Abstract
Mycobacterium tuberculosis has a relatively high resistance to killing by hydrogen peroxide and organic peroxides. Resistance may be mediated by mycobacterial catalase-peroxidase (KatG) and possibly by alkyl hydroperoxide reductase (AhpC). To determine the interrelationship between sensitivity to H2O2, catalase and peroxidase activities, and bacillary growth rates measured both intracellularly in human monocytes and in culture medium, we examined one laboratory strain, two clinical isolates, and three recombinant strains of M. tuberculosis with differing levels of KatG and AhpC. Five of the mycobacterial strains had intracellular doubling times of 27 to 32 h, while one KatG-deficient clinical isolate (ATCC 35825) doubled in ∼76 h. Killing of mycobacteria by exogenously added H2O2 was more pronounced for intracellular bacilli than for those bacilli derived from disrupted monocytes. Strains with no detectable KatG expression or catalase activity were relatively sensitive to killing (43 to 67% killing) by exogenous H2O2. However, once even minimal catalase activity was present, mycobacterial catalase activity over a 10-fold range (0.56 to 6.2 U/mg) was associated with survival of 85% of the bacilli. Peroxidase activity levels correlated significantly with resistance of the mycobacterial strains to H2O2-mediated killing. An endogenous oxidative burst induction by 4β-phorbol 12β-myristate 13α-acetate treatment of infected monocytes reduced the viability of the KatG null strain (H37Rv Inhr) but not the KatG-overexpressing strain [H37Rv(pMH59)]. These results suggest that mycobacterial resistance to oxidative metabolites (including H2O2 and other peroxides) may be an important mechanism of bacillary survival within the host phagocyte.
Mycobacterium tuberculosis is a facultative intracellular bacterium which has evolved sophisticated mechanisms to allow it to survive inside host mononuclear phagocytes. Once phagocytosed, the organism resides in a vacuole which does not fully mature along the endocytic pathway (6, 32, 33, 37, 40). Within the vacuole, the organism must protect itself against intracellular bactericidal mechanisms, including the production of reactive oxygen intermediates (ROI) and reactive nitrogen intermediates which diffuse freely through the cell (9, 11, 32).
M. tuberculosis has been shown to have a high resistance to killing by up to millimolar concentrations of H2O2 (15, 26). This resistance is believed to be mediated by the sole mycobacterial catalase-peroxidase protein (KatG) and the alkyl hydroperoxide reductase protein (AhpC), encoded by the genes katG and ahpC, respectively (13). Isoniazid, a widely used frontline antimycobacterial agent, requires activation by KatG before exerting a lethal effect (1, 41). Isoniazid resistance in a majority of clinical isolates results from point mutations in katG (2). Isoniazid-resistant mutants selected in vitro frequently lose KatG entirely. The availability of KatG mutant organisms has facilitated investigations of the role of catalase-peroxidase in the virulence of M. tuberculosis. These studies have produced conflicting results. Some investigators have observed no correlation between loss of KatG activity and virulence of M. tuberculosis in mice and guinea pigs (12, 16, 31) and no correlation between KatG levels and susceptibility to killing by hydrogen peroxide (15). However, others have found a strong apparent correlation between KatG status and M. tuberculosis virulence (26, 29). More recently, the loss of catalase and peroxidase activities in Mycobacterium bovis has been shown to correlate with the lack of virulence of M. bovis in guinea pigs (39). Reintroduction of a functional katG into this strain restored both isoniazid sensitivity and virulence in the host animal.
Isoniazid-resistant mutant strains of M. tuberculosis which have no detectable KatG activity acquire a compensatory mutation resulting in an upregulation of expression of AhpC (36). It has been suggested that this protein confers protection against H2O2-mediated damage even in the absence of adequate catalase and peroxidase activities, thus promoting survival of the organism in the environment of the phagocyte oxidative burst (36).
To gain a better understanding of the role of the mycobacterial catalase-peroxidase and alkyl hydroperoxide reductase enzyme activities in resistance to host cell ROI defensive mechanisms, we utilized a series of clinical isolates and recombinant mutant strains of M. tuberculosis with varying levels of expression of KatG and AhpC. We measured M. tuberculosis resistance to killing by H2O2 and studied the interrelationship between this resistance, the levels of expression of KatG and AhpC, and survival within human monocytes in vitro.
MATERIALS AND METHODS
Bacterial strains.
The strains of M. tuberculosis used in this study, and their patterns of KatG gene expression, are summarized in Table 1. The strains used include the laboratory strains H37Rv (Trudeau Institute, Saranac Lake, N.Y.), CDC 1551 (T. M. Shinnick, Centers for Disease Control and Prevention, Atlanta, Ga.), ATCC 35825, and H37Rv Inhr, which was selected from H37Rv by direct plating onto 10 μg of isoniazid per ml (loss of KatG was verified by [i] activity assay, [ii] Western blotting using anti-KatG antipeptide antisera, and [iii] Southern blot analysis using the katG probe and restriction digestion as described previously [25]), as well as the recombinant strain H37Rv(pMH59) and the recombinant strain H37Rv(pMH91), which have been previously described (36). The KatG-overexpressing construct consists of a 2.9-kb EcoRI fragment carrying the katG gene of H37Rv cloned into the polylinker site of pMV306 (36). This construct contains about 85 bp upstream of the GTG start codon of katG. The vector system also has a synthetic promoter element incorporated into the polylinker driving expression of KatG (10). Mycobacterial strains were grown for 7 days in Middlebrook 7H9 liquid medium (Difco, Detroit, Mich.) containing 0.05% Tween 80 (Sigma Chemical Co., St. Louis, Mo.) at 37°C with daily agitation. For the recombinant strains, isoniazid, kanamycin, and hygromycin B were added to the culture medium at final concentrations of 10, 25, and 50 μg/ml, respectively. For stock preparation, aliquots of 108 bacilli/ml were frozen at −70°C. The culture medium was lipopolysaccharide free and without reactivity in the Limulus amoebocyte lysis assay (Whittaker Bioproducts, Walkersville, Md.).
TABLE 1.
M. tuberculosis strains
| Mycobacterial strain | Protein expressiona
|
|
|---|---|---|
| KatG | AhpC | |
| Reference laboratory strain H37Rv | + | +/− |
| Clinical isolates | ||
| CDC 1551 | + | NDb |
| ATCC 35825 (Inhr) | − | + |
| Recombinant strains | ||
| H37Rv Inhr | − | +/− |
| H37Rv(pMH59) | +++ | +/− |
| H37Rv(pMH91) | + | +++ |
+/−, detectable by Western blotting but not by Coomassie blue staining of the gel; +, detectable by Coomassie blue staining; +++, overexpressed as Coomassie blue staining.
ND, not determined.
Reagents.
H2O2 at 3%, 4β-phorbol 12β-myristate 13α-acetate (PMA), and antibiotics (isoniazid, kanamycin, and hygromycin B) were obtained from Sigma Chemical Co. OADC supplement (sodium chloride, bovine albumin fraction V, dextrose, catalase, oleic acid) was obtained from Becton Dickinson (Cockeysville, Md.); human serum (pooled AB positive) was obtained from Biocell (Carson, Calif.).
Human monocytes.
Peripheral blood mononuclear cells were isolated from fresh human blood (buffy coat) (New York Blood Center, New York, N.Y.) by centrifugation on Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) and then diluted 1:1 with RPMI 1640 (Gibco BRL, Gaithersburg, Md.) culture medium as described previously (27). The cells were resuspended in RPMI 1640 medium supplemented with 1% human serum (R1) and counted in a hemacytometer. For culture, peripheral blood mononuclear cell density was adjusted to 6 × 106/ml, and 0.5 ml was plated in 24-well Falcon tissue culture dishes (Becton Dickinson Labware, Lincoln Park, N.J.). The cells were left to adhere for 1 to 1.5 h at 37°C and 5% CO2. Nonadherent cells were removed by three washes with R1 at 37°C, and the remaining adherent cells were incubated in 0.5 to 1 ml of RPMI 1640 with 20% human serum (R20) at 37°C and 5% CO2 depending on the assay (20).
Infection of human monocytes.
Monocytes were infected with different strains of M. tuberculosis on day 0, immediately after removal of nonadherent cells. For infection, aliquots of bacteria were thawed at 37°C and then sonicated for 20 s at a low-output power setting (model 60 Sonic Dismembrator; Fisher Scientific, Springfield, N.J.) to disperse clumped bacilli. The bacterial suspension, diluted in R20, was added to freshly isolated adherent monocytes at a multiplicity of infection of one viable bacillus per cell. All M. tuberculosis strains used in these experiments were phagocytosed efficiently; less than 1% of infecting bacilli were subsequently cultured from the extracellular medium at 4 h postinfection.
CFU assay.
Adherent monolayers of 3 × 105 infected monocytes (21) in R20 were cultured for 4 days. To facilitate release of the mycobacteria from the cells, phosphate-buffered saline containing 0.016% digitonin and 0.25% Tween 80 (Sigma Chemical Co.) was added to each well. After 10 min at 37°C, the organisms were processed for the CFU assay as previously described (28). Briefly, the cultures were sonicated for four pulses of 5 s each at a low-output power setting to disperse clumps of bacilli. Serial 10-fold dilutions of the bacterial suspension were made and plated on Middlebrook 7H10 agar supplemented with OADC and antibiotics when necessary. After 14 to 21 days of incubation, plates containing 10 to 100 colonies were counted with the aid of a dissecting microscope. For each culture dilution, six replicate samples were plated and the mean number of colonies was calculated. To determine the generation time of the mycobacterial strains in Middlebrook 7H9 medium, 1-ml aliquots of growing cells were removed daily, sonicated for 20 s at a low-output power setting to disperse clumps of bacilli, and then processed for the CFU assay as described above. None of the M. tuberculosis strains replicated in cell-free cultured medium. Data for the growth curves of each strain are the results of three independent experiments.
Intracellular and extracellular killing assays.
To determine intracellular killing by exogenously added H2O2, 4-day-infected monocytes in R20 were treated for 6 h with 10 mM H2O2. The cells were then disrupted by sonication for 20 s at a low-output power setting, and the culture was processed for the CFU assay as described above. For determination of extracellular killing by exogenous H2O2, the cell monolayers were disrupted before the addition of H2O2. H2O2 was then added for 6 h, and the cultures were processed for the CFU assay as described above. Results for the killing assays represent the mean ± standard error of the mean (SEM) of three to six independent experiments.
Intracellular killing by PMA-induced endogenous H2O2 production.
Fresh human monocytes in R20 were infected on day 0 with recombinant strain H37Rv(pMH59) or H37Rv Inhr at a multiplicity of infection of one viable bacillus per cell. To facilitate the adhesion of the bacteria to the cells, the infected monolayers were centrifuged for 10 min at 369 × g at room temperature and then incubated for 1 h at 37°C to allow phagocytosis. Infected monocytes were then treated with PMA at a final concentration of 100 ng/ml for either 2 or 6 h. To determine direct toxicity of PMA, bacterial suspensions at the same density in culture medium alone (no monocytes) were incubated in the presence and absence of PMA. After preparation of single-cell suspensions by sonication as follows, the CFU were quantified.
Detection of catalase and peroxidase enzymatic activities.
A 200-ml culture of each strain was grown in Middlebrook 7H9 medium to a final optical density (OD) at 650 nm of 0.2 to 0.3. Bacterial cells were collected by centrifugation at 5,000 × g for 25 min at 4°C, and the pellet was resuspended in 50 mM triethylamine (pH 7.8) containing 0.1 mM phenylmethylsulfonyl flouride to a final OD (at 650 nm) of 25. The suspension (1.5 ml) was lysed by adding 0.5 g of 0.1-mm-diameter glass beads and homogenizing with a BioSpec (Bartlesville, Okla.) Mini Bead-Beater for four 30-s bursts with cooling on ice for at least 1 min between homogenizations. The glass beads and cell wall pellet were removed by centrifugation at 10,000 × g for 30 min at 4°C, and the supernatants were transferred to fresh tubes. Catalase assays were performed as described previously (3). Briefly, 100 μl of the bacillary extract was added to 3 ml of 5 mM H2O2 in 50 mM potassium phosphate (pH 7.0). The decrease in absorbance at 240 nm was monitored for 10 min, and the linear part of the curve was used to quantitate the rate of decrease by using an extinction coefficient of H2O2 at 240 nm of 0.0435 OD/mM · cm. Peroxidase activity was determined as previously described (17). Briefly, 100 μl of the bacillary extract was added to 50 mM potassium phosphate (pH 7.0) containing 0.1 mM O-dianisidine and 23 mM t-butylhydroperoxide; absorbance at 460 nm was monitored, and rate calculations were performed with an extinction coefficient of 11.3 OD/mM · cm. Protein concentrations were determined by the method of Lowry et al. (24). Specific activities were calculated by dividing the observed rate of decrease by the protein concentration in the crude extracts.
RESULTS
Intracellular and extracellular mycobacterial growth rates.
We examined the growth rates, both intracellularly in monocytes and extracellularly in the bacterial liquid growth medium (Middlebrook 7H9), for each strain. For the three recombinant strains, the doubling time (30 to 31 h) within monocytes was close to the doubling time of the laboratory strain H37Rv (32 h) (Table 2). The clinical isolate CDC 1551 grew slightly faster than the reference strain both within monocytes (27 h) and in Middlebrook 7H9 medium (16 h). In contrast, the clinical isolate ATCC 35825 showed a dramatically lower replication rate within monocytes (76 h) and a somewhat lower extracellular growth rate in Middlebrook 7H9 medium (28 h) (Table 2). Thus, with the exception of ATCC 35825, all the strains studied had similar growth rates. Therefore, by using similar inocula for infection of the monocytes with five different mycobacterial strains, we achieved similar numbers of intracellular bacilli throughout the experiments. This enabled us to compare the effects of both exogenously added H2O2 and endogenous H2O2-induced killing on similar numbers of bacilli.
TABLE 2.
Growth rate of M. tuberculosis strains (in vitro)
| Mycobacterial strain | Generation time (h)
|
|
|---|---|---|
| Intracellular in monocytesa | Extracellular in Middlebrook 7H9 mediumb | |
| Reference laboratory strain H37Rv | 32 ± 2.6 | 20 |
| Clinical isolates | ||
| CDC 1551 | 27 ± 3.5 | 16 |
| ATCC 35825 (Inhr) | 76 ± 1.2 | 28 |
| Recombinant strains | ||
| H37Rv Inhr | 31 ± 2.9 | 18 |
| H37Rv (pMH59) | 30 ± 2.3 | 20 |
| H37Rv (pMH91) | 31 ± 1.4 | 22 |
Mean of three experiments ± SEM.
Results from one 7-day growth experiment.
Sensitivity of M. tuberculosis to exogenously added H2O2 in Middlebrook 7H9 medium.
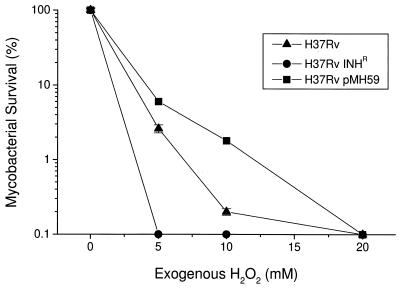
To select a suitable concentration of H2O2 for our studies, we tested the sensitivity of three bacillary strains to 5, 10, and 20 mM H2O2 in Middlebrook 7H9 medium. Previous studies of clinical isolates from a variety of geographic locations have demonstrated hydrogen peroxide-induced killing in vitro by using similar concentrations of H2O2 (3). H37Rv Inhr (katG null [Table 1]) was killed (100%) by 5 mM H2O2, while H37Rv (pMH59) (high KatG) expression [Table 1]) showed 100% killing only at 20 mM H2O2 (Fig. 1). H37Rv (wild-type levels of katG expression [Table 1]) showed an intermediate level of sensitivity to exogenously added H2O2. Thus, sensitivity to H2O2 in these strains correlated with levels of expression of KatG (Table 1). The concentration of 10 mM H2O2 was used for further experiments because it gave the best differential killing effect among the three strains tested.
FIG. 1.
Effect of exogenous H2O2 on mycobacterial viability in cell-free Middlebrook 7H9 medium. H2O2 (at the concentrations shown) was added to growing bacteria, and the number of CFU was determined as described in Materials and Methods. Results are expressed as percent survival relative to baseline ± SEM and are from one representative experiment with six replicate cultures. The numbers of CFU used for the experiment (and expressed as 100% in the figure) were 4.7 × 105 for H37Rv, 3.2 × 105 for H37Rv Inhr, and 2.8 × 105 for H37Rv(pMH59).
Effect of exogenous H2O2 on M. tuberculosis intracellular and extracellular survival.
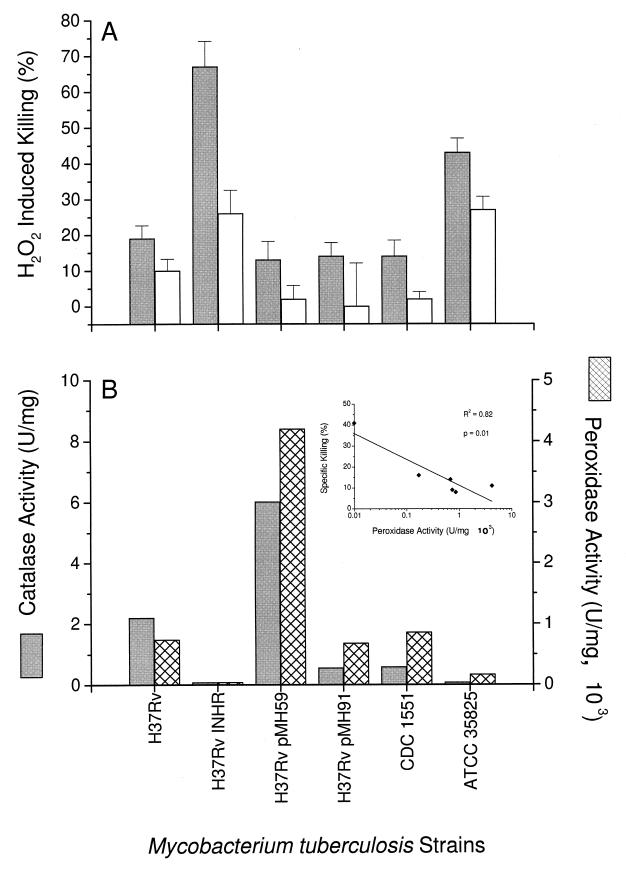
We next compared the intracellular and extracellular sensitivities of H37Rv and the two clinical and three recombinant strains of M. tuberculosis to exogenous H2O2 (10 mM). The results, expressed as percent killing, are shown in Fig. 2A. As expected, the two strains lacking in KatG and thus deficient in catalase activity (H37Rv Inhr and ATCC 35825) showed the greatest sensitivities to H2O2-mediated killing (67 and 43% killing, respectively). Surprisingly, overexpression of KatG had only a very small protective effect on intracellular survival following the addition of exogenous peroxide. Sensitivity to H2O2 in cell-free culture after disruption of the monocyte monolayer was proportional to the sensitivity within monocytes but lower. The level of AhpC did not affect sensitivity to H2O2. This is in spite of the fact that the extremely high level of AhpC overexpression in H37Rv(pMH91) is sufficient to confer protection to micromolar concentrations of cumene hydroperoxide (36).
FIG. 2.
(A) Killing of mycobacteria following treatment with exogenous H2O2. Fresh human monocytes were infected with M. tuberculosis, and 10 mM H2O2 was added to intact monolayers. After 6 h of treatment, numbers of CFU were determined (intracellular killing) (closed bars). Alternatively, the infected monocytes were disrupted by sonication before 6 h of treatment with H2O2. Cultures were then harvested for the CFU assay (extracellular killing) (open bars). Results are expressed as percent of mycobacteria killed and are the mean of three to six experiments, each done in triplicate, plus 1 SEM. (B) Mycobacterial catalase and peroxidase activities. Mycobacteria in log phase were harvested and homogenized, and the enzymatic activities were determined as described in Materials and Methods. The enzymatic activities are expressed as units per milligram. Results are means of one representative experiment carried out in quadruplicate.
Enzymatic activity levels of catalase and peroxidase.
The enzymatic activities of the mycobacterial catalase and peroxidase were determined as described in Materials and Methods. Lysates prepared from the recombinant strain H37Rv Inhr (katG null) had no detectable catalase and peroxidase enzymatic activities (Fig. 2B). In contrast H37Rv(pMH59) (with overexpression of KatG) showed the highest catalase and peroxidase activities (6.02 × 103 and 4.20 × 103 U/mg, respectively). All the other strains showed intermediate levels of these enzymatic activities. In general, the enzymatic activities were inversely associated with the H2O2-induced killing (compare Fig. 2B and A). A statistically significant inverse correlation was noted for specific killing versus peroxidase activity but not versus catalase activity (Fig. 2B, inset).
Killing mediated by the PMA-induced endogenous oxidative burst.
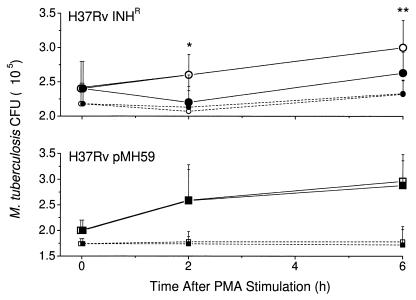
To further explore the relationship between KatG expression and the ability of the mycobacteria to withstand ROI-mediated killing, PMA was used to induce an endogenous monocyte oxidative burst. For these experiments, PMA was added to monocytes infected with either H37Rv Inhr (katG null) or H37Rv(pMH59) (KatG hyperexpressed), which are the two strains of M. tuberculosis with the lowest and highest catalase and peroxidase activities, respectively. A 20% specific killing of H37Rv Inhr was observed 2 h after the addition of PMA to the infected monocytes (Fig. 3). In contrast, H37Rv(pMH59) (KatG hyperexpressed) was resistant to the PMA-induced oxidative burst. After the initial reduction in CFU seen at 2 h, the H37Rv Inhr mycobacteria resumed replication at a similar rate to the growth rate of H37Rv(pMH59). No further killing was observed 2 to 6 h after PMA treatment. Exposure to PMA did not affect the survival of the control cell-free bacilli.
FIG. 3.
PMA-induced killing of intracellular mycobacteria. Monocytes were infected with H37Rv Inhr (circles) or H37Rv(pMH59) (squares). Intracellular bacilli (solid lines) and cell free bacilli (broken lines) were treated with PMA at a final concentration of 100 ng/ml. The open symbols indicate controls not treated with PMA. Results are expressed as mean CFU ± standard deviation of three experiments, each done in triplicate. Data were analyzed with a paired t test to compare control and PMA-treated cells. Significantly different results for controls versus corresponding PMA-treated cells are indicated by ∗ (P = 0.02) and ∗∗ (P = 0.04).
DISCUSSION
Survival of M. tuberculosis within the host macrophage phagosome requires that the bacilli be capable of resisting the normal microbicidal mechanisms of these potent leukocytes. The catalase-peroxidase protein (KatG) was implicated early in studies of mycobacterial pathogenesis because of the availability of mutants which had lost KatG function through the acquisition of isoniazid resistance. Even with these mutants, the relative contribution of the enzyme to mycobacterial survival within the host phagocyte has remained unclear. Some of the disparity in the published studies may be attributable to the use of a wide variety of model systems for evaluating virulence in which the actual effector molecules involved in mycobacterial killing may differ (e.g., nitric oxide synthase-mediated killing in murine macrophages [5, 7, 8] versus ROI-mediated killing in human mononuclear phagocytes [21]). Conflicting results may also be due to the comparison of nonisogenic strains of bacteria which may have acquired undefined compensatory mutations. However, in some systems, catalase and peroxidase activities have been clearly implicated as a virulence factor of mycobacteria. For example, the addition of exogenous catalase during in vitro infection of murine macrophages with an atypical mycobacterium such as M. avium has been shown to enhance the survival of the mycobacteria (21, 34).
The relative role of KatG in the protection of mycobacteria against intracellular killing within human monocytes has not been previously evaluated. Our present results provide a clear correlation between the peroxidase activity of M. tuberculosis strains and the ability of the bacilli (whether laboratory recombinants or clinical isolates) to survive intracellular killing in human monocytes in vitro. These findings are supported by the observation that in patients with a genetic inability to generate peroxides, a rare, fatal disseminated M. bovis BCG infection often occurs following BCG immunization (23, 35).
The role of upregulation of the AhpC protein is considerably less clear. AhpC of yeast requires reduced thioredoxin for catalysis, and in vitro detection of catalytic activity of AhpC (a complex three-protein system) is exceptionally difficult (4). Similarly, it is difficult to determine whether the overexpressed enzyme in H37Rv(pMH91) is functional or whether function is limited by the availability of the reduced thioredoxin cofactor. However, the finding that H37Rv(pMH91) has elevated resistance to killing in vitro by organic hydroperoxides such as cumene hydroperoxide suggests that AhpC may indeed be active in this recombinant strain (36). In addition, the recent observation that there is increased intracellular survival of Mycobacterium smegmatis containing the Mycobacterium leprae thioredoxin-thioredoxin reductase gene suggests that this protein may contribute to mycobacterial survival (38). In contrast to the in vitro observations suggesting a role for AhpC intracellular survival, in our experiments, AhpC hyperexpression did not appear to affect survival of M. tuberculosis following exposure to exogenous H2O2. This is consistent with other reported results indicating that AhpC overexpression does not alter the growth rate or virulence of M. tuberculosis in mice (14). Thus, the role of AhpC compensatory upregulation in virulence and intracellular growth of M. tuberculosis remains unclear.
Our experiments indicate that in human monocytes in vitro, the ability of M. tuberculosis to withstand killing by exogenously added or endogenously stimulated H2O2 is important for bacillary survival. This suggests that KatG-negative (isoniazid-resistant) M. tuberculosis should be less capable of withstanding the in vivo oxidative burst in human mononuclear phagocytes and therefore less pathogenic in humans. Nevertheless, KatG-negative (isoniazid-resistant) mycobacteria can cause disease in humans. This may be due to insufficient levels of ROI in the infected lung. It is not known what the extent of the oxidative burst is in macrophages in vivo. The levels of ROI achieved within the granuloma may differ from individual to individual.
Gamma interferon (IFN-γ) has been shown to induce an increased oxidative burst in human monocytes in vitro (30). It is therefore possible that within the granuloma in the presence of an efficient Th1 response and continuous local production of IFN-γ and interleukin-12, the oxidative burst associated with phagocytosis of organisms and cellular debris may be sufficient to contribute to killing of the intracellular bacilli. On the other hand, if there is a defect in the Th1 response and IFN-γ is not produced or is not functional in the lungs because of a lack of receptors for IFN-γ (18, 19), there may be lower amounts of ROI generated, leading to decreased killing of the infecting organisms and worse mycobacterial disease. Thus, the regulation of the oxidative burst within the infected lung, as well as the sensitivity of the mycobacteria to these toxic intermediates, will determine the outcome in patients.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants AI 22616 and AI 42056 from the NIAID.
We thank Pairote Laochumroonvorapong for helpful discussions, Judy Adams for preparation of the figures, and Marguerite Nulty for secretarial assistance. We also thank Mark Hickey and David Sherman of Pathogenesis Corporation, Seattle, Wash., for pMH59 and helpful discussions.
REFERENCES
- 1.Barry C E., III New horizons in the treatment of tuberculosis. Biochem Pharmacol. 1997;54:1165–1172. doi: 10.1016/s0006-2952(97)00163-9. [DOI] [PubMed] [Google Scholar]
- 2.Barry C E, III, Slayden R A, Mdluli K. Mechanisms of isoniazid resistance in Mycobacterium tuberculosis. Drug Res Updates. 1998;1:128–134. doi: 10.1016/s1368-7646(98)80028-9. [DOI] [PubMed] [Google Scholar]
- 3.Beers R F, Sizer I W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133–140. [PubMed] [Google Scholar]
- 4.Chae H Z, Chung S J, Rhee S G. Thioredoxin-dependent peroxide reductase from yeast. J Biol Chem. 1994;269:27670–27678. [PubMed] [Google Scholar]
- 5.Chan J, Xing Y, Magliozzo R S, Bloom B R. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175:1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemens D L, Horwitz M A. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J Exp Med. 1995;181:257–270. doi: 10.1084/jem.181.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng W, Thiel B, Tannenbaum C S, Hamilton T A, Stuehr D J. Synergistic cooperation between T cell lymphokines for induction of the nitric oxide synthase gene in murine peritoneal macrophages. J Immunol. 1993;151:322–329. [PubMed] [Google Scholar]
- 8.Denis M. Interferon gamma treated murine macrophages inhibit growth of tubercle bacilli via generation of reactive nitrogen intermediates. Cell Immunol. 1991;132:150–157. doi: 10.1016/0008-8749(91)90014-3. [DOI] [PubMed] [Google Scholar]
- 9.Fels A O, Cohn Z A. The alveolar macrophage. J Appl Physiol. 1986;60:353–369. doi: 10.1152/jappl.1986.60.2.353. [DOI] [PubMed] [Google Scholar]
- 10.George K M, Yuan Y, Sherman D R, Barry C E., III The biosynthesis of cyclopropanated mycolic acids in Mycobacterium tuberculosis. Identification and functional analysis of CMAS-2. J Biol Chem. 1995;270:27292–27298. doi: 10.1074/jbc.270.45.27292. [DOI] [PubMed] [Google Scholar]
- 11.Gordon A H, Hart P D. Stimulation or inhibition of the respiratory burst in cultured macrophages in a mycobacterium model: initial stimulation is followed by inhibition after phagocytosis. Infect Immun. 1994;62:4650–4651. doi: 10.1128/iai.62.10.4650-4651.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goulding R, King M B, Knox R, Robson J M. Relation between in vitro and in vivo resistance to isoniazid. Lancet. 1952;ii:69–70. doi: 10.1016/s0140-6736(52)92110-7. [DOI] [PubMed] [Google Scholar]
- 13.Heym B, Zhang Y, Poulet S, Young D, Cole S T. Characterization of the katG gene encoding a catalase-peroxidase required for the isoniazid susceptibility of Mycobacterium tuberculosis. J Bacteriol. 1993;175:4255–4259. doi: 10.1128/jb.175.13.4255-4259.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heym B, Stavropoulos E, Honore N, Domenech P, Saint-Joanis B, Wilson T M, Collins D M, Colston M J, Cole S T. Effects of overexpression of the alkyl-hydroperoxide reductase AhpC on the virulence and isoniazid resistance of Mycobacterium tuberculosis. Infect Immun. 1997;65:1395–1401. doi: 10.1128/iai.65.4.1395-1401.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackett P S, Aber V R, Lowrie D B. Virulence and resistance to superoxide, low pH and hydrogen peroxide among strains of Mycobacterium tuberculosis. J Gen Microbiol. 1978;104:37–45. doi: 10.1099/00221287-104-1-37. [DOI] [PubMed] [Google Scholar]
- 16.Jackett P S, Aber V R, Lowrie D B. Virulence of Mycobacterium tuberculosis and susceptibility to peroxidative killing systems. J Gen Microbiol. 1978;107:273–278. doi: 10.1099/00221287-107-2-273. [DOI] [PubMed] [Google Scholar]
- 17.Johnsson K, Froland W A, Schultz P G. Overexpression, purification and characterization of the catalase-peroxidase KatG from Mycobacterium tuberculosis. J Biol Chem. 1997;272:2834–2840. doi: 10.1074/jbc.272.5.2834. [DOI] [PubMed] [Google Scholar]
- 18.Jouanguy E, Altare F, Lamhamedi S, Revy P, Emile J F, Newport M, Levin M, Blanche S, Seboun E, Fischer A, Casanova J L. Interferon-gamma receptor deficiency in an infant with fatal Calmette-Guerin infection. N Engl J Med. 1996;335:1956–1961. doi: 10.1056/NEJM199612263352604. [DOI] [PubMed] [Google Scholar]
- 19.Jouanguy E, Lamhamedi-Cherradi S, Altare F, Fondaneche M C, Tuerlinckx D, Blanche S, Emile J F, Gaillard J L, Schreiber R, Levin M, Fischer A, Hivroz C, Casanova J L. Partial interferon-gamma receptor 1 deficiency in a child with tuberculoid bacillus Calmette-Guerin infection and sibling with clinical tuberculosis. J Clin Invest. 1997;100:2658–2664. doi: 10.1172/JCI119810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laochumroonvorapong P, Paul S, Elkon K B, Kaplan G. H2O2 induces monocyte apoptosis and reduces the viability of Mycobacterium avium-M. intracellulare within cultured human monocytes. Infect Immun. 1996;64:452–459. doi: 10.1128/iai.64.2.452-459.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laochumroonvorapong P, Paul S, Manca C, Freedman V H, Kaplan G. Mycobacterial growth and sensitivity to H2O2 killing in human monocytes in vitro. Infect Immun. 1997;65:4850–4857. doi: 10.1128/iai.65.11.4850-4857.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Locksley R M, Klebanoff S J. Oxygen-dependent microbicidal systems of phagocytes and host defense against intracellular protozoa. J Cell Biochem. 1983;22:173–185. doi: 10.1002/jcb.240220306. [DOI] [PubMed] [Google Scholar]
- 23.Lowrie D B, Andrew P W. Macrophage antimycobacterial mechanisms. Br Med Bull. 1988;44:624–634. doi: 10.1093/oxfordjournals.bmb.a072272. [DOI] [PubMed] [Google Scholar]
- 24.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Mdluli K, Swanson J, Fischer E, Lee R E, Barry C E., III Mechanisms involved in the intrinsic isoniazid resistance of Mycobacterium avium. Mol Microbiol. 1998;27:1223–1233. doi: 10.1046/j.1365-2958.1998.00774.x. [DOI] [PubMed] [Google Scholar]
- 26.Mitchison D A, Selkon J B, Lloyd J. Virulence in the guinea-pig, susceptibility to hydrogen peroxide, and catalase activity of isoniazid-sensitive tubercle bacilli from South Indian and British patients. J Pathol Bacteriol. 1963;86:377–386. doi: 10.1002/path.1700860213. [DOI] [PubMed] [Google Scholar]
- 27.Molloy A, Meyn P A, Smith K D, Kaplan G. Recognition and destruction of bacillus Calmette-Guerin-infected monocytes. J Exp Med. 1993;177:1691–1698. doi: 10.1084/jem.177.6.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molloy A, Laochumroonvorapong P, Kaplan G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guerin. J Exp Med. 1994;180:1499–1509. doi: 10.1084/jem.180.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morse W C, Weiser O L, Kuhns D M, Fusillo M, Dail M C, Evans J R. Study of the virulence of isoniazid-resistant tubercle bacilli in guinea pigs and mice. Am Rev Tuberc. 1954;69:464–468. doi: 10.1164/art.1954.69.3.464. [DOI] [PubMed] [Google Scholar]
- 30.Nathan C F, Murray H W, Wiebe M E, Rubin B Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983;158:670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Brien S, Jackett P S, Lowrie D B, Andrew P W. Guinea-pig alveolar macrophages kill Mycobacterium tuberculosis in vitro, but killing is independent of susceptibility to hydrogen peroxide or triggering of the respiratory burst. Microb Pathog. 1991;10:199–207. doi: 10.1016/0882-4010(91)90054-e. [DOI] [PubMed] [Google Scholar]
- 32.Russell D G, Dant J, Sturgill-Koszycki S. Mycobacterium avium- and Mycobacterium tuberculosis-containing vacuoles are dynamic, fusion-competent vesicles that are accessible to glycosphingolipids from the host cell plasmalemma. J Immunol. 1996;156:4764–4773. [PubMed] [Google Scholar]
- 33.Russell D G, Sturgill-Koszycki S, Vanheyningen T, Collins H, Schaible U E. Why intracellular parasitism need not be a degrading experience for mycobacterium. Philos Trans R Soc Lond Biol Sci. 1997;352:1303–1310. doi: 10.1098/rstb.1997.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarmento A, Appelberg R. Involvement of reactive oxygen intermediates in tumor necrosis factor alpha-dependent bacteriostasis of Mycobacterium avium. Infect Immun. 1996;64:3224–3230. doi: 10.1128/iai.64.8.3224-3230.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scoazec J Y, Fischer A, Nezelof C. Les infections generalisees a BCG, expression d’un deficit multi-factoriel de la bactericidie intramacrophagique. Etude anatomo-clinique de 11 cas. Arch Fr Pediatr. 1984;41:681–687. [PubMed] [Google Scholar]
- 36.Sherman D R, Mdluli K, Hickey M J, Arain T M, Morris S L, Barry III C E, Stover C K. Compensatory ahpC gene expression in isoniazid-resistant Mycobacterium tuberculosis. Science. 1996;272:1641–1643. doi: 10.1126/science.272.5268.1641. [DOI] [PubMed] [Google Scholar]
- 37.Sturgill-Koszycki S, Schaible U E, Russell D G. Mycobacterium-containing phagosomes are accessible to early endosomes and reflect a transitional state in normal phagosome biogenesis. EMBO J. 1996;15:6960–6968. [PMC free article] [PubMed] [Google Scholar]
- 38.Wieles B, Ottenhoff T H, Steenwijk T M, Franken K L, de Vries R R, Langermans J A. Increased intracellular survival of Mycobacterium smegmatis containing the Mycobacterium leprae thioredoxin-thioredoxin reductase gene. Infect Immun. 1997;65:2537–2541. doi: 10.1128/iai.65.7.2537-2541.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson T M, de Lisle G W, Collins D M. Effect of inhA and katG on isoniazid resistance and virulence of Mycobacterium bovis. Mol Microbiol. 1995;15:1009–1015. doi: 10.1111/j.1365-2958.1995.tb02276.x. [DOI] [PubMed] [Google Scholar]
- 40.Xu S, Cooper A, Sturgill-Koszycki S, van Heyningen T, Chatterjee D, Orme I, Allen P, Russell D G. Intracellular trafficking in Mycobacterium tuberculosis and Mycobacterium avium-infected macrophages. J Immunol. 1994;153:2568–2578. [PubMed] [Google Scholar]
- 41.Zhang Y, Heym B, Allen B, Young D, Cole S. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature (London) 1992;358:591–593. doi: 10.1038/358591a0. [DOI] [PubMed] [Google Scholar]