Abstract
Background
Clinical advantages of sutureless rapid-deployment (RD) aortic valve replacement (AVR) for severe aortic valve stenosis (AS) have not been elucidated compared with surgical (SAVR) or transcatheter (TAVR) aortic valve replacement.
Objectives
This study sought to investigate comparative effectiveness and safety of RD-AVR compared with SAVR and TAVR in a prospective cohort of patients with severe AS.
Methods
The primary outcome was a composite of death, stroke, or rehospitalization at 12 months. Propensity score matching was used to assemble a cohort of patients with similar baseline characteristics.
Results
Among 1,020 eligible patients, 107 (10.5%) underwent RD-AVR, 437 (42.8%) underwent SAVR, and 476 (46.7%) underwent TAVR. In the matched cohorts of RD-AVR and SAVR (n = 107), the incidence of primary composite outcome at 12 months was similar between the 2 groups (8.0% vs 10.8%, respectively; hazard ratio [HR]: 0.74; 95% confidence interval [CI]: 0.30-1.84; P = 0.52). In the matched cohorts of RD and TAVR (n = 58), the incidence of primary composite outcome at 12 months did not statistically differ between the 2 groups (9.4% vs 16.2%, respectively; HR: 0.53; 95% CI: 0.18-1.57; P = 0.25).
Conclusions
In this propensity-matched cohort of patients who underwent AVR for severe AS, we did not detect significant differences in the rates of the primary composite of death, stroke, or rehospitalization at 12 months when comparing RD-AVR with SAVR and TAVR. Because the study was underpowered, the results should be considered as hypothesis generating highlighting the need for further research. (ASAN Medical Center Aortic Valve Replacement Registry [ASAN-AVR]; NCT03298178)
Key Words: heart valves, mortality, surgery, transcatheter aortic valve replacement
Abbreviations and Acronyms: AS, aortic valve stenosis; ASAN-AVR, ASAN Medical Center Aortic Valve Replacement; AVR, aortic valve replacement; GARY, German Aortic Valve Registry; RCT, randomized clinical trial; RD, rapid deployment; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement
Central Illustration
Aortic valve stenosis (AS) is associated with high mortality after the appearance of cardiac symptoms (1,2), and conventional surgical aortic valve replacement (SAVR) has been the standard of care for treatment of severe symptomatic AS (3). The increasing age of patients combined with increasing comorbidities has led to the development of less invasive procedures to minimize surgery-related risks and reduce operative times. Over the past decade, transcatheter aortic valve replacement (TAVR) has become an alternative to SAVR on the basis of clinical evidence from multiple randomized clinical trials (RCTs) (4, 5, 6, 7, 8, 9), and the application of this innovative treatment has been widely expanded to patients with low surgical risk. During the same time period, sutureless bioprosthetic valves (so called rapid-deployment [RD] valves) have been introduced as a new technology for surgery, leveraging the technology of intravascular stents and transcatheter valves (10).
Sutureless AVR has a practical advantage of minimal invasive incisions and rapid deployment leading to reduced aortic cross-clamp time and cardiopulmonary bypass time compared with standard SAVR (11). Also, compared with TAVR, it has a benefit of excision of the calcified aortic valve and annulus, minimizing concerns regarding long-term durability and possible thromboembolic complications (11). However, while there are some data supporting reduced operative times with RD-AVR (12,13), whether the use of this technology results in improved clinical outcomes remains uncertain. Also, unlike TAVR devices, RD valves have not been extensively tested in RCTs.
Until recently, data have been limited regarding head-to-head comparison of RD-AVR with conventional SAVR or less invasive TAVR. We therefore evaluated the efficacy and safety of valve replacement with the use of RD bioprosthetic valves compared with SAVR and TAVR in patients with symptomatic severe AS who were prospectively enrolled in the ASAN-AVR (ASAN Medical Center Aortic Valve Replacement) registry.
Methods
Study population and procedures
The ASAN-AVR registry is a prospective, single-center, real-world registry that includes all consecutive patients with severe symptomatic AS who have undergone surgical or percutaneous AVR at the Asan Medical Center (Seoul, Korea). The current study population included consecutive patients with symptomatic severe AS who underwent isolated SAVR with a bioprosthetic valve (either conventional or RD) or isolated TAVR from January 1, 2010, to December 31, 2018. Patients who received concomitant coronary artery bypass grafting or percutaneous coronary intervention, those who underwent additional procedures (mitral, tricuspid, or pulmonic valve replacement, repair, or valvulotomy; replacement of the ascending aorta; atrial ablation for arrhythmia; and other rare procedures), and those who underwent emergency or urgent SAVR or TAVR procedures were excluded. This study was approved by the Institutional Review Board of Asan Medical Center, and all of the patients provided written informed consent.
The decision for SAVR or TAVR procedure was made by the multidisciplinary heart team, composed of cardiovascular surgeons, interventional cardiologists, imaging cardiologists, and anesthesiologists (14). SAVR was performed through either median full sternotomy or a minimally invasive approach. Myocardial protection was performed with the antegrade infusion of cardioplegic solution under mild systemic hypothermia or normothermia. The choice of SAVR style (either conventional or RD) as well as the size and type of surgically implanted valve were at the discretion of the surgeon. After SAVR, patients received aspirin (100 mg daily) or warfarin for at least 3 to 6 months. The details of TAVR procedures, such as valve type, valve size, and access route, were determined by using contrast-enhanced gated computed tomography and transesophageal echocardiography. Balloon aortic valvuloplasty before and after TAVR was performed at the operator’s discretion. After the TAVR procedure, patients were prescribed dual antiplatelet therapy (DAPT) with aspirin (100 mg once daily) and clopidogrel (75 mg once daily) for at least 6 months (15).
Study end point and follow-up
The primary outcome of the study was a composite of death from any cause, stroke, or rehospitalization at 12 months after the procedure. Various secondary outcomes were also assessed, including the rates of each component of the primary end point and postprocedural other major adverse events (ie, life-threatening or surgery-requiring bleeding, acute kidney injury requiring renal replacement therapy, new permanent pacemaker insertion, new-onset atrial fibrillation, and moderate or severe paravalvular regurgitation) at 30 days and at 12 months. All study end points were defined according to the criteria of the Valve Academic Research Consortium 2 (16). All stroke events were confirmed by a trained neurologist or stroke specialist. Rehospitalization was defined as any hospitalization related to the procedure, the valve, or heart failure. All components of the primary and secondary clinical outcomes were adjudicated by an independent group of clinicians who were unaware of the treatment arms.
Baseline clinical, laboratory, echocardiographic, and computed tomographic data as well as procedural and clinical follow-up data were collected with the use of a dedicated electronic case report form. Clinical follow-up was performed via clinical visit and/or telephone interview at 1, 6, and 12 months and then every 6 months thereafter. At each time of follow-up contact, data pertaining to patients’ clinical status and occurrence of any adverse clinical events were collected. If necessary, referring cardiologists, general practitioners, and patients were contacted as necessary to obtain further information.
Statistical analysis
The primary purpose of the study was to evaluate the effectiveness and safety outcomes of RD-AVR compared with conventional SAVR and TAVR. Given the differences in the baseline characteristics among eligible participants in the treatment groups, propensity score matching was used to identify a cohort of patients with similar baseline characteristics (17). In each cohort for comparison (RD-AVR vs SAVR and RD-AVR vs TAVR), the propensity score is a conditional probability of having a particular exposure (RD) given a set of baseline measured covariates. The propensity score was estimated with the use of a nonparsimonious logistic regression model, with the treatment group of RD as the dependent variable and the baseline characteristics outlined in Table 1 as covariates. Matching was performed with the use of a 1:1 matching protocol without replacement (greedy-matching algorithm), with a caliper width equal to 0.2 of the standard deviation of the logit of the propensity score. Balance between the groups was assessed by calculating standardized differences, for which a difference of <0.10 was considered to indicate good balance (18, 19, 20). The comparative risks of efficacy and safety outcomes were compared with the use of Cox proportional hazard regression models with robust standard errors that accounted for the clustering of matched pairs to preserve the benefit of matching. Kaplan-Meier survival curves were estimated in each matched cohort, and the survival curves were compared according to methods appropriate for matched data (21). As sensitivity analyses, we also performed multivariable Cox regression models for comparisons of RD-AVR vs SAVR and TAVR.
Table 1.
Characteristics of the Patients at Baseline
| RD-AVR (n = 107) | SAVR (n = 437) | TAVR (n = 476) |
P Value |
|||
|---|---|---|---|---|---|---|
| Overall Comparison | RD-AVR vs SAVR | RD-AVR vs TAVR | ||||
| Age, y | 70.3 ± 7.2 | 72.73 ± 6.4 | 79.1 ± 5.1 | <0.001 | <0.001 | <0.001 |
| Male | 57 (53.3) | 234 (53.6) | 232 (48.7) | 0.32 | >0.99 | 0.79 |
| Body mass index, kg/m2 | 25.0 ± 3.6 | 24.6 ± 3.3 | 24.0 ± 3.3 | 0.002 | 0.34 | 0.006 |
| STS score, %a | 2.1 ± 1.6 | 2.1 ± 1.2 | 4.0 ± 2.8 | <0.001 | >0.99 | <0.001 |
| NYHA III or IV | 29 (27.1) | 80 (18.3) | 206 (43.3) | <0.001 | 0.08 | 0.004 |
| Diabetes mellitus | 30 (28.0) | 104 (23.8) | 151 (31.7) | 0.03 | 0.72 | 0.91 |
| Hypertension | 75 (70.1) | 281 (64.3) | 380 (79.8) | <0.001 | 0.52 | 0.06 |
| Current smoker | 8 (7.5) | 44 (10.1) | 42 (8.8) | 0.65 | 0.83 | >0.99 |
| Hyperlipidemia | 68 (63.6) | 279 (63.8) | 280 (58.8) | 0.27 | >0.99 | 0.74 |
| Previous MI | 2 (1.9) | 2 (0.5) | 20 (4.2) | 0.001 | 0.35 | 0.80 |
| Previous PCI | 11 (10.3) | 30 (6.9) | 109 (22.9) | <0.001 | 0.46 | 0.007 |
| Previous stroke | 9 (8.4) | 36 (8.2) | 56 (11.8) | 0.18 | >0.99 | 0.64 |
| Peripheral vascular disease | 5 (4.7) | 7 (1.6) | 24 (5.0) | 0.02 | 0.13 | >0.99 |
| Chronic lung disease | 8 (7.5) | 76 (17.4) | 101 (21.2) | 0.003 | 0.02 | 0.002 |
| Renal failureb | 6 (5.6) | 16 (3.7) | 27 (5.7) | 0.34 | 0.72 | >0.99 |
| Atrial fibrillation | 1 (0.9) | 16 (3.7) | 66 (13.9) | <0.001 | 0.43 | <0.001 |
| Pulmonary hypertensionc | 12 (11.2) | 85 (19.5) | 123 (25.8) | 0.001 | 0.09 | 0.002 |
| Aortic valve area, cm2 | 0.62 ± 0.15 | 0.63 ± 0.16 | 0.61 ± 0.16 | 0.27 | 0.90 | 0.65 |
| Aortic valve gradient, mm Hg | 62.7 ± 19.0 | 64.4 ± 21.8 | 60.0 ± 21.4 | 0.009 | 0.63 | 0.33 |
| Bicuspid aortic valve | 43 (40.19) | 156 (35.7) | 56 (11.76) | <0.001 | 0.78 | <0.001 |
| LVEF, % | 61.7 ± 9.2 | 59.4 ± 11.5 | 58.5 ± 11.1 | 0.006 | 0.16 | 0.005 |
| Moderate or severe regurgitation | ||||||
| Aortic valve | 23 (21.5) | 100 (22.9) | 52 (10.9) | <0.001 | >0.99 | 0.006 |
| Mitral valve | 2 (1.9) | 28 (6.4) | 29 (6.1) | 0.18 | 0.13 | 0.16 |
| Tricuspid valve | 1 (0.9) | 7 (1.6) | 15 (3.2) | 0.18 | >0.99 | 0.66 |
Values are mean ± SD or n (%). Categoric variables were compared by means of chi-square test or Fisher exact test (with post hoc Bonferroni test) and continuous variables were compared by means of analysis of variance (with post hoc Dunnett test) or Kruskal-Wallis test (with post hoc Bonferroni test).
AVR = aortic valve replacement; CABG = coronary artery bypass graft; COPD = chronic obstructive pulmonary disease; LVEF = left ventricular ejection fraction; MI = myocardial infarction; NYHA = New York Heart Association functional class; PCI = percutaneous coronary intervention; RD = rapid-deployment; SAVR = surgical aortic valve replacement; TAVR = transcatheter aortic valve replacement.
Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) scores range from 0 to 100%, with higher scores indicating a greater risk of death within 30 days after the procedure. STS-PROM uses an algorithm that is based on the presence of coexisting illnesses to predict 30-day operative mortality. The STS-PROM score equals the predicted mortality expressed as a percentage.
Renal failure was defined as serum creatinine ≥2 mg/dL or on dialysis.
Pulmonary hypertension was indicated by pulmonary artery systolic pressure ≥50 mm Hg.
All reported P values were 2-sided, and P < 0.05 was considered to be significant for all tests. No adjustment for multiple testing was undertaken. Because of the potential for type I error due to multiple comparisons, all findings of this study should be interpreted as exploratory. All statistical analyses were performed with the use of SAS version 9.3 and R version 3.3.2 (R Foundation for Statistical Computing). SAS Proc MI was used for multiple imputation, Proc Phreg for the Cox regression model, and Proc logistics for propensity score calculation.
Results
Baseline characteristics
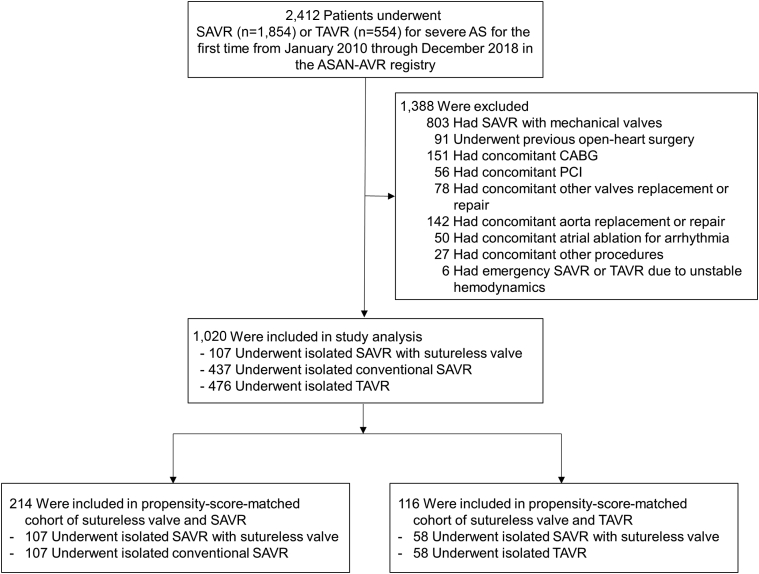
From January 1, 2010, through December 31, 2018, a total of 2,412 patients underwent SAVR or TAVR for treatment of symptomatic severe AS and were enrolled in the ASAN-AVR registry. Among them, we identified 1,020 patients with symptomatic severe AS who met our inclusion and exclusion criteria (Figure 1), of whom 107 (10.5%) underwent isolated RD-AVR, 437 (42.8%) underwent isolated conventional SAVR with bioprosthetic valve, and 476 (46.7%) underwent isolated TAVR. Baseline characteristics of these patients are summarized in Table 1. Before propensity score matching, there were considerable between-group differences in several of the baseline variables, especially between the RD-AVR and TAVR groups. Overall, patients receiving TAVR were significantly older and had substantially higher Society of Thoracic Surgeons Predicted Risk of Mortality scores than those receiving RD or conventional AVR. Compared with patients receiving SAVR, those undergoing RD-AVR were younger and less often had a history of chronic lung disease. Compared with TAVR patients, those undergoing RD-AVR were significantly younger, had a higher body mass index, less often presented with severe dyspnea, and had lower risk profiles of clinical and anatomic characteristics. The proportion of bicuspid valve was significantly higher in the surgical AVR group (either RD or conventional) than in the TAVR group.
Figure 1.
Study Flow Chart
AS = aortic valve stenosis; ASAN-AVR = ASAN Medical Center Aortic Valve Replacement; CABG = coronary artery bypass grafting; PCI = percutaneous coronary intervention; SAVR = surgical aortic-valve replacement; TAVR = transcatheter aortic-valve replacement.
Detailed information on operative and procedural characteristics is provided in Supplemental Table 1. Among 107 patients who received RD valves, 68 (63.6%) were received the balloon-expandable Intuity valve and 37 (34.6%) the self-expandable Perceival valve. Compared with conventional SAVR, the use of RD valves was associated with reduced procedure time, total aortic cross-clamp time, and total pump time, and they were substantially more often implanted through minimal-access sites other than conventional full sternotomy. Among 476 patients who underwent TAVR, 335 (70.4%) were received a balloon-expandable valve and 141 (29.6%) a self-expandable valve. Conscious sedation was used in 60.5% of the TAVR patients.
Postprocedural echocardiographic data are summarized in Supplemental Table 2. The indexed effective orifice area was smallest (0.84 cm2/m2) in the SAVR group, largest (0.96 cm2/m2) in the TAVR group, and intermediate (0.91 cm2/m2) in the RD-AVR group. Therefore, average values of postprocedure peak velocity or pressure gradient were intermediate in the RD-AVR group, lowest in the TAVR group, and highest in the SAVR group.
In the stratum of comparison between RD-AVR and conventional SAVR, we identified 107 pairs for comparing RD-AVR and SAVR (Figure 1). The C-statistic for the model was 0.75. After matching, the standardized differences were <0.10 for all variables, indicating only small differences between the 2 groups (Table 2). In the stratum of comparison between RD-AVR and TAVR, owing to substantial differences in baseline characteristics we identified only 58 pairs for comparing RD-AVR and TAVR (Figure 1). The C-statistic for the model was 0.89. After matching, the standardized differences were also <0.10 for most of variables (Table 2).
Table 2.
Baseline Characteristics of Patients, According to Treatment Group, After Propensity Score Matching
| Matched Cohorts of RD-AVR vs SAVR |
Matched Cohorts of RD-AVR vs TAVR |
|||||
|---|---|---|---|---|---|---|
| RD-AVR (n = 107) | SAVR (n = 107) | Standardized Differencea | RD-AVR (n = 58) | TAVR (n = 58) | Standardized Differencea | |
| Age, y | 70.3 ± 7.2 | 70.6 ± 8.3 | 0.04 | 74.9 ± 5.8 | 75.4 ± 6.6 | 0.09 |
| Male | 57 (53.27) | 56 (52.34) | 0.02 | 24 (41.38) | 25 (43.1) | 0.03 |
| Body mass index, kg/m2 | 25 ± 3.6 | 25.0 ± 2.9 | <0.001 | 24.7 ± 3.9 | 24.4 ± 2.9 | 0.10 |
| STS score,%b | 2.1 ± 1.6 | 2.1 ± 1.4 | 0.02 | 2.7 ± 1.8 | 2.6 ± 1.2 | 0.08 |
| NYHA functional class III or IV | 29 (27.1) | 30 (28.0) | 0.02 | 20 (34.5) | 18 (31.0) | 0.07 |
| Diabetes mellitus | 30 (28.0) | 32 (29.9) | 0.04 | 19 (32.8) | 18 (31.0) | 0.04 |
| Hypertension | 75 (70.1) | 79 (73.8) | 0 | 46 (79.3) | 45 (77.6) | 0.04 |
| Current smoker | 8 (7.5) | 7 (6.5) | 0.04 | 5 (8.6) | 6 (10.3) | 0.06 |
| Hyperlipidemia | 68 (63.6) | 64 (59.8) | 0.08 | 35 (60.3) | 36 (62.1) | 0.04 |
| Previous MI | 2 (1.9) | 1 (0.9) | 0.08 | 2 (3.5) | 1 (1.7) | 0.10 |
| Previous PCI | 11 (10.3) | 12 (11.2) | 0.03 | 6 (10.3) | 6 (10.3) | 0 |
| Previous stroke | 9 (8.4) | 7 (6.5) | 0.07 | 6 (10.3) | 8 (13.8) | 0.10 |
| Peripheral vascular disease | 5 (4.7) | 4 (3.7) | 0.05 | 2 (3.5) | 1 (1.7) | 0.10 |
| COPD | 8 (7.5) | 9 (8.4) | 0.03 | 7 (12.1) | 5 (8.6) | 0.11 |
| Renal failurec | 6 (5.6) | 4 (3.7) | 0 | 2 (3.5) | 2 (3.5) | 0 |
| Atrial fibrillation | 1 (0.9) | 1 (0.9) | 0 | 1 (1.7) | 2 (3.5) | 0.10 |
| Pulmonary hypertensiond | 12 (11.2) | 11 (10.3) | 0.03 | 7 (12.1) | 6 (10.3) | 0.05 |
| Aortic valve area, cm2 | 0.62 ± 0.15 | 0.62 ± 0.15 | 0.03 | 0.61 ± 0.13 | 0.60 ± 0.16 | 0.11 |
| Aortic valve gradient, mm Hg | 62.7 ± 19.0 | 63.3 ± 19.6 | 0.03 | 61.2 ± 18.7 | 63.8 ± 25.8 | 0.11 |
| Bicuspid aortic valve | 43 (40.2) | 44 (41.1) | 0.02 | 14 (24.1) | 15 (25.9) | 0.04 |
| LVEF, % | 61.7 ± 9.2 | 61.7 ± 8.3 | 0.004 | 60.8 ± 10.2 | 60.2 ± 10.2 | 0.07 |
| Moderate or severe regurgitation | ||||||
| Aortic valve | 23 (21.5) | 25 (23.4) | 0.04 | 13 (22.4) | 12 (20.7) | 0.04 |
| Mitral valve | 2 (1.9) | 3 (2.8) | 0.06 | 1 (1.7) | 2 (3.5) | 0.10 |
| Tricuspid valve | 1 (0.9) | 1 (0.9) | 0 | 1 (1.7) | 1 (1.7) | 0 |
Values are mean ± SD or n (%).
Abbreviations as in Table 1.
Balance between the groups was assessed by calculating standardized differences, for which a difference of <0.10 was considered to indicate good balance.
Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) scores range from 0 to 100%, with higher scores indicating a greater risk of death within 30 days after the procedure. STS-PROM uses an algorithm that is based on the presence of coexisting illnesses to predict 30-day operative mortality. The STS-PROM score equals the predicted mortality expressed as a percentage.
Renal failure was defined as serum creatinine ≥2 mg/dL or on dialysis.
Pulmonary hypertension was indicated by pulmonary artery systolic pressure ≥50 mm Hg.
Clinical outcomes
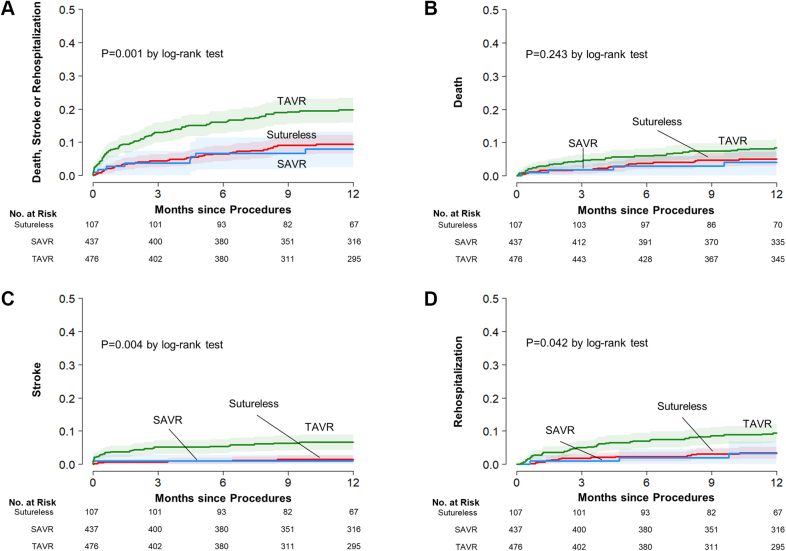
Observed rates of clinical events at 30 days and at 12 months are summarized in Table 3. At 30 days, unadjusted rates of composite of death, stroke, and rehospitalization were significantly higher in the TAVR group than in the RD-AVR and SAVR groups. After the procedures, the incidences of new permanent pacemaker insertion were higher in the TAVR group than in the RD-AVR and SAVR groups (9.1% in the TAVR group, 2.8% in the RD-AVR group, and 1.1% in the SAVR group; P < 0.001). However, the incidences of postprocedure new-onset atrial fibrillation was substantially higher in the surgery groups than in the TAVR group (29.9% in the RD-AVR group, 31.8% in the SAVR group, and 2.3% in the TAVR group; P < 0.001). At 12 months, complete follow-up was available for 98.6%. The observed rates of primary composite of death, stroke, rehospitalization at 12 months were 8.0% in the RD-AVR group, 9.3% in the SAVR group, and 20.0% in the TAVR group (Figure 2). The observed incidences of the individual components were also higher in the TAVR group.
Table 3.
Clinical Outcomes at 30 Days and at 12 Months
| RD-AVR (n = 107) | SAVR (n = 437) | TAVR (n = 476) | P Value | |
|---|---|---|---|---|
| 30-day outcomes | ||||
| Composite outcome of death, stroke, or rehospitalization | 3 (2.8) | 12 (2.8) | 38 (8.0) | 0.001 |
| Death | 1 (0.9) | 6 (1.4) | 13 (2.7) | 0.24 |
| Cardiac death | 1 (0.9) | 3 (0.7) | 8 (1.7) | 0.37 |
| Noncardiac death | 0 (0) | 3 (0.7) | 5 (1.1) | 0.51 |
| Stroke | 1 (0.9) | 3 (0.7) | 18 (3.8) | 0.004 |
| Disabling stroke | 0 (0) | 1 (0.2) | 7 (1.5) | 0.07 |
| Nondisabling stroke | 1 (0.9) | 2 (0.5) | 11 (2.3) | 0.05 |
| Death or stroke | 2 (1.9) | 9 (2.1) | 28 (5.9) | 0.006 |
| Rehospitalizationa | 1 (0.9) | 3 (0.7) | 13 (2.8) | 0.04 |
| Life-threatening or surgery-requiring bleeding | 6 (5.6) | 19 (4.3) | 34 (7.1) | 0.20 |
| Acute kidney injury requiring renal replacement therapy | 1 (0.9) | 14 (3.2) | 5 (1.1) | 0.05 |
| New permanent pacemaker | 3 (2.8) | 5 (1.1) | 43 (9.1) | <0.001 |
| New-onset atrial fibrillation | 32 (29.9) | 139 (31.8) | 11 (2.3) | <0.001 |
| Moderate or severe paravalvular regurgitation | 2 (1.9) | 0 (0) | 41 (8.6) | <0.001 |
| 12-month outcomes | ||||
| Primary composite outcome of death, stroke, or rehospitalization | 8 (8.0) | 39 (9.3) | 91 (20.0) | <0.001 |
| Death | 4 (4.1) | 21 (5.1) | 39 (8.7) | 0.054 |
| Cardiac death | 3 (3.1) | 12 (3.0) | 15 (3.4) | 0.91 |
| Noncardiac death | 1 (1) | 9 (2.2) | 24 (5.5) | 0.01 |
| Stroke | 1 (0.9) | 6 (1.5) | 30 (6.7) | <0.001 |
| Disabling stroke | 0 (0) | 2 (0.5) | 11 (2.4) | 0.02 |
| Nondisabling stroke | 1 (0.9) | 4 (1) | 19 (4.3) | 0.004 |
| Death or stroke | 5 (4.8) | 26 (6.2) | 60 (13.1) | <0.001 |
| Rehospitalizationa | 3 (3.3) | 14 (3.4) | 41 (9.5) | <0.001 |
| Life-threatening or surgery-requiring bleeding | 6 (5.6) | 19 (4.3) | 41 (8.8) | 0.03 |
| Acute kidney injury requiring renal replacement therapy | 2 (2.2) | 14 (3.2) | 5 (1.1) | 0.07 |
| New permanent pacemaker | 4 (3.8) | 5 (1.1) | 46 (9.8) | <0.001 |
| Moderate or severe paravalvular regurgitation | 6 (5.6) | 1 (0.2) | 51 (10.7) | <0.001 |
Values are n (%). Percentages were calculated by means of Kaplan-Meier estimates.
Abbreviations as in Table 1.
Valve-related or procedure-related and including heart failure.
Figure 2.
Unadjusted Cumulative Risks of the Study Outcomes
Event rates are shown with the use of Kaplan-Meier estimates of the rates of (A) the primary composite end point and the individual components of the primary end point, ie, (B) death from any cause, (C) stroke, and (D) rehospitalization, in patients who underwent rapid-deployment aortic-valve replacement (Sutureless/RD-AVR), surgical aortic valve replacement (SAVR), and transcatheter aortic valve replacement (TAVR).
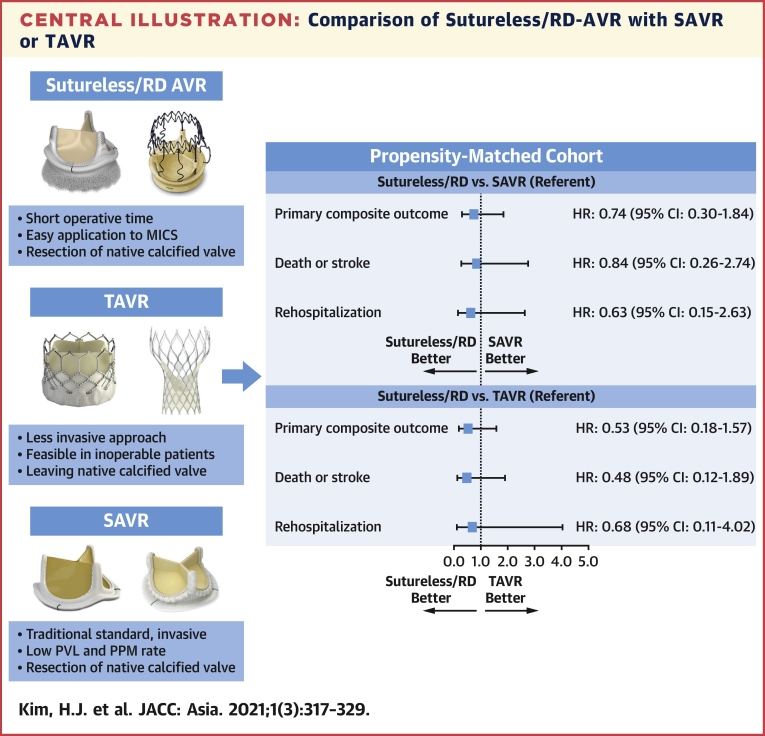
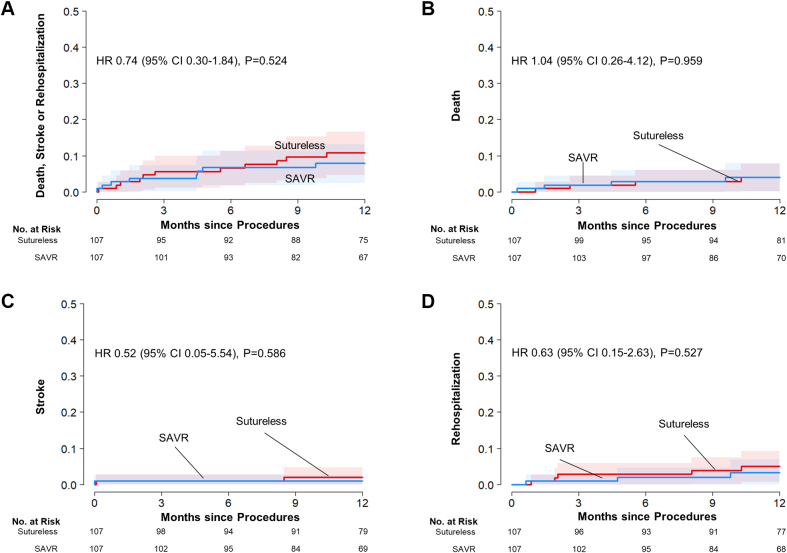
In the matched cohort of RD-AVR and SAVR, the rates of the composite of death, stroke, or rehospitalization at 12 months were similar between the 2 groups (8.0% in the RD group and 10.8% in the SAVR group; hazard ratio [HR]: 0.74; 95% confidence interval [CI]: 0.30-1.84; P = 0.52) (Table 4, Figure 3). The risks for the individual components of the primary outcome and other secondary outcomes were also similar between the 2 surgery groups. The incidence of moderate/severe paravalvular regurgitation was higher (but not statistically significantly, owing to small number of events) in the RD-AVR group than in the SAVR group (5.6% vs 0.9%; HR: 6.30; 95% CI: 0.73-54.19; P = 0.09).
Table 4.
Clinical Outcomes at 30 Days and at 12 Months in the Propensity Score–Matched Cohorts
| Matched Cohorts of AD-AVR vs SAVR |
Matched Cohorts of AD-AVR vs TAVR |
|||||||
|---|---|---|---|---|---|---|---|---|
| RD-AVR (n = 107) | SAVR (n = 107) | HR (95% CI)a | P Value | RD-AVR (n = 58) | TAVR (nN = 58) | HR (95% CI)a | P Value | |
| 30-day outcomes | ||||||||
| Composite outcome of death, stroke, or rehospitalization | 3 (2.8) | 2 (1.9) | 1.51 (0.26-8.96) | 0.65 | 1 (1.7) | 3 (5.2) | 0.32 (0.03-3.03) | 0.32 |
| Death | 1 (0.9) | 0 (0) | –b | 0 (0) | 1 (1.7) | –b | ||
| Cardiac death | 1 (0.9) | 0 (0) | –b | 0 (0) | 1 (1.7) | –b | ||
| Noncardiac death | 0 (0) | 0 (0) | –b | 0 (0) | 0 (0) | –b | ||
| Stroke | 1 (0.9) | 1 (0.9) | 1.00 (0.06-15.86) | >0.99 | 0 (0) | 2 (3.4) | –b | |
| Disabling stroke | 0 (0) | 0 (0) | –b | 0 (0) | 2 (3.4) | –b | ||
| Nondisabling stroke | 1 (0.9) | 1 (0.9) | 1.00 (0.06-15.86) | >0.99 | 0 (0) | 0 (0) | –b | |
| Death or stroke | 2 (1.9) | 1 (0.9) | 2.01 (0.18-21.9) | 0.57 | 0 (0) | 3 (5.2) | –b | |
| Rehospitalizationc | 1 (0.9) | 1 (0.9) | 1.02 (0.07-15.98) | 0.99 | 1 (1.7) | 0 (0) | –b | |
| Life-threatening or surgery-requiring bleeding | 6 (5.6) | 2 (1.9) | 3.04 (0.61-15.06) | 0.17 | 1 (1.7) | 5 (8.6) | 0.20 (0.02-1.69) | 0.14 |
| Acute kidney injury requiring renal replacement therapy | 1 (0.9) | 5 (4.7) | 0.20 (0.02-1.71) | 0.14 | 1 (1.7) | 0 (0) | –b | |
| New permanent pacemaker | 3 (2.8) | 1 (0.9) | 3.02 (0.31-29.13) | 0.34 | 1 (1.7) | 5 (8.6) | 0.19 (0.02-1.61) | 0.13 |
| New-onset atrial fibrillation | 32 (29.9) | 27 (25.2) | 1.26 (0.71-2.24) | 0.42 | 19 (32.8) | 1 (1.7) | 27.77 (3.96-194.96) | 0.001 |
| Moderate or severe paravalvular regurgitation | 2 (1.9) | 0 (0) | –b | 1 (1.7) | 2 (3.4) | 0.49 (0.04-5.73] | 0.577 | |
| 12-month outcomes | ||||||||
| Composite outcome of death, stroke, or rehospitalization | 8 (8.0) | 11 (10.8) | 0.74 (0.30-1.84) | 0.52 | 5 (9.4) | 9 (16.2) | 0.53 (0.18-1.57) | 0.25 |
| Death | 4 (4.1) | 4 (4) | 1.04 (0.26-4.12) | 0.96 | 3 (5.7) | 3 (5.6) | 0.99 (0.20-4.88) | 0.99 |
| Cardiac death | 3 (3.1) | 2 (2.1) | 1.59 (0.27-9.32) | 0.61 | 2 (3.9) | 3 (5.6) | 0.66 (0.11-3.91) | 0.65 |
| Noncardiac death | 1 (0.9) | 2 (2.1) | 0.50 (0.05-5.44) | 0.57 | 1 (1.9) | 0 (0) | –b | |
| Stroke | 1 (0.9) | 2 (2.1) | 0.52 (0.05-5.54) | 0.59 | 0 (0) | 4 (7.1) | –b | |
| Disabling stroke | 0 (0) | 0 (0) | –b | 0 (0) | 3 (5.3) | –b | ||
| Nondisabling stroke | 1 (0.9) | 2 (2.1) | 0.52 (0.05-5.54) | 0.59 | 0 (0) | 1 (1.8) | –b | |
| Death or stroke | 5 (4.8) | 6 (5.9) | 0.84 (0.26-2.74) | 0.78 | 3 (5.4) | 6 (10.8) | 0.48 (0.12-1.89) | 0.29 |
| Rehospitalizationc | 3 (3.3) | 5 (4.7) | 0.63 (0.15-2.63) | 0.53 | 2 (4.1) | 3 (5.5) | 0.68 (0.11-4.02) | 0.67 |
| Life-threatening or surgery-requiring bleeding | 6 (5.6) | 2 (1.9) | 3.04 (0.61-15.06) | 0.17 | 1 (1.7) | 6 (10.4) | 0.16 (0.02-1.36) | 0.09 |
| Acute kidney injury requiring renal replacement therapy | 2 (2.2) | 5 (4.7) | 0.40 (0.08-2.10) | 0.28 | 2 (4.1) | 0 (0) | –b | |
| New permanent pacemaker | 4 (3.8) | 1 (0.9) | 4.04 (0.45-36.31) | 0.21 | 1 (1.7) | 5 (8.6) | 0.19 (0.02-1.61) | 0.13 |
| Moderate or severe paravalvular regurgitation | 6 (5.6) | 1 (0.9) | 6.30 (0.73-54.19) | 0.09 | 4 (6.9) | 9 (15.5) | 0.40 (0.12-1.34) | 0.14 |
Values are mean ± SD or n (%).
CI = confidence interval; HR = hazard ratio; other abbreviations as in Table 1.
Hazard ratios are for the RD-AVR group compared with the SAVR or TAVR group (reference).
Not estimated.
Valve-related or procedure-related and including heart failure.
Figure 3.
Cumulative Risks of the Study Outcomes in the Matched Cohorts of Sutureless/RD-AVR and SAVR
Event rates are shown with the use of Kaplan-Meier estimates of the rates of (A) the primary composite end point and the individual components of the primary end point, ie, (B) death from any cause, (C) stroke, and (D) rehospitalization, in patients who underwent sutureless/AD-AVR and those who underwent SAVR. CI = confidence interval; HR = hazard ratio; other abbreviations as in Figure 1.
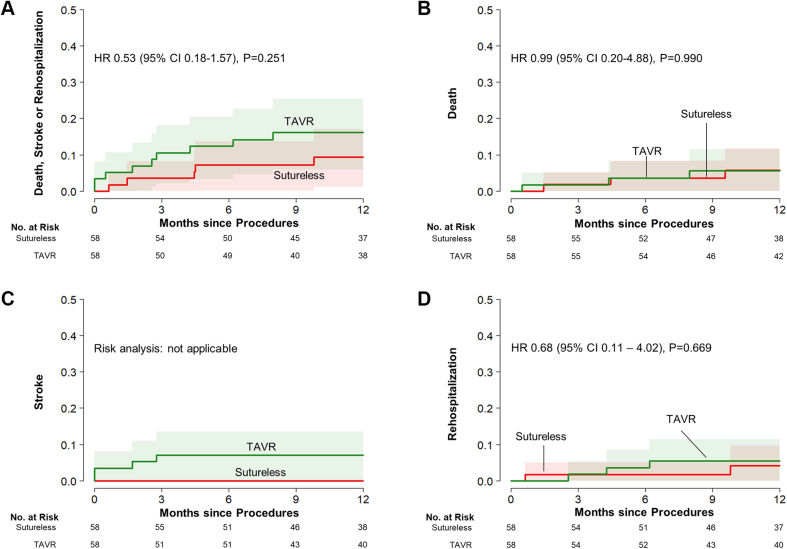
In the matched cohort of RD-AVR and TAVR, the rates of the composite of death, stroke, or rehospitalization did not significant differ between the 2 groups (9.4% in the RD-AVR group and 16.2% in the TAVR group; HR: 0.53; 95% CI: 0.18-1.57; P = 0.25) (Table 4, Figure 4). Also, the risks for the individual components of the primary outcome and other secondary outcomes were not significantly different between the RD-AVR group and the TAVR group.
Figure 4.
Cumulative Risks of the Study Outcomes in the Matched Cohorts of Sutureless/RD-AVR and TAVR
Event rates were shown by use of Kaplan-Meier estimates of the rates of (A)the primary composite end point and the individual components of the primary end point, ie, (B) death from any cause, (C) stroke, and (D) rehospitalization), in patients who underwent rapid deployment aortic-valve replacement (Sutureless/RD-AVR) and those who underwent transaortic aortic-valve replacement (SAVR). Abbreviations as in Figures 1 and 2.
When we performed sensitivity analyses including all patients with the use of multivariable Cox regression models, overall results for the study outcomes were consistent (Supplemental Table 3).
Discussion
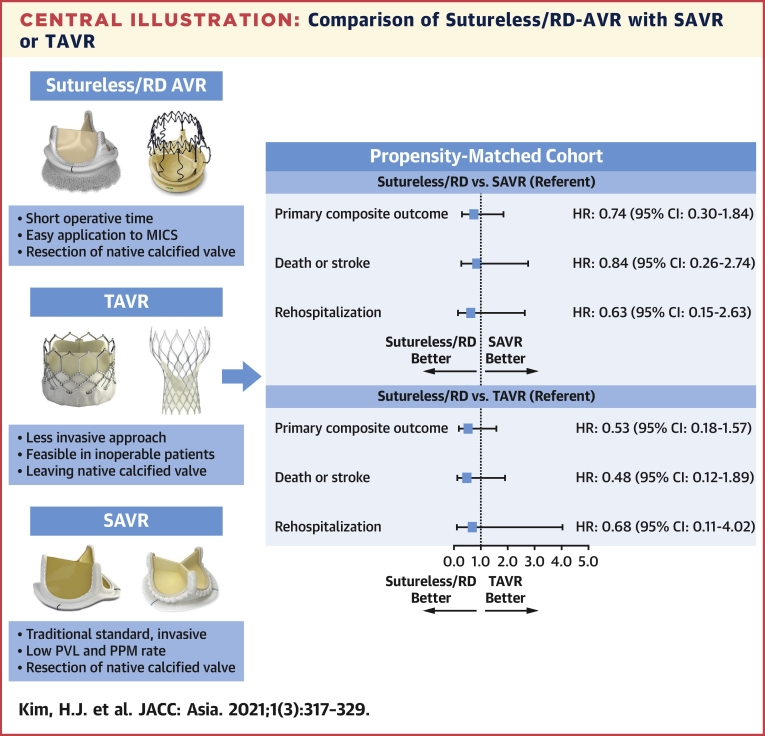
In this prospective cohort of patients with severe AS who underwent surgical or percutaneous AVR, we evaluated the effectiveness and safety of RD-AVR compared with conventional SAVR and TAVR. The major findings are that: 1) compared with SAVR, RD-AVR was associated with significantly reduced operating time (ie, procedure time, total aortic cross-clamp time, and total pump time) through more minimally invasive approaches; 2) the RD valve has intermediate levels of postprocedure hemodynamic measurements (indexed effective orifice area, peak velocity or pressure gradient), which was inferior to those of TAVR and superior to those SAVR; and 3) there was no statistically significant between-group difference with respect to the primary composite of death, stroke, or rehospitalization at 12 months in each propensity score–matched cohort of RD-AVR with SAVR or TAVR (Central Illustration).
Central Illustration.
Comparison of Sutureless/RD-AVR with SAVR or TAVR
Adjusted HRs (95% CIs) were derived from the propensity score–matched cohorts of RD-AVR with SAVR or TAVR. AVR = aortic valve replacement; RD = rapid-deployment; MICS = minimally invasive cardiac surgery; PPM = permanent pacemaker; PVL = paravalvular leak; SAVR = surgical aortic valve replacement; TAVR = transcatheter aortic valve replacement.
The technology of RD-AVR is novel and evolving, and it is still undetermined whether outcomes of RD-AVR are equivalent to those of SAVR. Also, specific patient indication for RD-AVR compared with conventional SAVR and less invasive TAVR is still unknown. Despite this, with a growing interest in minimally invasive procedures, RD-AVR is being adopted more and more. In this context, our study provides important insights into the effectiveness of RD-AVR compared with SAVR and TAVR, which could aid in decision making for the optimal AVR strategy in contemporary practice.
Several prior studies suggested that RD-AVR with Perceval and Intuity valves was associated with a less invasive approach and appeared to be safe and clinically feasible (13,22,23). Favorable findings were subsequently found in meta-analyses (24, 25, 26). However, in the large-sized GARY (German Aortic Valve Registry; NCT01165827) study, RD-AVR was associated with increased rates of disabling stroke, new pacemaker implantation, and elevated mean pressure gradient, but similar in-hospital mortality, compared with conventional SAVR (27). The small-sized (n = 100) RCT CADENCE-MIS (Edwards Intuity Valve System CADENCE-MIS, NCT02672553) showed that RD-AVR with Intuity valves was associated with significantly reduced cross-clamp time and better valvular hemodynamics than SAVR, but associated with higher rates of paravalvular leak rates (28). Some observational studies and meta-analyses comparing RD-AVR and TAVR showed conflicting results for hemodynamic performance and clinical outcomes (29, 30, 31). In a subsequent large-sized report from GARY (32), RD-AVR was associated with poorer in-hospital outcomes (higher rates of mortality, stroke, blood transfusion, and new renal dialysis) but a lower rate of new pacemaker compared with TAVR. In our study, we did not detect significant difference between RD-AVR vs conventional SAVR or TAVR regarding the rate of the primary composite of death, stroke, or rehospitalization at 12 months. Despite this, contemporary clinical evidence on RD-AVR might suffer from a limited number of patients, substantial selection biases or unmeasured confounders, as well as limited follow-up duration. Therefore, further research is needed in this area, particularly through large RCTs with longer-term follow-up, such as the PERSIST-AVR (Perceval Sutureless Implant Versus Standard Aortic Valve Replacement, NCT02673697) trial (33).
From the clinical viewpoint, specific indications for RD valves are not yet determined. Potential optimal indication of RD-AVR may include patients with a small and/or calcified aortic root, as well as patients requiring concomitant surgery as well as AVR (11). Some studies suggest that RD valves may be beneficial in patients with a small aortic root, owing to better hemodynamic performance and results in a lower incidence of patient-prothesis mismatch than with conventional SAVR (25,28). In addition, compared with SAVR, RD-AVR may be more beneficial in patients requiring combined cardiac surgery or complete aortic root replacement (27,34). Compared with TAVR, RD-AVR may be particularly beneficial in patients with very severe valve or annulus calcification, especially for severe calcified bicuspid valve (which was mostly excluded in pivotal RCTs of TAVR). Because we excluded patients with concomitant procedures for a fair comparison of isolated RD-AVR with isolated SAVR and TAVR, further studies are needed to adequately determine the optimal indication and positioning of RD valves in the contemporary clinical setting. Also, RD valves had an intermediate status of postprocedure hemodynamic measurements (indexed effective orifice area, peak velocity or pressure gradient), superior to those after SAVR, but inferior to those after TAVR. The impact of such hemodynamic findings on long-term clinical outcomes according to different AVR strategies should be evaluated via further research.
Interestingly, patients who underwent RD-AVR were substantially younger than those reported in studies from Western countries (23,27). Although the exact reasons for such substantial difference in patients’ ages are unclear, it might be explained in part by differences in operating surgeons’ or patients’ preference, surgical practice patterns, reimbursement policy, or race or ethnicity between our study population and those enrolled in other studies. Because the choice of RD-AVR at the time of SAVR was left to the discretion of the operating surgeons or the patients, we acknowledge that potential selection bias could exist. Therefore, the particulars of clinical practice in our institution, as well as the specific expertise of cardiac surgeons who performed the procedures, may differ from those of other institutions and practitioners, potentially limiting the reproducibility of our results in other clinical settings.
Study limitations
First, it has the inherent limitations of an observational study, and study findings should therefore be considered as hypothetical and hypothesis generating only. Second, the choice of AVR modalities was at the discretion of the treating physician and/or patient, which might be vulnerable to serious selection bias. It is possible that the operators selected the more favorable anatomic subsets of AS for RD-AVR, patients with highest-risk anatomic features were treated with conventional SAVR, and TAVR was performed mainly for older patients who were at higher surgical risk. Although robust propensity score matching was used to adjust for differences in baseline characteristics, unmeasured confounders still exist. Third, because the number of patients and clinical events was relatively low, this study was underpowered to draw any confirmative clinical message. Fourth, owing to the limited number of patients, we did not perform stratified analyses according to type of RV valves with different techniques and implantation mode (eg, Intuity vs Perceval). Finally, given that the follow-up duration of this study is limited, longer-term follow-up studies are required to address the long-term durability of RD-AVR.
Conclusions
In this prospective, registry-based, propensity score–matched cohort of patients undergoing AVR for severe AS, we did not detect significant differences in the rates of the primary composite outcome of death, stroke, or rehospitalization at 12 months between RD-AVR and SAVR or TAVR. However, because the study was underpowered, the results cannot be considered clinically directive and further RCTs are needed to adequately address the efficacy and safety of RD-AVR and its optimal indication in the contemporary clinical practice.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Data are limited regarding head-to-head comparison of RD-AVR with conventional SAVR or less invasive TAVR for treatment of severe symptomatic AS. In this prospective registry-based study of propensity score–matched patients who had undergone AVR for symptomatic severe AS, we did not detect significant differences in the rates of the primary composite outcome of death, stroke, or rehospitalization at 12 months between RD-AVR and conventional SAVR or TAVR.
TRANSLATIONAL OUTLOOK: Further randomized clinical trials are needed to adequately address the efficacy and safety of RD-AVR and its optimal indication in the contemporary clinical setting.
Funding Support and Author Disclosures
This study was partly supported by the CardioVascular Research Foundation (Seoul, South Korea) and the grant (2017IT1205) from the Asan Institute for Life Sciences, Asan Medical Center (Seoul, South Korea). The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Mao Chen, MD, served as Guest Associate Editor for this paper. D. Scott Lim, MD, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version of this paper.
Contributor Information
Duk-Woo Park, Email: dwpark@amc.seoul.kr.
Suk Jung Choo, Email: sjchoo@amc.seoul.kr.
Appendix
References
- 1.Ross J., Jr., Braunwald E. Aortic stenosis. Circulation. 1968;38:61–67. doi: 10.1161/01.cir.38.1s5.v-61. [DOI] [PubMed] [Google Scholar]
- 2.Turina J., Hess O., Sepulcri F., Krayenbuehl H.P. Spontaneous course of aortic valve disease. Eur Heart J. 1987;8:471–483. doi: 10.1093/oxfordjournals.eurheartj.a062307. [DOI] [PubMed] [Google Scholar]
- 3.Schwarz F., Baumann P., Manthey J., et al. The effect of aortic valve replacement on survival. Circulation. 1982;66:1105–1110. doi: 10.1161/01.cir.66.5.1105. [DOI] [PubMed] [Google Scholar]
- 4.Smith C.R., Leon M.B., Mack M.J., et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 5.Adams D.H., Popma J.J., Reardon M.J., et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790–1798. doi: 10.1056/NEJMoa1400590. [DOI] [PubMed] [Google Scholar]
- 6.Leon M.B., Smith C.R., Mack M.J., et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609–1620. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 7.Reardon M.J., Van Mieghem N.M., Popma J.J., et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321–1331. doi: 10.1056/NEJMoa1700456. [DOI] [PubMed] [Google Scholar]
- 8.Mack M.J., Leon M.B., Thourani V.H., et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 9.Popma J.J., Deeb G.M., Yakubov S.J., et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380:1706–1715. doi: 10.1056/NEJMoa1816885. [DOI] [PubMed] [Google Scholar]
- 10.Shrestha M., Khaladj N., Bara C., Hoeffler K., Hagl C., Haverich A. A staged approach toward interventional aortic valve implantation with a sutureless valve: initial human implants. Thorac Cardiovasc Surg. 2008;56:398–400. doi: 10.1055/s-2008-1038722. [DOI] [PubMed] [Google Scholar]
- 11.Bilkhu R., Borger M.A., Briffa N.P., Jahangiri M. Sutureless aortic valve prostheses. Heart. 2019;105:s16–s20. doi: 10.1136/heartjnl-2018-313513. [DOI] [PubMed] [Google Scholar]
- 12.Powell R., Pelletier M.P., Chu M.W.A., Bouchard D., Melvin K.N., Adams C. The Perceval sutureless aortic valve: review of outcomes, complications, and future direction. Innovations (Phila) 2017;12:155–173. doi: 10.1097/IMI.0000000000000372. [DOI] [PubMed] [Google Scholar]
- 13.Barnhart G.R., Accola K.D., Grossi E.A., et al. TRANSFORM (Multicenter Experience With Rapid Deployment Edwards Intuity Valve System for Aortic Valve Replacement) US clinical trial: performance of a rapid deployment aortic valve. J Thorac Cardiovasc Surg. 2017;153:241–251.e2. doi: 10.1016/j.jtcvs.2016.09.062. [DOI] [PubMed] [Google Scholar]
- 14.Oh J.K., Park S.J., Kim H.J., et al. Transcatheter versus surgical aortic valve replacement in low-risk, elderly patients with severe aortic stenosis. J Am Coll Cardiol. 2019;74:1514–1515. doi: 10.1016/j.jacc.2019.07.028. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura R.A., Otto C.M., Bonow R.O., et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2438–2488. doi: 10.1016/j.jacc.2014.02.537. [DOI] [PubMed] [Google Scholar]
- 16.Kappetein A.P., Head S.J., Genereux P., et al. Updated standardized end point definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol. 2012;60:1438–1454. doi: 10.1016/j.jacc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Rosenbaum P.R., Rubin D.B. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 18.Normand S.T., Landrum M.B., Guadagnoli E., et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 19.Bangalore S., Guo Y., Samadashvili Z., Blecker S., Xu J., Hannan E.L. Everolimus-eluting stents or bypass surgery for multivessel coronary disease. N Engl J Med. 2015;372:1213–1222. doi: 10.1056/NEJMoa1412168. [DOI] [PubMed] [Google Scholar]
- 20.Yun J.E., Kim Y.J., Park J.J., et al. Safety and effectiveness of contemporary P2Y12 inhibitors in an east Asian population with acute coronary syndrome: a nationwide population-based cohort study. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein J.P., Moeschberger M.L. Springer-Verlag; New York: 1997. Survival Analysis: Techniques for Censored and Truncated Data. [Google Scholar]
- 22.Shrestha M., Fischlein T., Meuris B., et al. European multicentre experience with the sutureless Perceval valve: clinical and haemodynamic outcomes up to 5 years in over 700 patients. Eur J Cardiothorac Surg. 2016;49:234–241. doi: 10.1093/ejcts/ezv040. [DOI] [PubMed] [Google Scholar]
- 23.Berretta P., Andreas M., Carrel T.P., et al. Minimally invasive aortic valve replacement with sutureless and rapid deployment valves: a report from an international registry (Sutureless and Rapid Deployment International Registry) Eur J Cardiothorac Surg. 2019;56:793–799. doi: 10.1093/ejcts/ezz055. [DOI] [PubMed] [Google Scholar]
- 24.Sohn S.H., Jang M.J., Hwang H.Y., Kim K.H. Rapid deployment or sutureless versus conventional bioprosthetic aortic valve replacement: a meta-analysis. J Thorac Cardiovasc Surg. 2018;155:2402–2412.e5. doi: 10.1016/j.jtcvs.2018.01.084. [DOI] [PubMed] [Google Scholar]
- 25.Meco M., Montisci A., Miceli A., et al. Sutureless perceval aortic valve versus conventional stented bioprostheses: meta-analysis of postoperative and midterm results in isolated aortic valve replacement. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.006091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lloyd D., Luc J.G.Y., Indja B.E., Leung V., Wang N., Phan K. Transcatheter, sutureless and conventional aortic-valve replacement: a network meta-analysis of 16,432 patients. J Thorac Dis. 2019;11:188–199. doi: 10.21037/jtd.2018.12.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ensminger S., Fujita B., Bauer T., et al. Rapid deployment versus conventional bioprosthetic valve replacement for aortic stenosis. J Am Coll Cardiol. 2018;71:1417–1428. doi: 10.1016/j.jacc.2018.01.065. [DOI] [PubMed] [Google Scholar]
- 28.Borger M.A., Dohmen P.M., Knosalla C., et al. Haemodynamic benefits of rapid deployment aortic valve replacement via a minimally invasive approach: 1-year results of a prospective multicentre randomized controlled trial. Eur J Cardiothorac Surg. 2016;50:713–720. doi: 10.1093/ejcts/ezw042. [DOI] [PubMed] [Google Scholar]
- 29.Kamperidis V., van Rosendael P.J., de Weger A., et al. Surgical sutureless and transcatheter aortic valves: hemodynamic performance and clinical outcomes in propensity score–matched high-risk populations with severe aortic stenosis. J Am Coll Cardiol Intv. 2015;8:670–677. doi: 10.1016/j.jcin.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 30.Meco M., Miceli A., Montisci A., et al. Sutureless aortic valve replacement versus transcatheter aortic valve implantation: a meta-analysis of comparative matched studies using propensity score matching. Interact Cardiovasc Thorac Surg. 2018;26:202–209. doi: 10.1093/icvts/ivx294. [DOI] [PubMed] [Google Scholar]
- 31.Takagi H., Umemoto T. Sutureless aortic valve replacement may improve early mortality compared with transcatheter aortic valve implantation: a meta-analysis of comparative studies. J Cardiol. 2016;67:504–512. doi: 10.1016/j.jjcc.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 32.Abdel-Wahab M., Fujita B., Frerker C., et al. Transcatheter versus rapid-deployment aortic valve replacement: a propensity-matched analysis from the German Aortic Valve Registry. J Am Coll Cardiol Intv. 2020;13:2642–2654. doi: 10.1016/j.jcin.2020.09.018. [DOI] [PubMed] [Google Scholar]
- 33.Lorusso R., Folliguet T., Shrestha M., et al. Sutureless versus stented bioprostheses for aortic valve replacement: the randomized PERSIST-AVR study design. Thorac Cardiovasc Surg. 2018 doi: 10.1055/s-0038-1675847. [DOI] [PubMed] [Google Scholar]
- 34.Akca F., Lam K., Ozdemir I., Tan E. Sutureless aortic valve replacement in a calcified homograft combined with mitral valve replacement. J Cardiothorac Surg. 2017;12:82. doi: 10.1186/s13019-017-0642-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.