Abstract
Background
Alcohol—a risk factor for atrial fibrillation (AF)—is metabolized by aldehyde dehydrogenase 2 (ALDH2). Dysfunctional alleles of ALDH2 (ALDH2-deficient variants) are prevalent among East Asians.
Objectives
Because the prevalence of AF is estimated to be high in ALDH2-deficient variant carriers, we investigated the correlation between AF and ALDH2-deficient variant carriers, including the association with habitual alcohol consumption.
Methods
A total of 656 consecutive patients were included in this investigation. ALDH2 genotypes were divided into ALDH2 homozygous wild-type (∗1/∗1), ALDH2 heterozygous-deficient allele (∗1/∗2), and ALDH2 homozygous-deficient allele (∗2/∗2). Multivariate analyses were applied to determine the correlation between ALDH2 genotype and AF.
Results
ALDH2∗1/∗2 and ALDH2∗2/∗2 carriers who were ALDH2-deficient variant carriers comprised 199 (30.3%) and 27 (4.1%) patients, respectively. Among these patients, the proportions of habitual alcohol consumption were 26.1% and 0%, respectively. Multivariate analysis revealed that ALDH2∗1/∗2 itself was not a risk factor for AF (odds ratio [OR]: 1.28; P = 0.21). However, habitual alcohol consumption in ALDH2∗1/∗2 carriers was an independent risk factor of AF (OR: 4.13; P = 0.001). Contrary to expectations, ALDH2∗2/∗2 itself had a lower incidence of AF among other risk factors (OR: 0.37; P = 0.03).
Conclusions
Although the ALDH2∗1/∗2 itself was not associated with AF, ALDH2∗1/∗2 carriers with habitual alcohol consumption could experience AF because of slow alcohol metabolism. In contrast, ALDH2∗2/∗2 itself had a lower incidence of AF. This might be related to the absence to habitual alcohol consumption in ALDH2∗2/∗2 carriers because of the negligible activity of ALDH2. Thus, abstaining from alcohol consumption might prevent the development of AF in patients who are ALDH2∗1/∗2 carriers.
Key Words: alcohol, aldehyde dehydrogenase, atrial fibrillation, East Asians
Abbreviations and Acronyms: AF, atrial fibrillation; ALDH2, aldehyde dehydrogenase 2; CI, confidence interval; ECG, electrocardiogram; OR, odds ratio
Central Illustration
Atrial fibrillation (AF) is the most common form of arrhythmia detected in clinical practice.1 AF increases the risk of stroke, cardiovascular disease, and all-cause mortality.2 Although it has been shown that a hyperadrenergic state and impairment of vagal tone are closely associated with AF,3,4 the precise mechanisms leading to AF remain unclear. Older age, male sex, hypertension, obesity, and diabetes mellitus have been shown to be risk factors for AF.5 Alcohol consumption, even at moderate levels, is also associated with this arrhythmia.6 Some reports have indicated that alcohol progresses atrial electrical remodeling and triggers AF.7 Another study showed that prohibition of alcohol consumption reduces AF attacks.8
Alcohol is metabolized in 2 steps. First, it is metabolized to acetaldehyde by an alcohol dehydrogenase subunit, and second, the acetaldehyde is metabolized to acetic acid by aldehyde dehydrogenase 2 (ALDH2).6 Dysfunctional alleles of the ALDH2 gene are prevalent among East Asian populations.9 ALDH2 genotypes consist of ALDH2 homozygous wild-type (∗1/∗1), ALDH2 heterozygous-deficient allele (∗1/∗2), and ALDH2 homozygous-deficient allele (∗2/∗2). The carriers of ALDH2∗1/∗2 and ALDH2∗2/∗2 are ALDH2-deficient variant carriers. Compared with the ALDH2 wild-type, ALDH2∗1/∗2 and ALDH2∗2/∗2 represent reduced and negligible activity of ALDH2, respectively.9 These findings imply that increased prevalence of AF in patients with ALDH2∗1∗2 who habitually consume alcohol is caused by the low metabolic activity and accumulation of toxic acetaldehyde. In contrast, there is decreased prevalence of AF in patients with ALDH2∗2/∗2, because these patients are not able to consume alcohol because of the negligible activity of ALDH2. In this study, we investigated the relationship between ALDH2 genotypes and the prevalence of AF in association with habitual alcohol consumption.
Methods
Patient population and data collection
Between January 2010 and June 2019, 10,603 patients were admitted to the Cardiovascular Center of Kumamoto University. Among these, 656 patients were enrolled in our study retrospectively. The purpose of admission was catheter ablation for AF in 385 patients, catheter ablation for other arrhythmias in 196 patients, investigation of aortic disease in 49 patients, and coronary angiography in 26 patients. The absence of coronary artery disease, moderate or severe valve disease, cardiomyopathy, and hyperthyroidism was confirmed in all patients. The genomic information of each patient was preserved at the time of blood sampling and obtained from our institution’s biobank. This study complied with the Declaration of Helsinki regarding investigation in humans and was approved by the Human Research Committee of Kumamoto University. Written informed consent was obtained from each patient.
The data collected from the study population, including vital signs, internal medicine history, and history and preference of alcohol, were obtained from the patient’s medical records.
In 385 patients who underwent catheter ablation for AF, AF was confirmed using surface electrocardiography (ECG) or Holter ECG. In the other patients, new-onset AF was confirmed using surface ECG and by monitoring the ECG during admission. Previously documented AF was confirmed using surface ECG or Holter ECG performed by their primary care doctor. AF was defined by the absence of P waves, presence of fibrillatory waves, and an irregular ventricular rate in patients without conduction disorders that lasted >30 s. Following this, AF was diagnosed by ≥2 cardiologists.
ALDH2 genotype and the allele ratio
Genomic DNA used for genotyping of ALDH2 was extracted from whole blood using a DNA purification kit (Flex Gene DNA kit, Qiagen). The ALDH2 rs671 (Glu504Lys; ALDH2∗2) genotypes were determined by performing a real-time TaqMan allelic discrimination assay (Step One Plus Real-Time PCR system version 2.1, Applied Biosystems) according to the manufacturer’s protocols (assay no. C_11703892_10). The allele ratio was analyzed using this method.
Variables
The following variables were analyzed: age, sex, hypertension, diabetes mellitus, obesity, alcohol consumption habit, and ALDH2 genotypes. We defined older age as older than 60 years, because the prevalence of AF increases in individuals above this age.10 Diabetes mellitus was defined based on the casual plasma glucose concentration of >200 mg/dl, fasting plasma glucose concentration of >126 mg/dl, 2-h plasma glucose concentration of >200 mg/dl in a 75-g oral glucose tolerance test, and administration of diabetes mellitus medication. Hypertension was defined in patients with blood pressure of >140/90 mm Hg or administration of antihypertensive medication. Obesity was categorized in subjects with a body mass index of >25 kg/m2. Habitual alcohol consumption was defined as ≥3 drinking opportunities per week, according to the Ministry of Health, Labour and Welfare in Japan.
Data analysis
To determine the correlation between the ALDH2∗2 allele and AF, univariate and multivariate analyses were performed to identify factors associated with AF.
In addition, we evaluated the relationship between AF and habitual alcohol consumption, based not only on categorical diagnosis, but also with regard to total alcohol consumption in relation to the ALDH2 genotype. Average alcohol intake volume was obtained from patients’ self-report. The volume was represented in standard drinks per week. One standard drink was defined as 12-g alcohol. According to this definition, alcohol intake volume was divided into the following groups: nondrinkers: 0-1 drinks/week; mild drinkers: 2-7 drinks/week; moderate drinkers: 8-21 drinks/week; and heavy drinkers: ≥22 drinks/week.11
Statistical analysis
All continuous data showed a normal distribution according to the Shapiro-Wilk test; continuous data were expressed as mean ± SD. One-way analysis of variance with Tukey’s test was used for multiple comparisons of data. Continuous data that showed a non-normal distribution using the Shapiro-Wilk test were expressed as the median (interquartile range). The Kruskal-Wallis with Steel-Dwass test was used for multiple comparisons of the data. Categorical data were presented as numbers or percentages. Differences between multiple groups were tested using Fisher’s exact test with Bonferroni’s correction. Multiple logistic regression analysis was performed to determine the risk factors for AF. Propensity-score matching and inversed probability treatment weighting analyses were performed to balance the bias of background. Cox regression analysis was performed to determine the risk factors for new-onset AF. A P value <0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 25 (IBM), except for propensity-score matching, which was performed using R (The R Foundation for Statistical Computing).
Results
Patient characteristics
Among the 656 patients, the distribution of the genotypes ALDH2∗1/∗1 (ALDH2 wild-type) and ALDH2-deficient variant carriers, which included ALDH2∗1/∗2 and ALDH2∗2/∗2 carriers, were 430, 199, and 27 patients, respectively. This proportion of ADLH2 genotypes was similar to the reports in Japanese and East Asian populations.9,12
Table 1 lists patient characteristics. There was no significant difference among ALDH2 wild-type carriers and ALDH2∗1∗2 and ALDH2∗2/∗2 allele carriers except for left atrial dimensions, drinking habits, and AF. The differences of left atrial dimensions between each genotype were not significantly different. However, prevalence of habitual alcohol consumption in ALDH2∗2/∗2 carriers was significantly less than that of ALDH2 wild-type and ALDH2∗1/∗2 carriers (0 of 27, 0% vs 231 of 430, 53.7%; P < 0.001; 0 of 27, 0% vs 51 of 199, 25.6%; P = 0.003). In addition, prevalence of habitual alcohol consumption in ALDH2∗1/∗2 carriers was significantly less than that of ALDH2 wild-type carriers (51 of 199, 25.6% vs 231 of 430, 53.7%; P < 0.001). Prevalence of ALDH2∗2/∗2 carriers’ history of AF was also significantly less than those of ALDH2 wild-type and ALDH2∗1/∗2 carriers (9 of 27, 33.3% vs 261 of 430, 60.7%; P = 0.02; 9 of 27, 33.3% vs 122 of 199, 61.3%; P = 0.02). However, there was no significant difference between ALDH2 wild-type and ALDH2∗1/∗2 carriers.
Table 1.
Patient Characteristics
| All (N = 656) | ALDH2 Wild (n = 430) | ALDH2∗1/∗2 (n = 199) | ALDH2∗2/∗2 (n = 27) | P Value | |
|---|---|---|---|---|---|
| Age, y | 64 (55-71) | 63 (53-71) | 65 (58-71) | 68 (57-72) | 0.42 |
| Male | 403 (61.4) | 273 (63.5) | 112 (56.3) | 18 (66.7) | 0.20 |
| BMI, kg/m2 | 23.2 (20.9-25.6) | 23.2 (21.1-25.7) | 23.2 (20.8-25.4) | 23.1 (21.8-26.2) | 0.65 |
| BNP, pg/mL | 28.3 (12.9-66.4) | 28.5 (13.6-65.9) | 28.0 (12.6-69.1) | 17.3 (10.4-50.3) | 0.60 |
| EF, % | 63.3 (60.0-66.2) | 63.5 (60.0-66.7) | 62.9 (59.8-65.6) | 64.5 (61.1-67.4) | 0.13 |
| LADs, mm | 35.9 ± 6.3 | 36.3 ± 6.3 | 35.2 ± 6.3 | 34.3 ± 6.5 | 0.03 |
| eGFR, mL/min/1.73 m2 | 72.0 (62.0-85.0) | 72.0 (63.0-85.0) | 70.0 (60.0-84.0) | 65.0 (56.5-81.0) | 0.09 |
| Atrial fibrillation | 392 (59.8) | 261 (60.7) | 122 (61.3) | 9 (33.3) | 0.02 |
| Hypertension | 343 (52.2) | 236 (54.9) | 96 (48.2) | 11 (40.7) | 0.14 |
| Diabetes mellitus | 92 (14.0) | 54 (12.3) | 35 (17.6) | 3 (11.1) | 0.23 |
| Drinking habit | 282 (43.0) | 231 (53.7) | 51 (25.6) | 0 (0) | <0.001 |
| Current smoking | 129 (19.7) | 88 (20.4) | 36 (18.0) | 5 (18.5) | 0.78 |
Values are median (interquartile range), n (%), or mean ± SD.
ALDH2 = aldehyde dehydrogenase; BMI = body mass index; BNP = brain natriuretic peptide; EF = ejection fraction; eGFR = estimated glomerular filtration rate; LAD = left atrial dimension.
Table 2 shows the relationship between ALDH2 genotypes and type of AF. As shown, the proportion of paroxysmal, persistent, and longstanding persistent AF was not significantly different between each ALDH2 genotype.
Table 2.
Relationship Proportion of AF Types of Each ALDH2 Genotypes
| ALDH2 Wild (n = 430) | ALDH2∗1/∗2 (n = 199) | ALDH2∗2/∗2 (n = 27) | P Value | |
|---|---|---|---|---|
| Paroxysmal AF | 197 (45.8) | 93 (46.7) | 8 (29.6) | 0.24 |
| Persistent AF | 62 (14.4) | 29 (14.6) | 1 (3.7) | 0.32 |
| Longstanding persistent AF | 2 (0.5) | 0 (0.0) | 0 (0.0) | 1.00 |
Values are n (%).
AF = atrial fibrillation.
Relationship between habitual alcohol consumption and prevalence of AF
Table 3 shows the relationship between AF and examined variables. Older age, hypertension, being male, and a pattern of habitual alcohol consumption were significantly correlated with AF. In addition, habitual alcohol consumption also exhibited a high odds ratio (OR) based on univariate (OR: 2.31; P < 0.001) and multivariate (OR: 1.75; P < 0.001) analyses.
Table 3.
Relationship Between AF and Variables
| Univariate Analysis |
Multivariate Analysis |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Age >60 y | 2.49 | 1.79-3.46 | <0.001 | 2.60 | 1.78-3.80 | <0.001 |
| Hypertension | 2.34 | 1.70-3.22 | <0.001 | 1.68 | 1.17-2.41 | 0.005 |
| Obesity | 1.11 | 0.79-1.56 | 0.55 | 1.02 | 0.70-1.50 | 0.91 |
| Diabetes mellitus | 0.92 | 0.58-1.41 | 0.65 | 0.63 | 0.38-1.02 | 0.06 |
| Male | 2.12 | 1.54-2.93 | <0.001 | 2.03 | 1.38-2.98 | <0.001 |
| Alcohol | 2.31 | 1.67-3.21 | <0.001 | 1.75 | 1.18-2.61 | 0.005 |
| ALDH2 genotype | ||||||
| ALDH2 wild | Reference | Reference | ||||
| ALDH2∗1/∗2 | 1.03 | 0.73-1.45 | 0.88 | 1.28 | 0.87-1.89 | 0.21 |
| ALDH2∗2/∗2 | 0.32 | 0.14-0.74 | 0.007 | 0.37 | 0.15-0.91 | 0.03 |
With respect to the relationship between ADLH2 genotypes and AF history, ALDH2∗1/∗2 carriers were not significantly correlated with AF on univariate (OR: 1.03; P = 0.88) and multivariate analyses (OR: 1.28; P = 0.21). Rather, contrary to expectations, ALDH2∗2/∗2 carriers had a lower incidence of AF on univariate (OR: 0.32; P = 0.007) and multivariate analyses (OR: 0.37; P = 0.03).
Relationship between habitual alcohol consumption and AF prevalence among ALDH2 genotypes
However, the relationship between AF and ALDH2 genotypes in regard to habitual alcohol consumption was lacking in this analysis. Therefore, variables in ALDH2 genotypes and habitual alcohol consumption were divided into 5 categories to understand the importance of ALDH2 genotypes and habitual alcohol consumption for the risk of developing AF. These categories were ALDH2 wild-type carriers who did not consume alcohol habitually, ALDH2 wild-type carriers who consumed alcohol habitually, ALDH2∗1/∗2 allele carriers who did not consume alcohol habitually, ALDH2∗1/∗2 allele carriers who consumed alcohol habitually, and ALDH2∗2/∗2 allele carriers (because of the absence of habitual alcohol consumption in ALDH2∗2/∗2 allele carriers). Multivariate analysis was performed as the reference for ALDH2 wild-type carriers who did not consume alcohol habitually by including other variables. Table 4 shows the relationship between AF and the variables studied. This multivariate analysis was adjusted for covariates such as age, hypertension, obesity, diabetes mellitus, and being male. As shown in Table 4, age older than 60 years (OR: 2.71; P < 0.001), hypertension (OR: 1.64; P = 0.007), being male (OR: 1.86; P < 0.001), ALDH2 wild-type carriers with habitual alcohol consumption (OR: 1.64; P = 0.037), and ALDH2∗1/∗2 allele carriers with habitual alcohol consumption (OR: 4.13; P = 0.001) positively correlated with the risk of AF. Particularly, ALDH2∗1/∗2 allele carriers who consumed alcohol habitually showed the highest OR among these variables. In addition, ALDH2∗2/∗2 allele carriers had a lower incidence of AF (OR: 0.35; P = 0.02).
Table 4.
Relationship Between AF and Variables Using Multivariate Analysis
| Habitual Alcohol Consumption | OR | 95% CI | P Value | |
|---|---|---|---|---|
| Age >60 y | 2.71 | 1.84-3.98 | <0.001 | |
| Hypertension | 1.64 | 1.14-2.36 | 0.007 | |
| Obesity | 1.03 | 0.70-1.51 | 0.88 | |
| Diabetes mellitus | 0.61 | 0.37-1.00 | 0.05 | |
| Male | 1.96 | 1.33-2.88 | <0.001 | |
| ALDH2 genotype | ||||
| ALDH2 wild | No | Reference | ||
| ALDH2 wild | Yes | 1.60 | 1.03-2.50 | 0.037 |
| ALDH2∗1/∗2 | No | 1.06 | 0.68-1.68 | 0.800 |
| ALDH2∗1/∗2 | Yes | 4.13 | 1.76-9.71 | 0.001 |
| ALDH2∗2/∗2 | No | 0.35 | 0.14-0.87 | 0.020 |
The previously mentioned results were based on the data of all included patients. Therefore, we performed these analyses between ALDH2 wild-type and ALDH2∗1/∗2 carriers using propensity-score matching analysis and inversed probability treatment weighting analyses. In this analysis, AF history and habitual alcohol consumption were included in the propensity score model as covariates. The values changed following propensity-score matching, but the overall meaning and significant difference remained the same (Supplemental Tables 1-4).
Correlation between volume of alcohol intake and prevalence of AF among ALDH2 wild-type and ALDH2∗1∗2 allele carriers
Table 5 shows the relationship between AF and the alcohol intake volume categories in ALDH2 wild-type and ALDH2∗1/∗2 allele carriers. Moderate and heavy drinkers with the ALDH2 wild-type allele (OR: 1.79; 95% confidence interval [CI]: 1.12-2.85; P = 0.01; OR: 3.07; 95% CI: 1.77-5.34; P < 0.001) and moderate drinkers with the ALDH2∗1/∗2 allele (OR: 5.07; 95% CI: 2.03-12.70; P < 0.001) were positively correlated with AF risk. ALDH2∗1∗2 allele carriers with moderate alcohol consumption showed the highest OR. ORs of heavy drinkers in ALDH2∗1/∗2 allele carriers was not obtained because the proportion of AF was 100%.
Table 5.
Relationship Between AF and Alcohol Consumption Volume in Each Genotype
| n | AF Patients | OR | 95% CI | P Value | |
|---|---|---|---|---|---|
| ALDH2 wild-type | 430 | 261 (60.7) | |||
| Nondrinkers | 199 | 102 (51.2) | Reference | ||
| Mild drinkers | 17 | 9 (52.9) | 1.07 | 0.40-2.89 | 0.89 |
| Moderate drinkers | 121 | 79 (65.3) | 1.79 | 1.12-2.85 | 0.01 |
| Heavy drinkers | 93 | 71 (76.3) | 3.07 | 1.77-5.34 | <0.001 |
| ALDH2∗1∗2 allele | 199 | 122 (61.3) | |||
| Nondrinkers | 148 | 80 (54.1) | Reference | ||
| Mild drinkers | 7 | 4 (57.1) | 1.27 | 0.28-5.81 | 0.76 |
| Moderate drinkers | 38 | 32 (84.2) | 5.07 | 2.03-12.70 | <0.001 |
| Heavy drinkers | 6 | 6 (100) | NA | NA | NA |
Follow-up after discharge and new-onset AF in patients without a history of AF
AF history was not observed at admission in 264 patients in the present study. Among these patients, 174 patients were followed up at our hospital. However, the follow-up was short, being only 10 months (range: 2.0-25.3 months). The number of patients who were ALDH2 wild-type carriers, ALDH2∗1/∗2 carriers, and ALDH2∗2/∗2 carriers were 111, 51, and 12, respectively. During follow-up, new-onset AF was observed in only 6 patients (in each genotype, new-onset AF was observed in 2 patients). Cox regression analysis did not show the correlation between ALDH2 genotypes and new-onset AF (Supplemental Table 4). In addition, ALDH2 genotypes with habitual alcohol consumption also showed an absence of correlation (Supplemental Tables 5 and 6).
Power analysis with respect to ALDH2/∗2/∗2 CARRIERS
There were only a few ALDH2∗2/∗2 carriers (n = 27); therefore, we conducted a power analysis with 27 ALDH2∗2/∗2 carriers and 430 ALDH2 wild-type carriers as control subjects. In this present study, proportion of ALDH2 wild-type carriers with a history of AF was 0.607, and the relative risk of AF in ALDH2∗2/∗2 carriers was 0.32. Under the type I error probability associated with this test of this null hypothesis of 0.05, the calculated power was 0.994. We used Fisher’s exact test to evaluate this null hypothesis. Therefore, we analyzed the data incorporating ALDH2∗2/∗2 carriers as well.
Discussion
Main findings
In this present study, we found 3 main findings. First, the ALDH2∗1/∗2 allele itself, which leads to the slow metabolism of alcohol, was not a risk factor for AF. However, the ALDH2∗1/∗2 allele carriers with habitual alcohol consumption had the strongest risk factor for AF compared with older age, hypertension, and male sex. Second, with increased alcohol consumption volume, the OR of AF risk increases in both ALDH2 wild-type and ALDH2∗1/∗2 allele carriers. This phenomenon appears prominently in ALDH2∗1/∗2 allele carriers. Third, the ALDH2∗2/∗2 allele itself, which negligibly metabolizes alcohol, correlated with lower incidence of AF.
Habitual alcohol consumption and AF
In this study, habitual alcohol consumption was one of the risk factors for AF according to the multivariate analysis.
A mechanism of developing AF based on habitual alcohol consumption may consist of various factors. First, alcohol has 2-sided effect in the atrium associated with AF, including autonomic modulation, represented by increased β-receptor density,3 and vagal inhibition, which leads to shortened atrial refractoriness.4 This is followed by atrial electrical remodeling, which is represented by lower atrial voltage and conduction slowing.13 These effects lead to shortened atrial and pulmonary vein action potentials, a shortened atrial effective refractory period, and slow intra-atrial and interatrial conduction. Second, it has been shown that habitual alcohol consumption and sleep apnea syndrome have a close relationship.14 Alcohol consumption can cause upper airway obstruction that leads to sleep apnea syndrome, and sleep apnea syndrome has been connected with developing AF because of hypoxia, hypercapnia, negative intrathoracic pressure generation, and alteration of autonomic nervous activity.7,15 As such, substrates of AF may be produced (7); therefore, habitual alcohol consumption is a risk factor for AF.6,7 These findings are supported by the positive correlation between alcohol consumption and AF observed in this study.
Prevalence of ALDH2 variant carriers
In this study, the distribution of the genotypes of ALDH2∗1/∗1 (ALDH2 wild-type) and ALDH2-deficient variant carriers, which included ALDH2∗1/∗2 and ALDH2∗2/∗2 carriers, was 430, 199, and 27 patients, respectively, among all 656 patients. Although the worldwide prevalence of ALDH2∗2 allele carriers is rather small,16 patients who were enrolled in this present study were all Japanese. In addition, the proportion of ALDH2∗1/∗2 and ALDH2∗2/∗2 carriers was similar to past reports in Japanese and East Asian populations.9,12
Effect of ALDH2-deficient variant allele on alcohol metabolism
The ALDH2-deficient variant allele has reduced enzyme activity.12,17 Therefore, low ALDH2 activity leads to accumulation of toxic acetaldehyde in ALDH2-deficient variant carriers, causing an alcohol flushing syndrome in ALDH2-deficient variant allele carriers after alcohol consumption.18 In addition, some reports have suggested that the ALDH2-deficient variant allele contributes to cardiovascular disease, diabetes, stroke, and cancer.19
Interaction between ALDH2-deficient variant allele carriers with habitual alcohol consumption and AF
In this study, we found that the ALDH2∗1/∗2 allele itself was not a risk factor for AF. Consistent with the data in this study, Nakano et al20 reported that the ALDH2-deficient variant allele itself was negatively associated with AF. However, the study did not focus on habitual alcohol consumption in ALDH2 genotypes. Being an ALDH2∗1/∗2 allele carrier with habitual alcohol consumption was the strongest risk factor for AF in the multivariate analysis in this present study. In addition, increased alcohol consumption volume, increased the OR of AF risk, especially in ALDH2∗1/∗2 allele carriers. It was shown that alcohol induced autonomic effects and atrial electrical remodeling, both of which are associated with the generation of the AF substrate.7 Following the generation of the AF substrate, atrial contraction, including triggered activity, could cause AF. In that state, accumulation of toxic acetaldehyde in ALDH2-deficient variant carriers could play an important role for developing AF. It was shown that overdrive pacing fails to induce triggered activity in the presence of ethanol. However, triggered activity was induced by overdrive pacing in the presence of acetaldehyde.21
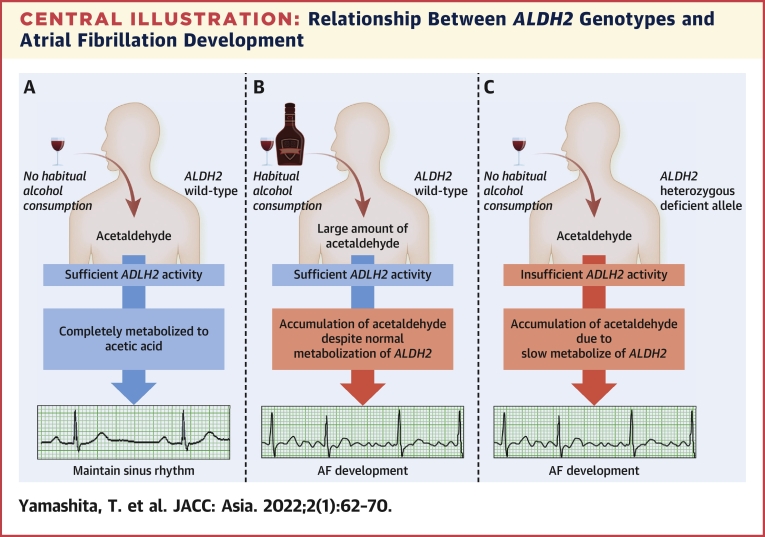
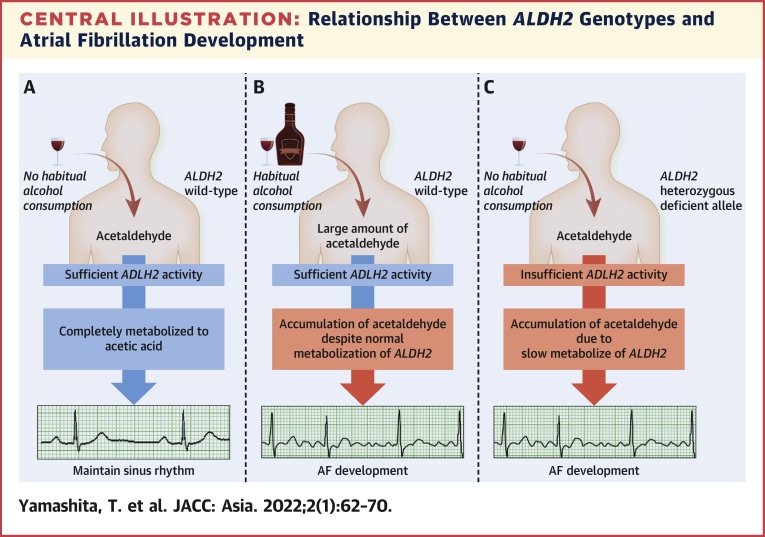
With respect to ALDH2∗2/∗2 allele carriers, symptoms of the alcohol flushing syndrome could be more severe because of the negligible activity of ALDH2 compared with that of ALDH2∗1/∗2 allele carriers. Therefore, ALDH2∗2/∗2 allele carriers cannot consume alcohol.18 This was consistent with this present study. In addition, this phenomenon might help to reduce developing AF. The Central Illustration shows the relationship between ALDH2 genotypes and AF development. As shown, although small amounts of alcohol did not lead to AF development in ALDH2 wild-type carriers, large amounts of alcohol led to AF development despite, normal ALDH2 metabolism. However, even small amounts of alcohol led to development of AF in ALDH2-deficient variant carriers because of low ALDH2 metabolism activity.
Central Illustration.
Relationship Between ALDH2 Genotypes and Atrial Fibrillation Development
(A) Consumption of a normal amount of alcohol in ALDH2 wild-type. (B) Consumption of a large amount of alcohol in ALDH2 wild-type. (C) Consumption of a normal amount of alcohol in ALDH2-deficient variant. AF = atrial fibrillation.
Therefore, it might be important to identify whether an individual is an ALDH2∗1/∗2 allele carrier, because both ALDH2 wild-type and ALDH2∗1/∗2 allele carriers can consume alcohol. Symptoms of alcohol flushing syndrome could be a predictor to differentiate between ALDH2 wild-type and ALDH2∗1/∗2 allele carriers, because in the ALDH2∗1/∗2 allele carriers, facial flushing, headache, nausea, and palpitations (the typical symptoms of alcohol flushing syndrome) have been observed even with small amount of alcohol.17
STUDY LIMITATIONS
This study’s population only included patients who were admitted to the Cardiovascular Center of Kumamoto University. In addition, the 385 patients of 656 patients underwent catheter ablation for AF. Although the proportion of the ALDH2 genotype was consistent with that in a previous report, it might not represent the general population.
Although the relationship between AF and sleep apnea syndrome was not evaluated, obesity was not a risk factor of AF in this study. This might be because most patients with the sleep apnea syndrome in the Japanese population develop the condition from having a small jaw and not because of obesity.22,23
Socioeconomics factors, educational attainment, and regional customs demonstrated a close relationship with habitual alcohol consumption.24,25 Although we could not gather enough information related to these data, they might be important predictors.
Although a correlation between ALDH2 genotypes and new-onset AF was not observed, the follow-up period was short because of retrospective study design. Increasing the follow-up period and patient numbers might clarify this correlation.
The prevalence of ALDH2∗2 allele carriers is extremely rare in Europeans14; therefore, the same result might not be obtained in a European cohort.
The duration from beginning habitual alcohol consumption to developing AF might be different for each ALDH2 genotype. However, the exact starting time of habitual alcohol consumption, especially the starting time of the current drinking volume, was obscure. In addition, some patients were asymptomatic for AF. Therefore, this evaluation could not be conducted.
Conclusions
Although the ALDH2∗1/∗2 itself was not associated with AF, ALDH2∗1/∗2 carriers with habitual alcohol consumption might experience AF because of slow alcohol metabolism. In contrast, ALDH2∗2/∗2 carriers had a lower incidence of AF. This might be related to the absence of habitual alcohol consumption in ALDH2∗2/∗2 carriers because of the negligible activity of ALDH2. Thus, abstaining from alcohol consumption could prevent the development of AF in patients who are ALDH2∗1/∗2 allele carriers in the Japanese population.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Alcohol—a risk factor for AF—is metabolized by ALDH2. Dysfunctional alleles of the ALDH2 (ALDH2-deficient variants) are prevalent among East Asians. In this study, we revealed that habitual alcohol consumption in ALDH2-deficient variant carriers is an independent risk factor for AF because of the presence of slow alcohol metabolism.
TRANSLATIONAL OUTLOOK: It should be noted that habitual alcohol consumption in ALDH2-deficient variant carriers is an independent risk factor for AF because of the presence of slow alcohol metabolism. Therefore, abstaining from alcohol consumption, particularly by ALDH2-deficient variant carriers, may suppress AF onset.
Funding Support and Author Disclosures
This work was supported by JSPS KAKENHI Grant Number JP20K22878. Drs Hoshiyama and Kanazawa have received grants from Medtronic Japan, Nihon Kohden, Abbott Medical Japan, Fukuda Denshi, Boston Scientific Japan, Japan Lifeline, Nipro, and Biotronik Japan. Dr Tsujita has received honoraria from Bayer Yakuhin, Daiichi-Sankyo, Kowa, MSD, Sanofi, and Takeda Pharmaceutical; and has received grants from Astellas Pharma, Abbott Vascular Japan, Bayer Yakuhin, Boehringer Ingelheim Japan, Boston Scientific Japan, Bristol Myers, Chugai Pharmaceutical, Daiichi-Sankyo, Goodman, Japan Lifeline, Medtronic Japan, Mitsubishi Tanabe Pharma, MSD, Novartis Pharma, Otsuka Pharmaceutical, Sanofi, Takeda Pharmaceutical, and Terumo. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version of this paper.
Appendix
References
- 1.Chugh S.S., Havmoeller R., Narayanan K., et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohsawa M., Okayama A., Okamura T., et al. Mortality risk attributable to atrial fibrillation in middle-aged and elderly people in the Japanese general population: Nineteen-year follow-up in NIPPON DATA80. Circ J. 2007;71:814–819. doi: 10.1253/circj.71.814. [DOI] [PubMed] [Google Scholar]
- 3.Mäki T., Toivonen L., Koskinen P., Näveri H., Härkönen M., Leinonen H. Effect of ethanol drinking, hangover, and exercise on adrenergic activity and heart rate variability in patients with a history of alcohol-induced atrial fibrillation. Am J Cardiol. 1998;82:317–322. doi: 10.1016/s0002-9149(98)00299-9. [DOI] [PubMed] [Google Scholar]
- 4.Anadon M.J., Almendral J., González P., Zaballos M., Delcan J.L., De Guevara J.L. Alcohol concentration determines the type of atrial arrhythmia induced in a porcine model of acute alcoholic intoxication. Pacing Clin Electrophysiol. 1996;19:1962–1967. doi: 10.1111/j.1540-8159.1996.tb03262.x. [DOI] [PubMed] [Google Scholar]
- 5.Zoni-Berisso M., Lercari F., Carazza T., Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 2014;6:213–220. doi: 10.2147/CLEP.S47385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallagher C., Hendriks J.M.L., Elliott A.D., et al. Alcohol and incident atrial fibrillation - a systematic review and meta-analysis. Int J Cardiol. 2017;246:46–52. doi: 10.1016/j.ijcard.2017.05.133. [DOI] [PubMed] [Google Scholar]
- 7.Voskoboinik A., Prabhu S., Ling L.H., Kalman J.M., Kistler P.M. Alcohol and atrial fibrillation: a sobering review. J Am Coll Cardiol. 2016;68:2567–2576. doi: 10.1016/j.jacc.2016.08.074. [DOI] [PubMed] [Google Scholar]
- 8.Voskoboinik A., Kalman J.M., De Silva A., et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382:20–28. doi: 10.1056/NEJMoa1817591. [DOI] [PubMed] [Google Scholar]
- 9.Mizuno Y., Harada E., Morita S., et al. East Asian variant of aldehyde dehydrogenase 2 is associated with coronary spastic angina: possible roles of reactive aldehydes and implications of alcohol flushing syndrome. Circulation. 2015;131:1665–1673. doi: 10.1161/CIRCULATIONAHA.114.013120. [DOI] [PubMed] [Google Scholar]
- 10.Feinberg W.M., Blackshear J.L., Laupacis A., Kronmal R., Hart R.G. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med. 1995;155:469–473. [PubMed] [Google Scholar]
- 11.Quintana D.S., Guastella A.J., McGregor I.S., Hickie I.B., Kemp A.H. Moderate alcohol intake is related to increased heart rate variability in young adults: implications for health and well-being. Psychophysiology. 2013;50:1202–1208. doi: 10.1111/psyp.12134. [DOI] [PubMed] [Google Scholar]
- 12.Li H., Borinskaya S., Yoshimura K., et al. Refined geographic distribution of the oriental aldh2∗504lys (nee 487lys) variant. Ann Hum Genet. 2009;73:335–345. doi: 10.1111/j.1469-1809.2009.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voskoboinik A., Wong G., Lee G., et al. Moderate alcohol consumption is associated with atrial electrical and structural changes: insights from high-density left atrial electroanatomic mapping. Heart Rhythm. 2019;16:251–259. doi: 10.1016/j.hrthm.2018.10.041. [DOI] [PubMed] [Google Scholar]
- 14.Gami A.S., Hodge D.O., Herges R.M., et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565–571. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 15.Shantha G., Pelosi F., Morady F. Relationship between obstructive sleep apnoea and AF. Arrhythm Electrophysiol Rev. 2019;8:180–183. doi: 10.15420/aer.2019.35.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goedde H.W., Agarwal D.P., Fritze G., et al. Distribution of ADH2 and ALDH2 genotypes in different populations. Hum Genet. 1992;88:344–346. doi: 10.1007/BF00197271. [DOI] [PubMed] [Google Scholar]
- 17.Yukawa Y., Muto M., Hori K., et al. Combination of ADH1b∗2/ALDH2∗2 polymorphisms alters acetaldehyde-derived DNA damage in the blood of Japanese alcoholics. Cancer Sci. 2012;103:1651–1655. doi: 10.1111/j.1349-7006.2012.02360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harada S., Agarwal D.P., Goedde H.W. Aldehyde dehydrogenase deficiency as cause of facial flushing reaction to alcohol in Japanese. Lancet. 1981;2:982. doi: 10.1016/s0140-6736(81)91172-7. [DOI] [PubMed] [Google Scholar]
- 19.Chen C.H., Ferreira J.C., Gross E.R., Mochly-Rosen D. Targeting aldehyde dehydrogenase 2: new therapeutic opportunities. Physiol Rev. 2014;94:1–34. doi: 10.1152/physrev.00017.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakano Y., Ochi H., Onohara Y., et al. Genetic variations of aldehyde dehydrogenase 2 and alcohol dehydrogenase 1b are associated with the etiology of atrial fibrillation in Japanese. J Biomed Sci. 2016;23:89. doi: 10.1186/s12929-016-0304-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallardo-Carpentier A., Aileru A.A., Carpentier R.G. Arrhythmogenic and antiarrhythmic actions of substances of abuse: effects on triggered activity. J Electrocardiol. 1997;30:137–142. doi: 10.1016/s0022-0736(97)80022-2. [DOI] [PubMed] [Google Scholar]
- 22.Ohdaira F., Nakamura K., Nakayama H., et al. Demographic characteristics of 3,659 Japanese patients with obstructive sleep apnea-hypopnea syndrome diagnosed by full polysomnography: associations with apnea-hypopnea index. Sleep Breath. 2007;11:93–101. doi: 10.1007/s11325-006-0087-5. [DOI] [PubMed] [Google Scholar]
- 23.Aihara K., Oga T., Harada Y., et al. Analysis of anatomical and functional determinants of obstructive sleep apnea. Sleep Breath. 2012;16:473–481. doi: 10.1007/s11325-011-0528-7. [DOI] [PubMed] [Google Scholar]
- 24.Grant B.F., Chou S.P., Saha T.D., et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on alcohol and related conditions. JAMA Psychiatry. 2017;74:911–923. doi: 10.1001/jamapsychiatry.2017.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grittner U., Kuntsche S., Gmel G., Bloomfield K. Alcohol consumption and social inequality at the individual and country levels--results from an international study. Eur J Public Health. 2013;23:332–339. doi: 10.1093/eurpub/cks044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.