personal fees from AstraZeneca; received grants, personal fees, and other from AstraZeneca; received grants and personal fees from Boehringer Ingelheim; and served as consultant for Vifor Pharma and personal fees from Sanofi, Amgen, Novo Nordisk, Merck (Diabetes), Janssen, Bayer, GlaxoSmithKline, Glytec, Novartis, Applied Therapeutics, Amarin, and Eli Lilly. Dr Martinez has received personal fees from AstraZeneca. Drs Bengtsson, Langkilde, and Sjostrand are full-time employees of AstraZeneca. Dr Ponikowski has received personal fees and other from AstraZeneca, Boehringer Ingelheim, Bayer, BMS, Cibiem, Novartis, and RenalGuard; personal fees from Pfizer, Servier, Respicardia, and Berlin-Chemie; other from Amgen; and grants, personal fees, and other from Vifor Pharma. Dr Sabatine has received grants from Bayer, Daiichi Sankyo, Eisai, GlaxoSmithKline, Pfizer, Poxel, Quark Pharmaceuticals, and Takeda; grants and personal fees from Amgen, AstraZeneca, Intarcia, Janssen Research and Development, The Medicines Company, MedImmune, Merck, and Novartis; and personal fees from Anthos Therapeutics, Bristol Myers Squibb, CVS Caremark, DalCor, Dyrnamix, Esperion, IFM Therapeutics, and Ionis. Dr Sabatine has served as a member of the TIMI Study Group, which has also received institutional research grant support through Brigham and Women’s Hospital from Abbott, Aralez, Roche, and Zora Biosciences. Dr Solomon has received grants from AstraZeneca, Bellerophon, Celladon, Ionis, Lone Star Heart, Mesoblast, National Institutes of Health/National Heart, Lung, and Blood Institute, Sanofi Pasteur, and Eidos; grants and personal fees from Alnylam, Amgen, AstraZeneca, BMS, Gilead, GSK, MyoKardia, Novartis, Theracos, Bayer, and Cytokinetics; and personal fees from Akros, Corvia, Ironwood, Merck, Roche, Takeda, Quantum Genomics, AoBiome, Janssen, Cardiac Dimensions, Tenaya, and Daiichi Sankyo. Dr Jhund has received other from AstraZeneca; personal fees from Novartis and Cytokinetics; and grants from Boehringer Ingelheim. Dr McMurray has received nonfinancial support and other from AstraZeneca, Cardiorentis, Amgen, Oxford University/Bayer, Theracos, Abbvie, Novartis, GlaxoSmithKline, Vifor-Fresenius, Kidney Research UK, and Novartis; and other support from Bayer, DalCor, Pfizer, Merck, and Bristol Myers Squibb.
Footnotes
Gregg Fonarow, MD, served as the Guest Associate Editor for this paper.
Nathan Wong, PhD, served as the Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Zinman B., Wanner C., Lachin J.M., et al. EMPA-REG OUTCOME Investigators Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 2.Neal B., Perkovic V., Mahaffey K.W., et al. CANVAS Program Collaborative Group Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 3.Wiviott S.D., Raz I., Bonaca M.P., et al. DECLARE–TIMI 58 Investigators Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 4.Perkovic V., Jardine M.J., Neal B., et al. CREDENCE Trial Investigators Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 5.Heerspink H.J.L., Stefánsson B.V., Correa-Rotter R., et al. DAPA-CKD Trial Committees and Investigators Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 6.McMurray J.J.V., Solomon S.D., Inzucchi S.E., et al. DAPA-HF Trial Committees and Investigators Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 7.Packer M., Anker S.D., Butler J., et al. EMPEROR-Reduced Trial Investigators Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 8.Bhatt D.L., Szarek M., Pitt B., et al. SCORED Investigators Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2021;384(2):129–139. doi: 10.1056/NEJMoa2030186. [DOI] [PubMed] [Google Scholar]
- 9.Bhatt D.L., Szarek M., Steg P.G., et al. SOLOIST-WHF Trial Investigators Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384(2):117–128. doi: 10.1056/NEJMoa2030183. [DOI] [PubMed] [Google Scholar]
- 10.Chan J.C., Malik V., Jia W., et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301(20):2129–2140. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 11.Ma R.C., Chan J.C. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013;1281(1):64–91. doi: 10.1111/nyas.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nanditha A., Ma R.C., Ramachandran A., et al. Diabetes in Asia and the Pacific: implications for the global epidemic. Diabetes Care. 2016;39(3):472–485. doi: 10.2337/dc15-1536. [DOI] [PubMed] [Google Scholar]
- 13.Yang L., Zhang L., He H., Zhang M., An Z. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in East Asians with type 2 diabetes: a systematic review and meta-analysis. Diabetes Ther. 2019;10(5):1921–1934. doi: 10.1007/s13300-019-0674-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh A.K., Singh R. Cardiovascular outcomes with SGLT-2 inhibitors and GLP-1 receptor agonist in Asians with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Diabetes Metab Syndr. 2020;14(4):715–722. doi: 10.1016/j.dsx.2020.04.051. [DOI] [PubMed] [Google Scholar]
- 15.Fujita Y., Inagaki N. Update on the efficacy and safety of sodium-glucose cotransporter 2 inhibitors in Asians and non-Asians. J Diabetes Investig. 2019;10(6):1408–1410. doi: 10.1111/jdi.13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang W., Ji L., Zhou Z., Cain V.A., Johnsson K.M., Sjöström C.D. Efficacy and safety of dapagliflozin in Asian patients: a pooled analysis. J Diabetes. 2017;9(8):787–799. doi: 10.1111/1753-0407.12484. [DOI] [PubMed] [Google Scholar]
- 17.Cai X., Gao X., Yang W., et al. No disparity of the efficacy and all-cause mortality between Asian and non-Asian type 2 diabetes patients with sodium-glucose cotransporter 2 inhibitors treatment: a meta-analysis. J Diabetes Investig. 2018;9(4):850–861. doi: 10.1111/jdi.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalra S., Ghosh S., Aamir A.H., et al. Safe and pragmatic use of sodium-glucose co-transporter 2 inhibitors in type 2 diabetes mellitus: South Asian Federation of Endocrine Societies consensus statement. Indian J Endocrinol Metab. 2017;21(1):210–230. doi: 10.4103/2230-8210.196029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramachandran A., Ma R.C., Snehalatha C. Diabetes in Asia. Lancet. 2010;375(9712):408–418. doi: 10.1016/S0140-6736(09)60937-5. [DOI] [PubMed] [Google Scholar]
- 20.Lam C.S., Teng T.K., Tay W.T., et al. Regional and ethnic differences among patients with heart failure in Asia: the Asian sudden cardiac death in heart failure registry. Eur Heart J. 2016;37(41):3141–3153. doi: 10.1093/eurheartj/ehw331. [DOI] [PubMed] [Google Scholar]
- 21.Dewan P., Docherty K.F., McMurray J.J.V. Sacubitril/valsartan in Asian patients with heart failure with reduced ejection fraction. Korean Circ J. 2019;49(6):469–484. doi: 10.4070/kcj.2019.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dewan P., Jhund P.S., Shen L., et al. Heart failure with reduced ejection fraction: comparison of patient characteristics and clinical outcomes within Asia and between Asia, Europe and the Americas. Eur J Heart Fail. 2019;21(5):577–587. doi: 10.1002/ejhf.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacDonald M.R., Tay W.T., Teng T.K., et al. ASIAN-HF investigators Regional variation of mortality in heart failure with reduced and preserved ejection fraction across Asia: outcomes in the ASIAN-HF registry. J Am Heart Assoc. 2020;9(1) doi: 10.1161/JAHA.119.012199. Erratum in: J Am Heart Assoc. 2020;9(5):e014512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajadurai J., Tse H.F., Wang C.H., Yang N.I., Zhou J., Sim D. Understanding the epidemiology of heart failure to improve management practices: an Asia-Pacific Perspective. J Card Fail. 2017;23(4):327–339. doi: 10.1016/j.cardfail.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Sato N. Epidemiology of heart failure in Asia. Heart Fail Clin. 2015;11(4):573–579. doi: 10.1016/j.hfc.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Mentz R.J., Roessig L., Greenberg B.H., et al. Heart failure clinical trials in East and Southeast Asia: understanding the importance and defining the next steps. J Am Coll Cardiol HF. 2016;4(6):419–427. doi: 10.1016/j.jchf.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tromp J., Tay W.T., Ouwerkerk W., et al. ASIAN-HF authors Multimorbidity in patients with heart failure from 11 Asian regions: a prospective cohort study using the ASIAN-HF registry. PLoS Med. 2018;15(3) doi: 10.1371/journal.pmed.1002541. Erratum in: PLoS Med. 2018;15(5):e1002583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scheen A.J. Pharmacokinetic/pharmacodynamic properties and clinical Use of SGLT2 inhibitors in non-Asian and Asian patients with type 2 diabetes and chronic kidney disease. Clin Pharmacokinet. 2020;59(8):981–994. doi: 10.1007/s40262-020-00885-z. [DOI] [PubMed] [Google Scholar]
- 29.Khoo C.M., Deerochanawong C., Chan S.P., et al. Use of sodium-glucose co-transporter-2 inhibitors in Asian patients with type 2 diabetes and kidney disease: An Asian perspective and expert recommendations. Diabetes Obes Metab. 2021;23(2):299–317. doi: 10.1111/dom.14251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee M.J., Ha K.H., Kim D.J., Park I. Trends in the incidence, prevalence, and mortality of end-stage kidney disease in South Korea. Diabetes Metab J. 2020;44(6):933–937. doi: 10.4093/dmj.2020.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liew A., Bavanandan S., Prasad N., et al. Asian Pacific Society of Nephrology Clinical Practice Guideline on Diabetic Kidney Disease - an executive summary. Nephrology (Carlton) 2020;25(11):809–817. doi: 10.1111/nep.13804. [DOI] [PubMed] [Google Scholar]
- 32.Chan J.C., Wat N.M., So W.Y., et al. Asian RENAAL Study Investigators Renin angiotensin aldosterone system blockade and renal disease in patients with type 2 diabetes. An Asian perspective from the RENAAL Study. Diabetes Care. 2004;27(4):874–879. doi: 10.2337/diacare.27.4.874. [DOI] [PubMed] [Google Scholar]
- 33.Tan E.S.J., Tay W.T., Teng T.K., et al. Ethnic differences in atrial fibrillation in patients with heart failure from Asia-Pacific. Heart. 2019;105(11):842–847. doi: 10.1136/heartjnl-2018-314077. [DOI] [PubMed] [Google Scholar]
- 34.Lo C., Nguyen S., Yang C., et al. Pharmacogenomics in Asian subpopulations and impacts on commonly prescribed medications. Clin Transl Sci. 2020;13(5):861–870. doi: 10.1111/cts.12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magavern E.F., Kaski J.C., Turner R.M., et al. The Role of pharmacogenomics in contemporary cardiovascular therapy: A position statement from the European Society of Cardiology Working Group on Cardiovascular Pharmacotherapy. Eur Heart J Cardiovasc Pharmacother. 2022;8(1):85–99. doi: 10.1093/ehjcvp/pvab018. [DOI] [PubMed] [Google Scholar]
- 36.McMurray J.J.V., DeMets D.L., Inzucchi S.E., et al. DAPA-HF Committees and Investigators A trial to evaluate the effect of the sodium-glucose co-transporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA-HF) Eur J Heart Fail. 2019;21(5):665–675. doi: 10.1002/ejhf.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McMurray J.J.V., DeMets D.L., Inzucchi S.E., et al. DAPA-HF Committees and Investigators The Dapagliflozin And Prevention of Adverse-outcomes in Heart Failure (DAPA-HF) trial: baseline characteristics. Eur J Heart Fail. 2019;21(11):1402–1411. doi: 10.1002/ejhf.1548. [DOI] [PubMed] [Google Scholar]
- 38.Kosiborod M.N., Jhund P.S., Docherty K.F., et al. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: results from the DAPA-HF trial. Circulation. 2020;141(2):90–99. doi: 10.1161/CIRCULATIONAHA.119.044138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inzucchi S.E., Docherty K.F., Køber L., et al. DAPA-HF Investigators and Committees dapagliflozin and the incidence of type 2 diabetes in patients with heart failure and reduced ejection fraction: an exploratory analysis from DAPA-HF. Diabetes Care. 2021;44(2):586–594. doi: 10.2337/dc20-1675. [DOI] [PubMed] [Google Scholar]
- 40.Teng T.K., Tromp J., Tay W.T., et al. ASIAN-HF investigators Prescribing patterns of evidence-based heart failure pharmacotherapy and outcomes in the ASIAN-HF registry: a cohort study. Lancet Glob Health. 2018;6(9):e1008–e1018. doi: 10.1016/S2214-109X(18)30306-1. [DOI] [PubMed] [Google Scholar]
- 41.Tahhan A.S., Vaduganathan M., Greene S.J., et al. Enrollment of older patients, women, and racial and ethnic minorities in contemporary heart failure clinical trials: a systematic review. JAMA Cardiol. 2018;3(10):1011–1019. doi: 10.1001/jamacardio.2018.2559. [DOI] [PubMed] [Google Scholar]
- 42.Martinez-Amezcua P., Haque W., Khera R., et al. The upcoming epidemic of heart failure in South Asia. Circ Heart Fail. 2020;13(10) doi: 10.1161/CIRCHEARTFAILURE.120.007218. [DOI] [PubMed] [Google Scholar]
- 43.Editors, Rubin E Striving for diversity in research studies. N Engl J Med. 2021;385(15):1429–1430. doi: 10.1056/NEJMe2114651. [DOI] [PubMed] [Google Scholar]