Abstract
Tumors are comprised of both cancer cells and surrounding stromal components. As an essential part of the tumor microenvironment, the tumor stroma is highly dynamic, heterogeneous and commonly tumor-type specific, and it mainly includes noncellular compositions such as the extracellular matrix and the unique cancer-associated vascular system as well as a wide variety of cellular components including activated cancer-associated fibroblasts, mesenchymal stromal cells, pericytes. All these elements operate with each other in a coordinated fashion and collectively promote cancer initiation, progression, metastasis and therapeutic resistance. Over the past few decades, numerous studies have been conducted to study the interaction and crosstalk between stromal components and neoplastic cells. Meanwhile, we have also witnessed an exponential increase in the investigation and recognition of the critical roles of tumor stroma in solid tumors. A series of clinical trials targeting the tumor stroma have been launched continually. In this review, we introduce and discuss current advances in the understanding of various stromal elements and their roles in cancers. We also elaborate on potential novel approaches for tumor-stroma-based therapeutic targeting, with the aim to promote the leap from bench to bedside.
Keywords: Tumor stroma, Extracellular matrix, cancer-associated fibroblasts, Mesenchymal stromal cells, Pericytes, cancer therapy, Targeted therapy, Clinical trial
Introduction
Although tremendous progress has been achieved, cancer remains a multifactorial disease with limited therapeutic strategies and one of the leading causes of premature death. In 2020, there were an estimated 19.3 million new cancer cases and approximately 10.0 million deaths caused by cancer worldwide [1], which indicated that malignant tumors seriously threaten public health. Therefore, it is necessary to comprehensively investigate the sophisticated pathogenesis of malignancies and develop effective approaches for cancer treatment.
Dating back to the 1880s, Stephen Paget proposed the “seed and soil” hypothesis and revealed that certain tumor cells displayed preferential affinity to invade specific organs, highlighting the critical role of the microenvironment in regulating metastasis growth [2, 3]. Nowadays, it is widely accepted that the tumor microenvironment (TME) constitutes the immediate niche surrounding tumor tissues and is implicated in tumorigenesis [4, 5]. As an essential element of the TME, the tumor stroma affects tumor biology and contributes to cancer initiation, progression, metastasis, and therapeutic resistance [6].
The tumor stroma is highly dynamic, heterogeneous and commonly tumor-type specific. It is mainly composed of noncellular compositions such as the extracellular matrix (ECM) and the unique cancer-associated vascular system as well as a diverse cellular components including, but not limited to, activated cancer-associated fibroblasts (CAFs), mesenchymal stromal cells (MSCs), pericytes [7–12]. These abundant stromal components form a dynamic milieu to support cancer progression and can potentially be regarded as biomarkers in cancer [13]. Importantly, the low tumor-stroma ratio (TSR) is remarkably correlated with poorer survival outcomes, and the TSR can be a valuable predictor for evaluating the prognosis and treatment outcome of cancer patients [14–18]. Except for tumor-promoting actions, stromal components can also restrain tumor growth, especially in pancreatic ductal adenocarcinoma, because the complete ablation of stroma resulted in a more invasive tumor phenotype and reduced overall survival [19–22]. In the early stages of tumorigenesis or metastatic dissemination, the stroma can be considered tumor suppressive [6]. However, the tumor stroma is constantly changing rather than a static entity, and our researches mainly focus on the roles and mechanisms by which stromal elements accelerate cancer initiation and progression, aiming at providing theoretical rationales and preclinical evidence for tumor-stroma-targeted therapy [23–25].
Over the past few decades, we have witnessed an exponential increase in the investigation and recognition of the critical role of stroma in solid tumors. Coupled with the significant progress of new insights to explore intrastromal communication, we are beginning to see the deployment of stroma-targeted cancer therapy. A series of clinical trials targeting the tumor stroma have been launched continually. In this review, we detailed introduce current advances in the understanding of various stromal elements and their roles in cancer. Furthermore, we summarize recent knowledge regarding the interplay between those various stromal compartment and elaborate on potential approaches for tumor-stroma-based therapeutic targeting.
Components of the tumor stroma
Tumor tissue is a heterogeneous mixture of both cancer cells and various stromal components. In solid tumors, stromal elements interact with neoplastic cells to influence tumor behavior. Tumor cells can alter their surrounding stroma, forming a permissive microenvironment to support their growth. Interestingly, tumor cells can also transdifferentiate into stromal-like cells through different signal transduction pathways to enhance tumor angiogenesis and facilitate cancer development [26–28].
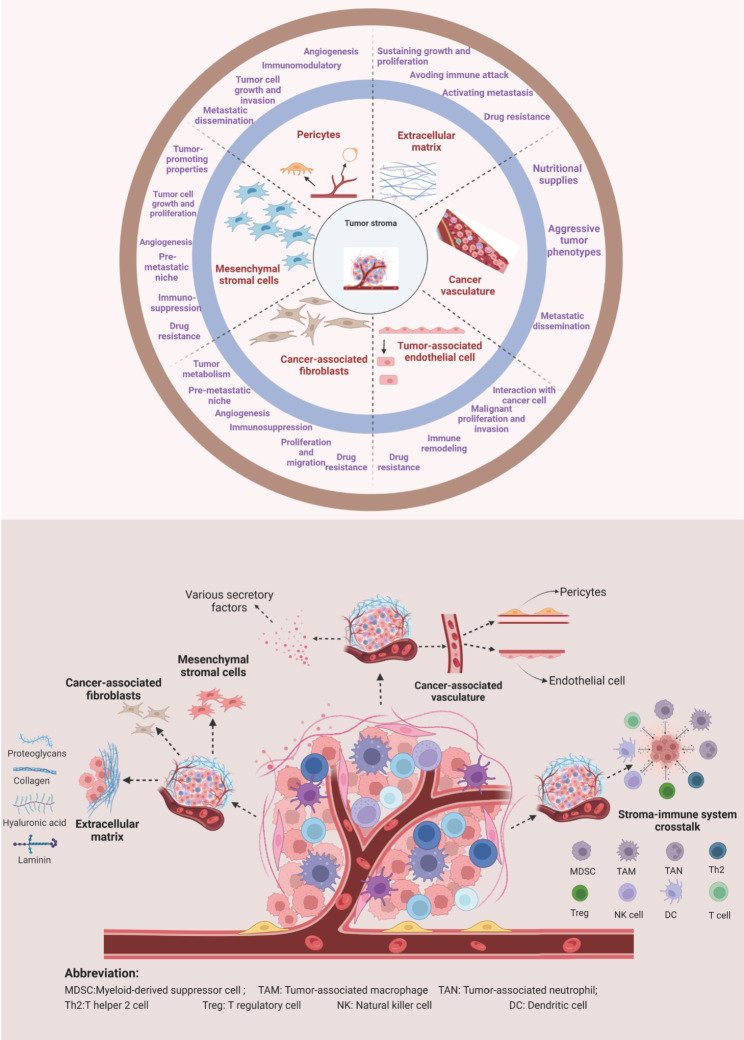
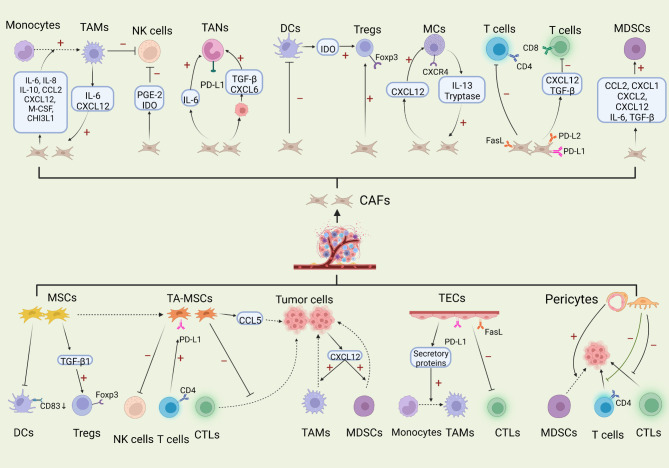
The tumor stroma participates in tumorigenesis, cancer progression, and therapy resistance, and it also profoundly affects many hallmarks of cancer [29–31]. Stromal elements contain the ECM, vasculature, and various cellular components such as activated CAFs, MSCs, pericytes, and osteoblasts. These components affect anti-tumor immune and determine neoplastic progression (Fig. 1). For example, osteoblasts are responsible for attracting cancer cells to bone marrow and driving malignant cells’ bone metastasis [32]. Adipocytes, as a population of active facilitators, affect cancer metabolism and are involved in tumor establishment, progression, and therapeutic resistance [33–36]. In recent years, oncologists have investigated the functions of osteoblasts and adipocytes in cancer, but their detailed description is beyond this Review’s scope. Herein, we mainly focus on the functions of ECM, stromal vasculature, tumor-associated endothelial cells, CAFs, MSCs, and pericytes.
Fig. 1.
Major components of the tumor stroma and their tumor-promoting functions
Extracellular matrix (ECM)
The extracellular matrix has a pivotal role in modulating and maintaining tissue development and homeostasis, but the dysregulation and mechanical features of ECM can determine cancer aggressiveness and impact the sensitivity to drug therapy [37–39]. The altered and stiffened ECM affects virtually every facet of cancer hallmarks including avoiding immune destruction, tumor-promoting inflammation, activating invasion and metastasis, and inducing angiogenesis [29, 40–42]. Therefore, the ECM not only influences the tumor behavior and histopathology but also be regarded as an integral and remarkable feature of cancer [43].
The ECM is an intricate and dynamic structure that is constantly remodeled by the synthesis and degradation of numerous ECM proteins [44, 45]. In general, the complex ECM network consists of fibrillar or non-fibrillar collagens, proteoglycans, glycoproteins, laminins, fibronectins and other macromolecules. Among them, collagens are the most abundant components of ECM [46]. Commonly, the deregulation of ECM homeostasis leads to cancer evolvement through two distinct mechanisms. On the one hand, ample molecules mainly derived from CAFs induce the pro-fibrotic response and result in excessive deposition of ECM, thereby protecting tumor cells from immune destruction and mediating therapeutic resistance. On the other hand, continuous ECM breakdown contributes to reducing the cancerous cell-ECM adhesion, promoting tumor cells’ invasive and migratory abilities, and inducing malignant cells intravasation via the regulation of invadopodia formation [47–51].
It is now accepted that excessive deposition of collagen and crosslinking of fibrillar collagens and elastin result in the dense ECM and increase the stroma stiffness, which has profound impacts on cancer progression [52]. Increased ECM deposition represents a crucial physical barrier that inhibits antitumor immunity [53]. Apart the formation of a natural barrier, the stiff ECM can also increase the expression of PD-L1 in lung cancer cells in a actin-dependent manner, thereby protecting tumor cells from the host immune attack [54]. The ECM together with tumor cell’s architecture also constitute a physical barrier for drug delivery [55]. In PDAC, stiffened ECM can reduce vascular density and induce epithelial-mesenchymal-transition, which results in the embeddedness of vessels into the matrix and subsequently creates a tough barrier to prevent drug perfusion [56, 57]. The stiff matrix can compress the micro blood vessels, and thus impedes the successful access of anti-tumor drugs into core tumor tissues through the vasculature [58, 59]. Tumor cells surrounded by the stroma can adhere to various ECM proteins, which decreases the chemotherapy efficacy, known as cell adhesion-mediated drug resistance [60]. Intriguingly, stiffened ECM can mechanoactivate glycolysis and glutamine metabolism to coordinate the flux of nonessential amino acid in the tumor tissue, which modulates tumor metabolism and potentially provides energies for tumor growth and aggressiveness [61]. Moreover, abundant ECM deposition potentiates the adhesion of metastatic malignant cells to the tumor endothelium, thus promoting cancer intravasation and subsequent metastasis [62].
Tumor cells often exhibit higher mobility in the remodeled ECM. Simultaneously, the remodeled ECM facilitates cancer cell-directed migration toward the vasculature, favoring the metastatic dissemination of these cells [63, 64]. When integrin binds to its ECM ligand, the FAK/Src complex is assembled at the cytoplasmic tail of integrin, which promotes the activation of downstream signals PI3K/AKT and RAS/MEK/ERK circuits to maintain cell survival and migration [65, 66]. Ras Suppressor-1 (RSU-1) is a cell-ECM protein and is obviously upregulated in breast cancer cells embedded in stiffer 3D collagen I gels. RSU-1 silencing resulted in the inhibition of MMP-13 and urokinase plasminogen activator, thereby reducing cancer cell invasion and migration [67]. Furthermore, some matrix metalloproteinases (MMPs) can degrade the ECM network, which mediates the pro-invasive phenotype of cancer cell and augments the cell mobility throughout the ECM [68, 69].
MMPs belong to ECM proteins that are involved in nearly all important steps during carcinogenesis and progression. More than 20 MMPs have been identified so for and most of them exist in the human proteome [70]. The activity of these enzymes is low under normal circumstances, but in the setting of tumor development their activity can be increased. Among all these MMP family numbers, MMP9 and MMP2 are perhaps the best-studied type and they can degrade the IV collagen to regulate ECM remodeling [71, 72]. MMP9 can also accelerate angiogenesis, tumor invasion and metastasis. Given its important role in tumorigenesis, MMP9 is currently considered as a biomarker and a legitimate therapeutic target for many cancer types [73]. Homoplastically, MMP2 induces tumor neovascularization through the activation of pro-angiogenic factors such as vascular endothelial growth factor (VEGF) and TGF-β, and it also promotes the proteolytic degradation of extracellular proteins to drive tumor metastasis [74, 75]. Apart from MMP9 and MMP2, other MMPs that are present in ECM also have tight association with oncogenic advancement. For example, MMP1, MMP3 and MMP10 have been found to promote cancer cell initial invasion and distant dissemination. MMP7 and MMP13 contribute to tumor cell growth and proliferation. Moreover, some MMPs can prevent the apoptosis of early cancer cell, such as MMP7, MMP10 and MMP11 [74]. An alternative key function of MMPs in cancer metastasis is to regulate the formation of invadopodia that is implicated in breaching basement membrane ao as to allow the extravasation and movement of tumor cell through tissues [76–78]. The targeted delivery and exocytosis of MMP2, MMP9 and MMP14 is required for invadopodia maturation, and thus the presence of these MMPs is usually regarded as one of the marks of functional mature invadopodia. In addition, the potency of invadopodia to degrade ECM and facilitate invasion is partially attributed to MMPs appearance [79, 80].
The ECM serves as an indispensable reservoir for many growth factors and cytokines that orchestrate diverse developmental processes and can trigger a series of signal transduction to induce sustained malignant transformation [59, 81]. As such, the degradation of ECM also contributes to tumor development by these secretory factors. For example, transforming growth factor-β (TGF-β), an essential cytokine for the activation of tumor stroma, is significantly overexpressed in the dysregulation ECM and induces immunosuppression within the TME [82]. The release of VEGF is sometimes accompanied by the remodeling ECM and further contributes to angiogenesis [83]. Hepatocyte growth factor (HGF) is a pleiotropic cytokine. Mature HGF retained in the ECM is able to bind its receptor c-MET to mediate cancer progression [84]. Furthermore, Oncostatin M (OSM), a proinflammatory cytokine, was demonstrated to induce the expression of lysyl oxidase like-2 (LOXL2) that catalyzed ECM transformation by crosslinking collagen I. The overexpressed OSM and LOXL2 had an evident correlation with a worse prognosis in patients with breast invasive ductal carcinoma [85]. The dynamic ECM also promotes the presentation of growth factors to their receptors [86, 87].
Cancer-associated vasculature
During malignant transformation, tumor tissues establish sophisticated compositions to support their growth. These compositions include an immunosuppressive TME, a nutritional environment suitable for tumor growth, and the unique cancer-associated vascular system. Angiogenesis is central to the growth and survival of tumor cells and is also the main conduit for tumor metastasis [88]. Approximately five decades ago, Folkman described that neovascularization promoted tumorigenesis and malignant progression. He held the view that destroying tumor angiogenesis could restrict nutrient supplies to malignancies and speculated that anti-angiogenic drugs would have potential therapeutic value for cancer [89].
Tumor tissue’s vascularization is a multidimensional process orchestrated by various molecular and cellular effectors [90]. Compared with normal stroma, the tumor stroma has abundant vasculature. Pancreatic ductal adenocarcinoma (PDAC) represents one of the most stroma-rich cancer types. Cancer-associated vasculature constitutes an integral part of the stroma in PDAC [91]. Clinically, the intratumoral microvessel density (MVD) was associated with adverse prognosis and could be regarded as an independent prognostic factor [92, 93].
The initial step of angiogenesis usually involves the action of diverse angiogenic stimuli such as hypoxia [94]. Under hypoxic conditions, tumor cells constantly consume glucose and then secrete lactate to create an acidic stromal environment that favors angiogenesis. Hypoxia-inducible factor (HIF) has a pivotal role in the responses of tumor cells and stromal cells to hypoxia [95]. HIF-1 was upregulated within a hypoxic environment, which further resulted in increased expression of VEGF and positively affected cancer metastasis [96, 97]. The downregulation of HIF-1α through the CRISPR/Cas9 technique was found to dramatically inhibited the migration of BxPC-3 cells achieved by decreased expression of MMP-9 and VEGF [98]. Therapeutic modalities based on anti-VEGF can repress human PDAC cells’ growth in murine models and reduce microvessel density, ultimately leading to depleted tumor angiogenesis [12].
A wide spectrum of pro-angiogenic factors and related cognate receptors partake in the activation of “angiogenic switch” and the formation of tumor vasculature [99]. Among all pro-angiogenic factors, VEGFs represent one of the most potent angiogenesis inducers and function by binding to their specific receptors VEGFR or co-receptors. VEGFA is the key angiogenesis regulator and the most investigated member of VEGF family [100]. Other key secretory factors involved in abnormal angiogenesis include fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), HGF and angiopoietins [88]. Furthermore, several chemokine signaling axes also contribute to tumor vasculature generation such as CXCLs/CXCR2, SDF1/CXCR4, and CCL2/CCR2 axis directly or indirectly [88].
Cancer-associated vasculature not only provides nutritional supplies for tumor tissues but also accelerates the transformation of pre-malignant to malignant and aggressive tumor phenotypes. Continuous vascular remodeling is an important characteristic of the established microvasculature of growing tumors. During cancer progression, host vasculature can be used as trails for the invasion of glioma cells into adjacent tissues, which make tumor cells acquire an aggressive character [101].
Taken as a whole, while the stromal composition vary across distinct cancer types, some major components are indispensable for solid tumors, especially the cancer-associated vasculature that has been shown to promote tumor growth and mediate the invasive tumor phenotype [23, 102]. All the above preclinical studies indicate that targeting stromal vasculature may be an effective tactic for cancer treatment. Unfortunately, the use of anti-angiogenic drugs to treat cancer patients often shows limited benefit and even has been a clinical failure, which poses a significant challenge in terms of how best to design this therapeutic option to ultimately elicit an efficacious antitumor response [99].
Tumor-associated endothelial cells
Recent studies have identified the central roles of tumor-associated endothelial cells (TECs) in instigating cancer initiation and progression. TECs usually exhibit phenotypes distinct from normal endothelial cells, because they are aneuploid and their centrosomes are abnormal [103]. Functionally, TECs actively promote the proliferative and aggressive capacity of cancer cells, as well as induce resistance to anti-tumor agents [104, 105].
TECs acquire high proliferative and invasive abilities and accelerate tumor cell growth by secreting soluble factors in a paracrine manner [106, 107]. Furthermore, TECs support malignant cell aggressive behavior by mediating the epigenetic dysregulation of secreted molecules and activating metastasis-associated signaling circuits [108, 109]. Activated TECs can also be released in the blood circulation system from the primary tumor mass and accompany with cancer cells to migrate to distant secondary sites [110]. Compared with low-metastatic tumor-derived TECs, those high-metastatic TECs possess higher mRNA expression level of stemness-related gene stem cell antigen and mesenchymal marker CD90, as well as higher levels of vascular secretion factors [111].
TECs mediate drug resistance and convert naive cancer cells into chemoresistant tumor stem-like cells [112, 113]. For a long time, TECs have been recognized as a group of normal diploid cells that would not induce therapeutic resistance. However, mounting researches found that TECs usually showed aneuploidy and had hallmarks of chromosomal instability, which may contribute to therapeutic resistance in antitumor treatment [103, 114, 115]. Indeed, scientists unveiled their drug resistance ability in different cancer types, such as renal carcinoma-derived TECs inducing vincristine resistance and hepatocellular carcinoma-derived TECs resistance to adriamycin, 5-fluorouracil and sorafenib [115–117]. It was also elucidated that TECs could upregulate the expression of p-glycoprotein (p-gp), one of the ABC transporters, to impair antitumor therapy. Inhibition of p-gp with verapamil abrogated TECs resistance, restored the chemosensitivity of tumor cell to paclitaxel and depleted tumor angiogenesis in the mouse model [118, 119].
Altogether, in addition to promoting angiogenesis, TECs serve as key players to partake in various steps of malignant transformation, and this is a relatively unexplored field that can potentially provide crucial insights into tumor progression.
Cancer-associated fibroblasts
Among all stromal cellular components, cancer-associated fibroblasts (CAFs) are one of the prominent and abundant cell populations. Activated fibroblasts found in primary or metastatic tumors are referred to as CAFs. They provide a physical support for cancerous cells and affect cancer initiation, progression and metastasis (Fig. 2) [120–122]. Of note, CAFs exert both protumorigenic and antitumorigenic effects during disparate stages of oncogenic advancement in an organ or context-specific manner, which brings challenges to the area of CAFs-targeted therapy for cancer treatment [123, 124].
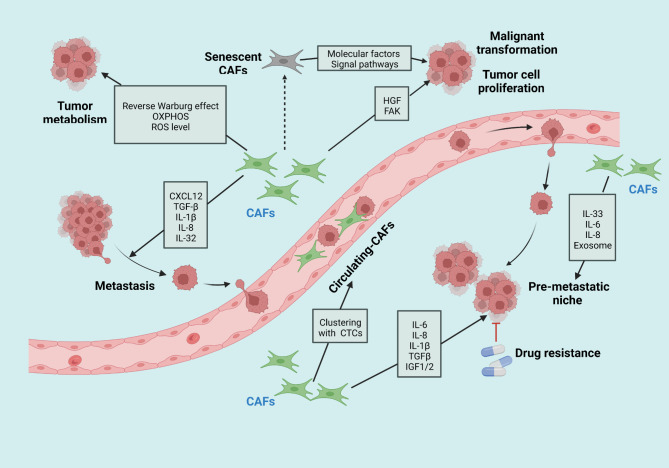
Fig. 2.
A brief summary of CAFs functions and related mechanisms in cancer initiation and progression. Activated CAFs are involved in nearly all stadges of cancer development through diverse means. By releasing numerous secretory factors and activating signaling pathways, CAFs contribute to maliganant transformantion, tumor growth and proliferation, cancer cell invasion, and the establishment of pre-metaststic niche. These pro-tumorigenic cells also affect tumor metabolism in distinct manners as shown in the figure. All these functions collectively determain tumor development and drug resistance
CAFs tend to exhibit heterogeneity and complexity with distinct origins, cellular states and functions. In contrast to normal fibroblasts, CAFs have been described as proliferative, migratory, and highly secretory cells [125]. Although it is generally accepted that most CAFs possibly originate from the activation of local tissue-resident fibroblasts [126], researchers have also identified other major cellular origins of CAFs including endothelial cells [127], adipocytes [128], bone marrow-derived mesenchymal stem cells [129–131], and pancreatic or hepatic stellate cells [132, 133]. Hence, it is difficult to precisely define where these cells originate from.
After being activated by diverse signaling pathways, CAFs derived from various cellular precursors exert many of their functions in cancer [134]. More recently, Silvia Affo et al. discovered that HGF produced by CAFs together with its receptor MET highly expressed in cancer cells instigated the proliferative activity of intrahepatic cholangiocarcinoma (ICC) tumor cells, which is primarily associated with the ERK and AKT phosphorylation. Furthermore, hyaluronan synthase 2 derived from CAFs, but not type I collagen, could effectually regulate the promoted effect on ICC [135]. Intriguingly, tumor cells not only attract fibroblasts and transform them into CAFs but can also be regulated by CAFs to sustain their proliferation and migration [136]. Cancer cell usually undergo metabolic reprogramming during tumorigenesis that can be modulated by CAFs [137]. As opposed to normal cells, tumor cell utilize glycolysis as their preferred energy source, which is often accompanied by increased production of lactate, known as the “Warburg effect” [138, 139]. Recently, Pavlides et al. proposed a novel hypothesis termed as “reverse Warburg effect” that might represent a general feature of CAFs. Tumor cells induce the Warburg effect in adjacent stromal CAFs. Then, in response to changes in the TME, these CAFs secrete pyruvate and lactate that can be used by epithelial cancerous cells to generate energy and enhance their proliferative capacity via oxidative phosphorylation (OXPHOS) [140]. The metabolism of tumor cells is also influenced by CAFs-modulated autophagy and oxidative stress pathway to promote tumor cell proliferation and drug resistance [141–145].
CAFs mediate the invasion and migration of malignant cell and are positively associated with the dedifferentiation and aggressiveness of cancers [136]. Recently, four CAFs subsets, named CAF-S1 to -S4, were identified in metastatic lymph nodes (LNs) of breast cancer (BC). Among them, both CAF-S1 and CAF-S4 subpopulations could be preferentially detected in tumor tissues and were proven to be closely related to tumor cell invasion in a complementary manner [146, 147]. CAF-S1 stimulated BC cell motility and epithelial-mesenchymal transition (EMT) initiation via CXCL12/TGF-β signal whereas CAF-S4 remodeled the matrix and promoted BC cell invasiveness in 3-dimensions via NOTCH-mediated pathways [147]. Currently, our studies about the function of CAFs subsets mainly converge on CAF-S1. In addition, the enrichment of CAF-S1 was correlated positively with PD-1+ and CTLA-4+ CD4+ T cell content but negatively with CD8+ T cells infiltration in tumor. CAF-S1 subset also can enhance the expression of PD-1 and CTLA-4 at the surface of CD4+ CD25+ FOXP3+ T lymphocytes to participate in the formation of immunosuppressive environment within tumor mass[148]. Clinically, the enrichment of CAF-S1 in stroma was significantly correlated with cancer recurrence [149]. Additionally, CAF-S3 subset was mainly detected in juxta-tumors whereas CAF-S2 equally distributed between the tumor mass and juxta-tumors [146]. While the distribution of CAF-S2 and CAF-S3 in tumors have been reported, their specific effects of CAF-S2 and CAF-S3 in tumorigenicity remain to be fully characterized and identified.
Secretory proteins derived from CAFs also partake in tumor cells invasion in an autocrine or paracrine manner. For instance, Lumican, an ECM protein expressed in human gastric CAFs, was found to have promoting effect on GC cells growth and migration in vitro by activating the integrin β1-FAK signal [150]. A great variety of soluble paracrine growth factors, cytokines, and exosomes secreted by CAFs also profoundly impacted malignant cells migratory and aggressive capacity in established tumors. These factors comprise, but are not restricted to, interleukin‑1β (IL-1β), IL-8, IL-32, CXCL12, and TGF-β [151–156].
In metastatic process, the pre-metastatic niche (PMN) acts as a fertile “soil” that supports the homing and engraftment of circulating tumor cells. CAFs are actively involved in the formation of PMN [157, 158]. CAFs-derived IL-33 was responsible for establishing the PMN in lung that facilitated pulmonary metastasis of breast cancer. This promoting effect was associated with type-2 inflammation and the recruitment of diverse immune cells such as eosinophils, neutrophils and inflammatory monocyte to the lung microenvironment [159]. Likewise, in the lung metastatic niche, high-metastatic hepatocellular carcinoma cells typically exhibited great ability to convert normal fibroblasts into CAFs, which was mediated by exosomal miR-1247-3p derived from HCC cells that activated CAFs via the B4GALT3-β1-integrin-NF-κB axis. And then, activated CAFs further accelerated cancer cells diffusion and metastasis by secreting pro-inflammatory IL-6 and IL-8 [160].
Drug resistance remains one of the major hurdles in cancer management. Stromal CAFs have been associated with resistance to anticancer agents by secreting numerous proteins, cytokines and extracellular vesicles. These factors can activate different signaling cascades to protect cancer cells from elimination and possibly cause recurrence [161, 162]. High levels of IL-8 released by CAFs have been identified to be associated with poor response to neoadjuvant chemotherapy. Mechanistically, IL-8-mediated resistance to cisplatin was achieved by NF-κB activation and ATP-binding cassette subfamily B member 1 upregulation [163]. CAFs-derived exosomal miR-98-5p was reported to suppress ovarian cancer (OC) cells apoptosis and promote their proliferative capacity by targeting cyclin-dependent kinase inhibitor 1 A that contributed to the sensitivity of OC cells to cisplatin [164]. Another secretome makes contribution to chemotherapeutic resistance, such as IL-1β [165], IL-6 [166], insulin-like growth factors (IGF) 1 and 2 [167], TGF-β [168], etc. Radiation therapy also leads to expansion and survival of stromal CAFs, which conversely provides signals stimulating malignant cell proliferation and enhances radioresistance.This radioresistance involved various mechanisms including paracrine IGF-1/IGF-1R signaling initiated by CAFs, signaling transduction regulated by exosomal miRNAs or exosomes derived from CAFs, and increased level of ROS mediated by CAFs-derived molecules [169–172].
Circulating-CAFs (cCAFs), similar to circulating tumor cells (CTCs), can be detected in approximately 88% of patients with metastatic breast cancer (BC) and 23% of patients with localized BC. These circulating-CAFs exist as homotypic cCAF clusters individually or present as heterotypic clusters together with CTCs and leukocytes [173]. Sharma et al. unveiled that CD44 acted as an indispensable mediator in cCAF-CTC heterotypic clustering and the cCAF-CTC clusters existed in nearly all clinical stages of BC [174]. Circulating fibroblast-like cells were also detected in blood of metastatic prostate cancer patients and could potentially serve as a prognostic marker [175].
Senescence is another characteristic of CAFs. Senescent CAFs usually acquire tumor-promoting properties, termed as senescence-associated secretory phenotype, which promotes malignant transformation by secreting molecular factors or driving downstream signal pathways [176]. By activating JAK/STAT3 signaling, senescent CAFs (s-CAFs) enhanced GC cells proliferative activity and contributed to peritoneal tumor formation of GC in vivo [177]. Specific induction of s-CAFs apoptosis remarkably enhanced radiosensitivity of non-small cell lung cancer cells [178].
To date, studies on the role of CAFs in cancer progression have gained momentum with increasing attention. Our understanding of their definitive functions in various cancer types will quickly evolve in the near future. The continuous exploration of new pro-tumorigenic molecular mechanisms for CAFs is likely to have profound implications for anticancer therapy.
Mesenchymal stromal cells (MSCs)
Mesenchymal stromal cells (MSCs) represent a group of pluripotent nonhematopoietic stem cells that have self-renewal ability and play substantial roles in tissue regeneration. MSCs have capacity to differentiate into osteoblasts, chondrocytes, or adipocytes in culture and then perform their different functions depending on the circumstances [179, 180]. Notably, MSCs can migrate to tumor tissue where they further evolve into tumorassociated MSCs (TAMSCs) that probably are distinct from those of normal tissue MSCs and have a pro-tumorigenic phenotype [181, 182] (Fig. 3).
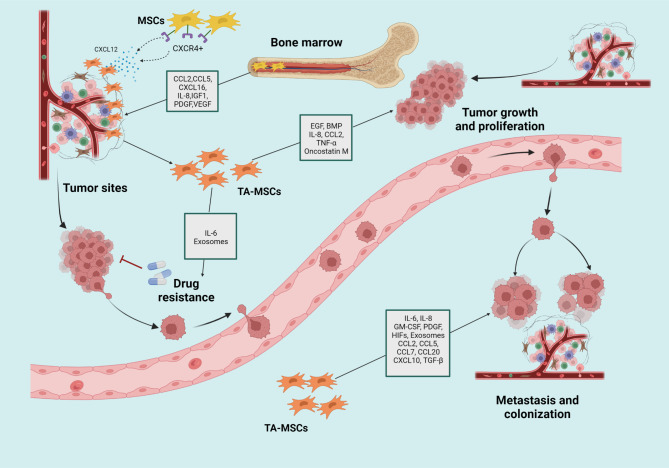
Fig. 3.
The tumor-promoting functions and related mechanisms of MSCs and TA-MSCs
The recruitment and migration of MSCs to tumor sites are affected by chemokines and growth factors, which in turn promotes cancerous development. The CXCL12/CXCR4 axis represents one of the most intensively studied pathways in the tumor tropism of MSCs [183, 184]. Tumor conditioned medium could upregulate CXCL12 expression that facilitated the migration of human BM-MSCs to tumor sites by activating JAK2/STAT3 and MEK/ERK1/2 pathways [185]. The capacity of MSCs to move toward tumor tissue is also mediated by other factors, such as chemokines CCL2, CCL5, CXCL16 [186–188], diffusible cytokine IL-8, as well as growth factors IGF1, PDGF, VEGF, and TGF-β [189, 190].
Compared with normal MSCs, TA-MSCs appear to transform into an “activated” state and undergo epigenetic reprogramming modulated by the TME. This reprogramming mediates a partial mesenchymal-to-epithelial transition that results in enhanced binding of TA-MSCs to cancer cells, thereby effectively favoring the colonization of TA-MSCs and tumor cell complex in metastatic sites [191]. In a mouse lymphoma model, following co-culturing with TA-MSCs, BM-MSCs acquire a tumorpromoting phenotype that depends on the recruitment of macrophages to tumor sites mediated by CCR2 [192]. However, the ability to become TA-MSCs might depend on a particular tissue or cancer type because the observation that breast cancer TME could reprogram BM-MSCs into TA-MSCs that dramatically promoted cancer cells growth whereas the ovarian TME could not [193].
After reaching at the tumor niche, TA-MSCs influence tumor development through direct and indirect manners. The direct cell-to-cell interplay between co-injected MSCs and MDA-MB-231 significantly increased intratumoral cancer cells viability and promoted their proliferation [194]. Following isolating and identifying from the primary tumor tissue, TA-MSCs manifested the homogenous immunophenotype and were shown to have differentiation potential. Furthermore, TA-MSCs could secrete epidermal growth factor (EGF) which activated the downstream PI3K/AKT signaling to modulate cancer cells proliferative activity [195]. TA-MSCs also activate neutrophils with enhanced expression of IL-8, CCL2, TNFα, and oncostatin M, as well as affect the chemotaxis of neutrophils to protect them from spontaneous apoptosis. TA-MSCs-educated neutrophils promote GC cells growth and migration in a cell contact-dependent manner [196]. Moreover, TA-MSCs were determined to facilitate tumor cell proliferation by increasing cancer stem cell numbers and augmenting BMP production [197]. Except for assisting malignantly transformed cell growth as mentioned above, TA-MSCs help cancer cells defend against senescence via the P53/P21 pathway and then prolong their survival cycle [198].
TA-MSCs also play critical roles in creating a favorable condition for successful metastasis of tumor cells. After being isolated from human colon cancer, TA-MSCs were shown to dramatically enhance the invasive activity of HCT-116 cells in vitro. IL-6 existing in the TA-MSCs-conditioned medium induced the enhanced surface expression level of CD44 in HCT-116 and HT-29 cell lines via Notch signaling to promote colon cancer progression [199]. On the basis of the initial inspection that visible tumor metastasis occurred in the tumor cell plus TA-MSCs group rather than the tumor cell-only group, Waghray et al. found that GM-CSF was the only cytokine secreted by the TA-MSCs in all tested patient samples and it can induce tumor cells EMT to drive metastasis [200]. Analogously, TA-MSCs contributed toward the M2 polarization of macrophages, which further significantly augmented the EMT process of GC cells [201]. The mutual transcriptome modulation between MSCs and tumor cells also impacts metastasis process. Tumor cells along with stromal factors partake in promoting normal MSCs conversion toward TA-MSCs, and in turn, TA-MSCs cause the upregulation of tumor metastasis-associated genes in primary lung cancer cells and selectively foster their migration and dissemination [202].
After being stimulated by cancer cells, TA-MSCs can produce the chemokine CCL5, which in turn acts on tumor cells in a paracrine manner to induce their motility and metastatic ability [203]. Other chemokines or cytokines secreted by TA-MSCs have also been shown to drive metastasis, such as CCL2, CCL7, CCL20, CXCL10, TGF-β, PDGF, IL-8, IL-6, and HIFs [204–212]. They collectively act as an extra driving force to support the successful dissemination of tumor cells from primary mass to metastatic sites. Furthermore, TA-MSCs-derived exosomes facilitated cancer cell growth and migration as well as potentially could served as biomarkers for GC [213]. Some scientists have suggested that TA-MSCs play a role in preparing PMNs for cancer cells, but the underlying mechanisms are not fully understood and merit further study for years to come [190].
TA-MSCs render tumor cell resistant to chemotherapy. In addition to sustaining tumor cells growth and assisting their metastatic ability, IL-6 was also reported to reduce cisplatin-triggered apoptosis in breast cancer cells via the STAT3 pathway [214]. The drug resistance effect and stemness of cancer cell is also expedited by TA-MSCs mediated-LncRNA secretion. There are reports suggesting that MSC-associated MSC-AS1 and AGAP2-AS1 mediate drug resistance through the PI3K/Akt signaling pathway and regulation of CPT1 expression, respectively [215, 216]. Targeting these MSCs or suppressing the cytokines and LncRNA expression they adjust may be a optimal approach to resensitize the tumors to anticancer therapy.
In short, TA-MSCs perform their tumor-promoting properties by sustaining tumor cell growth and proliferation, altering tumor cell phenotype and conferring them an aggressive or migratory ability, decreasing treatment response to various drugs, and even preparing a pre-metastatic niche for circulating tumor cells. Accumulating evidence unraveling the role of TA-MSCs in multiple cancer types at different steps of tumor development provides a novel insight for us to understand the important function of stroma in cancer.
Pericytes
Long known as regulators of vascular morphogenesis and function, pericytes represent a cell type neighboring the microvascular periendothelial mesenchyme and have been reported to participate in multiple pathological processes, especially malignant tumors [217]. Pericytes can interact with tumor cells or stromal cells to alter the TME and exert immunomodulatory functions, thereby contributing to tumorigenic processes and metastatic dissemination [218, 219]. Interestingly, tumor cells have potential to generate pericytes, since the observation that, in glioblastoma xenografts, approximately 89% of vascular pericytes originate from glioblastoma stem cells [220].
It has been shown that a pericyte co-culture system promoted ovarian cancer cells’ growth and invasion in vitro. In the xenograft model, tumor cell OVCAR-5 or OVCAR-8 along with pericytes were co-injected into nude mice, which resulted in accelerated tumor growth and invasive metastasis compared with injection of cancer cell alone, without altering and affecting tumor vasculature. The high pericyte score was highly predictive for poor prognosis of cancer patients [221]. Some secreted factors from pericyte were involved in oncogenic development. For example, pericytes secreted insulin-like growth factor 2 was found to have a pro-proliferative effect on breast cancer cell and contributed toward the formation of brain metastasis [222]. It was also found that deletion of β3 integrin in pericytes accelerated primary tumor growth and exacerbated cancer progression without influencing tumoral angiogenesis [223]. As for the pro-metastatic effects, preparing a PMN for cancer cells is a key section mediated by pericytes [224]. Furthermore, pericyte can be induced by PDGF-BB to transdifferentiate into fibroblast, which is important to facilitate tumor metastasis and offers a novel targeted option for anti-metastasis therapy [225].
Even though these valuable results have been obtained, it should be noted that our current comprehension regarding the concrete roles of pericytes in cancer is still relatively insufficient, and additional exploration is also warranted.
Interplay and crosstalk between intrastromal components
The tumor stroma is not a quiescent entity. Instead, it is a highly dynamic and constantly changing environment with complex elements that can interact with cancer cells to affect tumor behavior. Apart from directly interplaying with malignancies, the stromal components work in concert with one another, presumably impacting the unrestricted growth, invasion and propagation of a tumor through the body. The intrastromal crosstalk orchestrates multiple biological processes (Fig. 4), and a better understanding of their reciprocities is expected to shed substantial light on the investigation of tumor stroma and their roles in cancer.
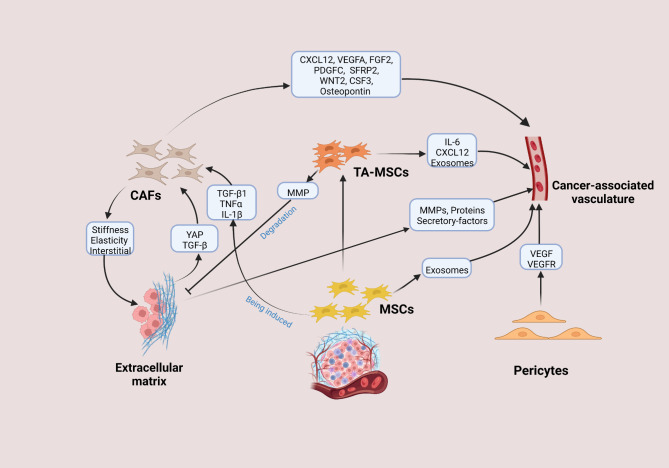
Fig. 4.
Interactions between stromal elements and each others
Interaction between stromal cellular elements and angiogenesis
During malignant transformation, tumor cells acquire a capacity to reshape and educate surrounding stroma to meet their nutrient requirements, which eventually induces unremitting angiogenesis. Simultaneously, the mobilization and activation of stromal cells and the infiltration of capillaries into tumor tissues are thought to be a prerequisite for tumor growth and metastasis. Because ECM usually serves as an essential repository of diverse effector molecules, it is not surprising that ECM profoundly impacts on the formation of cancer-associated vasculature. Following being affected by a series of pro-angiogenic signals, endothelial cells tend to migrate into the interstitial matrix and release MMP that can remodel the basement membrane surrounding the vasculature. Moreover, considerable tenascin, fibronectin, remodeled type I and III collagens existing in ECM stimulate angiogenesis [37, 226]. The stiffened ECM also drives angiogenesis by facilitating the activation of splicing factors to enhance the production of PKC βII and VEGF 165b alternative splice variant in endothelial cells [227]. The ECM also contributes to the expression of VEGFR and its internalization in endothelial cells, which helps endothelial cells survive by sustaining the ERK signaling [228].
The stimulative effects of CAFs on neovascularization are principally achieved by many secretory pro-angiogenic factors including CXCL12, WNT2, VEGFA, FGF2, PDGFC, secreted frizzled-related protein 2 (SFRP2), CSF3, and osteopontin [90, 229–231]. Also, CAFs can indirectly attune tumor vascularization via the biomechanical modulation of ECM stiffness, elasticity and interstitial fluid pressure [90, 232, 233]. Analogously, extensive studies regarding the role of TA-MSCs in building cancer-associated vasculature largely concentrate on pro-angiogenic chemokines or growth factors such as VEGF, IL-6, and the CXCL12/CXCR4 axis [234–236]. It has also been demonstrated that TA-MSCs drive angiogenesis through transdifferentiation into endothelial cells or the recruitment of endothelial progenitors [190]. Furthermore, MSCs can release exosomes that transfer miRNA to endothelial cells and contribute to angiogenesis in vitro, but whether the semblable result exists in the context of tumor deserves to further verify [237]. The recruitment of pericytes is indispensable for vasculature formation and maturation, since they can interact with endothelial cells to stimulate basement membrane matrix assembly, relay growth factors such as VEGF to modulate the survival of endothelial cells, and respond to VEGF by expressing VEGFR1 [238, 239].
Interaction between CAFs, the ECM and MSCs
Nowadays, mounting evidence has linked CAFs with the tumor ECM. On the one hand, CAFs may be the most effective cell type in building up and remodeling the structure of ECM, which is partially attributed to their ability of assisting tumor cells to migrate through the stroma and interact with other stromal elements. CAFs can synthesize and release many ECM proteins including collagens, laminin and fibronectin. Moreover, matrix-crosslinking enzymes produced by CAFs along with force-mediated ECM reconstitution are responsible for the enhancive stiffness of tumor tissues [121, 126]. On the other hand, the activation of CAFs is affected by some physical changes in the ECM. For example, one of the signature features of CAFs is to activate YAP transcription factor required for CAFs to induce increased matrix stiffness, and intriguingly, stiffened ECM in turn sustains CAFs phenotype by promoting the activation of YAP [240]. Additionally, the convergence of both ECM composition and elasticity together with TGF-β can influence the phenotypic heterogeneity of CAFs, which has potential value for further development of stroma-targeted treatment [241]. It has also been implied that TA-MSCs are capable of producing MMPs and then degrading ECM to impact the configuration of pro-metastatic tumor ECM [190].
During the interplay with cancer cells, MSCs can be induced to differentiate into CAFs. In the setting of prolonged exposure to cancer-conditioned medium, human MSCs could possess up-regulation of CAFs-associated genes and display functional properties of CAFs characterized by consistent expression of SDF-1 and higher expressed levels of α-SMA, vimentin, and fibroblast surface protein [131]. Specifically, the mobilization of MSCs to tumor sites and the transdifferentiation of MSCs into CAF-like cells are partially mediated by TGF-β1 derived from both cancer cells and tumor-educated-stromal cells [242]. Additionally, under the sustained stimulation with pro-inflammatory cytokines TNFα and IL-1β, MSCs converted into CAFs, and importantly, these CAFs release diverse factors to stimulate CCR2, CCR5, CXCR1/2 and Ras-activating receptors existing in cancer cell surfaces, thereby enhancing cancer cell dispersion and metastasis [243].
Stromal elements and the immune system
The immune system is typically thought to be a master mediator for cancer and plays crucial roles throughout the tumor initiation and progression. Arguably, immune cells exist in large quantities in the TME and attune the body’s response to malignant tumors. Most of stromal elements, if not all, jointly contribute toward forming of an immunosuppressive TME that enables cancer cells to evade surveillance and attack from body’s immune system (Fig. 5) [244].
Fig. 5.
Interactions between stromal cells and diverse immune cells
The ECM and the immune system
It has been illustrated that the ECM participated in modulating the differentiation, migration, infiltration and polarization of immune cells residing in the TME, and therefore supporting or compromising antitumor immunity. The ECM not only provides crucial migratory cues for immune cells but also also serves to affect their function [245–247]. Loose regions of fibronectin and collagen assist T cell motility and migration in chemokine-dependent ways, whereas dense ECM areas impede T cell trafficking and lead to reduced number of infiltrating CD8+ T-cells, suggesting that thickened ECM interferes with antitumor responses by governing the motility and positioning of T cell [248–250]. Furthermore, a recent study uncovered that interfering with collagen stabilization could deplete the content and stiffness of ECM, resulting in increased efficacy of anti-PD-1 therapy and effective T cell infiltration [251]. As for the contribution of ECM to immune cell’s function, an important aspect is their repressive role on T cell. In regard to this, stiffened ECM can impair the antigen presentation by APCs and decrease the production of IL-2 that is responsible for promoting Th1 cells’ differentiation and T cell’s proliferation [41, 252]. Furthermore, the ECM protein Tenascin-C can interact with α5β1 integrin on the T cell surface to impair reorganization of the actin-based cytoskeleton that is necessary for T cell activation [253].
Tumor areas that exhibit the highest levels of collagen cross-linking tend to demonstrate ample macrophage infiltration in the condition of breast cancer. Therapeutic ablation of these accumulated macrophages can reduce metastasis and stromal stiffening, which indicated that collagen cross-linking likely contributed to the recruitment of macrophages and drove tumor metastasis [254]. The tumor ECM also favors the infiltration of macrophages within tumor tissue and drives their polarization to M2-like phenotype to exert immunosuppressive function [255–257].
Cancer-associated vasculature and the immune system
Cancer-associated vasculature not only provides nutrient supply for tumor growth but also impedes effective drug delivery to tumor sites sometimes because of its abnormal structure. Importantly, tumor vasculature contributes to the formation of an immunosuppressive TME by limiting entry of effector T cells [258]. Also, hypoxic surroundings within a tumor caused by abnormal blood perfusion can accelerate the differentiation of tumor-infiltrating myeloid cells to M2-like tumor-associated macrophages (TAMs)[259, 260]. Meanwhile, hypoxia also supports the differentiation and function of MDSCs and Tregs via various immunosuppressive molecules to mediate antitumor immune escape [261]. Combination of vasculature targeting and immune checkpoint inhibitor was demonstrate to elicit potent antitumor response in preclinical study, which endow the further application of inhibiting vasculature plus immunotherapy high promise [262].
TECs and the immune system
TECs are responsible for protecting tumor cells from the host immune attack[263]. TECs-derived secreted protein mediated the M2 polarization of macrophages by activating the PI3K/AKT/mTOR pathway [264]. Notably, TECs can express the death mediator Fas ligand following the cooperatively inducing by several factors including VEGF-A, IL-10, and prostaglandin E2 (PGE2), thus obtaining the ability to kill effector CD8+ T cells rather than regulatory T cells (Treg) to enhance tumor cell escape [265]. TECs also induce CD8+ T cell infiltration and exhaustion via the expression of glycoprotein nonmetastatic melanoma protein B in hepatocellular carcinoma [266]. Moreover, TECs tend to exhibit elevated PD-L1 phenotype, so as to bind to programmed death 1(PD-1) in activated lymphocytes and hinder the body’s immune response [267, 268].
Activated CAFs and the immune system
Activated CAFs play structural and functional roles within the immune system through diverse manners including remodeling the ECM to create a physical immune barrier, regulating the antitumor activity of tumor-infiltrating immune cells, and facilitating the expression level of immune checkpoint molecules [269–271].
In the innate immune response, TAMs are perhaps the most predominant cells neighboring CAF-populated areas and have multidimensional interactions with CAFs. CAFs actively promote the recruitment of monocytes into tumor areas where they further evolve into the protumorigenic M2 macrophage subset [272–274]. Specifically, CAFs attract monocytes and promote their M2 polarization via the secretion of IL-8. This M2-like polarization can synergize with CAFs to restrain natural killer cells function [275]. CAFs also produce CCL2, CXCL12, IL-6, IL-10, glycoprotein CHI3L1, macrophage colony-stimulating factor to promote the migration of monocytes into tumor tissue and support their transdifferentiation into the M2 phenotype [276–282]. Interestingly, TAMs are reported to regulate the activation of CAFs by releasing CXCL12 and IL-6, thereby forming a positive loop to endorse cancer progression [282].
Analogous to the phenotypic macrophages, neutrophils can be roughly separated into two different polarized populations: N1 neutrophils with antitumor phenotype and N2 neutrophils with pro-tumor phenotype [283]. CAFs-derived IL-6 participated in the activation of STAT3 signal in tumor‑associated neutrophils (TANs), which sustained the survival and function of TANs and inhibited T cell’s attack ability via the PD1/PD-L1 signaling [284]. Cardiotrophin-like cytokine factor 1 derived from CAFs upregulated the CXCL6 and TGF-β expression levels in cancer cells, which promoted the polarization of the N2 neutrophil phenotype [285].
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous cell population that consists of immature myeloid cells and myeloid progenitor cells, with immunosuppressive activity in tumor development. MDSCs can regulate both innate and adaptive immune responses [286, 287]. They are influenced by CAFs to inhibit the antitumor activity of effector T cell. In short, CAFs facilitate MDSCs generation and infiltration mainly by releasing multiple secretory factors including chemokine CCL2, CXCL1, CXCL2, CXCL12 and cytokine IL-6, TGF-β, etc. [288]
As an indispensable population of antigen-presenting cells, dendritic cells (DCs) are affected by CAFs to induce tumor cells immune evasion, usually accompanied by impaired DCs maturation and blocked antigen presentation [289]. CAFs are found to recruit DCs and confer them a capacity to produce indoleamine 2,3-dioxygenase (IDO). These DCs inhibit T cells proliferative ability and upregulate the production of Treg in a IL-6-STAT3-dependent manner [290]. Furthermore, CAFs assist the proliferation and migration of mast cells (MCs) by the CXCL12/CXCR4 axis and potentiate MCs protumorigenic function [291]. The CAFs precursors, stellate cells, can stimulate MCs to secrete IL-13 and tryptase, which creates a fibrotic TME and mediates restrained antitumor immunity [292]. Reciprocally, tryptase derived from MCs potentiates CAFs-induced early malignant morphology changes of prostate epithelial cells [293]. CAFs also induce natural killer (NK) cells dysfunction and mediate their functional and phenotypic alterations by releasing PGE2 and IDO [294, 295].
The implications of CAFs on adaptive immunity are mainly achieved by regulating T lymphocytes activity. The antigen crosspresentation driven by CAFs could negatively modulate T cells function and survival [296]. Mechanistically, PD-1 ligand 2 (PD-L2) expressed by CAFs induces T cell anergy and even death through the interaction with PD-1. CAFs also express FAS ligand (FASL) to induce the apoptosis of CD8+ T cell expressing FAS [297]. In addition, TGF-β was uncovered to abate the antitumoral immune via the exclusion of CD8+ T cells [298, 299]. CAFs-derived CXCL12 is also necessary for blocking the access of CD8+ T cell and the failure of the treatment of T-cell checkpoint antagonists [300, 301].
CAFs can directly interfere with T cell’s activity by regulating the expression of immune checkpoint molecules including PD-L1, PD-L2, B7-H3, and B7-H4. Among them, PD-L1 and PD-L2 are the best-studied types. They can bind to the PD-1 receptor on T cell surface to impair T cell’s function. [297, 302–304]. CAFs also affect Th cell subsets, mainly Th2 cell subpopulations, and Treg transformation to inhibit antitumor response [270].
TA-MSCs and the immune system
MSCs are tightly correlated with both innate and adaptive immune, in particular mediating antitumor immune response. The immune modulatory functions of MSCs are mainly attribute to their capacity to block effector cells’ activated surface receptors expression, support regulatory cells expansion, and impair the maturation of antigen-presenting cells [305–308].
TA-MSCs isolated from cervical cancer dramatically repressed antigen-specific T cell recognition of tumor cells by cytotoxic T lymphocytes (CTLs) and provided immune protection for tumor cells growth. Mechanistically, TA-MSCs induced the downregulation of HLA class I molecules on cancer cells membrane in an IL-10-mediated manner, whereas HLA class I is important for the recognition by CTLs [309]. After co-culturing with MSCs, the proliferative potential of FoxP3+Treg was significantly enhanced, accompanied by the reduction of antitumor Th1 cytokines and the increase of Th2 cytokines, which mediated cancer cells immune evasion and contributed to disease progression. This finding can be partially explained by the elevated levels of MSC-generated TGF-β1 [310]. Another mechanism regarding MSCs restraining effective immune response is to destroy DCs mature as shown by the decreased expression of CD83 on DCs surface [311]. Furthermore, TA-MSCs usually exhibit a remarkable antiproliferative effect on mononuclear cells and abate NK cell activity [312].
Numerous chemokines participate in the communication between TA-MSCs, tumor cells and macrophages, which is driven by the HIF signal and substantially stimulate the invasion and metastasis of MDA-MB-231 cell [313]. On the one hand, CXCL10 secreted by TA-MSCs bind to its cognate receptors CXCR3 presented in cancer cells, and simultaneously, CXCL16 derived from cancer cell bind to CXCR6 on TA-MSCs surface, eventually potentiating the recruitment of TA-MSCs into tumor areas. On the other hand, TA-MSCs release CCL5 to bind to CCR5 on breast cancer cells, and then, signal-received cancer cells express CXCL12 to drive the migration and recruitment of TAMs and MDSCs [313]. Interestingly, there are bidirectional interactions between TA-MSCs and immune cells. TA-MSCs are plastic and can be modified by CD4+ T cell to induce tumor growth [314]. Following stimulating by CD4+ T cell, the immunophenotype of TA-MSCs undergoes significant changes, as they acquire the ability to overexpress PD-L1 in a STAT3-dependent manner and subsequently activate cancer cell-intrinsic PD-1/mTOR signaling to assist gastric cancer development [314].
Pericytes and the immune system
Pericytes exert their immunosuppressive functions by releasing multiple factors such as nitric oxide, IL-6, IL-33, CXCL12, PGE2 and TGFβ [218, 315]. Furthermore, the accumulation of pericytes affects cytotoxic lymphocytes activity, as they hinder allogeneic and mitogen-activated T cell responses in vitro[316]. Meanwhile, Bose et al. firstly confirmed that tumor-derived pericytes had a negative influence on the proliferation and activation of CD4+ T cell as well as resulted in CD4+ T cell dysfunction even anergy in response to antigen in an IL-6-dependent manner, which possibly hampered effective antitumor immune responses and shielded tumor cells from the host immune attack [317]. Pericytes are also responsible for recruiting MDSCs into the stroma to create an immunosuppressive surrounding that is favorable for tumor growth [318].
Targeted therapy based on tumor stroma
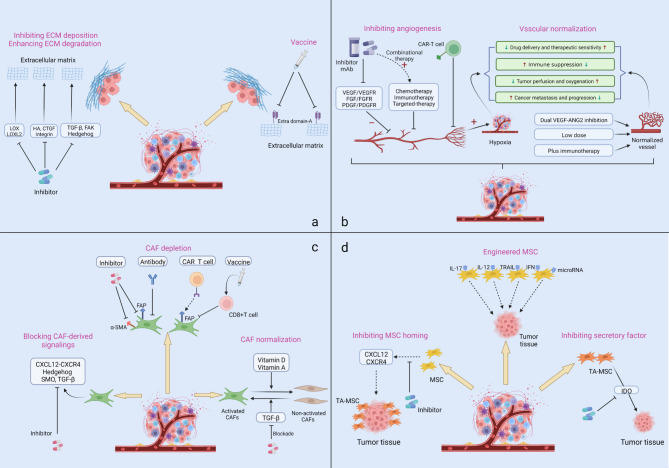
Traditionally, the rationale for anticancer therapies mainly focuses on eliminating tumor cells only while largely ignoring the ambient non-malignant-cell components of a tumor. In recent years, we have witnessed a great upgrade of precision medicine, and among all, molecular targeted therapy has been widely developed and introduced into clinical practice. Also, cancer initiation, progression and metastasis usually elicit a broad spectrum of dynamic evolutions and alterations in host tissues, which contributes to establishing complicated stromal surroundings that in turn cover a wide range of tumor cell activities and support cancer development. Accordingly, tumor stroma may be a fertile ground for developing effective therapeutic strategies to hopefully augment existing treatment options and realize personalized cancer therapy, especially for those stromal-rich and refractory cancers.
Approaches to targeting the tumor stroma include directly targeting both cellular or noncellular elements located in the stroma, disrupting and inhibiting related secretory factors and signaling pathways, and recently proposed reshaping or normalizing the tumor stroma that aims to slow or reverse tumor progression (Fig. 6). Herein, we summarize recent advancements targeting stromal components and highlight related potential therapeutic values, with the aim to promote the leap from bench to bedside.
Fig. 6.
Therapeutic approaches based on the stromal components
Targeting the ECM
Compared with the normal ECM, tumor ECM is more abundant, denser and stiffer. The tumor ECM typically undergoes a series of changes such as deposition, degradation, and post-translational modification [319]. To date, several strategies have been designed to inhibit or decrease the ECM with tumor-promoting functions, such as inhibiting the ECM synthesis and deposition, enhancing the degradation of different ECM components, and blocking signaling molecules that contribute toward cell-matrix interactions and protumorigenic feedback. Some targeted drugs are being assessed in clinical trials (Table 1).
Table 1.
Clinical trials targeting the ECM
| Target | Drug | Combination | Condition | Phase | Status | Trial number |
|---|---|---|---|---|---|---|
| LOXL2 | Simtuzumab | Gemcitabine | Pancreatic Cancer | II | Completed | NCT01472198 |
| FOLFIRI | Colorectal Cancer | II | Terminated | NCT01479465 | ||
| PAT-1251 | No | Healthy | I | Completed | NCT02852551 | |
| LOX | PXS-5382A | No | Healthy | I | Completed | NCT04183517 |
| Hyaluronic acid | PEGPH20 | Pembrolizumab | Pancreatic Cancer/Pancreatic Ductal Adenocarcinoma | II | Recruiting | NCT03634332 |
| Gemcitabine | Pancreatic Adenocarcinoma Non-resectable | II | Terminated | NCT02910882 | ||
| Pembrolizumab | NSCLC/Gastric Cancer | I | Completed | NCT02563548 | ||
| Cetuximab | Pancreatic Cancer | Not Applicable | Completed | NCT02241187 | ||
| Avelumab | Pancreatic Ductal Adenocarcinoma/Pancreatic Cancer | I | Terminated | NCT03481920 | ||
| Docetaxel | Non-small Cell Lung Cancer | I | Terminated | NCT02346370 | ||
| Eribulin mesylate | Metastatic Breast Cancer | I/II | Terminated | NCT02753595 | ||
| No | Solid Tumor | I | Completed | NCT01170897 | ||
| Gemcitabine | Pancreatic Cancer | I/II | Completed | NCT01453153 | ||
| FOLFIRINOX | Metastatic Pancreatic Adenocarcinoma | I/II | not recruiting | NCT01959139 | ||
| No | Solid Tumor | I | Completed | NCT00834704 | ||
| CIS/GEM/Atezolizumab | Cholangiocarcinoma | I | Terminated | NCT03267940 | ||
| Nabpaclitaxel/Gemcitabine | Metastatic Pancreatic Cancer | II | Completed | NCT01839487 | ||
| Atezolizumab | Pancreatic Ductal Adenocarcinoma | II | Terminated | NCT03979066 | ||
| Gemcitabine/Nab-paclitaxel | Adenocarcinoma | II | Terminated | NCT02487277 | ||
| NabPaclitaxel/Gemcitabine | Pancreatic Ductal Carcinoma | III | Terminated | NCT02715804 | ||
| CTGF | Pamrevlumab | Gemcitabine combined with nab-paclitaxel | Metastatic Pancreatic Cancer | III | Recruiting | NCT04229004 |
| Pamrevlumab | Pamrevlumab,Gemcitabine, Nab-paclitaxel, or Pamrevlumab, FOLFIRINOX | Pancreatic Cancer Non-resectable | III | Active, not recruiting | NCT03941093 | |
| Integrin | Cilengitide | Temozolomide,radiotherapy | Glioblastoma | III | NCT00689221 | |
| ATN-161 | Carboplatin | Malignant glioma | I/II | Completed | NCT04177108 | |
| MEDI-522 | Dacarbazine | Metastatic melanoma | II | Completed | NCT00066196 | |
| TGF-β | Fresolimumab | Radiation Therapy | Metastatic Breast Cancer | II | Completed | NCT01401062 |
| Radiation Therapy | Non-Small Cell Lung Carcinoma | I/II | Completed | NCT02581787 | ||
| FAK | Defactinib/PF-04554878 | No | Malignant Pleural Mesothelioma | II | Terminated | NCT01870609 |
| No | Non-Small Cell Lung Cancer | II | Completed | NCT01951690 | ||
| No | Solid Tumor | I | Completed | NCT00787033 | ||
| No | Non-Hematologic Malignancies | I | Completed | NCT01943292 | ||
| No | Advanced tumor | II | Active, not recruiting | NCT04439331 | ||
| VS-6766 | Ovarian Cancer | II | Recruiting | NCT04625270 | ||
| Paclitaxel | Ovarian Cancer | I | Completed | NCT01778803 | ||
| VS-6766 | Lung cancer/ ovarian cancer/ endometrioid carcinoma / pancreatic cancer | I | Recruiting | NCT03875820 | ||
| VS-6766 | Non-Small Cell Lung Cancer | II | Recruiting | NCT04620330 | ||
| Pembrolizumab, Gemcitabine | Solid tumors / pancreatic cancer | I | Completed | NCT02546531 | ||
| Paclitaxel, carboplatin | Ovarian cancer | I/II | Recruiting | NCT03287271 | ||
| Hedgehog | Sonidegib (LDE225) | Docetaxel | Triple Negative (TN) Advanced Breast Cance | I | Completed | NCT02027376 |
| No | Basal Cell Carcinoma | II | Completed | NCT01327053 | ||
| Gemcitabine | Pancreatic Cancer | I | Completed | NCT01487785 | ||
| No | Hepatocellular Carcinoma | I | Completed | NCT02151864 | ||
| Etoposide or Cisplatin | Small Cell Lung Cancer | I | Completed | NCT01579929 | ||
| Pembrolizumab | Advanced Solid Tumors | I | Recruiting | NCT04007744 | ||
| Fluorouracil, Leucovorin, Oxaliplatin, Irinotecan | Pancreatic Cancer | I | Completed | NCT01485744 | ||
| Vismodegib | No | Basal Cell Carcinoma | II | Completed | NCT03035188 | |
| Modified FOLFOX or FOLFIRI | Metastatic Colorectal Cancer | II | Completed | NCT00636610 | ||
| Gemcitabine Hydrochloride | Pancreatic Cancer | II | Completed | NCT01195415 | ||
| Gemcitabine or nab-Paclitaxel | Pancreatic Cancer | II | Completed | NCT01088815 |
One of the promising options for inhibiting ECM deposition is to disrupt its crosslinking and stabilization. Among these strategies, targeting lysyl oxidase (LOX) activity that is frequently upregulated in diverse cancer types and responsible for catalyzing collagen crosslinking is emerging as a optimal one, which can reduce the stroma density and consequently enhance the outcome of anticancer treatment [320–326]. Simtuzumab is an antibody targeting LOXL2 and has been tested clinically to appraise its efficacy and safety. A phase II trial of simtuzumab combined with gemcitabine was conducted to treat adult patients with metastatic pancreatic adenocarcinoma. Although this therapeutic regimen was tolerable, the progression-free survival (PFS), overall survival (OS) or objective response rate(ORR) in patients have not been improved [327] (NCT01472198). Simtuzumab in combination with FOLFIRI was also used to treat patients with colorectal cancer, and the ultimate result suggested that addition of simtuzumab did not improve the clinical outcome [328] (NCT01479465). PAT-1251 and PXS-5382 A are developed to target LOXL2 and LOX respectively, and clinical trials have studied their safety and tolerability in healthy adult subjects. Their anticancer potencies need to be rigorously explored (NCT02852551, NCT04183517).
Another rational approach to targeting ECM deposition or degradation is to degrade hyaluronic acid (HA) that typically accumulates cancer and can mechanically increase the ECM elastoviscosity [329–332]. PEGPH20 was designed to inhibit HA and underwent clinical trials as a single agent or in combination with other therapeutic drugs. Two similar studies have been conducted to evaluate its safety, tolerability and pharmacokinetics in patients with solid tumor, but the results have not been disclosed (NCT01170897, NCT00834704). A phase Ib study reported the effect of docetaxel in combination with PEGPH20 in patients with lung cancer. This strategy seemed to manifest an acceptable safety profile [333] (NCT02346370). In a randomized phase II trial, researchers investigated the effects of PEGPH20 in combination with standard nab-paclitaxel plus gemcitabine (PAG) to treat pancreatic cancer patients. The results showed that patients with HA-high tumors who received PAG had the largest FAS improvement, and importantly, the related clinical data also supported the potential application of tumor HA as a predictive biomarker for cancer patients [334] (NCT01839487). Notwithstanding, the results have been mixed. Owing to the negative trial outcome that didn’t meet its primary end point of OS, a similarly subsequent phase III study had to be terminated [335] (NCT02715804). Another phase IB/II randomized study tested the clinical efficacy of PEGPH20 with modified fluorouracil, leucovorin, irinotecan, and oxaliplatin (mFOLFIRINOX) to treat patients with metastatic pancreatic cancer. Unfortunately, compared with mFOLFIRINOX alone, this therapeutic scheme led to increased toxicity and decreased treatment duration, suggesting that the addition of PEGPH20 yielded detrimental effect in patients unselected for tumor HA status [336] (NCT01959139).
The connective tissue growth factor (CTGF) is responsible for enhancing matrix deposition in cancers, and anti-CTGF therapy can reduce matrix deposition in murine pancreatic cancer model [337]. To date, using pamrevlumab to target CTGF in patients with pancreatic cancer have entered phase III clinical trials (NCT03941093, NCT04229004). Integrin is a critical mechanosignal transducer that can perceive the ECM mechanical force and mediate signal transductions to intracellular proteins. Hence, targeting integrin may be a promising approach to delaying tumor progression, and meanwhile, a series of clinical trials have been launched continually to evaluate its therapeutic prospects (NCT00689221, NCT04177108, NCT00066196).
Among all signaling molecules that are involved in ECM deposition, TGF-β represent an optimal target to inhibit collagen synthesis and subsequently ECM deposition. Several TGF-β-targeted drugs have been actively assessed in clinic to potentiate antitumor effects [338, 339] (NCT01401062, NCT02581787). An alternative method is to target FAK, an important downstream effector of integrins [340]. FAK inhibitors have shown antitumor activity in preclinical studies [341–343]. Based on the above successful practices, defactinib (also known as PF-04554878) has been tested in phase clinical trials, mainly in malignant pleural mesothelioma and advanced solid tumors. Even though this drug was well tolerated, using defactinib alone or in combination with other therapies to treat patients with different cancers showed limited outcome or even failed to show clinical benefits [344–347] (NCT01951690, NCT00787033, NCT01870609). Furthermore, a previous study uncovered that blocking the fibrotic Hedgehog signaling pathway could decrease fibrosis in cancer, which enhanced the delivery of chemotherapy and contributed to prolonged survival times in tumor-bearing mice [56]. Hitherto, several Hedgehog inhibitors such as vismodegib and sonidegib (LDE225) have been studied in clinical trials to mainly treat patients with basal cell carcinoma and solid tumors, which are summarized in Table 1.
Cancer vaccine is emerging as a promising therapeutic strategy for solid tumors and being intensively evaluated in both preclinical and clinical studies [348]. Several ECM components have recently been used as antigens for designing cancer vaccine. During tumor matrix remodeling, the alternatively spliced extra domain-A (ED-A) of fibronectin was reported to reexpress, which enabled them to become an ideal target. Targeting ED-A with immunization in the therapeutic condition could inhibit cancer metastasis and decrease the tumor burden, which suggested that the ECM might behave as a suitable candidate for designing effective cancer vaccines and warranted further study in clinical trials [349].
Targeting cancer vasculature
In 2004, the American FDA granted an unprecedented approval to a humanized anti-VEGFA monoclonal antibody, named as bevacizumab, to treat patients with metastatic colorectal cancer [350]. Since then, targeting cancer vessels has aroused great interest of an increasing number of scientists and been utilized in clinical practices. The conventional tactic is to inhibit proangiogenic signaling or factors activity, but in some conditions, this application has not yielded long-term clinically survival benefits and even unexpectedly promotes drug resistance or limits agent delivery, ultimately leading to tumor metastasis [351, 352]. As such, an attractive possibility is remodeling aberrant tumor blood vessels, which can restore the structure and function of vasculature and then improve the drug penetration, as well as achieve better outcomes, currently known as “vascular normalization” [351, 353]. In this section, we summarize the clinical trial progress in antiangiogenic therapies and the strategies for vascular normalization (Table 2).
Table 2.
Some major clinical trials targeting cancer vasculature
| Target | Drug | Combination | Condition | Phase | Status | Trial number |
|---|---|---|---|---|---|---|
| VEGF/VEGFR | Bevacizumab | No | Solid tumors | IV | Completed | NCT01588184 |
| Bevacizumab | Chemotherapies | Ovarian cancer | III | Active, not recruiting | NCT00565851 | |
| Bevacizumab | Erlotinib | Lung cancer | II | Completed | NCT01562028 | |
| Bevacizumab | Erlotinib | Hepatocellular Carcinoma | II | Completed | NCT01180959 | |
| Bevacizumab | Niraparib | Ovarian Cancer | I/II | Completed | NCT02354131 | |
| Olaparib | Chemotherapies | Ovarian Cancer | III | Active, not recruiting | NCT02477644 | |
| Olaparib | Enzalutamide, abiraterone acetate | Prostate Cancer | III | Active, not recruiting | NCT02987543 | |
| Bevacizumab | Osimertinib | Lung Cancer | I/II | Completed | NCT02803203 | |
| Bevacizumab | Osimertinib | Lung Cancer | III | Recruiting | NCT04181060 | |
| Ramucirumab | Erlotinib, Gefitinib, Osimertinib | Metastatic NSCLC | III | Active, not recruiting | NCT02411448 | |
| Ramucirumab | Paclitaxel | Gastric Adenocarcinoma | III | Completed | NCT01170663 | |
| Ramucirumab | No | Gastric Cancer and Adenocarcinoma | III | Completed | NCT00917384 | |
| Ramucirumab | No | Hepatocellular Carcinoma | III | Completed | NCT01140347 | |
| Ramucirumab | No | Hepatocellular Carcinoma | III | Completed | NCT02435433 | |
| Ramucirumab | No | Hepatocellular Carcinoma | III | Completed | NCT02435433 | |
| Aflibercept | FOLFIRI | Metastatic Colorectal Cancer | III | Completed | NCT00561470 | |
| Aflibercept | Levofolinate, Irinotecan, 5-FU | Metastatic Colorectal Cancer | II | Completed | NCT01882868 | |
| Aflibercept | Capecitabine | Metastatic Colorectal Cancer | I/II | Completed | NCT01661972 | |
| TKI | Sorafenib | No | Hepatocellular Carcinoma | III | Completed | NCT00692770 |
| Sunitinib | AGS-003 | Kidney Cancer | II | Completed | NCT00678119 | |
| Sunitinib | Nivolumab, Pazopanib, Ipilimumab | Renal Cell Carcinoma | I | Completed | NCT01472081 | |
| Pazopanib | No | Ovarian Cancer | I/II | Completed | NCT01238770 | |
| Pazopanib | No | Renal Cell Carcinoma | IV | Completed | NCT01521715 | |
| Pazopanib | Paclitaxel | Ovarian Cancer | II | Completed | NCT01644825 | |
| Pazopanib | GSK1120212 | Solid Tumors, Thyroid Cancer | I | Completed | NCT01438554 | |
| ANG-VEGF | Vanucizumab | Atezolizumab | Solid Tumors | I | Completed | NCT01688206 |
| Vanucizumab | Bevacizumab, Selicrelumab | Solid Tumors | I | Completed | NCT02665416 | |
| Plus immunotherapy | Bevacizumab | Nivolumab, Rucaparib | Peritoneal Cancer,Ovarian Cancer, Fallopian Tube Cancer | II | Recruiting | NCT02873962 |
| Bevacizumab | Nivolumab and chemotherapies | Non-small Cell Lung Cancer | I | Completed | NCT01454102 | |
| Bevacizumab | Pembrolizumab | Clear Cell Renal Carcinoma | I/II | Completed | NCT02348008 | |
| Bevacizumab | Pembrolizumab | Glioblastoma | II | Completed | NCT02337491 | |
| Bevacizumab | Pembrolizumab | Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | II | Completed | NCT02853318 | |
| Bevacizumab | Durvalumab | Glioblastoma | II | Completed | NCT02336165 | |
| Bevacizumab | Durvalumab | Metastatic Breast Cancer | I | Completed | NCT02802098. | |
| Bevacizumab | Tremelimumab | Colorectal Cancer With Liver Metastases | I | Active, not recruiting | NCT02754856 | |
| Vanucizumab | Atezolizumab | Metastatic Solid Tumors | I | Completed | NCT01688206 |
Among all proangiogenic signalings, VEGF/VEGFR is the best-studied pathway, and related mAb or inhibitors have been widely used in clinic. Bevacizumab that can target VEGF-A and inhibit its interaction with VEGFR-1 and − 2 has been tested in various human cancer types, both as monotherapy and in combination with other antitumor drugs [354, 355]. A recent clinical study evaluated the safety of long-term administration of bevacizumab in patients with solid tumors. No treatment-related adverse effect was happened and patients obtained clinical benefit over an extended period (NCT01588184) [356]. However, previous studies indicated that side effects usually increased when bevacizumab was combined with chemotherapies [357, 358]. These opposite results indicate that the clinical responses and toxic side effects may depend on the specific therapeutic schemes and conditions [359]. As for its therapeutic outcomes in combination with chemotherapies, some published meta-analyses assessed the additional effect of chemotherapy plus bevacizumab, and the results indicated that, compared to chemotherapy alone, the combinational strategy improved the PFS and OS in cancer patients [360–363]. Nonetheless, disappointing outcomes are still existed. In a large randomized phase III trial, researchers assessed the effect of standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer, and the antitumor response was not as promising as initially hoped with no increased OS in the study population was observed [364]. This contradictory phenomenon may be attributed to the different dose usage and particular cancer types [365].
Aside from in combination with chemotherapy, bevacizumab plus targeted therapy often exhibits antitumor activity and yields clinical benefits in cancer patients. For example, erlotinib, an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, has shown synergistic effects when combined with anti-VEGF therapies, mainly in patients with advanced non-small cell lung cancer and colorectal cancer [366–370]. Compared to erlotinib alone in EGFR-positive NSCLC patients, the combinational utilization of bevacizumab plus erlotinib brings clinical benefits to patients with the improvement of their progression-free survival (NCT02759614, NCT01562028) [371–373]. Furthermore, this combined regimen has also been verified in liver cancer patients and has shown a signal of survival benefit, which supports the further clinical studies of this strategy (NCT01180959) [374]. In addition to erlotinib, many other molecular targeted drugs such as olaparib, niraparib and osimertinib in combination with bevacizumab have been approved and introduced across several indications in the clinic, and it was feasible to expand the application of these dual-targeted therapies owing to the observation of progression-free survival benefits in patients (NCT02477644, NCT02354131, NCT02987543, NCT02803203 NCT04181060) [375–378].
Other anti-VEGF signaling drugs have also been assessed in the clinic, both as single agents and in combination with chemotherapy or targeted therapy. Ramucirumab, also known as Cyramza, is a humanized antibody approved by the FDA that targets the VEGFR-2 extracellular domain, and exhibits some degree of efficiency in prolonging PFS and OS in patients with lung cancer, gastro-oesophageal junction adenocarcinoma, and liver cancer (NCT02411448, NCT01170663, NCT01140347) [379–382]. Aflibercept is a recombinant fusion protein that can inhibit the combination of VEGF and VEGFR, and has been approved in combination with FOLFIRI to treat patients with metastatic colorectal cancer (NCT00561470, NCT01882868, NCT01661972) [383–386].
Alternative options that may represent attractive therapies are using tyrosine kinase inhibitors (TKIs) and targeting other proangiogenic signalings, such as inhibiting FGF/FGFR and PDGF/PDGFR, which have been tested or are undergoing phase clinical trials [387]. Among those approved TKIs, sorafenib has gradually become the research hot spot for the treatment or alleviation of a variety of cancer conditions, especially in liver cancer [388, 389]. Other TKIs such as sunitinib and pazopanib, together with inhibitors of FGF/FGFR and PDGF/PDGFR axis have also generated some degree of clinical benefits [387], as listed in Table 2.
In spite of some promising results have been observed, the clinical activity of anti-angiogenic therapies is usually partial and eventually, followed by relapse. Mechanistically, previous methods that inhibit angiogenesis are cutting off their blood supply, but this strategy simultaneously exacerbates the formation of an anoxic TME, leads to the increased compensatory proangiogenic factors production, and creates an immunosuppressive circumstances, thus facilitating pathological angiogenesis and disease progression [390–393]. Therefore, “vascular normalization” that aims to judiciously use antiangiogenesis treatment rather entirely destruction or excessive pruning and keep the balance between proangiogenic and antiangiogenic signalings becomes an advisable direction and is hopefully to accelerate the development of antitumor therapy due to its potential to improve tumor oxygenation and perfusion, enhance the efficiency of drug delivery and delay tumor progression [392].
Long-term vascular-targeted therapies in high doses usually give rise to tumor hypoxia, and meanwhile, some specific cancer types are susceptible to anti-VEGF treatments, which underscores the importance of selecting appropriate doses and time ranges of anti-angiogenic drugs’ administration to achieve vascular normalization as initially expected, termed as “normalization window” [394, 395]. High-dose administration of anti-VEGF agents cannot lead to beneficial outcomes, and instead forms an immunosuppressive TME accompanied by the recruitment of nonclassical Ly6Clow monocytes, which also promotes the occurrence of therapeutic resistance to anti-VEGF [396–398]. Clinically, patients with rectal cancer receiving a relatively low dose of bevacizumab (5 mg/kg) had favorable delivery efficiencies and enhanced pericyte coverage of blood vessels, whereas patients receiving higher dosage of bevacizumab (10 mg/kg) did not exhibit the same benefits, suggesting that the choice of drug dosage is a key consideration in cancer treatment [399]. Likewise, low-dose intensity of bevacizumab confers a greater survival benefit than the usage of high-dose and can be regarded as a significant independent prognostic survival factor in glioblastoma patients [400–402]. In the context of the “normalization window”, antiangiogenesis therapies have synergistic and reinforced effects with other anti-cancer therapeutic modalities including immunotherapy and chemotherapy [403]. However, it has been recognized that the dosage of anti-VEGF targeted drugs that can achieve the window is relatively narrow and their effects often vary among different cancer types [232, 404]. Hence, how to best utilize the anti-angiogenic strategy to benefit patients with cancer is the chief question we need to overcome and merits further mechanistic and functional investigation.
In addition to legitimately identifying the dosage of anti-angiogenic drugs, another noteworthy question is that the process of vascular normalization is transient and reversible, with lasting for several days or months after therapy began in diverse cancer types. In this regard, researchers unexpectedly found a compensatory mechanism in which ectopic expression of angiopoietin (Ang)-2 could inhibit vessel normalization to diminish the beneficial effects of VEGF-VEGFR signaling blockade [405]. Dual inhibition of VEGF-ANG2 has been explored and exhibited prolonged normalization window in mouse model of glioblastoma. Dual VEGF-ANG2 blockade also contributed to antitumor immunity [406–408]. This finding motivated the initiation of multiple clinical trials using dual anti-VEGF and ANG2 inhibitors for tumors, with some trials have shown clinically survival benefits.
Immunotherapy is gradually becoming a central focus of cancer therapy and represents a suitable method in advanced solid tumors. Interestingly, tumoral vascular normalization has the potential to improve the infiltration of diverse immune effector cells, and vice versa as the discovery that the functional stimulation of immune cells can normalize tumor vessels, which establishes a bidirectionally positive feedback loop for antitumor effects and provides a novel combined option for antitumor treatment [409–412]. A phase II trial assessed the effect of nivolumab (anti-PD-1 mAb) in combination with bevacizumab in patients with different cancer types and showed clinical benefit with improved ORR or durable response (NCT02873962, NCT01454102) [413, 414]. Some others anti-PD-1 mAb, such as pembrolizumab and durvalumab in combination with bevacizumab, have also been tested in clinical trials, as summarized in Table 2. In addition to anti-PD-1 drugs, anti-CTLA-4-mAb plus vascular-targeted agents are also utilized to treat patients with cancers, but the related results have not been disclosed yet (NCT02754856, NCT01688206). Noteworthy, based on the advances and successful practices of engineered chimeric antigen receptor (CAR) T cells therapy, different CAR designs have been exploited to fight against various diseases, in particular malignancies. Therefore, using CAR-T cells to target multiple antigens on tumor vasculature may provide new opportunities for the development of anti-angiogenic therapy [415].
Taken together, since the first angiogenesis inhibitor bevacizumab was approved for cancer treatment, numerous vascular-targeted drugs have been designed and exploited in clinic. However, anti-VEGF or targeting other proangiogenic signalings as monotherapy sometimes yields limited clinical outcomes or even results in metastasis, and therefore caution must be taken. Furthermore, realizing vascular normalization without excessive pruning opened new avenues for cancer therapy. One of these approaches is to choose a rational dosage and time range of anti-angiogenic drugs. Further prospective and randomized trials using lower dose of vascular-targeted drugs are warranted. It is also worth noting that judicious use of immune checkpoint blockade together with angiogenesis inhibitors has potential to improve cancer treatment. The next goal of both preclinical and clinical studies is finding the most reasonable combinations to exert more robust anti-tumor immune responses and reduce toxic side effects. The possibilities of this field are virtually endless.
Targeting CAFs for cancer therapy
Numerous previous researches identified various mechanisms of CAFs tumor-promoting functions. Clinically, it was confirmed that the infiltration of activated CAFs was closely related to worse prognosis, resistance to multiple therapies, and even disease recurrence in cancer patients [416–420]. Hence, targeting CAFs has evolved as one of the appealing strategies for cancer intervention and is expected to provide oncologists with clinical decision-making (Table 3).
Table 3.
Clinical trials targeting the CAFs
| Target | Drug | Combination | Condition | Phase | Status | Trial number |
|---|---|---|---|---|---|---|
| FAP-expressing cells | Simlukafusp alfa (RO6874281) | Atezolizumab and chemotherapy | Advanced and/or Metastatic Solid Tumors | II | Completed | NCT03386721 |
| Simlukafusp alfa (RO6874282) | Trastuzumab and Cetuximab | Solid tumor | I | Active, not recruiting | NCT02627274 | |
| Sibrotuzumab (BIBH 1) | No | Metastatic Colorectal Cancer | II | Completed | NCT02198274 | |
| FAP-specific CAR-T | No | FAP-Positive Malignant Pleural Mesothelioma | I | Completed | NCT01722149 | |
| No | Malignant Solid Tumors | I | Recruiting | NCT03932565 | ||
| Vitamin D receptor | Calcipotriol | 5-fluorouracil | Skin cancer precursor immunotherapy | I | Completed | NCT02019355 |
| Paricalcitol | Docetaxel, ixabepilone, and paclitaxel | Metastatic Breast Cancer | I | Completed | NCT00637897 | |
| Paricalcitol | Gemcitabine, Nab-paclitaxel | Metastatic Pancreatic Cancer | I/II | Recruiting | NCT03520790 | |
| Vitamin A metabolism | ATRA | Gemcitabine and Nab-paclitaxel | Pancreatic Adenocarcinoma | I | Completed | NCT03307148 |
| No | Advanced Adenoid Cystic Carcinoma | II | Completed | NCT03999684 | ||
| Paclitaxel and Cisplatin | Non-small Cell Lung Cancer | III | Unknown | NCT01041833 | ||
| Interferon-Alpha 2a | Recurrent Neuroblastoma or Wilms’ Tumor | II | Completed | NCT00001509 | ||
| TGFβ | LY2157299(galunisertib) | Nivolumab |
Solid Tumor; Non-Small Cell Lung Cancer; Hepatocellular Carcinoma Recurrent |
I/II | Completed | NCT02423343 |
| Gemcitabine | Advanced or Metastatic Unresectable Pancreatic Cancer | I/II | Completed | NCT01373164 | ||
| Sorafenib, Ramucirumab | Hepatocellular Carcinoma | II | Completed | NCT01246986 | ||
| Durvalumab | Metastatic Pancreatic Cancer | I | Completed | NCT02734160 | ||
| Radiation, Temozolomide | Malignant Glioma | I/II | Completed | NCT01220271 | ||
| Capecitabine, Fluorouracil | Rectal Cancer | II | Active, not recruiting | NCT02688712 | ||
| Lomustine | Glioblastoma | II | Active, not recruiting | NCT01582269 | ||
| Minnelide | No | Advanced Gastrointestinal Tumors | I | Completed | NCT01927965 | |
| No | Pancreatic Cancer | II | Completed | NCT03117920 | ||
| AP 12,009 | No | Pancreatic Neoplasms, Melanoma, Colorectal Neoplasms | I | Completed | NCT00844064 | |
| CXCR4 | Motixafortide (BL-8040) | Pembrolizumab, chemotherapy | Metastatic Pancreatic Cancer | II | Active, not recruiting | NCT02826486 |
| AMD3100 (plerixafor) | No | Advanced Pancreatic, Ovarian and Colorectal Cancers | I | Completed | NCT02179970 | |
| Mozobil | Children Cancer, Solid Tumor | II | Completed | NCT01225419 | ||
| Hedgehog | Itraconazole | No | Basal Cell Carcinoma (BCC), Skin Cancer | II | Completed | NCT01108094 |
| Vismodegib | No | Basal Cell Carcinoma | II | Completed | NCT01700049 | |
| Gemcitabine hydrochloride | Pancreatic Cancer | I/II | Completed | NCT01064622 | ||
| Gemcitabine Hydrochloride | Pancreatic Cancer | II | Completed | NCT01195415 | ||
| Cisplatin, Cixutumumab, Etoposide | Lung cancer | II | Completed | NCT00887159 | ||
| LDE225 (sonidegib) | Etoposide and Cisplatin | Lung cancer | I | Completed | NCT01579929 | |
| Paclitaxel | Solid Tumor | I | Completed | NCT01954355 | ||
| Docetaxel | Breast cancer | I | Completed | NCT02027376 |
At present, one strategy that has already been tested is CAFs-depletion by targeting their cell surface markers. Fibroblast activation protein-α (FAP), an integral serine protease specifically expressed by CAFs, participates in nearly all steps of the carcinogenic process [421–423]. High-level expression of FAP can predict poor prognosis in high-grade serous ovarian cancer [424]. The depletion of FAP+ cells inhibits tumor growth primarily achieved by augmenting anti-tumor immunity [425, 426]. Meanwhile, widespread efforts are underway to realize the translation of this plausible approach into practice. It has been established that a DNA vaccine exclusively targeting FAP could suppress primary and metastatic tumor growth, promote the uptake of chemotherapeutic drugs and prolong the survival of tumor-bearing mice primarily by inducing CD8+ T cell-mediated killing of CAFs [427, 428]. The combinational use of FAP-DNA vaccine and other tumor antigen-specific DNA vaccines showed synergic effects of anti-tumor immunity characterized by increased CD8+ T cell infiltration and decreased macrophage infiltration [429]. Additionally, FAP-CAR-T cell therapies have been engineered to treat solid tumors in preclinical studies [430]. In the mouse model, adoptively transferred FAP-specific CAR-T cells inhibited FAP+ CAFs activity and delayed the proliferation and growth of multiple types of subcutaneously transplanted tumors without the observation of distinct toxic signs [431]. A phase I trial was conducted to evaluate the safety of a fixed single dose of 1 × 106 adoptively transferred FAP-specific CAR-T cells given directly in the pleural effusion (NCT01722149), but the result was still unavailable [432]. A bispecific FAP-CD40 antibody that could induce CD40 stimulation solely in the presence of FAP was designed, which induced predominantly intratumoral immune activation and exhibited well tolerance [433]. Furthermore, FAP-targeted in combination with near-infrared photoimmunotherapy were shown to recover the sensitivity to chemotherapy in CAF-rich tumors and induce tumor regression [434]. Some others FAP-targeted drugs or inhibitors such as RO6874281, PT630 and UAMC-1110 have been verified in several preclinical studies [423, 435, 436], and currently some of these agents have been advanced to testing in clinical trials (NCT03386721, NCT02627274 and NCT02198274).
The high expression of α-smooth muscle actin (α-SMA) is another prominent characteristic of CAFs, and its expression level has been identified as a novel biomarker of resistance to trastuzumab early-stage of HER2-positive breast cancer [437]. Specific targeting α-SMA with docetaxel-conjugate nanoparticles increased drug delivery efficiency by enhancing vascular perfusion and reduced cancer metastases [438]. In the myofibroblast-depleted mouse PDAC model, selective depletion of the α-SMA+ fibroblasts suppressed angiogenesis but led to enhanced tumor hypoxia and induced cancer stem cell-like phenotype. This selective depletion also contributed toward CD3+Foxp3+Treg cells infiltration into the tumor stroma, which ultimately increased tumor aggressiveness and reduced animal survival [20]. These contradictory findings emphasize the importance of targeting stromal cells with caution, and suitable targeting α-SMA rather than complete depletion may deserve further exploration.
Instead of direct CAFs depletion through their cell surface markers, the normalization of activated CAFs that aims to reprogram pro-tumorigenic CAFs into a non-activated or quiescent state represents a plausible option. It has been shown that using vitamin D receptor (VDR) ligand calcipotriol or all-transretinoic acid (ATRA) could achieve CAFs normalization. Specifically, VDR servers as a master genomic suppressor of pancreatic stellate cells (PSC) activation state and has the potential to revert PSCs into quiescent state. In this context, calcipotriol destroyed several tumor-supporting signaling pathways and enhanced the effects of chemotherapy in multiple mouse tumor models, which hindered the tumor-stroma interplay and tumor proliferation [439–442]. Some vitamin D analogues (e.g. calcipotriol and paricalcitol) are being or have been tested in the clinic (NCT02019355 [443], NCT00637897, NCT03520790). Moreover, ATRA is an active metabolite of vitamin A and is intensively studied because of its ability to restore the mechanical quiescence of PSCs and to inhibit aggressive tumors progression. The use of ATRA can suppress force-mediated extracellular matrix remodeling, block pro-tumorigenic signaling pathways and assist the migration of CD8+ T cells to tumor sites and juxtatumoral stromal compartments [300, 444–446]. Currently, ATRA has been evaluated in several clinical trials to treat patients with solid cancers, whether in combination or not with chemotherapies (NCT03307148, NCT03999684, NCT01041833) [447–450].
TGF-β also plays crucial roles in CAFs activation and affects cancer progression. The blockade of TGF-β signaling is excepted to realize CAFs normalization [416]. LY2157299 (galunisertib) is an oral small-molecule inhibitor of TGF-β receptor I kinase and can prevent the activation of CAFs and immunosuppression. Several trials have tested its safety and effectiveness in multiple human cancer types, both as monotherapy or in combination with other treatments, but the results have been mixed (NCT01220271, NCT01373164, NCT01246986, NCT02734160, NCT01582269) [451–458]. Another agent, minnelide, has also been studied in phased trials due to its capacity to suppress the TGF-β signaling (NCT01927965, NCT03117920).
In addition to elimination and normalization of activated CAFs, blockade of CAF-derived signalings may contributes to the acquisition of clinical benefits. FAP+ CAFs are identified as the primary source of a chemokine CXCL12 that exerts immunosuppressive function by binding to its receptor CXCR4 [301]. Some CXCR4 antagonists or inhibitors have been developed from bench to bed [459]. Motixafortide (BL-8040) has already been investigated in combination with pembrolizumab and/or chemotherapy in pancreatic cancer patients, showing some degree of efficacy signs (NCT02826486) [460]. AMD3100 is a CXCR4 inhibitor with the potential to reverse tumor immunosuppression and has been utilized in clinic (NCT02179970, NCT01225419). An alternative approach is to target the sonic hedgehog (SHH)-smoothened (SMO) signaling axis responsible for tumor formation and growth [461]. In several clinical trials, although a SMO inhibitor itraconazole showed antitumor activity with inhibited neoplastic growth (NCT01108094) [462], another SMO inhibitor vismodegib yielded limited or even disappointing clinical outcomes (NCT01700049, NCT01064622, NCT01195415, NCT00887159) [463–466]. LDE225 (sonidegib) is an oral small-molecule SMO inhibitor and are currently undergoing clinical assessment (NCT01579929, NCT01954355, NCT02027376) [467–469].
Overall, CAFs are increasingly recognized as an attractive target that can be clinically intervened for therapeutic benefit in cancer patients. Nevertheless, the clinical effects of targeting CAFs are not extremely encouraging and satisfactory, and no CAF-specific mAb or inhibitor has been approved for standardized cancer treatment thus for. Notably, we face numerous challenges in this field, and more in-depth investigations are still needed. First, CAFs are populations with heterogeneity and plasticity and lack definitive surface biomarkers, so it is difficult to precisely and roundly target these cells. Second, CAFs have been confirmed to possess both tumor-promoting and tumor-restraining functions depending on the TME to which they are exposed, which may account for the clinical failure of CAF-targeted therapy, or in other words, targeting CAFs in cancer is a double-edged sword and sometimes cannnot enhance tumor control as initially hoped. In this condition, developing an approach specifically targeting the tumor-promoting CAFs subtypes may be valuable. Finally, our current researches are largely at the preclinical stages, and it is clear that, to expedite the leap from bench to bedside, we still have a long way to go.
MSCs as potential therapeutic target
TA-MSCs are widely appreciated for playing multiple roles in tumorigenesis and malignant progression, which theoretically provides new opportunities for designing feasible anticancer therapies. However, similar to CAFs populations, the lack of specific cell surface markers and controversial roles with both pro- and anti-tumorigenic functions make it challenging to target TA-MSCs precisely. Alternative strategies have been developed, among which, inhibiting MSC-related signaling pathways or secretory factors and using MSCs as a vehicle for therapeutic delivery represent promising directions [190].
The homing of TA-MSCs into the stroma can accelerate tumor growth and metastasis, and thus inhibiting TA-MSCs aggregation might potentially aid tumor control. The CXCL12/CXCR4 axis is a classic signaling that governs the homing of MSCs and upregulates the expression of PD-L1 to mediate selective immunosuppression within a tumor [470–473]. Hence, much attention has been paid to blocking this pathway with various methods. Olaptesed pegol (ola-PEG) is a high-affinity L-RNA Spiegelmer to CXCL12 with the ability to neutralize CXCL12 activity. The use of ola-PEG delays tumor growth and distant colonization of multiple myeloma cell [474]. Another approach is to target CXCR4 with specific antagonists such as AMD3100. The administration of AMD3100 not only reduced the migration potential of MSCs but also significantly enhanced the effects of anti-PD-L1 treatment [301, 475, 476].
Several previous studies determined the immunosuppressive peculiarity of TA-MSCs that exert profound influence on the growth and aggressive behavior of cancer cells, which is mainly achieved by producing immunoregulatory factors [477]. Among those secretory factors, IDO was found to be overexpressed in tumor, mediate immune escape by reducing both tumor-infiltrating CD8+ T cells and B cells, and contribute to the resistance of anti-CTLA-4 therapy [478–481]. Accumulated preclinical evidence is paving the way for future clinical evaluation, and currently using IDO inhibitors (e.g. navoximod and 1-methyl-DL-tryptophan) to treat cancer patients is undergoing phase clinical trials [482, 483].
An emerging therapeutic paradigm is to develop MACs as carriers for anti-tumor payloads due to their inherent tumor-homing capacity, which can also potentially attenuate their viability and invasive characteristics [484]. Genetically modified MSCs can realize the delivery of therapeutic proteins, cytokines as well as micro RNAs and has manifested obvious antitumor effects in preclinical studies. Based on the significant success of using IFNs, a class of cytokines with antitumor properties, to fight against various cancer types, engineered MSCs with the transfection IFN-α or IFN-β have been designed and exhibited varying degrees of anti-tumor activity. This strategy restricted tumor growth by inducing apoptosis and enhanced both NK cells and CD8+ T cells activity to reinforce antitumor immune responses [485, 486]. Other cytokines have also been stably transduced in MSCs and yielded similar antitumor outcomes, such as IL-12 and IL-17 [487, 488]. Tumor necrosis factor related apoptosis-inducing ligand (TRAIL) is a type II membrane-bound protein capable of inducing apoptosis in various cancer cells. TRAIL-expressing MSCs exhibited directional migration and infiltration toward tumor tissues, extended animal survival and contributed to overcoming drug resistance [489–491]. Furthermore, exogenous microRNAs delivered by MSCs also assist in antitumor therapy, which deserves further clinical evaluation [492–494]. Another valid use of modified MSCs is to load various anti-tumor drugs, which has been extensively tested in numerous cancer types with significant inhibiting tumor growth and improving the anti-cancer efficacy of chemotherapeutic drugs [495–497].
Although it is difficult to deplete TAMSCs directly, the preclinical studies regarding TA-MSCs-associated factors or modified MSCs vastly motivated the initiation of a series of clinical trials (Supplementary Table 4). The clinical trials registered on ClinicalTrials.gov. involve inhibiting secretory factors derived from TA-MSCs, using MSCs as therapeutic agents to treat cancer patients directly and using MSCs as carrier for delivering therapeutic cytokines or proteins. Most of these studies are aimed to evaluate the safety, maximal tolerated and anti-tumor activity of MSCs loaded with different drugs in patients with several cancer types. The others tested the homing of BM-MSCs to tumor sites and the capacity of MSCs to improve the overall survival of patients (NCT01045382, NCT01983709).
In short, TA-MSCs have a multifaceted involvement in cancer, which leads to the springing up of a broader range of studies with respect to MSCs-based anticancer therapies. However, trying to realize and accelerate the clinical transformation of MSCs-based therapies remains a challenge we need to solve. Future research may focus on understanding the interplay of tumor cells and TA-MSCs for better improving clinical safety and outcomes of MSCs-based treatment (Table 4).
Table 4.
Clinical trials based on MSCs
| Target | Drug or intervation | Combination | Condition | Phase | Status | Trial number |
|---|---|---|---|---|---|---|
| IDO | Navoximod | No | Solid Tumor | I | Completed | NCT02048709 |
| Navoximod | Atezolizumab | Locally Advanced or Metastatic Solid Tumors | I | Completed | NCT02471846 | |
| Engineered MSC | BM-MSC-INFβ | No | Ovarian cancer | I | Completed | NCT02530047 |
| MSC-TRAIL | No | Adenocarcinoma of lung | I/II | Recruiting | NCT03298763 | |
| GX-051 | No | Head and neck cancer | I | Unknown | NCT02079324 | |
| CELYVIR | No | Metastatic and refractory tumors | I/II | Completed | NCT01844661 | |
| AdMSC-MV-NIS | No | Ovarian, primary peritoneal or fallopian tube cancer | I/II | Recruiting | NCT02068794 | |
| AloCELYVIR | No | Diffuse Intrinsic Pontine Glioma | I/II | Recruiting | NCT04758533 | |
| MSC-derived exosome | iExosomes | No | Metastatic pancreas cancer | I | Recruiting | NCT03608631 |
| Tissue-derived MSC | HB-adMSCs | No | Pancreatic cancer | I | Unknown | NCT04087889 |
| EB-CMF | No | Mandible tumor | I/II | Recruiting | NCT03678467 | |
| Cord blood MSCs | NeuroRegen Scaffold™ | Rectal cancer | I/II | Recruiting | NCT02648386 | |
| MSCs | Hematopoietic stem cells | Leukemia, lymphoma, and myeloma | II | Terminated | NCT01045382 | |
| IFNγ-primed bone marrow MSCs | No | Acute leukemia | I | Recruiting | NCT04328714 |
Targeting pericytes
Apart from their acknowledged participation in maintaining the integrity of blood vessels, pericytes also act as important regulators of cancer initiation and progression. Targeting pericytes for cancer treatment is growing vigorously and exhibits some degree of benefits in preclinical studies.
One approach to target pericytes is the use of ibrutinib that not only improves the permeability of blood-brain barrier but also prolongs animal survival by enhancing chemotherapeutic effectiveness [498]. Some other investigations have confirmed that the inhibition of PDGFRβ+ pericytes with imatinib (a specific tyrosine kinase inhibitor) achieved pericytes depletion and delayed lymphoma growth in both murine allograft and human xenograft models [499, 500]. Clinically, the main purpose of applying imatinib is to inhibit tumor angiogenesis, but this scheme often showed modest or no effect as a single agent or in combination with chemotherapy (NCT01738139, NCT00785785) [501, 502]. In this context, dual-targeting VEGFR and PDGFR-β blockade may have better outcomes for cancer treatment and needs further clinical verification [503]. Furthermore, pericytes can protect tumor cells from immune surveillance and attack, which suggests that pericytes can potentially be viewed as a novel target or combined option for cancer immunotherapy. Designing novel agents addressing pericytes and rationally choosing therapeutic combinations are expected to improve a better cancer control and remission.
Conclusions and perspectives
It is traditionally recognized that cancer is a malignant cell-centric disease, and with the rapid advances in the knowledge of the TME, this view is currently being replaced by the understanding of dependency and interplay between cancer cells and the tumor stroma. Specifically, cancer initiation, progression and metastasis usually elicit a broad spectrum of dynamic evolutions and alterations in host tissues, which contributes to the establishment of complicated stromal surroundings that are a prerequisite for tumor cell invasion and metastasis. These abundant stromal elements operate with each other in a coordinated fashion. Some stromal components and their molecular changes can also be considered potential biomarkers for diagnosis, prognosis, and response to treatment in cancer, which endows them with clinical significance as the abundance of stromal cells is typically related to unfavorable prognosis.
The tumor stroma participates in nearly all stages of malignant disease progression, and thereby constituting legitimate targets for therapeutic intervention. At present, a great variety of stroma-targeted modalities that aim to reduce or deprive the pro-tumorigenic functions of stroma have been developed and tested in both preclinical studies and clinical trials. Furthermore, it is possible to hopefully slow or reverse tumor development by “normalizing” the tumor stroma, such as vascular normalization and CAFs normalization. Despite these advances and findings, stromal targeting approaches can only reduce tumor growth rates or slightly extend patients survival, and are rarely curative. Simultaneously, there are still several unresolved aspects deserving further exploration. First, the stroma has both pro- and anti-tumorigenic properties since complete ablation of the stroma leads to a more invasive tumor phenotype and reduces animal survival, and thus a crucial consideration that cannot be ignored is to suitably target tumor-promoting stromal populations without hurting healthy tissues. Second, some cellular components of stroma such as CAFs and TA-MSCs lack specific cell surface markers and even possess diverse subtypes. The next goal of preclinical studies is to identify the relevant stromal cells with a specific biomarker and explore the totality tumor-promoting or tumor-restraining functions of a given stromal cell type, which will dramatically motivate the development of stroma-based therapies. Third, current mechanistic and functional investigations regarding roles of stromal elements in cancer largely rely on xenograft or syngeneic animal models, and in this condition, measures should be taken in future studies to improve the model design and try to expedite the leap from bench to bedside. Finally, in the field of cancer targeted therapies, combinational strategies with chemotherapy or immunotherapy typically exhibit more beneficial and effective outcomes. Furthermore, interfering with cancer-stromal interactions should choose optimal opportunity such as earlier phases of carcinogenesis rather than only the invasive stage, which contributes to therapeutic intervention and reduces deleterious effects. Consequently, searching for the best administration regimens with distinct combinations together with legitimate sequence and time is expected to yield significant clinical benefits for patients.
In summary, all cellular and noncellular components of the tumor stroma can interact and engage in highly regulated reciprocal dialogues, which contributes toward cancer initiation, progression and therapeutic resistance. Importantly, these findings and insights bring stroma-targeted therapies for cancer treatment onto the agenda. An in-depth understanding of the crosstalk between stroma and cancer cells is crucial for designing novel strategies for new therapeutic interventions, especially for those stroma-rich cancer types such as pancreatic carcinoma. Moving forwards, despite much work remaining to be done, it can be anticipated that, as an emerging strategy for cancer treatment, stroma-targeted therapy will open a new avenue of research in the management of malignancy and reshape the therapeutic landscape with the potential to bring more clinical benefits for cancer patients.
Acknowledgements
Not applicable.
Abbreviations
- TME
Tumor microenvironment
- ECM
Extracellular matrix
- CAFs
Cancer-associated fibroblasts
- MSCs
Mesenchymal stromal cells
- TAMSCs
Tumorassociated MSCs
- TGF-β
Transforming growth factor-β
- VEGF
Vascular endothelial growth factor
- HGF
Hepatocyte growth factor
- OSM
Oncostatin M
- LOX
Lysyl oxidase
- LOXL2
Lysyl oxidase like-2
- RSU-1
Ras Suppressor-1
- MMPs
Matrix metalloproteinases
- PDAC
Pancreatic ductal adenocarcinoma
- MVD
Microvessel density
- HIF
Hypoxia-inducible factor
- TECs
Tumor-associated endothelial cells
- PGE2
Prostaglandin E2
- Treg
Regulatory T cell
- PD-1
Programmed death 1
- PD-L1
PD-1 ligand 1
- PD-L2
PD-1 ligand 2
- ICC
Intrahepatic cholangiocarcinoma
- OXPHOS
Oxidative phosphorylation
- BC
Breast cancer
- GC
Gastric cancer
- IL-1β
Interleukin‑1β
- TNF
Tumor necrosis factor
- PMN
Pre-metastatic niche
- IGF
Insulin-like growth factor
- CTCs
Circulating tumor cells
- cCAFs
Circulating-CAFs
- PDGF
Platelet-derived growth factor
- MDSCs
Myeloid-derived suppressor cells
- SFRP2
Secreted frizzled-related protein 2
- TAMs
Tumor‑associated macrophages
- TANs
Tumor‑associated neutrophils
- DCs
Dendritic cells
- MCs
Mast cells
- NK
Natural killer
- IDO
Indoleamine 2,3-dioxygenase
- CTLs
Cytotoxic T lymphocytes
- PFS
Progression-free survival
- OS
Overall survival
- ORR
Objective response rate
- CTGF
Connective tissue growth factor
- ED-A
Extra domain-A
- EGFR
Epidermal growth factor receptor
- TKIs
Tyrosine kinase inhibitors
- Ang
Angiopoietin
- CAR
Chimeric antigen receptor
- VDR
Vitamin D receptor
- ATRA
All-transretinoic acid
- PSC
Pancreatic stellate cells
Authors’ contributions
Yuquan Wei and Xiawei Wei offered main direction and significant guidance of this manuscript. Maosen Xu and Tao Zhang drafted the manuscript. Ruolan Xia illustrated the figures, made the tables for the manuscript and critically revised the manuscript. All authors approved the final manuscript.
Funding
This work was supported by the National Science Fund for Excellent Young Scholars National Science Fund for Excellent Young Scholars (No. 32122052), National Natural Science Foundation Regional Innovation and Development (No. U19A2003).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors consent to publication.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Maosen Xu, Tao Zhang and Ruolan Xia contributed equally to the work.
Contributor Information
Maosen Xu, Email: maosenxu1997@163.com.
Tao Zhang, Email: 2015181622062@stu.scu.edu.cn.
Ruolan Xia, Email: 130931980@qq.com.
Yuquan Wei, Email: yuquanwei@scu.edu.cn.
Xiawei Wei, Email: xiaweiwei@scu.edu.cn.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Paget S. PAGET,STEPHEN PAPER REPRODUCED FROM THE LANCET, 1889. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 3.Langley RR, Fidler IJ. The seed and soil hypothesis revisited–the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer. 2011;128:2527–35. doi: 10.1002/ijc.26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elia I, Haigis MC. Metabolites and the tumour microenvironment: from cellular mechanisms to systemic metabolism. Nat Metab. 2021;3:21–32. doi: 10.1038/s42255-020-00317-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arneth B. Tumor Microenvironment. Medicina (Kaunas) 2019, 56. [DOI] [PMC free article] [PubMed]
- 6.Valkenburg KC, de Groot AE, Pienta KJ. Targeting the tumour stroma to improve cancer therapy. Nat Rev Clin Oncol. 2018;15:366–81. doi: 10.1038/s41571-018-0007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plava J, Cihova M, Burikova M, Matuskova M, Kucerova L, Miklikova S. Recent advances in understanding tumor stroma-mediated chemoresistance in breast cancer. Mol Cancer. 2019;18:67. doi: 10.1186/s12943-019-0960-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.RonnovJessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: Importance of the stromal reaction. Physiol Rev. 1996;76:69–125. doi: 10.1152/physrev.1996.76.1.69. [DOI] [PubMed] [Google Scholar]
- 9.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–33. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 10.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–50. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 11.Bejarano L, Jordao MJC, Joyce JA. Therapeutic Targeting of the Tumor Microenvironment. Cancer Discov. 2021;11:933–59. doi: 10.1158/2159-8290.CD-20-1808. [DOI] [PubMed] [Google Scholar]
- 12.Hosein AN, Brekken RA, Maitra A. Pancreatic cancer stroma: an update on therapeutic targeting strategies. Nat Rev Gastroenterol Hepatol. 2020;17:487–505. doi: 10.1038/s41575-020-0300-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sund M, Kalluri R. Tumor stroma derived biomarkers in cancer. Cancer Metastasis Rev. 2009;28:177–83. doi: 10.1007/s10555-008-9175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mesker WE, Junggeburt JMC, Szuhai K, de Heer P, Morreau H, Tanke HJ, Tollenaar R. The carcinoma-stromal ratio of colon carcinoma is an independent factor for survival compared to lymph node status and tumor stage. Cell Oncol. 2007;29:387–98. doi: 10.1155/2007/175276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Almangush A, Alabi RO, Troiano G, Coletta RD, Salo T, Pirinen M, Makitie AA, Leivo I. Clinical significance of tumor-stroma ratio in head and neck cancer: a systematic review and meta-analysis. BMC Cancer. 2021;21:480. doi: 10.1186/s12885-021-08222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagenaars SC, de Groot S, Cohen D, Dekker TJA, Charehbili A, Meershoek-Klein Kranenbarg E, Duijm-de Carpentier M, Pijl H, Putter H, Tollenaar R, et al. Tumor-stroma ratio is associated with Miller-Payne score and pathological response to neoadjuvant chemotherapy in HER2-negative early breast cancer. Int J Cancer. 2021;149:1181–8. doi: 10.1002/ijc.33700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He R, Li D, Liu B, Rao J, Meng H, Lin W, Fan T, Hao B, Zhang L, Lu Z, et al. The prognostic value of tumor-stromal ratio combined with TNM staging system in esophagus squamous cell carcinoma. J Cancer. 2021;12:1105–14. doi: 10.7150/jca.50439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu Y, Jin Z, Qian Y, Shen Y, Wang Z. Prognostic Value of Tumor-Stroma Ratio in Rectal Cancer: A Systematic Review and Meta-analysis. Front Oncol. 2021;11:685570. doi: 10.3389/fonc.2021.685570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kota J, Hancock J, Kwon J, Korc M. Pancreatic cancer: Stroma and its current and emerging targeted therapies. Cancer Lett. 2017;391:38–49. doi: 10.1016/j.canlet.2016.12.035. [DOI] [PubMed] [Google Scholar]
- 20.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–34. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP, Tattersall IW, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–47. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–37. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–49. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 24.Spaw M, Anant S, Thomas SM. Stromal contributions to the carcinogenic process. Mol Carcinog. 2017;56:1199–213. doi: 10.1002/mc.22583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nwabo Kamdje H, Seke Etet AF, Tagne Simo P, Vecchio R, Erique Lukong L, Krampera K. Emerging data supporting stromal cell therapeutic potential in cancer: reprogramming stromal cells of the tumor microenvironment for anti-cancer effects. Cancer Biology and Medicine. 2020;17:828–41. doi: 10.20892/j.issn.2095-3941.2020.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bussard KM, Mutkus L, Stumpf K, Gomez-Manzano C, Marini FC. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res. 2016;18:84. doi: 10.1186/s13058-016-0740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scully S, Francescone R, Faibish M, Bentley B, Taylor SL, Oh D, Schapiro R, Moral L, Yan W, Shao R. Transdifferentiation of glioblastoma stem-like cells into mural cells drives vasculogenic mimicry in glioblastomas. J Neurosci. 2012;32:12950–60. doi: 10.1523/JNEUROSCI.2017-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen HF, Huang CH, Liu CJ, Hung JJ, Hsu CC, Teng SC, Wu KJ. Twist1 induces endothelial differentiation of tumour cells through the Jagged1-KLF4 axis. Nat Commun. 2014;5:4697. doi: 10.1038/ncomms5697. [DOI] [PubMed] [Google Scholar]
- 29.Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 30.Massague J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature. 2016;529:298–306. doi: 10.1038/nature17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engels B, Rowley DA, Schreiber H. Targeting stroma to treat cancers. Semin Cancer Biol. 2012;22:41–9. doi: 10.1016/j.semcancer.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shupp AB, Kolb AD, Mukhopadhyay D, Bussard KM. Cancer Metastases to Bone: Concepts, Mechanisms, and Interactions with Bone Osteoblasts. Cancers (Basel) 2018, 10. [DOI] [PMC free article] [PubMed]
- 33.Lyu X, Zhang Q, Fares HM, Wang Y, Han Y, Sun L. Contribution of adipocytes in the tumor microenvironment to breast cancer metabolism. Cancer Lett. 2022;534:215616. doi: 10.1016/j.canlet.2022.215616. [DOI] [PubMed] [Google Scholar]
- 34.Choi J, Cha YJ, Koo JS. Adipocyte biology in breast cancer: From silent bystander to active facilitator. Prog Lipid Res. 2018;69:11–20. doi: 10.1016/j.plipres.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Cai Z, Liang Y, Xing C, Wang H, Hu P, Li J, Huang H, Wang W, Jiang C. Cancerassociated adipocytes exhibit distinct phenotypes and facilitate tumor progression in pancreatic cancer. Oncol Rep. 2019;42:2537–49. doi: 10.3892/or.2019.7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71:2455–65. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 37.Kai F, Drain AP, Weaver VM. The Extracellular Matrix Modulates the Metastatic Journey. Dev Cell. 2019;49:332–46. doi: 10.1016/j.devcel.2019.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mammoto T, Ingber DE. Mechanical control of tissue and organ development. Development. 2010;137:1407–20. doi: 10.1242/dev.024166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garde A, Sherwood DR. Fueling Cell Invasion through Extracellular Matrix. Trends Cell Biol. 2021;31:445–56. doi: 10.1016/j.tcb.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 41.Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014;15:1243–53. doi: 10.15252/embr.201439246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 43.Petersen EV, Chudakova DA, Skorova EY, Anikin V, Reshetov IV, Mynbaev OA. The Extracellular Matrix-Derived Biomarkers for Diagnosis, Prognosis, and Personalized Therapy of Malignant Tumors. Front Oncol. 2020;10:575569. doi: 10.3389/fonc.2020.575569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195–200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karamanos NK, Theocharis AD, Piperigkou Z, Manou D, Passi A, Skandalis SS, Vynios DH, Orian-Rousseau V, Ricard-Blum S, Schmelzer CEH, et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021;288:6850–912. doi: 10.1111/febs.15776. [DOI] [PubMed] [Google Scholar]
- 46.Cox TR. The matrix in cancer. Nat Rev Cancer. 2021;21:217–38. doi: 10.1038/s41568-020-00329-7. [DOI] [PubMed] [Google Scholar]
- 47.Eble JA, Niland S. The extracellular matrix in tumor progression and metastasis. Clin Exp Metastasis. 2019;36:171–98. doi: 10.1007/s10585-019-09966-1. [DOI] [PubMed] [Google Scholar]
- 48.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cavaco ACM, Darnas S, Casimiro S, Costa L. Collagen biology making inroads into prognosis and treatment of cancer progression and metastasis. Cancer Metastasis Rev. 2020;39:603–23. doi: 10.1007/s10555-020-09888-5. [DOI] [PubMed] [Google Scholar]
- 50.Poltavets V, Kochetkova M, Pitson SM, Samuel MS. The Role of the Extracellular Matrix and Its Molecular and Cellular Regulators in Cancer Cell Plasticity. Front Oncol. 2018;8:431. doi: 10.3389/fonc.2018.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuzhalin AE, Lim SY, Kutikhin AG, Gordon-Weeks AN. Dynamic matrisome: ECM remodeling factors licensing cancer progression and metastasis. Biochim Biophys Acta Rev Cancer. 2018;1870:207–28. doi: 10.1016/j.bbcan.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 52.Xu S, Xu H, Wang W, Li S, Li H, Li T, Zhang W, Yu X, Liu L. The role of collagen in cancer: from bench to bedside. J Transl Med. 2019;17:309. doi: 10.1186/s12967-019-2058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ahmad RS, Eubank TD, Lukomski S, Boone BA: Immune Cell Modulation of the Extracellular Matrix Contributes to the Pathogenesis of Pancreatic Cancer. Biomolecules 2021, 11. [DOI] [PMC free article] [PubMed]
- 54.Miyazawa A, Ito S, Asano S, Tanaka I, Sato M, Kondo M, Hasegawa Y. Regulation of PD-L1 expression by matrix stiffness in lung cancer cells. Biochem Biophys Res Commun. 2018;495:2344–9. doi: 10.1016/j.bbrc.2017.12.115. [DOI] [PubMed] [Google Scholar]
- 55.Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60:2497–503. [PubMed] [Google Scholar]
- 56.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, et al. Inhibition of Hedgehog Signaling Enhances Delivery of Chemotherapy in a Mouse Model of Pancreatic Cancer. Science. 2009;324:1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rice AJ, Cortes E, Lachowski D, Cheung BCH, Karim SA, Morton JP. Del Rio Hernandez A: Matrix stiffness induces epithelial-mesenchymal transition and promotes chemoresistance in pancreatic cancer cells. Oncogenesis. 2017;6:e352. doi: 10.1038/oncsis.2017.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Piersma B, Hayward MK, Weaver VM. Fibrosis and cancer: A strained relationship. Biochim Biophys Acta Rev Cancer. 2020;1873:188356. doi: 10.1016/j.bbcan.2020.188356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Najafi M, Farhood B, Mortezaee K. Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J Cell Biochem. 2019;120:2782–90. doi: 10.1002/jcb.27681. [DOI] [PubMed] [Google Scholar]
- 60.Hazlehurst LA, Dalton WS. Mechanisms associated with cell adhesion mediated drug resistance (CAM-DR) in hematopoietic malignancies. Cancer Metastasis Rev. 2001;20:43–50. doi: 10.1023/A:1013156407224. [DOI] [PubMed] [Google Scholar]
- 61.Bertero T, Oldham WM, Grasset EM, Bourget I, Boulter E, Pisano S, Hofman P, Bellvert F, Meneguzzi G, Bulavin DV, et al. Tumor-Stroma Mechanics Coordinate Amino Acid Availability to Sustain Tumor Growth and Malignancy. Cell Metab. 2019;29:124–40 e110. doi: 10.1016/j.cmet.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reid SE, Kay EJ, Neilson LJ, Henze AT, Serneels J, McGhee EJ, Dhayade S, Nixon C, Mackey JB, Santi A, et al. Tumor matrix stiffness promotes metastatic cancer cell interaction with the endothelium. EMBO J. 2017;36:2373–89. doi: 10.15252/embj.201694912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rammal H, Saby C, Magnien K, Van-Gulick L, Garnotel R, Buache E, El Btaouri H, Jeannesson P, Morjani H. Discoidin Domain Receptors: Potential Actors and Targets in Cancer. Front Pharmacol. 2016;7:55. doi: 10.3389/fphar.2016.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miroshnikova YA, Rozenberg GI, Cassereau L, Pickup M, Mouw JK, Ou G, Templeman KL, Hannachi EI, Gooch KJ, Sarang-Sieminski AL, et al. alpha 5 beta 1-Integrin promotes tension-dependent mammary epithelial cell invasion by engaging the fibronectin synergy site. Mol Biol Cell. 2017;28:2958–77. doi: 10.1091/mbc.e17-02-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vachon PH. Integrin signaling, cell survival, and anoikis: distinctions, differences, and differentiation. J Signal Transduct. 2011;2011:738137. doi: 10.1155/2011/738137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Woods EC, Kai F, Barnes JM, Pedram K, Pickup MW, Hollander MJ, Weaver VM, Bertozzi CR. A bulky glycocalyx fosters metastasis formation by promoting G1 cell cycle progression. Elife 2017, 6. [DOI] [PMC free article] [PubMed]
- 67.Gkretsi V, Stylianou A, Louca M, Stylianopoulos T. Identification of Ras suppressor-1 (RSU-1) as a potential breast cancer metastasis biomarker using a three-dimensional in vitro approach. Oncotarget. 2017;8:27364–79. doi: 10.18632/oncotarget.16062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alexander J, Cukierman E. Stromal dynamic reciprocity in cancer: intricacies of fibroblastic-ECM interactions. Curr Opin Cell Biol. 2016;42:80–93. doi: 10.1016/j.ceb.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sottnik JL, Dai JL, Zhang HL, Campbell B, Keller ET. Tumor-Induced Pressure in the Bone Microenvironment Causes Osteocytes to Promote the Growth of Prostate Cancer Bone Metastases. Cancer Res. 2015;75:2151–8. doi: 10.1158/0008-5472.CAN-14-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mondal S, Adhikari N, Banerjee S, Amin SA, Jha T. Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: A minireview. Eur J Med Chem. 2020;194:112260. doi: 10.1016/j.ejmech.2020.112260. [DOI] [PubMed] [Google Scholar]
- 71.Jablonska-Trypuc A, Matejczyk M, Rosochacki S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J Enzyme Inhib Med Chem. 2016;31:177–83. doi: 10.3109/14756366.2016.1161620. [DOI] [PubMed] [Google Scholar]
- 72.Quintero-Fabian S, Arreola R, Becerril-Villanueva E, Torres-Romero JC, Arana-Argaez V, Lara-Riegos J, Ramirez-Camacho MA, Alvarez-Sanchez ME. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front Oncol. 2019;9:1370. doi: 10.3389/fonc.2019.01370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang H. Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors (Basel) 2018, 18. [DOI] [PMC free article] [PubMed]
- 74.Siddhartha R, Garg M. Molecular and clinical insights of matrix metalloproteinases into cancer spread and potential therapeutic interventions. Toxicol Appl Pharmacol. 2021;426:115593. doi: 10.1016/j.taap.2021.115593. [DOI] [PubMed] [Google Scholar]
- 75.Webb AH, Gao BT, Goldsmith ZK, Irvine AS, Saleh N, Lee RP, Lendermon JB, Bheemreddy R, Zhang Q, Brennan RC, et al. Inhibition of MMP-2 and MMP-9 decreases cellular migration, and angiogenesis in in vitro models of retinoblastoma. BMC Cancer. 2017;17:434. doi: 10.1186/s12885-017-3418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leong HS, Robertson AE, Stoletov K, Leith SJ, Chin CA, Chien AE, Hague MN, Ablack A, Carmine-Simmen K, McPherson VA, et al. Invadopodia are required for cancer cell extravasation and are a therapeutic target for metastasis. Cell Rep. 2014;8:1558–70. doi: 10.1016/j.celrep.2014.07.050. [DOI] [PubMed] [Google Scholar]
- 77.Kumar S, Das A, Barai A, Sen S. MMP Secretion Rate and Inter-invadopodia Spacing Collectively Govern Cancer Invasiveness. Biophys J. 2018;114:650–62. doi: 10.1016/j.bpj.2017.11.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miyazawa Y, Uekita T, Ito Y, Seiki M, Yamaguchi H, Sakai R. CDCP1 regulates the function of MT1-MMP and invadopodia-mediated invasion of cancer cells. Mol Cancer Res. 2013;11:628–37. doi: 10.1158/1541-7786.MCR-12-0544. [DOI] [PubMed] [Google Scholar]
- 79.Jacob A, Prekeris R. The regulation of MMP targeting to invadopodia during cancer metastasis. Front Cell Dev Biol. 2015;3:4. doi: 10.3389/fcell.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grauzam S, Brock AM, Holmes CO, Tiedeken JA, Boniface SG, Pierson BN, Patterson DG, Coaxum SD, Neskey DM, Rosenzweig SA. NEDD9 stimulated MMP9 secretion is required for invadopodia formation in oral squamous cell carcinoma. Oncotarget. 2018;9:25503–16. doi: 10.18632/oncotarget.25347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.da Silva PHR, Borges BC, Uehara IA, Soldi LR, de Araujo RA, Silva MJB. Chemokines and the extracellular matrix: Set of targets for tumor development and treatment. Cytokine. 2021;144:155548. doi: 10.1016/j.cyto.2021.155548. [DOI] [PubMed] [Google Scholar]
- 82.Chakravarthy A, Khan L, Bensler NP, Bose P, De Carvalho DD. TGF-beta-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat Commun. 2018;9:4692. doi: 10.1038/s41467-018-06654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hutchings H, Ortega N, Plouet J. Extracellular matrix-bound vascular endothelial growth factor promotes endothelial cell adhesion, migration, and survival through integrin ligation. Faseb J. 2003;17:1520-+. doi: 10.1096/fj.02-0691fje. [DOI] [PubMed] [Google Scholar]
- 84.Noriega-Guerra H, Freitas VM. Extracellular Matrix Influencing HGF/c-MET Signaling Pathway: Impact on Cancer Progression. Int J Mol Sci 2018, 19. [DOI] [PMC free article] [PubMed]
- 85.Dinca SC, Greiner D, Weidenfeld K, Bond L, Barkan D, Jorcyk CL. Novel mechanism for OSM-promoted extracellular matrix remodeling in breast cancer: LOXL2 upregulation and subsequent ECM alignment. Breast Cancer Res. 2021;23:56. doi: 10.1186/s13058-021-01430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang J, Zhang L, Wan D, Zhou L, Zheng S, Lin S, Qiao Y. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct Target Ther. 2021;6:153. doi: 10.1038/s41392-021-00544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Capurro MI, Xiang YY, Lobe C, Filmus J. Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res. 2005;65:6245–54. doi: 10.1158/0008-5472.CAN-04-4244. [DOI] [PubMed] [Google Scholar]
- 88.Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci. 2020;77:1745–70. doi: 10.1007/s00018-019-03351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Folkman J, Bach M, Rowe JW, Davidoff F, Lambert P, Hirsch C, Goldberg A, Hiatt HH, Glass J, Henshaw E. TUMOR ANGIOGENESIS - THERAPEUTIC IMPLICATIONS. N Engl J Med. 1971;285:1182. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 90.De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17:457–74. doi: 10.1038/nrc.2017.51. [DOI] [PubMed] [Google Scholar]
- 91.Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266–76. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ikeda N, Adachi M, Taki T, Huang C, Hashida H, Takabayashi A, Sho M, Nakajima Y, Kanehiro H, Hisanaga M, et al. Prognostic significance of angiogenesis in human pancreatic cancer. Br J Cancer. 1999;79:1553–63. doi: 10.1038/sj.bjc.6690248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nishida T, Yoshitomi H, Takano S, Kagawa S, Shimizu H, Ohtsuka M, Kato A, Furukawa K, Miyazaki M. Low Stromal Area and High Stromal Microvessel Density Predict Poor Prognosis in Pancreatic Cancer. Pancreas. 2016;45:593–600. doi: 10.1097/MPA.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 94.Teleanu RI, Chircov C, Grumezescu AM, Teleanu DM. Tumor Angiogenesis and Anti-Angiogenic Strategies for Cancer Treatment. J Clin Med 2019, 9. [DOI] [PMC free article] [PubMed]
- 95.LaGory EL, Giaccia AJ. The ever-expanding role of HIF in tumour and stromal biology. Nat Cell Biol. 2016;18:356–65. doi: 10.1038/ncb3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Buchler P, Reber HA, Buchler M, Shrinkante S, Buchler MW, Friess H, Semenza GL, Hines OJ. Hypoxia-inducible factor 1 regulates vascular endothelial growth factor expression in human pancreatic cancer. Pancreas. 2003;26:56–64. doi: 10.1097/00006676-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 97.Matsuo Y, Ding Q, Desaki R, Maemura K, Mataki Y, Shinchi H, Natsugoe S, Takao S. Hypoxia inducible factor-1 alpha plays a pivotal role in hepatic metastasis of pancreatic cancer: an immunohistochemical study. J Hepatobiliary Pancreat Sci. 2014;21:105–12. doi: 10.1002/jhbp.6. [DOI] [PubMed] [Google Scholar]
- 98.Li M, Xie H, Liu Y, Xia C, Cun X, Long Y, Chen X, Deng M, Guo R, Zhang Z, He Q. Knockdown of hypoxia-inducible factor-1 alpha by tumor targeted delivery of CRISPR/Cas9 system suppressed the metastasis of pancreatic cancer. J Control Release. 2019;304:204–15. doi: 10.1016/j.jconrel.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 99.Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Cancer. 2010;10:505–14. doi: 10.1038/nrc2868. [DOI] [PubMed] [Google Scholar]
- 100.Claesson-Welsh L, Welsh M. VEGFA and tumour angiogenesis. J Intern Med. 2013;273:114–27. doi: 10.1111/joim.12019. [DOI] [PubMed] [Google Scholar]
- 101.Vajkoczy P, Farhadi M, Gaumann A, Heidenreich R, Erber R, Wunder A, Tonn JC, Menger MD, Breier G. Microtumor growth initiates angiogenic sprouting with simultaneous expression of VEGF, VEGF receptor-2, and angiopoietin-2. J Clin Invest. 2002;109:777–85. doi: 10.1172/JCI0214105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schito L, Rey S. Hypoxia: Turning vessels into vassals of cancer immunotolerance. Cancer Lett. 2020;487:74–84. doi: 10.1016/j.canlet.2020.05.015. [DOI] [PubMed] [Google Scholar]
- 103.Hida K, Hida Y, Amin DN, Flint AF, Panigrahy D, Morton CC, Klagsbrun M. Tumor-associated endothelial cells with cytogenetic abnormalities. Cancer Res. 2004;64:8249–55. doi: 10.1158/0008-5472.CAN-04-1567. [DOI] [PubMed] [Google Scholar]
- 104.Maishi N, Annan DA, Kikuchi H, Hida Y, Hida K. Tumor Endothelial Heterogeneity in Cancer Progression. Cancers (Basel) 2019, 11. [DOI] [PMC free article] [PubMed]
- 105.Chen WZ, Jiang JX, Yu XY, Xia WJ, Yu PX, Wang K, Zhao ZY, Chen ZG. Endothelial cells in colorectal cancer. World J Gastrointest Oncol. 2019;11:946–56. doi: 10.4251/wjgo.v11.i11.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tokumoto MW, Tanaka H, Tauchi Y, Kasashima H, Kurata K, Yashiro M, Sakurai K, Toyokawa T, Kubo N, Amano R, et al. Identification of tumour-reactive lymphatic endothelial cells capable of inducing progression of gastric cancer. Br J Cancer. 2015;113:1046–54. doi: 10.1038/bjc.2015.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Butler JM, Kobayashi H, Rafii S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat Rev Cancer. 2010;10:138–46. doi: 10.1038/nrc2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maishi N, Ohba Y, Akiyama K, Ohga N, Hamada J, Nagao-Kitamoto H, Alam MT, Yamamoto K, Kawamoto T, Inoue N, et al. Tumour endothelial cells in high metastatic tumours promote metastasis via epigenetic dysregulation of biglycan. Sci Rep. 2016;6:28039. doi: 10.1038/srep28039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wieland E, Rodriguez-Vita J, Liebler SS, Mogler C, Moll I, Herberich SE, Espinet E, Herpel E, Menuchin A, Chang-Claude J, et al. Endothelial Notch1 Activity Facilitates Metastasis. Cancer Cell. 2017;31:355–67. doi: 10.1016/j.ccell.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 110.Yadav A, Kumar B, Yu JG, Old M, Teknos TN, Kumar P. Tumor-Associated Endothelial Cells Promote Tumor Metastasis by Chaperoning Circulating Tumor Cells and Protecting Them from Anoikis. PLoS ONE. 2015;10:e0141602. doi: 10.1371/journal.pone.0141602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ohga N, Ishikawa S, Maishi N, Akiyama K, Hida Y, Kawamoto T, Sadamoto Y, Osawa T, Yamamoto K, Kondoh M, et al. Heterogeneity of tumor endothelial cells: comparison between tumor endothelial cells isolated from high- and low-metastatic tumors. Am J Pathol. 2012;180:1294–307. doi: 10.1016/j.ajpath.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 112.Hida K, Akiyama K, Ohga N, Maishi N, Hida Y. Tumour endothelial cells acquire drug resistance in a tumour microenvironment. J Biochem. 2013;153:243–9. doi: 10.1093/jb/mvs152. [DOI] [PubMed] [Google Scholar]
- 113.Cao Z, Scandura JM, Inghirami GG, Shido K, Ding BS, Rafii S. Molecular Checkpoint Decisions Made by Subverted Vascular Niche Transform Indolent Tumor Cells into Chemoresistant Cancer Stem Cells. Cancer Cell. 2017;31:110–26. doi: 10.1016/j.ccell.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Akino T, Hida K, Hida Y, Tsuchiya K, Freedman D, Muraki C, Ohga N, Matsuda K, Akiyama K, Harabayashi T, et al. Cytogenetic Abnormalities of Tumor-Associated Endothelial Cells in Human Malignant Tumors. Am J Pathol. 2009;175:2657–67. doi: 10.2353/ajpath.2009.090202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hida K, Maishi N, Annan DA, Hida Y. Contribution of Tumor Endothelial Cells in Cancer Progression. Int J Mol Sci 2018, 19. [DOI] [PMC free article] [PubMed]
- 116.Bussolati B, Deambrosis I, Russo S, Deregibus MC, Camussi G. Altered angiogenesis and survival in human tumor-derived endothelial cells. Faseb J. 2003;17:1159-+. doi: 10.1096/fj.02-0557fje. [DOI] [PubMed] [Google Scholar]
- 117.Xiong YQ, Sun HC, Zhang W, Zhu XD, Zhuang PY, Zhang JB, Wang L, Wu WZ, Qin LX, Tang ZY. Human hepatocellular carcinoma tumor-derived endothelial cells manifest increased angiogenesis capability and drug resistance compared with normal endothelial cells. Clin Cancer Res. 2009;15:4838–46. doi: 10.1158/1078-0432.CCR-08-2780. [DOI] [PubMed] [Google Scholar]
- 118.Akiyama K, Maishi N, Ohga N, Hida Y, Ohba Y, Alam MT, Kawamoto T, Ohmura H, Yamada K, Torii C, et al. Inhibition of multidrug transporter in tumor endothelial cells enhances antiangiogenic effects of low-dose metronomic paclitaxel. Am J Pathol. 2015;185:572–80. doi: 10.1016/j.ajpath.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 119.Hida K, Maishi N, Sakurai Y, Hida Y, Harashima H. Heterogeneity of tumor endothelial cells and drug delivery. Adv Drug Deliv Rev. 2016;99:140–7. doi: 10.1016/j.addr.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 120.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–98. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 121.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 122.Franco OE, Shaw AK, Strand DW, Hayward SW. Cancer associated fibroblasts in cancer pathogenesis. Semin Cell Dev Biol. 2010;21:33–9. doi: 10.1016/j.semcdb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cox TR, Erler JT. Fibrosis and Cancer: Partners in Crime or Opposing Forces? Trends Cancer. 2016;2:279–82. doi: 10.1016/j.trecan.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 124.Meng Q, Luo X, Chen J, Wang D, Chen E, Zhang W, Zhang G, Zhou W, Xu J, Song Z. Unmasking carcinoma-associated fibroblasts: Key transformation player within the tumor microenvironment. Biochim Biophys Acta Rev Cancer. 2020;1874:188443. doi: 10.1016/j.bbcan.2020.188443. [DOI] [PubMed] [Google Scholar]
- 125.Maia A, Wiemann S. Cancer-Associated Fibroblasts: Implications for Cancer Therapy. Cancers (Basel) 2021, 13. [DOI] [PMC free article] [PubMed]
- 126.Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, Fearon D, Greten FR, Hingorani SR, Hunter T, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020;20:174–86. doi: 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123–8. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 128.Bochet L, Lehuede C, Dauvillier S, Wang YY, Dirat B, Laurent V, Dray C, Guiet R, Maridonneau-Parini I, Le Gonidec S, et al. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 2013;73:5657–68. doi: 10.1158/0008-5472.CAN-13-0530. [DOI] [PubMed] [Google Scholar]
- 129.Raz Y, Cohen N, Shani O, Bell RE, Novitskiy SV, Abramovitz L, Levy C, Milyavsky M, Leider-Trejo L, Moses HL, et al. Bone marrow-derived fibroblasts are a functionally distinct stromal cell population in breast cancer. J Exp Med. 2018;215:3075–93. doi: 10.1084/jem.20180818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–72. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mishra PJ, Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, Ganesan S, Glod JW, Banerjee D. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008;68:4331–9. doi: 10.1158/0008-5472.CAN-08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Apte MV, Haber PS, Darby SJ, Rodgers SC, McCaughan GW, Korsten MA, Pirola RC, Wilson JS. Pancreatic stellate cells are activated by proinflammatory cytokines: implications for pancreatic fibrogenesis. Gut. 1999;44:534–41. doi: 10.1136/gut.44.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yin CY, Evason KJ, Asahina K, Stainier DYR. Hepatic stellate cells in liver development, regeneration, and cancer. J Clin Invest. 2013;123:1902–10. doi: 10.1172/JCI66369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu F, Yang J, Liu J, Wang Y, Mu J, Zeng Q, Deng S, Zhou H. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct Target Ther. 2021;6:218. doi: 10.1038/s41392-021-00641-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Affo S, Nair A, Brundu F, Ravichandra A, Bhattacharjee S, Matsuda M, Chin L, Filliol A, Wen W, Song X, et al. Promotion of cholangiocarcinoma growth by diverse cancer-associated fibroblast subpopulations. Cancer Cell. 2021;39:866–82 e811. doi: 10.1016/j.ccell.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Asif PJ, Longobardi C, Hahne M, Medema JP. The Role of Cancer-Associated Fibroblasts in Cancer Invasion and Metastasis. Cancers (Basel) 2021, 13. [DOI] [PMC free article] [PubMed]
- 137.Vander Heiden MG, DeBerardinis RJ. Understanding the Intersections between Metabolism and Cancer Biology. Cell. 2017;168:657–69. doi: 10.1016/j.cell.2016.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8:519–30. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vaupel P, Multhoff G. Revisiting the Warburg effect: historical dogma versus current understanding. J Physiol. 2021;599:1745–57. doi: 10.1113/JP278810. [DOI] [PubMed] [Google Scholar]
- 140.Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S, et al. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 141.Zhou WJ, Xu G, Wang YQ, Xu Z, Liu XF, Xu X, Ren GJ, Tian KL. Oxidative stress induced autophagy in cancer associated fibroblast enhances proliferation and metabolism of colorectal cancer cells. Cell Cycle. 2017;16:73–81. doi: 10.1080/15384101.2016.1252882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wu X, Zhou Z, Xu S, Liao CL, Chen X, Li B, Peng JW, Li D, Yang LF. Extracellular vesicle packaged LMP1-activated fibroblasts promote tumor progression via autophagy and stroma-tumor metabolism coupling. Cancer Lett. 2020;478:93–106. doi: 10.1016/j.canlet.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 143.Jena BC, Rout L, Dey A, Mandal M. Active autophagy in cancer-associated fibroblasts: Recent advances in understanding the novel mechanism of tumor progression and therapeutic response. J Cell Physiol. 2021;236:7887–902. doi: 10.1002/jcp.30419. [DOI] [PubMed] [Google Scholar]
- 144.Chaudhri VK, Salzler GG, Dick SA, Buckman MS, Sordella R, Karoly ED, Mohney R, Stiles BM, Elemento O, Altorki NK, McGraw TE. Metabolic Alterations in Lung Cancer-Associated Fibroblasts Correlated with Increased Glycolytic Metabolism of the Tumor. Mol Cancer Res. 2013;11:579–92. doi: 10.1158/1541-7786.MCR-12-0437-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wei JR, Dong J, Li L. Cancer-associated fibroblasts-derived gamma-glutamyltransferase 5 promotes tumor growth and drug resistance in lung adenocarcinoma. Aging-Us. 2020;12:13220–33. doi: 10.18632/aging.103429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Costa A, Kieffer Y, Scholer-Dahirel A, Pelon F, Bourachot B, Cardon M, Sirven P, Magagna I, Fuhrmann L, Bernard C, et al. Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell. 2018;33:463–79 e410. doi: 10.1016/j.ccell.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 147.Pelon F, Bourachot B, Kieffer Y, Magagna I, Mermet-Meillon F, Bonnet I, Costa A, Givel AM, Attieh Y, Barbazan J, et al. Cancer-associated fibroblast heterogeneity in axillary lymph nodes drives metastases in breast cancer through complementary mechanisms. Nat Commun. 2020;11:404. doi: 10.1038/s41467-019-14134-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kieffer Y, Hocine HR, Gentric G, Pelon F, Bernard C, Bourachot B, Lameiras S, Albergante L, Bonneau C, Guyard A, et al. Single-Cell Analysis Reveals Fibroblast Clusters Linked to Immunotherapy Resistance in Cancer. Cancer Discov. 2020;10:1330–51. doi: 10.1158/2159-8290.CD-19-1384. [DOI] [PubMed] [Google Scholar]
- 149.Bonneau C, Elies A, Kieffer Y, Bourachot B, Ladoire S, Pelon F, Hequet D, Guinebretiere JM, Blanchet C, Vincent-Salomon A, et al. A subset of activated fibroblasts is associated with distant relapse in early luminal breast cancer. Breast Cancer Res. 2020;22:76. doi: 10.1186/s13058-020-01311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang X, Zhou Q, Yu Z, Wu X, Chen X, Li J, Li C, Yan M, Zhu Z, Liu B, Su L. Cancer-associated fibroblast-derived Lumican promotes gastric cancer progression via the integrin beta1-FAK signaling pathway. Int J Cancer. 2017;141:998–1010. doi: 10.1002/ijc.30801. [DOI] [PubMed] [Google Scholar]
- 151.Zhang X, Hwang YS. Cancer-associated fibroblast stimulates cancer cell invasion in an interleukin-1 receptor (IL-1R)-dependent manner. Oncol Lett. 2019;18:4645–50. doi: 10.3892/ol.2019.10784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ji Z, Tian W, Gao W, Zang R, Wang H, Yang G. Cancer-Associated Fibroblast-Derived Interleukin-8 Promotes Ovarian Cancer Cell Stemness and Malignancy Through the Notch3-Mediated Signaling. Front Cell Dev Biol. 2021;9:684505. doi: 10.3389/fcell.2021.684505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wen S, Hou Y, Fu L, Xi L, Yang D, Zhao M, Qin Y, Sun K, Teng Y, Liu M. Cancer-associated fibroblast (CAF)-derived IL32 promotes breast cancer cell invasion and metastasis via integrin beta3-p38 MAPK signalling. Cancer Lett. 2019;442:320–32. doi: 10.1016/j.canlet.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 154.Sugihara H, Ishimoto T, Yasuda T, Izumi D, Eto K, Sawayama H, Miyake K, Kurashige J, Imamura Y, Hiyoshi Y, et al. Cancer-associated fibroblast-derived CXCL12 causes tumor progression in adenocarcinoma of the esophagogastric junction. Med Oncol. 2015;32:618. doi: 10.1007/s12032-015-0618-7. [DOI] [PubMed] [Google Scholar]
- 155.Shi X, Luo J, Weigel KJ, Hall SC, Du D, Wu F, Rudolph MC, Zhou H, Young CD, Wang XJ. Cancer-Associated Fibroblasts Facilitate Squamous Cell Carcinoma Lung Metastasis in Mice by Providing TGFbeta-Mediated Cancer Stem Cell Niche. Front Cell Dev Biol. 2021;9:668164. doi: 10.3389/fcell.2021.668164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li C, Teixeira AF, Zhu HJ, Ten Dijke P. Cancer associated-fibroblast-derived exosomes in cancer progression. Mol Cancer. 2021;20:154. doi: 10.1186/s12943-021-01463-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9:285–93. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang Z, Liu J, Huang H, Ye M, Li X, Wu R, Liu H, Song Y. Metastasis-associated fibroblasts: an emerging target for metastatic cancer. Biomark Res. 2021;9:47. doi: 10.1186/s40364-021-00305-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shani O, Vorobyov T, Monteran L, Lavie D, Cohen N, Raz Y, Tsarfaty G, Avivi C, Barshack I, Erez N. Fibroblast-Derived IL33 Facilitates Breast Cancer Metastasis by Modifying the Immune Microenvironment and Driving Type 2 Immunity. Cancer Res. 2020;80:5317–29. doi: 10.1158/0008-5472.CAN-20-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fang T, Lv H, Lv G, Li T, Wang C, Han Q, Yu L, Su B, Guo L, Huang S, et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat Commun. 2018;9:191. doi: 10.1038/s41467-017-02583-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bu L, Baba H, Yasuda T, Uchihara T, Ishimoto T. Functional diversity of cancer-associated fibroblasts in modulating drug resistance. Cancer Sci. 2020;111:3468–77. doi: 10.1111/cas.14578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Feng B, Wu J, Shen B, Jiang F, Feng J. Cancer-associated fibroblasts and resistance to anticancer therapies: status, mechanisms, and countermeasures. Cancer Cell Int. 2022;22:166. doi: 10.1186/s12935-022-02599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhai J, Shen J, Xie G, Wu J, He M, Gao L, Zhang Y, Yao X, Shen L. Cancer-associated fibroblasts-derived IL-8 mediates resistance to cisplatin in human gastric cancer. Cancer Lett. 2019;454:37–43. doi: 10.1016/j.canlet.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 164.Guo H, Ha C, Dong H, Yang Z, Ma Y, Ding Y. Cancer-associated fibroblast-derived exosomal microRNA-98-5p promotes cisplatin resistance in ovarian cancer by targeting CDKN1A. Cancer Cell Int. 2019;19:347. doi: 10.1186/s12935-019-1051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang D, Li L, Jiang H, Li Q, Wang-Gillam A, Yu J, Head R, Liu J, Ruzinova MB, Lim KH. Tumor-Stroma IL1beta-IRAK4 Feedforward Circuitry Drives Tumor Fibrosis, Chemoresistance, and Poor Prognosis in Pancreatic Cancer. Cancer Res. 2018;78:1700–12. doi: 10.1158/0008-5472.CAN-17-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Qiao Y, Zhang C, Li A, Wang D, Luo Z, Ping Y, Zhou B, Liu S, Li H, Yue D, et al. IL6 derived from cancer-associated fibroblasts promotes chemoresistance via CXCR7 in esophageal squamous cell carcinoma. Oncogene. 2018;37:873–83. doi: 10.1038/onc.2017.387. [DOI] [PubMed] [Google Scholar]
- 167.Ireland L, Santos A, Ahmed MS, Rainer C, Nielsen SR, Quaranta V, Weyer-Czernilofsky U, Engle DD, Perez-Mancera PA, Coupland SE, et al. Chemoresistance in Pancreatic Cancer Is Driven by Stroma-Derived Insulin-Like Growth Factors. Cancer Res. 2016;76:6851–63. doi: 10.1158/0008-5472.CAN-16-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang HF, Xie CH, Yue J, Jiang ZZ, Zhou RJ, Xie RF, Wang Y, Wu SX. Cancer-associated fibroblasts mediated chemoresistance by a FOXO1/TGF1 signaling loop in esophageal squamous cell carcinoma. Mol Carcinog. 2017;56:1150–63. doi: 10.1002/mc.22581. [DOI] [PubMed] [Google Scholar]
- 169.Tommelein J, De Vlieghere E, Verset L, Melsens E, Leenders J, Descamps B, Debucquoy A, Vanhove C, Pauwels P, Gespach CP, et al. Radiotherapy-Activated Cancer-Associated Fibroblasts Promote Tumor Progression through Paracrine IGF1R Activation. Cancer Res. 2018;78:659–70. doi: 10.1158/0008-5472.CAN-17-0524. [DOI] [PubMed] [Google Scholar]
- 170.Chen X, Liu Y, Zhang Q, Liu B, Cheng Y, Zhang Y, Sun Y, Liu J. Exosomal miR-590-3p derived from cancer-associated fibroblasts confers radioresistance in colorectal cancer. Mol Ther Nucleic Acids. 2021;24:113–26. doi: 10.1016/j.omtn.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liu L, Zhang Z, Zhou L, Hu L, Yin C, Qing D, Huang S, Cai X, Chen Y. Cancer associated fibroblasts-derived exosomes contribute to radioresistance through promoting colorectal cancer stem cells phenotype. Exp Cell Res. 2020;391:111956. doi: 10.1016/j.yexcr.2020.111956. [DOI] [PubMed] [Google Scholar]
- 172.Wang Y, Gan G, Wang B, Wu J, Cao Y, Zhu D, Xu Y, Wang X, Han H, Li X, et al. Cancer-associated Fibroblasts Promote Irradiated Cancer Cell Recovery Through Autophagy EBioMedicine. 2017;17:45–56. doi: 10.1016/j.ebiom.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ao Z, Shah SH, Machlin LM, Parajuli R, Miller PC, Rawal S, Williams AJ, Cote RJ, Lippman ME, Datar RH, El-Ashry D. Identification of Cancer-Associated Fibroblasts in Circulating Blood from Patients with Metastatic Breast Cancer. Cancer Res. 2015;75:4681–7. doi: 10.1158/0008-5472.CAN-15-1633. [DOI] [PubMed] [Google Scholar]
- 174.Sharma U, Medina-Saenz K, Miller PC, Troness B, Spartz A, Sandoval-Leon A, Parke DN, Seagroves TN, Lippman ME, El-Ashry D. Heterotypic clustering of circulating tumor cells and circulating cancer-associated fibroblasts facilitates breast cancer metastasis. Breast Cancer Res Treat. 2021;189:63–80. doi: 10.1007/s10549-021-06299-0. [DOI] [PubMed] [Google Scholar]
- 175.Jones ML, Siddiqui J, Pienta KJ, Getzenberg RH. Circulating fibroblast-like cells in men with metastatic prostate cancer. Prostate. 2013;73:176–81. doi: 10.1002/pros.22553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pazolli E, Alspach E, Milczarek A, Prior J, Piwnica-Worms D, Stewart SA. Chromatin remodeling underlies the senescence-associated secretory phenotype of tumor stromal fibroblasts that supports cancer progression. Cancer Res. 2012;72:2251–61. doi: 10.1158/0008-5472.CAN-11-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yasuda T, Koiwa M, Yonemura A, Miyake K, Kariya R, Kubota S, Yokomizo-Nakano T, Yasuda-Yoshihara N, Uchihara T, Itoyama R, et al. Inflammation-driven senescence-associated secretory phenotype in cancer-associated fibroblasts enhances peritoneal dissemination. Cell Rep. 2021;34:108779. doi: 10.1016/j.celrep.2021.108779. [DOI] [PubMed] [Google Scholar]
- 178.Meng J, Li Y, Wan C, Sun Y, Dai X, Huang J, Hu Y, Gao Y, Wu B, Zhang Z, et al: Targeting senescence-like fibroblasts radiosensitizes non-small cell lung cancer and reduces radiation-induced pulmonary fibrosis. JCI Insight 2021, 6. [DOI] [PMC free article] [PubMed]
- 179.Friedenstein AJ, Chailakhjan RK, Lalykina KS, DEVELOPMENT OF FIBROBLAST COLONIES IN MONOLAYER CULTURES OF GUINEA-PIG BONE MARROW AND SPLEEN CELLS Cell and Tissue Kinetics. 1970;3:393-+. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 180.Zhou T, Yuan Z, Weng J, Pei D, Du X, He C, Lai P. Challenges and advances in clinical applications of mesenchymal stromal cells. J Hematol Oncol. 2021;14:24. doi: 10.1186/s13045-021-01037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ren G, Zhao X, Wang Y, Zhang X, Chen X, Xu C, Yuan ZR, Roberts AI, Zhang L, Zheng B, et al. CCR2-dependent recruitment of macrophages by tumor-educated mesenchymal stromal cells promotes tumor development and is mimicked by TNFalpha. Cell Stem Cell. 2012;11:812–24. doi: 10.1016/j.stem.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Frisbie L, Buckanovich RJ, Coffman L. Carcinoma Associated Mesenchymal Stem/Stromal Cells - Architects of the Pro-tumorigenic tumor microenvironment. Stem Cells 2022. [DOI] [PMC free article] [PubMed]
- 183.Hmadcha A, Martin-Montalvo A, Gauthier BR, Soria B, Capilla-Gonzalez V. Therapeutic Potential of Mesenchymal Stem Cells for Cancer Therapy. Front Bioeng Biotechnol. 2020;8:43. doi: 10.3389/fbioe.2020.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ryan D, Koziol J, ElShamy WM. Targeting AXL and RAGE to prevent geminin overexpression-induced triple-negative breast cancer metastasis. Scientific Reports 2019, 9. [DOI] [PMC free article] [PubMed]
- 185.Gao H, Priebe W, Glod J, Banerjee D. Activation of signal transducers and activators of transcription 3 and focal adhesion kinase by stromal cell-derived factor 1 is required for migration of human mesenchymal stem cells in response to tumor cell-conditioned medium. Stem Cells. 2009;27:857–65. doi: 10.1002/stem.23. [DOI] [PubMed] [Google Scholar]
- 186.Dwyer RM, Potter-Beirne SM, Harrington KA, Lowery AJ, Hennessy E, Murphy JM, Barry FP, O’Brien T, Kerin MJ. Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin Cancer Res. 2007;13:5020–7. doi: 10.1158/1078-0432.CCR-07-0731. [DOI] [PubMed] [Google Scholar]
- 187.Yu PF, Huang Y, Han YY, Lin LY, Sun WH, Rabson AB, Wang Y, Shi YF. TNFalpha-activated mesenchymal stromal cells promote breast cancer metastasis by recruiting CXCR2(+) neutrophils. Oncogene. 2017;36:482–90. doi: 10.1038/onc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jung Y, Kim JK, Shiozawa Y, Wang J, Mishra A, Joseph J, Berry JE, McGee S, Lee E, Sun H, et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat Commun. 2013;4:1795. doi: 10.1038/ncomms2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Spaeth E, Klopp A, Dembinski J, Andreeff M, Marini F. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 2008;15:730–8. doi: 10.1038/gt.2008.39. [DOI] [PubMed] [Google Scholar]
- 190.Shi Y, Du L, Lin L, Wang Y. Tumour-associated mesenchymal stem/stromal cells: emerging therapeutic targets. Nat Rev Drug Discov. 2017;16:35–52. doi: 10.1038/nrd.2016.193. [DOI] [PubMed] [Google Scholar]
- 191.Fan H, Atiya HI, Wang Y, Pisanic TR, Wang TH, Shih IM, Foy KK, Frisbie L, Buckanovich RJ, Chomiak AA, et al. Epigenomic Reprogramming toward Mesenchymal-Epithelial Transition in Ovarian-Cancer-Associated Mesenchymal Stem Cells Drives Metastasis. Cell Rep. 2020;33:108473. doi: 10.1016/j.celrep.2020.108473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ren G, Liu Y, Zhao X, Zhang J, Zheng B, Yuan ZR, Zhang L, Qu X, Tischfield JA, Shao C, Shi Y. Tumor resident mesenchymal stromal cells endow naive stromal cells with tumor-promoting properties. Oncogene. 2014;33:4016–20. doi: 10.1038/onc.2013.387. [DOI] [PubMed] [Google Scholar]
- 193.Coffman LG, Pearson AT, Frisbie LG, Freeman Z, Christie E, Bowtell DD, Buckanovich RJ. Ovarian Carcinoma-Associated Mesenchymal Stem Cells Arise from Tissue-Specific Normal Stroma. Stem Cells. 2019;37:257–69. doi: 10.1002/stem.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mandel K, Yang Y, Schambach A, Glage S, Otte A, Hass R. Mesenchymal stem cells directly interact with breast cancer cells and promote tumor cell growth in vitro and in vivo. Stem Cells Dev. 2013;22:3114–27. doi: 10.1089/scd.2013.0249. [DOI] [PubMed] [Google Scholar]
- 195.Yan XL, Fu CJ, Chen L, Qin JH, Zeng Q, Yuan HF, Nan X, Chen HX, Zhou JN, Lin YL, et al. Mesenchymal stem cells from primary breast cancer tissue promote cancer proliferation and enhance mammosphere formation partially via EGF/EGFR/Akt pathway. Breast Cancer Res Treat. 2012;132:153–64. doi: 10.1007/s10549-011-1577-0. [DOI] [PubMed] [Google Scholar]
- 196.Zhu Q, Zhang X, Zhang L, Li W, Wu H, Yuan X, Mao F, Wang M, Zhu W, Qian H, Xu W. The IL-6-STAT3 axis mediates a reciprocal crosstalk between cancer-derived mesenchymal stem cells and neutrophils to synergistically prompt gastric cancer progression. Cell Death Dis. 2014;5:e1295. doi: 10.1038/cddis.2014.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.McLean K, Gong Y, Choi Y, Deng N, Yang K, Bai S, Cabrera L, Keller E, McCauley L, Cho KR, Buckanovich RJ. Human ovarian carcinoma-associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. J Clin Invest. 2011;121:3206–19. doi: 10.1172/JCI45273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Li G, Zhang R, Zhang X, Shao S, Hu F, Feng Y. Human colorectal cancer derived-MSCs promote tumor cells escape from senescence via P53/P21 pathway. Clin Transl Oncol. 2020;22:503–11. doi: 10.1007/s12094-019-02152-5. [DOI] [PubMed] [Google Scholar]
- 199.Lin JT, Wang JY, Chen MK, Chen HC, Chang TH, Su BW, Chang PJ. Colon cancer mesenchymal stem cells modulate the tumorigenicity of colon cancer through interleukin 6. Exp Cell Res. 2013;319:2216–29. doi: 10.1016/j.yexcr.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 200.Waghray M, Yalamanchili M, Dziubinski M, Zeinali M, Erkkinen M, Yang H, Schradle KA, Urs S, Di PascaMagliano M, Welling TH, et al. GM-CSF Mediates Mesenchymal-Epithelial Cross-talk in Pancreatic Cancer. Cancer Discov. 2016;6:886–99. doi: 10.1158/2159-8290.CD-15-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Li W, Zhang X, Wu F, Zhou Y, Bao Z, Li H, Zheng P, Zhao S. Gastric cancer-derived mesenchymal stromal cells trigger M2 macrophage polarization that promotes metastasis and EMT in gastric cancer. Cell Death Dis. 2019;10:918. doi: 10.1038/s41419-019-2131-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Fregni G, Quinodoz M, Moller E, Vuille J, Galland S, Fusco C, Martin P, Letovanec I, Provero P, Rivolta C, et al. Reciprocal modulation of mesenchymal stem cells and tumor cells promotes lung cancer metastasis. EBioMedicine. 2018;29:128–45. doi: 10.1016/j.ebiom.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mi Z, Bhattacharya SD, Kim VM, Guo H, Talbot LJ, Kuo PC. Osteopontin promotes CCL5-mesenchymal stromal cell-mediated breast cancer metastasis. Carcinogenesis. 2011;32:477–87. doi: 10.1093/carcin/bgr009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chen K, Liu Q, Tsang LL, Ye Q, Chan HC, Sun Y, Jiang X. Human MSCs promotes colorectal cancer epithelial-mesenchymal transition and progression via CCL5/beta-catenin/Slug pathway. Cell Death Dis. 2017;8:e2819. doi: 10.1038/cddis.2017.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lou Q, Zhao M, Xu Q, Xie S, Liang Y, Chen J, Yuan L, Wang L, Jiang L, Mou L, et al. Retinoic Acid Inhibits Tumor-Associated Mesenchymal Stromal Cell Transformation in Melanoma. Front Cell Dev Biol. 2021;9:658757. doi: 10.3389/fcell.2021.658757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Xu Z, Gao H, Zhang Y, Feng W, Miao Y, Xu Z, Li W, Chen F, Lv Z, Huo J, et al: CCL7 and TGF-β secreted by MSCs play opposite roles in regulating CRC metastasis in a KLF5/CXCL5-dependent manner. Molecular Therapy 2022. [DOI] [PMC free article] [PubMed]
- 207.Huang F, Wang M, Yang T, Cai J, Zhang Q, Sun Z, Wu X, Zhang X, Zhu W, Qian H, Xu W. Gastric cancer-derived MSC-secreted PDGF-DD promotes gastric cancer progression. J Cancer Res Clin Oncol. 2014;140:1835–48. doi: 10.1007/s00432-014-1723-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Li W, Zhou Y, Yang J, Zhang X, Zhang H, Zhang T, Zhao S, Zheng P, Huo J, Wu H. Gastric cancer-derived mesenchymal stem cells prompt gastric cancer progression through secretion of interleukin-8. J Exp Clin Cancer Res. 2015;34:52. doi: 10.1186/s13046-015-0172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zhang X, Hu F, Li G, Li G, Yang X, Liu L, Zhang R, Zhang B, Feng Y. Human colorectal cancer-derived mesenchymal stem cells promote colorectal cancer progression through IL-6/JAK2/STAT3 signaling. Cell Death Dis. 2018;9:25. doi: 10.1038/s41419-017-0176-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Chaturvedi P, Gilkes DM, Wong CC, Kshitiz, Luo W, Zhang H, Wei H, Takano N, Schito L, Levchenko A, Semenza GL. Hypoxia-inducible factor-dependent breast cancer-mesenchymal stem cell bidirectional signaling promotes metastasis. J Clin Invest. 2013;123:189–205. doi: 10.1172/JCI69244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ma X, Liu J, Yang X, Fang K, Zheng P, Liang X, Liu J. Mesenchymal stem cells maintain the stemness of colon cancer stem cells via interleukin-8/mitogen-activated protein kinase signaling pathway. Exp Biol Med (Maywood) 2020;245:562–75. doi: 10.1177/1535370220910690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Raghavan S, Snyder CS, Wang A, McLean K, Zamarin D, Buckanovich RJ, Mehta G. Carcinoma-Associated Mesenchymal Stem Cells Promote Chemoresistance in Ovarian Cancer Stem Cells via PDGF Signaling. Cancers (Basel) 2020, 12. [DOI] [PMC free article] [PubMed]
- 213.Wang M, Zhao C, Shi H, Zhang B, Zhang L, Zhang X, Wang S, Wu X, Yang T, Huang F, et al. Deregulated microRNAs in gastric cancer tissue-derived mesenchymal stem cells: novel biomarkers and a mechanism for gastric cancer. Br J Cancer. 2014;110:1199–210. doi: 10.1038/bjc.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Xu H, Zhou Y, Li W, Zhang B, Zhang H, Zhao S, Zheng P, Wu H, Yang J. Tumor-derived mesenchymal-stem-cell-secreted IL-6 enhances resistance to cisplatin via the STAT3 pathway in breast cancer. Oncol Lett. 2018;15:9142–50. doi: 10.3892/ol.2018.8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Han J, Qu H, Han M, Ding Y, Xie M, Hu J, Chen Y, Dong H. MSC-induced lncRNA AGAP2-AS1 promotes stemness and trastuzumab resistance through regulating CPT1 expression and fatty acid oxidation in breast cancer. Oncogene. 2021;40:833–47. doi: 10.1038/s41388-020-01574-8. [DOI] [PubMed] [Google Scholar]
- 216.Li C, Feng S, Chen L. MSC-AS1 knockdown inhibits cell growth and temozolomide resistance by regulating miR-373-3p/CPEB4 axis in glioma through PI3K/Akt pathway. Mol Cell Biochem. 2021;476:699–713. doi: 10.1007/s11010-020-03937-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 218.Sun R, Kong X, Qiu X, Huang C, Wong PP. The Emerging Roles of Pericytes in Modulating Tumor Microenvironment. Front Cell Dev Biol. 2021;9:676342. doi: 10.3389/fcell.2021.676342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Fujimoto T, Nakagawa S, Morofuji Y, Watanabe D, Ujifuku K, Horie N, Izumo T, Niwa M, Banks WA, Deli MA, Matsuo T. Pericytes Suppress Brain Metastasis from Lung Cancer In Vitro. Cell Mol Neurobiol. 2020;40:113–21. doi: 10.1007/s10571-019-00725-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Cheng L, Huang Z, Zhou W, Wu Q, Donnola S, Liu JK, Fang X, Sloan AE, Mao Y, Lathia JD, et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell. 2013;153:139–52. doi: 10.1016/j.cell.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sinha D, Chong L, George J, Schluter H, Monchgesang S, Mills S, Li J, Parish C, Bowtell D, Kaur P. Australian Ovarian Cancer Study G: Pericytes Promote Malignant Ovarian Cancer Progression in Mice and Predict Poor Prognosis in Serous Ovarian Cancer Patients. Clin Cancer Res. 2016;22:1813–24. doi: 10.1158/1078-0432.CCR-15-1931. [DOI] [PubMed] [Google Scholar]
- 222.Molnar K, Meszaros A, Fazakas C, Kozma M, Gyori F, Reisz Z, Tiszlavicz L, Farkas AE, Nyul-Toth A, Hasko J, et al. Pericyte-secreted IGF2 promotes breast cancer brain metastasis formation. Mol Oncol. 2020;14:2040–57. doi: 10.1002/1878-0261.12752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wong PP, Munoz-Felix JM, Hijazi M, Kim H, Robinson SD, De Luxan-Delgado B, Rodriguez-Hernandez I, Maiques O, Meng YM, Meng Q, et al. Cancer Burden Is Controlled by Mural Cell-beta3-Integrin Regulated Crosstalk with Tumor Cells. Cell. 2020;181:1346–63 e1321. doi: 10.1016/j.cell.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 224.Paiva AE, Lousado L, Guerra DAP, Azevedo PO, Sena IFG, Andreotti JP, Santos GSP, Goncalves R, Mintz A, Birbrair A. Pericytes in the Premetastatic Niche. Cancer Res. 2018;78:2779–86. doi: 10.1158/0008-5472.CAN-17-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hosaka K, Yang Y, Seki T, Fischer C, Dubey O, Fredlund E, Hartman J, Religa P, Morikawa H, Ishii Y, et al. Pericyte-fibroblast transition promotes tumor growth and metastasis. Proc Natl Acad Sci U S A. 2016;113:E5618–27. doi: 10.1073/pnas.1608384113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Neve A, Cantatore FP, Maruotti N, Corrado A, Ribatti D. Extracellular matrix modulates angiogenesis in physiological and pathological conditions. Biomed Res Int. 2014;2014:756078. doi: 10.1155/2014/756078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Bordeleau F, Califano JP, Negron Abril YL, Mason BN, LaValley DJ, Shin SJ, Weiss RS, Reinhart-King CA. Tissue stiffness regulates serine/arginine-rich protein-mediated splicing of the extra domain B-fibronectin isoform in tumors. Proc Natl Acad Sci U S A. 2015;112:8314–9. doi: 10.1073/pnas.1505421112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.LaValley DJ, Zanotelli MR, Bordeleau F, Wang WJ, Schwager SC, Reinhart-King CA. Matrix stiffness enhances VEGFR-2 internalization, signaling, and proliferation in endothelial cells. Convergent Science Physical Oncology 2017, 3. [DOI] [PMC free article] [PubMed]
- 229.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 230.Unterleuthner D, Neuhold P, Schwarz K, Janker L, Neuditschko B, Nivarthi H, Crncec I, Kramer N, Unger C, Hengstschlager M, et al. Cancer-associated fibroblast-derived WNT2 increases tumor angiogenesis in colon cancer. Angiogenesis. 2020;23:159–77. doi: 10.1007/s10456-019-09688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu NF, Selig M, Nielsen G, Taksir T, Jain RK, Seed B. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715–25. doi: 10.1016/S0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 232.Viallard C, Larrivee B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. 2017;20:409–26. doi: 10.1007/s10456-017-9562-9. [DOI] [PubMed] [Google Scholar]
- 233.Egeblad M, Rasch MG, Weaver VM. Dynamic interplay between the collagen scaffold and tumor evolution. Curr Opin Cell Biol. 2010;22:697–706. doi: 10.1016/j.ceb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Li P, Gong Z, Shultz LD, Ren G. Mesenchymal stem cells: From regeneration to cancer. Pharmacol Ther. 2019;200:42–54. doi: 10.1016/j.pharmthera.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Beckermann BM, Kallifatidis G, Groth A, Frommhold D, Apel A, Mattern J, Salnikov AV, Moldenhauer G, Wagner W, Diehlmann A, et al. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br J Cancer. 2008;99:622–31. doi: 10.1038/sj.bjc.6604508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Huang WH, Chang MC, Tsai KS, Hung MC, Chen HL, Hung SC. Mesenchymal stem cells promote growth and angiogenesis of tumors in mice. Oncogene. 2013;32:4343–54. doi: 10.1038/onc.2012.458. [DOI] [PubMed] [Google Scholar]
- 237.Gong M, Yu B, Wang JC, Wang YG, Liu M, Paul C, Millard RW, Xiao DS, Ashraf M, Xu MF. Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget. 2017;8:45200–12. doi: 10.18632/oncotarget.16778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Picoli CC, Goncalves BOP, Santos GSP, Rocha BGS, Costa AC, Resende RR, Birbrair A. Pericytes cross-talks within the tumor microenvironment. Biochim Biophys Acta Rev Cancer. 2021;1876:188608. doi: 10.1016/j.bbcan.2021.188608. [DOI] [PubMed] [Google Scholar]
- 239.Chen Z, Xu XH, Hu J. Role of pericytes in angiogenesis: focus on cancer angiogenesis and anti-angiogenic therapy. Neoplasma. 2016;63:173–82. doi: 10.4149/201_150704N369. [DOI] [PubMed] [Google Scholar]
- 240.Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary E, Charras G, Sahai E. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol. 2013;15:637–46. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Avery D, Govindaraju P, Jacob M, Todd L, Monslow J, Pure E. Extracellular matrix directs phenotypic heterogeneity of activated fibroblasts. Matrix Biol. 2018;67:90–106. doi: 10.1016/j.matbio.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Barcellos-de-Souza P, Comito G, Pons-Segura C, Taddei ML, Gori V, Becherucci V, Bambi F, Margheri F, Laurenzana A, Del Rosso M, Chiarugi P. Mesenchymal Stem Cells are Recruited and Activated into Carcinoma-Associated Fibroblasts by Prostate Cancer Microenvironment-Derived TGF-beta1. Stem Cells. 2016;34:2536–47. doi: 10.1002/stem.2412. [DOI] [PubMed] [Google Scholar]
- 243.Rubinstein-Achiasaf L, Morein D, Ben-Yaakov H, Liubomirski Y, Meshel T, Elbaz E, Dorot O, Pichinuk E, Gershovits M, Weil M, Ben-Baruch A. Persistent Inflammatory Stimulation Drives the Conversion of MSCs to Inflammatory CAFs That Promote Pro-Metastatic Characteristics in Breast Cancer Cells. Cancers (Basel) 2021, 13. [DOI] [PMC free article] [PubMed]
- 244.Mun JY, Leem SH, Lee JH, Kim HS. Dual Relationship Between Stromal Cells and Immune Cells in the Tumor Microenvironment. Front Immunol. 2022;13:864739. doi: 10.3389/fimmu.2022.864739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Gordon-Weeks A, Yuzhalin AE. Cancer Extracellular Matrix Proteins Regulate Tumour Immunity. Cancers (Basel) 2020, 12. [DOI] [PMC free article] [PubMed]
- 246.Mushtaq MU, Papadas A, Pagenkopf A, Flietner E, Morrow Z, Chaudhary SG, Asimakopoulos F. Tumor matrix remodeling and novel immunotherapies: the promise of matrix-derived immune biomarkers. J Immunother Cancer. 2018;6:65. doi: 10.1186/s40425-018-0376-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kolesnikoff N, Chen CH, Samuel MS. Interrelationships between the extracellular matrix and the immune microenvironment that govern epithelial tumour progression. Clin Sci (Lond) 2022;136:361–77. doi: 10.1042/CS20210679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean MC, Validire P, Trautmann A, Mami-Chouaib F, Donnadieu E. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest. 2012;122:899–910. doi: 10.1172/JCI45817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Hartmann N, Giese NA, Giese T, Poschke I, Offringa R, Werner J, Ryschich E. Prevailing Role of Contact Guidance in Intrastromal T-cell Trapping in Human Pancreatic Cancer. Clin Cancer Res. 2014;20:3422–33. doi: 10.1158/1078-0432.CCR-13-2972. [DOI] [PubMed] [Google Scholar]
- 250.Kuczek DE, Larsen AMH, Thorseth ML, Carretta M, Kalvisa A, Siersbaek MS, Simoes AMC, Roslind A, Engelholm LH, Noessner E, et al. Collagen density regulates the activity of tumor-infiltrating T cells. J Immunother Cancer. 2019;7:68. doi: 10.1186/s40425-019-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Nicolas-Boluda A, Vaquero J, Vimeux L, Guilbert T, Barrin S, Kantari-Mimoun C, Ponzo M, Renault G, Deptula P, Pogoda K, et al: Tumor stiffening reversion through collagen crosslinking inhibition improves T cell migration and anti-PD-1 treatment. Elife 2021, 10. [DOI] [PMC free article] [PubMed]
- 252.O’Connor RS, Hao X, Shen K, Bashour K, Akimova T, Hancock WW, Kam LC, Milone MC. Substrate rigidity regulates human T cell activation and proliferation. J Immunol. 2012;189:1330–9. doi: 10.4049/jimmunol.1102757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Jachetti E, Caputo S, Mazzoleni S, Brambillasca CS, Parigi SM, Grioni M, Piras IS, Restuccia U, Calcinotto A, Freschi M, et al. Tenascin-C Protects Cancer Stem-like Cells from Immune Surveillance by Arresting T-cell Activation. Cancer Res. 2015;75:2095–108. doi: 10.1158/0008-5472.CAN-14-2346. [DOI] [PubMed] [Google Scholar]
- 254.Maller O, Drain AP, Barrett AS, Borgquist S, Ruffell B, Zakharevich I, Pham TT, Gruosso T, Kuasne H, Lakins JN, et al. Tumour-associated macrophages drive stromal cell-dependent collagen crosslinking and stiffening to promote breast cancer aggression. Nat Mater. 2021;20:548–59. doi: 10.1038/s41563-020-00849-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Deligne C, Midwood KS. Macrophages and Extracellular Matrix in Breast Cancer: Partners in Crime or Protective Allies? Front Oncol. 2021;11:620773. doi: 10.3389/fonc.2021.620773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zhang G, Guo L, Yang C, Liu Y, He Y, Du Y, Wang W, Gao F. A novel role of breast cancer-derived hyaluronan on inducement of M2-like tumor-associated macrophages formation. Oncoimmunology. 2016;5:e1172154. doi: 10.1080/2162402X.2016.1172154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Larsen AMH, Kuczek DE, Kalvisa A, Siersbaek MS, Thorseth ML, Johansen AZ, Carretta M, Grontved L, Vang O, Madsen DH. Collagen Density Modulates the Immunosuppressive Functions of Macrophages. J Immunol. 2020;205:1461–72. doi: 10.4049/jimmunol.1900789. [DOI] [PubMed] [Google Scholar]
- 258.Fabian KL, Storkus WJ. Immunotherapeutic Targeting of Tumor-Associated Blood Vessels. Adv Exp Med Biol. 2017;1036:191–211. doi: 10.1007/978-3-319-67577-0_13. [DOI] [PubMed] [Google Scholar]
- 259.Huang YH, Goel S, Duda DG, Fukumura D, Jain RK. Vascular Normalization as an Emerging Strategy to Enhance Cancer Immunotherapy. Cancer Res. 2013;73:2943–8. doi: 10.1158/0008-5472.CAN-12-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Chanmee T, Ontong P, Konno K, Itano N. Tumor-Associated Macrophages as Major Players in the Tumor Microenvironment. Cancers. 2014;6:1670–90. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Barsoum IB, Koti M, Siemens DR, Graham CH. Mechanisms of hypoxia-mediated immune escape in cancer. Cancer Res. 2014;74:7185–90. doi: 10.1158/0008-5472.CAN-14-2598. [DOI] [PubMed] [Google Scholar]
- 262.Pierini S, Mishra A, Perales-Linares R, Uribe-Herranz M, Beghi S, Giglio A, Pustylnikov S, Costabile F, Rafail S, Amici A, et al: Combination of vasculature targeting, hypofractionated radiotherapy, and immune checkpoint inhibitor elicits potent antitumor immune response and blocks tumor progression. J Immunother Cancer 2021, 9. [DOI] [PMC free article] [PubMed]
- 263.Nagl L, Horvath L, Pircher A, Wolf D. Tumor Endothelial Cells (TECs) as Potential Immune Directors of the Tumor Microenvironment - New Findings and Future Perspectives. Front Cell Dev Biol. 2020;8:766. doi: 10.3389/fcell.2020.00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Zhou J, Zhang A, Fan L. HSPA12B Secreted by Tumor-Associated Endothelial Cells Might Induce M2 Polarization of Macrophages via Activating PI3K/Akt/mTOR Signaling. Onco Targets Ther. 2020;13:9103–11. doi: 10.2147/OTT.S254985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Motz GT, Santoro SP, Wang LP, Garrabrant T, Lastra RR, Hagemann IS, Lal P, Feldman MD, Benencia F, Coukos G. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med. 2014;20:607–15. doi: 10.1038/nm.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Sakano Y, Noda T, Kobayashi S, Sasaki K, Iwagami Y, Yamada D, Tomimaru Y, Akita H, Gotoh K, Takahashi H, et al. Tumor endothelial cell-induced CD8(+) T-cell exhaustion via GPNMB in hepatocellular carcinoma. Cancer Sci. 2022;113:1625–38. doi: 10.1111/cas.15331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Jennewein L, Bartsch G, Gust K, Kvasnicka HM, Haferkamp A, Blaheta R, Mittelbronn M, Harter PN, Mani J. Increased tumor vascularization is associated with the amount of immune competent PD-1 positive cells in testicular germ cell tumors. Oncol Lett. 2018;15:9852–60. doi: 10.3892/ol.2018.8597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Bagaria SP, Gatalica Z, Maney T, Serie D, Parasramka M, Attia S, Krishna M, Joseph RW. Association Between Programmed Death-Ligand 1 Expression and the Vascular Endothelial Growth Factor Pathway in Angiosarcoma. Front Oncol. 2018;8:71. doi: 10.3389/fonc.2018.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Yamauchi M, Gibbons DL, Zong C, Fradette JJ, Bota-Rabassedas N, Kurie JM. Fibroblast heterogeneity and its impact on extracellular matrix and immune landscape remodeling in cancer. Matrix Biol 2020, 91–92:8–18. [DOI] [PubMed]
- 270.Mhaidly R, Mechta-Grigoriou F. Role of cancer-associated fibroblast subpopulations in immune infiltration, as a new means of treatment in cancer. Immunol Rev. 2021;302:259–72. doi: 10.1111/imr.12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Klemm F, Joyce JA. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2015;25:198–213. doi: 10.1016/j.tcb.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Komohara Y, Takeya M. CAFs and TAMs: maestros of the tumour microenvironment. J Pathol. 2017;241:313–5. doi: 10.1002/path.4824. [DOI] [PubMed] [Google Scholar]
- 273.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Gunaydin G. CAFs Interacting With TAMs in Tumor Microenvironment to Enhance Tumorigenesis and Immune Evasion. Front Oncol. 2021;11:668349. doi: 10.3389/fonc.2021.668349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Zhang R, Qi F, Zhao F, Li G, Shao S, Zhang X, Yuan L, Feng Y. Cancer-associated fibroblasts enhance tumor-associated macrophages enrichment and suppress NK cells function in colorectal cancer. Cell Death Dis. 2019;10:273. doi: 10.1038/s41419-019-1435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Ksiazkiewicz M, Gottfried E, Kreutz M, Mack M, Hofstaedter F, Kunz-Schughart LA. Importance of CCL2-CCR2A/2B signaling for monocyte migration into spheroids of breast cancer-derived fibroblasts. Immunobiology. 2010;215:737–47. doi: 10.1016/j.imbio.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 277.Gok Yavuz B, Gunaydin G, Gedik ME, Kosemehmetoglu K, Karakoc D, Ozgur F, Guc D. Cancer associated fibroblasts sculpt tumour microenvironment by recruiting monocytes and inducing immunosuppressive PD-1(+) TAMs. Sci Rep. 2019;9:3172. doi: 10.1038/s41598-019-39553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Kuen J, Darowski D, Kluge T, Majety M. Pancreatic cancer cell/fibroblast co-culture induces M2 like macrophages that influence therapeutic response in a 3D model. PLoS ONE. 2017;12:e0182039. doi: 10.1371/journal.pone.0182039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Takahashi H, Sakakura K, Kudo T, Toyoda M, Kaira K, Oyama T, Chikamatsu K. Cancer-associated fibroblasts promote an immunosuppressive microenvironment through the induction and accumulation of protumoral macrophages. Oncotarget. 2017;8:8633–47. doi: 10.18632/oncotarget.14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Cohen N, Shani O, Raz Y, Sharon Y, Hoffman D, Abramovitz L, Erez N. Fibroblasts drive an immunosuppressive and growth-promoting microenvironment in breast cancer via secretion of Chitinase 3-like 1. Oncogene. 2017;36:4457–68. doi: 10.1038/onc.2017.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Zhang A, Qian Y, Ye Z, Chen H, Xie H, Zhou L, Shen Y, Zheng S. Cancer-associated fibroblasts promote M2 polarization of macrophages in pancreatic ductal adenocarcinoma. Cancer Med. 2017;6:463–70. doi: 10.1002/cam4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Comito G, Giannoni E, Segura CP, Barcellos-de-Souza P, Raspollini MR, Baroni G, Lanciotti M, Serni S, Chiarugi P. Cancer-associated fibroblasts and M2-polarized macrophages synergize during prostate carcinoma progression. Oncogene. 2014;33:2423–31. doi: 10.1038/onc.2013.191. [DOI] [PubMed] [Google Scholar]
- 283.Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16:431–46. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- 284.Cheng Y, Li H, Deng Y, Tai Y, Zeng K, Zhang Y, Liu W, Zhang Q, Yang Y. Cancer-associated fibroblasts induce PDL1 + neutrophils through the IL6-STAT3 pathway that foster immune suppression in hepatocellular carcinoma. Cell Death Dis. 2018;9:422. doi: 10.1038/s41419-018-0458-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Song M, He J, Pan QZ, Yang J, Zhao J, Zhang YJ, Huang Y, Tang Y, Wang Q, He J, et al. Cancer-Associated Fibroblast-Mediated Cellular Crosstalk Supports Hepatocellular Carcinoma Progression. Hepatology. 2021;73:1717–35. doi: 10.1002/hep.31792. [DOI] [PubMed] [Google Scholar]
- 286.Li K, Shi H, Zhang B, Ou X, Ma Q, Chen Y, Shu P, Li D, Wang Y. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct Target Ther. 2021;6:362. doi: 10.1038/s41392-021-00670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Mao X, Xu J, Wang W, Liang C, Hua J, Liu J, Zhang B, Meng Q, Yu X, Shi S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Molecular Cancer 2021, 20. [DOI] [PMC free article] [PubMed]
- 289.Hsu YL, Hung JY, Chiang SY, Jian SF, Wu CY, Lin YS, Tsai YM, Chou SH, Tsai MJ, Kuo PL. Lung cancer-derived galectin-1 contributes to cancer associated fibroblast-mediated cancer progression and immune suppression through TDO2/kynurenine axis. Oncotarget. 2016;7:27584–98. doi: 10.18632/oncotarget.8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Cheng Jt D, Yn Y, Hm W, Gy Fu, Bs C, Wj, Liu W, Tai Y, Peng Yw, Zhang Q. Hepatic carcinoma-associated fibroblasts induce IDO-producing regulatory dendritic cells through IL-6-mediated STAT3 activation. Oncogenesis. 2016;5:e198–8. doi: 10.1038/oncsis.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Ellem SJ, Taylor RA, Furic L, Larsson O, Frydenberg M, Pook D, Pedersen J, Cawsey B, Trotta A, Need E, et al. A pro-tumourigenic loop at the human prostate tumour interface orchestrated by oestrogen, CXCL12 and mast cell recruitment. J Pathol. 2014;234:86–98. doi: 10.1002/path.4386. [DOI] [PubMed] [Google Scholar]
- 292.Ma Y, Hwang RF, Logsdon CD, Ullrich SE. Dynamic mast cell-stromal cell interactions promote growth of pancreatic cancer. Cancer Res. 2013;73:3927–37. doi: 10.1158/0008-5472.CAN-12-4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Pereira BA, Lister NL, Hashimoto K, Teng L, Flandes-Iparraguirre M, Eder A, Sanchez-Herrero A, Niranjan B. Melbourne Urological Research A: Tissue engineered human prostate microtissues reveal key role of mast cell-derived tryptase in potentiating cancer-associated fibroblast (CAF)-induced morphometric transition in vitro. Biomaterials. 2019;197:72–85. doi: 10.1016/j.biomaterials.2018.12.030. [DOI] [PubMed] [Google Scholar]
- 294.Li T, Yang Y, Hua X, Wang G, Liu W, Jia C, Tai Y, Zhang Q, Chen G. Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer Lett. 2012;318:154–61. doi: 10.1016/j.canlet.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 295.Balsamo M, Scordamaglia F, Pietra G, Manzini C, Cantoni C, Boitano M, Queirolo P, Vermi W, Facchetti F, Moretta A, et al. Melanoma-associated fibroblasts modulate NK cell phenotype and antitumor cytotoxicity. Proc Natl Acad Sci USA. 2009;106:20847–52. doi: 10.1073/pnas.0906481106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Jenkins L, Jungwirth U, Avgustinova A, Iravani M, Mills AP, Haider S, Harper J, Isacke CM. Cancer-associated fibroblasts suppress CD8 + T cell infiltration and confer resistance to immune checkpoint blockade. Cancer research 2022. [DOI] [PMC free article] [PubMed]
- 297.Lakins MA, Ghorani E, Munir H, Martins CP, Shields JD. Cancer-associated fibroblasts induce antigen-specific deletion of CD8 (+) T Cells to protect tumour cells. Nat Commun. 2018;9:948. doi: 10.1038/s41467-018-03347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, Kadel EE, III, Koeppen H, Astarita JL, Cubas R, et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–8. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, Sevillano M, Ibiza S, Canellas A, Hernando-Momblona X, et al. TGFbeta drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554:538–43. doi: 10.1038/nature25492. [DOI] [PubMed] [Google Scholar]
- 300.Ene-Obong A, Clear AJ, Watt J, Wang J, Fatah R, Riches JC, Marshall JF, Chin-Aleong J, Chelala C, Gribben JG, et al. Activated pancreatic stellate cells sequester CD8 + T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma. Gastroenterology. 2013;145:1121–32. doi: 10.1053/j.gastro.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110:20212–7. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Gorchs L, Fernandez Moro C, Bankhead P, Kern KP, Sadeak I, Meng Q, Rangelova E, Kaipe H. Human Pancreatic Carcinoma-Associated Fibroblasts Promote Expression of Co-inhibitory Markers on CD4(+) and CD8(+) T-Cells. Front Immunol. 2019;10:847. doi: 10.3389/fimmu.2019.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Zhan S, Liu Z, Zhang M, Guo T, Quan Q, Huang L, Guo L, Cao L, Zhang X. Overexpression of B7-H3 in alpha-SMA-Positive Fibroblasts Is Associated With Cancer Progression and Survival in Gastric Adenocarcinomas. Front Oncol. 2019;9:1466. doi: 10.3389/fonc.2019.01466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Li Q, Yang Y, Jiang X, Jin YF, Wu JY, Qin Y, Qi XW, Cheng Y, Mao Y, Hua D. The combined expressions of B7H4 and ACOT4 in cancer-associated fibroblasts are related to poor prognosis in patients with gastric carcinoma. Int J Clin Exp Pathol. 2019;12:2672–81. [PMC free article] [PubMed] [Google Scholar]
- 305.Jiang W, Xu J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020;53:e12712. doi: 10.1111/cpr.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383–96. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 307.Hass R. Role of MSC in the Tumor Microenvironment. Cancers (Basel) 2020, 12. [DOI] [PMC free article] [PubMed]
- 308.Poggi A, Varesano S, Zocchi MR. How to Hit Mesenchymal Stromal Cells and Make the Tumor Microenvironment immunostimulant Rather Than immunosuppressive. Frontiers in Immunology 2018, 9. [DOI] [PMC free article] [PubMed]
- 309.Montesinos JJ, Mora-Garcia Mde L, Mayani H, Flores-Figueroa E, Garcia-Rocha R, Fajardo-Orduna GR, Castro-Manrreza ME, Weiss-Steider B, Monroy-Garcia A. In vitro evidence of the presence of mesenchymal stromal cells in cervical cancer and their role in protecting cancer cells from cytotoxic T cell activity. Stem Cells Dev. 2013;22:2508–19. doi: 10.1089/scd.2013.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Patel SA, Meyer JR, Greco SJ, Corcoran KE, Bryan M, Rameshwar P. Mesenchymal stem cells protect breast cancer cells through regulatory T cells: role of mesenchymal stem cell-derived TGF-beta. J Immunol. 2010;184:5885–94. doi: 10.4049/jimmunol.0903143. [DOI] [PubMed] [Google Scholar]
- 311.Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–6. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 312.Johann PD, Vaegler M, Gieseke F, Mang P, Armeanu-Ebinger S, Kluba T, Handgretinger R, Muller I. Tumour stromal cells derived from paediatric malignancies display MSC-like properties and impair NK cell cytotoxicity. Bmc Cancer 2010, 10. [DOI] [PMC free article] [PubMed]
- 313.Chaturvedi P, Gilkes DM, Takano N, Semenza GL. Hypoxia-inducible factor-dependent signaling between triple-negative breast cancer cells and mesenchymal stem cells promotes macrophage recruitment. Proc Natl Acad Sci U S A. 2014;111:E2120–9. doi: 10.1073/pnas.1406655111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Xu R, Zhao X, Zhao Y, Chen B, Sun L, Xu C, Shen B, Wang M, Xu W, Zhu W. Enhanced gastric cancer growth potential of mesenchymal stem cells derived from gastric cancer tissues educated by CD4(+) T cells. Cell Prolif. 2018;51:e12399. doi: 10.1111/cpr.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Turley SJ, Cremasco V, Astarita JL. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat Rev Immunol. 2015;15:669–82. doi: 10.1038/nri3902. [DOI] [PubMed] [Google Scholar]
- 316.Ochs K, Sahm F, Opitz CA, Lanz TV, Oezen I, Couraud PO, von Deimling A, Wick W, Platten M. Immature mesenchymal stem cell-like pericytes as mediators of immunosuppression in human malignant glioma. J Neuroimmunol. 2013;265:106–16. doi: 10.1016/j.jneuroim.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 317.Bose A, Barik S, Banerjee S, Ghosh T, Mallick A, Bhattacharyya Majumdar S, Goswami KK, Bhuniya A, Banerjee S, Baral R, et al. Tumor-derived vascular pericytes anergize Th cells. J Immunol. 2013;191:971–81. doi: 10.4049/jimmunol.1300280. [DOI] [PubMed] [Google Scholar]
- 318.Hong J, Tobin NP, Rundqvist H, Li T, Lavergne M, Garcia-Ibanez Y, Qin H, Paulsson J, Zeitelhofer M, Adzemovic MZ, et al: Role of Tumor Pericytes in the Recruitment of Myeloid-Derived Suppressor Cells. J Natl Cancer Inst 2015, 107. [DOI] [PMC free article] [PubMed]
- 319.Lampi MC, Reinhart-King CA. Targeting extracellular matrix stiffness to attenuate disease: From molecular mechanisms to clinical trials. Science Translational Medicine 2018, 10. [DOI] [PubMed]
- 320.Cox TR, Bird D, Baker AM, Barker HE, Ho MW, Lang G, Erler JT. LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res. 2013;73:1721–32. doi: 10.1158/0008-5472.CAN-12-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Miller BW, Morton JP, Pinese M, Saturno G, Jamieson NB, McGhee E, Timpson P, Leach J, McGarry L, Shanks E, et al. Targeting the LOX/hypoxia axis reverses many of the features that make pancreatic cancer deadly: inhibition of LOX abrogates metastasis and enhances drug efficacy. EMBO Mol Med. 2015;7:1063–76. doi: 10.15252/emmm.201404827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Boufraqech M, Nilubol N, Zhang L, Gara SK, Sadowski SM, Mehta A, He M, Davis S, Dreiling J, Copland JA, et al. miR30a inhibits LOX expression and anaplastic thyroid cancer progression. Cancer Res. 2015;75:367–77. doi: 10.1158/0008-5472.CAN-14-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Liu X, Liu T, Hu L, Jiang T, Liu H, Wang Y, Lei Y, Zhu J, Bu Y. Identification and characterization of the promoter of cancer-related gene LOXL2. Exp Cell Res. 2020;387:111786. doi: 10.1016/j.yexcr.2019.111786. [DOI] [PubMed] [Google Scholar]
- 324.Minna E, Brich S, Todoerti K, Pilotti S, Collini P, Bonaldi E, Romeo P, Cecco L, Dugo M, Perrone F, et al: Cancer Associated Fibroblasts and Senescent Thyroid Cells in the Invasive Front of Thyroid Carcinoma. Cancers (Basel) 2020, 12. [DOI] [PMC free article] [PubMed]
- 325.Salvador F, Martin A, Lopez-Menendez C, Moreno-Bueno G, Santos V, Vazquez-Naharro A, Santamaria PG, Morales S, Dubus PR, Muinelo-Romay L, et al. Lysyl Oxidase-like Protein LOXL2 Promotes Lung Metastasis of Breast Cancer. Cancer Res. 2017;77:5846–59. doi: 10.1158/0008-5472.CAN-16-3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Wen B, Xu LY, Li EM. LOXL2 in cancer: regulation, downstream effectors and novel roles. Biochim Biophys Acta Rev Cancer. 2020;1874:188435. doi: 10.1016/j.bbcan.2020.188435. [DOI] [PubMed] [Google Scholar]
- 327.Benson AB, Wainberg ZA, Hecht JR, Vyushkov D, Dong H, Bendell J, Kudrik F. A Phase II Randomized, Double-Blind, Placebo-Controlled Study of Simtuzumab or Placebo in Combination with Gemcitabine for the First-Line Treatment of Pancreatic Adenocarcinoma. Oncologist. 2017;22:241-e215. doi: 10.1634/theoncologist.2017-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Hecht JR, Benson AB, Vyushkov D, Yang YS, Bendell J, Verma U: A Phase II, Randomized, Double-Blind, Placebo-Controlled Study of Simtuzumab in Combination with FOLFIRI for the Second-Line Treatment of Metastatic KRAS Mutant Colorectal Adenocarcinoma. Oncologist 2017, 22:243-+. [DOI] [PMC free article] [PubMed]
- 329.Bayer IS. Hyaluronic Acid and Controlled Release: A Review. Molecules 2020, 25. [DOI] [PMC free article] [PubMed]
- 330.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic Targeting of the Stroma Ablates Physical Barriers to Treatment of Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2012;21:418–29. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Wong KM, Horton KJ, Coveler AL, Hingorani SR, Harris WP. Targeting the Tumor Stroma: the Biology and Clinical Development of Pegylated Recombinant Human Hyaluronidase (PEGPH20). Current Oncology Reports 2017, 19. [DOI] [PubMed]
- 332.Li XM, Shepard HM, Cowell JA, Zhao CM, Osgood RJ, Rosengren S, Blouw B, Garrovillo SA, Pagel MD, Whatcott CJ, et al. Parallel Accumulation of Tumor Hyaluronan, Collagen, and Other Drivers of Tumor Progression. Clin Cancer Res. 2018;24:4798–807. doi: 10.1158/1078-0432.CCR-17-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Heineman T, Baumgart M, Nanavati C, Gabrail N, Van Wart SA, Mager DE, Maneval DC, Fathallah AM, Sekulovich RE. Safety and pharmacokinetics of docetaxel in combination with pegvorhyaluronidase alfa in patients with non-small cell lung cancer. Cts-Clinical and Translational Science. 2021;14:1875–85. doi: 10.1111/cts.13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Hingorani SR, Zheng L, Bullock AJ, Seery TE, Harris WP, Sigal DS, Braiteh F, Ritch PS, Zalupski MM, Bahary N, et al. HALO 202: Randomized Phase II Study of PEGPH20 Plus Nab- Paclitaxel/Gemcitabine Versus Nab- Paclitaxel/Gemcitabine in Patients With Untreated, Metastatic Pancreatic Ductal Adenocarcinoma. J Clin Oncol. 2018;36:359-+. doi: 10.1200/JCO.2017.74.9564. [DOI] [PubMed] [Google Scholar]
- 335.Doherty GJ, Tempero M, Corrie PG. HALO-109-301: a Phase III trial of PEGPH20 (with gemcitabine and nab-paclitaxel) in hyaluronic acid-high stage IV pancreatic cancer. Future Oncol. 2018;14:13–22. doi: 10.2217/fon-2017-0338. [DOI] [PubMed] [Google Scholar]
- 336.Ramanathan RK, McDonough S, Philip PA, Hingorani SR, Lacy J, Kortmansky JS, Thumar J, Chiorean EG, Shields AF, Behl D, et al. Phase IB/II Randomized Study of FOLFIRINOX Plus Pegylated Recombinant Human Hyaluronidase Versus FOLFIRINOX Alone in Patients With Metastatic Pancreatic Adenocarcinoma: SWOG S1313. J Clin Oncol. 2019;37:1062-+. doi: 10.1200/JCO.18.01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Neesse A, Frese KK, Bapiro TE, Nakagawa T, Sternlicht MD, Seeley TW, Pilarsky C, Jodrell DI, Spong SM, Tuveson DA. CTGF antagonism with mAb FG-3019 enhances chemotherapy response without increasing drug delivery in murine ductal pancreas cancer. Proc Natl Acad Sci USA. 2013;110:12325–30. doi: 10.1073/pnas.1300415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Formenti SC, Hawtin RE, Dixit N, Evensen E, Lee P, Goldberg JD, Li XC, Vanpouille-Box C, Schaue D, McBride WH, Demaria S. Baseline T cell dysfunction by single cell network profiling in metastatic breast cancer patients. Journal for Immunotherapy of Cancer 2019, 7. [DOI] [PMC free article] [PubMed]
- 339.Sato M, Kadota M, Tang B, Yang HH, Yang YA, Shan M, Weng J, Welsh MA, Flanders KC, Nagano Y, et al: An integrated genomic approach identifies persistent tumor suppressive effects of transforming growth factor-beta in human breast cancer. Breast Cancer Research 2014, 16. [DOI] [PMC free article] [PubMed]
- 340.Roy-Luzarraga M, Hodivala-Dilke K. Molecular Pathways: Endothelial Cell FAK-A Target for Cancer Treatment. Clin Cancer Res. 2016;22:3718–24. doi: 10.1158/1078-0432.CCR-14-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Zhang LY, Zhao D, Wang Y, Zhang WM, Zhang J, Fan JW, Zhan QM, Chen J. Focal adhesion kinase (FAK) inhibitor-defactinib suppresses the malignant progression of human esophageal squamous cell carcinoma (ESCC) cells via effective blockade of PI3K/AKT axis and downstream molecular network. Mol Carcinog. 2021;60:113–24. doi: 10.1002/mc.23273. [DOI] [PubMed] [Google Scholar]
- 342.Lin HM, Lee BY, Castillo L, Spielman C, Grogan J, Yeung NK, Kench JG, Stricker PD, Haynes AM, Centenera MM, et al. Effect of FAK inhibitor VS-6063 (defactinib) on docetaxel efficacy in prostate cancer. Prostate. 2018;78:308–17. doi: 10.1002/pros.23476. [DOI] [PubMed] [Google Scholar]
- 343.Liu JY, Xue L, Xu X, Luo JH, Zhang SJ. FAK-targeting PROTAC demonstrates enhanced antitumor activity against KRAS mutant non-small cell lung cancer. Experimental Cell Research 2021, 408. [DOI] [PubMed]
- 344.Gerber DE, Camidge DR, Morgensztern D, Cetnar J, Kelly RJ, Ramalingam SS, Spigel DR, Jeong W, Scaglioni PP, Zhang S, et al. Phase 2 study of the focal adhesion kinase inhibitor defactinib (VS-6063) in previously treated advanced KRAS mutant non-small cell lung cancer. Lung Cancer. 2020;139:60–7. doi: 10.1016/j.lungcan.2019.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Jones SF, Siu LL, Bendell JC, Cleary JM, Razak AR, Infante JR, Pandya SS, Bedard PL, Pierce KJ, Houk B, et al. A phase I study of VS-6063, a second-generation focal adhesion kinase inhibitor, in patients with advanced solid tumors. Invest New Drugs. 2015;33:1100–7. doi: 10.1007/s10637-015-0282-y. [DOI] [PubMed] [Google Scholar]
- 346.Shimizu T, Fukuoka K, Takeda M, Iwasa T, Yoshida T, Horobin J, Keegan M, Vaickus L, Chavan A, Padval M, Nakagawa K. A first-in-Asian phase 1 study to evaluate safety, pharmacokinetics and clinical activity of VS-6063, a focal adhesion kinase (FAK) inhibitor in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol. 2016;77:997–1003. doi: 10.1007/s00280-016-3010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Fennell DA, Baas P, Taylor P, Nowak AK, Gilligan D, Nakano T, Pachter JA, Weaver DT, Scherpereel A, Pavlakis N, et al. Maintenance Defactinib Versus Placebo After First-Line Chemotherapy in Patients With Merlin-Stratified Pleural Mesothelioma: COMMAND-A Double-Blind, Randomized, Phase II Study. J Clin Oncol. 2019;37:790-+. doi: 10.1200/JCO.2018.79.0543. [DOI] [PubMed] [Google Scholar]
- 348.Liu J, Fu M, Wang M, Wan D, Wei Y, Wei X. Cancer vaccines as promising immuno-therapeutics: platforms and current progress. Journal of Hematology & Oncology 2022, 15. [DOI] [PMC free article] [PubMed]
- 349.Femel J, Huijbers EJM, Saupe F, Cedervall J, Zhang L, Roswall P, Larsson E, Olofsson H, Pietras K, Dimberg A, et al. Therapeutic vaccination against fibronectin ED-A attenuates progression of metastatic breast cancer. Oncotarget. 2014;5:12418–27. doi: 10.18632/oncotarget.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 351.Zheng R, Li F, Li F, Gong A. Targeting tumor vascularization: promising strategies for vascular normalization. J Cancer Res Clin Oncol. 2021;147:2489–505. doi: 10.1007/s00432-021-03701-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17:1359–70. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 353.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 354.Zhao Y, Adjei AA. Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor. Oncologist. 2015;20:660–73. doi: 10.1634/theoncologist.2014-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Liu G, Chen T, Ding Z, Wang Y, Wei Y, Wei X. Inhibition of FGF-FGFR and VEGF-VEGFR signalling in cancer treatment. Cell Prolif. 2021;54:e13009. doi: 10.1111/cpr.13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Oza AM, Dubois F, Hegg R, Hernandez CA, Finocchiaro G, Ghiringhelli F, Zamagni C, Nick S, Irahara N, Perretti T, Colombo N. A Long-Term Extension Study of Bevacizumab in Patients With Solid Tumors. Oncologist. 2021;26:e2254–64. doi: 10.1002/onco.13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, Griffing S, Bergsland E. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU) leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–5. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 358.Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, Langer CJ, DeVore RF, Gaudreault J, Damico LA, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–91. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 359.Dang A, Jagan Mohan Venkateswara Rao P, Kishore R, Vallish BN. Real world safety of bevacizumab in cancer patients: A systematic literature review of case reports. Int J Risk Saf Med. 2021;32:163–73. doi: 10.3233/JRS-194051. [DOI] [PubMed] [Google Scholar]
- 360.Miyashita M, Hattori M, Takano T, Toyama T, Iwata H. Risks and benefits of bevacizumab combined with chemotherapy for advanced or metastatic breast cancer: a meta-analysis of randomized controlled trials. Breast Cancer. 2020;27:347–54. doi: 10.1007/s12282-020-01052-9. [DOI] [PubMed] [Google Scholar]
- 361.Tomasello G, Petrelli F, Ghidini M, Russo A, Passalacqua R, Barni S. FOLFOXIRI Plus Bevacizumab as Conversion Therapy for Patients With Initially Unresectable Metastatic Colorectal Cancer: A Systematic Review and Pooled Analysis. JAMA Oncol. 2017;3:e170278. doi: 10.1001/jamaoncol.2017.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Rosen VM, Guerra I, McCormack M, Nogueira-Rodrigues A, Sasse A, Munk VC, Shang AJ. Systematic Review and Network Meta-Analysis of Bevacizumab Plus First-Line Topotecan-Paclitaxel or Cisplatin-Paclitaxel Versus Non-YBevacizumab-Containing Therapies in Persistent, Recurrent, or Metastatic Cervical Cancer. Int J Gynecol Cancer. 2017;27:1237–46. doi: 10.1097/IGC.0000000000001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Chen H, Liang M, Min J. Efficacy and Safety of Bevacizumab-Combined Chemotherapy for Advanced and Recurrent Endometrial Cancer: A Systematic Review and Meta-Analysis. Balkan medical journal 2020. [DOI] [PMC free article] [PubMed]
- 364.Oza AM, Cook AD, Pfisterer J, Embleton A, Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P, Cervantes A, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015;16:928–36. doi: 10.1016/S1470-2045(15)00086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Yang T, Xiao H, Liu X, Wang Z, Zhang Q, Wei N, Guo X. Vascular Normalization: A New Window Opened for Cancer Therapies. Front Oncol. 2021;11:719836. doi: 10.3389/fonc.2021.719836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Wu SG, Shih JY. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol Cancer. 2018;17:38. doi: 10.1186/s12943-018-0777-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Wang CC, Chiu LC, Tung PH, Kuo SC, Chu CH, Huang AC, Wang CL, Chen CH, Yang CT, Hsu PC. A Real-World Analysis of Patients with Untreated Metastatic Epidermal Growth Factor Receptor (EGFR)-Mutated Lung Adenocarcinoma Receiving First-Line Erlotinib and Bevacizumab Combination Therapy. Oncol Ther. 2021;9:489–503. doi: 10.1007/s40487-021-00152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Kaveh S, Ebrahimi P, Rezapour A, Mozafari M, Sayehmiri K. Bevacizumab and erlotinib versus bevacizumab for colorectal cancer treatment: systematic review and meta-analysis. Int J Clin Pharm. 2019;41:30–41. doi: 10.1007/s11096-018-0754-1. [DOI] [PubMed] [Google Scholar]
- 369.Erlotinib plus Bevacizumab Is Safe and Effective in EGFR-Mutant NSCLC. Cancer Discov. 2021;11:OF6. doi: 10.1158/2159-8290.CD-RW2021-120. [DOI] [PubMed] [Google Scholar]
- 370.Liu Y, Li Y, Wang Y, Lin C, Zhang D, Chen J, Ouyang L, Wu F, Zhang J, Chen L. Recent progress on vascular endothelial growth factor receptor inhibitors with dual targeting capabilities for tumor therapy. J Hematol Oncol. 2022;15:89. doi: 10.1186/s13045-022-01310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Zhou Q, Xu CR, Cheng Y, Liu YP, Chen GY, Cui JW, Yang N, Song Y, Li XL, Lu S, et al. Bevacizumab plus erlotinib in Chinese patients with untreated, EGFR-mutated, advanced NSCLC (ARTEMIS-CTONG1509): A multicenter phase 3 study. Cancer Cell. 2021;39:1279–91 e1273. doi: 10.1016/j.ccell.2021.07.005. [DOI] [PubMed] [Google Scholar]
- 372.Saito H, Fukuhara T, Furuya N, Watanabe K, Sugawara S, Iwasawa S, Tsunezuka Y, Yamaguchi O, Okada M, Yoshimori K, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20:625–35. doi: 10.1016/S1470-2045(19)30035-X. [DOI] [PubMed] [Google Scholar]
- 373.Rosell R, Dafni U, Felip E, Curioni-Fontecedro A, Gautschi O, Peters S, Massutí B, Palmero R, Aix SP, Carcereny E, et al. Erlotinib and bevacizumab in patients with advanced non-small-cell lung cancer and activating EGFR mutations (BELIEF): an international, multicentre, single-arm, phase 2 trial. The Lancet Respiratory Medicine. 2017;5:435–44. doi: 10.1016/S2213-2600(17)30129-7. [DOI] [PubMed] [Google Scholar]
- 374.Kaseb AO, Morris JS, Iwasaki M, Al-Shamsi HO, Raghav KP, Girard L, Cheung S, Nguyen V, Elsayes KM, Xiao L, et al. Phase II trial of bevacizumab and erlotinib as a second-line therapy for advanced hepatocellular carcinoma. Onco Targets Ther. 2016;9:773–80. doi: 10.2147/OTT.S91977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Ray-Coquard I, Pautier P, Pignata S, Perol D, Gonzalez-Martin A, Berger R, Fujiwara K, Vergote I, Colombo N, Maenpaa J, et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N Engl J Med. 2019;381:2416–28. doi: 10.1056/NEJMoa1911361. [DOI] [PubMed] [Google Scholar]
- 376.Mirza MR, Åvall Lundqvist E, Birrer MJ, dePont Christensen R, Nyvang G-B, Malander S, Anttila M, Werner TL, Lund B, Lindahl G, et al. Niraparib plus bevacizumab versus niraparib alone for platinum-sensitive recurrent ovarian cancer (NSGO-AVANOVA2/ENGOT-ov24): a randomised, phase 2, superiority trial. Lancet Oncol. 2019;20:1409–19. doi: 10.1016/S1470-2045(19)30515-7. [DOI] [PubMed] [Google Scholar]
- 377.de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, Chi KN, Sartor O, Agarwal N, Olmos D, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020;382:2091–102. doi: 10.1056/NEJMoa1911440. [DOI] [PubMed] [Google Scholar]
- 378.Yu HA, Schoenfeld AJ, Makhnin A, Kim R, Rizvi H, Tsui D, Falcon C, Houck-Loomis B, Meng F, Yang JL, et al. Effect of Osimertinib and Bevacizumab on Progression-Free Survival for Patients With Metastatic EGFR-Mutant Lung Cancers: A Phase 1/2 Single-Group Open-Label Trial. JAMA Oncol. 2020;6:1048–54. doi: 10.1001/jamaoncol.2020.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Nakagawa K, Garon EB, Seto T, Nishio M, Ponce Aix S, Paz-Ares L, Chiu C-H, Park K, Novello S, Nadal E, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:1655–69. doi: 10.1016/S1470-2045(19)30634-5. [DOI] [PubMed] [Google Scholar]
- 380.Wilke H, Muro K, Van Cutsem E, Oh S-C, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim T-Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–35. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 381.Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, dos Santos LV, Aprile G, Ferry DR, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. The Lancet. 2014;383:31–9. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 382.Zhu AX, Nipp RD, Finn RS, Galle PR, Llovet JM, Blanc JF, Okusaka T, Chau I, Cella D, Girvan A, et al: Ramucirumab in the second-line for patients with hepatocellular carcinoma and elevated alpha-fetoprotein: patient-reported outcomes across two randomised clinical trials. ESMO Open 2020, 5. [DOI] [PMC free article] [PubMed]
- 383.Chau I, Fakih M, Garcia-Alfonso P, Linke Z, Ruiz Casado A, Marques EP, Picard P, Celanovic M, Cartwright T: Safety and Effectiveness of Aflibercept + Fluorouracil, Leucovorin, and Irinotecan (FOLFIRI) for the Treatment of Patients with Metastatic Colorectal Cancer (mCRC) in Current Clinical Practice: OZONE Study. Cancers (Basel) 2020, 12. [DOI] [PMC free article] [PubMed]
- 384.Ruff P, Van Cutsem E, Lakomy R, Prausova J, van Hazel GA, Moiseyenko VM, Soussan-Lazard K, Dochy E, Magherini E, Macarulla T, Papamichael D. Observed benefit and safety of aflibercept in elderly patients with metastatic colorectal cancer: An age-based analysis from the randomized placebo-controlled phase III VELOUR trial. J Geriatr Oncol. 2018;9:32–9. doi: 10.1016/j.jgo.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 385.Denda T, Sakai D, Hamaguchi T, Sugimoto N, Ura T, Yamazaki K, Fujii H, Kajiwara T, Nakajima TE, Takahashi S, et al. Phase II trial of aflibercept with FOLFIRI as a second-line treatment for Japanese patients with metastatic colorectal cancer. Cancer Sci. 2019;110:1032–43. doi: 10.1111/cas.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Strickler JH, Rushing CN, Niedzwiecki D, McLeod A, Altomare I, Uronis HE, Hsu SD, Zafar SY, Morse MA, Chang DZ, et al. A phase Ib study of capecitabine and ziv-aflibercept followed by a phase II single-arm expansion cohort in chemotherapy refractory metastatic colorectal cancer. BMC Cancer. 2019;19:1032. doi: 10.1186/s12885-019-6234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Mashreghi M, Azarpara H, Bazaz MR, Jafari A, Masoudifar A, Mirzaei H, Jaafari MR. Angiogenesis biomarkers and their targeting ligands as potential targets for tumor angiogenesis. J Cell Physiol. 2018;233:2949–65. doi: 10.1002/jcp.26049. [DOI] [PubMed] [Google Scholar]
- 388.Pinyol R, Montal R, Bassaganyas L, Sia D, Takayama T, Chau GY, Mazzaferro V, Roayaie S, Lee HC, Kokudo N, et al. Molecular predictors of prevention of recurrence in HCC with sorafenib as adjuvant treatment and prognostic factors in the phase 3 STORM trial. Gut. 2019;68:1065–75. doi: 10.1136/gutjnl-2018-316408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Huang A, Yang XR, Chung WY, Dennison AR, Zhou J. Targeted therapy for hepatocellular carcinoma. Signal Transduct Target Ther. 2020;5:146. doi: 10.1038/s41392-020-00264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Kuczynski EA, Vermeulen PB, Pezzella F, Kerbel RS, Reynolds AR. Vessel co-option in cancer. Nat Rev Clin Oncol. 2019;16:469–93. doi: 10.1038/s41571-019-0181-9. [DOI] [PubMed] [Google Scholar]
- 391.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–74. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 392.Martin JD, Seano G, Jain RK. Normalizing Function of Tumor Vessels: Progress, Opportunities, and Challenges. Annu Rev Physiol. 2019;81:505–34. doi: 10.1146/annurev-physiol-020518-114700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–31. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.De Bock K, Mazzone M, Carmeliet P. Antiangiogenic therapy, hypoxia, and metastasis: risky liaisons, or not? Nat Rev Clin Oncol. 2011;8:393–404. doi: 10.1038/nrclinonc.2011.83. [DOI] [PubMed] [Google Scholar]
- 395.Ebos JM, Kerbel RS. Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat Rev Clin Oncol. 2011;8:210–21. doi: 10.1038/nrclinonc.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Jung K, Heishi T, Incio J, Huang Y, Beech EY, Pinter M, Ho WW, Kawaguchi K, Rahbari NN, Chung E, et al. Targeting CXCR4-dependent immunosuppressive Ly6C(low) monocytes improves antiangiogenic therapy in colorectal cancer. Proc Natl Acad Sci U S A. 2017;114:10455–60. doi: 10.1073/pnas.1710754114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Jung K, Heishi T, Khan OF, Kowalski PS, Incio J, Rahbari NN, Chung E, Clark JW, Willett CG, Luster AD, et al. Ly6Clo monocytes drive immunosuppression and confer resistance to anti-VEGFR2 cancer therapy. J Clin Invest. 2017;127:3039–51. doi: 10.1172/JCI93182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15:325–40. doi: 10.1038/nrclinonc.2018.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–7. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Lu-Emerson C, Duda DG, Emblem KE, Taylor JW, Gerstner ER, Loeffler JS, Batchelor TT, Jain RK. Lessons from anti-vascular endothelial growth factor and anti-vascular endothelial growth factor receptor trials in patients with glioblastoma. J Clin Oncol. 2015;33:1197–213. doi: 10.1200/JCO.2014.55.9575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Levin VA, Mendelssohn ND, Chan J, Stovall MC, Peak SJ, Yee JL, Hui RL, Chen DM. Impact of bevacizumab administered dose on overall survival of patients with progressive glioblastoma. J Neurooncol. 2015;122:145–50. doi: 10.1007/s11060-014-1693-x. [DOI] [PubMed] [Google Scholar]
- 402.Lorgis V, Maura G, Coppa G, Hassani K, Taillandier L, Chauffert B, Apetoh L, Ladoire S, Ghiringhelli F. Relation between bevacizumab dose intensity and high-grade glioma survival: a retrospective study in two large cohorts. J Neurooncol. 2012;107:351–8. doi: 10.1007/s11060-011-0748-5. [DOI] [PubMed] [Google Scholar]
- 403.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapy. Nat Med. 2001;7:987–9. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 404.Wang K, Chen Q, Liu N, Zhang J, Pan X. Recent advances in, and challenges of, anti-angiogenesis agents for tumor chemotherapy based on vascular normalization. Drug Discov Today. 2021;26:2743–53. doi: 10.1016/j.drudis.2021.07.024. [DOI] [PubMed] [Google Scholar]
- 405.Chae SS, Kamoun WS, Farrar CT, Kirkpatrick ND, Niemeyer E, de Graaf AM, Sorensen AG, Munn LL, Jain RK, Fukumura D. Angiopoietin-2 interferes with anti-VEGFR2-induced vessel normalization and survival benefit in mice bearing gliomas. Clin Cancer Res. 2010;16:3618–27. doi: 10.1158/1078-0432.CCR-09-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Peterson TE, Kirkpatrick ND, Huang Y, Farrar CT, Marijt KA, Kloepper J, Datta M, Amoozgar Z, Seano G, Jung K, et al. Dual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages. Proc Natl Acad Sci U S A. 2016;113:4470–5. doi: 10.1073/pnas.1525349113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Schmittnaegel M, Rigamonti N, Kadioglu E, Cassara A, Rmili CW, Kiialainen A, Kienast Y, Mueller HJ, Ooi CH, Laoui D, De Palma M. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Science Translational Medicine 2017, 9. [DOI] [PubMed]
- 408.Mueller T, Freystein J, Lucas H, Schmoll HJ. Efficacy of a Bispecific Antibody Co-Targeting VEGFA and Ang-2 in Combination with Chemotherapy in a Chemoresistant Colorectal Carcinoma Xenograft Model. Molecules 2019, 24. [DOI] [PMC free article] [PubMed]
- 409.Huang Y, Kim BYS, Chan CK, Hahn SM, Weissman IL, Jiang W. Improving immune-vascular crosstalk for cancer immunotherapy. Nat Rev Immunol. 2018;18:195–203. doi: 10.1038/nri.2017.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Melo V, Bremer E, Martin JD. Towards Immunotherapy-Induced Normalization of the Tumor Microenvironment. Frontiers in Cell and Developmental Biology 2022, 10. [DOI] [PMC free article] [PubMed]
- 411.Matuszewska K, Pereira M, Petrik D, Lawler J, Petrik J. Normalizing Tumor Vasculature to Reduce Hypoxia, Enhance Perfusion, and Optimize Therapy Uptake. Cancers (Basel) 2021, 13. [DOI] [PMC free article] [PubMed]
- 412.Liu Z, Wang Y, Huang Y, Kim BYS, Shan H, Wu D, Jiang W. Tumor Vasculatures: A New Target for Cancer Immunotherapy. Trends Pharmacol Sci. 2019;40:613–23. doi: 10.1016/j.tips.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Liu JF, Herold C, Gray KP, Penson RT, Horowitz N, Konstantinopoulos PA, Castro CM, Hill SJ, Curtis J, Luo W, et al. Assessment of Combined Nivolumab and Bevacizumab in Relapsed Ovarian Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 2019;5:1731–8. doi: 10.1001/jamaoncol.2019.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Hellmann MD, Rizvi NA, Goldman JW, Gettinger SN, Borghaei H, Brahmer JR, Ready NE, Gerber DE, Chow LQ, Juergens RA, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017;18:31–41. doi: 10.1016/S1470-2045(16)30624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Akbari P, Katsarou A, Daghighian R, van Mil L, Huijbers EJM, Griffioen AW, van Beijnum JR. Directing CAR T cells towards the tumor vasculature for the treatment of solid tumors. Biochim Biophys Acta Rev Cancer. 2022;1877:188701. doi: 10.1016/j.bbcan.2022.188701. [DOI] [PubMed] [Google Scholar]
- 416.Belhabib I, Zaghdoudi S, Lac C, Bousquet C, Jean C. Extracellular Matrices and Cancer-Associated Fibroblasts: Targets for Cancer Diagnosis and Therapy? Cancers (Basel) 2021, 13. [DOI] [PMC free article] [PubMed]
- 417.Knops AM, South A, Rodeck U, Martinez-Outschoorn U, Harshyne LA, Johnson J, Luginbuhl AJ, Curry JM. Cancer-Associated Fibroblast Density, Prognostic Characteristics, and Recurrence in Head and Neck Squamous Cell Carcinoma: A Meta-Analysis. Front Oncol. 2020;10:565306. doi: 10.3389/fonc.2020.565306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Heichler C, Scheibe K, Schmied A, Geppert CI, Schmid B, Wirtz S, Thoma OM, Kramer V, Waldner MJ, Buttner C, et al. STAT3 activation through IL-6/IL-11 in cancer-associated fibroblasts promotes colorectal tumour development and correlates with poor prognosis. Gut. 2020;69:1269–82. doi: 10.1136/gutjnl-2019-319200. [DOI] [PubMed] [Google Scholar]
- 419.Herrera M, Berral-Gonzalez A, Lopez-Cade I, Galindo-Pumarino C, Bueno-Fortes S, Martin-Merino M, Carrato A, Ocana A, De La Pinta C, Lopez-Alfonso A, et al. Cancer-associated fibroblast-derived gene signatures determine prognosis in colon cancer patients. Mol Cancer. 2021;20:73. doi: 10.1186/s12943-021-01367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Wu Z, Shi J, Lai C, Li K, Li K, Li Z, Tang Z, Liu C, Xu K. Clinicopathological significance and prognostic value of cancer-associated fibroblasts in prostate cancer patients. Urol Oncol 2021, 39:433 e417-433 e423. [DOI] [PubMed]
- 421.Xin L, Gao JF, Zheng ZL, Chen YY, Lv SX, Zhao ZK, Yu CH, Yang XT, Zhang RP. Fibroblast Activation Protein-alpha as a Target in the Bench-to-Bedside Diagnosis and Treatment of Tumors: A Narrative Review. Frontiers in Oncology 2021, 11. [DOI] [PMC free article] [PubMed]
- 422.Lo A, Wang LCS, Scholler J, Monslow J, Avery D, Newick K, O’Brien S, Evans RA, Bajor DJ, Clendenin C, et al. Tumor-Promoting Desmoplasia Is Disrupted by Depleting FAP-Expressing Stromal Cells. Cancer Res. 2015;75:2800–10. doi: 10.1158/0008-5472.CAN-14-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Huang Y, Simms AE, Mazur A, Wang S, Leon NR, Jones B, Aziz N, Kelly T. Fibroblast activation protein-alpha promotes tumor growth and invasion of breast cancer cells through non-enzymatic functions. Clin Exp Metastasis. 2011;28:567–79. doi: 10.1007/s10585-011-9392-x. [DOI] [PubMed] [Google Scholar]
- 424.Li M, Cheng X, Rong R, Gao Y, Tang XW, Chen YG. High expression of fibroblast activation protein (FAP) predicts poor outcome in high-grade serous ovarian cancer. Bmc Cancer 2020, 20. [DOI] [PMC free article] [PubMed]
- 425.Arnold JN, Magiera L, Kraman M, Fearon DT. Tumoral Immune Suppression by Macrophages Expressing Fibroblast Activation Protein-alpha and Heme Oxygenase-1. Cancer Immunol Res. 2014;2:121–6. doi: 10.1158/2326-6066.CIR-13-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, Gopinathan A, Tuveson DA, Fearon DT. Suppression of Antitumor Immunity by Stromal Cells Expressing Fibroblast Activation Protein-alpha. Science. 2010;330:827–30. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]
- 427.Loeffler M, Kruger JA, Niethammer AG, Reisfeld RA. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest. 2006;116:1955–62. doi: 10.1172/JCI26532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Wen YA, Wang CT, Ma TT, Li ZY, Zhou LN, Mu B, Leng F, Shi HS, Li YO, Wei YQ. Immunotherapy targeting fibroblast activation protein inhibits tumor growth and increases survival in a murine colon cancer model. Cancer Sci. 2010;101:2325–32. doi: 10.1111/j.1349-7006.2010.01695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Duperret EK, Trautz A, Ammons D, Perales-Puchalt A, Wise MC, Yan J, Reed C, Weiner DB. Alteration of the Tumor Stroma Using a Consensus DNA Vaccine Targeting Fibroblast Activation Protein (FAP) Synergizes with Antitumor Vaccine Therapy in Mice. Clin Cancer Res. 2018;24:1190–201. doi: 10.1158/1078-0432.CCR-17-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Bughda R, Dimou P, D’Souza RR, Klampatsa A. Fibroblast Activation Protein (FAP)-Targeted CAR-T Cells: Launching an Attack on Tumor Stroma. Immunotargets and Therapy. 2021;10:313–23. doi: 10.2147/ITT.S291767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Wang LCS, Lo A, Scholler J, Sun J, Majumdar RS, Kapoor V, Antzis M, Cotner CE, Johnson LA, Durham AC, et al. Targeting Fibroblast Activation Protein in Tumor Stroma with Chimeric Antigen Receptor T Cells Can Inhibit Tumor Growth and Augment Host Immunity without Severe Toxicity. Cancer Immunol Res. 2014;2:154–66. doi: 10.1158/2326-6066.CIR-13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Petrausch U, Schuberth PC, Hagedorn C, Soltermann A, Tomaszek S, Stahel R, Weder W, Renner C. Re-directed T cells for the treatment of fibroblast activation protein (FAP)-positive malignant pleural mesothelioma (FAPME-1). Bmc Cancer 2012, 12. [DOI] [PMC free article] [PubMed]
- 433.Sum E, Rapp M, Frobel P, Le Clech M, Durr H, Giusti AM, Perro M, Speziale D, Kunz L, Menietti E, et al. Fibroblast Activation Protein alpha-Targeted CD40 Agonism Abrogates Systemic Toxicity and Enables Administration of High Doses to Induce Effective Antitumor Immunity. Clin Cancer Res. 2021;27:4036–53. doi: 10.1158/1078-0432.CCR-20-4001. [DOI] [PubMed] [Google Scholar]
- 434.Katsube R, Noma K, Ohara T, Nishiwaki N, Kobayashi T, Komoto S, Sato H, Kashima H, Kato T, Kikuchi S, et al. Fibroblast activation protein targeted near infrared photoimmunotherapy (NIR PIT) overcomes therapeutic resistance in human esophageal cancer. Sci Rep. 2021;11:1693. doi: 10.1038/s41598-021-81465-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Waldhauer I, Gonzalez-Nicolini V, Freimoser-Grundschober A, Nayak TK, Fahrni L, Hosse RJ, Gerrits D, Geven EJW, Sam J, Lang S, et al. Simlukafusp alfa (FAP-IL2v) immunocytokine is a versatile combination partner for cancer immunotherapy. MAbs. 2021;13:1913791. doi: 10.1080/19420862.2021.1913791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Gunderson AJ, Yamazaki T, McCarty K, Phillips M, Alice A, Bambina S, Zebertavage L, Friedman D, Cottam B, Newell P, et al. Blockade of fibroblast activation protein in combination with radiation treatment in murine models of pancreatic adenocarcinoma. PLoS ONE. 2019;14:e0211117. doi: 10.1371/journal.pone.0211117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Vathiotis IA, Moutafi MK, Divakar P, Aung TN, Qing T, Fernandez A, Yaghoobi V, El-Abed S, Wang Y, Guillaume S, et al. Alpha-smooth Muscle Actin Expression in the Stroma Predicts Resistance to Trastuzumab in Patients with Early-stage HER2-positive Breast Cancer. Clin Cancer Res. 2021;27:6156–63. doi: 10.1158/1078-0432.CCR-21-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Murakami M, Ernsting MJ, Undzys E, Holwell N, Foltz WD, Li SD. Docetaxel conjugate nanoparticles that target alpha-smooth muscle actin-expressing stromal cells suppress breast cancer metastasis. Cancer Res. 2013;73:4862–71. doi: 10.1158/0008-5472.CAN-13-0062. [DOI] [PubMed] [Google Scholar]
- 439.Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H, Collisson EA, Connor F, Van Dyke T, Kozlov S, et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159:80–93. doi: 10.1016/j.cell.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Zhao ZX, Zhang YQ, Sun H, Chen ZQ, Chang JJ, Wang X, Wang X, Tan C, Ni SJ, Weng WW, et al: Calcipotriol abrogates cancer-associated fibroblast-derived IL-8-mediated oxaliplatin resistance in gastric cancer cells via blocking PI3K/Akt signaling. Acta Pharmacologica Sinica. [DOI] [PMC free article] [PubMed]
- 441.Milczarek M, Psurski M, Kutner A, Wietrzyk J. Vitamin D analogs enhance the anticancer activity of 5-fluorouracil in an in vivo mouse colon cancer model. Bmc Cancer 2013, 13. [DOI] [PMC free article] [PubMed]
- 442.Li X, Yong T, Wei Z, Bie N, Zhang X, Zhan G, Li J, Qin J, Yu J, Zhang B, et al. Reversing insufficient photothermal therapy-induced tumor relapse and metastasis by regulating cancer-associated fibroblasts. Nat Commun. 2022;13:2794. doi: 10.1038/s41467-022-30306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Cunningham TJ, Tabacchi M, Eliane JP, Tuchayi SM, Manivasagam S, Mirzaalian H, Turkoz A, Kopan R, Schaffer A, Saavedra AP, et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127:106–16. doi: 10.1172/JCI89820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Chronopoulos A, Robinson B, Sarper M, Cortes E, Auernheimer V, Lachowski D, Attwood S, Garcia R, Ghassemi S, Fabry B. Del Rio Hernandez A: ATRA mechanically reprograms pancreatic stellate cells to suppress matrix remodelling and inhibit cancer cell invasion. Nat Commun. 2016;7:12630. doi: 10.1038/ncomms12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Carapuca EF, Gemenetzidis E, Feig C, Bapiro TE, Williams MD, Wilson AS, Delvecchio FR, Arumugam P, Grose RP, Lemoine NR, et al. Anti-stromal treatment together with chemotherapy targets multiple signalling pathways in pancreatic adenocarcinoma. J Pathol. 2016;239:286–96. doi: 10.1002/path.4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Froeling FE, Feig C, Chelala C, Dobson R, Mein CE, Tuveson DA, Clevers H, Hart IR, Kocher HM. Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wnt-beta-catenin signaling to slow tumor progression. Gastroenterology. 2011;141:1486–97. doi: 10.1053/j.gastro.2011.06.047. [DOI] [PubMed] [Google Scholar]
- 447.North B, Kocher HM, Sasieni P. A new pragmatic design for dose escalation in phase 1 clinical trials using an adaptive continual reassessment method. BMC Cancer 2019, 19. [DOI] [PMC free article] [PubMed]
- 448.Kocher HM, Basu B, Froeling FEM, Sarker D, Slater S, Carlin D, deSouza NM, De Paepe KN, Goulart MR, Hughes C, et al. Phase I clinical trial repurposing all-trans retinoic acid as a stromal targeting agent for pancreatic cancer. Nat Commun. 2020;11:4841. doi: 10.1038/s41467-020-18636-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Hanna GJ, Cutler AON, Flynn JM, Vijaykumar M, Clark T, Wirth JR, Lorch LJ, Park JH, Mito JC, et al. A phase II trial of all-trans retinoic acid (ATRA) in advanced adenoid cystic carcinoma. Oral Oncol. 2021;119:105366. doi: 10.1016/j.oraloncology.2021.105366. [DOI] [PubMed] [Google Scholar]
- 450.Arrieta O, Gonzalez-De la Rosa CH, Arechaga-Ocampo E, Villanueva-Rodriguez G, Ceron-Lizarraga TL, Martinez-Barrera L, Vazquez-Manriquez ME, Rios-Trejo MA, Alvarez-Avitia MA, Hernandez-Pedro N, et al. Randomized phase II trial of All-trans-retinoic acid with chemotherapy based on paclitaxel and cisplatin as first-line treatment in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:3463–71. doi: 10.1200/JCO.2009.26.6452. [DOI] [PubMed] [Google Scholar]
- 451.Wick A, Desjardins A, Suarez C, Forsyth P, Gueorguieva I, Burkholder T, Cleverly AL, Estrem ST, Wang S, Lahn MM, et al. Phase 1b/2a study of galunisertib, a small molecule inhibitor of transforming growth factor-beta receptor I, in combination with standard temozolomide-based radiochemotherapy in patients with newly diagnosed malignant glioma. Invest New Drugs. 2020;38:1570–9. doi: 10.1007/s10637-020-00910-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Harding JJ, Do RK, Yaqubie A, Cleverly A, Zhao Y, Gueorguieva I, Lahn M, Benhadji KA, Kelley RK, Abou-Alfa GK. Phase 1b study of galunisertib and ramucirumab in patients with advanced hepatocellular carcinoma. Cancer Med. 2021;10:3059–67. doi: 10.1002/cam4.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Faivre S, Santoro A, Kelley RK, Gane E, Costentin CE, Gueorguieva I, Smith C, Cleverly A, Lahn MM, Raymond E, et al. Novel transforming growth factor beta receptor I kinase inhibitor galunisertib (LY2157299) in advanced hepatocellular carcinoma. Liver Int. 2019;39:1468–77. doi: 10.1111/liv.14113. [DOI] [PubMed] [Google Scholar]
- 454.Giannelli G, Santoro A, Kelley RK, Gane E, Paradis V, Cleverly A, Smith C, Estrem ST, Man M, Wang S, et al. Biomarkers and overall survival in patients with advanced hepatocellular carcinoma treated with TGF-betaRI inhibitor galunisertib. PLoS ONE. 2020;15:e0222259. doi: 10.1371/journal.pone.0222259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Melisi D, Garcia-Carbonero R, Macarulla T, Pezet D, Deplanque G, Fuchs M, Trojan J, Kozloff M, Simionato F, Cleverly A, et al. TGFbeta receptor inhibitor galunisertib is linked to inflammation- and remodeling-related proteins in patients with pancreatic cancer. Cancer Chemother Pharmacol. 2019;83:975–91. doi: 10.1007/s00280-019-03807-4. [DOI] [PubMed] [Google Scholar]
- 456.Melisi D, Garcia-Carbonero R, Macarulla T, Pezet D, Deplanque G, Fuchs M, Trojan J, Oettle H, Kozloff M, Cleverly A, et al. Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer. Br J Cancer. 2018;119:1208–14. doi: 10.1038/s41416-018-0246-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Kelley RK, Gane E, Assenat E, Siebler J, Galle PR, Merle P, Hourmand IO, Cleverly A, Zhao Y, Gueorguieva I, et al. A Phase 2 Study of Galunisertib (TGF-beta1 Receptor Type I Inhibitor) and Sorafenib in Patients With Advanced Hepatocellular Carcinoma. Clin Transl Gastroenterol. 2019;10:e00056. doi: 10.14309/ctg.0000000000000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Brandes AA, Carpentier AF, Kesari S, Sepulveda-Sanchez JM, Wheeler HR, Chinot O, Cher L, Steinbach JP, Capper D, Specenier P, et al. A Phase II randomized study of galunisertib monotherapy or galunisertib plus lomustine compared with lomustine monotherapy in patients with recurrent glioblastoma. Neuro Oncol. 2016;18:1146–56. doi: 10.1093/neuonc/now009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Martin M, Mayer IA, Walenkamp AME, Lapa C, Andreeff M, Bobirca A. At the Bedside: Profiling and treating patients with CXCR4-expressing cancers. J Leukoc Biol. 2021;109:953–67. doi: 10.1002/JLB.5BT1219-714R. [DOI] [PubMed] [Google Scholar]
- 460.Bockorny B, Semenisty V, Macarulla T, Borazanci E, Wolpin BM, Stemmer SM, Golan T, Geva R, Borad MJ, Pedersen KS, et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: the COMBAT trial. Nat Med. 2020;26:878–85. doi: 10.1038/s41591-020-0880-x. [DOI] [PubMed] [Google Scholar]
- 461.Chen Y, McAndrews KM, Kalluri R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol. 2021;18:792–804. doi: 10.1038/s41571-021-00546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Kim DJ, Kim J, Spaunhurst K, Montoya J, Khodosh R, Chandra K, Fu T, Gilliam A, Molgo M, Beachy PA, Tang JY. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745–51. doi: 10.1200/JCO.2013.49.9525. [DOI] [PubMed] [Google Scholar]
- 463.Fosko SW, Chu MB, Armbrecht E, Galperin T, Potts GA, Mattox A, Kurta A, Polito K, Slutsky JB, Burkemper NM, Hurley MY. Efficacy, rate of tumor response, and safety of a short course (12–24 weeks) of oral vismodegib in various histologic subtypes (infiltrative, nodular, and superficial) of high-risk or locally advanced basal cell carcinoma, in an open-label, prospective case series clinical trial. J Am Acad Dermatol. 2020;82:946–54. doi: 10.1016/j.jaad.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 464.Catenacci DV, Junttila MR, Karrison T, Bahary N, Horiba MN, Nattam SR, Marsh R, Wallace J, Kozloff M, Rajdev L, et al. Randomized Phase Ib/II Study of Gemcitabine Plus Placebo or Vismodegib, a Hedgehog Pathway Inhibitor, in Patients With Metastatic Pancreatic Cancer. J Clin Oncol. 2015;33:4284–92. doi: 10.1200/JCO.2015.62.8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Kim EJ, Sahai V, Abel EV, Griffith KA, Greenson JK, Takebe N, Khan GN, Blau JL, Craig R, Balis UG, et al. Pilot clinical trial of hedgehog pathway inhibitor GDC-0449 (vismodegib) in combination with gemcitabine in patients with metastatic pancreatic adenocarcinoma. Clin Cancer Res. 2014;20:5937–45. doi: 10.1158/1078-0432.CCR-14-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Belani CP, Dahlberg SE, Rudin CM, Fleisher M, Chen HX, Takebe N, Velasco MR, Jr, Tester WJ, Sturtz K, Hann CL, et al. Vismodegib or cixutumumab in combination with standard chemotherapy for patients with extensive-stage small cell lung cancer: A trial of the ECOG-ACRIN Cancer Research Group (E1508) Cancer. 2016;122:2371–8. doi: 10.1002/cncr.30062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Pietanza MC, Litvak AM, Varghese AM, Krug LM, Fleisher M, Teitcher JB, Holodny AI, Sima CS, Woo KM, Ng KK, et al. A phase I trial of the Hedgehog inhibitor, sonidegib (LDE225), in combination with etoposide and cisplatin for the initial treatment of extensive stage small cell lung cancer. Lung Cancer. 2016;99:23–30. doi: 10.1016/j.lungcan.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Stathis A, Hess D, von Moos R, Homicsko K, Griguolo G, Joerger M, Mark M, Ackermann CJ, Allegrini S, Catapano CV, et al. Phase I trial of the oral smoothened inhibitor sonidegib in combination with paclitaxel in patients with advanced solid tumors. Investig New Drugs. 2017;35:766–72. doi: 10.1007/s10637-017-0454-z. [DOI] [PubMed] [Google Scholar]
- 469.Ruiz-Borrego M, Jimenez B, Antolin S, Garcia-Saenz JA, Corral J, Jerez Y, Trigo J, Urruticoechea A, Colom H, Gonzalo N, et al. A phase Ib study of sonidegib (LDE225), an oral small molecule inhibitor of smoothened or Hedgehog pathway, in combination with docetaxel in triple negative advanced breast cancer patients: GEICAM/2012-12 (EDALINE) study. Invest New Drugs. 2019;37:98–108. doi: 10.1007/s10637-018-0614-9. [DOI] [PubMed] [Google Scholar]
- 470.Kalimuthu S, Oh JM, Gangadaran P, Zhu L, Lee HW, Rajendran RL, Baek SH, Jeon YH, Jeong SY, Lee SW, et al. In Vivo Tracking of Chemokine Receptor CXCR4-Engineered Mesenchymal Stem Cell Migration by Optical Molecular Imaging. Stem Cells Int. 2017;2017:8085637. doi: 10.1155/2017/8085637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Ho IA, Yulyana Y, Sia KC, Newman JP, Guo CM, Hui KM, Lam PY. Matrix metalloproteinase-1-mediated mesenchymal stem cell tumor tropism is dependent on crosstalk with stromal derived growth factor 1/C-X-C chemokine receptor 4 axis. FASEB J. 2014;28:4359–68. doi: 10.1096/fj.14-252551. [DOI] [PubMed] [Google Scholar]
- 472.Luz-Crawford P, Noel D, Fernandez X, Khoury M, Figueroa F, Carrion F, Jorgensen C, Djouad F. Mesenchymal stem cells repress Th17 molecular program through the PD-1 pathway. PLoS ONE. 2012;7:e45272. doi: 10.1371/journal.pone.0045272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Biasci D, Smoragiewicz M, Connell CM, Wang Z, Gao Y, Thaventhiran JED, Basu B, Magiera L, Johnson TI, Bax L, et al. CXCR4 inhibition in human pancreatic and colorectal cancers induces an integrated immune response. Proc Natl Acad Sci U S A. 2020;117:28960–70. doi: 10.1073/pnas.2013644117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Roccaro AM, Sacco A, Purschke WG, Moschetta M, Buchner K, Maasch C, Zboralski D, Zollner S, Vonhoff S, Mishima Y, et al. SDF-1 inhibition targets the bone marrow niche for cancer therapy. Cell Rep. 2014;9:118–28. doi: 10.1016/j.celrep.2014.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Wobus M, List C, Dittrich T, Dhawan A, Duryagina R, Arabanian LS, Kast K, Wimberger P, Stiehler M, Hofbauer LC, et al. Breast carcinoma cells modulate the chemoattractive activity of human bone marrow-derived mesenchymal stromal cells by interfering with CXCL12. Int J Cancer. 2015;136:44–54. doi: 10.1002/ijc.28960. [DOI] [PubMed] [Google Scholar]
- 476.Zhang XH, Jin X, Malladi S, Zou Y, Wen YH, Brogi E, Smid M, Foekens JA, Massague J. Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell. 2013;154:1060–73. doi: 10.1016/j.cell.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Harrell CR, Volarevic A, Djonov VG, Jovicic N, Volarevic V. Mesenchymal Stem Cell: A Friend or Foe in Anti-Tumor Immunity. Int J Mol Sci 2021, 22. [DOI] [PMC free article] [PubMed]
- 478.Lob S, Konigsrainer A, Rammensee HG, Opelz G, Terness P. Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: can we see the wood for the trees? Nat Rev Cancer. 2009;9:445–52. doi: 10.1038/nrc2639. [DOI] [PubMed] [Google Scholar]
- 479.Ling W, Zhang J, Yuan Z, Ren G, Zhang L, Chen X, Rabson AB, Roberts AI, Wang Y, Shi Y. Mesenchymal stem cells use IDO to regulate immunity in tumor microenvironment. Cancer Res. 2014;74:1576–87. doi: 10.1158/0008-5472.CAN-13-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med. 2013;210:1389–402. doi: 10.1084/jem.20130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Gazdic M, Simovic Markovic B, Jovicic N, Misirkic-Marjanovic M, Djonov V, Jakovljevic V, Arsenijevic N, Lukic ML, Volarevic V. Mesenchymal Stem Cells Promote Metastasis of Lung Cancer Cells by Downregulating Systemic Antitumor Immune Response. Stem Cells Int. 2017;2017:6294717. doi: 10.1155/2017/6294717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Nayak-Kapoor A, Hao Z, Sadek R, Dobbins R, Marshall L, Vahanian NN, Jay Ramsey W, Kennedy E, Mautino MR, Link CJ, et al. Phase Ia study of the indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor navoximod (GDC-0919) in patients with recurrent advanced solid tumors. J Immunother Cancer. 2018;6:61. doi: 10.1186/s40425-018-0351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Stewart Coats AJ, Srinivasan V, Surendran J, Chiramana H, Vangipuram SR, Bhatt NN, Jain M, Shah S, Ali IA, Fuang HG, et al. The ACT-ONE trial, a multicentre, randomised, double-blind, placebo-controlled, dose-finding study of the anabolic/catabolic transforming agent, MT-102 in subjects with cachexia related to stage III and IV non-small cell lung cancer and colorectal cancer: study design. J Cachexia Sarcopenia Muscle. 2011;2:201–7. doi: 10.1007/s13539-011-0046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Babajani A, Soltani P, Jamshidi E, Farjoo MH, Niknejad H. Recent Advances on Drug-Loaded Mesenchymal Stem Cells With Anti-neoplastic Agents for Targeted Treatment of Cancer. Front Bioeng Biotechnol. 2020;8:748. doi: 10.3389/fbioe.2020.00748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Xu C, Lin L, Cao G, Chen Q, Shou P, Huang Y, Han Y, Wang Y, Shi Y. Interferon-alpha-secreting mesenchymal stem cells exert potent antitumor effect in vivo. Oncogene. 2014;33:5047–52. doi: 10.1038/onc.2013.458. [DOI] [PubMed] [Google Scholar]
- 486.Shen CJ, Chan TF, Chen CC, Hsu YC, Long CY, Lai CS. Human umbilical cord matrix-derived stem cells expressing interferon-beta gene inhibit breast cancer cells via apoptosis. Oncotarget. 2016;7:34172–9. doi: 10.18632/oncotarget.8997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Elzaouk L, Moelling K, Pavlovic J. Anti-tumor activity of mesenchymal stem cells producing IL-12 in a mouse melanoma model. Exp Dermatol. 2006;15:865–74. doi: 10.1111/j.1600-0625.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 488.Hombach AA, Geumann U, Gunther C, Hermann FG, Abken H: IL7-IL12 Engineered Mesenchymal Stem Cells (MSCs) Improve A CAR T Cell Attack Against Colorectal Cancer Cells. Cells 2020, 9. [DOI] [PMC free article] [PubMed]
- 489.Jiang X, Fitch S, Wang C, Wilson C, Li J, Grant GA, Yang F. Nanoparticle engineered TRAIL-overexpressing adipose-derived stem cells target and eradicate glioblastoma via intracranial delivery. Proc Natl Acad Sci U S A. 2016;113:13857–62. doi: 10.1073/pnas.1615396113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Mueller LP, Luetzkendorf J, Widder M, Nerger K, Caysa H, Mueller T. TRAIL-transduced multipotent mesenchymal stromal cells (TRAIL-MSC) overcome TRAIL resistance in selected CRC cell lines in vitro and in vivo. Cancer Gene Ther. 2011;18:229–39. doi: 10.1038/cgt.2010.68. [DOI] [PubMed] [Google Scholar]
- 491.Wang Z, Chen H, Wang P, Zhou M, Li G, Hu Z, Hu Q, Zhao J, Liu X, Wu L, Liang D. Site-Specific Integration of TRAIL in iPSC-Derived Mesenchymal Stem Cells for Targeted Cancer Therapy. Stem Cells Transl Med. 2022;11:297–309. doi: 10.1093/stcltm/szab031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Li X, Liu LL, Yao JL, Wang K, Ai H: Human Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles Inhibit Endometrial Cancer Cell Proliferation and Migration through Delivery of Exogenous miR-302a. Stem Cells Int 2019, 2019:8108576. [DOI] [PMC free article] [PubMed]
- 493.Lang FM, Hossain A, Gumin J, Momin EN, Shimizu Y, Ledbetter D, Shahar T, Yamashita S, Parker Kerrigan B, Fueyo J, et al. Mesenchymal stem cells as natural biofactories for exosomes carrying miR-124a in the treatment of gliomas. Neuro Oncol. 2018;20:380–90. doi: 10.1093/neuonc/nox152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Weng ZJ, Zhang BW, Wu CZ, Yu FY, Han B, Li B, Li LJ. Therapeutic roles of mesenchymal stem cell-derived extracellular vesicles in cancer. Journal of Hematology & Oncology 2021, 14. [DOI] [PMC free article] [PubMed]
- 495.Layek B, Shetty M, Nethi SK, Sehgal D, Starr TK, Prabha S. Mesenchymal Stem Cells As Guideposts for Nanoparticle-Mediated Targeted Drug Delivery in Ovarian Cancer. Cancers (Basel) 2020, 12. [DOI] [PMC free article] [PubMed]
- 496.Layek B, Sadhukha T, Panyam J, Prabha S. Nano-Engineered Mesenchymal Stem Cells Increase Therapeutic Efficacy of Anticancer Drug Through True Active Tumor Targeting. Mol Cancer Ther. 2018;17:1196–206. doi: 10.1158/1535-7163.MCT-17-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Wang X, Gao J, Ouyang X, Wang J, Sun X, Lv Y. Mesenchymal stem cells loaded with paclitaxel-poly(lactic-co-glycolic acid) nanoparticles for glioma-targeting therapy. Int J Nanomedicine. 2018;13:5231–48. doi: 10.2147/IJN.S167142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Guerra DAP, Paiva AE, Sena IFG, Azevedo PO, Silva WN, Mintz A, Birbrair A. Targeting glioblastoma-derived pericytes improves chemotherapeutic outcome. Angiogenesis. 2018;21:667–75. doi: 10.1007/s10456-018-9621-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Ruan J, Luo M, Wang C, Fan L, Yang SN, Cardenas M, Geng H, Leonard JP, Melnick A, Cerchietti L, Hajjar KA. Imatinib disrupts lymphoma angiogenesis by targeting vascular pericytes. Blood. 2013;121:5192–202. doi: 10.1182/blood-2013-03-490763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Chute JP, Himburg HA. Imatinib tackles lymphoma via the PDGFR beta(+) pericyte. Blood. 2013;121:5107–8. doi: 10.1182/blood-2013-05-501205. [DOI] [PubMed] [Google Scholar]
- 501.Reilley MJ, Bailey A, Subbiah V, Janku F, Naing A, Falchook G, Karp D, Piha-Paul S, Tsimberidou A, Fu S, et al. Phase I clinical trial of combination imatinib and ipilimumab in patients with advanced malignancies. J Immunother Cancer. 2017;5:35. doi: 10.1186/s40425-017-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Blay J-Y, Shen L, Kang Y-K, Rutkowski P, Qin S, Nosov D, Wan D, Trent J, Srimuninnimit V, Pápai Z, et al. Nilotinib versus imatinib as first-line therapy for patients with unresectable or metastatic gastrointestinal stromal tumours (ENESTg1): a randomised phase 3 trial. Lancet Oncol. 2015;16:550–60. doi: 10.1016/S1470-2045(15)70105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Sennino B. Two is better than one: benefits of VEGF and PDGF inhibition in ovarian cancer. Cancer Biol Ther. 2010;9:183–5. doi: 10.4161/cbt.9.3.11117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.