Abstract
Type 2 diabetes is a major threat to human health in the 21st century. More than half a billion people may suffer from this pandemic disease in 2030, leading to a huge burden of cardiovascular complications. Recently, 2 novel antidiabetic agents, glucagon-like peptide 1 receptor agonists and sodium-glucose cotransporter 2 inhibitors, reduced cardiovascular complications in a number of randomized control trials. To integrate new information and to achieve a streamlined process for better patient care, a working group was appointed by the Taiwan Society of Cardiology to formulate a stepwise consensus pathway for these therapies to reduce cardiovascular events in patients with type 2 diabetes. This consensus pathway is complementary to clinical guidelines, acting as a reference to improve patient care.
Key Words: antidiabetic agents, chronic kidney disease, heart failure, Taiwan Society of Cardiology, type 2 diabetes
Abbreviations and Acronyms: ASCVD, atherosclerotic cardiovascular disease; CKD, chronic kidney disease; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HF, heart failure; GLP-1RA, glucagon-like peptide 1 receptor agonist; MACE, major adverse cardiovascular events; SGLT2, sodium-glucose cotransporter 2
Central Illustration
Highlights
-
•
SGLT2 inhibitors and GLP-1 RAs decreased cardiovascular endpoints in a majority of clinical trials.
-
•
SGLT2 inhibitors are more effective in preventing and reducing renal endpoints and heart failure, while GLP-1 RAs are more effective in preventing and reducing stroke.
We have developed a stepwise algorithm, using 5 clinical parameters, to prioritize specific medication in different clinical settings, including patients with ASCVD or risk factors alone.
The Taiwan Society of Cardiology has published several clinical practice guidelines or consensuses to provide guidance on the management of cardiovascular (CV) diseases since 2010 (1, 2, 3, 4, 5, 6). To integrate information in time and to achieve a streamlined process for better patient care, The Taiwan Society of Cardiology has recently appointed a working group to formulate consensus pathways to address key questions and to provide potential solutions for high-value clinical topics. The first challenge is to provide a consensus pathway for novel antidiabetic agents to reduce CV events in patients with type 2 diabetes. These novel antidiabetic agents, namely glucagon-like peptide 1 receptor agonists (GLP-1RAs) and sodium-glucose cotransporter 2 (SGLT2) inhibitors, reduced CV complications in a number of randomized control trials. This consensus pathway is complementary to clinical guidelines, acting as a reference to improve patient care.
Introduction
Type 2 diabetes has become a major threat to human health in the 21st century (7). More than half a billion people may suffer from this pandemic disease in 2030 and more than 60% of patients with diabetes will experience macrovascular complications, including myocardial infarction, stroke, peripheral artery disease, and CV death (8). Despite a linear relationship between glycated hemoglobin (HbA1c) and macrovascular complications has been demonstrated for years, traditional antidiabetic agents, such as sulfonylurea or insulin, were unable to decrease these complications (9, 10, 11, 12). Before 2015, metformin was the only drug proven to be effective in reducing myocardial infarction and death in patients with type 2 diabetes (13).
Based on a single meta-analysis claiming that rosiglitazone was associated with significant increases in the risk of myocardial infarction and CV death (14), the U.S. Food and Drug Administration issued a mandate on December 17, 2008, that CV outcome trials were requested for all novel antidiabetic drugs to confirm their CV safety. Rosiglitazone is actually safe, confirmed by the late-coming RECORD (Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes) trial (15,16), but more than 200,000 patients have been enrolled in more than 20 CV outcome trials since 2008 (17). Surprisingly, 2 classes of these novel antidiabetic agents, namely GLP-1RAs and SGLT2 inhibitors, were effective in reducing CV end points. In the 2019 European Society of Cardiology/European Association for the Study of Diabetes guidelines on diabetes, prediabetes, and cardiovascular diseases (18), GLP-1RAs and SGLT2 inhibitors were recommended as the frontline therapy ahead of metformin in drug-naïve patients with atherosclerotic cardiovascular disease (ASCVD) or with high or very high CV risks.
The mechanisms of CV protection for these 2 classes of drugs are different. The CV outcome trials confirm that GLP-1RAs are effective in reducing major adverse cardiovascular events (MACE) and that SGLT2 inhibitors mainly decrease heart failure (HF) and end-stage renal disease, although MACE may also be reduced. But how to prioritize these 2 agents is quite difficult in some clinical settings, because patients with diabetes may have multiple concomitant diseases, including HF, chronic kidney disease (CKD), and ASCVD. In the American College of Cardiology 2020 expert consensus decision pathway on novel therapies for CV risk reduction in patients with type 2 diabetes (19), GLP-1RAs and SGLT2 inhibitors were given different decision pathways for high-risk patients. But how to decide which one should be the first-line therapy in patients with multiple concomitant diseases has not been mentioned and there has been no clear suggestion for patients with risk factors alone but devoid of documented CV disease (19). Since the initiative of the 2020 American College of Cardiology decision pathway, 5 more trials and other meta-analyses have been published (20, 21, 22, 23, 24, 25, 26, 27), rendering an even stronger support to update an evidence-based decision pathway. A working group was recently appointed by Taiwan Society of Cardiology to formulate a consensus pathway as guidance to these novel therapies to reduce CV events in patients with type 2 diabetes. All the available randomized control trials, meta-analyses, and network meta-analyses were carefully reviewed by the working group in an attempt to finalize a timely decision pathway for these 2 novel agents.
GLP-1RAs and SGLT2 Inhibitors
The first approved GLP-1RA for the treatment of type 2 diabetes was exenatide, a synthetic exendin-4 originated from saliva of a lizard from Arizona, Heloderma suspectum (28). Exenatide has a limited sequence homology of 53% to human GLP-1 and a very short half-life of approximately 2-3 hours (29). In contrast, GLP-1RAs with human backbone structure, such as liraglutide, semaglutide, albiglutide, and dulaglutide, have more than 90% homology to human GLP-1 (29). Except for liraglutide, whose half-life is around 13 hours, their half-lives are around 5-7 days (29). GLP-1RAs induced glucose-dependent insulin secretion and inhibited glucagon release (30). GLP-1 receptors are widely expressed in various CV tissues (31, 32, 33). Clinical trials of human-backbone GLP-1RAs demonstrated remarkable CV benefits (34).
French chemists isolated phlorizin in 1835, a substance from the bark of apple trees (35). Chronic administration of phlorizin in a canine model produced many symptoms similar to those observed in human diabetes (glucosuria, polyuria, and weight loss) (35). This observation led to the discovery of SGLT2 inhibitors 150 years later (35). SGLT2 inhibitors blocked SGLT2 in the proximal tubule in the kidney (36), resulting in glucosuria, blood glucose lowering, and body weight loss (37). SGLT2 inhibitors also caused osmotic diuresis and natriuresis (38), decreased blood pressure (39), and decreased left ventricular mass and improved left ventricular diastolic function (40) in patients with diabetes. Moreover, SGLT2 inhibitors inhibited Na+/H+ exchanger 1 in the myocardium and reduced cytoplasmic concentrations of sodium and calcium (41,42), leading to a reduction in intracellular calcium overload and cardiac protection (43).
Search Strategy and Selection Criteria
We searched all randomized CV outcome trials of dipeptidyl peptidase 4 inhibitors, GLP-1RAs, and SGLT2 inhibitors from January 1, 2012, to February 1, 2021, with the use of PubMed. The search algorithm is provided in the Supplemental Methods. We restricted our search to trials including more than 1,000 patients. Data search and extraction were performed by 2 independent reviewers (S.-H.S. and H.-M.C.), and any discrepancies were resolved by consensus or by consulting a third reviewer (C.-E.C). The PRISMA flow chart is shown in Supplemental Figure 1. We identified a total of 22 trials (Table 1). All trials met criteria for being well conducted and had low risk of bias according to the Cochrane tool for assessing risk of bias in randomized clinical trials (44) (Supplemental Figure 2).
Table 1.
Effects of Novel Antidiabetic Drugs on Cardiovascular Outcomes in Clinical Trials
| First Author (Ref. #) | Trial Name (n) | Drug Name | Target Patients | Outcome |
|||||
|---|---|---|---|---|---|---|---|---|---|
| MACE | Composite Renal Including Albuminuria | Composite Renal Excluding Albuminuria | Heart Failure | CV Death | All-Cause Death | ||||
| DPP-4 inhibitors | |||||||||
| Scirica et al (49) | SAVOR-TIMI 53 (n = 16,492) | Saxagliptin | Diabetes | ↔ | ↔ | ↔ | ↑ | ↔ | ↔ |
| Green et al (94) | TECOS (n = 14,671) | Sitagliptin | Diabetes | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ |
| White et al (95) | EXAMINE (n = 5,380) | Alogliptin | Diabetes | ↔ | ↔ | ↔ | ↑ | ↔ | ↔ |
| Rosenstock et al (96) | CAROLINA (n = 6,042) | Linagliptin | Diabetes | ↔ | NR | NR | ↔ | ↔ | ↔ |
| Rosenstock et al (97) | CARMELINA (n = 6,991) | Linagliptin | DKD | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ |
| GLP-1 receptor agonists | |||||||||
| Pfeffer et al (77) | ELIXA (n = 6,068) | Lixisenatide | Diabetes | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ |
| Holman et al (98) | EXSCEL (n = 14,752) | Exenatide-SR | Diabetes | ↔ | ↔ | ↔ | ↔ | ↔ | ↓ |
| Marso et al (51) | LEADER (n = 9,340) | Liraglutide | Diabetes | ↓ | ↓ | ↔ | ↔ | ↓ | ↓ |
| Hernandez et al (52) | HARMONY (n = 9,463) | Albiglutide | Diabetes | ↓ | NR | NR | ↔ | ↔ | ↔ |
| Marso et al (53) | SUSTAIN-6 (n = 3,297) | Semaglutide | Diabetes | ↓ | ↓ | ↔ | ↔ | ↔ | ↔ |
| Husain et al (99) | PIONEER-6 (n = 3,183) | Semaglutide (oral) | Diabetes | ↔ | NR | NR | ↔ | ↓ | ↓ |
| Gerstein et al (54) | REWIND (n = 9,901) | Dulaglutide | Diabetes | ↓ | ↓ | ↔ | ↔ | ↔ | ↔ |
| SGLT2 inhibitors | |||||||||
| Zinman et al (55) | EMPA-REG OUTCOME (n = 7,020) | Empagliflozin | Diabetes | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ |
| Neal et al (56) | CANVAS Program (n = 10,142) | Canagliflozin | Diabetes | ↓ | ↓ | ↓ | ↓ | ↔ | ↔ |
| Wiviott et al (57) | DECLARE-TIMI 58 (n = 17,160) | Dapagliflozin | Diabetes | ↔ | NR | ↓ | ↓ | ↔ | ↔ |
| Cannon et al (20) | VERTIS CV (n = 8,246) | Ertugliflozin | Diabetes | ↔ | NR | ↔ | ↓ | ↔ | ↔ |
| Perkovic et al (58) | CREDENCE (n = 4,401) | Canagliflozin | DKD | ↓ | ↓ | ↓ | ↓ | ↔ | ↔ |
| Heerspink et al (22) | DAPA-CKD (n = 4,304) | Dapagliflozin | CKD | NR | NR | ↓ | ↓ | ↔ | ↓ |
| Bhatt et al (23) | SCORED (n = 10,584) | Sotagliflozin | DKD | ↓ | NR | ↔ | ↓ | ↔ | ↔ |
| McMurray et al (60) | DAPA-HF (n = 4,744) | Dapagliflozin | HF | NR | NR | ↔ | ↓ | ↓ | ↓ |
| Packer et al (21) | EMPEROR-Reduced (n = 3,730) | Empagliflozin | HF | NR | NR | ↓ | ↓ | ↔ | ↔ |
| Bhatt et al (24) | SOLOIST-WHF (n = 1,222) | Sotagliflozin | HF | NR | NR | NR | ↓ | ↔ | ↔ |
Arrows indicate risk: decreased, neutral, or increased.
CKD = chronic kidney disease; CV = cardiovascular; DKD = diabetic kidney disease; DPP-4 = dipeptidyl peptidase 4; GLP-1 = glucagon-like peptide 1; HF = heart failure; MACE = major adverse cardiovascular events; NR = not reported; SGLT2 = sodium-glucose cotransporter 2.
We also searched PubMed for meta-analyses of outcome trials of GLP-1RAs and SGLT2 inhibitors. Meta-analyses that involved other types of antidiabetic agents were not included. Network meta-analyses that compared GLP-1RA and SGLT2 inhibitor were included. We limited our search from January 1, 2019, to February 15, 2021, to take advantage of more recent trials. Meta-analyses that included the same trials but were published in different journals were evaluated and selected, based on the consensus of the 3 reviewers (S.-H.S., H.-M.C., and C.-E.C). We identified 5 meta-analyses (26,45, 46, 47, 48) and 2 network meta-analyses (25,27).
Paradigm Shift in Antidiabetic Management
Since the US Food and Drug Administration issued the mandate in 2008 that novel antidiabetic agents should be tested for safety by means of randomized control trials, 22 randomized control trials have been completed (Table 1). In general, DPP-4 inhibitors had neutral effects on CV and renal outcomes, with the exception of saxagliptin and alogliptin, which increased the risk of HF (49,50). GLP-1RAs that belong to the exendine-4–backbone group (lixisenatide and exenatide) had no beneficial effect on CV and renal outcomes. The human-backbone group (liraglutide, albiglutide, semaglutide, and dulaglutide) reduced MACE and the composite renal end point that included albuminuria (51, 52, 53, 54). However, they did not reduce the composite renal end point when albuminuria was excluded in the analysis, nor could they prevent HF. When SGLT2 inhibitors were tested in patients with diabetes, the rates of composite renal end points and HF were reduced (55, 56, 57). Ertugliflozin was an exception in that primary MACE end points and 2 secondary end points (composite of CV death and hospitalization for HF, and composite renal end points) all failed to reach significant differences (20). Among the 3 CKD trials of SGLT2 inhibitors, CREDENCE (Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy) (58), DAPA-CKD (A Study to Evaluate the Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients With Chronic Kidney Disease) (22), and SCORED (Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients With Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk) (23), 2 of them (CREDENCE and DAPA-CKD) demonstrated significant reduction in renal end points and HF (22,58). The SCORED trial was prematurely terminated owing to a funding issue (23). The primary end points were modified and the CV events were unadjudicated. The results were therefore less convincing (23). In addition, sotagliflozin is a dual SGLT2 and SGLT-1 inhibitor (59). Its mechanism of action and adverse effects are different from other SGLT2 inhibitors. We did not include it in the present consensus pathway. Finally, among the 3 trials dedicated to HF patients, DAPA-HF (Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients With Chronic Heart Failure) (60), EMPEROR-Reduced (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction) (21), and SOLOIST-WHF (Effect of Sotagliflozin on Cardiovascular Events in Patients With Type 2 Diabetes Post Worsening Heart Failure) (24), SGLT2 inhibitors consistently reduced the HF end point. Similarly to the SCORED trial, SOLOIST-WHF was prematurely terminated because of a funding issue, and the primary end points were modified and the events were unadjudicated (24).
Based on these randomized control trials, the recent 2019 European Society of Cardiology/European Association for the Study of Diabetes guidelines (18), the 2019 update of American Diabetes Association/European Association for the Study of Diabetes consensus on the management of hyperglycemia in type 2 diabetes (61), and the 2021 American Diabetes Association guidelines (62) have changed management from “glucose-driven strategy” to “event-driven strategy” for patients with diabetes and high CV risk. For patients with established ASCVD or high ASCVD risk, either GLP-1RA or SGLT2 inhibitor should be considered, while SGLT2 inhibitor should be the first choice for CKD patients or patients with HF with reduced ejection fraction.
Methods for Formation of the Consensus Pathway
Novel antidiabetic agents with demonstrated benefit on primary end points in randomized control trials were discussed and included in this consensus pathway. We excluded agents that failed to achieve significant effects on primary end points in randomized control trials, such as lixisenatide, exenatide-SR, and ertugliflozin. Albiglutide was not discussed as it had been withdrawn from the market.
The working group of the consensus pathway systemically analyzed the following important clinical evidence to support the use of SGLT2 inhibitors and GLP-1RAs:
-
•
Asian subgroup data versus global data (checking the P value for interaction) in randomized control trials.
-
•Placebo-controlled randomized control trials:
-
○Effect size (relative risk reduction).
-
○Significance level (P value).
-
○
-
•Meta-analyses of placebo-controlled randomized control trials:
-
○Effect size (relative risk reduction).
-
○Significance level (P value).
-
○
-
•Network meta-analyses comparing SGLT2 inhibitors to GLP-1RAs:
-
○Effect size (relative risk reduction).
-
○Significance level (P value).
-
○
The working group focused on the following end points: 3-point MACE (CV death, nonfatal myocardial infarction, and nonfatal stroke) and their individual components, HF, and renal events. For patients with type 2 diabetes, concomitant HF increases total death by about 3-fold, the highest compared with other comorbidities (63). Therefore, HF was our first consideration when formulating the stepwise consensus pathway. Patients with diabetic kidney disease have higher mortality rate than patients with diabetes who have ASCVD (64). Second to HF, CKD was our next consideration, followed by ASCVD. Patients with risk factors alone were our last consideration, because they have lower mortality rates than those with ASCVD (54,57). The working group members convened twice (February 20, 2021, and March 13, 2021) to review all evidence extensively. Each suggestion was reached by consensus.
Asian Subgroup in Randomized Control Trials of SGLT2 Inhibitor and GLP-1RA
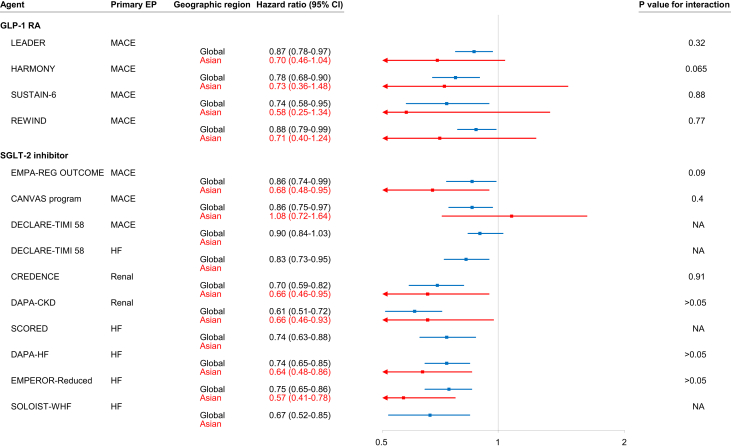
Proportions of Asians in these randomized control trials are shown in Supplemental Figure 3. Twelve Asian countries/territories recruited patients for these trials (Supplemental Table 1). In general, efficacy on the primary end points for Asian subgroups was consistent with that of the whole study population. Figure 1 is a forest plot of primary end points for Asian patients versus the whole study population in individual trials. Except the unreported ones, the P values for interaction were >0.05 in all randomized control trials, suggesting that the efficacy of these agents in Asian patients was consistent with that in the whole study population. In a recent meta-analysis comparing Asians with Whites in randomized control trials of SGLT2 inhibitors and GLP-1RAs, Asian patients with HF with reduced ejection fraction derived greater benefit in CV death/hospitalization for HF from SGLT2 inhibitors, and Asian patients with type 2 diabetes derived greater MACE benefit from GLP-1RAs (65). Although there is no specific Asian outcome trial, findings from these global trials can be reasonably applied to Asian patients.
Figure 1.
Forest Plot of End Points for Asians Versus Whole Study Population
Clinical trials with positive findings are shown. In general, the efficacy of GLP-1 receptor agonists and SGLT2 inhibitors in Asians were consistent with those in the whole study population. There were 4 trials for GLP-1RAs, including LEADER (51), HARMONY (52), SUSTAIN-6 (53), and REWIND (54); 9 trials for SGLT2 inhibitors, including EMPA-REG OUTCOME (55), CANVAS Program (56), DECLARE-TIMI 58 (57), CREDENCE (58), DAPA-CKD (22), the SCORED (23), DAPA-HF (60), EMPEROR-Reduced (21), and SOLOIST-WHF (24). CI = confidence interval; EP = end point; GLP-1RA = glucagon-like peptide 1 receptor agonist; HF = heart failure; MACE = major adverse cardiovascular events; NA = not available; SGLT2 = sodium-glucose cotransporter 2.
Primary and Secondary Prevention of MACE
Liraglutide, semaglutide, and dulaglutide are GLP-1RAs with demonstrated benefits in reducing 3-point MACE in patients with diabetes (Table 1). A meta-analysis including all 7 trials of GLP-1RAs further provided solid evidence to support the role of GLP-1RA in reducing 3-point MACE (47). The efficacy was consistent in patients with ASCVD (secondary prevention) or with risk factors alone (primary prevention), with a P value for interaction of 0.24 (47) (Table 2). GLP-1RAs also reduced the risk of each component of the 3-point MACE, ie, CV death, myocardial infarction, and stroke (Table 2). More details regarding the differential effects on each MACE component in patients with ASCVD (secondary prevention) or with risk factors alone (primary prevention) were not provided.
Table 2.
Effects of GLP-1RAs and SGLT2 Inhibitors on MACE in Meta-analyses
| First Author (Ref. #) | N (Ref. #) | HR (95% CI) | P Value | P for Interaction |
|---|---|---|---|---|
| MACE | ||||
| Kristensen et al (47) | 56,004 | |||
| GLP-1RA vs placebo | 0.88 (0.82-0.94) | <0.0001 | ||
| Previous ASCVD | 0.86 (0.80-0.93) | 0.24 | ||
| Risk factors alone | 0.94 (0.83-1.07) | |||
| Zelniker et al (45,46) | 77,242 (45), 34,322 (46) | |||
| SGLT2i vs placebo | 0.89 (0.83-0.96) | 0.0014 | ||
| Previous ASCVD | 0.86 (0.80-0.93) | 0.05 | ||
| Risk factors alone | 1.00 (0.87-1.16) | |||
| CV death | ||||
| Kristensen et al (47) | 56,004 | |||
| GLP-1RA vs placebo | 0.88 (0.81-0.96) | 0.003 | ||
| Zelniker et al (46) | 34,322 | |||
| SGLT2i vs placebo (46) | 0.84 (0.75-0.94) | 0.0023 | ||
| Previous ASCVD | 0.80 (0.71-0.91) | 0.0005 | 0.31 | |
| Risk factors alone | 1.02 (0.80-1.30) | 0.89 | ||
| Myocardial infarction | ||||
| Kristensen et al (47) | 56,004 | |||
| GLP-1RA vs placebo | 0.91 (0.84-1.00) | 0.043 | ||
| Zelniker et al (46) | 34,322 | |||
| SGLT2i vs placebo | 0.89 (0.80-0.98) | 0.0177 | ||
| Previous ASCVD | 0.85 (0.76-0.95) | 0.00045 | 0.17 | |
| Risk factors alone | 0.99 (0.79-1.24) | 0.92 | ||
| Stroke | ||||
| Kristensen et al (47) | 56,004 | |||
| GLP-1RA vs placebo | 0.84 (0.76-0.93) | <0.0001 | ||
| Zelniker et al (46) | 34,322 | |||
| SGLT2i vs placebo | 0.97 (0.86-1.10) | 0.64 | ||
| Previous ASCVD | 0.98 (0.84-1.14) | 0.78 | 0.83 | |
| Risk factors alone | 1.01 (0.80-1.28) | 0.94 | ||
ASCVD = atherosclerotic cardiovascular disease; CI = confidence interval; GLP-1RA = glucagon-like peptide 1 receptor agonist; HR = hazard ratio; MACE = major adverse cardiovascular events; SGLT2i = sodium-glucose cotransporter 2 inhibitor.
Empagliflozin, canagliflozin, and dapagliflozin are SGLT2 inhibitors with demonstrated benefits in reducing primary end points in patients with diabetes (Table 1). DECLARE (Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events) had 2 coprimary end points, MACE and CV death/hospitalization for HF (57). Dapagliflozin decreased CV death/hospitalization for HF, but not MACE (57). Two meta-analyses demonstrated the beneficial effects of SGLT2 inhibitors on MACE and other CV end points (45,46). The differential effects on MACE, CV death, myocardial infarction, and stroke in patients with ASCVD or with risk factors alone were also provided (45,46). Table 2 presents a summary of their effects on MACE, CV death, myocardial infarction, and stroke. SGLT2 inhibitors, compared with placebo, reduced MACE only in patients with ASCVD (secondary prevention), not in patients with risk factors alone (primary prevention), with a P value for interaction of 0.05. A more recent meta-analysis included the CREDENCE (Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy) trial and concluded that the effect of SGLT2 inhibitors on the reduction of MACE was consistent for primary prevention and secondary prevention (48). However, this particular meta-analysis was not included in our analysis in this section because the CREDENCE trial exclusively enrolled patients with diabetic kidney disease, not the full spectrum of patients with diabetes. The CREDENCE trial is discussed in the section dealing with renal events.
As presented in Table 2, both GLP-1RAs and SGLT2 inhibitors reduce CV death and myocardial infarction. Only GLP-1RAs reduce stroke, whereas SGLT2 inhibitors have no effect on stroke. In the REWIND (Researching Cardiovascular Events With a Weekly Incretin in Diabetes) trial, dulaglutide decreased fatal and nonfatal stroke by 26% (hazard ratio [HR] 0.74, 95% confidence interval [CI] 0.62-0.94; P = 0.0096), and the effect was consistent in patients with or without previous stroke (P for interaction = 0.83) (66). Therefore, it is reasonable to advocate GLP-1RA exclusively in patients with a history of stroke.
-
•
For primary prevention of MACE: Only GLP-1RAs significantly reduced MACE compared with placebo.
-
•
For secondary prevention of MACE: Both GLP-1RAs and SGLT2 inhibitors significantly reduced MACE compared with placebo.
-
•
For primary and secondary prevention of stroke: Only GLP-1RAs significantly reduced stroke compared with placebo.
Primary and Secondary Prevention of HF With Reduced Ejection Fraction
In general, GLP-1RAs have no effect on HF prevention, as presented in Table 1. Nevertheless, a meta-analysis of the 7 GLP-1RA trials showed a marginal effect on HF (HR 0.91, 95% CI 0.83-0.99; P = 0.028) (26) (Table 3). The effect of HF reduction was mainly observed in patients without HF (primary prevention), given that only about 20% of patients had a history of HF in these trials. Moreover, there is no GLP-1RA trial dedicated specifically for HF patients (secondary prevention). In contrast, SGLT2 inhibitors decreased the risk of HF in all randomized control trials (Table 1), including those trials for patients with diabetes as a whole (55, 56, 57) and those dedicated for HF patients (21,60). DAPA-HF and EMPEROR-Reduced exclusively enrolled patients with HF with reduced ejection fraction (21,60). Patients with diabetes and without diabetes were enrolled. In both trials, SGLT2 inhibitors significantly reduced CV death and hospitalization for HF (21,60). CV death and all-cause death were also reduced by dapagliflozin (60). Randomized control trials testing SGLT2 inhibitors in patients with HF with preserved ejection fraction are still ongoing and final results are worth waiting for. Two meta-analyses demonstrated the effects of SGLT2 inhibitors on HF with reduced ejection fraction (26,46). One meta-analysis included exclusively diabetic trials (55, 56, 57), and only 10%-20% patients in these trials had previous history of HF (46). The other (26) included most recent trials, such as DAPA-HF (60) and EMPEROR-Reduced (21). As presented in Table 3, SGLT2 inhibitors significantly reduce the risk of HF in both meta-analyses, regardless of history of previous HF.
-
•
For primary prevention of HF with reduced ejection fraction: GLP-1RAs had marginal effects compared with placebo, and SGLT2 inhibitors significantly reduced HF compared with placebo.
-
•
For secondary prevention of HF with reduced ejection fraction: Only SGLT2 inhibitors significantly reduced HF compared with placebo.
Table 3.
Effects of GLP-1RAs and SGLT2 Inhibitors on Hospitalization for Heart Failure in Meta-analyses
| First Author (Ref. #) | N | HR (95% CI) | P Value | P for Interaction |
|---|---|---|---|---|
| Kristensen et al (47) | 56,004 | |||
| GLP-1RA vs placebo | 0.91 (0.83-0.99) | 0.028 | ||
| Zelniker et al (46) | 34,322 | |||
| SGLT2i vs placebo | 0.69 (0.61-0.79) | <0.001 | ||
| History of HF | 0.68 (0.55-0.83) | 0.0002 | 0.76 | |
| No history of HF | 0.71 (0.60-0.83) | <0.0001 | ||
| Salah et al (26) | 59,747 | |||
| SGLT2i vs placebo (all patients) | 0.69 (0.64-0.74) | <0.000001 | ||
| SGLT2i vs placebo (patients with DM and HF) | 0.71 (0.61-0.83) | <0.0001 | ||
| SGLT2i vs placebo (patients with HF ± DM) | 0.69 (0.62-0.76) | <0.00001 |
DM = diabetes mellitus; HF = heart failure; other abbreviations as in Table 2.
Primary and Secondary Prevention of Renal Events
Among GLP-1RAs, liraglutide, semaglutide, and dulaglutide reduced composite renal end points that included albuminuria in the analysis, regardless of history of CKD (67,68); yet none of them achieved significant reduction in composite renal end points when albuminuria was excluded from the analysis (Table 1). More specifically, GLP-1RAs were effective in reducing albuminuria, but were unable to halt the deterioration of estimated glomerular filtration rate (eGFR) or to decrease the risk of end-stage renal disease (53,67,68). Meta-analyses demonstrated similar findings (25,26,45) (Table 4). A trial of semaglutide dedicated for diabetic kidney disease is ongoing (A Research Study to See How Semaglutide Works Compared to Placebo in People With Type 2 Diabetes and Chronic Kidney Disease [FLOW]; NCT03819153).
Table 4.
Effects of GLP-1RAs and SGLT2 Inhibitors on Renal End Points in Meta-analyses
| First Author (Ref. #) | N | HR (95% CI) | P Value |
|---|---|---|---|
| Composite renal end point including albuminuria | |||
| Zelniker et al (45) | 77,242 | ||
| GLP-1RA vs placebo | 0.82 (0.75-0.89) | <0.001 | |
| SGLT2i vs placebo | 0.62 (0.58-0.67) | <0.001 | |
| Kristensen et al (47) | 56,004 | ||
| GLP-1RA vs placebo | 0.83 (0.78-0.89) | <0.001 | |
| Composite renal end point excluding albuminuria | |||
| Zelniker et al (45) | 77,242 | ||
| GLP-1RA vs placebo | 0.92 (0.80-1.06) | 0.24 | |
| SGLT2i vs placebo | 0.55 (0.48-0.64) | <0.001 | |
| Zelniker et al (46) | 34,322 | ||
| SGLT2i vs placebo | |||
| All patients | 0.55 (0.48-0.64) | <0.001 | |
| Patients with kidney disease | 0.67 (0.51-0.89) | <0.001 | |
| Kristensen et al (47) | 56,004 | ||
| GLP-1RA vs placebo | 0.87 (0.73-1.03) | 0.098 | |
| Yamada et al (network meta-analysis) (25) | 32,949 | ||
| GLP-1RA vs placebo | 0.86 (0.72-1.03) | NR | |
| Salah et al (26) | 59,747 | ||
| SGLT2i vs placebo | |||
| Patients with kidney disease | 0.68 (0.48-0.95) | 0.03 | |
NR = not reported; other abbreviations as in Table 2.
Reduction in albuminuria is not a reliable surrogate for clinical kidney end point. For example, in the blood pressure study of the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial (69), the baseline mean eGFR was 91.6 mL/min/1.73 m2 in the intensive arm (target systolic blood pressure <120 mm Hg) versus 91.7 mL/min/1.73 m2 in the standard arm (target systolic blood pressure <140 mm Hg) (P = 0.93), while the baseline median urinary albumin/creatinine ratio was 14.6 mg/g versus 14.0 mg/g (P > 0.05). At the end of study, the urinary albumin/creatinine ratio was 12.6 mg/g versus 14.9 mg/g (P < 0.001), and the new onset of albuminuria was 6.6% versus 8.7% (P = 0.009), suggesting that intensive blood pressure control was more effective in reducing albuminuria than standard blood pressure control. However, the eGFR decreased to 74.8 mL/min/1.73 m2 versus 80.6 mL/min/1.73 m2 (P < 0.001) (69). Similar findings were observed in the ACCOMPLISH (Avoiding Cardiovascular Events Through Combination Therapy in Patients Living With Systolic Hypertension) trial in which the combination of benazepril plus hydrochlorothiazide was compared with the combination of benazepril plus amlodipine (70). The former was more effective in reducing urinary albumin/creatinine ratio, but the event rates of the renal end point (composite of doubling of serum creatinine concentration, eGFR <15 mL/min/1.73 m2, or need for dialysis) were double (70). Therefore, a reduction in albuminuria is not a surrogate for kidney protection in this consensus pathway.
In contrast, most SGLT2 inhibitors, except ertugliflozin, reduced all composite renal end points in diabetes trials (Table 1). More specifically, SGLT2 inhibitors not only reduced albuminuria, but also preserved eGFR and decreased the risk of end-stage renal disease (56,71,72). The CREDENCE trial enrolled only patients with diabetic kidney disease, and demonstrated that canagliflozin significantly reduced the components of all renal end points (58). The DAPA-CKD trial recruited CKD patients with and without diabetes (22). Dapagliflozin decreased the decline of eGFR and reduced the risk of end-stage renal disease, although the effect on albuminuria was not reported (73). The benefits were consistent in patient with and without diabetes. Meta-analyses demonstrated similar findings that SGLT2 inhibitors significantly reduce all composite renal end points in patients with and without previous CKD (Table 4).
-
•
For both primary and secondary prevention of CKD: GLP-1RAs, compared with placebo, reduce composite renal end points that include albuminuria, but not when albuminuria is excluded from the analysis.
-
•
For both primary and secondary prevention of CKD: SGLT2 inhibitors, compared with placebo, reduce all composite renal end points.
Network Meta-Analysis
There is no existing randomized control trial published comparing SGLT2 inhibitors with GLP-1RAs at the present time. Adapting data from network meta-analysis is feasible for comparison. Two network meta-analyses focusing on comparison of SGLT2 inhibitors versus GLP-RAs are cited here (25,27). The network meta-analysis by Yamada et al investigated the effects of these drugs on MACE and renal events (25). SGLT2 inhibitors shared similar effects with GLP-1RAs on MACE (relative risk: 0.94, 95% CI: 0.78-1.12) but were associated with a lower risk of renal events than GLP-1RAs (relative risk: 0.79, 95% CI: 0.63-0.99) (25).
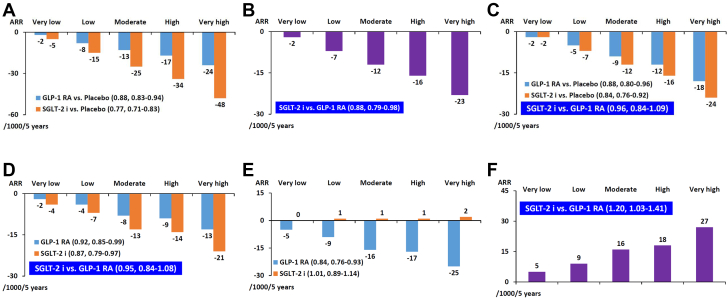
A more comprehensive network meta-analysis updated to August 11, 2020, with data sourced from Medline, Embase, and Cochrane Central, was published by Parmer et al (27) and included a total of 421,346 patients from 764 trials, but 5 randomized control trials published after August 11, 2020, namely, VERTIS CV (Cardiovascular Outcomes Following Ertugliflozin Treatment in Type 2 Diabetes Mellitus Participants With Vascular Disease) (20), EMPEROR-Reduced (21), DAPA-CKD (22), SCORED (23), and SOLOIST-WHF (24), were not included. The investigators estimated absolute effects of treatment per 1,000 patients treated for 5 years in patients at very low risk (no CV risk factors), low risk (3 or more CV risk factors), moderate risk (ASCVD), high risk (CKD), and very high risk (ASCVD + CKD). Six end points of interested were examined: all-cause death, CV death, nonfatal myocardial infarction, nonfatal stroke, kidney failure, and hospitalization for HF. The results are summarized in Figures 2 and 3. Both SGLT2 inhibitors and GLP-1RAs reduced all-cause death compared with placebo (HR: 0.77, 95% CI: 0.71-0.83 and HR: 0.88, 95% CI: 0.83-0.94, respectively). However, SGLT2 inhibitors were more effective than GLP-1RAs in reducing all-cause death (HR: 0.88, 95% CI: 0.79-0.98). For CV death and nonfatal myocardial infarction, both agents were effective compared with placebo (CV death: HR: 0.84, 95% CI: 0.76-0.92 and HR: 0.88, 95% CI: 0.80-0.96, respectively; nonfatal myocardial infarction: HR: 0.87, 95% CI: 0.79-0.97 and HR: 0.92, 95% CI: 0.85-0.99, respectively). SGLT2 inhibitors shared similar effects with GLP-1RAs in CV death and nonfatal myocardial infarction (CV death: HR: 0.96, 95% CI: 0.84-1.09; nonfatal myocardial infarction: HR: 0.85, 95% CI: 0.84-1.08). For nonfatal stroke, only GLP-1RAs were effective compared with placebo (SGLT2 inhibitors: HR: 1.01, 95% CI: 0.89-1.14; GLP-1RAs: HR: 0.84, 95% CI: 0.76-0.93). SGLT2 inhibitors caused more nonfatal stroke compared with GLP-1RAs (HR: 1.20, 95% CI: 1.03-1.41). For kidney failure, both agents were effective compared with placebo (HR: 0.71, 95% CI: 0.57-0.89 and HR: 0.78, 95% CI: 0.67-0.92, respectively). SGLT2 inhibitors shared similar effects with GLP-1RAs in kidney failure (HR: 0.90, 95% CI: 0.69-1.20). SGLT2 inhibitors, but not GLP-1RAs, reduced the risk of hospitalization for HF compared with placebo (HR: 0.70, 95% CI: 0.63-0.77 and HR: 0.94, 95% CI: 0.85-1.03, respectively). SGLT2 inhibitors were more effective than GLP-1RAs in reducing hospitalization for HF (HR: 0.74, 95% CI: 0.65-0.85). Although GLP-1RAs shared similar efficacy with SGLT2 inhibitors in reducing kidney failure in this analysis, DAPA-CKD (22) and other trials (20,21,23,24) were not included in this network meta-analysis. It is generally thought that SGLT2 inhibitors are more effective than GLP-1RAs in renal protection.
Figure 2.
SGLT2 Inhibitors Versus GLP-1RAs on Cardiovascular Outcomes
The bars represent absolute effects of treatment per 1,000 patients treated for 5 years for patients at very low risk (no cardiovascular risk factors), low risk (3 or more cardiovascular risk factors), moderate risk (atherosclerotic cardiovascular disease), high risk (chronic kidney disease), and very high risk (atherosclerotic vascular disease plus chronic kidney disease). (A) All-cause death: SGLT2 inhibitor and GLP-1RA compared with placebo. (B) All-cause death: SGLT2 inhibitor compared with GLP-1RA. (C) Cardiovascular death: SGLT2 inhibitor and GLP-1RA compared with placebo. (D) Nonfatal myocardial infarction: SGLT2 inhibitor and GLP-1RA compared with placebo. (E) Nonfatal stroke: SGLT2 inhibitor and GLP-1RA compared with placebo. (F) Nonfatal stroke: SGLT2 inhibitor compared with GLP-1RA. Adapted from Palmer et al (27) with permission. ARR = absolute risk reduction; other abbreviations as in Figure 1.
Figure 3.
SGLT2 Inhibitors Versus GLP-1RAs on Kidney and Heart Failure
The bars represent absolute effects of treatment per 1,000 patients treated for 5 years for patients at very low risk (no cardiovascular risk factors), low risk (3 or more cardiovascular risk factors), moderate risk (atherosclerotic cardiovascular disease), high risk (chronic kidney disease), and very high risk (atherosclerotic vascular disease plus chronic kidney disease). (A) Kidney failure: SGLT2 inhibitor and GLP-1RA compared with placebo. (B) Kidney failure: SGLT2 inhibitor compared with GLP-1RA. (C) Hospitalization for heart failure: SGLT2 inhibitor and GLP-1RA compared with placebo. (D) Hospitalization for heart failure: SGLT2 inhibitor compared with GLP-1RA. Data adapted from Palmer et at (27) with permission. Abbreviations as in Figures 1 and 2.
Patients With Multiple Comorbid Diseases
HF, CKD, and ASCVD are the most important comorbid diseases in patients with type 2 diabetes and are the main consideration in this consensus pathway. Patients with HF have the highest risk of all-cause death, followed by those with CKD and with ASCVD (Table 5). In the DAPA-HF trial, 56.4% had ischemic etiology, ie, they had HF with reduced ejection fraction and ASCVD (60,74). Compared with placebo, dapagliflozin reduced the primary end point to a similar extent in patients with and without ischemic etiology (HR: 0.77, 95% CI: 0.65-0.92 and HR: 0.71, 95% CI: 0.58-0.87, respectively; P for interaction = 0.55). Consistent benefits were observed for the components of the primary outcome and all-cause mortality (74). In the EMPEROR-Reduced trial, 51.8% had ischemic etiology (21). Compared with placebo, empagliflozin reduced the primary end point to a similar extent in both patients with and without ischemic etiology (HR: 0.82, 95% CI: 0.68-0.99 and HR: 0.67, 95% CI: 0.55-0.82, respectively; P for interaction > 0.05). In the DAPA-HF trial, 41% had eGFR <60 mL/min/1.73 m2, ie, they had HF with reduced ejection fraction and CKD (75). The effect of dapagliflozin on the primary and secondary outcomes did not differ by eGFR category or by examining eGFR as a continuous measurement (75). The HR for the primary end point in patients with eGFR <60 mL/min/1.73 m2 was 0.71 (95% CI: 0.59-0.86) versus 0.77 (95% CI: 0.64-0.93) in those with eGFR ≥60 mL/min/1.73 m2 (P for interaction = 0.54) (75). In the EMPEROR-Reduced trial, 48.3% had eGFR <60 mL/min/1.73 m2 (21). Compared with placebo, empagliflozin reduced the primary end point to a similar extent in patients with eGFR below and above 60 mL/min/1.73 m2 (HR: 0.78, 95% CI: 0.65-0.93 and HR: 0.72, 95% CI: 0.58-0.90, respectively; P for interaction = 0.63) (76). Taken together, in patients with HF with reduced ejection fraction plus ASCVD or CKD, SGLT2 inhibitors consistently reduced CV death and hospitalization for HF. Therefore, HF is taken as the first priority in the present stepwise consensus pathway for patients who have multiple comorbidities, and SGLT2 inhibitor is the treatment of choice.
Table 5.
All-Cause Death in Patients With Diabetes With Different Comorbidities in Clinical Trials
| First Author (Ref. #) | Trial Name (n) | All-Cause Death, Placebo Group, per 100 Person-Years |
|---|---|---|
| DM + ASCVD | ||
| Zinman et al (55) | EMPA-REG OUTCOME (n = 7,020) | 2.86 |
| Hernandez et al (52) | HARMONY (n = 9,463) | 2.56 |
| Pfeffer et al (77) | ELIXA (n = 6,068) | 3.3 |
| DM + CKD | ||
| Perkovic et al (58) | CREDENCE (n = 4,401) | 3.5 |
| Heerspink et al (22) | DAPA-CKD (n = 4,304) | 3.1 |
| Bhatt et al (23) | SCORED (n = 10,584) | 3.5 |
| DM + HFrEF | ||
| McMurray et al (60) | DAPA-HF (n = 4,744) | 9.5 |
| Packer et al (21) | EMPEROR-Reduced (n = 3,730) | 10.7 |
| Bhatt et al (24) | SOLOIST-WHF (n = 1,222) | 16.3 |
ASCVD = atherosclerotic cardiovascular disease; CKD = chronic kidney disease; CV = cardiovascular; DM = diabetes mellitus; HFrEF = heart failure with reduced ejection fraction.
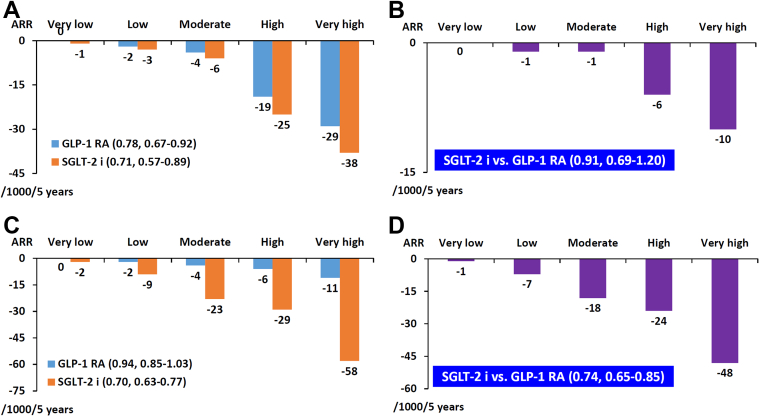
The mortality rates in patients with CKD were generally higher than those with ASCVD (Table 5). The ELIXA (Evaluation of Cardiovascular Outcomes in Patients With Type 2 Diabetes After Acute Coronary Syndrome During Treatment With AVE0010 (Lixisenatide)) trial enrolled patients with acute coronary syndrome (77), and the mortality rate in this trial was higher than in other randomized control trials that enrolled patients with chronic ASCVD (52,55). SGLT2 inhibitors reduced all-cause death in CKD trials. For example, dapagliflozin decreased all-cause death by 26% versus placebo (HR: 0.74, 95% CI: 0.56-0.98) in the DAPA-CKD trial (73), in contrast to lack of evidence for GLP-1RAs. In DAPA-CKD, 37.4% had both CKD and ASCVD (78). Dapagliflozin reduced the primary composite end point (a composite of sustained decline in eGFR of ≥50%, end-stage renal disease, and death from renal or CV causes) to a similar extent in patients with (HR: 0.61, 95% CI: 0.47-0.79) and without (HR: 0.61, 95% CI: 0.48-0.78) ASCVD, with a P for interaction of 0.90. This was also true for the composite of hospitalization for HF and CV death (HR: 0.70, 95% CI: 0.52-0.94 versus HR: 0.67, 95% CI: 0.40-1.13; P for interaction = 0.88) and for all-cause death (HR: 0.70, 95% CI: 0.51-0.95 versus HR: 0.63, 95% CI: 0.41-0.98; P for interaction = 0.71) (78). For patients with ASCVD and CKD included in the network meta-analysis by Palmer et al, SGLT2 inhibitors were more effective than GLP-1RAs in reducing all-cause death and hospitalization for HF, whereas GLP-1RAs were more effective than SGLT2 inhibitors in reducing nonfatal stroke (27) (Figure 4). Taken together, in patients with CKD + ASCVD, SGLT2 inhibitors consistently reduced composite renal end points, CV death/hospitalization for HF, and all-cause death. Therefore, CKD sits in the second place in this stepwise consensus pathway for patients with multiple comorbidities, and SGLT2 inhibitors are preferred.
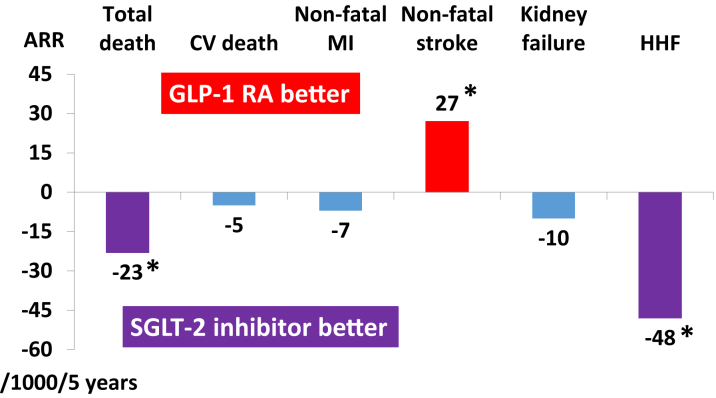
Figure 4.
SGLT2 Inhibitors Versus GLP-1RAs in Patients With ASCVD and CKD
The bars represent absolute effects of treatment per 1,000 patients treated for 5 years in patients with ASCVD and CKD. SGLT2 inhibitors were more effective than GLP-1RAs in reducing total death and hospitalization due to heart failure, whereas GLP-1RAs were more effective than SGLT2 inhibitors in reducing stroke. Both drugs shared similar efficacy in CV death, nonfatal MI, and kidney failure. ∗P < 0.05. Data adapted from Palmer et at (27) with permission. ASCVD = atherosclerotic cardiovascular disease; CKD = chronic kidney disease; CV = cardiovascular; HHF = hospitalization for heart failure; MI = myocardial infarction; other abbreviations as in Figures 1 and 2.
Prediction of ASCVD and HF in Patients With Risk Factors Alone
Patients with diabetes may present with risk factors alone without ASCVD or HF. In a recent nationwide survey from Taiwan, more than 60% of patients with diabetes had risk factors alone without any history of CV disease (79). For patients with risk factors alone, equations for prediction of future risk of ASCVD and HF are required for the consensus pathway. We considered several factors in our selection of risk equations: 1) the risk equations should be based on the same derivative cohorts that can predict 10-year risk of both ASCVD and HF; 2) the total risk of ASCVD, including coronary heart disease and stroke, should be included in the calculation; 3) the risk equations have been widely adopted in major treatment guidelines; and 4) the risk equations have web-based tools. Several risk equations have been developed specifically for diabetes population, including the UK Prospective Diabetes Study risk engine (80), the model from the Swedish National Diabetes Register (81), and an Australian cardiovascular risk equation (82). However, these prediction equations can predict the risk of coronary heart disease, but not the risks of stroke and HF. The Thrombolysis in Myocardial Infarction risk score can predict the risk of HF in patients with diabetes (83), but a risk score for ASCVD in patients with diabetes is lacking. Several prediction models were developed from general population, such as the Framingham Risk Score (84), the Prospective Cardiovascular Münster (PROCAM) score (85), the Pooled Cohort Equation (86), etc. The Framingham Risk Score and the PROCAM score can predict the risk of coronary heart disease, but not the risk of stroke (84,85). The Pooled Cohort Equation can predict 10-year risk of the first episode of ASCVD, including coronary heart disease and stroke, and has been widely accepted (86). It has been adopted by the recent U.S. lipid guidelines (87) and the US blood pressure guidelines (88). The Pooled Cohort Equation has been validated in patients with diabetes (86). A different formulation of Pooled Cohort Equation can also predict 10-year risk of the first incidence of HF (89), and has been validated in patients with diabetes as well (90). Web-based tools of the Pooled Cohort Equations are available for ASCVD and HF. The working group adopted the Pooled Cohort Equations in the present consensus to predict 10-year risks of ASCVD and HF in patients with risk factors alone (86,89). Table 6 presents the baseline characteristics that are required for these 2 risk equations. Figure 5 demonstrates 2 different scenarios for clinical applications of these 2 risk equations.
Table 6.
Baseline Characteristics for Pooled Cohort Equation to Predict 10-Year Risk of ASCVDa (86) and HFb (89)
| ASCVD | HF | |
|---|---|---|
| Sex | ✓ | ✓ |
| Age | ✓ | ✓ |
| Race | ✓ | ✓ |
| Total cholesterol | ✓ | ✓ |
| HDL cholesterol | ✓ | ✓ |
| Systolic blood pressure | ✓ | ✓ |
| Hypertension treatment | ✓ | ✓ |
| Smoker | ✓ | ✓ |
| Diabetes | ✓ | ✓ |
| Fasting glucose | ✓ | |
| Body mass index | ✓ | |
| QRS duration | ✓ |
ASCVD = atherosclerotic cardiovascular disease; HDL = high-density lipoprotein; HF = heart failure.
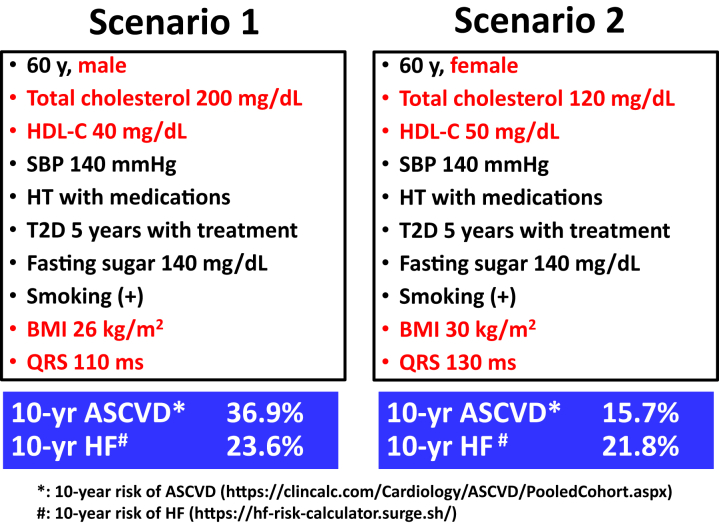
Figure 5.
Two Scenarios for Clinical Applications of Risk Equations
Pooled Cohort Equations are used to predict 10-year risk of atherosclerotic cardiovascular disease and 10-year risk of heart failure (86,89). In scenario 1, 10-year risk of ASCVD is higher than 10-year risk of HF. Therefore, GLP-1RA stands out as the first-line therapy, followed by SGLT2 inhibitor. In scenario 2, 10-year risk of HF is higher than 10-yr risk of ASCVD. Therefore, SGLT2 inhibitor is the first-line therapy, followed by GLP-1RA. BMI = body mass index (kg/m2); HDL-C = high-density lipoprotein cholesterol; HF = heart failure; HT = hypertension; SBP = systolic blood pressure; T2D = type 2 diabetes; other abbreviations as in Figures 1, 2, and 4.
The websites of the online calculators for these 2 risk equations are https://clincalc.com/Cardiology/ASCVD/PooledCohort.aspx for 10-year risk of ASCVD and https://hf-risk-calculator.surge.sh for 10-year risk of HF.
Adverse Events
Adverse events of these 2 classes of drugs should be taken into account when making the appropriate choice from the decision pathway. Table 7 presents the adverse events of these 2 classes of drugs (27). Except for 2 adverse events, the safety profiles of SGLT2 inhibitors were similar to GLP-1RAs. Genital tract infection was more common for SGLT2 inhibitors compared with placebo and GLP-1RAs. Severe gastrointestinal events were more common for GLP-1RAs compared with placebo, whereas data for SGLT2 inhibitors were limited. Pancreatitis was slightly less common for SGLT2 inhibitors compared with GLP-1RAs, albeit the absolute risk was very low. Patients with previous history of repeated genital tract infections should be educated to improve personal hygiene before starting SGLT2 inhibitors. In patients planned for initiation of GLP-1RAs, gastrointestinal adverse events should be educated to improve gastrointestinal tolerability.
Table 7.
Adverse Events of SGLT2 Inhibitors and GLP-1RAs
| SGLT2i vs Placebo | GLP-1RA vs Placebo | SGLT2i vs GLP-1RA | Anticipated Absolute Effects (95% CI), SGLT2i vs GLP-1RAs Over 5 Years per 1,000 Patients | |
|---|---|---|---|---|
| Severe hypoglycemia | 0.90 (0.70-1.16) | 0.92 (0.79-1.08) | 0.98 (0.73-1.31) | 0 (−6 to +7) |
| Blindness | 0.17 (0.01-4.07) | 1.00 (0.23-4.41) | 0.99 (0.57-1.73) | 0 (0 to +1) |
| Amputation | 1.14 (0.96-1.35) | 0.33 (0.01-8.18) | 3.43 (0.14-84.5) | 72 (−27 to +1,000) |
| Diabetic ketoacidosis | 1.04 (0.61-1.78) | 0.61 (0.33-1.11) | 1.71 (0.79-3.69) | 1 (0 to +3) |
| Genital tract infection | 3.50 (3.01-4.07) | 0.70 (0.34-1.44) | 5.00 (2.45-10.2) | 158 (+64 to +299) |
| Fournier gangrene | 0.56 (0.16-19.2) | NA | NA | NA |
| Severe gastrointestinal events | NA | 2.46 (1.22-4.97) | NA | NA |
| Pancreatic cancer | 1.77 (0.55-5.75) | 1.19 (0.78-1.81) | 1.49 (0.43-5.19) | +2 (−3 to +21) |
| Pancreatitis | 0.64 (0.39-1.05) | 1.18 (0.90-1.56) | 0.54 (0.31-0.94) | −3 (−4 to 0) |
Values are odds ratio (95% confidence interval). Adapted from Palmer et al (27) with permission.
GLP-1RA = glucagon-like peptide 1 receptor agonists; NA = not available; SGLT2i = sodium-glucose cotransporter 2 inhibitor.
Consensus Pathway
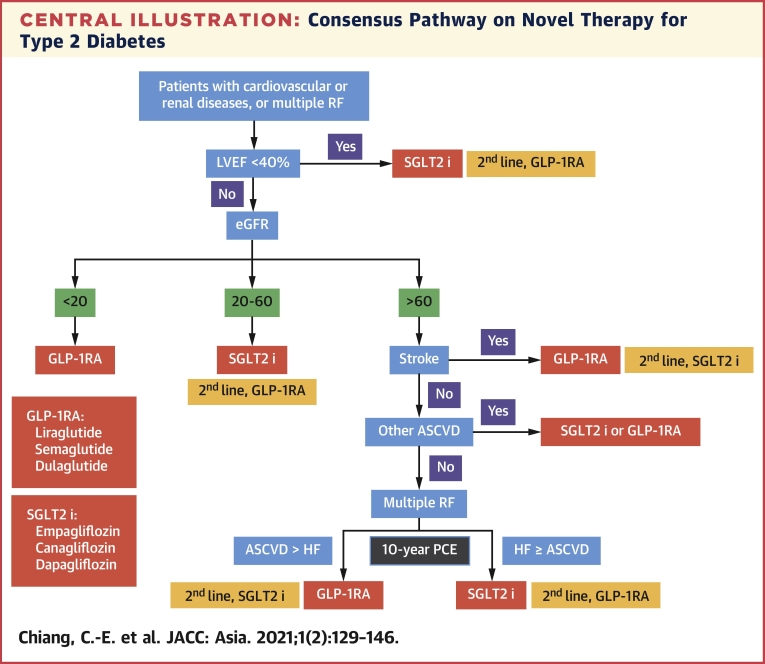
Patients with diabetes who have comorbid CV or renal diseases have higher risk of death and CV events compared with those who do not have these comorbidities. Therefore, the first step in the consensus pathway is to check for comorbid CV or renal diseases. As presented in Table 5, HF is associated with a higher risk of all-cause death than are CKD and ASCVD, and only SGLT2 inhibitors, not GLP-1RAs, can reduce CV death and hospitalization for HF. CKD ranks second in the consensus pathway, because it causes higher risk of death than ASCVD does. SGLT2 inhibitors, but not GLP-1RAs, reduce the composite renal end points, including eGFR deterioration and end-stage renal disease, in patients with CKD. Stroke should be the third consideration in the consensus pathway, where GLP-1RAs prevail.
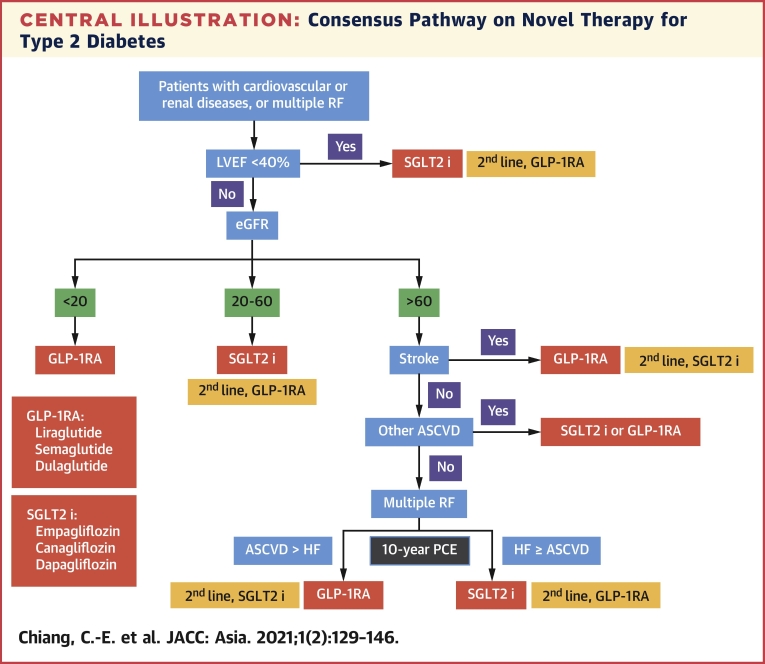
Following are the 5 steps in our consensus pathway.
Step 1: Patients with HF with reduced ejection fraction (<40%) should be identified. SGLT2 inhibitors are recommended first and GLP-1RAs second.
Step 2: Patients with CKD with an eGFR ≤60 mL/min/1.73 m2 should be next of interest. SGLT2 inhibitors are still the first recommendation and GLP-1RAs the second. When eGFR is <20 mL/min/1.73 m2, SGLT2 inhibitors are contraindicated and GLP-1RAs remain as the choice.
Step 3: Patients with a history of stroke should be the next group of interest, because only GLP-1RAs are effective in reducing stroke.
Step 4: Patients with other types of ASCVD, excluding stroke, should be recognized. Either SGLT2 inhibitors or GLP-1RAs can be used, because both are equally effective in reducing MACE in patients with preexisting ASCVD.
Step 5: Patients with multiple risk factors are the final group of interest. Ten-year risk of HF and ASCVD should be calculated with the use of the Pooled Cohort Equations. SGLT2 inhibitors are preferred if the 10-year risk of HF is greater than or equal to that of ASCVD, and GLP-1RAs remain as the second choice. The opposite leading role of GLP-1RAs emerges when the 10-year risk of ASCVD is greater than that of HF, and SGLT2 inhibitors step down. Central Illustration shows the consensus pathway.
Central Illustration.
Consensus Pathway on Novel Therapy for Type 2 Diabetes
SGLT2 inhibitors include those with proven efficacy, including empagliflozin, canagliflozin, and dapagliflozin. GLP-1RAs include those with proven efficacy, including liraglutide, semaglutide, and dulaglutide. ASCVD = atherosclerotic cardiovascular disease; eGFR = estimated glomerular filtration rate (mL/min/1.73 m2); GLP-1 RA = glucagon-like peptide 1 receptor agonist; HF = heart failure; LVEF = left ventricular ejection fraction; PCE = Pooled Cohort Equation; RF = risk factor; SGLT2 i = sodium-glucose cotransporter 2 inhibitor.
Glycemic Management
Standard-of-care therapies for glycemic management also should be considered in patients with type 2 diabetes. We suggest a target HbA1c of <7.0% for most adults (6,91). A more stringent HbA1c target of <6.5% may be considered if this can be achieved without significant hypoglycemia or other adverse events (91). Metformin should be added if the HbA1c target cannot be achieved with the use of SGLT2 inhibitors or GLP-1RAs (6,18). Other antidiabetic agents, such as pioglitazone, dipeptidyl peptidase 4 inhibitors, and sulfonylurea can be added after metformin when appropriate (6,18).
Management of Other CV Risk Factors
For individuals with diabetes and a higher CV risk (existing ASCVD or 10-year ASCVD risk ≥15% as defined by Pooled Cohort Equation) (86), intensive blood pressure and lipid control, together with antiplatelet therapies, also are required (4,92,93). Table 8 shows the treatment targets and standard of care therapies for patients with high CV risk. Details about these strategies are beyond the scope of the present consensus.
Table 8.
Management of Other Risk Factors in Patients With High Cardiovascular Riska
| Target | Standard-of-Care Therapies | |
|---|---|---|
| Blood pressure | <130/80 mm Hg | ACEI/ARB, CCB, thiazide, or in combination if required |
| LDL-C | <70 mg/dL | High-intensity statins, or in combination with ezetimibe and PCSK9 inhibitors if required |
| Antiplatelet | Aspirin, or clopidogrel if aspirin intolerance |
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; ASCVD = atherosclerotic cardiovascular disease; CCB = calcium channel blocker; LDL-C = low-density lipoprotein cholesterol; PCSK9 = proprotein convertase subtilisin/kexin type 9.
Existing ASCVD or 10-year ASCVD risk ≥15% (86).
Summary
The working group of the Taiwan Society of Cardiology has formulated a new stepwise consensus pathway. This consensus pathway provides health care workers a complement to guidelines in choosing novel antidiabetic agents in their daily practice. The consensus pathway was based on the most updated evidence from recent randomized control trials, meta-analyses, and network meta-analyses. Nevertheless, final decisions regarding use of these therapies may still need to be individualized and based on clinicians’ discretion.
Funding Support and Author Disclosures
This work was supported, in part, by grants from the Ministry of Health and Welfare (MOHW110-TDU-B-211-124001) and intramural grants from the Taipei Veterans General Hospital (V110C-181). D. C.-E.Chiang has received honoraria from AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, Merck Sharpe & Dohme, Novartis, Pfizer, and Sanofi. Dr T.-H. Chao has received honoraria from AstraZeneca, Boehringer Ingelheim, Bayer, Daiichi-Sankyo, Merck Sharp & Dohme, Novartis, Pfizer, Sanofi, and Orient EuroPharma. Dr T.-H. Lin has received honoraria from AstraZeneca, Boehringer Ingelheim, Tanabe, Merck Sharpe & Dohme, Novartis, Pfizer, Takeda, Sanofi, Novo Nordisk, and Lilly. Dr Y.-J. Wu has received honoraria from Johnson & Johnson, Pfizer, GlaxoSmithKline, AstraZeneca, and Boehringer Ingelheim. Dr K.-L. Wang has received honoraria from Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Novartis, and Pfizer. Dr H.-I. Yeh has received honoraria from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Lilly, Mitsubishi Tanabe, Novartis, Merck Sharpe & Dohme, Orient EuroPharma, Pfizer, and Sanofi. Dr Y.-H. Li has received honoraria from Pfizer, AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, Sanofi, Bayer, and Orient EuroPharma. Dr P.-Y. Liu has received honoraria from AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, Merck Sharpe & Dohme, Novartis, Pfizer, and Sanofi. Dr K.-C. Chang has received honoraria from AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, Bayer, Tanabe, Novartis, Pfizer, and Sanofi. Dr K.-G. Shyu has received honoraria from Pfizer, Daiichi-Sankyo, Bayer, AstraZeneca, Boehringer Ingelheim, Orient EuroPharma, and Eli Lilly. Dr J.-L. Huang has received honoraria from Abbott, Bayer, Biotronik, Boehringer Ingelheim, Daiichi-Sankyo, Medtronic, Novartis, and Pfizer. Dr C-D. Tsai has received honoraria from Pfizer, Daiichi-Sankyo, and Novartis. Dr M.-E. Liu has received honoraria from AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, Merck Sharpe & Dohme, Novartis, and Pfizer. Dr T.-F. Chao has received honoraria from Abbott, Bayer, Biotronik, Boehringer Ingelheim, Daiichi-Sankyo, Medtronic, Novartis, and Pfizer. Dr H.-M. Cheng has received honoraria from AstraZeneca, Pfizer, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Novartis, Servier, Eli Lilly, Sanofi, and Takeda; and has received grants for clinical research from Microlife and Intelligent Vision Technology. Dr P.-H. Chu has received honoraria from AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, Novartis, and Pfizer. Dr Y.-W. Wu has received honoraria from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Eli Lilly, Menarini, Mitsubishi Tanabe, Novartis, Merck Sharp & Dohme, Pfizer, and Sanofi. Dr W.-T. Lai has received honoraria from AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, Novartis, Pfizer, and Sanofi. Dr S.-J. Yeh has received honoraria from AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, Merck Sharp & Dohme, Novartis, Pfizer, Sanofi, and Tanabe. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Laurence S. Sperling, MD, served as Guest Associate Editor. Nathan Wong, PhD, as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental methods, figures, and a table, please see the online version of this paper.
Appendix
References
- 1.Chiang C.E., Wang T.D., Li Y.H., et al. 2010 guidelines of the Taiwan Society of Cardiology for the management of hypertension. J Formos Med Assoc. 2010;109:740–773. doi: 10.1016/S0929-6646(10)60120-9. [DOI] [PubMed] [Google Scholar]
- 2.Chiang C.E., Wang T.D., Ueng K.C., et al. 2015 Guidelines of the Taiwan Society of Cardiology and the Taiwan Hypertension Society for the Management of Hypertension. J Chin Med Assoc. 2015;78:1–47. doi: 10.1016/j.jcma.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Chiang C.E., Wu T.J., Ueng K.C., et al. 2016 Guidelines of the Taiwan Heart Rhythm Society and the Taiwan Society of Cardiology for the management of atrial fibrillation. J Formos Med Assoc. 2016;115:893–952. doi: 10.1016/j.jfma.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Chiang C.E., Wang T.D., Lin T.H., et al. The 2017 focused update of the guidelines of the Taiwan Society of Cardiology (TSOC) and the Taiwan Hypertension Society (THS) for the management of hypertension. Acta Cardiol Sin. 2017;33:213–225. doi: 10.6515/ACS20170421A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiang C.E., Lin S.Y., Lin T.H., et al. 2018 consensus of the Taiwan Society of Cardiology and the Diabetes Association of Republic of China (Taiwan) on the pharmacological management of patients with type 2 diabetes and cardiovascular diseases. J Chin Med Assoc. 2018;81:189–222. doi: 10.1016/j.jcma.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Chiang C.E., Ueng K.C., Chao T.H., et al. 2020 Consensus of Taiwan Society of Cardiology on the pharmacological management of patients with type 2 diabetes and cardiovascular diseases. J Chin Med Assoc. 2020;83:587–621. doi: 10.1097/JCMA.0000000000000359. [DOI] [PubMed] [Google Scholar]
- 7.NCD Risk Factor Collaboration Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513–1530. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatterjee S., Khunti K., Davies M.J. Type 2 diabetes. Lancet. 2017;389:2239–2251. doi: 10.1016/S0140-6736(17)30058-2. [DOI] [PubMed] [Google Scholar]
- 9.Turner R.C., Holman R.R., Stratton I.M., et al. UK Prospective Diabetes Study Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 10.Gerstein H.C., Miller M.E., Byington R.P., et al. ACCORD Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel A., MacMahon S., Chalmers J., et al. Advance Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 12.Duckworth W., Abraira C., Moritz T., et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 13.Turner R.C., Holman R.R., Stratton I.M., et al. UK Prospective Diabetes Study Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 14.Nissen S.E., Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 15.Home P.D., Pocock S.J., Beck-Nielsen H., et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373:2125–2135. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 16.Mahaffey K.W., Hafley G., Dickerson S., et al. Results of a reevaluation of cardiovascular outcomes in the RECORD trial. Am Heart J. 2013;166:240–249.e1. doi: 10.1016/j.ahj.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Holman R.R., Sourij H., Califf R.M. Cardiovascular outcome trials of glucose-lowering drugs or strategies in type 2 diabetes. Lancet. 2014;383:2008–2017. doi: 10.1016/S0140-6736(14)60794-7. [DOI] [PubMed] [Google Scholar]
- 18.Cosentino F., Grant P.J., Aboyans V., et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 19.Das S.R., Everett B.M., Birtcher K.K., et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76:1117–1145. doi: 10.1016/j.jacc.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cannon C.P., Pratley R., Dagogo-Jack S., et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383:1425–1435. doi: 10.1056/NEJMoa2004967. [DOI] [PubMed] [Google Scholar]
- 21.Packer M., Anker S.D., Butler J., et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 22.Heerspink H.J.L., Stefánsson B.V., Correa-Rotter R., et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 23.Bhatt D.L., Szarek M., Pitt B., et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2020;384:129–139. doi: 10.1056/NEJMoa2030186. [DOI] [PubMed] [Google Scholar]
- 24.Bhatt D.L., Szarek M., Steg P.G., et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2020;384:117–128. doi: 10.1056/NEJMoa2030183. [DOI] [PubMed] [Google Scholar]
- 25.Yamada T., Wakabayashi M., Bhalla A., et al. Cardiovascular and renal outcomes with SGLT-2 inhibitors versus GLP-1 receptor agonists in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and network meta-analysis. Cardiovasc Diabetol. 2021;20:14. doi: 10.1186/s12933-020-01197-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salah H.M., Al’Aref S.J., Khan M.S., et al. Effect of sodium-glucose cotransporter 2 inhibitors on cardiovascular and kidney outcomes—systematic review and meta-analysis of randomized placebo-controlled trials. Am Heart J. 2021;232:10–22. doi: 10.1016/j.ahj.2020.10.064. [DOI] [PubMed] [Google Scholar]
- 27.Palmer S.C., Tendal B., Mustafa R.A., et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2021;372:m4573. doi: 10.1136/bmj.m4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eng J., Kleinman W.A., Singh L., Singh G., Raufman J.P. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem. 1992;267:7402–7405. [PubMed] [Google Scholar]
- 29.Nauck M.A., Meier J.J. Management of endocrine disease: are all GLP-1 agonists equal in the treatment of type 2 diabetes? Eur J Endocrinol. 2019;181:R211–R234. doi: 10.1530/EJE-19-0566. [DOI] [PubMed] [Google Scholar]
- 30.Bajaj H.S., Al-Jabri B., Verma S. Glucagon-like peptide-1 receptor agonists and cardiovascular protection in type 2 diabetes: a pathophysiology-based review of clinical implications. Curr Opin Cardiol. 2018;33:665–675. doi: 10.1097/HCO.0000000000000562. [DOI] [PubMed] [Google Scholar]
- 31.Pyke C., Heller R.S., Kirk R.K., et al. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014;155:1280–1290. doi: 10.1210/en.2013-1934. [DOI] [PubMed] [Google Scholar]
- 32.Wallner M., Kolesnik E., Ablasser K., et al. Exenatide exerts a PKA-dependent positive inotropic effect in human atrial myocardium: GLP-1R mediated effects in human myocardium. J Mol Cell Cardiol. 2015;89:365–375. doi: 10.1016/j.yjmcc.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 33.Ban K., Noyan-Ashraf M.H., Hoefer J., Bolz S.S., Drucker D.J., Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340–2350. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- 34.Drucker D.J. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 2016;24:15–30. doi: 10.1016/j.cmet.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 35.Ehrenkranz J.R., Lewis N.G., Kahn C.R., Roth J. Phlorizin: a review. Diabetes Metab Res Rev. 2005;21:31–38. doi: 10.1002/dmrr.532. [DOI] [PubMed] [Google Scholar]
- 36.Bays H. From victim to ally: the kidney as an emerging target for the treatment of diabetes mellitus. Curr Med Res Opin. 2009;25:671–681. doi: 10.1185/03007990802710422. [DOI] [PubMed] [Google Scholar]
- 37.Ferrannini E., Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. 2012;8:495–502. doi: 10.1038/nrendo.2011.243. [DOI] [PubMed] [Google Scholar]
- 38.Bakris G.L., Fonseca V.A., Sharma K., Wright E.M. Renal sodium-glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int. 2009;75:1272–1277. doi: 10.1038/ki.2009.87. [DOI] [PubMed] [Google Scholar]
- 39.Kario K., Okada K., Kato M., et al. 24-Hour blood pressure-lowering effect of an SGLT-2 inhibitor in patients with diabetes and uncontrolled nocturnal hypertension: results from the randomized, placebo-controlled SACRA study. Circulation. 2018;139:2089–2097. doi: 10.1161/CIRCULATIONAHA.118.037076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verma S., Garg A., Yan A.T., et al. Effect of empagliflozin on left ventricular mass and diastolic function in individuals with diabetes: an important clue to the EMPA-REG OUTCOME trial? Diabetes Care. 2016;39:e212–e213. doi: 10.2337/dc16-1312. [DOI] [PubMed] [Google Scholar]
- 41.Baartscheer A., Schumacher C.A., Wüst R.C.I., et al. Empagliflozin decreases myocardial cytoplasmic Na+ through inhibition of the cardiac Na+/H+ exchanger in rats and rabbits. Diabetologia. 2017;60:568–573. doi: 10.1007/s00125-016-4134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verma S., McMurray J.J.V. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61:2108–2117. doi: 10.1007/s00125-018-4670-7. [DOI] [PubMed] [Google Scholar]
- 43.Packer M. Reconceptualization of the molecular mechanism by which sodium-glucose cotransporter 2 inhibitors reduce the risk of heart failure events. Circulation. 2019;140:443–445. doi: 10.1161/CIRCULATIONAHA.119.040909. [DOI] [PubMed] [Google Scholar]
- 44.Higgins J.P., Altman D.G., Gøtzsche P.C., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zelniker T.A., Wiviott S.D., Raz I., et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139:2022–2031. doi: 10.1161/CIRCULATIONAHA.118.038868. [DOI] [PubMed] [Google Scholar]
- 46.Zelniker T.A., Wiviott S.D., Raz I., et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 47.Kristensen S.L., Rørth R., Jhund P.S., et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776–785. doi: 10.1016/S2213-8587(19)30249-9. [DOI] [PubMed] [Google Scholar]
- 48.McGuire D.K., Shih W.J., Cosentino F., et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol. 2021;6:148–158. doi: 10.1001/jamacardio.2020.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scirica B.M., Bhatt D.L., Braunwald E., et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 50.Zannad F., Cannon C.P., Cushman W.C., et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067–2076. doi: 10.1016/S0140-6736(14)62225-X. [DOI] [PubMed] [Google Scholar]
- 51.Marso S.P., Daniels G.H., Brown-Frandsen K., et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hernandez A.F., Green J.B., Janmohamed S., et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (HARMONY Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519–1529. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 53.Marso S.P., Bain S.C., Consoli A., et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 54.Gerstein H.C., Colhoun H.M., Dagenais G.R., et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 55.Zinman B., Wanner C., Lachin J.M., et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 56.Neal B., Perkovic V., Mahaffey K.W., et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 57.Wiviott S.D., Raz I., Bonaca M.P., et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 58.Perkovic V., Jardine M.J., Neal B., et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 59.Zelniker T.A., Braunwald E. Clinical benefit of cardiorenal effects of sodium-glucose cotransporter 2 inhibitors: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:435–447. doi: 10.1016/j.jacc.2019.11.036. [DOI] [PubMed] [Google Scholar]
- 60.McMurray J.J.V., Solomon S.D., Inzucchi S.E., et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 61.Buse J.B., Wexler D.J., Tsapas A., et al. 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2020;63:221–228. doi: 10.1007/s00125-019-05039-w. [DOI] [PubMed] [Google Scholar]
- 62.American Diabetes Association 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2021. Diabetes Care. 2021;44:S111–S124. doi: 10.2337/dc21-S009. [DOI] [PubMed] [Google Scholar]
- 63.Zareini B., Blanche P., D’Souza M., et al. Type 2 diabetes mellitus and impact of heart failure on prognosis compared to other cardiovascular diseases. Circ Cardiovasc Qual Outcomes. 2020;13 doi: 10.1161/CIRCOUTCOMES.119.006260. [DOI] [PubMed] [Google Scholar]
- 64.Tonelli M., Muntner P., Lloyd A., et al. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet. 2012;380:807–814. doi: 10.1016/S0140-6736(12)60572-8. [DOI] [PubMed] [Google Scholar]
- 65.Lee M.M.Y., Ghouri N., McGuire D.K., Rutter M.K., Sattar N. Meta-analyses of results from randomized outcome trials comparing cardiovascular effects of SGLT2is and GLP-1RAs in Asian versus White patients with and without type 2 diabetes. Diabetes Care. 2021;44:1236–1241. doi: 10.2337/dc20-3007. [DOI] [PubMed] [Google Scholar]
- 66.Gerstein H.C., Hart R., Colhoun H.M., et al. The effect of dulaglutide on stroke: an exploratory analysis of the REWIND trial. Lancet Diabetes Endocrinol. 2020;8:106–114. doi: 10.1016/S2213-8587(19)30423-1. [DOI] [PubMed] [Google Scholar]
- 67.Mann J.F.E., Ørsted D.D., Brown-Frandsen K., et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377:839–848. doi: 10.1056/NEJMoa1616011. [DOI] [PubMed] [Google Scholar]
- 68.Gerstein H.C., Colhoun H.M., Dagenais G.R., et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019;394:131–138. doi: 10.1016/S0140-6736(19)31150-X. [DOI] [PubMed] [Google Scholar]
- 69.ACCORD Study Group. Cushman M.C., Evans G.W., Byington R.P., et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jamerson K., Weber M.A., Bakris G.L., et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417–2428. doi: 10.1056/NEJMoa0806182. [DOI] [PubMed] [Google Scholar]
- 71.Wanner C., Inzucchi S.E., Lachin J.M., et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 72.Mosenzon O., Wiviott S.D., Cahn A., et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7:606–617. doi: 10.1016/S2213-8587(19)30180-9. [DOI] [PubMed] [Google Scholar]
- 73.Wheeler D.C., Stefánsson B.V., Jongs N., et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and nondiabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021;9:22–31. doi: 10.1016/S2213-8587(20)30369-7. [DOI] [PubMed] [Google Scholar]
- 74.Butt J.H., Nicolau J.C., Verma S., et al. Efficacy and safety of dapagliflozin according to aetiology in heart failure with reduced ejection fraction: insights from the DAPA-HF trial. Eur J Heart Fail. 2021;23:601–613. doi: 10.1002/ejhf.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jhund P.S., Solomon S.D., Docherty K.F., et al. Efficacy of dapagliflozin on renal function and outcomes in patients with heart failure with reduced ejection fraction: results of DAPA-HF. Circulation. 2021;143:298–309. doi: 10.1161/CIRCULATIONAHA.120.050391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zannad F., Ferreira J.P., Pocock S.J., et al. Cardiac and kidney benefits of empagliflozin in heart failure across the spectrum of kidney function: insights from EMPEROR-Reduced. Circulation. 2021;143:310–321. doi: 10.1161/CIRCULATIONAHA.120.051685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pfeffer M.A., Claggett B., Diaz R., et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 78.McMurray J.J.V., Wheeler D.C., Stefánsson B.V., et al. Effect of dapagliflozin on clinical outcomes in patients with chronic kidney disease, with and without cardiovascular disease. Circulation. 2021;143:438–448. doi: 10.1161/CIRCULATIONAHA.120.051675. [DOI] [PubMed] [Google Scholar]
- 79.Lee C.H., Wu Y.L., Kuo J.F., Chen J.F., Chin M.C., Hung Y.J. Prevalence of diabetic macrovascular complications and related factors from 2005 to 2014 in Taiwan: a nationwide survey. J Formos Med Assoc. 2019;118(suppl 2):S96–S102. doi: 10.1016/j.jfma.2019.08.035. [DOI] [PubMed] [Google Scholar]
- 80.Stevens R.J., Kothari V., Adler A.I., Stratton I.M. The UKPDS risk engine: a model for the risk of coronary heart disease in type II diabetes (UKPDS 56) Clin Sci. 2001;101:671–679. [PubMed] [Google Scholar]
- 81.Zethelius B., Eliasson B., Eeg-Olofsson K., Svensson A.M., Gudbjörnsdottir S., Cederholm J. A new model for 5-year risk of cardiovascular disease in type 2 diabetes, from the Swedish National Diabetes Register (NDR) Diabetes Res Clin Pract. 2011;93:276–284. doi: 10.1016/j.diabres.2011.05.037. [DOI] [PubMed] [Google Scholar]
- 82.Davis W.A., Knuiman M.W., Davis T.M. An Australian cardiovascular risk equation for type 2 diabetes: the Fremantle Diabetes Study. Intern Med J. 2010;40:286–292. doi: 10.1111/j.1445-5994.2009.01958.x. [DOI] [PubMed] [Google Scholar]
- 83.Berg D.D., Wiviott S.D., Scirica B.M., et al. Heart failure risk stratification and efficacy of sodium-glucose cotransporter-2 inhibitors in patients with type 2 diabetes mellitus. Circulation. 2019;140:1569–1577. doi: 10.1161/CIRCULATIONAHA.119.042685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Anderson K.M., Odell P.M., Wilson P.W., Kannel W.B. Cardiovascular disease risk profiles. Am Heart J. 1991;121:293–298. doi: 10.1016/0002-8703(91)90861-b. [DOI] [PubMed] [Google Scholar]
- 85.Assmann G., Cullen P., Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the Prospective Cardiovascular Münster (PROCAM) study. Circulation. 2002;105:310–315. doi: 10.1161/hc0302.102575. [DOI] [PubMed] [Google Scholar]
- 86.Goff D.C., Jr., Lloyd-Jones D.M., Bennett G., et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grundy S.M., Stone N.J., Bailey A.L., et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285–e350. doi: 10.1016/j.jacc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 88.Whelton P.K., Carey R.M., Aronow W.S., et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–e248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 89.Khan S.S., Ning H., Shah S.J., et al. 10-year risk equations for incident heart failure in the general population. J Am Coll Cardiol. 2019;73:2388–2397. doi: 10.1016/j.jacc.2019.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bavishi A., Lloyd-Jones D.M., Ning H., et al. Systematic examination of a heart failure risk prediction tool: the pooled cohort equations to prevent heart failure. PLoS One. 2020;15 doi: 10.1371/journal.pone.0240567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.American Diabetes Association 6. Glycemic targets: standards of medical care in diabetes—2021. Diabetes Care. 2021;44:S73–S84. doi: 10.2337/dc21-S006. [DOI] [PubMed] [Google Scholar]
- 92.American Diabetes Association 10. Cardiovascular disease and risk management: standards of medical care in diabetes—2021. Diabetes Care. 2021;44:S125–S150. doi: 10.2337/dc21-S010. [DOI] [PubMed] [Google Scholar]
- 93.Li Y.H., Ueng K.C., Jeng J.S., et al. 2017 Taiwan lipid guidelines for high risk patients. J Formos Med Assoc. 2017;116:217–248. doi: 10.1016/j.jfma.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 94.Green J.B., Bethel M.A., Armstrong P.W., et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 95.White W.B., Cannon C.P., Heller S.R., et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 96.Rosenstock J., Kahn S.E., Johansen O.E., et al. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. JAMA. 2019;322:1155–1166. doi: 10.1001/jama.2019.13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rosenstock J., Perkovic V., Johansen O.E., et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321:69–79. doi: 10.1001/jama.2018.18269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Holman R.R., Bethel M.A., Mentz R.J., et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228–1239. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Husain M., Birkenfeld A.L., Donsmark M., et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841–851. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.