Abstract
Background
The influence of baseline HbA1c levels on vein graft outcomes post coronary artery bypass grafting (CABG) remains unclear.
Objective
The purpose of this study was to assess the association between baseline HbA1c and 1-year vein graft patency, and the effects of antiplatelet therapy on the 1-year vein graft patency after CABG in patients with baseline HbA1c <6.5% vs ≥6.5%.
Methods
We examined the subgroups with baseline HbA1c <6.5% vs ≥6.5% from the DACAB trial (NCT02201771), in which 500 patients were randomly allocated to receive ticagrelor plus aspirin (T+A), ticagrelor alone (T), or aspirin alone (A) for 1 year after CABG. The primary outcome was the vein graft patency (FitzGibbon grade A) at 1 year.
Results
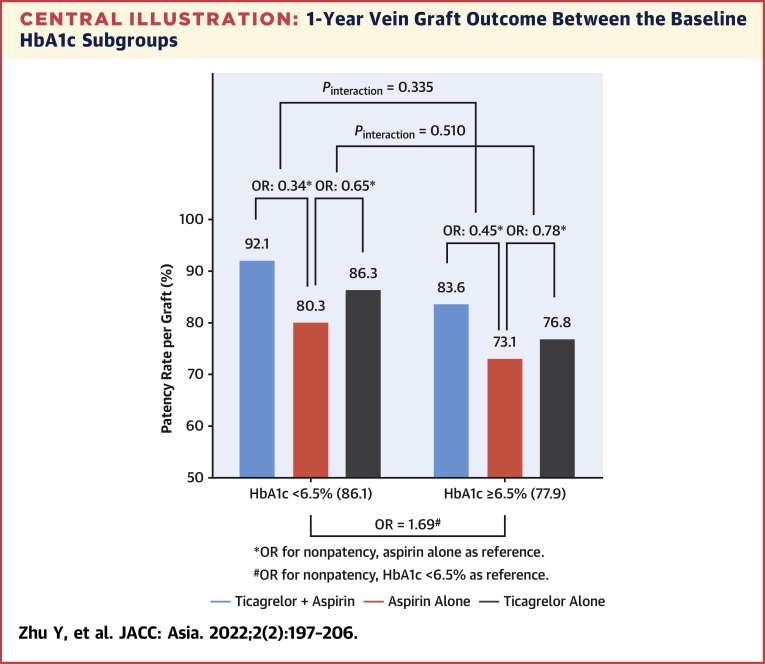
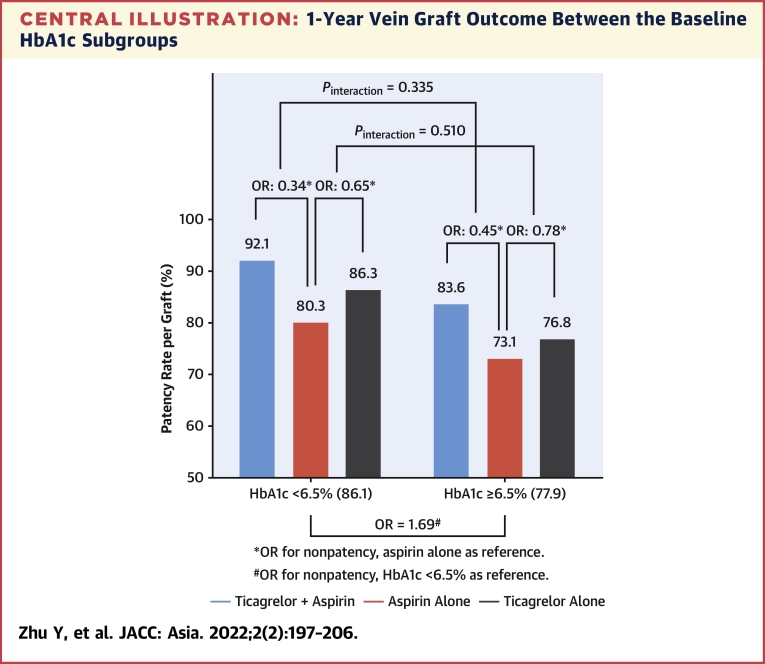
A total of 405 patients with available baseline HbA1c data were included in this subgroup analysis. Of them, there were 233 patients (678 vein grafts) with baseline HbA1c <6.5% and 172 patients (512 vein grafts) with baseline HbA1c ≥6.5%. Compared with the HbA1c <6.5% subgroup, the HbA1c ≥6.5% subgroup showed worse 1-year vein graft patency (adjusted odds ratio [OR] for nonpatency: 1.69, 95% confidence interval [CI]: 1.08-2.64). T+A showed higher vein graft patency than A in both HbA1c <6.5% (adjusted OR for nonpatency: 0.34, 95% CI: 0.15-0.75) and HbA1c ≥6.5% subgroups (adjusted OR for nonpatency: 0.45, 95% CI: 0.19-1.09), without an interaction effect (P for interaction = 0.335), whereas T did not show more significant improvement than A in both subgroups.
Conclusions
In the DACAB trial, lower baseline HbA1c was associated with higher vein graft patency 1 year after CABG. T+A improved 1-year vein graft patency vs A, irrespective of baseline HbA1c.
Key Words: aspirin, coronary artery bypass grafting, glycated hemoglobin A, patency, ticagrelor, vein graft
Abbreviations and Acronyms: CABG, coronary artery bypass graft; CAD, coronary artery disease; CI, confidence interval; DM, diabetes mellitus; HbA1c, glycated hemoglobin; ITT, intention to treat; MACE, major adverse cardiovascular event; OR, odds ratio; T+A, ticagrelor plus aspirin
Central Illustration
Diabetes mellitus (DM) is associated with a worse prognosis of coronary artery diseases (CAD).1 A large study of 1.9 million people showed that type 2 diabetes was positively associated with the incidence of stable angina and myocardial infarction.2 Approximately two-thirds of the deaths in patients with DM are attributable to CAD,1 and CAD occurs approximately 15 years earlier in patients with DM than those without.3 A systematic review indicated that 32.2% of patients with DM have CAD.4 Approximately 70% of the patients 65 years or older and with DM will die of CAD or stroke.1
For diabetic patients with 1- or 2-vessel disease, including the proximal left anterior descending artery, 3-vessel disease, or left main coronary artery disease, the current European guidelines made a Class I recommendation on coronary artery bypass grafting (CABG) as the gold standard for myocardial revascularization.5 The internal mammary artery graft is often considered the first choice because of its higher 10-year patency rate (>90%) and improved survival compared with any other grafts.6 Other arterial grafts (especially the radial artery) should be preferred as the second choice.7 Nevertheless, the saphenous vein grafts remain the most commonly used graft worldwide, with occlusion rates of 11% within 1 year after surgery and 40% to 50% at 10 years.8
Although blood glucose levels are punctual and vary greatly within a given day, glycated hemoglobin (HbA1c) levels represent the glucose levels over the past 90 days and can be used to evaluate glycemic control.9 The DCCT (Diabetes Control and Complications Trial) showed that high HbA1c levels were a predictor of microvascular complications of DM and that maintaining HbA1c at <7% decreased the occurrence of diabetic retinopathy, nephropathy, and neuropathy, as well as cardiovascular risk and mortality.10, 11, 12 Recently, several studies revealed that higher preoperative HbA1c levels were associated with a higher cardiovascular risk and long-term mortality after CABG, irrespective of DM.13, 14, 15 However, the effect of HbA1c control on vein graft outcome is still unclear, especially in the early term.
Therefore, this post hoc subgroup analysis of the DACAB (Different Antiplatelet Therapy Strategy After Coronary Artery Bypass Graft Surgery) trial aimed to assess the effects of ticagrelor with or without aspirin vs aspirin alone on the 1-year vein graft patency after CABG according to baseline HbA1c levels. The results could provide additional data for the personalization of antiplatelet therapy after CABG.
Methods
Data source
The DACAB trial (NCT02201771) was a multicenter, open-label, randomized trial of ticagrelor with or without aspirin vs aspirin alone that enrolled patients undergoing elective CABG (75% off-pump) in China.16 Briefly, it was a randomized, multicenter, open-label trial that enrolled 500 patients scheduled to undergo elective CABG surgery at 1 of 6 hospitals in China. The eligible patients were randomized 1:1:1 to receive antiplatelet therapy within 24 hours after CABG and for 1 year: 1) ticagrelor (90 mg twice daily) plus aspirin (100 mg once daily); 2) ticagrelor alone (90 mg twice daily); or 3) aspirin alone (100 mg once daily). Lipid-lowering and antidiabetic drugs were at the discretion of their treating physicians. For the present post hoc analysis, baseline HbA1c levels were available for only 405 participants; hence 95 participants were excluded. The patients were grouped according to baseline HbA1c (<6.5% and ≥6.5%).17
The present analysis was approved by the ethics committee of the Ruijin Hospital Shanghai Jiao Tong University School of Medicine. All participants or their legal representatives provided written informed consent before study enrollment. This consent included the post hoc analyses of the trial data.
Primary outcome/assessment
The outcome of the grafts was assessed by computed tomography angiography or coronary angiography and graded as described by FitzGibbon et al.18 The primary outcome was defined as patency (FitzGibbon grade A) vs nonpatency (FitzGibbon grade B+O).
Statistical analysis
All 405 patients (intent-to-treat [ITT] population) with 1,190 vein grafts from the original DACAB trial and available baseline HbA1c data were included in the present analysis. In the ITT analysis, all grafts with missing outcome assessments were considered FitzGibbon grade O.
Continuous variables were presented as means ± standard deviations or medians with interquartile ranges. Differences in baseline characteristics between treatment groups within baseline HbA1c <6.5% vs ≥6.5% and among subgroups were compared using the analysis of variance test. Categorical variables were presented as numbers and percentages and were analyzed using the chi-square test or Fisher’s exact test. The analyses were performed on a per-graft and per-patient basis. In the per-graft analysis, as each patient received multiple vein grafts, a generalized estimating equation model was used to analyze the repeated measures of outcomes. A logit link function was applied, and an independent covariance structure was used to model the correlation of the responses from the same patients. The stability of the model was verified using unstructured and exchangeable covariance structures. The estimation of the between-subgroup differences in vein graft patency was presented as odds ratios (ORs) and 95% confidence intervals (CIs). In the per-patient analysis, the patient outcome was classified according to the worst graft outcome in the patient. Logistic regression was used to obtain ORs and corresponding 95% CIs. Confounders were selected based on data availability and the literature, including age, sex, medical history of hypertension and hyperlipidemia, SYNTAX score, target vessel distribution, antiplatelet therapy, and statin use at 1-year after CABG. The consistency of the treatment effect among the subgroups was explored using a treatment × subgroup interaction. An HbA1c cutoff point of 6.5% was used in the analyses. Besides, HbA1c cutoff point of 7%, 7.5%, and 8% was used as sensitive analysis.
A 2-sided level of significance of 0.05 was applied. All analyses were performed using SAS 9.4 (SAS Institute).
Results
Characteristics of the patients
According to available baseline HbA1c data, there are 233 patients (678 vein grafts) in the <6.5% subgroup and 172 patients (512 vein grafts) in the ≥6.5% subgroup (Table 1). The baseline characteristics were generally comparable between the baseline HbA1c <6.5% vs ≥6.5% subgroups, except for sex (85.0% male in HbA1c <6.5% subgroup vs 76.7% male in ≥6.5% subgroup, P = 0.035) and statin use at 1 year post CABG (98.3% in HbA1c <6.5% subgroup vs 93.0% in ≥6.5% subgroup, P = 0.007). Supplemental Table 1 presents the characteristics of the patients according to baseline HbA1c <6.5% vs ≥6.5% and antiplatelet treatment.
Table 1.
Characteristics of the Patients Between Baseline HbA1c ≥6.5% and <6.5%
| HbA1c <6.5% (n = 233) | HbA1c ≥6.5% (n = 172) | P Value | |
|---|---|---|---|
| Age, y | 63.8 ± 8.1 | 64.2 ± 8.0 | 0.622 |
| Male | 198 (85.0) | 132 (76.7) | 0.035 |
| Clinical status | |||
| Stable angina | 80 (34.3) | 71 (41.3) | 0.321 |
| Unstable angina | 146 (62.7) | 95 (55.2) | |
| NSTEMI | 7 (3.0) | 6 (3.5) | |
| NYHA functional class III-IV | 89 (38.2) | 63 (36.6) | 0.671 |
| Medical history | |||
| Myocardial infarction | 77 (33.1) | 54 (31.4) | 0.725 |
| Stroke | 32 (13.7) | 20 (11.6) | 0.531 |
| Hypertension | 173 (74.3) | 135 (78.5) | 0.323 |
| Hyperlipidemiaa | 166 (71.2) | 123 (71.5) | 0.953 |
| Peripheral vascular disease | 43 (18.5) | 34 (19.8) | 0.739 |
| Smoking | 127 (54.5) | 77 (44.8) | 0.053 |
| COPD | 22 (9.4) | 12 (7.0) | 0.377 |
| LVEDD, mm | 49 (46, 53) | 49 (46, 52) | 0.206 |
| LVEF, % | 63 (57, 68) | 62 (55, 68) | 0.649 |
| SYNTAX score | |||
| Low (0-22) | 33 (14.2) | 21 (12.2) | 0.767 |
| Intermediate (23-32) | 137 (58.8) | 100 (58.1) | |
| High (≥33) | 63 (27.0) | 51 (29.6) | |
| EuroSCORE | |||
| Low (0-2) | 99 (42.5) | 74 (43.0) | 0.559 |
| Medium (3-5) | 104 (44.6) | 70 (40.7) | |
| High (≥6) | 30 (12.9) | 28 (16.3) | |
| Medication use, baseline | |||
| Beta blocker | 213 (91.4) | 160 (93.0) | 0.553 |
| ACEI/ARB | 150 (64.4) | 110 (64.0) | 0.930 |
| Statins | 226 (97.0) | 163 (94.8) | 0.255 |
| Medication use, 1 y | |||
| Beta blocker | 220 (94.4) | 162 (94.2) | 0.920 |
| ACEI/ARB | 121 (51.9) | 100 (58.1) | 0.215 |
| Statins | 229 (98.3) | 160 (93.0) | 0.007 |
| Surgical procedure | |||
| Pump use | 35 (15.0) | 32 (18.6) | 0.337 |
| Total grafts, n | 877 | 657 | - |
| Mean grafts/patient, n | 3.8 | 3.8 | - |
| IMA use | 192 (82.4) | 144 (83.7) | 0.727 |
Values are mean ± SD, n (%), median (Q1, Q3), or n.
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; COPD = chronic obstructive pulmonary disease; EuroSCORE = logistic European System for Cardiac Operative Risk Evaluation; HbA1c = glycated hemoglobin; IMA = Internal mammary artery; LVEDD = left ventricular end-diastolic diameter; LVEF = left ventricular ejection fraction; NSTEMI = non-ST-segment elevation myocardial infarction; NYHA = New York Heart Association; SYNTAX = Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery.
Defined as baseline low-density lipoprotein cholesterol ≥1.8 mmol/L.
Graft outcome between the baseline HbA1c subgroups
In the per-graft analysis, the 1-year vein graft patency (FitzGibbon grade A) and nonocclusion (FitzGibbon grade A+B) rates were 86.1% and 88.9% in the HbA1c <6.5% subgroup and 77.9% and 81.6% in the HbA1c ≥6.5% subgroup, respectively. Compared with the HbA1c <6.5% subgroup, the HbA1c ≥6.5% subgroup showed worse patency (adjusted OR for nonpatency: 1.69; 95% CI: 1.08-2.64; P = 0.021) and occlusion (adjusted OR for occlusion: 1.70; 95% CI: 1.04-2.79; P = 0.034). Similar results were observed for vein graft patency in the per-patient analysis (Table 2, Central Illustration). The results remained consistent in the sensitivity analyses using HbA1c <7.0% vs ≥7.0% (Supplemental Table 2), HbA1c <7.5% vs ≥7.5% (Supplemental Table 3), and HbA1c <8.0% vs ≥8.0% (Supplemental Table 4). When analyzed as a continuous variable, baseline HbA1c was associated with vein graft failure (for nonpatency per graft: adjusted OR: 1.25; 95% CI: 1.08-1.45; P = 0.003; for occlusion per graft: adjusted OR: 1.25; 95% CI: 1.07-1.46; P = 0.004) (Table 3).
Table 2.
One-Year Vein Graft Outcome Between Baseline HbA1c ≥6.5% and <6.5%
| 1-Year Outcome | HbA1c <6.5% | HbA1c ≥ 6.5% | HbA1c ≥6.5% vs. <6.5% Adjusted OR (95% CI) |
P Value |
|---|---|---|---|---|
| Per graft | n = 678 | n = 512 | ||
| FitzGibbon grade A | 584 (86.1) | 399 (77.9) | ||
| FitzGibbon grade B | 19 (2.8) | 19 (3.7) | ||
| FitzGibbon grade O | 75 (11.1) | 94 (18.4) | ||
| Patency (A) | 584 (86.1) | 399 (77.9) | 1.69 (1.08–2.64)a | 0.021 |
| Nonocclusion (A+B) | 603 (88.9) | 418 (81.6) | 1.70 (1.04–2.79)b | 0.034 |
| Per patient | n = 233 | n = 172 | ||
| FitzGibbon grade A | 177 (76.0) | 113 (65.7) | ||
| FitzGibbon grade B | 10 (4.3) | 13 (7.6) | ||
| FitzGibbon grade O | 46 (19.7) | 46 (26.7) | ||
| Patency (A) | 177 (76.0) | 113 (65.7) | 1.62 (1.01–2.60)a | 0.048 |
| Nonocclusion (A+B) | 187 (80.3) | 126 (73.3) | 1.41 (0.86–2.32)b | 0.174 |
Values are n (%) unless otherwise noted.
CI = confidence interval; HbA1c = glycated hemoglobin; OR = odds ratio, OR was adjusted for age, sex, medical history of hypertension and hyperlipidemia, SYNTAX score, target vessel distribution, antiplatelet therapy, and statin use at 1 year after coronary artery bypass graft; SYNTAX = Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery.
With nonpatency (B+O) as outcome.
With occlusion (O) as outcome.
Central Illustration.
1-Year Vein Graft Outcome Between the Baseline HbA1c Subgroups
Odds ratio was adjusted for age, sex, medical history of hypertension and hyperlipidemia, SYNTAX score, target vessel distribution, antiplatelet therapy and statin use at 1-year after coronary artery bypass graft. HbA1c = glycated hemoglobin.
Table 3.
Baseline HbA1c Treated as Continuous Variable and Vein Graft Outcome 1 Year After CABG
| 1-Year Outcome | Patency (FitzGibbon Grade A) |
Nonocclusion (FitzGibbon Grade A+B) |
||
|---|---|---|---|---|
| OR Adjusted for Nonpatency (95% CI) | P Value | OR Adjusted for Occlusion (95% CI) | P Value | |
| Per graft | 1.25 (1.08–1.45) | 0.003 | 1.25 (1.07–1.46) | 0.004 |
| Per patient | 1.29 (1.10–1.52) | 0.002 | 1.26 (1.07–1.48) | 0.007 |
CI = confidence interval; HbA1c = glycated hemoglobin; OR = odds ratio, OR was adjusted for age, sex, medical history of hypertension and hyperlipidemia, SYNTAX score, target vessel distribution, antiplatelet therapy, and statin use at 1 year after coronary artery bypass graft; SYNTAX = Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery.
We also analyzed artery graft outcome between the baseline HbA1c <6.5% vs ≥6.5% subgroups. In the per-graft analysis, the 1-year artery graft patency (FitzGibbon grade A) and nonocclusion (FitzGibbon grade A+B) rates were 96.9% and 97.4% in the HbA1c <6.5% subgroup and 97.7% and 98.5% in the HbA1c ≥6.5% subgroup, respectively. There was no difference in artery graft outcomes between the baseline HbA1c <6.5% vs ≥6.5% subgroups (all P > 0.05) (Supplemental Table 5).
Vein graft outcome among randomized antiplatelet treatments in the HbA1c <6.5% and ≥6.5% subgroups
In the per-graft analysis, the 1-year vein graft patency rates were 88.5% with ticagrelor plus aspirin (T+A), 81.8% with ticagrelor alone, and 77.4% with aspirin alone. T+A had lower odds of nonpatency compared with aspirin alone in the HbA1c <6.5% subgroup (adjusted OR for nonpatency: 0.34; 95% CI: 0.15-0.75) and in the HbA1c ≥6.5% subgroup (adjusted OR for nonpatency: 0.45; 95% CI: 0.19-1.09). There was no interaction effect between the baseline HbA1c status and antiplatelet treatment (interaction P = 0.335). There were no significant differences in patency for ticagrelor alone vs aspirin alone in both HbA1c subgroups (all P > 0.05) and no interaction effect (interaction P = 0.510). Similar results were observed in the per-patient analysis (Table 4).
Table 4.
1-Year Vein Graft Outcome Among Randomized Antiplatelet Treatments in Baseline HbA1c Subgroups
| 1-Year Patency | T+A |
T |
A |
T+A vs A |
T vs A |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | n (%) | n | n (%) | n | n (%) | Adjusted ORa (95% CI) | Interaction P Value | Adjusted ORa (95% CI) | Interaction P Value | |
| Per graft | ||||||||||
| HbA1c<6.5% | 229 | 211 (92.1) | 211 | 182 (86.3) | 238 | 191 (80.3) | 0.34 (0.15-0.75) | 0.335 | 0.65 (0.30-1.40) | 0.510 |
| HbA1c≥6.5% | 171 | 143 (83.6) | 185 | 142 (76.8) | 156 | 114 (73.1) | 0.45 (0.19-1.09) | 0.78 (0.36-1.70) | ||
| Per patient | ||||||||||
| HbA1c<6.5% | 79 | 67 (84.8) | 73 | 55 (75.3) | 81 | 55 (67.9) | 0.43 (0.19-0.99) | 0.973 | 0.77 (0.35-1.69) | 0.269 |
| HbA1c≥6.5% | 59 | 46 (78.0) | 60 | 35 (58.3) | 53 | 32 (60.4) | 0.42 (0.16-1.10) | 1.03 (0.44-2.42) | ||
| 1-Year Nonocclusion | T+A |
T |
A |
T+A vs A |
T vs A |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | n (%) | n | n (%) | n | n (%) | Adjusted ORb (95% CI) | Interaction P Value | Adjusted ORb (95% CI) | Interaction P Value | |
| Per graft | ||||||||||
| HbA1c<6.5% | 229 | 213 (93.0) | 211 | 188 (89.1) | 238 | 202 (84.9) | 0.40 (0.17-0.94) | 0.664 | 0.69 (0.29-1.66) | 0.154 |
| HbA1c≥6.5% | 171 | 147 (86.0) | 185 | 150 (81.1) | 156 | 121 (77.6) | 0.49 (0.18-1.31) | 0.79 (0.34-1.84) | ||
| Per patient | ||||||||||
| HbA1c<6.5% | 79 | 68 (86.1) | 73 | 60 (82.2) | 81 | 59 (72.8) | 0.52 (0.22-1.21) | 0.818 | 0.67 (0.29-1.57) | 0.620 |
| HbA1c≥6.5% | 59 | 49 (83.1) | 60 | 40 (66.7) | 53 | 37 (69.8) | 0.51 (0.18-1.45) | 1.19 (0.49-2.92) | ||
A = aspirin; CI = confidence interval; HbA1c = glycated hemoglobin; OR = odds ratio, OR was adjusted for age, sex, medical history of hypertension and hyperlipidemia, SYNTAX score, target vessel distribution, antiplatelet therapy and statin use at 1-year after coronary artery bypass graft; SYNTAX = synergy between percutaneous coronary intervention with Taxus and cardiac surgery; T = ticagrelor.
With nonpatency as outcome.
With occlusion as outcome.
For comparison of occlusion outcome as sensitivity analysis, in the per-graft analysis, T+A also had lower odds of occlusion compared with aspirin alone in the HbA1c <6.5% subgroup (adjusted OR for occlusion: 0.40; 95% CI: 0.17-0.94) and in the ≥6.5% subgroup (adjusted OR for occlusion: 0.49; 95% CI: 0.18-1.31). There was no interaction effect between baseline HbA1c status and antiplatelet treatment (interaction P = 0.664). There were no significant differences in occlusion for ticagrelor alone vs aspirin alone in both subgroups (all P > 0.05) and no interaction effect (interaction P = 0.154). Similar results were observed in the per-patient analysis (Table 4).
Cardiovascular events and bleeding outcomes in patients with HbA1c <6.5% vs ≥6.5%
Thirteen major adverse cardiovascular events (MACEs), defined as a composite of cardiovascular death, myocardial infarction, and stroke, were observed within 1 year after CABG, with 6 (2.6%) in the HbA1c <6.5% subgroup and 7 (4.1%) in HbA1c ≥6.5% subgroup, respectively. Overall, the incidence of MACE was relatively low for all randomized antiplatelet treatments in both subgroups. There were no significant differences between the baseline HbA1c <6.5% and ≥6.5% subgroups and between antiplatelet treatments (all P > 0.05) (Table 5).
Table 5.
1-Year Cardiovascular and Bleeding in Baseline HbA1c Subgroups
| Events | HbA1c<6.5% |
HbA1c≥6.5% |
P Value |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T+A (n = 79) | T (n = 73) | A (n = 81) | Total (n = 233) | P Value | T+A (n = 59) | T (n = 60) | A (n = 53) | Total (n = 172) | P Value | HbA1c <6.5% vs ≥6.5% | |
| MACE | 0 | 2 (2.7) | 4 (4.9) | 6 (2.6) | 0.124 | 2 (3.4) | 1 (1.7) | 4 (7.5) | 7 (4.1) | 0.260 | 0.409 |
| Cardiovascular death | 0 | 0 | 0 | 0 | 1 (1.7) | 0 | 2 (3.8) | 3 (1.7) | 0.207 | ||
| Myocardial infarction | 0 | 1 (1.4) | 2 (2.5) | 3 (1.3) | 1 (1.7) | 0 | 0 | 1 (0.6) | |||
| Stroke | 0 | 1 (1.4) | 2 (2.5) | 3 (1.3) | 0 | 1 (1.7) | 2 (3.8) | 3 (1.7) | |||
| Bleeding | 31 (39.2) | 8 (11) | 6 (7.4) | 46 (19.8) | <0.001 | 15 (25.4) | 10 (16.7) | 5 (9.4) | 30 (17.5) | 0.082 | 0.632 |
| Major | 2 (2.5) | 1 (1.4) | 0 | 3 (1.3) | 0.419 | 0 | 0 | 0 | 0 | - | 0.265 |
| Minor | 2 (2.5) | 0 | 0 | 2 (0.9) | 0.211 | 0 | 0 | 1 (1.9) | 1 (0.6) | 0.308 | 1.000 |
| Minimum | 28 (35.4) | 7 (9.6) | 6 (7.4) | 41 (17.6) | <0.001 | 15 (25.4) | 10 (16.7) | 4 (7.5) | 29 (16.9) | 0.039 | 0.846 |
Values are n (%).
A = aspirin; MACE = major adverse cardiovascular events; T = ticagrelor.
The incidence of overall bleeding events was similar between the HbA1c <6.5% and ≥6.5% subgroups (19.8% vs 17.5%, P = 0.632). In both HbA1c subgroups, the incidence of bleeding, particularly minimal bleeding, was significantly different among the randomized antiplatelet treatments (T+A: 35.4% vs T: 9.6% vs A: 7.4% in the HbA1c <6.5% subgroup, P < 0.001; T+A: 25.4% vs T: 16.7% vs A: 7.5% in the HbA1c ≥6.5% subgroup; P = 0.039).
Discussion
This post hoc study of the DACAB trial aimed to assess the influence of baseline HbA1c levels on early (1-year) vein graft patency after CABG and the effects of a more aggressive antiplatelet therapy (ticagrelor with or without aspirin vs aspirin alone) on 1-year vein graft patency in patients with HbA1c <6.5% vs ≥6.5%. In the per-graft analysis, compared with the HbA1c <6.5% subgroup, the HbA1c ≥6.5% subgroup showed worse patency (adjusted OR for nonpatency: 1.69; 95% CI: 1.08-2.64; P = 0.021) and nonocclusion (adjusted OR for occlusion: 1.70; 95% CI: 1.04-2.79; P = 0.034). The association was also observed when HbA1c was analyzed as a continuous variable. T+A showed a higher vein graft patency rate than A in both HbA1c <6.5% subgroup (adjusted OR for nonpatency: 0.34; 95% CI: 0.15-0.75) and ≥6.5% subgroup (adjusted OR for nonpatency: 0.45; 95% CI: 0.19-1.09), without an interaction effect (P for interaction = 0.035). While, T did not show improvement compared with A in both subgroups. The results indicated that higher baseline HbA1c was associated with lower vein graft patency at 1 year after CABG. T+A improved 1-year vein graft patency vs A in patients who underwent CABG, irrespective of baseline HbA1c.
Previous studies showed that DM was not associated with the 1-year vein graft patency after CABG, including the BARI (Bypass Angioplasty Revascularization Investigation)19 and PREVENT IV (Prevention of Autogenous Vein Graft Failure in Coronary Artery Bypass Procedures)20 trials. A meta-analysis by Antonopoulos et al.8 showed that DM was independently associated with early (1-year) vein graft occlusion, with an OR of 1.43 (95% CI: 1.25-1.65). Nevertheless, using DM alone can bias the results because patients with DM with well-controlled glycemia will display a better microvascular and macrovascular status than patients with DM with poorly controlled glycemia.21 In the same line of thought, patients without DM with poorly controlled glycemia but not overt DM might have poorer outcomes than those with well-controlled glycemia.15
Hence, using HbA1c, which represents the overall glycemic control over the past 3 months,9 might yield more precise results and allow for more personalized medicine through better risk assessment. Maintaining low HbA1c levels has been shown to decrease the incidence of diabetic microvascular and macrovascular complications10, 11, 12 and cardiovascular death in general.14 Of note, Abu Tailakh et al.13 showed that patients with DM with high baseline HbA1c (>7%) were at higher risk of long-term complications and mortality after CABG. In patients with type 1 DM, high baseline HbA1c was associated with higher risks of long-term MACEs and death.22 A meta-analysis showed that after coronary interventions, baseline HbA1c levels were associated with renal failure and myocardial infarction in patients with DM and with mortality and renal failure in those without DM.15 Nevertheless, this meta-analysis also highlighted the lack of high-quality evidence. Another meta-analysis showed that the baseline HbA1c status was associated with morbidity and mortality after CABG, irrespective of DM status.23 However, all these studies examined morbidity and mortality after CABG, but none specifically examined the patency of the grafts.
A previous small-scale study suggested no differences in patency between patients with HbA1c <6.5% and ≥6.5% (96.9% vs 99.2%, P = 0.037),24 but the number of events was probably too small for reliable analyses. In the present study, compared with the HbA1c <6.5% subgroup, the HbA1c ≥6.5% subgroup showed worse patency (adjusted OR for nonpatency: 1.69; 95% CI: 1.08-2.64) and nonocclusion (adjusted OR for occlusion: 1.70; 95% CI: 1.04-2.79). We hypothesized that different HbA1c thresholds may influence the results. Still, the present study showed similar results when using 6.5%, 7.0%, 7.5%, and 8.0% as the cutoff HbA1c level or when analyzing HbA1c as a continuous variable.
Currently, the internal mammary artery is the golden standard of CABG because of high long-term patency rates and improved survival compared with any other grafts.25 Besides, Radial Artery Database International Alliance study and RAPCO (Radial Artery Patency and Clinical Outcomes) trial showed that radial artery grafts have better long-term patency outcome than vein grafts, which was considered as the second-choice graft.7,26 Lorusso et al. demonstrated that arterial grafts in patients with DM post CABG appear to sustain their biological integrity despite of the poor glycemic statues, which may explain the long-term patency rate. However, the same effect was not observed in the vein grafts.27 In our study, the 1-year graft outcome was obviously better in artery grafts than in vein grafts, which was consistent with the previous findings. Besides, there was no difference in 1-year artery graft outcome between patients with HbA1c <6.5% and ≥6.5%. Thus, for patients with poor glycemic control, autologous arteries might be more appropriate as grafts for CABG, which would be a testable opinion in the future.
Our study found that, in both per-graft and per patient analysis, T+A significantly prevented vein graft failure (nonpatency or occlusion) at 1 year in the HbA1c <6.5% subgroup and in the ≥6.5% subgroups, and there was no interaction between the HbA1c control and antiplatelet therapy. It suggests that a more aggressive dual antiplatelet therapy is sufficient to counteract the potential negative effects of HbA1c on thrombosis-related vein graft failure. Recently, the POPular CABG (Effect of Ticagrelor On Saphenous Vein Graft Patency in Patients Undergoing Coronary Artery Bypass Grafting Surgery) trial did not observe a decreased vein graft occlusion rate with T+A vs aspirin alone at 1 year after CABG. The OR for occlusion was 0.9 (95% CI: 0.3-2.7) in DM and 1.5 (95% CI: 0.7-2.9) in non-DM, with an interaction P = 0.49. Several potential reasons might explain this inconsistency. First, 35.3% of the patients in the POPular CABG trial had permanently discontinued study medication during the 1-year follow-up, including 8.0% who received study medication for <60 days. In the DACAB trial, only 3.4% of the patients discontinued study medication ≥60 days. The compliance was different in the 2 trials. Second, 94.6% of the patients in the POPular CABG trial underwent on-pump CABG, whereas the proportion was only 24.2% in the DACAB trial. Off-pump CABG had a negative effect on vein graft patency.28 In a post hoc analysis of the DACAB trial, more benefits of DAPT on vein graft patency were observed in the off-pump subgroup than the on-pump subgroup.29 Of note, the 1-year vein graft occlusion rate in the off-pump subgroup of the POPular CABG trial was 28.9%, which was much higher than the rate (8.9%) in the on-pump subgroup. Third, in the primary ITT analysis of the DACAB trial, 6.6% of the patients (6.2% of vein grafts) without primary outcome assessment at 1 year after CABG were imputed as occlusion, although in the POPular CABG trial, 10.6% of the patients without primary outcome assessment at 1 year after CABG were excluded from the ITT analysis. This difference in dealing with the missing data led to a higher 1-year vein graft occlusion rate in the DACAB trial (14.5%) than in the POPular CABG trial (9.9%). If that 6.6% of patients without primary outcome assessment were also excluded from the ITT analysis, the 1-year vein graft occlusion rate would be 8.8% in the DACAB trial, which was similar to that in the POPular CABG trial. In the prematurely terminated TiCAB (Ticagrelor in Coronary Artery Bypass) trial, ticagrelor monotherapy showed no advantage in preventing MACE or major bleeding than aspirin,30 which was in line with our results.
This study has limitations. First, baseline HbA1c levels were missing for 95 of 500 patients. The exclusion of these patients may cause data deviation. Second, most patients underwent off-pump CABG, which represents the current clinical practice status in China. Therefore, the conclusions may not apply to patients undergoing on-pump CABG. Third, we did not collect the data of type 1 and type 2 DM in the DACAB trial. Thus, the graft outcomes of patients with type 1 or type 2 DM were not compared. Fourth, the long-term outcomes are not yet available. Of note, a 5-year follow-up extension study is currently ongoing (NCT03987373). Fifth, we only analyzed the baseline HbA1c levels. The fluctuations in HbA1c levels during the intervention may occur, which was not considered in this post hoc analysis. Finally, the present study was a post hoc analysis of a completed trial, and the study design did not allow any causality determination. Future studies might be longitudinal in design and allow determination of the causes of vein graft failure. In addition, the power analysis of the original trial did not take into account baseline HbA1c.
Conclusions
In the DACAB trial, higher baseline HbA1c was associated with lower vein graft patency 1 year after CABG. T+A improved 1-year vein graft patency vs aspirin, irrespective of baseline HbA1c. Overall, multiple artery grafts might be a more appropriate choice for these patients with poor glycemic control.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: The influence of baseline HbA1c levels and the interaction effect of antiplatelet therapy on vein graft outcomes post CABG remains unclear. Our subgroup analysis of the DACAB trial showed higher baseline HbA1c was associated with worse vein graft patency 1 year after CABG. Ticagrelor plus aspirin showed higher 1-year vein graft patency compared with aspirin alone, irrespective of baseline HbA1c.
TRANSLATIONAL OUTLOOK: For patients with poor baseline glycemic control, the vein graft failure was a matter of concern even in the early term. Compared with aspirin alone, ticagrelor plus aspirin-based dual antiplatelet therapy could improve the 1-year vein graft patency, irrespective of baseline HbA1c. Thus, artery grafts might be a more appropriate choice for these patients, which needs further validation.
Funding Support and Author Disclosures
The DACAB was funded by AstraZeneca; this post hoc analysis was not. Dr Zhu has served as a speaker for AstraZeneca, Johnson & Johnson, Novartis, and Sanofi; and as an investigator on clinical trials sponsored by AstraZeneca, Bayer, Novartis, and Sanofi. Dr Liu has served as a speaker for Pfizer. Dr R. Wang has served as a speaker for AstraZeneca; and as an investigator on clinical trials sponsored by Bayer. Dr. X. Wang has served as a speaker for AstraZeneca and Johnson & Johnson. Dr Cheng has served as a speaker for Medtronic. Dr Zhao has served as a speaker for AstraZeneca, Johnson & Johnson, and Medtronic; and has been an investigator on clinical trials sponsored by AstraZeneca, Bayer, Novartis, and Sanofi. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The abstract of this study was presented orally at the ESC Congress 2019.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version of this paper.
Contributor Information
Zhaoyun Cheng, Email: chengzhy@zzu.edu.cn.
Qiang Zhao, Email: zq11607@rjh.com.cn.
Appendix
References
- 1.Low Wang C.C., Hess C.N., Hiatt W.R., Goldfine A.B. Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus - mechanisms, management, and clinical considerations. Circulation. 2016;133:2459–2502. doi: 10.1161/CIRCULATIONAHA.116.022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah A.D., Langenberg C., Rapsomaniki E., et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015;3:105–113. doi: 10.1016/S2213-8587(14)70219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Booth G.L., Kapral M.K., Fung K., Tu J.V. Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. Lancet. 2006;368:29–36. doi: 10.1016/S0140-6736(06)68967-8. [DOI] [PubMed] [Google Scholar]
- 4.Einarson T.R., Acs A., Ludwig C., Panton U.H. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol. 2018;17:83. doi: 10.1186/s12933-018-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cosentino F., Grant P.J., Aboyans V., et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 6.Caliskan E., de Souza D.R., Boning A., et al. Saphenous vein grafts in contemporary coronary artery bypass graft surgery. Nat Rev Cardiol. 2020;17:155–169. doi: 10.1038/s41569-019-0249-3. [DOI] [PubMed] [Google Scholar]
- 7.Gaudino M., Benedetto U., Fremes S., et al. Radial-artery or saphenous-vein grafts in coronary-artery bypass surgery. N Engl J Med. 2018;378:2069–2077. doi: 10.1056/NEJMoa1716026. [DOI] [PubMed] [Google Scholar]
- 8.Antonopoulos A.S., Odutayo A., Oikonomou E.K., et al. Development of a risk score for early saphenous vein graft failure: an individual patient data meta-analysis. J Thorac Cardiovasc Surg. 2020;160:116–127.e4. doi: 10.1016/j.jtcvs.2019.07.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilstrap L.G., Chernew M.E., Nguyen C.A., et al. Association between clinical practice group adherence to quality measures and adverse outcomes among adult patients with diabetes. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.9139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nathan D.M., Group D.E.R. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37:9–16. doi: 10.2337/dc13-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nathan D.M. Realising the long-term promise of insulin therapy: the DCCT/EDIC study. Diabetologia. 2021;64:1049–1058. doi: 10.1007/s00125-021-05397-4. [DOI] [PubMed] [Google Scholar]
- 12.Lachin J.M., Orchard T.J., Nathan D.M., Group D.E.R. Update on cardiovascular outcomes at 30 years of the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37:39–43. doi: 10.2337/dc13-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abu Tailakh M., Ishay S.Y., Awesat J., Poupko L., Sahar G., Novack V. Hemoglobin A1c in patients with diabetes predict long-term mortality following coronary artery surgery. J Clin Med. 2021;10:2739. doi: 10.3390/jcm10122739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavero-Redondo I., Peleteiro B., Alvarez-Bueno C., Rodriguez-Artalejo F., Martinez-Vizcaino V. Glycated haemoglobin A1c as a risk factor of cardiovascular outcomes and all-cause mortality in diabetic and non-diabetic populations: a systematic review and meta-analysis. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-015949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J., Luo X., Jin X., et al. Effects of preoperative HbA1c levels on the postoperative outcomes of coronary artery disease surgical treatment in patients with diabetes mellitus and nondiabetic patients: a systematic review and meta-analysis. J Diabetes Res. 2020;2020:3547491. doi: 10.1155/2020/3547491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Q., Zhu Y., Xu Z., et al. Effect of ticagrelor plus aspirin, ticagrelor alone, or aspirin alone on saphenous vein graft patency 1 year after coronary artery bypass grafting: a randomized clinical trial. JAMA. 2018;319:1677–1686. doi: 10.1001/jama.2018.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Introduction: standards of medical care in diabetes-2020. Diabetes Care. 2020;43:S1–S2. doi: 10.2337/dc20-Sint. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgibbon G.M., Kafka H.P., Leach A.J., et al. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol. 1996;28:616–626. doi: 10.1016/0735-1097(96)00206-9. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz L., Kip K.E., Frye R.L., et al. Coronary bypass graft patency in patients with diabetes in the Bypass Angioplasty Revascularization Investigation (BARI) Circulation. 2002;106:2652–2658. doi: 10.1161/01.cir.0000038885.94771.43. [DOI] [PubMed] [Google Scholar]
- 20.Koshizaka M., Lopes R.D., Reyes E.M., et al. Long-term clinical and angiographic outcomes in patients with diabetes undergoing coronary artery bypass graft surgery: results from the Project of Ex-vivo Vein Graft Engineering via Transfection IV trial. Am Heart J. 2015;169:175–184. doi: 10.1016/j.ahj.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Prattichizzo F., de Candia P., De Nigris V., Nicolucci A., Ceriello A. Legacy effect of intensive glucose control on major adverse cardiovascular outcome: systematic review and meta-analyses of trials according to different scenarios. Metabolism. 2020;110:154308. doi: 10.1016/j.metabol.2020.154308. [DOI] [PubMed] [Google Scholar]
- 22.Nystrom T., Holzmann M.J., Eliasson B., Kuhl J., Sartipy U. Glycemic control in type 1 diabetes and long-term risk of cardiovascular events or death after coronary artery bypass grafting. J Am Coll Cardiol. 2015;66:535–543. doi: 10.1016/j.jacc.2015.05.054. [DOI] [PubMed] [Google Scholar]
- 23.Tennyson C., Lee R., Attia R. Is there a role for HbA1c in predicting mortality and morbidity outcomes after coronary artery bypass graft surgery? Interact Cardiovasc Thorac Surg. 2013;17:1000–1008. doi: 10.1093/icvts/ivt351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuura K., Imamaki M., Ishida A., Shimura H., Niitsuma Y., Miyazaki M. Off-pump coronary artery bypass grafting for poorly controlled diabetic patients. Ann Thorac Cardiovasc Surg. 2009;15:18–22. [PubMed] [Google Scholar]
- 25.Martinez-Gonzalez B., Reyes-Hernandez C.G., Quiroga-Garza A., et al. Conduits used in coronary artery bypass grafting: a review of morphological studies. Ann Thorac Cardiovasc Surg. 2017;23:55–65. doi: 10.5761/atcs.ra.16-00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buxton B.F., Hayward P.A., Raman J., et al. Long-term results of the RAPCO trials. Circulation. 2020;142:1330–1338. doi: 10.1161/CIRCULATIONAHA.119.045427. [DOI] [PubMed] [Google Scholar]
- 27.Lorusso R., Pentiricci S., Raddino R., et al. Influence of type 2 diabetes on functional and structural properties of coronary artery bypass conduits. Diabetes. 2003;52:2814–2820. doi: 10.2337/diabetes.52.11.2814. [DOI] [PubMed] [Google Scholar]
- 28.Gaudino M., Angelini G.D., Antoniades C., et al. Off-pump coronary artery bypass grafting: 30 years of debate. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.009934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu Y., Xue Q., Zhang M., et al. Effect of ticagrelor with or without aspirin on vein graft outcome 1 year after on-pump and off-pump coronary artery bypass grafting. J Thorac Dis. 2020;12:4915–4923. doi: 10.21037/jtd-20-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schunkert H., Boening A., von Scheidt M., et al. Randomized trial of ticagrelor vs. aspirin in patients after coronary artery bypass grafting: the TiCAB trial. Eur Heart J. 2019;40:2432–2440. doi: 10.1093/eurheartj/ehz185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.