Abstract
Incubation of INT407 cells with various clinical isolates of Campylobacter jejuni resulted in secretion of interleukin-8 (IL-8) at levels ranging from 96 to 554 pg/ml at 24 h. The strains which produced the highest levels of IL-8 secretion were 81-176 and BT44. Induction of IL-8 secretion required live cells of 81-176 and was dependent on de novo protein synthesis. Site-specific mutants of 81-176, which were previously shown to be defective in adherence and invasion, resulted in reduced levels of secretion of IL-8, and cheY mutants of strains 81-176 and 749, which are hyperadherent and hyperinvasive, resulted in higher levels of IL-8 secretion. Another mutant of 81-176, which adheres at about 43% of the wild-type levels but is noninvasive, also showed marked reduction in IL-8 levels, suggesting that invasion is necessary for high levels of IL-8 secretion. When gentamicin was added to INT407 cells at 2 h after infection with 81-176, IL-8 secretion 22 h later was equivalent to that of controls without gentamicin, suggesting that the events which trigger induction and release of IL-8 occur early in the interactions of bacteria and eukaryotic cells.
Campylobacter jejuni is among the most frequently isolated causes of bacterial diarrhea worldwide (8, 37, 38). The diarrhea seen with campylobacters is usually of low volume and often is accompanied by occult or frank blood in stools. Human feeding studies have confirmed the importance of inflammation in the pathology of the disease (7). In those studies, done with two strains of C. jejuni, 81-176 and A3249, fever preceded diarrhea in most patients and all persons who became ill had fecal leukocytes. Moreover, rectal biopsy specimens showed inflammatory cells and edema. Little is understood about the mechanisms of campylobacter pathogenesis other than observations that motility and chemotaxis are absolutely required for campylobacters to colonize animals (9, 32). Many strains of campylobacters are invasive in vitro (17, 22, 23, 30, 40), and mutants defective in invasion have been shown to be reduced in virulence in a ferret diarrheal disease model (43). Motility and chemotaxis are also necessary for invasion in vitro (17, 40, 42, 43). There are numerous reports of cytotoxins in campylobacters, but only one, the cytolethal distending toxin (34), has been characterized in detail. Although this toxin has been shown to inhibit eukaryotic target cells in the G2 phase (41), the role of the cytolethal distending toxin in virulence in vivo has not been reported.
There are numerous reports on the ability of different pathogens to elicit proinflammatory cytokine release in tissue culture systems (1, 11, 14, 15, 19, 20, 35, 36). Most often, cytokine release requires invasion of the eukaryotic cell by the bacterium (14, 15), but there are exceptions (19, 35, 36). Helicobacter pylori, the primary cause of active chronic gastritis in humans, is known to induce interleukin-8 (IL-8) release from a variety of epithelial cells in vitro (19, 35). This ability of H. pylori to induce IL-8, a potent chemoattractant and cellular activator, is considered a major virulence determinant. One study of IL-8 induction by H. pylori in a gastric epithelial cell line also showed that C. jejuni 81-176 could induce some IL-8 secretion (35). In this study, we demonstrate that many strains of Campylobacter spp. can induce secretion of IL-8 by the intestinal epithelial cell line INT407. Moreover, the strains which produce the highest levels of IL-8 are the more invasive strains in vitro, and adherence and/or invasiveness of C. jejuni appears to be associated with induction of IL-8 secretion.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
For the bacterial strains used in this study, see Table 1. C. jejuni cells were routinely grown on Mueller-Hinton (MH) agar (Difco) under microaerobic conditions or in biphasic MH cultures; kanamycin was added to a final concentration of 50 μg/ml when appropriate. Escherichia coli DH5α was grown on Luria-Bertani medium.
TABLE 1.
Secretion of IL-8 by INT407 cells after exposure to C. jejuni for 24 ha
| Strain | Serotype
|
Origin (reference) | IL-8 (pg/ml)c | Invasion (%)d | |
|---|---|---|---|---|---|
| Lior | Pennerb | ||||
| 81-176 | 6 | 23, 36 | United States (7, 24) | 554 ± 156 | 2.1 ± 0.62 |
| BT44 | 9 | ND | Thailand (28) | 525 ± 113 | 0.41 ± 0.11 |
| PC72 | 4 | 02 | Canada (4) | 236 ± 81 | 0.01 ± 0.002 |
| BT43 | 11 | ND | Thailand (28) | 214 ± 22 | 0.007 ± 0.003 |
| MK104 | 4 | 19 | Canada (3) | 193 ± 128 | 0.0013 ± 0.000014 |
| 749 | 36 | ND | Egypt (6) | 161 ± 15 | 0.12 ± 0.03 |
| MSC57360 | 2 | 01 | Canada (5) | 96 ± 18 | 0.02 ± 0.004 |
Control experiments with E. coli DH5α resulted in secretion of 42.2 ± 16 pg of IL-8 per ml in 24 h.
ND, not determined.
Values are means and standard deviations from four experiments. For all values, P was <0.05 compared to a control experiment with E. coli DH5α.
Values are means and standard deviations from three experiments.
Cell cultures.
Human embryo intestinal epithelial (INT407) cells were maintained in minimal essential medium (MEM) supplemented with 5% fetal bovine serum and 0.5% l-glutamine (Gibco, Gaithersburg, Md.). INT407 cells were grown to a confluent monolayer in an 80-cm flask, washed, and released with trypsin-EDTA. The cells were diluted 1:39 in MEM plus fetal bovine serum and l-glutamine and seeded at 1 ml per well in 24-well plates. The monolayer was allowed to re-form during overnight incubation at 37°C.
Assay for IL-8 secretion.
Bacteria were added to the INT407 monolayers, gently shaken (2,500 rpm for 2 min), centrifuged at 1,000 rpm in a Sorvall RT600D centrifuge for 5 min, and incubated for various times at 37°C. Culture medium was then harvested and stored at −70°C until analyzed for IL-8 protein by enzyme-linked immunosorbent assay (ELISA). Phorbol ester (phorbol myristate acetate) and calcium ionophore (A23187) were each added to a final concentration of 100 ng/ml as positive controls.
Nunc Maxi-sorp plates were coated with 3 ng of rabbit anti-human IL-8 (Endogen, Cambridge, Mass.) per well overnight at 4°C. The plates were washed three times with phosphate-buffered saline (PBS) (pH 7.4) plus 0.1% Tween 20 (PBS-Tween) and then blocked with 3 mg of bovine serum albumin per ml in PBS-Tween for 1 h at 37°C. Culture supernatants were diluted 1:1 in PBS plus 3% bovine serum albumin and added to blocked and washed ELISA plates. Samples were incubated on the plates for 90 min at 37°C. Following five washes with PBS-Tween, biotin-linked anti-human IL-8 (0.5 μg/ml) was added to the plates and they were incubated at 37°C for 90 min. Avidin-peroxidase (500 μg/ml) (Gibco BRL) was added to the ELISA plates following five washes with PBS-Tween. The assay was developed with TMB (3,3′,5,5′-tetramethylbenzidine; Sigma, St. Louis, Mo.). Following 20-min incubations at room temperature, the A405 of reaction wells were determined in an ELISA plate reader.
Fractionation of C. jejuni cells.
Campylobacters were separated into membrane and soluble fractions by a modification of the method of Logan and Trust (26). Bacteria were harvested from confluent MH agar plates such that wet pellets weighed between 1 and 3 g. Bacterial pellets were resuspended in cold 20 mM Tris-HCl, pH 7.4. The suspension was supplemented with RNase and DNase and sonicated on ice. Whole cells were removed by centrifugation at 4,000 × g for 30 min. Cell membranes were sedimented via centrifugation at 40,000 × g for 30 min, and the supernatant (soluble fraction) was frozen at −20°C. The membrane pellet was washed three times in 20 mM Tris-HCl, pH 7.4, and resuspended in a final volume of 250 μl of the same buffer. Protein concentrations were determined via Bio-Rad protein assay, and fractions were adjusted to 1 mg/ml with 20 mM Tris-HCl, pH 7.4.
Formalin inactivation of C. jejuni cells.
C. jejuni cells were grown in biphasic MH culture flasks, washed in PBS, and resuspended to 1/10 of the original volume in 0.025 M formaldehyde. Following incubation at room temperature for 6 h, the cells were washed extensively in PBS and the optical density at 600 nm was adjusted to correspond to approximately 1.4 × 106 cells/μl.
Invasion assays.
Invasion assays were performed with INT407 monolayers and a slight modification of the procedure previously described (30, 42, 43). Typically, approximately 6 × 106 bacterial cells were added to a monolayer consisting of about 7 × 104 epithelial cells (about 100 bacteria/epithelial cell). Following centrifugation at 200 × g for 5 min, the assay mixtures were incubated for 2, 4, 8, or 24 h at 37°C in 5% CO2. Following the incubation period, monolayers were washed four times with strong agitation in Hanks balanced salt solution (HBSS). Gentamicin was added to a final concentration of 100 μg/ml, and the monolayers were reincubated for 2 h to kill extracellular bacteria. Following additional washes with HBSS, the epithelial cells were lysed with 0.01% Triton X-100 and the internalized bacteria were enumerated by plate count.
Natural transformation.
The cheY mutation in strain 81-176, as originally described (43), was moved into strain 749 by natural transformation with DNA from RY209 (43), as described previously (2, 18).
Statistical analyses.
Experimental results from independent tests were presented as mean IL-8 induction (in picograms per milliliter) ± 1 standard deviation. Mean IL-8 values were compared by using two-tailed t tests; sample variance determinations were based upon F-test analysis.
RESULTS
INT407 cells secrete IL-8 after exposure to C. jejuni 81-176.
Preliminary experiments indicated that exposure of INT407 cells to C. jejuni 81-176 resulted in secretion of IL-8 (data not shown). Different ratios of bacteria to INT407 cells were used to determine the time course of induction and to optimize the assay. Figure 1 shows that secretion of IL-8 was detected at the earliest time point tested (8 h) and that it continued to rise through 24 h, at which time >500 pg of IL-8 per ml was detected. Secretion of IL-8 was apparently dose related, since the amount secreted at all time points increased as the ratio of bacteria to INT407 cells increased to the maximum tested (100:1).
FIG. 1.
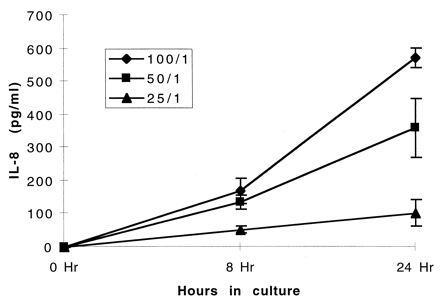
Kinetics of IL-8 secretion by INT407 monolayers after inoculation with C. jejuni 81-176. The epithelial monolayers were inoculated such that ratios of Campylobacter to monolayer cells of 100:1, 50:1, and 25:1 were obtained. Culture supernatants were assayed at 8 and 24 h for IL-8 via ELISA (Endogen). Values are means and standard deviations from two experiments.
Secretion of IL-8 by other strains of C. jejuni.
A number of other clinical isolates of C. jejuni were tested for the ability to induce IL-8 secretion by INT407 cells by using a 100:1 ratio of bacteria to epithelial cells. The results, shown in Table 1, indicated that there was considerable variability in the levels of IL-8 released following exposure to the different strains, and strains 81-176 and BT44, an isolate from Thailand, produced the highest levels of the strains tested (>500 pg/ml). Most strains induced secretion of IL-8 of between 160 and 236 pg/ml. The lowest level of IL-8 induced was by MSC57360, the type strain of the O:1 serotype (5), which produced only 96 ± 18 pg/ml. Exposure to the negative control, E. coli DH5α, resulted in secretion of 42.2 ± 16 pg of IL-8 per ml. INT407 cells incubated with phorbol ester (phorbol myristate acetate) and calcium ionophore (A23187) produced 4,461 ± 452 pg of IL-8 per ml at 24 h.
IL-8 secretion requires live, intact bacteria and de novo protein synthesis.
To determine if living cells were required to induce secretion of IL-8, 81-176 cells were inactivated with formalin, as described in Materials and Methods, and these killed cells were used in the IL-8 assay at the same concentration as live cells. The results, summarized in Fig. 2, indicate that the levels of IL-8 secreted following exposure to the formalin-fixed cells (40.5 ± 21 pg/ml) are similar to those with the medium control (64.0 ± 43 pg/ml).
FIG. 2.
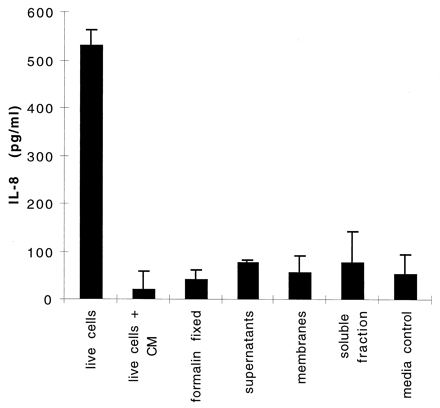
IL-8 secretion by INT407 cells treated with C. jejuni 81-176 cells and fractions. INT407 monolayers were exposed to either whole cells or various fractions of C. jejuni 81-176 cells for 24 h, and IL-8 levels were measured by ELISA. The various samples were live 81-176 cells, live 81-176 cells plus 100 μg of chloramphenicol (CM)/ml, formalin-killed 81-176 cells, 15 μl of filter-sterilized culture supernatants from 18-h biphasic cultures of 81-176 cells, total membrane fraction purified from 81-176 cells (10 μg of total protein), soluble fraction purified from 81-176 cells (10 μg of total protein), and a control of MEM alone. Values are means and standard deviations from two to six experiments.
C. jejuni 81-176 cells were also fractionated into soluble and membrane fractions, and samples of each fraction (10 μg of total protein) were added to INT407 monolayers. The levels of IL-8 detected following addition of the membrane fraction (57.1 ± 34.7 pg/ml) and soluble fraction (76.7 ± 67.8 pg/ml) were similar to that seen with the medium control. Similarly, 15 μl of supernatant from overnight biphasic cultures of 81-176 induced secretion of only 77.8 ± 4.3 pg of IL-8 per ml. Addition of chloramphenicol to the monolayer at a final concentration of 100 μg/ml immediately prior to addition of live 81-176 cells resulted in release of only 21.7 ± 37.6 pg of IL-8 per ml. This represents a >95% reduction in the amount of IL-8 released by live cells without chloramphenicol (531.7 ± 21.7 pg/ml) and indicates that C. jejuni requires de novo protein synthesis to induce IL-8 secretion.
Induction of IL-8 secretion is associated with adherence and/or invasion.
81-176 caused release of the highest levels of IL-8, and this strain invades tissue culture cells at levels which are higher than those of most other strains of campylobacters (30). Moreover, invasion of 81-176 and other strains of C. jejuni in vitro has been shown to require de novo synthesis of proteins (22, 30). To determine if invasion was associated with IL-8 secretion, we compared the invasiveness of the strains listed in Table 1 with the same multiplicity of infection as used in the cytokine assay. The results indicated that the two strains that produce the highest levels of IL-8 secretion are also the most invasive. Thus, 81-176, which invaded at 2.1%, resulted in secretion of 554 pg of IL-8 per ml and BT44, which invaded at 0.4%, resulted in secretion of 525 pg of IL-8 per ml. All of the other strains invaded at levels of ≤0.12% of the inoculum and induced IL-8 secretion of <250 pg/ml, suggesting a trend toward association of invasiveness with the amount of IL-8 secreted, without a strict correlation between the two.
To examine the role of invasion more specifically, various mutants of 81-176 which have been shown to be affected in both adherence and invasiveness in vitro (33, 42, 43) were tested in the IL-8 assay. The results, shown in Table 2, indicated that levels of adherence and/or invasion appear to be associated with the levels of IL-8 secreted. A flaA mutant of 81-176, K2-32, which has been shown to adhere to and invade INT407 cells at 1.5 and 0.5%, respectively, of the level of the wild type (42), induced 18% of the level of IL-8 induced by the wild type. Mutant RY213, which has two copies of the cheY gene and has been shown to be reduced in both adherence and invasion (43), resulted in release of about 30% of the level of IL-8 induced by the wild-type strain. Conversely, RY209, a cheY mutant which has been reported to adhere and invade at about three times the level of 81-176 (43), resulted in a 2.2-fold increase in IL-8 release. Mutant RY303, which is affected in the flagellar motor, has been shown to adhere at about 43% of the level of the wild type but to invade at only 1% of the level of the wild type (42). Interestingly, this mutant caused secretion of IL-8 at levels of about 27% of that of the wild type, similar to that of RY213 and K2-32, suggesting that invasion, rather than adherence, is necessary for IL-8 induction. Another mutation of 81-176 shown to affect adherence and invasion is found in the peb1A mutant described by Pei et al. (33). This mutation, in a gene encoding a protein which has significant sequence similarity to ATP-binding cassette (ABC) transporters, has been reported to cause adherence at 10 to 50% of the level of wild-type 81-176 and invasion at 5.5% of the level of the wild type (33). The peb1A mutant resulted in release of 18% of the level of IL-8 induced by the wild-type strain. Another mutant, RY224, which is unable to form pili under inducing conditions but which remains adherent and invasive in vitro (13), was not affected in IL-8 secretion.
TABLE 2.
IL-8 secretion by INT407 cells after 24 h of exposure to C. jejuni strains
| Strain | Genotype | Relevant features (reference) | IL-8 secretion
|
% of wild typeb
|
||
|---|---|---|---|---|---|---|
| IL-8 (pg/ml)a | % of wild type | Adherence | Invasion | |||
| 81-176 strains | ||||||
| 81-176 | Wild type | 652 ± 34 | 100 | 100 | 100 | |
| K2-32 | flaA | Mot− FlaA− Inv− (42) | 120 ± 48* | 18 | 1.5 | 0.5 |
| RY213 | cheY+ diploid | Mot+ Che+ Inv− (43) | 194 ± 24* | 30 | 7.3 | 4.8 |
| RY303 | pflA | Mot− Che+ Inv− (42) | 175 ± 52* | 27 | 43 | 1 |
| RY224 | pspA | Mot+ Che+ Inv+; nonpiliated (13) | 622 ± 67 | 95 | 103 | 103 |
| RY209 | cheY | Mot+ Che−; hyperinvasive (43) | 1,482 ± 78* | 227 | 305.2 | 284 |
| 81-176 | peb1A | Mot+ Che+ Inv− (33) | 120 ± 25* | 18 | 10–50 | 5.5 |
| 749 strains | ||||||
| 749 | Wild type | 189 ± 44 | 100 | 100 | 100 | |
| 749 (cheY) | cheY | Mot+ Che−; hyperinvasive | 1,148 ± 22* | 607 | 317 | 133 |
The IL-8 levels are means and standard deviations from three or four independent assays. *, P < 0.001 compared to the appropriate wild-type strain.
The percent adherence and invasion for the peb1A mutant are taken from Pei et al. (33), those for the pspA mutant are from Doig et al. (13), and those for all other mutants are from Yao et al. (42, 43); the actual adherence for wild-type 749 was 0.035% ± 0.007%, and the actual invasion for wild-type 749 was 0.10% ± 0.002%.
The cheY mutation was moved by natural transformation from RY209 into another strain of C. jejuni, 749, which showed low levels of both invasion and IL-8 secretion (see Table 1). The resulting mutant showed no zones of chemotaxis on motility agar but was motile by examination of wet mounts in the microscope. This mutant invaded at 13-fold-higher levels (1.37% ± 0.32%) than the 749 parent (0.103% ± 0.002%). The mutant resulted in release of over sixfold more IL-8 than did wild-type 749, as seen in Table 2.
Kinetics of invasion and IL-8 secretion.
The requirement for invasion was examined in more detail for strain 81-176. An assay was done in which bacteria were added to INT407 monolayers in triplicate at a ratio of 100 bacteria per eukaryotic cell. The mixtures were incubated for 2, 4, or 8 h prior to addition of gentamicin or for 24 h without gentamicin. Gentamicin killing was allowed to proceed for 2 h prior to subsequent enumeration of internalized bacteria in one set of wells. In the second set of wells, IL-8 was measured immediately following the 2-h gentamicin kill period. In the third set of wells, the cells were incubated in the presence of gentamicin overnight to allow for maximum production of IL-8. The results, shown in Table 3, indicate that the number of viable internalized bacteria peaked at 8 h (2.5%) but then dropped off by 24 h to only 0.3%, consistent with reports of little to no intracellular replication of campylobacters following invasion (30). IL-8 levels were not detectable when measured immediately after the 2-h gentamicin kill period, and only 29 pg/ml was detected immediately after the 4-h invasion period; this level increased to 78 pg/ml after 8 h of invasion and to 441 pg/ml after 24 h of incubation. Although no IL-8 was detectable immediately following the 2-h invasion period, if the cells were allowed to incubate overnight in the presence of gentamicin, 534 ± 19 pg of IL-8 per ml was detected. Similarly, when gentamicin was added at 4 and 8 h after invasion, followed by overnight incubation, the levels of IL-8 detected rose to 454 ± 30 and 435 ± 34 pg/ml, respectively. Cells not treated with gentamicin produced 441 ± 108 pg of IL-8 per ml after 24 h.
TABLE 3.
Association of invasion and IL-8 induction by C. jejuni 81-176a
| Time of invasion assay (h) | % of inoculum internalized | IL-8 production (pg/ml)
|
|
|---|---|---|---|
| End of invasion assay | Invasion assay + overnight incubation | ||
| 2 | 0.8 ± 0.2 | 0 | 534 ± 19 |
| 4 | 2.0 ± 1.2 | 29 ± 42 | 454 ± 30 |
| 8 | 2.5 ± 1.9 | 78 ± 110 | 435 ± 34 |
| 24 | 0.3 ± 0.07 | 441 ± 108 | ND |
Values are means and standard deviations from two experiments. ND, not done.
DISCUSSION
Recent studies have suggested that IL-8 secretion by epithelial cells may be an early signal for the acute inflammatory response following numerous bacterial infections. Although the data are difficult to compare directly because of differences in cell lines and kinetics of induction, the levels of IL-8 induced by C. jejuni appear to be comparable to those reported for other pathogens. For example, in one survey of different enteric pathogens, IL-8 induction in Caco-2 cells ranged from 115 pg/ml for Shigella dysenteriae to 1,412 pg/ml for Salmonella dublin (20). In the case of most enteric pathogens, IL-8 induction requires invasion (14, 15), although induction by enteroaggregative E. coli appears to require adherence only (36). H. pylori, which is not considered to be an invasive pathogen (10), can also induce IL-8 production in stomach cell lines in vitro, and this ability is thought to play a key role in triggering the disease process. Interestingly, as reported here for C. jejuni, there is considerable variation in the ability of different strains of H. pylori to induce IL-8. This variability in H. pylori is thought to represent differences among strains in virulence potential (35).
The studies reported here demonstrate that C. jejuni can induce secretion of IL-8 from INT407 intestinal epithelial cells, although there appears to be variability in the levels of IL-8 which are released following exposure to different clinical isolates. Those strains which are the highest invaders of intestinal epithelial cells are also those which induce the highest levels of IL-8 release. This association with adherence and/or invasion is most clearly demonstrated with site-specific mutants. Thus, a mutant defective in the major flagellin subunit, K2-32 (42), is reduced in adherence, invasion, and IL-8 induction. Similarly, a mutant, RY213, which contains two copies of a wild-type cheY gene (43), and the peb1A mutant (33) are both reduced in adherence, invasion, and IL-8 induction. Moreover, a cheY mutant of 81-176, which has been shown to be hyperadherent and hyperinvasive (43), shows a similar increase in IL-8 release. Another mutant strain of 81-176, RY303, is defective in a gene presumably involved in the function of the bacterial motor (pflA, or paralyzed flagella) and has been shown to invade at 1% of the level of the wild type but retains the ability to adhere at approximately 43% of the level of the wild type (43). Despite the ability of RY303 to adhere at these relatively high levels, the strain results in secretion of levels of IL-8 similar to those caused by RY213, suggesting that invasion, rather than adherence, is crucial to IL-8 release. This hypothesis is strengthened by the observation that addition of chloramphenicol, which has been shown to eliminate invasion of 81-176 (30), results in loss of IL-8 induction. However, in our hands, addition of chloramphenicol also prevented adherence of 81-176 to INT407 cells (data not shown). Moreover, since there are reports of multiple adhesins in C. jejuni (12, 23, 27, 34), it remains possible that the pflA mutant adheres via a secondary adhesin (perhaps flagellin) which is incapable of triggering IL-8 secretion. Thus, induction of IL-8 might require adherence via a specific bacterial ligand and release of proteins directly into the eukaryotic cell via a process which requires de novo protein synthesis. In addition, most of the mutants examined affect motility and/or chemotaxis, processes which appear to be coordinately regulated with virulence in campylobacters and numerous other pathogens (16, 28, 31, 43). Thus, these mutations could also be affecting other unidentified virulence factors, the expression of which may be coordinately regulated with motility and/or chemotaxis. The reduction in IL-8 induction by the peb1A mutant, which is defective in an adhesin described by Kervella et al. (21), would suggest that this may be the requisite adhesin. However, the insertion in the peb1A mutant is in a gene encoding a protein which resembles a component of an ABC transporter, and the role of this gene product in adherence and invasion remains unclear (33), especially in light of the requirement for de novo protein synthesis.
We have previously reported that changes in the levels of CheY in 81-176 alter adherence and invasion (43), presumably by changes in signal transduction affecting virulence factors which are coordinately regulated with motility and/or chemotaxis. The observation that a cheY mutant of 749 also becomes hyperinvasive and causes increased secretion of IL-8 strengthens previous suggestions that CheY somehow modulates expression of virulence factors. These results also suggest that the generally higher levels of invasion and IL-8 secretion observed for wild-type 81-176 than for most other strains may reflect differences in regulation of common virulence factors. Thus, expression of virulence factors may be repressed in most strains of C. jejuni under in vitro conditions, while those of 81-176 are relatively derepressed under the same growth conditions. Perturbation of signal transduction pathways may derepress expression of virulence factors and result in levels of invasion and IL-8 induction in other strains (such as 749) more comparable to those observed for 81-176.
Collectively, these data suggest that secretion of IL-8 by intestinal epithelial cells exposed to C. jejuni may be the initial signal for the acute inflammatory response. Interestingly, a recent study reported that levels of IL-8 in humans with campylobacter enteritis rose during the acute phase of the disease and fell with recovery (39). Studies are under way to confirm this by using animal models and samples from human volunteers fed 81-176. We are also examining induction of other inflammatory cytokines in intestinal lines following exposure to C. jejuni and are attempting to determine the bacterial components necessary to elicit cytokine induction.
ACKNOWLEDGMENTS
This work was supported by Naval Medical Research and Development Command work no. 61102A3M161102BS13 AK.111.
We thank Lan Fong Lee for helpful discussions.
REFERENCES
- 1.Agace W, Hedges S, Andersson U, Andersson J, Ceska M, Svanborg C. Selective cytokine production by epithelial cells following exposure to Escherichia coli. Infect Immun. 1993;61:602–609. doi: 10.1128/iai.61.2.602-609.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alm R A, Guerry P, Power M E, Lior H, Trust T J. Analysis of the role of flagella in the heat-labile Lior serotyping scheme of thermophilic campylobacters by mutant allele exchange. J Clin Microbiol. 1991;29:2438–2445. doi: 10.1128/jcm.29.11.2438-2445.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aspinall G O, McDonald A G, Pang H. Lipopolysaccharides of Campylobacter jejuni serotype O:19: structures of O antigen chains from the serostrain and two bacterial isolates from patients with the Guillain-Barre syndrome. Biochemistry. 1994;33:250–255. doi: 10.1021/bi00167a033. [DOI] [PubMed] [Google Scholar]
- 4.Aspinall G O, McDonald A G, Raju T S, Pang H, Kurjanczyk L A, Penner J L, Moran A P. Chemical structure of the core region of Campylobacter jejuni serotype O:2 lipopolysaccharide. Eur J Biochem. 1993;213:1029–1037. doi: 10.1111/j.1432-1033.1993.tb17850.x. [DOI] [PubMed] [Google Scholar]
- 5.Aspinall G O, McDonald A G, Raju T S, Pang H, Kurjanczyk L A, Moran A P, Penner J L. Chemical structures of the core regions of Campylobacter jejuni serotypes O:1, O:4, O:23 and O:36 lipopolysaccharides. Eur J Biochem. 1993;213:1017–1027. doi: 10.1111/j.1432-1033.1993.tb17849.x. [DOI] [PubMed] [Google Scholar]
- 6.Baqar S, Bourgeois A L, Applebee L A, Mourad A S, Kleinosky M T, Mohran Z, Murphy J R. Murine intranasal challenge model for the study of Campylobacter pathogenesis and immunity. Infect Immun. 1996;64:4933–4939. doi: 10.1128/iai.64.12.4933-4939.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black R E, Levine M M, Clements M I, Hughes T P, Blaser M J. Experimental Campylobacter jejuni infections in humans. J Infect Dis. 1988;157:472–479. doi: 10.1093/infdis/157.3.472. [DOI] [PubMed] [Google Scholar]
- 8.Butzler J P, Skirrow M B. Campylobacter enteritis. Clin Gastroenterol. 1979;8:737–765. [PubMed] [Google Scholar]
- 9.Caldwell M B, Guerry P, Lee E C, Burans J P, Walker R I. Reversible expression of flagella in Campylobacter jejuni. Infect Immun. 1985;50:941–943. doi: 10.1128/iai.50.3.941-943.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Couthesey-Theulaz I, Porta N, Pringault E, Racine L, Bogdanova A, Draehenbuhl J P, Blum A L, Michetti P. Adhesion of Helicobacter pylori to polarized T84 human intestinal cell monolayers is pH dependent. Infect Immun. 1996;64:3827–3832. doi: 10.1128/iai.64.9.3827-3832.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crowe S E, Alvarez L, Dytoc M, Hunt R H, Muller M, Sherman P, Patel J, Jin Y, Ernst P B. Expression of interleukin 8 and CD54 by human gastric epithelium after Helicobacter pylori infection in vitro. Gastroenterology. 1995;108:65–74. doi: 10.1016/0016-5085(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 12.de Melo M A, Pechère J-C. Identification of Campylobacter jejuni surface proteins that bind to eucaryotic cells in vitro. Infect Immun. 1990;58:1749–1756. doi: 10.1128/iai.58.6.1749-1756.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doig P, Yao R, Burr D H, Guerry P, Trust T J. An environmentally regulated pilus-like appendage involved in Campylobacter pathogenesis. Mol Microbiol. 1996;20:885–894. doi: 10.1111/j.1365-2958.1996.tb02526.x. [DOI] [PubMed] [Google Scholar]
- 14.Eckmann L, Kagnoff M F, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993;61:4569–4574. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fierer J, Eckmann L, Kagnoff M. IL-8 secreted by epithelial cells invaded by bacteria. Infect Agents Dis. 1994;2:255–258. [PubMed] [Google Scholar]
- 16.Gardel C L, Mekalanos J J. Alterations in Vibrio cholerae motility phenotypes correlate with changes in virulence factor expression. Infect Immun. 1996;64:2246–2255. doi: 10.1128/iai.64.6.2246-2255.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grant C C R, Konkel M E, Cieplak W, Jr, Tompkins L S. Role of flagella in adherence, internalization, and translocation of Campylobacter jejuni in nonpolarized and polarized epithelial cell cultures. Infect Immun. 1993;61:1764–1771. doi: 10.1128/iai.61.5.1764-1771.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guerry P, Yao R, Alm R A, Burr D H, Trust T J. Systems of experimental genetics for Campylobacter sp. Methods Enzymol. 1994;235:474–481. doi: 10.1016/0076-6879(94)35163-5. [DOI] [PubMed] [Google Scholar]
- 19.Huang J, O’Toole P W, Doig P, Trust T J. Stimulation of interleukin-8 production in epithelial cell lines by Helicobacter pylori. Infect Immun. 1995;63:1732–1738. doi: 10.1128/iai.63.5.1732-1738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung H C, Eckmann L, Yang S-K, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff M F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Investig. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kervella M, Pagès J-M, Pei Z, Grollier G, Blaser M J, Fauchère J-L. Isolation and characterization of two Campylobacter glycine-extracted proteins that bind to HeLa cell membranes. Infect Immun. 1993;61:3440–3448. doi: 10.1128/iai.61.8.3440-3448.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konkel M E, Cieplak W., Jr Altered synthetic response of Campylobacter jejuni to cocultivation with human epithelial cells is associated with enhanced internalization. Infect Immun. 1992;60:4945–4949. doi: 10.1128/iai.60.11.4945-4949.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konkel M E, Garvis S G, Tipton S L, Anderson D E, Jr, Cieplak W., Jr Identification and molecular cloning of a gene encoding a fibronectin-binding protein (CadF) from Campylobacter jejuni. Mol Microbiol. 1997;24:953–963. doi: 10.1046/j.1365-2958.1997.4031771.x. [DOI] [PubMed] [Google Scholar]
- 24.Konkel M E, Joens L A. Adhesion to and invasion of HEp-2 cells by Campylobacter spp. Infect Immun. 1989;57:2984–2990. doi: 10.1128/iai.57.10.2984-2990.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korlath J A, Osterholm M T, Judy L A, Forfang J C, Robinson R A. A point-source outbreak of campylobacteriosis associated with consumption of raw milk. J Infect Dis. 1985;152:592–596. doi: 10.1093/infdis/152.3.592. [DOI] [PubMed] [Google Scholar]
- 26.Logan S M, Trust T J. Molecular identification of surface protein antigens of Campylobacter jejuni. Infect Immun. 1983;42:675–682. doi: 10.1128/iai.42.2.675-682.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McSweegan E, Walker R I. Identification and characterization of two Campylobacter jejuni adhesins for cellular and mucous substrates. Infect Immun. 1986;53:141–148. doi: 10.1128/iai.53.1.141-148.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mekalanos J J. Environmental signals controlling expression of virulence determinants in bacteria. J Bacteriol. 1992;174:1–7. doi: 10.1128/jb.174.1.1-7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy G S, Jr, Echeverria P, Jackson L R, Arness M K, LeBron C, Pitarangsi C. Ciprofloxacin- and azithromycin-resistant Campylobacter causing travelers’ diarrhea in U.S. troops deployed to Thailand in 1994. Clin Infect Dis. 1996;22:868–869. doi: 10.1093/clinids/22.5.868. [DOI] [PubMed] [Google Scholar]
- 30.Oelschlaeger T A, Guerry P, Kopecko D J. Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc Natl Acad Sci USA. 1993;90:6884–6888. doi: 10.1073/pnas.90.14.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ottemann K M, Miller J F. Roles for motility in bacterial-host interactions. Mol Microbiol. 1997;24:1109–1117. doi: 10.1046/j.1365-2958.1997.4281787.x. [DOI] [PubMed] [Google Scholar]
- 32.Pavlovskis O R, Rollins D M, Haberberger R L, Jr, Green A E, Habash L, Stroko S, Walker R I. Significance of flagella in colonization resistance of rabbits immunized with Campylobacter spp. Infect Immun. 1991;59:2259–2264. doi: 10.1128/iai.59.7.2259-2264.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pei Z, Burucoa C, Grignon B, Baqar S, Huang X-Z, Kopecko D J, Bourgeois A L, Fauchere J-L, Blaser M J. Mutation in the peb1A locus of Campylobacter jejuni reduces interactions with epithelial cells and intestinal colonization of mice. Infect Immun. 1998;66:938–943. doi: 10.1128/iai.66.3.938-943.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pickett C L, Pesci E C, Cottle D L, Russell G, Erdem A N, Zeytin H. Prevalence of cytolethal distending toxin production in Campylobacter jejuni and relatedness of Campylobacter sp. cdtB genes. Infect Immun. 1996;64:2070–2078. doi: 10.1128/iai.64.6.2070-2078.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma S A, Tummuru M K R, Miller G G, Blaser M J. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect Immun. 1995;63:1681–1687. doi: 10.1128/iai.63.5.1681-1687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steiner T S, Lima A A M, Nataro J P, Guerrant R L. Enteroaggregative Escherichia coli produce intestinal inflammation and growth impairment and cause interleukin-8 release from intestinal epithelial cells. J Infect Dis. 1998;177:88–96. doi: 10.1086/513809. [DOI] [PubMed] [Google Scholar]
- 37.Tauxe R V. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations. In: Nachamkin I, Blaser M J, Tompkins L S, editors. Campylobacter jejuni: current status and future trends. Washington, D.C: American Society for Microbiology; 1992. pp. 9–19. [Google Scholar]
- 38.Taylor D N. Campylobacter infections in developing countries. In: Nachamkin I, Blaser M J, Tompkins L S, editors. Campylobacter jejuni: current status and future trends. Washington, D.C: American Society for Microbiology; 1992. pp. 20–30. [Google Scholar]
- 39.Thornley J P, Wright T, Neal K, Jenkins D, Spiller R. A prospective cohort study of campylobacter diarrhoea with the use of fecal inflammatory and leukocyte markers to investigate the resolution of disease. J Med Microbiol. 1998;47:463–470. [Google Scholar]
- 40.Wassenaar T M, Bleumink-Pluym N M C, van der Zeijst B A M. Inactivation of Campylobacter jejuni flagellin genes by homologous recombination demonstrates that flaA but not flaB is required for invasion. EMBO J. 1991;10:2055–2061. doi: 10.1002/j.1460-2075.1991.tb07736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whitehouse C A, Balbo P B, Pesci E C, Cottle D L, Mirabito P M, Pickett C L. Campylobacter jejuni cytolethal distending toxin causes a G2-phase cell cycle block. Infect Immun. 1998;66:1934–1940. doi: 10.1128/iai.66.5.1934-1940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao R, Burr D H, Doig P, Trust T J, Niu H, Guerry P. Isolation of motile and non-motile insertional mutants of Campylobacter jejuni defective in invasion of eukaryotic cells: the role of flagella in invasion. Mol Microbiol. 1994;14:883–893. doi: 10.1111/j.1365-2958.1994.tb01324.x. [DOI] [PubMed] [Google Scholar]
- 43.Yao R, Burr D H, Guerry P. CheY-mediated modulation of Campylobacter jejuni virulence. Mol Microbiol. 1997;23:1021–1032. doi: 10.1046/j.1365-2958.1997.2861650.x. [DOI] [PubMed] [Google Scholar]


