Abstract
Background
Despite a potential role of hemoglobin in atherosclerosis, data on coronary plaque volume changes (PVC) related to serum hemoglobin levels are limited.
Objectives
The authors sought to evaluate coronary atherosclerotic plaque burden changes related to serum hemoglobin levels using serial coronary computed tomographic angiography (CCTA).
Methods
A total of 830 subjects (age 61 ± 10 years, 51.9% male) who underwent serial CCTA were analyzed. The median interscan period was 3.2 (IQR: 2.5-4.4) years. Quantitative assessment of coronary plaques was performed at both scans. All participants were stratified into 4 groups based on the quartile of baseline hemoglobin levels. Annualized total PVC (mm3/year) was defined as total PVC divided by the interscan period.
Results
Baseline total plaque volume (mm3) was not different among all groups (group I [lowest]: 34.1 [IQR: 0.0-127.4] vs group II: 28.8 [IQR: 0.0-123.0] vs group III: 49.9 [IQR: 5.6-135.0] vs group IV [highest]: 34.3 [IQR: 0.0-130.7]; P = 0.235). During follow-up, serum hemoglobin level changes (Δ hemoglobin; per 1 g/dL) was related to annualized total PVC (β = −0.114) in overall participants (P < 0.05). After adjusting for age, sex, traditional risk factors, baseline hemoglobin and creatinine levels, baseline total plaque volume, and the use of aspirin, beta-blocker, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, and statin, Δ hemoglobin significantly affected annualized total PVC in only the composite of groups I and II (β = −2.401; P = 0.004).
Conclusions
Serial CCTA findings suggest that Δ hemoglobin has an independent effect on coronary atherosclerosis. This effect might be influenced by baseline hemoglobin levels. (Progression of Atherosclerotic Plaque Determined by Computed Tomographic Angiography Imaging [PARADIGM]; NCT02803411)
Key Words: atherosclerosis, coronary computed tomography angiography, hemoglobin
Abbreviations and Acronyms: Δ hemoglobin, hemoglobin level changes; CCTA, coronary computed tomographic angiography; CV, cardiovascular; PVC, plaque volume changes
Central Illustration
Low levels of hemoglobin are associated with an increased risk of cardiovascular (CV) disease.1 Moreover, it is an independent predictor for adverse clinical outcomes, especially in patients with established CV disease.2, 3, 4, 5, 6 The change of hemoglobin levels could affect the progression of atherosclerosis, considering its significant role in inflammatory mediators.7,8 However, little is known regarding the relation of serum hemoglobin level changes (Δ hemoglobin) with coronary atherosclerosis according to baseline hemoglobin status. Recently, coronary computed tomographic angiography (CCTA) has been established as a useful noninvasive imaging tool with a strong predictive value for major CV events.9, 10, 11, 12 The present study from the PARADIGM (Progression of Atherosclerotic Plaque Determined by Computed Tomographic Angiography Imaging) registry aimed to evaluate the association between Δ hemoglobin and coronary plaque volume change (PVC), focusing on baseline hemoglobin levels using serial CCTA.
Methods
Study design and populations
The study design of PARADIGM was previously described in detail.13 Briefly, the PARADIGM is a prospective, international, and multicenter observational registry designed to evaluate associations between clinical variables and coronary atherosclerotic change using serial CCTA. Between 2003 and 2015, a total of 2,252 consecutive subjects underwent serial CCTA at 13 centers in 7 countries. Among these subjects, 830 with available hemoglobin data at both baseline and follow-up CCTA were included in the present study. All blood samples were obtained at visit for each CCTA examination. All participants were categorized into 4 groups based on the quartile of baseline hemoglobin levels. Hemoglobin levels of <12.0 mg/dL was defined as low hemoglobin status. Hypertension, diabetes, hyperlipidemia, overweight or obesity, and current smoking were considered as traditional risk factors in the present study. The study protocol was approved by the institutional review boards of all participating centers.
Acquisition and interpretation of CCTA
The data acquisition and post-processing of CCTA were in accordance with the Society of Cardiovascular Computed Tomography guidelines.14,15 CCTA was conducted using a scanner with ≥64-detector rows in all centers. All datasets from each center were transferred to an offline workstation for analysis using the semiautomated plaque analysis software (QAngioCT Research Edition v2.1.9.1, Medis Medical Imaging Systems, Leiden, the Netherlands) with manual correction. Segments with a diameter >2 mm were evaluated using a modified 17-segment American Heart Association model.14,15 Regardless of the presence of atherosclerotic plaque, plaque volumes (mm3) of every coronary segment were obtained and summated to generate the total plaque volume on a per-patient level. Coronary plaques were further classified by composition according to the predefined intensity cutoffs in Hounsfield units (HU) for necrotic core (−30 to 30 HU), fibrofatty plaque (31 to 130 HU), fibrous plaque (131 to 350 HU), and calcified plaque (≥351 HU).16,17 To compare longitudinal CCTA images, all baseline and follow-up coronary segments were registered together with fiduciary landmarks, including branch vessel takeoffs or distance from ostia. PVC was defined as plaque volume at follow-up CCTA minus plaque volume at baseline CCTA on a per-patient level. Annualized PVC (mm3/year) was defined as PVC divided by interscan period. Plaque progression was defined as the difference in plaque volume between follow-up and baseline CCTA >0.
Statistical analysis
Continuous variables are expressed as mean ± SD or median (interquartile range). Categorical variables are presented as absolute values and proportions. The characteristics of participants across the quartile groups were compared using 1-way analysis of variance or the Kruskal-Wallis test for continuous variables, as appropriate, and the chi-square test was used for categorical variables. In subgroup analysis, continuous variables were compared using the independent t-test or Mann-Whitney U test, as appropriate, and the categorical variables were compared using a chi-square test. Univariate linear and logistic regression analyses were performed to evaluate the relation of clinical variables to annualized PVC and plaque progression, respectively. Multiple linear regression models were used to identify the association of Δ hemoglobin with annualized PVC according to the median baseline hemoglobin levels. In multiple regression models, all independent variables entered into the analyses irrespective of statistical significance in univariate regression analysis. All statistical analyses were performed using the Statistical Package for the Social Sciences version 19 (SPSS). A P value <0.05 was considered significant for all analyses.
Results
Baseline characteristics
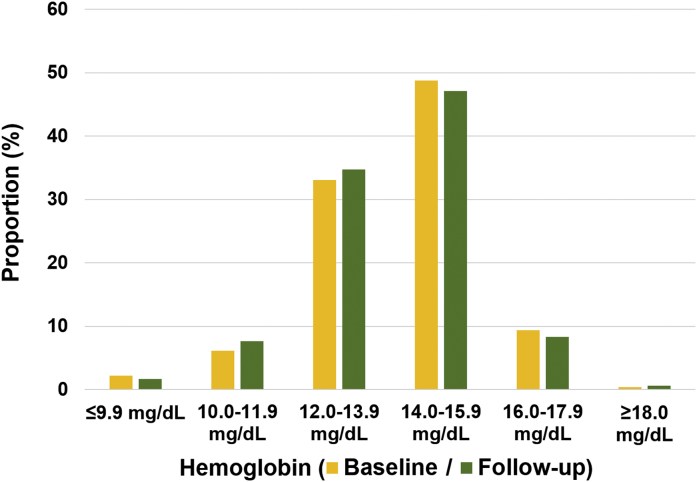
Table 1 shows the clinical characteristics of participants according to the quartile of baseline hemoglobin levels. The mean age was 61 ± 10 years, and 431 participants (51.9%) were male. Age decreased steadily with increasing quintiles of hemoglobin. The levels of total cholesterol, triglyceride, and low-density lipoprotein increased steadily with increasing quintiles of hemoglobin. A significant difference in the incidence of male sex and current smoker with an increasing tendency and in the incidence of hypertension, diabetes, and medications including aspirin, beta-blocker, and angiotensin-converting enzyme inhibitor or angiotensin receptor blocker with a decreasing tendency were noted. Baseline plaque characteristics across the quartile of hemoglobin levels at baseline are presented in Table 2. There were no significant differences in total plaque volume among the quartile groups. Regarding the plaque subtypes, there was significant difference in the fibrous-fatty and necrotic-core plaque volume among the quartile groups. The distribution of hemoglobin levels at baseline and follow-up is presented in Figure 1.
Table 1.
Clinical Characteristics
| Quartiles of Baseline Hemoglobin |
P Value | ||||
|---|---|---|---|---|---|
| I (Lowest) (5.5−13.2 g/dL) (n = 210) | II (13.3−14.3 g/dL) (n = 223) | III (14.4−15.2 g/dL) (n = 205) | IV (Highest) (15.3−18.2 g/dL) (n = 192) | ||
| Age, y | 64.2 ± 9.7 | 61.9 ± 9.6 | 59.9 ± 8.9 | 56.1 ± 8.7 | <0001 |
| Male | 67 (31.9) | 99 (44.4) | 142 (69.3) | 123 (64.1) | <0.001 |
| Systolic blood pressure, mm Hg | 126.1 ± 16.1 | 124.5 ± 16.2 | 122.8 ± 15.4 | 125.1 ± 16.4 | 0.285 |
| Diastolic blood pressure, mm Hg | 75.5 ± 10.2 | 76.3 ± 10.3 | 76.3 ± 10.0 | 77.7 ± 11.6 | 0.288 |
| Body mass index, kg/m2 | 24.3 ± 3.1 | 24.5 ± 3.0 | 25.3 ± 3.2 | 25.6 ± 2.6 | <0.001 |
| Traditional risk factors | |||||
| Hypertension | 135 (65.2) | 135 (60.5) | 105 (51.5) | 95 (49.5) | 0.003 |
| Diabetes | 57 (27.5) | 44 (19.7) | 40 (19.6) | 28 (15.1) | 0.020 |
| Hyperlipidemia | 64 (30.8) | 74 (33.2) | 70 (34.3) | 62 (32.3) | 0.889 |
| Overweight or obesity | 81 (38.9) | 85 (38.6) | 102 (50.5) | 99 (53.8) | 0.002 |
| Current smoking | 16 (7.7) | 23 (10.3) | 53 (26.0) | 48 (25.0) | <0.001 |
| Family history of coronary artery disease | 55 (26.2) | 65 (29.1) | 66 (32.2) | 58 (30.2) | 0.597 |
| Cerebrovascular disease | 22 (10.7) | 17 (7.7) | 14 (6.9) | 10 (5.2) | 0.210 |
| Peripheral arterial disease | 6 (2.9) | 3 (1.4) | 3 (1.5) | 0 (0.0) | 0.115 |
| Medication | |||||
| Aspirin | 119 (56.9) | 99 (44.4) | 89 (43.4) | 77 (40.1) | 0.004 |
| Beta-blocker | 85 (40.7) | 67 (30.0) | 56 (27.3) | 51 (26.8) | 0.008 |
| ACE inhibitor/ARB | 87 (41.6) | 70 (31.4) | 61 (29.8) | 50 (26.3) | 0.007 |
| Statin | 98 (47.6) | 94 (42.9) | 80 (40.6) | 85 (45.0) | 0.539 |
| Laboratory | |||||
| Total cholesterol, mg/dL | 171.3 ± 37.8 | 186.2 ± 39.5 | 186.1 ± 39.5 | 188.3 ± 36.2 | <0.001 |
| Triglyceride, mg/dL | 125.5 ± 70.9 | 138.0 ± 93.9 | 140.6 ± 83.0 | 153.1 ± 80.6 | 0.013 |
| High-density lipoprotein cholesterol, mg/dL | 48.5 ± 13.4 | 49.5 ± 11.9 | 48.4 ± 12.9 | 47.8 ± 11.3 | 0.572 |
| Low-density lipoprotein cholesterol, mg/dL | 103.4 ± 32.1 | 114.0 ± 34.0 | 114.7 ± 34.9 | 115.8 ± 32.9 | 0.001 |
| Glucose, mg/dL | 109.3 ± 37.8 | 105.3 ± 30.4 | 109.1 ± 29.9 | 107.0 ± 28.7 | 0.526 |
| Creatinine, mg/dL | 1.1 ± 1.2 | 0.9 ± 0.2 | 1.0 ± 0.2 | 1.1 ± 0.2 | 0.043 |
| Hemoglobin, g/dL | 12.0 ± 1.5 | 13.8 ± 0.3 | 14.8 ± 0.3 | 16.0 ± 0.6 | <0.001 |
| Δ Hemoglobin, g/dL | 0.5 ± 1.7 | 0.0 ± 1.1 | −0.1 ± 1.0 | −0.6 ± 1.0 | <0.001 |
Values are mean ± SD or n (%).
Δ hemoglobin = hemoglobin level changes; ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker.
Table 2.
Baseline Plaque Characteristics
| Quartiles of Baseline Hemoglobin |
P Value | ||||
|---|---|---|---|---|---|
| I (Lowest) (5.5−13.2 g/dL) (n = 210) | II (13.3−14.3 g/dL) (n = 223) | III (14.4−15.2 g/dL) (n = 205) | IV (Highest) (15.3−18.2 g/dL) (n = 192) | ||
| Total, mm3 | 34.1 (0.0-127.4) | 28.8 (0.0-123.0) | 49.9 (5.6-135.0) | 34.3 (0.0-130.7) | 0.235 |
| Fibrous, mm3 | 15.9 (0.0-55.5) | 12.1 (0.0-51.1) | 23.2 (2.4-59.1) | 15.7 (0.0-56.2) | 0.118 |
| Fibrous-fatty, mm3 | 1.1 (0.0-13.5) | 0.9 (0.0-13.0) | 5.1 (0.0-22.5) | 3.3 (0.0-21.2) | 0.006 |
| Necrotic core, mm3 | 0.0 (0.0-0.5) | 0.0 (0.0-0.5) | 0.0 (0.0-1.4) | 0.0 (0.0-1.1) | 0.036 |
| Dense calcium, mm3 | 7.2 (0.0-46.2) | 4.3 (0.0-38.3) | 5.0 (0.0-33.6) | 3.1 (0.0-32.7) | 0.398 |
Values are median (interquartile range).
Figure 1.
Distribution of Hemoglobin at Baseline and Follow-Up
The proportion of categorical hemoglobin levels at baseline and follow-up is presented.
Association of clinical variables with annualized total PVC and plaque progression
The median interscan period was 3.2 (2.5 to 4.4) years. Univariate linear regression analysis showed that age, hypertension, diabetes, overweight or obesity, creatinine levels, and baseline plaque volume were positively related to annualized total PVC; however, Δ hemoglobin had an inverse relation with annualized total PVC (P < 0.05, respectively). In univariate logistic regression analysis, age, male sex, hypertension, diabetes, hyperlipidemia, overweight or obesity, baseline hemoglobin, and baseline plaque volume were associated with the increased risk of plaque progression; by contrast, Δ hemoglobin was associated with the decreased risk of plaque progression (P < 0.05, respectively) (Table 3). Results of subgroup analyses regarding the association of Δ hemoglobin with annualized total PVC and plaque progression are present in Table 4. In subgroups with age ≥65 years, female, and overweight or obesity and those without diabetes and hyperlipidemia, Δ hemoglobin had a significant association with both annualized total PVC and plaque progression (P < 0.05, respectively). Significant interaction for plaque progression related to Δ hemoglobin was observed between male and female groups.
Table 3.
Association of Clinical Variables With Annualized Total PVC and Plaque Progression
| Annualized Total PVC |
Plaque Progression |
||||
|---|---|---|---|---|---|
| β (95% CI) | SE | P Value | OR (95% CI) | P Value | |
| Age, per 1-y increase | 0.448 (0.277 to 0.620) | 0.087 | <0.001 | 1.031 (1.014 to 1.048) | <0.001 |
| Male | 2.523 (−0.847 to 5.894) | 1.717 | 0.142 | 1.513 (1.102 to 2.077) | 0.010 |
| Hypertension | 8.217 (4.846 to 11.588) | 1.717 | <0.001 | 1.778 (1.302 to 2.457) | <0.001 |
| Diabetes | 6.691 (2.530 to 10.851) | 2.120 | 0.002 | 1.557 (1.021 to 2.374) | 0.040 |
| Hyperlipidemia | 1.235 (−2.370 to 4.839) | 1.836 | 0.502 | 1.501 (1.056 to 2.134) | 0.024 |
| Overweight or obesity | 4.081 (0.652 to 7.511) | 1.747 | 0.020 | 1.470 (1.064 to 2.033) | 0.020 |
| Current smoking | 4.472 (−0.023 to 8.967) | 2.290 | 0.051 | 1.143 (0.743 to 1.757) | 0.544 |
| Creatinine, per 1 mg/dL increase | 4.263 (1.714 to 6.811) | 1.298 | 0.001 | 1.269 (0.788 to 2.043) | 0.328 |
| Hemoglobin, per 1 g/dL increase | 0.644 (−0.371 to 1.658) | 0.517 | 0.213 | 1.109 (1.012 to 1.215) | 0.027 |
| Δ Hemoglobin, per 1 g/dL increase | −2.173 (−3.459 to −0.887) | 0.655 | 0.001 | 0.868 (0.770 to 0.978) | 0.020 |
| Baseline total plaque volume, per-1 mm3 increase | 0.073 (0.064 to 0.082) | 0.005 | <0.001 | 1.010 (1.007 to 1.012) | <0.001 |
Δ hemoglobin = hemoglobin level changes; PVC = plaque volume changes.
Table 4.
Subgroup Analysis for the Association of Δ Hemoglobin (per 1 g/dL Increase) With Coronary Atherosclerotic Changes
| n | Annualized Total PVC |
Plaque Progression |
P for Interaction | ||||
|---|---|---|---|---|---|---|---|
| β (95% CI) | SE | P Value | OR (95% CI) | P Value | |||
| Age | |||||||
| <65 y | 517 | −0.279 (−1.721 to 1.164) | 0.734 | 0.704 | 0.950 (0.817 to 1.106) | 0.510 | 0.062 |
| ≥65 y | 313 | −4.666 (−7.018 to −2.314) | 1.195 | <0.001 | 0.746 (0.609 to 0.914) | 0.005 | |
| Sex | |||||||
| Female | 399 | −2.244 (−4.177 to −0.310) | 0.984 | 0.023 | 0.696 (0.575 to 0.843) | <0.001 | 0.003 |
| Male | 431 | −2.212 (−3.941 to −0.483) | 0.880 | 0.012 | 1.028 (0.864 to 1.223) | 0.755 | |
| Hypertension | |||||||
| No hypertension | 356 | −0.359 (−1.924 to 1.206) | 0.796 | 0.652 | 0.850 (0.708 to 1.020) | 0.080 | 0.757 |
| Hypertension | 470 | −3.196 (−5.051 to −1.341) | 0.944 | 0.001 | 0.883 (0.751 to 1.039) | 0.134 | |
| Diabetes | |||||||
| No diabetes | 656 | −1.974 (−3.361 to −0.586) | 0.707 | 0.005 | 0.859 (0.750 to 0.984) | 0.028 | 0.785 |
| Diabetes | 170 | −2.829 (−5.977 to 0.320) | 1.595 | 0.078 | 0.896 (0.686 to 1.170) | 0.418 | |
| Hyperlipidemia | |||||||
| No hyperlipidemia | 557 | −2.770 (−4.290 to −1.250) | 0.774 | <0.001 | 0.859 (0.747 to 0.988) | 0.033 | 0.667 |
| Hyperlipidemia | 270 | −0.658 (−3.096 to 1.781) | 1.239 | 0.596 | 0.914 (0.717 to 1.163) | 0.464 | |
| Overweight or obesity | |||||||
| No overweight or obesity | 447 | −1.101 (−2.537 to 0.335) | 0.731 | 0.133 | 0.915 (0.790 to 1.058) | 0.231 | 0.249 |
| Overweight or obesity | 367 | −3.928 (−6.345 to −1.511) | 1.229 | 0.002 | 0.788 (0.640 to 0.969) | 0.024 | |
| Current smoking | |||||||
| No current smoking | 688 | −2.672 (−4.022 to −1.321) | 0.688 | <0.001 | 0.868 (0.761 to 0.991) | 0.037 | 0.985 |
| Current smoking | 140 | −0.044 (−3.697 to 3.609) | 1.847 | 0.981 | 0.866 (0.652 to 1.149) | 0.319 | |
Abbreviations as in Table 3.
Association between Δ hemoglobin and annualized total PVC according to baseline hemoglobin levels
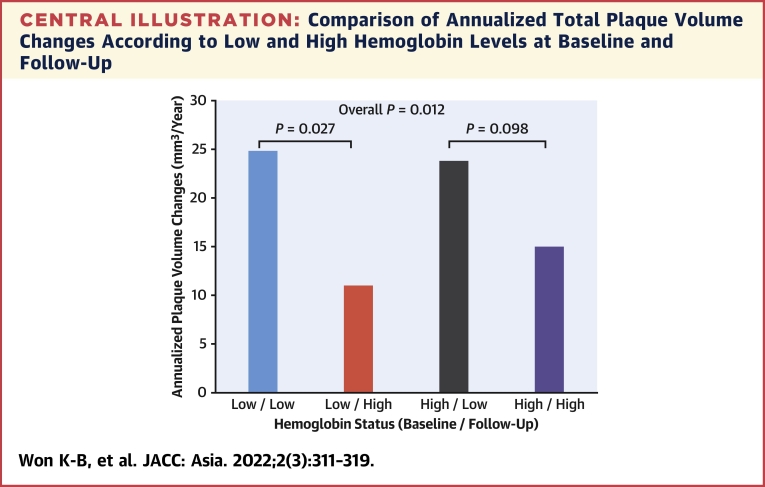
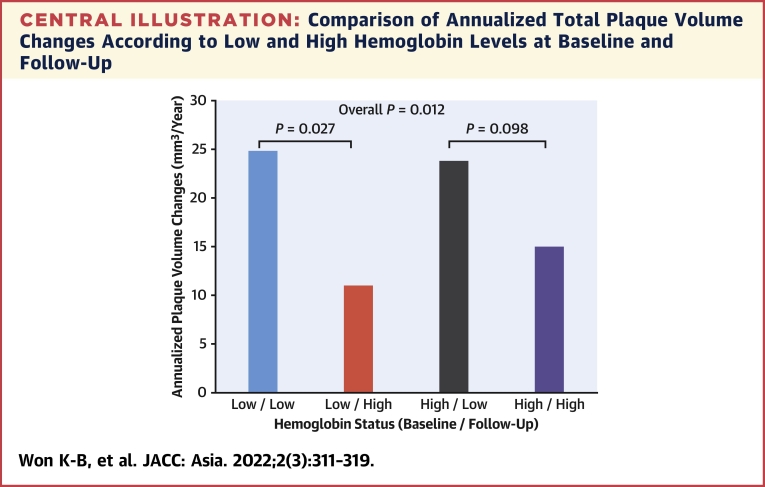
Among participants with a low hemoglobin level at baseline (n = 69), the annualized total PVC were significantly lower in subjects with a hemoglobin level of ≥12.0 g/dL (n = 34) than in those with a level <12.0 g/dL (n = 35) during follow-up (11.0 ± 16.2 vs 24.7 ± 31.7 mm3/year; P = 0.027). By contrast, the annualized total PVC were not significantly different between subjects with a hemoglobin level of ≥12.0 g/dL (n = 719) and those with a level <12.0 g/dL (n = 42) during follow-up among participants without a low hemoglobin level at baseline (n = 761) (15.0 ± 24.0 vs. 23.8 ± 33.0 mm3/year; p = 0.098) (Central Illustration).
Central Illustration.
Comparison of Annualized Total Plaque Volume Changes According to Low and High Hemoglobin Levels at Baseline and Follow-Up
The median interscan period was 3.2 (2.5 to 4.4) years. Among subjects with a low hemoglobin level (defined as a hemoglobin level of <12.0 g/dL) at baseline, the annualized total PVC were significantly higher in subjects with a low hemoglobin level than in those with a high level (defined as a hemoglobin level of ≥12.0 g/dL) at follow-up (24.7 ± 31.7 vs 11.0 ± 16.2 mm3/year; P = 0.027). By contrast, the annualized total PVC were not significantly different between low and high hemoglobin levels at follow-up in subjects with a high hemoglobin level at baseline (23.8 ± 33.0 vs 15.0 ± 24.0 mm3/year; P = 0.098). This finding might support the hypothesis that the association between Δ hemoglobin and coronary PVC is different according to baseline hemoglobin levels. PVC = plaque volume changes.
In multiple linear regression models, Δ hemoglobin was independently associated with a decrease in annualized total PVC in the composite of groups I and II. However, significant relation between Δ hemoglobin and annualized total PVC was not observed in the composite of groups III and IV after adjusting for other clinical factors (Table 5). Similarly, Δ hemoglobin, the percent change of hemoglobin, defined as Δ hemoglobin divided by baseline hemoglobin multiplied by 100, showed a consistent result according to the composite groups (Supplemental Table 1). Regarding the association of Δ hemoglobin with annualized PVC of plaque subtypes (Supplemental Table 2), Δ hemoglobin was inversely associated with annualized calcified PVC, especially in the composite of groups I and II. The results of receiver-operating characteristic analysis regarding the predictive value of Δ hemoglobin for plaque progression in each composite group are present in Supplemental Figure 1.
Table 5.
Association of Δ Hemoglobin (per-1 g/dL Increase) With Coronary Atherosclerotic Changes According to Baseline Hemoglobin
| Annualized Total PVC |
|||
|---|---|---|---|
| β (95% CI) | SE | P Value | |
| Overall | |||
| Model 1 | −2.173 (−3.459 to −0.887) | 0.655 | 0.001 |
| Model 2 | −1.999 (−3.270 to −0.728) | 0.647 | 0.002 |
| Model 3 | −1.642 (−2.924 to −0.360) | 0.653 | 0.012 |
| Model 4 | −1.707 (−2.975 to −0.439) | 0.646 | 0.008 |
| The composite of I and II | |||
| Model 1 | −2.045 (−3.669 to −0.421) | 0.826 | 0.014 |
| Model 2 | −2.251 (−3.903 to −0.599) | 0.841 | 0.008 |
| Model 3 | −2.226 (−3.852 to −0.600) | 0.827 | 0.007 |
| Model 4 | −2.401 (−4.053 to −0.749) | 0.840 | 0.004 |
| The composite of III and IV | |||
| Model 1 | −2.297 (−4.576 to −0.017) | 1.159 | 0.048 |
| Model 2 | −1.242 (−3.509 to 1.024) | 1.153 | 0.282 |
| Model 3 | −0.628 (−2.767 to 1.511) | 1.088 | 0.564 |
| Model 4 | −0.549 (−2.647 to 1.548) | 1.066 | 0.607 |
Model 1: Unadjusted. Model 2: Adjusted for age, sex, and traditional risk factors including hypertension, diabetes, hyperlipidemia, overweight or obesity, and current smoking. Model 3: Adjusted for age, sex, traditional risk factors including hypertension, diabetes, hyperlipidemia, overweight or obesity, current smoking, baseline hemoglobin levels, and baseline total plaque volume. Model 4: Adjusted for age, sex, traditional risk factors including hypertension, diabetes, hyperlipidemia, overweight or obesity, current smoking, baseline hemoglobin and creatinine levels, baseline total plaque volume, and the use of aspirin, beta-blocker, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, and statin.
Discussion
This prospective, international, and multicenter study using serial CCTA found that Δ hemoglobin was independently related to coronary atherosclerotic burden beyond traditional risk factors and baseline plaque burden. However, this relation could be strongly influenced by baseline hemoglobin levels.
Previous studies reported that low hemoglobin levels were strongly associated with adverse clinical outcomes in patients with high CV risk such as acute coronary syndrome2,4,6,18, 19, 20 or heart failure.3,5,21 Also, this association was consistently observed in patients with stable coronary artery disease.22,23 Recently, the CLARIFY (Prospective Observational Longitudinal Registry of Patients With Stable Coronary Artery Disease) study reported that low hemoglobin levels were an independent predictor of mortality, CV events, and major bleedings in 21,829 patients with stable coronary artery disease.23
Hypoxia caused by a low hemoglobin level can cause both hemodynamic and non-hemodynamic compensatory changes, such as increased erythropoietin production, decreased affinity of hemoglobin for oxygen, activation of the sympathetic nervous system, stimulation of the renin-angiotensin-aldosterone system, increased oxidative stress, and increased chronic inflammation.24,25 Tissue hypoxia and blood flow pattern changes resulting from these compensatory consequences may play a substantial role in atherosclerosis. Although a recent cross-sectional cohort study reported that serum hemoglobin levels had a significant association with atherosclerosis independent of metabolic abnormalities,26 there is a paucity of longitudinal data on the association between Δ hemoglobin and coronary atherosclerosis.
In the present study, the baseline characteristics of participants were different among the hemoglobin quartile groups. As in previous studies,20,27 participants with lower baseline hemoglobin levels were older and more likely to be female. Comorbidities were more prevalent in participants with lower hemoglobin levels, especially noticeable in hypertension and diabetes. It was challenging to properly evaluate the impact of newly developed low hemoglobin on the change in coronary plaque volume, because the incidence of new-onset low hemoglobin was very low (5.5%) among participants without low hemoglobin at baseline in this study. In addition, subgroup analysis showed the possibility of a sex difference in coronary plaque progression related to Δ hemoglobin. However, considering the inverse association of Δ hemoglobin with total PVC in participants with low to normal hemoglobin levels at baseline, the endeavor to elevate hemoglobin levels might be substantially attenuated with the progression of coronary atherosclerosis in this population.
A recent PARADIGM substudy has identified that quantitative atherosclerosis characterization is the most important factor for predicting coronary atherosclerosis progression beyond the clinical, laboratory, and qualitative coronary features.28 In the present study, total coronary plaque volume at baseline was not significantly different across the baseline hemoglobin quartile groups. This study assessed the association between Δ hemoglobin and annualized total PVC based on the median baseline hemoglobin cutoffs under the hypothesis that the relation of Δ hemoglobin with coronary PVC could be influenced by the baseline hemoglobin status. In accordance with our hypothesis, we identified that Δ hemoglobin had an independent association with annualized PVC in participants with baseline hemoglobin lower than the median value. This result implied that coronary PVC might be more affected by Δ hemoglobin in condition with low to normal hemoglobin levels. Although baseline hemoglobin level had a significant and positive association with the risk of coronary plaque progression in the overall population, this association was not observed after adjusting for confounding clinical factors according to median baseline hemoglobin cutoffs (Supplemental Table 3). Further longitudinal prospective studies with larger sample sizes should be required to confirm the effect of hemoglobin on the quantitative and qualitative aspects of coronary atherosclerosis and optimal hemoglobin levels for preventing coronary atherosclerosis progression.
Study limitations
First, only baseline and final hemoglobin data were available; therefore, longitudinal changes in hemoglobin level between CCTA scans could not be confirmed. Second, we could not identify the impact of new-onset low hemoglobin levels on coronary PVC because of its low incidence after the initial CCTA. This might be associated with stricter health care after the initial CCTA as reported in the previous PARADIGM study. Third, although we applied strict criteria for CCTA, atherosclerotic findings could be affected by the Hounsfield unit density, which is influenced by a myriad of factors. Fourth, we could not consider hematologic comorbidities and medical treatment for anemia because of a paucity of information in the present study. Fifth, the overall incidence of plaque progression was 75.2% in the present study. Considering that the aim of present study is to evaluate the changes of coronary atherosclerotic plaque burden related to serum hemoglobin levels, the definition of plaque progression has a limitation to identify this issue. Thus, we focused on analyzing the association of hemoglobin levels with coronary plaque burden changes beyond traditional risk factors using the annualized PVC. Finally, the major proportion of the overall PARADIGM registry was of East Asian ethnicity. In this PARADIGM substudy, 747 participants (90.0%) were East Asians (Supplemental Table 4); hence, this might limit the generalizability of the findings. However, the results of present study might provide substantial data for an Asian population. Despite these limitations of the present study, the study is unique in that we longitudinally assessed the association of Δ hemoglobin on coronary atherosclerosis using serial CCTA.
Conclusions
Based on serial CCTA findings, Δ hemoglobin has an independent effect on coronary atherosclerosis beyond traditional risk factors and baseline plaque burden. This effect might be influenced by baseline hemoglobin levels. The endeavor to avoid low hemoglobin status might be helpful for attenuating coronary atherosclerosis progression in clinical practice.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Based on serial coronary computed tomographic angiography findings, serum hemoglobin level changes (Δ hemoglobin) were inversely associated with annualized plaque volume changes. Interestingly, this association beyond traditional risk factors and baseline plaque burden was only observed in conditions with lower than median hemoglobin levels at baseline.
TRANSLATIONAL OUTLOOK: Our analysis highlights a different association of Δ hemoglobin with coronary atherosclerotic changes according to baseline serum hemoglobin levels. This finding suggests that the optimal serum hemoglobin levels for attenuating coronary atherosclerosis progression are present beyond traditional risk factors. Further prospective and randomized studies are necessary to confirm this issue.
Funding Support and Author Disclosures
This work was supported by the Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety) (Project Number: 1711139017). Dr. Leipsic has served as a consultant for and has stock options in HeartFlow and Circle Cardiovascular Imaging; has received grant support from GE Healthcare; and has received speaker fees from Philips. Dr. Samady has equity interest in Covanos. Dr. Berman receives software royalties from Cedars-Sinai Medical Center. Dr. Min has received funding from the Dalio Foundation, National Institutes of Health, and GE Healthcare. Dr. Min has served on scientific advisory boards for Arineta and GE Healthcare; and has an equity interest in Cleerly. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental figure and tables, please see the online version of this paper.
Appendix
References
- 1.Sarnak M.J., Tighiouart H., Manjunath G., et al. Anemia as a risk factor for cardiovascular disease in the Atherosclerosis Risk in Communities (ARIC) study. J Am Coll Cardiol. 2002;40:27–33. doi: 10.1016/s0735-1097(02)01938-1. [DOI] [PubMed] [Google Scholar]
- 2.van Straten A.H., Hamad M.A., van Zundert A.J., et al. Preoperative hemoglobin level as a predictor of survival after coronary artery bypass grafting: a comparison with the matched general population. Circulation. 2009;120:118–125. doi: 10.1161/CIRCULATIONAHA.109.854216. [DOI] [PubMed] [Google Scholar]
- 3.Mozaffarian D., Benjamin E.J., Go A.S., et al. Heart disease and stroke statistics--2016 update: a report from the American Heart Association. Circulation. 2016;133 doi: 10.1161/CIR.0000000000000350. e38–360. [DOI] [PubMed] [Google Scholar]
- 4.Lawler P.R., Filion K.B., Dourian T., et al. Anemia and mortality in acute coronary syndromes: a systematic review and meta-analysis. Am Heart J. 2013;165:143–153. doi: 10.1016/j.ahj.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 5.He S.W., Wang L.X. The impact of anemia on the prognosis of chronic heart failure: a meta-analysis and systemic review. Congest Heart Fail. 2009;15:123–130. doi: 10.1111/j.1751-7133.2008.00030.x. [DOI] [PubMed] [Google Scholar]
- 6.Wester A., Attar R., Mohammad M.A., et al. Impact of baseline anemia in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a prespecified analysis from the VALIDATE-SWEDEHEART trial. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss G., Goodnough L.T. Anemia of chronic disease. N Engl J Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 8.Libby P., Hansson G.K. Inflammation and immunity in diseases of the arterial tree: players and layers. Circ Res. 2015;116:307–311. doi: 10.1161/CIRCRESAHA.116.301313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meijboom W.B., Meijs M.F., Schuijf J.D., et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52:2135–2144. doi: 10.1016/j.jacc.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 10.Budoff M.J., Dowe D., Jollis J.G., et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52:1724–1732. doi: 10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 11.Chow B.J., Small G., Yam Y., et al. Incremental prognostic value of cardiac computed tomography in coronary artery disease using CONFIRM: COroNary Computed Tomography Angiography Evaluation for Clinical Outcomes: an InteRnational Multicenter registry. Circ Cardiovasc Imaging. 2011;4:463–472. doi: 10.1161/CIRCIMAGING.111.964155. [DOI] [PubMed] [Google Scholar]
- 12.Min J.K., Dunning A., Lin F.Y., et al. Age- and sex related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol. 2011;58:849–860. doi: 10.1016/j.jacc.2011.02.074. [DOI] [PubMed] [Google Scholar]
- 13.Lee S.E., Chang H.J., Rizvi A., et al. Rationale and design of the Progression of AtheRosclerotic PlAque DetermIned by Computed TomoGraphic Angiography IMaging (PARADIGM) registry: a comprehensive exploration of plaque progression and its impact on clinical outcomes from a multicenter serial coronary computed tomographic angiography study. Am Heart J. 2016;182:72–79. doi: 10.1016/j.ahj.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Leipsic J., Abbara S., Achenbach S., et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2014;8:342–358. doi: 10.1016/j.jcct.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Abbara S., Blanke P., Maroules C.D., et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: a report of the society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2016;10:435–449. doi: 10.1016/j.jcct.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Achenbach S., Moselewski F., Ropers D., et al. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography a segment-based comparison with intravascular ultrasound. Circulation. 2004;109:14–17. doi: 10.1161/01.CIR.0000111517.69230.0F. [DOI] [PubMed] [Google Scholar]
- 17.de Graaf M.A., Broersen A., Kitslaar P.H., et al. Automatic quantification and characterization of coronary atherosclerosis with computed tomography coronary angiography: cross-correlation with intravascular ultrasound virtual histology. Int J Cardiovasc Imaging. 2013;29:1177–1190. doi: 10.1007/s10554-013-0194-x. [DOI] [PubMed] [Google Scholar]
- 18.Mamas M.A., Kwok C.S., Kontopantelis E., et al. Relationship between anemia and mortality outcomes in a national acute coronary syndrome cohort: insights from the UK Myocardial Ischemia National Audit Project Registry. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moghaddam N., Wong G.C., Cairns J.A., et al. Association of anemia with outcomes among ST-segment-elevation myocardial infarction patients receiving primary percutaneous coronary intervention. Circ Cardiovasc Interv. 2018;11 doi: 10.1161/CIRCINTERVENTIONS.118.007175. [DOI] [PubMed] [Google Scholar]
- 20.Guedeney P., Sorrentino S., Claessen B., et al. The link between anemia and adverse outcomes in patients with acute coronary syndrome. Expert Rev Cardiovasc Ther. 2019;17:151–159. doi: 10.1080/14779072.2019.1575729. [DOI] [PubMed] [Google Scholar]
- 21.Gupta K., Kalra R., Rajapreyar I., et al. Anemia, mortality, and hospitalizations in heart failure with a preserved ejection fraction (from the TOPCAT trial) Am J Cardiol. 2020;125:1347–1354. doi: 10.1016/j.amjcard.2020.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muzzarelli S., Pfisterer M. Anemia as independent predictor of major events in elderly patients with chronic angina. Am Heart J. 2006;152:991–996. doi: 10.1016/j.ahj.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Kalra P.R., Greenlaw N., Ferrari R., et al. Hemoglobin and change in hemoglobin status predict mortality, cardiovascular events, and bleeding in stable coronary artery disease. Am J Med. 2017;130:720–730. doi: 10.1016/j.amjmed.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Metivier F., Marchais S.J., Guerin A.P., et al. Pathophysiology of anaemia: focus on the heart and blood vessels. Nephrol Dial Transplant. 2000;15(suppl 3):14–18. doi: 10.1093/oxfordjournals.ndt.a027970. [DOI] [PubMed] [Google Scholar]
- 25.Anand I.S., Chandrashekhar Y., Wander G.S., et al. Endothelium-derived relaxing factor is important in mediating the high output state in chronic severe anemia. J Am Coll Cardiol. 1995;25:1402–1407. doi: 10.1016/0735-1097(95)00007-Q. [DOI] [PubMed] [Google Scholar]
- 26.Choi Y., Won K.B., Kang H.H., et al. Association of serum hemoglobin level with the risk of carotid plaque beyond metabolic abnormalities among asymptomatic adults without major adverse clinical events: a cross-sectional cohort study. BMC Cardiovasc Disord. 2021;21:35. doi: 10.1186/s12872-021-01852-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pilgrim T., Rothenbuhler M., Kalesan B., et al. Additive effect of anemia and renal impairment on long-term outcome after percutaneous coronary intervention. PLoS One. 2014;9 doi: 10.1371/journal.pone.0114846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han D., Kolli K.K., Al'Aref S.J., et al. Machine learning framework to identify individuals at risk of rapid progression of coronary atherosclerosis: from the PARADIGM registry. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.013958. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.