Abstract
Background
Drug-coated balloons (DCBs) offer an effective treatment for in-stent restenosis (ISR). The Genoss DCB is a novel paclitaxel-coated balloon with a shellac plus vitamin E excipient that enhances drug delivery to the target lesion, minimizing restenosis.
Objectives
This study aimed to investigate the angiographic efficacy, clinical safety, and effectiveness of the novel shellac plus vitamin E–based DCB in a randomized controlled trial designed to enable regulatory approval of this new device in South Korea.
Methods
This noninferiority trial randomized patients experiencing their first ISR to the novel shellac plus vitamin E–based DCB or the reference SeQuent Please iopromide-based DCB in a 1:1 ratio. All patients underwent planned angiographic and clinical follow-up at 6 months. The study was powered for the primary endpoint of 6 months in-segment late lumen loss (LLL).
Results
A total of 82 patients from 7 centers were randomized to either the novel shellac plus vitamin E–based DCB group (n = 41) or the reference iopromide-based DCB group (n = 41). The 6-month in-segment LLL was 0.15 ± 0.43 mm with the novel DCB compared with 0.24 ± 0.39 mm with the reference device. The 1-sided 97.5% upper confidence limit of the difference was 0.13 mm, lower than the noninferiority limit of 0.29 mm, achieving noninferiority (P for noninferiority = 0.001). Major cardiovascular events were comparable between 2 groups at 6 months (7.7% for the novel DCB vs 10.3% for the reference DCB; P = 0.692).
Conclusions
In this multicenter, head-to-head comparison randomized trial, the novel shellac plus vitamin E–based DCB showed a comparable result to the reference iopromide-based device for the primary endpoint of 6-month in-segment LLL for the treatment of coronary ISR. (Compare the Safety and Efficacy of Genoss® DCB and SeQuent® Please in Korean Patient With Coronary In-stent Restenosis; NCT04405063)
Key Words: cardiovascular events, drug-coated balloon, in-segment late lumen loss, major adverse
Abbreviations and Acronyms: DCB, drug-coated balloon; DES, drug-eluting stent; DS, diameter stenosis; ISR, in-stent restenosis; LLL, late lumen loss; MLD, minimal lumen diameter; MACE, major adverse cardiovascular event; PCB, paclitaxel-coated balloon; TLR, target lesion revascularization
Central Illustration
Although drug-eluting stents (DES) have improved the clinical outcomes of patients with in-stent restenosis (ISR), the optimal treatment strategy for ISR remains unclear. Drug-coated balloons (DCBs), which are a recommended treatment option for ISR with a Class I, Level of Evidence: A recommendation in the European guidelines for revascularization, importantly avoid the need for a second layer of a stent.1, 2, 3 Paclitaxel remains the preferred drug for the balloon coating because of its irreversible binding to microtubules,4 resulting in effective inhibition of neointimal proliferation.5 Most randomized studies that compare DCBs with alternative percutaneous therapies for the treatment of ISR are centered on the iopromide-based SeQuent Please (B. Braun); however, alternative paclitaxel-coated balloons (PCBs) using different excipients are available for clinical applications. Several prospective randomized studies have shown comparable angiographic and clinical outcomes among different types of DCBs for patients with coronary ISR.6,7 The Genoss PCB (Genoss DCB; GENOSS) is a new DCB with a different excipient, ie, shellac plus vitamin E, which is designed to enhance drug delivery to the target lesion. This study aimed to evaluate the angiographic efficacy, clinical safety, and effectiveness of this new DCB in a randomized trial designed to enable regulatory approval of the new device in South Korea.
Methods
Study design and patient population
This prospective, multicenter, randomized controlled trial compared the new shellac plus vitamin E–based DCB with the iopromide-based DCB in patients with ISR (NCT04405063). The protocol was approved by all ethics committees of all participating centers. Patients ≥19 years of age with clinical evidence of stable or unstable angina, silent myocardial ischemia, or a positive functional study were considered for enrollment. Clinical inclusion criteria required lesions to be Mehran type I to III ISR with at least 50% diameter stenosis (DS), occurring >90 days after coronary stent implantation. Major clinical exclusion criteria were infarct-related artery lesions in patients with acute myocardial infarction; restenosis lesions with thrombosis or bypass grafts; target vessels with complete occlusion (Mehran type IV); known hypersensitivity or contraindications to aspirin, heparin, clopidogrel, and paclitaxel; sensitivity to contrast media not amenable to pre-medication; lesion length >40 mm; or vessel diameter <2.0 mm.
Eligible patients who provided written informed consent were enrolled from 7 university hospitals in South Korea. We randomly stratified patients on the basis of the lesion site and used an interactive Web response system to randomize patients. Study coordination, data management, and on-site monitoring support were provided by an independent contract research organization company (Synex Consulting). The study sponsor did not have any role in the analysis and interpretation of data or writing of the manuscript and did not participate in the decision to submit the paper for publication.
Study devices and procedures
The SeQuent Please (reference device) is an iopromide-based DCB that is coated with 3 μg paclitaxel/mm2 of balloon surface and uses iopromide as a hydrophilic excipient. The Genoss DCB (tested device) is also coated with 3 μg paclitaxel/mm2 of balloon surface but uses a wax-free shellac and vitamin E as an excipient, which is designed to enhance drug delivery to the target lesion and prevent restenosis.
Randomization was performed when the ISR lesion was judged to be capable of DCB treatment. Pre-dilatation of the target lesion was mandatory using a standard balloon catheter, followed by full plaque modification. The DCB was selected using a balloon-to-artery ratio of 1.0, which is longer than the lesion segment. The delivery time of the DCB catheter from the vascular access to the vessel wall and expansion to nominal pressure was minimized to <3 minutes. If more than 3 minutes had elapsed, the failed DCB was removed and replaced with a new product for retreatment. The recommended DCB balloon inflation time was at least 60 seconds with nominal pressure, but if the patient could not tolerate this duration, inflation was performed twice for 30 seconds. All patients were preloaded with clopidogrel and already on aspirin before coronary angioplasty. Unfractionated heparin was administered according to the standard hospital practice, and most of the procedures were performed via radial access. Vascular sheaths, either through the transradial or transfemoral route, were removed according to the usual hospital practice.
Quantitative coronary angiography
Angiography before and after all interventions and at angiographic follow-up was performed using identical projections and analyses. All coronary angiographic images were analyzed by an expert (Yoonha Noh) in the core laboratory at the Cardiovascular Center of the Genome Research Foundation. Measurements were obtained in the stented area with measurement shoulder to shoulder (in stent; narrowing size and references were automatically identified by the system) and in the total stented area plus 5.0 mm proximally and distally (in segment; lesion + complete treated segment + 5 mm adjacent margins). The following parameters were analyzed: reference vessel diameter, minimal lumen diameter (MLD), percent DS, acute lumen gain (defined as the difference between MLD after DCB treatment and MLD at baseline), late lumen loss (LLL) (defined as the difference between MLD after DCB treatment and MLD at follow-up), lesion length, binary restenosis, and persistence of dissection (National Heart, Lung, and Blood Institute classification).8 Patterns of ISR were defined according to the Mehran classification.9
Follow-up and endpoints
Dual antiplatelet therapy was continued orally for 6 months, and patients underwent follow-up angiography after 6 months. Clinical follow-up was performed at 30 ± 7 days and at 6 months ± 7 days post-procedure. All clinical endpoints and adverse events were evaluated with consensus of the investigators, and all events were cross-checked with the medical records by external monitors. Given the different packaging of the study balloon catheters, the investigators performing the study procedures were not blinded to the treatment assignment; however, statisticians were blinded. Angiographic in-segment LLL was the primary endpoint. Secondary endpoints were major adverse cardiovascular events (MACE) composed of the occurrence of cardiac death, target lesion myocardial infarction, stent thrombosis, and clinically driven target lesion revascularization (TLR) at 6 months, and these conditions were defined according to the Academic Research Consortium consensus document.10
Device success was defined as successful delivery and deployment of the study balloon. Lesion success was defined as the achievement of final residual stenosis <30% by visual estimation. Procedural success was defined as a target lesion DS <30% immediately after DCB treatment, Thrombolysis In Myocardial Infarction (TIMI) flow grade 3, and absence of in-hospital MACE. Binary restenosis was defined as a DS of at least 50% of the luminal diameter at angiographic follow-up.
Statistical analysis
The study hypothesis was that the novel shellac plus vitamin E–based DCB was noninferior to the iopromide-based DCB for the treatment of ISR, in terms of in-segment LLL. Accordingly, the power calculation of the present trial included the assumption of an LLL of 0.17 mm in both arms, with a delta of 0.00, alpha of 2.5%, power of 80%, SD of 0.4, and noninferiority margin of 0.29 mm. The estimation of 0.17 to 0.42 mm of LLL in the control group was derived from previous studies with the same device in a similar lesion setting.11, 12, 13, 14 Therefore, we calculated a population of 34 patients per group. With an attrition rate for angiographic follow-up of 17%, we decided to include a total population of 82 patients.
For the demographic information, continuous data were summarized by descriptive statistics (number of subjects, mean, SD, median, minimum, and maximum) and categorical data by frequency and fraction. Two-sided statistical tests were performed at a significance level of 5%, unless otherwise stated. The purpose of this study was to demonstrate the noninferiority of the shellac plus vitamin E–based DCB to the iopromide-based DCB in terms of in-segment LLL.
The primary endpoint, in-segment LLL, was analyzed in all patients who had undergone angiographic follow-up. For the comparison between the 2 groups, in-segment LLL values measured at 6 months were evaluated using the independent 2-sample Student's t-test or Wilcoxon rank sum test. At this time, if the upper limit of the 97.5% 1-sided CI between the shellac plus vitamin E–based DCB group and the iopromide-based DCB group was <0.29 mm (noninferiority limit), the shellac plus vitamin E–based DCB group was significantly noninferior to the iopromide-based DCB group. MLD and DS immediately after the procedure and at 6 months’ follow-up were evaluated using the 2-sample Student's t-test or Wilcoxon rank sum test.
If necessary, a chi-square test or Fisher’s exact test was performed to compare the 2 groups. All statistical analyses were performed at a 2-sided significance level of 0.05, using SPSS version 21.0 (IBM), NCSS (NCSS), and R version 3.6.3 (R Foundation for Statistical Computing).
Results
From November 2016 to July 2018, 82 patients with coronary ISR were randomly assigned to either the shellac plus vitamin E–based DCB (n = 41) or the iopromide-based DCB Please (n = 41) treatment group. Figure 1 shows the flowchart of the trial. Baseline characteristics between the 2 groups were generally balanced (Table 1), with no significant differences. Table 2 summarizes the angiographic and procedural characteristics, and Table 3 summarizes the quantitative coronary angiographic results. The mean SYNTAX (SYNergy between PCI with TAXUS and Cardiac Surgery) score was 9.6 ± 7.2 mm with the shellac plus vitamin E–based DCB compared with 8.9 ± 7.4 mm with the iopromide-based DCB. The device success rate was 100%. The lesion and procedural success rate was 85.9% (shellac plus vitamin E–based DCB at 82.1% vs iopromide-based DCB at 89.7%; P = 0.329). No procedural complications or bailout stenting occurred after any treatment, and all patients were successfully treated with the assigned DCB without flow-limiting dissection.
Figure 1.
Flow Chart of the Study
Between November 2016 and July 2018, a total of 82 patients with coronary in-stent restenosis (ISR) were randomized to the shellac plus vitamin E–based drug-coated balloon (DCB) (n = 41) or the iopromide-based DCB (n = 41). Two patients in each group were excluded caused by stenting after balloon angiography. The clinical follow-up was 100% complete, and the angiography follow-up was completed in the shellac plus vitamin E–based DCB group (n = 29) and the iopromide-based DCB group (n = 31) at 6 months.
Table 1.
Clinical Baseline Characteristics
| Shellac + Vitamin E–Based DCB (n = 39) | Iopromide-Based DCB (n = 39) | |
|---|---|---|
| Age, y | 67.8 ± 11.2 | 65.2 ± 9.7 |
| Male | 33 (84.6) | 29 (74.4) |
| Body mass index, kg/m2 | 26.8 ± 8.8 | 24.7 ± 2.5 |
| Hypertension | 32 (82.1) | 30 (76.9) |
| Diabetes mellitus | 21 (53.9) | 24 (61.5) |
| Insulin treatment | 7 (18.0) | 5 (12.8) |
| Hyperlipidemia | 12 (30.8) | 12 (30.8) |
| Current smoking | 5 (12.8) | 8 (20.5) |
| Prior myocardial infarction | 5 (12.8) | 8 (20.5) |
| Prior coronary artery bypass grafting | 1 (2.6) | 0 |
| Prior stroke | 0 | 0 |
| Stable angina pectoris status | 31 (79.5) | 29 (74.4) |
| Bare-metal stent ISR | 5 | 4 |
| Drug-eluting stent ISR | 34 | 35 |
Values are mean ± SD, n (%), or n.
DCB = drug-coated balloon; ISR = in-stent restenosis.
Table 2.
Angiographic and Procedural Characteristics
| Shellac + Vitamin E–Based DCB (n = 39) | Iopromide-Based DCB (n = 39) | |
|---|---|---|
| Multivessel disease | 11 (28.2) | 10 (25.6) |
| Lesion type, B2/C | 16 (41.0) | 21 (53.9) |
| Target vessel location | ||
| Left anterior descending artery | 18 (46.2) | 19 (48.7) |
| Left circumflex artery | 10 (25.6) | 9 (23.1) |
| Right coronary artery | 11 (28.2) | 11 (28.2) |
| Mehran type | ||
| I | 19 (48.7) | 17 (43.6) |
| II | 16 (41.0) | 16 (41.0) |
| III | 4 (10.3) | 5 (12.8) |
| IV | 0 | 1 (2.6) |
| TIMI flow grade 3 before procedure | 39 (100.0) | 39 (100.0) |
| Number of study balloons | 1.0 ± 0.2 (40) | 1.1 ± 0.3 (42) |
| Study balloon diameter, mm | 3.0 ± 0.4 | 3.0 ± 0.4 |
| Study balloon length, mm | 24.6 ± 5.1 | 27.2 ± 7.0 |
| Maximum study balloon pressure, atm | 10.3 ± 2.7 | 9.8 ± 3.0 |
| Study balloon inflation time, s | 54.6 ± 12.5 | 53.9 ± 12.3 |
| Delivery time to target lesion, s | 43.8 ± 39.1 | 42.5 ± 33.2 |
| TIMI flow grade 3 at end of procedure | 39 (100.0) | 39 (100.0) |
| Bailout strategy | 0 | 0 |
Values are n (%), mean ± SD (n), or mean ± SD.
DCB = drug-coated balloon, TIMI = Thrombolysis In Myocardial Infarction.
Table 3.
Serial Quantitative Coronary Angiographic Results
| Shellac + Vitamin E–Based DCB | Iopromide-Based DCB | P Value | |
|---|---|---|---|
| Pre-procedure | n = 39 | n = 39 | |
| LL, mm | 20.10 ± 6.03 | 21.79 ± 8.61 | 0.318 |
| RVD, mm | 2.41 ± 0.48 | 2.49 ± 0.54 | 0.466 |
| MLD, mm | 0.87 ± 0.28 | 0.87 ± 0.34 | 0.936 |
| DS, % | 62.18 ± 14.02 | 64.95 ± 10.72 | 0.331 |
| Post-balloon angioplasty | n = 39 | n = 39 | |
| RVD in-segment, mm | 2.64 ± 0.46 | 2.64 ± 0.47 | 0.980 |
| RVD in-stent, mm | 2.64 ± 0.46 | 2.64 ± 0.47 | 0.980 |
| MLD in-segment, mm | 1.92 ± 0.42 | 1.90 ± 0.40 | 0.821 |
| MLD in-stent, mm | 1.92 ± 0.41 | 1.90 ± 0.40 | 0.821 |
| DS in-segment, % | 27.32 ± 11.18 | 27.73 ± 10.81 | 0.874 |
| DS in-stent, % | 27.35 ± 11.16 | 27.73 ± 10.81 | 0.882 |
| Post-DCB | n = 39 | n = 39 | |
| RVD in-segment, mm | 2.69 ± 0.54 | 2.80 ± 0.46 | 0.343 |
| RVD in-stent, mm | 2.68 ± 0.54 | 2.80 ± 0.46 | 0.310 |
| MLD in segment, mm | 2.13 ± 0.44 | 2.24 ± 0.36 | 0.220 |
| MLD in-stent, mm | 2.13 ± 0.44 | 2.24 ± 0.36 | 0.220 |
| DS in-segment, % | 20.49 ± 8.76 | 19.51 ± 8.86 | 0.625 |
| DS in-stent, % | 20.49 ± 8.76 | 19.51 ± 8.86 | 0.625 |
| Acute lumen gain in-segment, mm | 1.26 ± 0.38 | 1.37 ± 0.43 | 0.250 |
| Acute lumen gain in-stent, mm | 1.26 ± 0.38 | 1.37 ± 0.43 | 0.249 |
| 6-mo follow-up | n = 29 | n = 31 | |
| Follow-up, d | 184.03 ± 24.48 | 187.77 ± 20.27 | 0.299 |
| RVD in-segment, mm | 2.71 ± 0.50 | 2.65 ± 0.43 | 0.640 |
| RVD in-stent, mm | 2.71 ± 0.50 | 2.66 ± 0.42 | 0.707 |
| MLD in-segment, mm | 2.07 ± 0.44 | 1.90 ± 0.55 | 0.196 |
| MLD in-stent, mm | 2.07 ± 0.44 | 1.87 ± 0.47 | 0.107 |
| DS in-segment, % | 23.53 ± 10.93 | 30.43 ± 11.17 | 0.020a |
| DS in-stent, % | 23.53 ± 10.93 | 30.23 ± 10.82 | 0.022a |
| LLL in-segment, mm | 0.15 ± 0.43 | 0.24 ± 0.39 | 0.299 |
| LLL in-stent, mm | 0.15 ± 0.43 | 0.26 ± 0.36 | 0.201 |
| Binary restenosis in-segment | 0 | 2 (6.45) | 0.164 |
| Binary restenosis rate in-stent | 0 | 1 (3.22) | 0.329 |
Values are mean ± SD or n (%).
DCB = drug-coated balloon; DS = diameter stenosis; LL = lesion length; LLL = late lumen loss; MLD = minimal lumen diameter; RVD = reference vessel diameter.
P < 0.05.
Angiographic outcomes
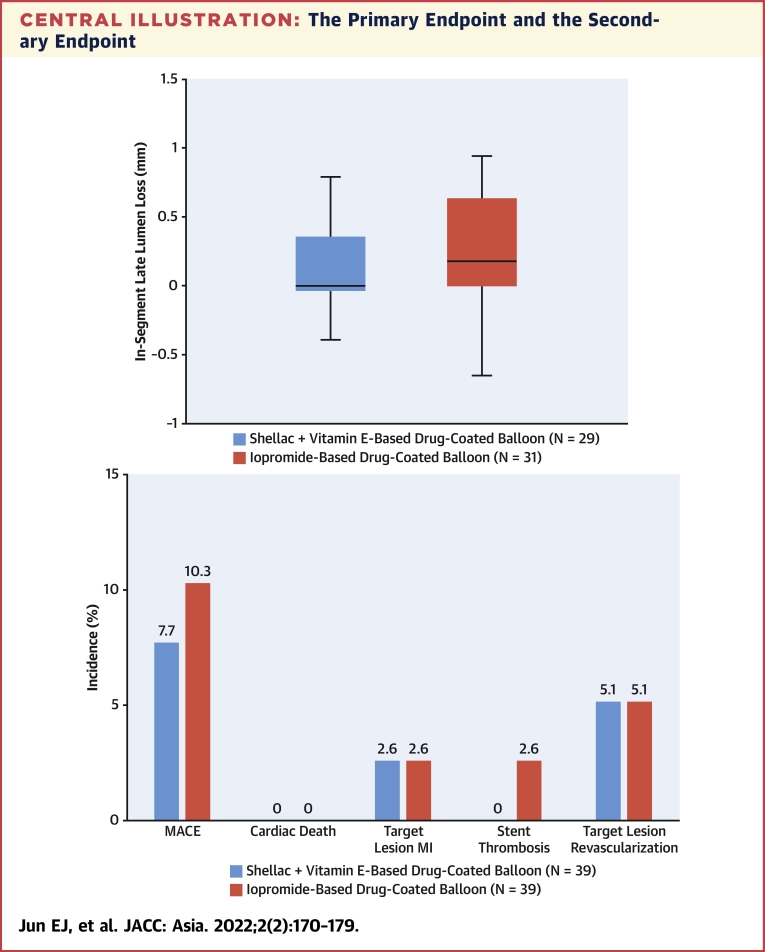
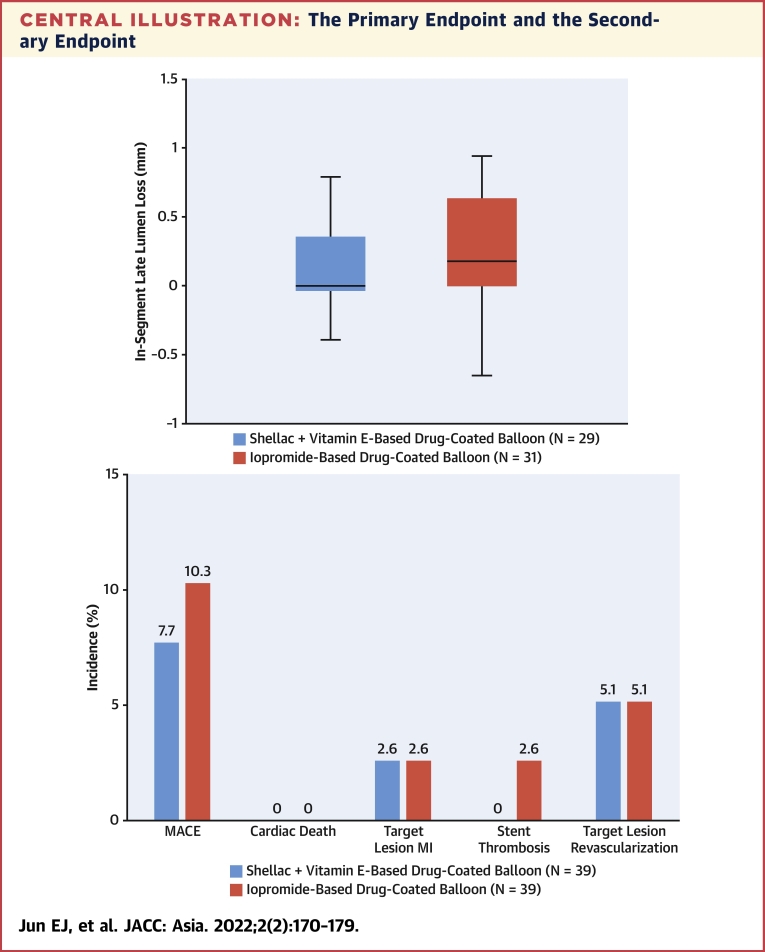
Angiographic follow-up data (Table 3) were available for 29 patients in the shellac plus vitamin E–based DCB group and 31 patients in the iopromide-based DCB group. The 6-month in-segment LLL was 0.15 ± 0.43 mm with the shellac plus vitamin E–based DCB compared with 0.24 ± 0.39 mm with the iopromide-based DCB (Central Illustration). The upper limit of the 97.5% one-sided CI for differences was 0.13 mm, lower than the noninferiority limit of 0.29 mm, achieving the noninferiority of the shellac plus vitamin E–based DCB to the iopromide-based DCB (P for noninferiority = 0.001).
Central Illustration.
The Primary Endpoint and the Secondary Endpoint
(A) The primary endpoint of in-segment late lumen loss and (B) the secondary endpoint of major adverse cardiovascular events (MACE) at 6 months. In this multicenter, head-to-head comparison randomized trial for coronary in-stent restenosis, the shellac plus vitamin E–based drug-coated balloon (DCB) shows a comparable result to the reference iopromide-based DCB for the primary endpoint of 6 months in-segment late lumen loss. Both DCBs had similar MACE rates at 6 months. MI = myocardial infarction; TLR = target lesion revascularization.
Immediately after DCB treatment in both groups, there were comparable increases in reference vessel diameter and MLD and decreases in DS. In addition, in-segment (shellac plus vitamin E–based DCB 1.26 ± 0.38 mm vs iopromide-based DCB 1.37 ± 0.43mm; P = 0.250) and in-stent (shellac plus vitamin E–based DCB 1.26 ± 0.38 mm vs iopromide-based DCB 1.37 ± 0.43 mm; P = 0.249) acute lumen gains were similar in both groups. At angiographic follow-up, no significant between-group differences were found, except for DS. In-segment (shellac plus vitamin E–based DCB 23.53 ± 10.93% vs iopromide-based DCB 30.43 ± 11.17%; P = 0.020) and in-stent (shellac plus vitamin E–based DCB 23.53 ± 10.93% vs iopromide-based DCB 30.23 ± 10.82%; P = 0.022) DS were significantly lower after DCB treatment than before the treatment. As shown in Table 3, no significant between-group differences were noted in either in-segment (shellac plus vitamin E–based DCB 0% vs iopromide-based DCB 6.45% [n = 2 of 31]; P = 0.164) or in-stent (shellac plus vitamin E–based DCB 0% vs iopromide-based DCB 3.22% [n = 1 of 31]; P = 0.329) binary restenosis. Figures 2 and 3 present the cumulative frequency distributions of in-segment MLD, DS, and LLL.
Figure 2.
Angiographic Outcomes
(A) Cumulative frequency distribution of in-segment minimal lumen diameter and (B) cumulative frequency distribution of in-segment diameter stenosis determined by quantitative coronary angiography. Both drug-coated balloons (DCBs) show comparable results of in-segment minimal lumen diameter and in-segment diameter stenosis.
Figure 3.
Cumulative Frequency Distribution of In-Segment Late Lumen Loss
Cumulative frequency distribution of in-segment late lumen loss determined by quantitative coronary angiography after drug-coated balloon (DCB) treatment. Both groups show comparable results.
Clinical outcomes
Clinical follow-up was completed for all patients in both groups at 6 months. Table 4 summarizes the clinical events at 6 months. There was no cardiac death, and the target lesion myocardial infarction rate was 2.6% (n = 1 of 39) in both groups. Stent thrombosis occurred in only 1 patient in the iopromide-based DCB group, and TLR was 5.1% (n = 2 of 39) in both groups. MACE at 6 months was 7.7% (n = 3 of 39) in the shellac plus vitamin E–based DCB group and 10.3% (n = 4 of 39) in the SeQuent Please group (Central Illustration).
Table 4.
Clinical Follow-Up at 6 Months
| Shellac + Vitamin E–Based DCB (n = 39) | Iopromide-Based DCB (n = 39) | |
|---|---|---|
| Major adverse cardiovascular events | 3 (7.7) | 4 (10.3) |
| Cardiac death | 0 | 0 |
| Myocardial infarction | 1 (2.6) | 1 (2.6) |
| Stent thrombosis | 0 | 1 (2.6) |
| Target lesion revascularization | 2 (5.1) | 2 (5.1) |
| Target vessel revascularization | 2 (5.1) | 2 (5.1) |
| Stroke | 0 | 0 |
| New vessel revascularization | 0 | 0 |
Values are n (%).
DCB = drug-coated balloon
Discussion
This trial demonstrated that the shellac plus vitamin E-based DCB was noninferior to the iopromide-based DCB with regard to the primary endpoint of 6-month in-segment LLL in treating coronary ISR. Additionally, the rates of adverse clinical events within 6 months were similar between the treatment groups. The results of this new, adequately powered, head-to-head randomized trial comparing 2 different DCBs in ISR add important insights to the available clinical evidence for treatment strategies for ISR.
Although the risk of restenosis after implantation of contemporary new-generation DES is considered very low, the number of complex coronary interventions is increasing. Consequently, the indication for approximately 10% of all coronary interventions remains the treatment of ISR.15 The main cause of ISR is excessive neointimal proliferation caused by the stimulus of the permanent implant. Therefore, ISR therapy typically involves local drug delivery to reduce the risk of neointimal proliferation.16
Although strategies for treating ISR varied and ranged from repeat balloon angioplasty to surgical revascularization, based on the available evidence, only 2 procedures could be considered effective, namely, implantation of another DES or use of a DCB.17 Ultimately, only these 2 device types have been endorsed in revascularization guidelines.1 Common to both procedures is local drug delivery to the restenotic area within the stent to address the mechanism of excessive neointimal proliferation. As an alternative to the implantation of another stent, the treatment of ISR with a DCB was proven to be clinically effective.18 After 1 year, similar recurrence rates were observed with DES and DCB.12,13,19,20 In addition, evidence presents that long-term hard clinical endpoints are positively influenced by the absence of a further layer of metal.21,22 A recent meta-analysis of 4,590 patients compared DCBs with alternative treatments, such as DES, for coronary ISR and de novo lesions and reported a significantly lower all-cause and cardiac mortality after 3 years when using a DCB.23 The suggested mechanism for this surprising finding is the avoidance of a permanent metal implant when treating patients with a DCB. For DES, a device-related annual event rate of up to 2% has been reported.24 For this reason, several authors suggest using a DCB as the primary strategy for treating ISR,25 even if the reduction in the risk of recurrent TLR appeared lower in repeat stenting with DES.26
Many randomized data on the comparison of DCBs with alternative percutaneous therapies for the treatment of ISR are based on the iopromide-based DCB. Thus, we designed this trial to provide evidence for regulatory approval in South Korea by comparing the safety and efficacy of the new shellac plus vitamin E–based DCB with that of the reference iopromide-based DCB. The hydrophilic shellac coating on the surface of the new DCB allows for rapid release and diffusion into the tissue.27,28 Added to the coating is the antioxidant vitamin E, which is known to be effective in preventing restenosis by reducing local plasminogen activator inhibitor-1 and by directly blocking the accumulation of smooth muscle cells, which are activated when the vascular wall is damaged after balloon angioplasty.29,30 At the 6-month follow-up, this study yielded comparable clinical and angiographic results between the iopromide-based and shellac plus vitamin E–based DCBs. Furthermore, in-segment and in-stent DS were significantly lower at follow-up angiography with the shellac plus vitamin E–based DCB than with the iopromide-based DCB, even though the baseline and post-DCB DS were comparable. Lesion preparation, followed by local drug delivery, represents the fundamental principle of any DCB therapy.3 Clinical outcomes after ISR therapy with a DCB depend on whether the quality criteria of the DCB consensus group are met.31,32 The primary goal of lesion preparation is to avoid flow-limiting dissections and to reduce the degree of residual stenosis to <30%. In our study, lesion preparation was mandatory. Bailout stent implantation after DCB was not needed. Although the procedural success rate was numerically lower in the shellac plus vitamin E–based DCB group compared with the iopromide-based DCB group (82.1% vs 89.7%; P = 0.329), their rates of MACE at 6 months were comparable. The results of this new head-to-head comparison randomized trial of 2 different PCBs in ISR provide important insights into the available clinical evidence for the treatment strategies for ISR. To our knowledge, this is the first evidence that shellac plus vitamin E–based DCB is effective and safe, at least for the treatment of ISR at 6 months’ follow-up. Following these results, we obtained regulatory approval for the device in South Korea.
Study limitations
First, it was difficult to ensure sufficient power to detect differences in clinical outcomes with the relatively low sample size. Thus, no further conclusions are possible on the clinical safety or efficacy of the shellac plus vitamin E–based DCB. However, angiographic surrogate endpoints such as LLL have been widely used and validated in other trials that evaluated the safety and efficacy of DCBs. Second, although the noninferior limit was met in this study, according to the study plan, the sample calculated to prove noninferiority required 80% case follow-up. Therefore, interpretation of the results required sufficient attention. The limited sample size and short follow-up period could have caused the absence of mortality in this study. Real-world evidence is important to confirm the clinical effectiveness of the shellac plus vitamin E–based DCB.
Conclusions
In this multicenter, head-to-head comparison, first-in-man randomized trial, the new DCB, with a shellac plus vitamin E excipient coating, shows comparable results to the reference iopromide-coated DCB for the primary endpoint of 6-month in-segment LLL.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: DCBs offer an effective treatment for ISR. With comparable clinical results, they do not require the implantation of an additional metal layer and may lead to better long-term results. In the present multicenter, head-to-head, randomized trial, the shellac plus vitamin E–based DCB was noninferior to the standard reference iopromide-coated DCB for the primary endpoint of 6-month in-segment LLL, with comparable clinical outcomes at 6 months.
TRANSLATIONAL OUTLOOK: Longer-term follow-up and large-scale studies with primary clinical endpoints are needed to evaluate clinical outcomes with the shellac plus vitamin E–based DCB in the treatment of coronary ISR. Furthermore, this new DCB should also be investigated in de novo lesions.
Funding Support and Author Disclosures
This study was supported by an unrestricted research grant from GENOSS through an institutional research grant. The executive committee, together with the sponsor, designed the clinical trial. The sponsor had no role in data collection, data analysis, data interpretation, writing the manuscript, or the decision to submit the manuscript for publication. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank the patients who participated in the Genoss DCB ISR South Korea trial and appreciate the dedicated efforts of the clinical research collaborators in the Genoss DCB ISR South Korea study organization, the contributions of the participating centers, and the CRO company (Synex Consulting).
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Windecker S., Neumann F.J., Jüni P., Sousa-Uva M., Falk V. Considerations for the choice between coronary artery bypass grafting and percutaneous coronary intervention as revascularization strategies in major categories of patients with stable multivessel coronary artery disease: an accompanying article of the task force of the 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:204–212. doi: 10.1093/eurheartj/ehy532. [DOI] [PubMed] [Google Scholar]
- 2.Kleber F.X., Mathey D.G., Rittger H., Scheller B., German Drug-eluting Balloon Consensus Group How to use the drug-eluting balloon: recommendations by the German consensus group. EuroIntervention. 2011;7(Suppl K):K125–K128. doi: 10.4244/EIJV7SKA21. [DOI] [PubMed] [Google Scholar]
- 3.Alfonso F., Scheller B. State of the art: balloon catheter technologies-drug-coated balloon. Eurointervention. 2017;13:680–695. doi: 10.4244/EIJ-D-17-00494. [DOI] [PubMed] [Google Scholar]
- 4.Rowinsky E.K., Donehower R.C. Paclitaxel (taxol) N Engl J Med. 1995;332:1004–1014. doi: 10.1056/NEJM199504133321507. [DOI] [PubMed] [Google Scholar]
- 5.Clever Y.P., Cremers B., Krauss B., et al. Paclitaxel and sirolimus differentially affect growth and motility of endothelial progenitor cells and coronary artery smooth muscle cells. EuroIntervention. 2011;7(Suppl K):K32–K42. doi: 10.4244/EIJV7SKA6. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y., Gao L., Qin Q., et al. RESTORE ISR China Investigators Comparison of 2 different drug-coated balloons in in-stent restenosis: the RESTORE ISR China Randomized Trial. J Am Coll Cardiol Intv. 2018;11:2368–2377. doi: 10.1016/j.jcin.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Ali R.M., Abdul Kader M.A.S.K., Wan Ahmad W.A., et al. Treatment of coronary drug-eluting stent restenosis by a sirolimus-or paclitaxel-coated balloon. J Am Coll Cardiol Intv. 2019;12:558–566. doi: 10.1016/j.jcin.2018.11.040. [DOI] [PubMed] [Google Scholar]
- 8.Eshtehardi P., Adorjan P., Togni M., et al. Iatrogenic left main coronary artery dissection: incidence, classification, management, and long-term follow-up. Am Heart J. 2010;159:1147–1153. doi: 10.1016/j.ahj.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Mehran R., Dangas G., Abizaid A.S., et al. Angiographic patterns of in-stent restenosis: classification and implications for long-term outcome. Circulation. 1999;100:1872–1878. doi: 10.1161/01.cir.100.18.1872. [DOI] [PubMed] [Google Scholar]
- 10.Cutlip D.E., Windecker S., Mehran R., et al. Academic Research Consortium Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 11.Xu B., Gao R., Wang J., et al. PEPCAD China ISR Trial Investigators A prospective, multicenter, randomized trial of paclitaxel-coated balloon versus paclitaxel-eluting stent for the treatment of drug-eluting stent in-stent restenosis: results from the PEPCAD China ISR trial. J Am Coll Cardiol Intv. 2014;7:204–211. doi: 10.1016/j.jcin.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Rittger H., Brachmann J., Sinha A.M., et al. A randomized, multicenter, single-blinded trial comparing paclitaxel-coated balloon angioplasty with plain balloon angioplasty in drug-eluting stent restenosis: the PEPCAD-DES study. J Am Coll Cardiol. 2012;59:1377–1382. doi: 10.1016/j.jacc.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Habara S., Mitsudo K., Kadota K., et al. Effectiveness of paclitaxel-eluting balloon catheter in patients with sirolimus-eluting stent restenosis. J Am Coll Cardiol Intv. 2011;4:149–154. doi: 10.1016/j.jcin.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Unverdorben M., Vallbracht C., Cremers B., et al. Paclitaxel-coated balloon catheter versus paclitaxel-coated stent for the treatment of coronary in-stent restenosis: the three-year results of the PEPCAD II ISR study. EuroIntervention. 2015;11:926–934. doi: 10.4244/EIJY14M08_12. [DOI] [PubMed] [Google Scholar]
- 15.Moussa I.D., Mohananey D., Saucedo J., et al. Trends and Outcomes of Restenosis After Coronary Stent Implantation in the United States. J Am Coll Cardiol. 2020;76:1521–1531. doi: 10.1016/j.jacc.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Alfonso F., Byrne R.A., Rivero F., Kastrati A. Current treatment of in-stent restenosis. J Am Coll Cardiol. 2014;63:2659–2673. doi: 10.1016/j.jacc.2014.02.545. [DOI] [PubMed] [Google Scholar]
- 17.Siontis G.C., Stefanini G.G., Mavridis D., et al. Percutaneous coronary interventional strategies for treatment of in-stent restenosis: a network meta-analysis. Lancet. 2015;386:655–664. doi: 10.1016/S0140-6736(15)60657-2. [DOI] [PubMed] [Google Scholar]
- 18.Scheller B., Hehrlein C., Bocksch W., et al. Treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. N Engl J Med. 2006;355:2113–2124. doi: 10.1056/NEJMoa061254. [DOI] [PubMed] [Google Scholar]
- 19.Byrne R.A., Neumann F.J., Mehilli J., et al. ISAR-DESIRE 3 investigators Paclitaxel-eluting balloons, paclitaxel-eluting stents, and balloon angioplasty in patients with restenosis after implantation of a drug-eluting stent (ISAR-DESIRE 3): a randomised, open-label trial. Lancet. 2013;381:461–467. doi: 10.1016/S0140-6736(12)61964-3. [DOI] [PubMed] [Google Scholar]
- 20.Baan J., Claessen B.E., Boerlage-van Dijk K., et al. A randomized comparison of paclitaxel-eluting balloon versus everolimus-eluting stent for the treatment of any in-stent restenosis: the DARE trial. J Am Coll Cardiol Intv. 2018;11:275–283. doi: 10.1016/j.jcin.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 21.Xu B., Qian J., Ge J., et al. PEPCAD China ISR investigators Two-year results and subgroup analyses of the P EPCAD China in-stent restenosis trial: A prospective, multicenter, randomized trial for the treatment of drug-eluting stent in-stent restenosis. Catheter Cardiovasc Interv. 2016;87:624–629. doi: 10.1002/ccd.26401. [DOI] [PubMed] [Google Scholar]
- 22.Kufner S., Cassese S., Valeskini M., et al. ISAR-DESIRE 3 Investigators Long-term efficacy and safety of paclitaxel-eluting balloon for the treatment of drug-eluting stent restenosis: 3-year results of a randomized controlled trial. J Am Coll Cardiol Intv. 2015;8:877–884. doi: 10.1016/j.jcin.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 23.Scheller B., Vukadinovic D., Jeger R., et al. Survival after coronary revascularization with paclitaxel-coated balloons. J Am Coll Cardiol. 2020;75:1017–1028. doi: 10.1016/j.jacc.2019.11.065. [DOI] [PubMed] [Google Scholar]
- 24.Madhavan M.V., Kirtane A.J., Redfors B., et al. Stent-related adverse events >1 year after percutaneous coronary intervention. J Am Coll Cardiol. 2020;75:590–604. doi: 10.1016/j.jacc.2019.11.058. [DOI] [PubMed] [Google Scholar]
- 25.Lansky A., Grubman D., Scheller B. Paclitaxel-coated balloons: a safe alternative to drug-eluting stents for coronary in-stent restenosis. Eur Heart J. 2020;41(38):3729–3731. doi: 10.1093/eurheartj/ehz731. [DOI] [PubMed] [Google Scholar]
- 26.Giacoppo D., Alfonso F., Xu B., et al. Paclitaxel-coated balloon angioplasty vs. drug-eluting stenting for the treatment of coronary in-stent restenosis: a comprehensive, collaborative, individual patient data meta-analysis of 10 randomized clinical trials (DAEDALUS study) Eur Heart J. 2020;41:3715–3728. doi: 10.1093/eurheartj/ehz594. Published correction appears in Eur Heart J. 2020;41(38):3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stella P.R., Belkacemi A., Waksman R., et al. Valentine Investigators. The Valentines Trial: results of the first one week worldwide multicentre enrolment trial, evaluating the real world usage of the second generation DIOR paclitaxel drug-eluting balloon for in-stent restenosis treatment. EuroIntervention. 2011;7:705–710. doi: 10.4244/EIJV7I6A113. [DOI] [PubMed] [Google Scholar]
- 28.Pósa A., Nyolczas N., Hemetsberger R., et al. Optimization of drug-eluting balloon use for safety and efficacy: evaluation of the 2nd generation paclitaxel-eluting DIOR-balloon in porcine coronary arteries. Catheter Cardiovasc Interv. 2010;76:395–403. doi: 10.1002/ccd.22468. [DOI] [PubMed] [Google Scholar]
- 29.Konneh M.K., Rutherford C., Li S.R., Anggård E.E., Ferns G.A. Vitamin E inhibits the intimal response to balloon catheter injury in the carotid artery of the cholesterol-fed rat. Atherosclerosis. 1995;113:29–39. doi: 10.1016/0021-9150(94)05423-g. [DOI] [PubMed] [Google Scholar]
- 30.Orbe J., Rodriguez J.A., Calvo A., et al. Vitamins C and E attenuate plasminogen activator inhibitor-1 (PAI-1) expression in a hypercholesterolemic porcine model of angioplasty. Cardiovasc Res. 2001;49:484–492. doi: 10.1016/s0008-6363(00)00260-1. [DOI] [PubMed] [Google Scholar]
- 31.Jeger R.V., Eccleshall S., Wan Ahmad W.A., et al. International DCB Consensus Group Drug-coated balloons for coronary artery disease: third report of the International DCB Consensus Group. J Am Coll Cardiol Intv. 2020;13:1391–1402. [Google Scholar]
- 32.Her A.Y., Shin E.S., Bang L.H., et al. Drug-coated balloon treatment in coronary artery disease: recommendations from an Asia-Pacific Consensus Group. Cardiol J. 2021;28(1):136–149. doi: 10.5603/CJ.a2019.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]