Abstract
Background
Waist circumference (WC), visceral adiposity index (VAI), lipid accumulation product (LAP), and Chinese visceral adiposity index (CVAI) are considered surrogate indicators of abdominal fat deposition, but the longitudinal association of these indices with cardiovascular (CV) events in adults with type 2 diabetes (T2D) remains unclear. Our study aimed to examine the associations between abdominal obesity indices and incident CV events among people with T2D and to compare their predictive performance in risk assessment.
Methods
The present study included 2328 individuals with T2D from the Xinjiang Multi-Ethnic Cohort. Multivariable Cox regression analyses were applied to assess the associations between abdominal obesity indices and CV events. Harrell's concordance statistic (C-statistic), net reclassification improvement (NRI) index, and integrated discrimination improvement (IDI) index were utilized to evaluate the predictive performance of each abdominal obesity index.
Results
At a median follow-up period of 59 months, 289 participants experienced CV events. After multivariable adjustment, each 1-SD increase in WC, VAI, LAP, and CVAI was associated with a higher risk of CV events in people with T2D, with adjusted hazard ratios (HRs) being 1.57 [95% CI (confidence interval): 1.39–1.78], 1.11 (95% CI 1.06–1.16), 1.46 (95% CI 1.36–1.57), and 1.78 (95% CI 1.57–2.01), respectively. In subgroup analyses, these positive associations appeared to be stronger among participants with body mass index (BMI) < 25 kg/m2 compared to overweight/obese participants. As for the predictive performance, CVAI had the largest C-statistic (0.700, 95% CI 0.672–0.728) compared to VAI, LAP, WC, and BMI (C-statistic: 0.535 to 0.670, all P for comparison < 0.05). When the abdominal obesity index was added to the basic risk model, the CVAI index also showed the greatest incremental risk stratification (C-statistic: 0.751 vs. 0.701, P < 0.001; IDI: 4.3%, P < 0.001; NRI: 26.6%, P < 0.001).
Conclusions
This study provided additional evidence that all abdominal obesity indices were associated with the risk of CV events and highlighted that CVAI might be a valuable abdominal obesity indicator for identifying the high risk of CV events in Chinese populations with T2D. These results suggest that proactive assessment of abdominal obesity could be helpful for the effective clinical management of the diabetic population.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-022-01670-x.
Keywords: Type 2 diabetes, Abdominal obesity, Cardiovascular disease, Visceral adiposity index, Lipid accumulation product, Chinese visceral adiposity index
Background
The International Diabetes Federation published a report in 2021 indicating that there are about 536 million people with diabetes globally, while China has 140 million diabetic patients, ranking first in the world [1]. Compared to those without type 2 diabetes (T2D), adults with T2D are at a twofold higher risk of incident cardiovascular disease (CVD), which occurs earlier and more severely [2, 3]. At the same time, the primary cause of hospital admissions and death in people with T2D is CVD, which poses a heavy economic healthcare burden [4]. A meta-analysis estimated that CVD impacted 32.2% of individuals with T2D and caused 9.9% of deaths in T2D patients (accounting for 50.3% of all deaths) [5]. Thus, it is vital to identify modifiable CVD risk factors among individuals with T2D to provide effective prevention strategies.
Obesity has been established as a crucial risk factor for cardiometabolic disease and premature death [6]. Body mass index (BMI) is frequently utilized to classify overweight or obesity, but the evidence from published studies on the relationship between BMI and CVD risk in people with T2D is inconsistent. Several studies indicated that BMI was positively associated with incident cardiovascular (CV) events among people with T2D [7–9], but others showed a negative [10, 11] or no association [12, 13]. Such inconsistent results suggest that BMI is not a perfect estimator of obesity, as it neither distinguishes between lean mass and total fat mass nor captures the distribution of body fat [14, 15]. Recent evidence demonstrated that adipose tissue distribution rather than overall adiposity is a significant factor in determining CVD risk [16]. Moreover, increasing studies have shown that abdominal fat deposition plays a crucial role in cardiometabolic diseases [16–18].
Radiological imaging techniques can accurately assess abdominal adiposity, but they are inappropriate for widespread epidemiological investigations due to their long duration, high price, and radiation hazards [19]. Hence, there is a need to establish reliable and easily accessible indices to evaluate abdominal adiposity. Waist circumference (WC) is a widely used indicator for abdominal obesity, but it has deficiencies in discriminating between subcutaneous and visceral adipose tissue. Several novel abdominal obesity indices established by combining anthropometric and lipid parameters were considered superior to traditional anthropometric parameters [20, 21]. These novel indices are the visceral adiposity index (VAI), lipid accumulation product (LAP), and Chinese visceral adiposity index (CVAI). Although several studies have suggested that these abdominal obesity indices could predict the occurrence of CVD in the general population [22–24], this issue is rarely examined in the T2D population. To date, only one cross-sectional study has revealed the relationship between abdominal obesity indicators and macrovascular complications of diabetes [25]. However, there is still unclear about the longitudinal association of these indices with CVD risk in people with T2D. In addition, although several studies have estimated and compared the predictive performances of abdominal obesity indices for chronic diseases in different populations [26–28], the findings were inconsistent. Thus, it is necessary to examine further which abdominal obesity index is the optimal predictor of CV events in people with T2D.
In this background, we explored the relationship between baseline abdominal obesity indices (WC, LAP, VAI, and CVAI) and incident CV events among people with T2D and compared their risk prediction performances.
Methods
Study design and participants
The Xinjiang Multi-Ethnic Cohort (XMC) is a longitudinal, population-based study in which participants were recruited from community health centers in three regions of Xinjiang (Urumqi, Hotan, and Yili), China. In brief, the XMC study was designed to explore the influences of genetics, environmental exposures, and lifestyle on health outcomes. Details of the XMC have been presented in a previous paper [29].
Between 2017 to 2018, 23,095 participants received a comprehensive health examination, and 3060 were identified as suffering from T2D. T2D was defined as any one of the following standards: (1) fasting blood glucose (FBG) level ≥ 7 mmol/L; (2) taking glucose-lowering agents; (3) previously diagnosed T2D by physicians. We excluded participants with a history of CVD (n = 576) at baseline, with incomplete data (n = 114), or without any follow-up information (n = 42). Ultimately, 2328 participants with T2D could be included in the present study (Fig. 1). Approval for this study protocol was granted by the Institutional Review Board of the Traditional Chinese Medical Hospital of Xinjiang Uygur Autonomous Region (2018XE0108). All participants signed an informed consent document.
Fig.1.
Flowchart of the study population
Baseline data collection and definitions
Demographic characteristics (age, gender, ethnicity, location, and education), medical history, use of antidiabetic agents, and lifestyle factors were collected by trained clinic staff through a standardized questionnaire. The ethnicity was classified as Han, Hui, Uyghur, and others (Kazakh, Mongolian, and Tibetan). Education status was categorized as primary school or below, middle school, and high school or further education. The location of residence was categorized as urban and rural. Smoking and drinking habits were classified as current and never or previous. Physical activity was defined as exercise more than once a week [30].
Trained physicians or nurses performed anthropometric measurements following standardized protocols. WC was checked at the middle point between the iliac crest and the lower rib margin. Weight and height were checked using an auto-anthropometer (SK-X80, China) with an accuracy of 0.1 kg and 0.1 cm, respectively. When participants were seated after a 10-min rest, blood pressure was assessed two times with a calibrated mercury sphygmomanometer, and the mean of the two assessments was applied for subsequent analysis. After an overnight fast, nurses collected participants' venous blood samples for laboratory tests. Triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and FBG levels were performed in the laboratories of the community health centers with automated analyzers (Hitachi, Tokyo, Japan).
The formulas for BMI, VAI [31], LAP [32], and CVAI [21] were:
Follow-up and outcomes assessment
Participants were followed up annually through health examinations and telephone interviews. In addition, Medical Record Information System, Medical Insurance System, Chronic Disease Management System, and Death Information Registration System were used to confirm health outcomes further. The primary endpoint was the time in days from admission to the study to the first occurrence of a CV event, including nonfatal myocardial infarction, nonfatal stroke, or CV death (fatal myocardial infarction, fatal stroke, and other CV death) [33]. Participants were tracked from baseline to date of death or CV events or June 2022, whichever occurred first.
Statistical analysis
Data were summarized as percentages, mean ± standard deviation (SD), or median (interquartile range) where appropriate. When comparing the differences between the two groups, the Chi-square test was performed for categorical data, and the Student's t-test or Mann-Whitney U-test was for continuous data. Kaplan–Meier curves were plotted to describe the cumulative rates of CV events by groups according to the quartiles of abdominal obesity indices and were compared using the log-rank test. Cox proportional-hazards regression analysis was utilized to obtain the hazard ratios (HRs) of CV events across the quartiles of the WC, VAI, LAP, and CVAI. We also evaluated the risk of CV events associated with one SD increase in abdominal obesity indices. Model 1 was unadjusted. Model 2 was adjusted for gender, age, ethnicity, education, smoking status, and drinking status. Then, the third model additionally adjusted for LDL-C, TC, FBG, systolic blood pressure, diastolic blood pressure, physical activity, antidiabetic agents, and diabetes duration. Furthermore, the restricted cubic spline analysis was conducted to examine the non-linear relationships between abdominal obesity indices and hazards of incident CV events. Subgroup analyses were conducted based on age, BMI, ethnicity, and usage of antidiabetic agents, and interactions between subgroups were examined.
Harrell's concordance statistic (C-statistic) was calculated to estimate the predictive performance of BMI, WC, VAI, LAP, and CVAI for incident CV events in people with T2D. Differences between the C-statistics of these abdominal obesity indices were compared using the DeLong test [34]. Also, the net reclassification improvement (NRI) index and integrated discrimination improvement (IDI) index were calculated to estimate the incremental predictive value of WC, VAI, LAP, and CVAI beyond the established risk factors for CV events. Considering that non-CVD-related death is a competing risk, we performed a sensitivity analysis by using Fine–Gray proportional sub-distribution hazards models to assess the robustness of associations between abdominal obesity indices and CV events. Moreover, we included participants who were excluded because of missing data and repeated the primary analyses to assess the robustness of the results. Multiple imputation by chained equations was performed to fill the missing covariates at baseline. Statistical analyses were done with STATA MP version 17.0 (Stata Corp) and R version 4.2.1. P value < 0.05 was considered statistical significance.
Results
Baseline characteristics
In total, 2328 participants (male, n = 976; female, n = 1352) with T2D were available for analysis, and the mean age was 59.30 ± 10.01 years at baseline. At a median follow-up of 59 months (interquartile range: 56–61), 289 (12.41%) participants experienced CV events (including 135 strokes and 176 myocardial infarctions), and 70 (3.01%) participants died. The baseline characteristics of participants by CVD status at follow-up are summarized in Table 1. Compared to those without CVD, individuals who developed CVD tended to be older, current smokers and drinkers, and less physically active. Moreover, they also had higher systolic blood pressure, diastolic blood pressure, BMI, LDL-C, TG, WC, VAI, LAP, and CVAI and lower HDL-C than those without CVD (all P-values < 0.05, Table 1).
Table 1.
Baseline characteristics of participants according to the cardiovascular disease status at follow-up
| Variables | Total (n = 2328) |
Incident CVD (n = 289) | Non-case (n = 2039) |
P-value |
|---|---|---|---|---|
| Age, years | 59.30 ± 10.01 | 65.58 ± 7.47 | 58.41 ± 10.00 | < 0.001 |
| Diabetes duration, years | 7.73 ± 3.17 | 7.94 ± 2.84 | 7.70 ± 3.21 | 0.227 |
| Gender, n (%) | 0.915 | |||
| Male | 976 (41.92) | 122 (42.21) | 854 (41.88) | |
| Female | 1352 (58.08) | 167 (57.79) | 1185 (58.12) | |
| Ethnicity, n (%) | 0.072 | |||
| Han | 1015 (43.60) | 128 (44.29) | 887 (43.50) | |
| Hui | 324 (13.92) | 29 (10.03) | 295 (14.47) | |
| Uyghur | 901 (38.70) | 125 (43.25) | 776 (38.06) | |
| Others | 88 (3.78) | 7 (2.42) | 81 (3.97) | |
| Education, n (%) | 0.098 | |||
| Primary school or below | 1508 (64.78) | 203 (70.24) | 1305 (64.00) | |
| Middle school | 548 (23.54) | 60 (20.76) | 488 (23.93) | |
| High school or further | 272 (11.68) | 26 (9.00) | 246 (12.06) | |
| Location, n (%) | ||||
| Urban | 1045 (44.89) | 136 (47.06) | 909 (44.58) | 0.428 |
| Rural | 1283 (55.11) | 153 (52.94) | 1130 (55.42) | |
| Current smoking, n (%) | 337 (14.48) | 62 (21.45) | 275 (13.49) | < 0.001 |
| Current drinking, n (%) | 224 (9.62) | 39 (13.49) | 185 (9.07) | 0.017 |
| Physical activity, n (%) | 676 (29.04) | 69 (23.88) | 607 (29.77) | 0.039 |
| Antidiabetic agents, n (%) | 1196 (51.37) | 162 (56.06) | 1034 (50.71) | 0.089 |
| SBP, mmHg | 133.47 ± 18.16 | 137.75 ± 20.42 | 132.86 ± 17.74 | < 0.001 |
| DBP, mmHg | 79.75 ± 10.84 | 81.43 ± 11.75 | 79.51 ± 10.69 | 0.005 |
| Total cholesterol, mmol/L | 4.91 ± 2.28 | 5.14 ± 2.63 | 4.87 ± 2.23 | 0.059 |
| HDL cholesterol, mmol/L | 1.44 ± 0.57 | 1.32 ± 0.45 | 1.45 ± 0.59 | < 0.001 |
| LDL cholesterol, mmol/L | 2.69 ± 1.02 | 2.80 ± 0.82 | 2.68 ± 1.04 | 0.044 |
| Triglyceride, mmol/L | 1.44 (1.04, 1.95) | 1.87 (1.24, 2.39) | 1.40 (1.00, 1.85) | < 0.001 |
| FBG, mmol/L | 7.28 ± 3.21 | 7.50 ± 3.25 | 7.25 ± 3.20 | 0.221 |
| Body mass index, kg/m2 | 26.42 ± 3.73 | 26.86 ± 3.81 | 26.36 ± 3.71 | 0.034 |
| Waist circumference, cm | 91.18 ± 10.95 | 95.76 ± 10.23 | 90.53 ± 10.90 | < 0.001 |
| VAI | 1.76 (1.14, 2.66) | 2.42 (1.64, 3.37) | 1.67 (1.08, 2.54) | < 0.001 |
| LAP | 41.15 (26.00, 65.01) | 60.20 (39.60, 89.10) | 38.72 (24.70, 60.72) | < 0.001 |
| CVAI | 121.08 ± 39.57 | 145.52 ± 33.89 | 117.61 ± 39.10 | < 0.001 |
Data are summarized as number (percentage), mean ± standard deviation, or median (interquartile range)
CVD cardiovascular disease, SBP systolic blood pressure, DBP diastolic blood pressure, HDL high-density lipoprotein, LDL low-density lipoprotein, FBG fasting blood glucose, VAI visceral adiposity index, LAP lipid accumulation product, CVAI Chinese visceral adiposity index
Relationship between abdominal obesity indices and cardiovascular events among people with type 2 diabetes
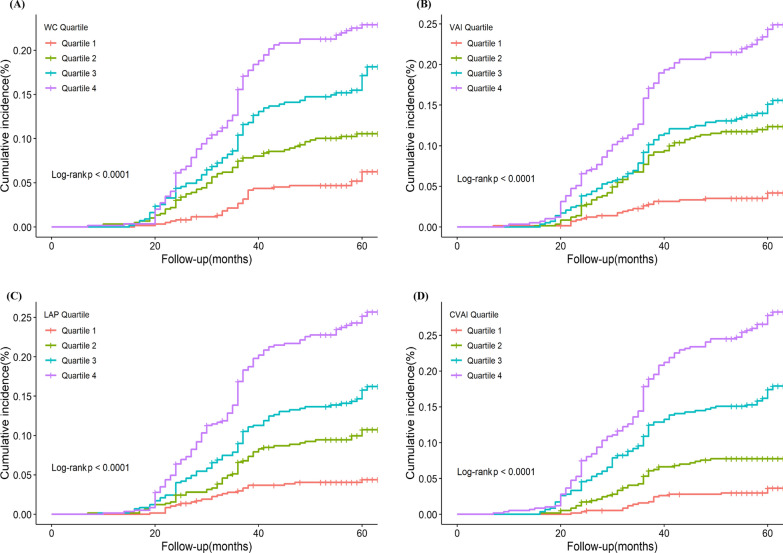
Kaplan–Meier curves for incident CV events among people with T2D by quartiles of abdominal obesity indices are presented in Fig. 2. Higher baseline WC, VAI, LAP, and CVAI were correlated with an elevated risk of CV events in people with T2D (all log-rank tests P < 0.001).
Fig.2.
Kaplan–Meier incidence rate of cardiovascular events in people with type 2 diabetes according to quartiles of abdominal obesity indices. A waist circumference (WC), B visceral adiposity index (VAI), C lipid accumulation product (LAP), and D Chinese visceral adiposity index (CVAI)
The Cox regression models were applied to investigate the association of abdominal obesity indices with the risk of CV events among people with T2D (Table 2). Compared to the lowest quartile group of baseline WC, VAI, LAP, and CVAI, the fully adjusted HRs (95%) for CV events in the highest quartile group were 3.92 (95% CI 2.62–5.88), 6.88 (95% CI 4.27–11.07), 6.03 (95% CI 3.85–9.47), and 5.67 (95% CI 3.47–9.27), respectively. Such associations remained significant when all abdominal obesity indices were estimated as continuous variables. After adjusting for all covariates, each 1-SD increase in WC, VAI, LAP, and CVAI was associated with a higher risk of CV events among people with T2D, with adjusted HR being 1.57 (95% CI 1.39–1.78), 1.11 (95% CI 1.06–1.16), 1.46 (95% CI 1.36–1.57), and 1.78 (95% CI 1.57–2.01), respectively. Furthermore, restricted cubic spline regression revealed the non-linear relationships between abdominal obesity indices and hazards of incident CV events in people with T2D (Additional file 1: Fig. S1).
Table 2.
Association between baseline abdominal obesity indices and incident cardiovascular events in people with type 2 diabetes
| Indices | Event/ Total |
Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| WC | |||||||
| Per 1 SD increase | 289/2328 | 1.52 (1.37–1.69) | < 0.001 | 1.59 (1.41–1.80) | < 0.001 | 1.57(1.39–1.78) | < 0.001 |
| Quartiles | |||||||
| Q1(≤ 84.10) | 33/614 | 1.00 | 1.00 | 1.00 | |||
| Q2(84.20–90.00) | 59/599 | 1.89 (1.23–2.89) | 0.003 | 1.75 (1.14–2.68) | 0.010 | 1.72 (1.12–2.65) | 0.013 |
| Q3(90.10–98.00) | 85/559 | 2.99 (2.00–4.47) | < 0.001 | 2.95 (1.97–4.43) | < 0.001 | 2.88 (1.92–4.32) | < 0.001 |
| Q4(> 98.00) | 112/556 | 4.05 (2.75–5.97) | < 0.001 | 4.10 (2.74–6.12) | < 0.001 | 3.92 (2.62–5.88) | < 0.001 |
| VAI | |||||||
| Per 1 SD increase | 289/2328 | 1.09 (1.05–1.13) | < 0.001 | 1.11 (1.07–1.16) | < 0.001 | 1.11 (1.06–1.16) | < 0.001 |
| Quartiles | |||||||
| Q1(≤ 1.14) | 22/582 | 1.00 | 1.00 | 1.00 | |||
| Q2(1.15–1.76) | 66/582 | 3.11 (1.92–5.04) | < 0.001 | 3.09 (1.90–5.02) | < 0.001 | 3.03 (1.86–4.93) | < 0.001 |
| Q3(1.77–2.66) | 79/582 | 3.77 (2.35–6.04) | < 0.001 | 4.10 (2.54–6.64) | < 0.001 | 3.97 (2.45–6.44) | < 0.001 |
| Q4(> 2.66) | 122/582 | 6.11 (3.88–9.62) | < 0.001 | 7.14 (4.46–11.41) | < 0.001 | 6.88 (4.27–11.07) | < 0.001 |
| LAP | |||||||
| Per 1 SD increase | 289/2328 | 1.41 (1.32–1.50) | < 0.001 | 1.47 (1.37–1.57) | < 0.001 | 1.46 (1.36–1.57) | < 0.001 |
| Quartiles | |||||||
| Q1(≤ 26.00) | 24/583 | 1.00 | 1.00 | 1.00 | |||
| Q2(26.01–41.15) | 56/581 | 2.40 (1.49–3.88) | < 0.001 | 2.51 (1.55–4.06) | < 0.001 | 2.50 (1.54–4.04) | < 0.001 |
| Q3(41.16–65.00) | 82/582 | 3.58 (2.27–5.64) | < 0.001 | 3.71 (2.35–5.88) | < 0.001 | 3.65 (2.30–5.80) | < 0.001 |
| Q4(> 65.00) | 127/582 | 5.84 (3.78–9.04) | < 0.001 | 6.18 (3.96–9.63) | < 0.001 | 6.03 (3.85–9.47) | < 0.001 |
| CVAI | |||||||
| Per 1 SD increase | 289/2328 | 1.87 (1.68–2.08) | < 0.001 | 1.80 (1.60–2.04) | < 0.001 | 1.78 (1.57–2.01) | < 0.001 |
| Quartiles | |||||||
| Q1(≤ 96.58) | 19/582 | 1.00 | 1.00 | 1.00 | |||
| Q2(96.59–121.59) | 43/582 | 2.34 (1.37–4.02) | 0.002 | 1.88 (1.09–3.24) | 0.023 | 1.91 (1.11–3.30) | 0.020 |
| Q3(121.60–146.38) | 89/582 | 5.05 (3.08–8.29) | < 0.001 | 3.81 (2.31–6.28) | < 0.001 | 3.71 (2.24–6.15) | < 0.001 |
| Q4(> 146.38) | 138/582 | 8.14 (5.04–13.15) | < 0.001 | 5.86 (3.61–9.53) | < 0.001 | 5.67 (3.47–9.27) | < 0.001 |
Model 1: crude model; Model 2: adjusted for gender, age, ethnicity, education, smoking status, and drinking status; Model 3: adjusted for gender, age, ethnicity, education, smoking status, drinking status, low-density lipoprotein cholesterol, total cholesterol, fasting blood glucose, systolic blood pressure, diastolic blood pressure, physical activity, antidiabetic agents, and diabetes duration
HR hazard ratio, CI confidence interval, SD standard deviation, WC waist circumference, VAI visceral adiposity index, LAP lipid accumulation product, CVAI Chinese visceral adiposity index
In subgroup analyses, the positive correlations between abdominal obesity indices and incident CV events among people with T2D were consistent across the subgroups, including age (< 60 years vs. ≥ 60 years), ethnicity (Han vs. minorities), BMI (< 25 kg/m2 vs. ≥ 25 kg/m2), and the usage of antidiabetic agents (no vs. yes) (Fig. 3). Notably, significant interactions existed between the abdominal obesity indicators and BMI regarding the risk of CV events among people with T2D (all P values for interaction < 0.05). Compared to the overweight/obese group, a stronger association of each abdominal obesity index with incident CV events was found in the subgroup with BMI < 25 kg/m2(Fig. 3).
Fig.3.
Association between abdominal obesity indices and cardiovascular events among people with type 2 diabetes in different subgroups. Each subgroup was adjusted for gender, age, ethnicity, education, smoking status, drinking status, low-density lipoprotein cholesterol, total cholesterol, fasting blood glucose, systolic blood pressure, diastolic blood pressure, physical activity, antidiabetic agents, and diabetes duration, except for stratification variables. Hazard ratios are presented as per 1 SD increase in the abdominal obesity indices for cardiovascular events. BMI, body mass index; CI, confidence interval; HR, hazard ratio; WC, waist circumference; VAI, visceral adiposity index; LAP, lipid accumulation product; CVAI, Chinese visceral adiposity index
Predictive performance of abdominal obesity indices for cardiovascular events in people with type 2 diabetes
Table 3 shows the predictive performance of BMI, WC, VAI, LAP, and CVAI for incident CV events in people with T2D. Our results indicated that the C-statistic of CVAI, VAI, LAP, WC, and BMI was 0.700 (95% CI 0.672–0.728), 0.656 (95% CI 0.627–0.685), 0.670 (95% CI 0.641–0.700), 0.638 (95% CI 0.608–0.668), and 0.535 (95% CI 0.501–0.569), respectively. Furthermore, CVAI had the largest C-statistic compared to VAI, LAP, WC, and BMI (all P for comparison < 0.05) (Table 3).
Table 3.
Predictive performance of abdominal obesity indices for incident cardiovascular events in people with type 2 diabetes
| Indices | C-statistic (95% CI) |
P-value | P for comparison |
|---|---|---|---|
| CVAI | 0.700 (0.672–0.728) | < 0.001 | Ref. |
| VAI | 0.656 (0.627–0.685) | < 0.001 | 0.012 |
| LAP | 0.670 (0.641–0.700) | < 0.001 | 0.035 |
| WC | 0.638 (0.608–0.668) | < 0.001 | < 0.001 |
| BMI | 0.535 (0.501–0.569) | < 0.001 | < 0.001 |
CI, confidence interval; C-statistic Harrell's concordance statistic, LAP lipid accumulation product, VAI visceral adiposity index, CVAI Chinese visceral adiposity index, WC waist circumference, BMI body mass index
Incremental predictive value of abdominal obesity indices in the risk assessment of cardiovascular events among people with type 2 diabetes
The incremental predictive values of abdominal obesity indices for CV events are summarized in Table 4. The C-statistics of the basic model were significantly enhanced by adding WC, LAP, or CVAI (all P < 0.05). Moreover, after adding WC, LAP, and CVAI to the basic model, IDI showed significant improvements of 2.6% (95% CI 0.012–0.046, P < 0.001), 4.2% (95% CI 0.026–0.062, P < 0.001), and 4.3% (95% CI 0.025–0.066, P < 0.001) respectively. Meanwhile, NRI was also significant for WC (0.217, 95% CI 0.133–0.273, P < 0.001), LAP (0.306, 95% CI 0.213–0.381, P < 0.001), and CVAI (0.266, 95% CI 0.187–0.332, P < 0.001) (Table 4). When added to the basic model, VAI did not significantly improve the C-statistics (P = 0.169) but had a relatively small incremental impact on discrimination and reclassification ability.
Table 4.
Improvement in discrimination and risk reclassification for cardiovascular events after adding abdominal obesity indices
| Model | C-statistic (95% CI) |
P-value | IDI (95% CI) |
P-value | NRI (95% CI) |
P-value |
|---|---|---|---|---|---|---|
| Basic model | 0.701 (0.672–0.731) | Ref. | Ref. | Ref. | ||
| + WC | 0.735 (0.708–0.762) | < 0.001 | 0.026 (0.012–0.046) | < 0.001 | 0.217 (0.133–0.273) | < 0.001 |
| + VAI | 0.709 (0.679–0.738) | 0.169 | 0.006 (0.001–0.013) | 0.014 | 0.249 (0.099–0.349) | 0.008 |
| + LAP | 0.740 (0.713–0.768) | < 0.001 | 0.042 (0.026–0.062) | < 0.001 | 0.306 (0.213–0.381) | < 0.001 |
| + CVAI | 0.751 (0.724–0.777) | < 0.001 | 0.043 (0.025–0.066) | < 0.001 | 0.266 (0.187–0.332) | < 0.001 |
The basic model included gender, age, ethnicity, education, smoking status, drinking status, low-density lipoprotein cholesterol, total cholesterol, fasting blood glucose, systolic blood pressure, diastolic blood pressure, physical activity, antidiabetic agents, and diabetes duration
CI confidence interval, C-statistic Harrell's concordance statistic, IDI integrated discrimination improvement, NRI net reclassification improvement, WC waist circumference, VAI visceral adiposity index, LAP lipid accumulation product, CVAI Chinese visceral adiposity index
Sensitive analyses
In the competing risk analysis, the associations of abdominal obesity indices with CV events in people with T2D remained unchanged (Additional file 1: Fig. S2). In addition, we repeated the primary analyses after multiple imputation of missing covariates at baseline (Additional file 1: Tables S1-S3). We found that the positive correlations between abdominal obesity indices and incident CV events among people with T2D remained significant (Additional file 1: Table S1). The CVAI index had the optimal predictive capacity (C-statistic: 0.701, 95% CI 0.674–0.729, all P for comparison < 0.05) for CV events in people with T2D, and showed the largest incremental predictive value beyond the conventional risk factors for CV events (C-statistic: 0.752 vs. 0.704, P < 0.001; IDI: 4.3%, P < 0.001; NRI: 25.5%, P < 0.001) (Additional file 1: Tables S2-S3).
Discussion
In this multiethnic cohort study, we investigated and compared the predictive performance of abdominal adiposity indices for incident CV events among people with T2D. Our results indicated that all abdominal obesity indicators were positively correlated with the risk of CV events among people with T2D. Further subgroup analysis revealed that these associations remained significant in different subgroups, including age, ethnicity, BMI, and usage of antidiabetic agents. Moreover, the CVAI index exhibited the greatest power in predicting CV events compared with BMI, WC, VAI, and LAP. When the abdominal obesity index was added to the basic risk model, the CVAI index showed the largest incremental effect on CV events risk stratification in people with T2D compared to WC, VAI, and LAP.
China has the largest obese population in the world, with more than 15% of adults suffering from overall obesity and more than 35% suffering from abdominal obesity from 2014 to 2018 [35]. Increasing evidence supported that abdominal fat accumulation was more strongly correlated with the risk of diabetes and CVD than overall obesity [36–38]. As an indicator of overall obesity, BMI fails to differentiate between muscle and fat mass, and its increase might be attributed to muscle formation rather than fat deposition from overeating [39]. Although previous studies revealed that the surrogate indicators of abdominal fat deposition have stronger predictive accuracy for CVD in the general population compared to BMI [22, 24, 40], this issue is unclear in the T2D population. Considering that people with T2D are more likely to be obese than non-T2D patients [41], studies from the general population may not be appropriate for people with T2D. Our study adds to the existing evidence indicating that abdominal adiposity indices are correlated with incident CV events and superior to BMI in predicting the risk of CV events among people with T2D. In addition, the subgroup analyses showed that WC, VAI, LAP, and CVAI had a stronger association with CV events in participants with BMI < 25 kg/m2 compared to overweight/obese participants. A previous study discovered that CVD mortality in adults with diabetes increased from a BMI greater than or equal to 24.8 kg/m2 [42], which might lead to a relatively lower additional risk of CVD caused by abdominal obesity in the overweight/obese population (BMI ≥ 25 kg/m2). Similarly, a prospective cohort study in Ommoord also found a stronger association of WC, VAI, and LAP with incident diabetes among non-obese individuals [19]. These data underline the importance of assessing the abdominal fat deposition in people with T2D, even among people with normal weight.
Our results indicated, among all abdominal adiposity indices, that CVAI not only had the best ability to predict incident CV events in people with T2D but also had the greatest incremental risk stratification when added to the conventional risk model. A potential explanation for these findings could be that the CVAI, constructed based on the visceral fat volume detected by computed tomography (CT) examination, is a reliable surrogate indicator to assess visceral fat mass in the Chinese population [21]. Abdominal fat accumulation includes both subcutaneous and visceral adipose tissue, of which visceral adiposity contributes more to the development of CVD than subcutaneous fat [43]. However, WC only reflects the amount of abdominal fat and fails to distinguish the amount of visceral and subcutaneous fat. LAP was established based on population frequency charts of WC and triglyceride concentrations, which could reflect central lipid accumulation and toxicity, but remained deficient in differentiating visceral from subcutaneous fat [25, 32]. In this study, although VAI was correlated with the development of CV events among people with T2D, it had a weaker predictive value for CV events than CVAI and LAP. In addition, we found that VAI did not significantly improve the C-statistic when added to the basic risk model of CV events. The VAI was designed to evaluate visceral adipose tissue in Caucasians [31], an index that combined anthropometric and metabolic measurements. Because of significant differences in adipose tissue distribution among ethnic groups [44], VAI might not accurately reflect the visceral adipose tissue of Chinese adults. Indeed, previous evidence demonstrated that VAI was inferior to CVAI, BMI, and WC in evaluating visceral adipose accumulation among Chinese adults [21].
Several underlying mechanisms were proposed to explain the association between abdominal obesity and CV events. First, macrophages often infiltrate abdominal adipose tissue, causing increased production of pro-inflammatory cytokines, such as interleukin-6 and tumor necrosis factor-α, which may induce oxidative stress and endothelial dysfunction, ultimately facilitating the development of atherosclerosis [45]. Second, visceral adipose tissue accumulation could promote changes in adipokines (leptin and adiponectin) expression, another factor contributing to inflammation and endothelial dysfunction. Available evidence indicated that leptin, a bioactive substance derived from visceral adipose tissue, could promote arterial thrombosis and vascular cell calcification [45]. Adiponectin is an anti-inflammatory adipokine, and excess visceral adipose decreases the secretion of adiponectin [46], which may enhance vascular reactive oxygen species production and impairment of endothelial function. Third, visceral adipose tissue accumulation increases the production of oxidized low-density lipoprotein (OxLDL) [45], which leads to monocyte chemotaxis, inflammation, endothelial injury, and vascular remodeling [47], thereby promoting atherosclerosis.
Some strengths in this study should be acknowledged. Primarily, our study is based on a prospective cohort of individuals with T2D, which could interpret the longitudinal associations of abdominal obesity indices with incident CV events. In addition, this study included a medium-sized, socio-economically diverse, and multiethnic Chinese population, which extends the population applicability of the findings. Finally, we adjusted for underlying confounding factors in analyses where possible, as well as performed subgroup and sensitivity analyses to guarantee the robustness of the results. However, the current study also had several limitations. First, we did not use CT and magnetic resonance imaging (MRI) to validate the consistency between the actual amount of visceral fat tissue and these abdominal obesity indices. As a result, values of abdominal obesity indices may be subject to measurement bias. Second, this observational study limits causal inferences between abdominal obesity indices and CV events in people with T2D. Third, despite adjusting for potential risk factors in multivariate analyses, some residual or unassessed confounding variables still could not be excluded. In addition, the number of outcomes events was small to establish a robust relationship between the abdominal obesity index and different subtypes of CVD, such as myocardial infarction and stroke. Finally, all participants in this study were Chinese, so our results might not be directly extendable to other populations.
Conclusions
Overall, this prospective cohort study showed that all abdominal obesity indicators were significantly associated with the risk of CV events among people with T2D. Among these abdominal obesity indicators, CVAI exhibited the best performance for predicting incident CV events. The evidence from this study suggested that CVAI might be a valuable abdominal obesity indicator for identifying the high risk of CV events in Chinese populations with T2D. In public health practice, we could consider reducing abdominal fat deposition in people with T2D as an intervention measure to prevent CV events, particularly in people with normal weight. Large randomized clinical trials are needed to confirm the hypothesis.
Supplementary Information
Additional file 1: Figure S1. Restricted cubic splines analysis of the relationship between abdominal obesity indices and the risk of cardiovascular events among people with type 2 diabetes. Figure S2. Subdistribution hazard ratios for the association between abdominal obesity indices and cardiovascular events in people with type 2 diabetes. Table S1. Association between baseline abdominal obesity indices and incident cardiovascular events in people with type 2 diabetes. Table S2. Predictive performance of abdominal obesity indices for incident cardiovascular events in people with type 2 diabetes. Table S3. Improvement in discrimination and risk reclassification for cardiovascular events after adding abdominal obesity indices. Table S4. Checklist of items that should be reported in cohort studies according to the STROBE statement.
Acknowledgements
The authors gratefully acknowledge all researchers and participants of the Xinjiang Multi-Ethnic Cohort study.
Abbreviations
- T2D
Type 2 diabetes
- CVD
Cardiovascular disease
- BMI
Body mass index
- WC
Waist circumference
- VAI
Visceral adiposity index
- LAP
Lipid accumulation product
- CVAI
Chinese visceral adiposity index
- XMC
The Xinjiang Multi-Ethnic Cohort
- FBG
Fasting blood glucose
- TG
Triglycerides
- TC
Total cholesterol
- HDL-C
High-density lipoprotein cholesterol
- LDL-C
Low-density lipoprotein cholesterol
- HR
Hazard ratio
- CI
Confidence interval
- SD
Standard deviation
- C-statistic
Harrell's concordance statistic
- NRI
Net reclassification improvement
- IDI
Integrated discrimination improvement
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- CT
Computed tomography
- OxLDL
Oxidized low-density lipoprotein
- MRI
Magnetic resonance imaging
Author contributions
TQ and JD designed the study. TQ and TL performed the data analyses. TQ, BY, AA, HZ, and DA collected the data. TQ, TL, and HP wrote the manuscript. TQ, DW, and JD revised the manuscript. JD supervised the study. All authors read and approved the final manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (NO. 82160640), the National Key Research and Development Program of China (NO. SQ2017YFSF090013 and 2017YFC0907203), and the Key Laboratory of Special Environment and Health Research in Xinjiang.
Availability of data and materials
The datasets analysed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of the Traditional Chinese Medical Hospital of Xinjiang Uygur Autonomous Region (2018XE0108). All participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tingting Qiao and Tao Luo contributed equally to this work
References
- 1.Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, et al. IDF diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah AD, Langenberg C, Rapsomaniki E, Denaxas S, Pujades-Rodriguez M, Gale CP, Deanfield J, Smeeth L, Timmis A, Hemingway H. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015;3(2):105–13. doi: 10.1016/S2213-8587(14)70219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 4.Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–41. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17(1):83. doi: 10.1186/s12933-018-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kivimaki M, Strandberg T, Pentti J, Nyberg ST, Frank P, Jokela M, Ervasti J, Suominen SB, Vahtera J, Sipila PN, et al. Body-mass index and risk of obesity-related complex multimorbidity: an observational multicohort study. Lancet Diabetes Endocrinol. 2022;10(4):253–263. doi: 10.1016/S2213-8587(22)00033-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costanzo P, Cleland JG, Pellicori P, Clark AL, Hepburn D, Kilpatrick ES, Perrone-Filardi P, Zhang J, Atkin SL. The obesity paradox in type 2 diabetes mellitus: relationship of body mass index to prognosis: a cohort study. Ann Intern Med. 2015;162(9):610–618. doi: 10.7326/M14-1551. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Y, Qie R, Han M, Huang S, Wu X, Zhang Y, Feng Y, Yang X, Li Y, Wu Y, et al. Association of BMI with cardiovascular disease incidence and mortality in patients with type 2 diabetes mellitus: a systematic review and dose-response meta-analysis of cohort studies. Nutr Metab Cardiovasc Dis. 2021;31(7):1976–1984. doi: 10.1016/j.numecd.2021.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Eeg-Olofsson K, Cederholm J, Nilsson PM, Zethelius B, Nunez L, Gudbjornsdottir S, Eliasson B. Risk of cardiovascular disease and mortality in overweight and obese patients with type 2 diabetes: an observational study in 13,087 patients. Diabetologia. 2009;52(1):65–73. doi: 10.1007/s00125-008-1190-x. [DOI] [PubMed] [Google Scholar]
- 10.Perotto M, Panero F, Gruden G, Fornengo P, Lorenzati B, Barutta F, Ghezzo G, Amione C, Cavallo-Perin P, Bruno G. Obesity is associated with lower mortality risk in elderly diabetic subjects: the Casale Monferrato study. Acta Diabetol. 2013;50(4):563–568. doi: 10.1007/s00592-011-0338-1. [DOI] [PubMed] [Google Scholar]
- 11.Shen Y, Shi L, Nauman E, Katzmarzyk PT, Price-Haywood EG, Bazzano AN, Nigam S, Hu G. Association between body mass index and stroke risk among patients with type 2 diabetes. J Clin Endocrinol Metab. 2020;105(1):96–105. doi: 10.1210/clinem/dgz032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu XM, Liu YJ, Zhan J, He QQ. Overweight, obesity and risk of all-cause and cardiovascular mortality in patients with type 2 diabetes mellitus: a dose-response meta-analysis of prospective cohort studies. Eur J Epidemiol. 2015;30(1):35–45. doi: 10.1007/s10654-014-9973-5. [DOI] [PubMed] [Google Scholar]
- 13.Polemiti E, Baudry J, Kuxhaus O, Jager S, Bergmann MM, Weikert C, Schulze MB. BMI and BMI change following incident type 2 diabetes and risk of microvascular and macrovascular complications: the EPIC-potsdam study. Diabetologia. 2021;64(4):814–825. doi: 10.1007/s00125-020-05362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandramouli C, Tay WT, Bamadhaj NS, Tromp J, Teng TK, Yap JJL, MacDonald MR, Hung CL, Streng K, Naik A, et al. Association of obesity with heart failure outcomes in 11 Asian regions: a cohort study. PLoS Med. 2019;16(9):e1002916. doi: 10.1371/journal.pmed.1002916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silveira EA, Kliemann N, Noll M, Sarrafzadegan N, de Oliveira C. Visceral obesity and incident cancer and cardiovascular disease: an integrative review of the epidemiological evidence. Obes Rev. 2021;22(1):e13088. doi: 10.1111/obr.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koenen M, Hill MA, Cohen P, Sowers JR. Obesity, adipose tissue and vascular dysfunction. Circ Res. 2021;128(7):951–968. doi: 10.1161/CIRCRESAHA.121.318093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, Santos RD, Arsenault B, Cuevas A, Hu FB, et al. Waist circumference as a vital sign in clinical practice: a consensus statement from the IAS and ICCR working group on visceral obesity. Nat Rev Endocrinol. 2020;16(3):177–189. doi: 10.1038/s41574-019-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilding JPH, Jacob S. Cardiovascular outcome trials in obesity: a review. Obes Rev. 2021;22(1):e13112. doi: 10.1111/obr.13112. [DOI] [PubMed] [Google Scholar]
- 19.Brahimaj A, Rivadeneira F, Muka T, Sijbrands EJG, Franco OH, Dehghan A, Kavousi M. Novel metabolic indices and incident type 2 diabetes among women and men: the Rotterdam study. Diabetologia. 2019;62(9):1581–1590. doi: 10.1007/s00125-019-4921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun K, Lin D, Feng Q, Li F, Qi Y, Feng W, Yang C, Yan L, Ren M, Liu D. Assessment of adiposity distribution and its association with diabetes and insulin resistance: a population-based study. Diabetol Metab Syndr. 2019;11:51. doi: 10.1186/s13098-019-0450-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia MF, Chen Y, Lin HD, Ma H, Li XM, Aleteng Q, Li Q, Wang D, Hu Y, Pan BS, et al. A indicator of visceral adipose dysfunction to evaluate metabolic health in adult Chinese. Sci Rep. 2016;6:38214. doi: 10.1038/srep38214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kyrou I, Panagiotakos DB, Kouli GM, Georgousopoulou E, Chrysohoou C, Tsigos C, Tousoulis D, Pitsavos C. Lipid accumulation product in relation to 10-year cardiovascular disease incidence in Caucasian adults: the ATTICA study. Atherosclerosis. 2018;279:10–16. doi: 10.1016/j.atherosclerosis.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 23.Kouli GM, Panagiotakos DB, Kyrou I, Georgousopoulou EN, Chrysohoou C, Tsigos C, Tousoulis D, Pitsavos C. Visceral adiposity index and 10-year cardiovascular disease incidence: the ATTICA study. Nutr Metab Cardiovasc Dis. 2017;27(10):881–889. doi: 10.1016/j.numecd.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Lee Y, Wu Y, Zhang X, Jin C, Huang Z, Wang Y, Wang Z, Kris-Etherton P, Wu S, et al. A prospective study of waist circumference trajectories and incident cardiovascular disease in China: the Kailuan Cohort study. Am J Clin Nutr. 2021;113(2):338–347. doi: 10.1093/ajcn/nqaa331. [DOI] [PubMed] [Google Scholar]
- 25.Wan H, Wang Y, Xiang Q, Fang S, Chen Y, Chen C, Zhang W, Zhang H, Xia F, Wang N, et al. Associations between abdominal obesity indices and diabetic complications: Chinese visceral adiposity index and neck circumference. Cardiovasc Diabetol. 2020;19(1):118. doi: 10.1186/s12933-020-01095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hosseinpanah F, Barzin M, Mirbolouk M, Abtahi H, Cheraghi L, Azizi F. Lipid accumulation product and incident cardiovascular events in a normal weight population: Tehran Lipid and Glucose Study. Eur J Prev Cardiol. 2016;23(2):187–193. doi: 10.1177/2047487314558771. [DOI] [PubMed] [Google Scholar]
- 27.Yi X, Ling J, Meng H, Wu L, Zhu S, Zhu L. Lipid accumulation product predicts diabetes remission after bariatric surgery in Chinese patients with BMI < 35 kg/m(2): a Multicenter Cohort Study. Obes Surg. 2022;32(6):1935–1943. doi: 10.1007/s11695-022-06003-1. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Hong F, Liu L, Nie F, Du L, Guan H, Wang Z, Zeng Q, Yang J, Wang J, et al. Lipid accumulation product is a reliable indicator for identifying metabolic syndrome: the China Multi-Ethnic Cohort (CMEC) Study. QJM. 2022;115(3):140–147. doi: 10.1093/qjmed/hcaa325. [DOI] [PubMed] [Google Scholar]
- 29.Tao L, Tian T, Liu L, Zhang Z, Sun Q, Sun G, Dai J, Yan H. Cohort profile: the Xinjiang multiethnic cohort (XMC) study. BMJ Open. 2022;12(5):e048242. doi: 10.1136/bmjopen-2020-048242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi Y, Choi JW. Association of sleep disturbance with risk of cardiovascular disease and all-cause mortality in patients with new-onset type 2 diabetes: data from the Korean NHIS-HEALS. Cardiovasc Diabetol. 2020;19(1):61. doi: 10.1186/s12933-020-01032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, Galluzzo A, AlkaMeSy Study G Visceral adiposity index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33(4):920–2. doi: 10.2337/dc09-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kahn HS. The "lipid accumulation product" performs better than the body mass index for recognizing cardiovascular risk: a population-based comparison. BMC Cardiovasc Disord. 2005;5:26. doi: 10.1186/1471-2261-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guasch-Ferre M, Liu G, Li Y, Sampson L, Manson JE, Salas-Salvado J, Martinez-Gonzalez MA, Stampfer MJ, Willett WC, Sun Q, et al. Olive oil consumption and cardiovascular risk in U.S Adults. J Am Coll Cardiol. 2020;75(15):1729–39. doi: 10.1016/j.jacc.2020.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
- 35.Mu L, Liu J, Zhou G, Wu C, Chen B, Lu Y, Lu J, Yan X, Zhu Z, Nasir K, et al. Obesity prevalence and risks among Chinese adults: findings from the China PEACE million persons project, 2014–2018. Circ Cardiovasc Qual Outcomes. 2021;14(6):e007292. doi: 10.1161/CIRCOUTCOMES.120.007292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cornier MA, Despres JP, Davis N, Grossniklaus DA, Klein S, Lamarche B, Lopez-Jimenez F, Rao G, St-Onge MP, Towfighi A, et al. Assessing adiposity: a scientific statement from the American heart association. Circulation. 2011;124(18):1996–2019. doi: 10.1161/CIR.0b013e318233bc6a. [DOI] [PubMed] [Google Scholar]
- 37.Decoda Study G. Nyamdorj R, Qiao Q, Lam TH, Tuomilehto J, Ho SY, Pitkaniemi J, Nakagami T, Mohan V, Janus ED, et al. BMI compared with central obesity indicators in relation to diabetes and hypertension in Asians. Obesity (Silver Spring) 2008;16(7):1622–1635. doi: 10.1038/oby.2008.73. [DOI] [PubMed] [Google Scholar]
- 38.Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366(9497):1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 39.Nevill AM, Stewart AD, Olds T, Holder R. Relationship between adiposity and body size reveals limitations of BMI. Am J Phys Anthropol. 2006;129(1):151–156. doi: 10.1002/ajpa.20262. [DOI] [PubMed] [Google Scholar]
- 40.Huang YC, Huang JC, Lin CI, Chien HH, Lin YY, Wang CL, Liang FW, Dai CY, Chuang HY. Comparison of innovative and traditional cardiometabolic indices in estimating atherosclerotic cardiovascular disease risk in adults. Diagnostics (Basel) 2021;11(4):603. doi: 10.3390/diagnostics11040603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xing Z, Pei J, Huang J, Peng X, Chen P, Hu X. Relationship of obesity to adverse events among patients with mean 10-year history of type 2 diabetes mellitus: results of the ACCORD Study. J Am Heart Assoc. 2018;7(22):e010512. doi: 10.1161/JAHA.118.010512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dahlstrom EH, Sandholm N, Forsblom CM, Thorn LM, Jansson FJ, Harjutsalo V, Groop PH. Body mass index and mortality in individuals with type 1 diabetes. J Clin Endocrinol Metab. 2019;104(11):5195–5204. doi: 10.1210/jc.2019-00042. [DOI] [PubMed] [Google Scholar]
- 43.Powell-Wiley TM, Poirier P, Burke LE, Despres JP, Gordon-Larsen P, Lavie CJ, Lear SA, Ndumele CE, Neeland IJ, Sanders P, et al. Obesity and cardiovascular disease: a scientific statement from the American heart association. Circulation. 2021;143(21):e984–e1010. doi: 10.1161/CIR.0000000000000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chirinos DA, Llabre MM, Goldberg R, Gellman M, Mendez A, Cai J, Sotres-Alvarez D, Daviglus M, Gallo LC, Schneiderman N. Defining abdominal obesity as a risk factor for coronary heart disease in the U.S: results from the hispanic community health study/study of Latinos (HCHS/SOL) Diabetes Care. 2020;43(8):1774–80. doi: 10.2337/dc19-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 46.Mathieu P, Lemieux I, Despres JP. Obesity, inflammation, and cardiovascular risk. Clin Pharmacol Ther. 2010;87(4):407–416. doi: 10.1038/clpt.2009.311. [DOI] [PubMed] [Google Scholar]
- 47.Couillard C, Ruel G, Archer WR, Pomerleau S, Bergeron J, Couture P, Lamarche B, Bergeron N. Circulating levels of oxidative stress markers and endothelial adhesion molecules in men with abdominal obesity. J Clin Endocrinol Metab. 2005;90(12):6454–6459. doi: 10.1210/jc.2004-2438. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Restricted cubic splines analysis of the relationship between abdominal obesity indices and the risk of cardiovascular events among people with type 2 diabetes. Figure S2. Subdistribution hazard ratios for the association between abdominal obesity indices and cardiovascular events in people with type 2 diabetes. Table S1. Association between baseline abdominal obesity indices and incident cardiovascular events in people with type 2 diabetes. Table S2. Predictive performance of abdominal obesity indices for incident cardiovascular events in people with type 2 diabetes. Table S3. Improvement in discrimination and risk reclassification for cardiovascular events after adding abdominal obesity indices. Table S4. Checklist of items that should be reported in cohort studies according to the STROBE statement.
Data Availability Statement
The datasets analysed during the current study are available from the corresponding author upon reasonable request.