Abstract
Background
Muscle dysfunction is prevalent in dialysis patients. Gait speed and handgrip strength are simple and reliable methods of assessing muscle function. Numerous observational studies have linked 25-hydroxy vitamin D[25(OH)D] status with gait speed and handgrip strength in populations without kidney diseases. This study aimed to evaluate the potential associations of 25(OH)D status with gait speed and handgrip strength in patients on hemodialysis.
Methods
In this observational cross-sectional study, demographic data, biological data, and dialysis parameters were collected. Gait speed and handgrip strength were measured. Multiple linear regression and logistic regression analysis were used to investigate the relationship of 25(OH)D status with gait speed and handgrip strength after adjusting for potential confounders.
Results
Overall, a total of 118 participants undergoing hemodialysis were included. Seventy-one (60.2%) participants were male. The median 25(OH)D status in participants was 11.58 (interquartile range: 8.51 to 15.41) ng/ml. When controlling for age, gender, dialysis vintage, and other confounders with a p-value < 0.15 in univariate analyses, 25(OH)D was significantly positively associated with gait speed (β = 0.16, 95% CI 0.05 to 0.28, p = 0.006) and handgrip strength (β = 3.83, 95% CI 1.09 to 6.56, p = 0.007).
Conclusion
Our study showed that 25(OH)D status seemed to be associated with gait speed and handgrip strength in patients on hemodialysis. However, these results were not robust. The relationships between 25(OH)D status and gait speed and handgrip should be further explored.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12882-022-02973-7.
Keywords: 25-hydroxy vitamin D, Handgrip strength, Gait speed, Hemodialysis
Introduction
Muscle dysfunction is a prevalent condition in dialysis patients [1, 2]. Gait speed and handgrip strength are simple and reliable methods to assess muscle function, which are commonly used [3]. Impaired gait speed and handgrip strength are strongly associated with high mortality in this population [2, 4, 5].
Vitamin D plays a vital role in regulating calcium phosphate homeostasis and mineral bone metabolism; meanwhile, there is emerging evidence that vitamin D is implicated in muscle strength and function [6]. There are several molecular mechanisms of vitamin D’s impact on muscle strength and function [7–9]. Vitamin D modulates muscle cell differentiation, intracellular calcium handling, and genomic activity [6, 9]. The deficiency of vitamin D leads to oxidative stress in skeletal muscle and has an effect on mitochondrial function and the development of skeletal muscle atrophy [8]. Numerous observational studies have linked vitamin D status with gait speed and handgrip in populations without kidney diseases.
Inadequate vitamin D status is common among patients undergoing dialysis [10]. However, the associations of vitamin D status with gait speed and handgrip strength have not been well-established in this population [11, 12]. The relationship between vitamin D status and gait speed was negative according to Kang et al [13]. However, Bucar Pajek et al.[14] found vitamin D status was significantly positively associated with 6-minute walk test results. Some studies focused on the associations between vitamin D status and handgrip strength, and these studies yielded inconsistent results [13–15].
As a result of the conflicting results, this study was conducted to investigate the potential relationship between vitamin D status and gait speed and handgrip strength.
Materials and methods
Study participants
This study was designed as a cross-sectional study recruiting patients on hemodialysis from two centers in China (Beijing Jishuitan Hospital and the Chinese PLA Strategic Support Force Medical Center). Ethical approval of this study was obtained from the local Ethics Committee (Ethics Committee of Beijing Jishuitan Hospital, No. 202104-56). All procedures in the study involving human participants were following the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was designed following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement [16].
Adult patients on hemodialysis (aged 18 years and over) were eligible for study participation if they had been treated with hemodialysis for more than three months and could walk independently. Exclusion criteria were as follows: (a) acute cardiovascular and cerebrovascular disease occurred within a month; (b) joint, muscle, neurological, or vascular and other diseases leading to difficulty in completing the tests; (c) native vitamin D supplementation (ergocalciferol, cholecalciferol or calcifediol) within 3 months; (d) pregnant. All participants provided their written informed consent. Patients all received dialysis three times a week. Dialysate A contained sodium chloride, potassium chloride, calcium chloride, magnesium chloride, glacial acetic acid, and an appropriate amount of dialysis water, and dialysate B contained sodium bicarbonate with an appropriate amount of dialysis water. The dialysate flow rate was 500 mL/min, the blood flow rate was 200 to 280 mL/min, and the dialysis duration was 4 h each time.
Demographic, clinical, and biochemical data
Demographic and clinical data, including age, sex, weight, height, dialysis vintage, causes of end-stage kidney disease, and comorbid conditions were obtained from participant interviews and a review of medical records. We calculated body-mass index (BMI; kg/m²) from dry weight and height.
Blood was collected before and after dialysis. The following biochemical variables were measured: hemoglobin, sodium, potassium, calcium, phosphate, creatinine, urea, intact parathyroid hormone (iPTH), bicarbonate, albumin, and high sensitivity C-reactive protein (hs-CRP) by an autoanalyzer with standard procedures. Albumin-adjusted calcium concentration was calculated using serum albumin and calcium concentrations. The conventional urea-kinetic measure known as single-pool Kt/V (spKt/V) was used to estimate the dialysis dose. In this term, K is dialyzer clearance of urea; t is dialysis time; V is the volume of distribution of urea. The spKt/V was estimated by calculation of Daugirdas single pool equation [17]
spKt/V = -ln(R − 0.008 × t) + (4–3.5R) × UF/W.
where R = post-dialysis/pre-dialysis blood urea nitrogen, t = dialysis hours, UF = pre-post-dialysis weight change, and W = post-dialysis weight.
We used 25-hydroxy vitamin D [25(OH)D] as the marker of vitamin D status. Serum 25(OH)D concentrations were measured in samples taken predialysis by electrochemiluminescence immunoassay (Vitamin D total, Roche Diagnostics, Germany).
Usual gait speed and handgrip strength measurement
Usual gait speed and handgrip strength were measured prior to a midweek dialysis session. Participants’ usual gait speed was assessed using 6-m walking tests [18]. Each participant was asked to complete two trials with a standing start. Participants were instructed to walk at their usual pace until they reach the finish line. One trained interviewer walking alongside the participants recorded the time from the start line to the stop line using a hand-held stopwatch following standardized procedures. Total course time was converted to meters/second (m/s). Participants could use walking aids if necessary but not the assistance of another person [19].
Handgrip strengths, in kilogram-force, were measured using a Jamar dynamometer in the dominant hand or the non-fistula hand if implanted before a dialysis session according to previous studies [4, 20, 21]. The dynamometer was calibrated before each measurement day. The standard positionings recommended are sitting with 90 degrees of elbow flexion [18]. In order to achieve the best performance, patients were instructed to adjust the dynamometer so it fits comfortably to their hand size by an experienced research staff blinded to all clinical and biochemical data of the patients. The arm should be extended sideways from the body, with the dynamometer facing away. Encouragement was given while doing so. Each trial was repeated three times with intervals of 60 s and the maximum value was used [18].
Statistical analysis
Characteristics of the study population were reported as mean ± standard deviation, median and interquartile range, or frequencies, as appropriate. Tests for normality were performed using Skewness and Kurtosis. Skewed continuous variables were logarithmically transformed to ensure normality and better fit a linear relationship between the variables. Bivariate analysis was first performed to evaluate the associations of 25(OH)D with gait speed and handgrip strength. Multiple linear regression analysis was used to investigate the relationship of 25(OH)D with gait speed and handgrip strength. Potential confounders with a p-value < 0.15 in univariate analyses were considered, based on previous studies [22, 23]. Model 1 was unadjusted and only included 25(OH)D. Model 2 included 25(OH)D and demographic variables with a p-value < 0.15 in univariate analyses as confounders. Model 3 included variables in Model 2 plus biological data and dialysis variables with a p-value < 0.15 in univariate analyses. Although 25(OH)D status is influenced by climate and season, laboratory and anthropometry data were collected during the same period. Hence, seasonal variations were not entered in adjusted models. Meanwhile, 25(OH)D status was dichotomized at a cutoff point of 10 ng/mL to assess the consistency of our results based on previous studies [24–26]. According to the Asian Working Group for Sarcopenia (AWGS) consensus [18], low handgrip strength is defined as < 28 kg for men and < 18 kg for women, and low gait speed is defined as 6-m walk speed < 1.0 m/s. By using these cut-off values, handgrip strength was dichotomized as low handgrip strength and normal handgrip strength; while, gait speed was dichotomized as low gait speed and normal gait speed. Then logistic regression analysis was performed to analyze the associations of low handgrip strength and gait speed with the 25(OH)D. A 2-sided P value of 0.05 was considered statistically significant. All analyses were done using STATA 15 (StataCorp LP, College Station, TX).
Results
Participants characteristics
A total of 206 patients were screened in our hemodialysis center. Finally, 118 patients participated in the study. The basic characteristics of the participants are presented in Table 1. In the present study, 71 (60.2%) participants were male. Causes of end-stage kidney disease were diabetes mellitus (31.4%), glomerulonephritis (28.8%), hypertensive nephrosclerosis (16.1%), and others/undetermined nephropathies (23.7%). The median 25(OH)D status was 11.58 (interquartile range: 8.51 to 15.41) ng/ml in participants. In this study, 40 (33.9%) of participants had serum 25(OH)D status less than 10 ng/ml, 66 (55.9%) had 25(OH)D status between 10 and 20 ng/ml, 9 (7.6%) had 25(OH)D status between 20 and 30 ng/ml, and 3 (2.5%) had a 25(OH)D status above 30 ng/ml. The mean gait speed was 0.95 ± 0.22 m/s (0.93 ± 0.2 for males and 0.98 ± 0.22 for females, Supplementary File S1) and the mean handgrip strength was 27.92 ± 9.12 kg (31.96 ± 8.93 for males and 21.68 ± 4.98 for females, Supplementary File S1).
Table 1.
Basic characteristics of study participants
| Parameters | Total | 25(OH)D ≤ 10.0 ng/ml | 25(OH)D > 10.0 ng/ml | P value |
|---|---|---|---|---|
| Men (%) | 71 (60.2%) | 21 (52.5%) | 50 (64.1%) | 0.22 |
| Age, year | 61.4 ± 12.8 | 61.5 ± 12.4 | 61.3 ± 13.0 | 0.93 |
| BMI, kg/m2 | 23.6 (20.8, 26.2) | 24.0 (20.7, 27.1) | 23.5 (20.8, 25.9) | 0.53 |
| Causes of end-stage kidney disease | 0.42 | |||
| Diabetes mellitus | 37 (31.4%) | 16 (40.0%) | 21 (27.0%) | |
| Glomerulonephritis | 34 (28.81%) | 9 (22.5%) | 25 (32.0%) | |
| Hypertensive nephrosclerosis | 19 (16.1%) | 5 (12.5%) | 14 (18.0%) | |
| Others and Undetermined | 28(23.73%) | 10 (25.0%) | 18 (23.1%) | |
| Hemoglobin, g/L | 112.7 ± 12.2 | 112.6 ± 12.1 | 112.8 ± 12.4 | 0.92 |
| Sodium, mmol/L | 140.0 (138.0, 141.9) | 139.2 (138.0, 141.4) | 140.0 (138.0, 142.0) | 0.52 |
| Potassium, mmol/L | 5.02 ± 0.76 | 4.88 ± 0.75 | 5.10 ± 0.76 | 0.14 |
| Phosphate, mmol/L | 1.53 ± 0.44 | 1.53 ± 0.43 | 1.53 ± 0.45 | 0.97 |
| Bicarbonate, mmol/L | 22.71 ± 3.02 | 23.32 ± 2.60 | 22.39 ± 3.18 | 0.11 |
| Albumin-adjusted calcium, mmol/L | 2.20 ± 0.20 | 2.23 ± 0.19 | 2.19 ± 0.21 | 0.28 |
| Albumin,g/L | 40.91 ± 2.67 | 40.89 ± 2.51 | 40.92 ± 2.77 | 0.94 |
| hs-CRP, mg/L | 2.20 (0.99, 6.67) | 1.83 (1.04, 5.42) | 2.39 (0.96, 6.92) | 0.54 |
| Predialysis creatinine, µmol/L | 921.6 ± 217.0 | 854.7 ± 216.5 | 956.0 ± 210.4 | 0.02 |
| Predialysis BUN, mmol/L | 24.9 (22.8, 27.3) | 24.1 (20.8, 26.3) | 25.0 (23.1, 27.7) | 0.13 |
| iPTH, pmol/L | 88.0 (23.2, 200.6) | 100.4 (27.6, 171.0) | 85.2 (22.2, 207.9) | 0.99 |
| 25(OH)D, ng/ml | 11.58 (8.51, 15.41) | 7.49 (5.56, 8.53) | 13.73 (11.59, 17.86) | < 0.001 |
| single-pool Kt/V | 1.43 (1.24, 1.66) | 1.51 (1.29, 1.64) | 1.36 (1.21, 1.66) | 0.46 |
| Dialysis vintage, months | 82 (52,135) | 81 (43,136) | 83 (52,130) | 0.76 |
| Gait speed, m/s | 0.95 ± 0.22 | 0.88 ± 0.21 | 0.99 ± 0.20 | 0.009 |
| Handgrip strength, kg | 27.92 ± 9.12 | 24.79 ± 7.10 | 29.54 ± 9.65 | 0.007 |
| Low gait speed | 69 (58.5%) | 28 (70.0%) | 41 (52.6%) | 0.07 |
| Low handgrip strengh | 28 (23.7%) | 12 (30.0%) | 16 (20.8%) | 0.27 |
Notes: Values are expressed as means ± standard deviation, medians (interquartile range), or numbers (percentages). To convert plasma iPTH in pmol/L to pg/ml multiply by 9.09; to convert 25(OH)D values in ng/ml to nmol/L multiply by 2.5. Abbreviations: BMI, body mass index; BUN, blood urea nitrogen; hs-CRP, high sensitivity C-reactive protein; iPTH, intact parathyroid hormone; 25(OH)D, 25-hydroxyvitamin D
A total of 69 (58.5%) participants had low gait speed and 28 (23.7%) participants had low handgrip strength. We further made comparisons between participants with 25(OH)D ≤ 10.0 ng/ml and 25(OH)D > 10.0 ng/ml. There was a significant difference in predialysis creatinine, gait speed, and handgrip strength between the two groups. In the participants with 25(OH)D ≤ 10.0 ng/ml, 28 (70.0%) had low gait speed and 12 (30.0%) had low handgrip strength. The difference did not reach statistically significant between the two groups.
Associations between 25(OH)D and gait speed and handgrip strength
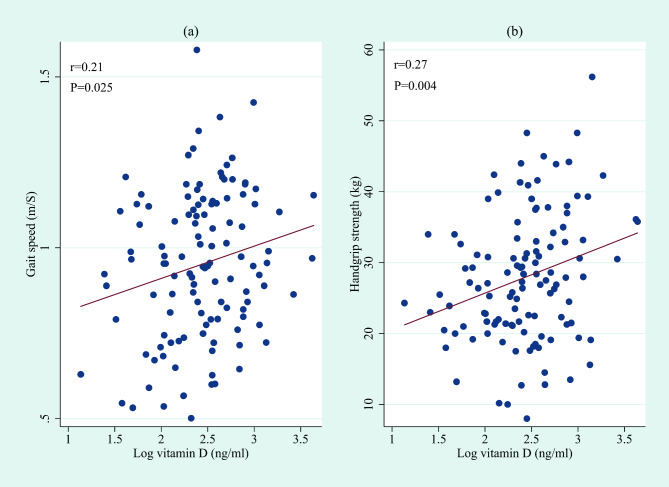
BMI, dialysis vintage, 25(OH)D, high sensitivity C-reactive protein (hs-CRP), sodium, intact parathyroid hormone (iPTH), and spKt/V with skewed distributions were log-transformed. Bivariate analysis showed that log-transformed 25(OH)D status was significantly correlated to gait speed (r = 0.21, p = 0.025, Fig. 1a) and handgrip strength (r = 0.27, p = 0.004, Fig. 1b).
Fig. 1.
The bivariate analysis showed a significant relationship of vitamin D status with gait speed and handgrip strength. (a) Association between 25(OH)D and gait speed. (b) Association between 25(OH)D and handgrip strength. 25(OH)D had positively skewed distributions and were log-transformed
Multiple linear regression analysis was further performed to investigate the associations of 25(OH)D status with gait speed and handgrip strength (Table 2). In the unadjusted model (Model 1), log-transformed 25(OH)D status was positively correlated to gait speed and handgrip strength. For gait speed, age, and BMI were included in Model 2 according to the results of univariate analyses (Details of univariate analyses are given in Supplementary File S2 and S3); for handgrip, age, gender, and BMI were included in Model 2. After adjusting these parameters, significant associations were still found. In Model 3, laboratory and dialysis parameters with a p-value < 0.15 in univariate analyses were additionally adjusted based on Model 2. For gait speed, albumin, potassium, serum creatinine, and iPTH were additionally adjusted on basis of Model 2. For handgrip strength, albumin, bicarbonate, potassium, corrected calcium, hs-CRP, serum creatinine, and spKt/V were identified as additional confounders. After controlling for all the potential confounders, 25(OH)D remained a significant independent predictor of gait speed (β = 0.16, 95% CI 0.05 to 0.28, p = 0.006) and handgrip strength (β = 3.83, 95% CI 1.09 to 6.56, p = 0.007). When 25(OH)D was dichotomized using a cutoff value of 10 ng/ml in the abovementioned models, the conclusion remained unchanged (Supplementary File S4 and S5). In addition, logistic regression analysis was also performed to analyze the association of low handgrip strength and gait speed with dichotomized 25(OH)D. However, the results of logistic regression analysis were nonsignificant (Supplementary File S6 and S7).
Table 2.
Associations of 25(OH)D status with gait speed and handgrip strength: multiple linear regression analysis
| Models | β | 95% CI | Standard error | P Value | |
|---|---|---|---|---|---|
| Gait speed | Model 1 | 0.094** | 0.012 to 0.177 | 0.0417 | 0.025 |
| Model 2 | 0.097** | 0.021 to 0.172 | 0.038 | 0.013 | |
| Model 3 | 0.161** | 0.048 to 0.275 | 0.057 | 0.006 | |
| Handgrip strength | Model 1 | 5.172** | 1.717 to 8.627 | 1.744 | 0.004 |
| Model 2 | 4.956** | 1.851 to 7.291 | 1.373 | 0.001 | |
| Model 3 | 3.825** | 1.093 to 6.556 | 1.378 | 0.007 |
For gait speed, Model 1: unadjusted model; Model 2: age, BMI, and 25(OH)D; Model 3: Model 2 + albumin, potassium, serum creatinine, and iPTH For handgrip strength, Model 1: unadjusted model; Model 2: age, gender, BMI, and 25(OH)D; Model 3: Model 2 + albumin, bicarbonate, potassium, albumin-adjusted calcium, hs-CRP, serum creatinine, and spKt/V Abbreviations: BMI, body mass index; hs-CRP, high sensitivity C-reactive protein; iPTH, intact parathyroid hormone; spKt/V, single-pool Kt/V; 25(OH)D, 25-hydroxyvitamin D; * P < 0.05; ** P < 0.01
Discussion
In this observational cross-sectional study, we investigated the associations of 25(OH)D with gait speed and handgrip strength in patients undergoing hemodialysis. The present study suggested that vitamin D status was an independent predictor of gait speed and handgrip strength when gait speed and handgrip strength as continuous variables.
In the current study, we found that vitamin D status seemed to be positively correlated to the usual gait speed in patients on hemodialysis, which has been confirmed in the general population. In the general population, numerous studies have found a positive association between vitamin D and gait speed [27–29]. One recent meta-analysis included 22 studies and provided robust evidence that circulating vitamin D concentrations were positively associated with usual and fast gait speed in the general population [28]. For patients on hemodialysis, Kand and his colleagues’ study [13] showed that the association between vitamin D and gait speed was not statistically significant. By contrast, another study [14] found that vitamin D status was significantly positively associated with 6-minute walk test results, which was consistent with ours.
Some cross-sectional studies examined the association between handgrip strength and vitamin D status in dialysis patients [13–15], and these studies yielded different results. Our result was similar to the study by Kang et al. who found that serum vitamin D is associated with handgrip in multivariate analysis in patients on hemodialysis [13]. By contrast, Bucar and his colleagues [14] showed that no association existed between vitamin D status and handgrip. In another study, Bataille and his colleagues [15] reported a positive association between plasma vitamin D level and handgrip strength. However, Bataille and his colleagues fail to find a significant association when 25(OH)D was analyzed as a continuous variable. In that study, there was a significant association only when 25(OH)D was analyzed as a dichotomous (<or ≥ 30 ng/mL) variable. The difference might be explained by the different vitamin D statuses of participants. In the study conducted by Bataille et al., the median value was 30.6 (22.5–38.8) ng/ml. In our study, 25(OH)D status was much lower with a median of 11.58 (8.51–15.41) ng/ml and only 3 (2.5%) patients had 25(OH)D status of more than 30 ng/ml. Association might only be true when 25(OH)D was less than 30 ng/ml [15].
Our study found that 25(OH)D was independently related to lower handgrip and gait speed when 25(OH)D was dichotomized into two groups using the cutoff value of 10 ng/ml. For categorical analysis, different cutoff values were used to explore the associations of low 25(OH)D status with health outcomes in chronic kidney disease patients [24–26, 30]. Some studies found that 25(OH)D < 10ng/ml was related to poor outcomes in dialysis patients [24, 25, 31]. So, we adopted the threshold of 10 ng/ml, referring to the previous study [24, 25]. Previous meta-analyses demonstrated a beneficial effect of vitamin D supplementation on muscle strength; this effect is more pronounced in older patients and those with lower 25(OH)D status in the general population [32–34]. Further study should explore the effect of vitamin D supplementation can improve muscle strength, especially in the subgroup with 25(OH)D < 10 ng/ml.
Several mechanisms may explain the link between vitamin D and muscle weakness and poor physical performance. First, vitamin D deficiency may directly affect muscle morphology. Vitamin D deficiency resulted in significant type II muscle fiber atrophy [35]. Moreover, vitamin D deficiency was associated with enlarged interfibrillar spaces and infiltration of fat, fibrosis, and glycogen granules [35]. Vitamin D may also interact with the vitamin D receptor in muscle cells by nongenomic effects. The nongenomic mechanism suggested that the active form of vitamin D acted upon calcium channels on the cell membrane, which in turn led to a rapid influx of calcium into the cell, improving muscle function and contractility [6].
Besides, vitamin D, as a neurosteroid hormone, plays an important role in nervous system function, which may affect gait speed in another way [36]. Vitamin D status was significantly associated with cognitive impairment in both the general population [37] and dialysis patients [38]. Low vitamin D status was associated with worse executive functioning, attention, and nerve conduction velocity, which were involved in gait control [39, 40].
Although some studies have demonstrated the association of vitamin D status with gait speed and handgrip strength, the exact effects of vitamin D supplementation remain uncertain. In a randomized controlled trial [41], participants undergoing hemodialysis were allocated to receive 50,000 IU cholecalciferol or placebo once weekly for eight weeks and then monthly for four months. Over the 6-month follow-up period, no significant differences were found in handgrip strength between the groups. Another self-controlled study [42] included patients on peritoneal dialysis who were prescribed cholecalciferol, 50,000 IU orally once per week for 4–8 weeks. The investigators found that vitamin D supplementation significantly improved handgrip strength from 26.0 to 27.7 kg and gait velocity test from 27.7 ± 3.2 to 25.8 ± 2.9 s. However, the study samples were both relatively small and only 60 and 47 dialysis patients were included, respectively. Furthermore, only 21 patients in the cholecalciferol group (70%) and 24 patients in the placebo group (80%) returned for scheduled 6-month follow-up evaluations in the randomized controlled trial [41]. A high withdrawal rate can lead to bias [43]. The effects of vitamin D on gait speed and handgrip strength still require further exploration.
There were also some limitations in our study. First, the conclusion of this study was not robust. Although the linear regression analysis showed a positive association between 25(OH)D and gait speed and handgrip strength, the logistic analysis yielded nonsignificant results. The nonsignificant results may partially be explained by low power due to the small sample size. Meanwhile, when dichotomizing a continuous outcome to a binary outcome, information is lost and power is reduced [44–46]. Second, when 25(OH)D status was above 30 ng/ml, the association was unknown due to the lower baseline 25(OH)D status in our study. Third, this was an observational study, and patients with native vitamin D supplementation (e.g., ergocalciferol or cholecalciferol) were excluded; consequently, the findings might not indicate a causal relationship. It is still unknown if 25(OH)D is merely a marker or a direct cause of impaired gait speed and handgrip strength. Fourth, a chance finding could not confidently be excluded due to the small sample size. A large-scale randomized controlled study is needed to evaluate the effects of vitamin D administration. Fifth, based on inclusion and exclusion criteria, some patients with certain characteristics were not included. Meanwhile, the participants in our study had a lower 25(OH)D status. Hence, a sampling bias could not be excluded and the study population might not represent all the population of patients on hemodialysis. Sixth, we only examined handgrip strength on the dominant hand, but the maximal handgrip strength may occur on the non-dominant hand. Moreover, data on dietary factors were not collected in our study, and these factors were not adjusted in our regression model. Due to these limitations, we should be cautious about the generalizability of research conclusions.
In conclusion, our study demonstrated that 25(OH)D status was positively associated with gait speed and handgrip strength when gait speed and handgrip strength as continuous variables in patients on hemodialysis. However, further large-scale studies are needed to determine whether a causal relationship exists between 25(OH)D and gait speed and handgrip strength. The exact effects of vitamin D supplementation in dialysis patients should be further explored.
Practical application
25(OH)D seemed to be associated with gait speed and muscle strength in patients on hemodialysis. In patients with lower 25(OH)D status, sarcopenia should be assessed. In future research, whether there is a cause-effect relationship between them needs to be investigated. Once a cause-effect relationship is confirmed, vitamin D supplementation might offer skeletal health benefits in hemodialysis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are extremely grateful to all the patients who took part in this study.
Abbreviations
- BMI
Body mass index.
- BUN
Blood urea nitrogen.
- hs-CRP
High sensitivity C-reactive protein.
- iPTH
intact parathyroid hormone.
- 25(OH)D
25-hydroxyvitamin D.
Authors’ contributions
Research idea and study design: Yongxing Xu, Yuehua Gao; data acquisition: Fengqin Wu, Enhong Han, Chen Fu, Fang Chen; statistical analysis: Yongxing Xu, Chen Fu; interpreted the data: Chen Fu, Yongxing Xu; wrote the paper: Yongxing Xu, Chen Fu, Yuehua Gao; Yongxing Xu had primary responsibility for final content. All authors read and approved the final manuscript.
Funding
Sources of Support: the project is supported by Beijing JST Research Funding (code: ZR-202210).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
All procedures in the study involving human participants were following the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethical approval for this study was obtained from the local Ethics Committee (Ethics Committee of Beijing Jishuitan Hospital, No. 202104-56). All participants provided their written informed consent.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yuehua Gao, Email: kidneygyh@163.com.
Yongxing Xu, Email: xuyongxingstar@163.com.
References
- 1.Bucar Pajek M, Pajek J. Characterization of deficits across the spectrum of motor abilities in dialysis patients and the impact of sarcopenic overweight and obesity. Clin Nutr. 2018;37(3):870–7. doi: 10.1016/j.clnu.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Shu X, Lin T, Wang H, Zhao Y, Jiang T, Peng X, Yue J: Diagnosis, prevalence, and mortality of sarcopenia in dialysis patients: a systematic review and meta-analysis. J Cachexia, Sarcopenia and Muscle. 2022;13(1):145–158. [DOI] [PMC free article] [PubMed]
- 3.Carrero JJ, Johansen KL, Lindholm B, Stenvinkel P, Cuppari L, Avesani CM. Screening for muscle wasting and dysfunction in patients with chronic kidney disease. Kidney Int. 2016;90(1):53–66. doi: 10.1016/j.kint.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 4.Isoyama N, Qureshi AR, Avesani CM, Lindholm B, Barany P, Heimburger O, Cederholm T, Stenvinkel P, Carrero JJ. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin J Am Soc Nephrol. 2014;9(10):1720–8. doi: 10.2215/CJN.10261013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribeiro HS, Neri SGR, Oliveira JS, Bennett PN, Viana JL, Lima RM. Association between sarcopenia and clinical outcomes in chronic kidney disease patients: A systematic review and meta-analysis. Clin Nutr. 2022;41(5):1131–40. doi: 10.1016/j.clnu.2022.03.025. [DOI] [PubMed] [Google Scholar]
- 6.Girgis CM, Clifton-Bligh RJ, Hamrick MW, Holick MF, Gunton JE. The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocr Rev. 2013;34(1):33–83. doi: 10.1210/er.2012-1012. [DOI] [PubMed] [Google Scholar]
- 7.Bollen SE, Bass JJ, Fujita S, Wilkinson D, Hewison M, Atherton PJ. The Vitamin D/Vitamin D receptor (VDR) axis in muscle atrophy and sarcopenia. Cell Signal. 2022;96:110355. doi: 10.1016/j.cellsig.2022.110355. [DOI] [PubMed] [Google Scholar]
- 8.Dzik KP, Kaczor JJ. Mechanisms of vitamin D on skeletal muscle function: oxidative stress, energy metabolism and anabolic state. Eur J Appl Physiol. 2019;119(4):825–39. doi: 10.1007/s00421-019-04104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia M, Seelaender M, Sotiropoulos A, Coletti D, Lancha AH., Jr Vitamin D, muscle recovery, sarcopenia, cachexia, and muscle atrophy. Nutrition. 2019;60:66–9. doi: 10.1016/j.nut.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 10.Jean G, Souberbielle JC, Chazot C. Vitamin D in Chronic Kidney Disease and Dialysis Patients. Nutrients. 2017;9(4). [DOI] [PMC free article] [PubMed]
- 11.Vogt BP, Caramori JCT: Vitamin D and skeletal muscle: A narrative review focusing on chronic kidney disease and dialysis. Hemodialysis international International Symposium on Home Hemodialysis. 2021. [DOI] [PubMed]
- 12.Molina P, Carrero JJ, Bover J, Chauveau P, Mazzaferro S, Torres PU. Vitamin D, a modulator of musculoskeletal health in chronic kidney disease. J cachexia sarcopenia muscle. 2017;8(5):686–701. doi: 10.1002/jcsm.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang SH, Do JY, Cho JH, Jeong HY, Yang DH, Kim JC. Association between Vitamin D Level and Muscle Strength in Patients Undergoing Hemodialysis. Kidney Blood Press Res. 2020;45(3):419–30. doi: 10.1159/000506986. [DOI] [PubMed] [Google Scholar]
- 14.Bucar Pajek M, Cuk I, Mlinsek G, Osredkar J, Pajek J. Physical function and 25-hydroxyvitamin D in dialysis patients - lessons learned from the Slovenian DIAGIB study. Clin Nephrol. 2017;88(13):48–52. doi: 10.5414/CNP88FX12. [DOI] [PubMed] [Google Scholar]
- 15.Bataille S, Landrier JF, Astier J, Giaime P, Sampol J, Sichez H, Ollier J, Gugliotta J, Serveaux M, Cohen J, et al. The “Dose-Effect” Relationship Between 25-Hydroxyvitamin D and Muscle Strength in Hemodialysis Patients Favors a Normal Threshold of 30 ng/mL for Plasma 25-Hydroxyvitamin D. J Ren nutrition: official J Council Ren Nutr Natl Kidney Foundation. 2016;26(1):45–52. doi: 10.1053/j.jrn.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 16.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet (London England) 2007;370(9596):1453–7. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 17.Daugirdas JT. Second generation logarithmic estimates of single-pool variable volume Kt/V: an analysis of error. J Am Soc Nephrology: JASN. 1993;4(5):1205–13. doi: 10.1681/ASN.V451205. [DOI] [PubMed] [Google Scholar]
- 18.Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc. 2020;21(3):300–7.e302. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Krasnoff JB, Basaria S, Pencina MJ, Jasuja GK, Vasan RS, Ulloor J, Zhang A, Coviello A, Kelly-Hayes M, D’Agostino RB, et al. Free testosterone levels are associated with mobility limitation and physical performance in community-dwelling men: the Framingham Offspring Study. J Clin Endocrinol Metab. 2010;95(6):2790–9. doi: 10.1210/jc.2009-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogt BP, Borges MCC, Goes CR, Caramori JCT. Handgrip strength is an independent predictor of all-cause mortality in maintenance dialysis patients. Clin Nutr. 2016;35(6):1429–33. doi: 10.1016/j.clnu.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 21.Lee YH, Kim JS, Jung SW, Hwang HS, Moon JY, Jeong KH, Lee SH, Lee SY, Ko GJ, Lee DY, et al. Gait speed and handgrip strength as predictors of all-cause mortality and cardiovascular events in hemodialysis patients. BMC Nephrol. 2020;21(1):166. doi: 10.1186/s12882-020-01831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sicherer SH, Wood RA, Perry TT, Jones SM, Leung DYM, Henning AK, Dawson P, Burks AW, Lindblad R, Sampson HA. Clinical factors associated with peanut allergy in a high-risk infant cohort. Allergy. 2019;74(11):2199–211. doi: 10.1111/all.13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Vilsteren M, Boot CR, Knol DL, van Schaardenburg D, Voskuyl AE, Steenbeek R, Anema JR. Productivity at work and quality of life in patients with rheumatoid arthritis. BMC Musculoskelet Disord. 2015;16:107. doi: 10.1186/s12891-015-0562-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drechsler C, Verduijn M, Pilz S, Dekker FW, Krediet RT, Ritz E, Wanner C, Boeschoten EW, Brandenburg V: Vitamin D status and clinical outcomes in incident dialysis patients: results from the NECOSAD study. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2011;26(3):1024–1032. [DOI] [PubMed]
- 25.Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, Steele D, Chang Y, Camargo CA, Jr, Tonelli M, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72(8):1004–13. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 26.Hsu HJ, Wu IW, Hsu KH, Sun CY, Chen CY, Lee CC. Vitamin D deficiency, cardiothoracic ratio, and long-term mortality in hemodialysis patients. Sci Rep. 2020;10(1):7533. doi: 10.1038/s41598-020-64359-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaes AMM, Brouwer-Brolsma EM, Toussaint N, de Regt M, Tieland M, van Loon LJC, de Groot L: The association between 25-hydroxyvitamin D concentration, physical performance and frailty status in older adults. European J Nutrition. 2018. [DOI] [PMC free article] [PubMed]
- 28.Annweiler C, Henni S, Walrand S, Montero-Odasso M, Duque G, Duval GT. Vitamin D and walking speed in older adults: Systematic review and meta-analysis. Maturitas. 2017;106:8–25. doi: 10.1016/j.maturitas.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Mendes J, Santos A, Borges N, Afonso C, Moreira P, Padrão P, Negrão R, Amaral TF. Vitamin D status and functional parameters: A cross-sectional study in an older population. PLoS ONE. 2018;13(8):e0201840. doi: 10.1371/journal.pone.0201840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pilz S, Iodice S, Zittermann A, Grant WB, Gandini S. Vitamin D status and mortality risk in CKD: a meta-analysis of prospective studies. Am J kidney diseases: official J Natl Kidney Foundation. 2011;58(3):374–82. doi: 10.1053/j.ajkd.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 31.Drechsler C, Pilz S, Obermayer-Pietsch B, Verduijn M, Tomaschitz A, Krane V, Espe K, Dekker F, Brandenburg V, März W, et al. Vitamin D deficiency is associated with sudden cardiac death, combined cardiovascular events, and mortality in haemodialysis patients. Eur Heart J. 2010;31(18):2253–61. doi: 10.1093/eurheartj/ehq246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beaudart C, Buckinx F, Rabenda V, Gillain S, Cavalier E, Slomian J, Petermans J, Reginster JY, Bruyère O. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2014;99(11):4336–45. doi: 10.1210/jc.2014-1742. [DOI] [PubMed] [Google Scholar]
- 33.Halfon M, Phan O, Teta D. Vitamin D: a review on its effects on muscle strength, the risk of fall, and frailty. Biomed Res Int. 2015;2015:953241. doi: 10.1155/2015/953241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muir SW, Montero-Odasso M. Effect of vitamin D supplementation on muscle strength, gait and balance in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2011;59(12):2291–300. doi: 10.1111/j.1532-5415.2011.03733.x. [DOI] [PubMed] [Google Scholar]
- 35.Ceglia L, Harris SS. Vitamin D and its role in skeletal muscle. Calcif Tissue Int. 2013;92(2):151–62. doi: 10.1007/s00223-012-9645-y. [DOI] [PubMed] [Google Scholar]
- 36.Landel V, Annweiler C, Millet P, Morello M, Feron F. Vitamin D, Cognition and Alzheimer’s Disease: The Therapeutic Benefit is in the D-Tails. J Alzheimer’s disease: JAD. 2016;53(2):419–44. doi: 10.3233/JAD-150943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller JW, Harvey DJ, Beckett LA, Green R, Farias ST, Reed BR, Olichney JM, Mungas DM, DeCarli C. Vitamin D Status and Rates of Cognitive Decline in a Multiethnic Cohort of Older Adults. JAMA Neurol. 2015;72(11):1295–303. doi: 10.1001/jamaneurol.2015.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaffi K, Tighiouart H, Scott T, Lou K, Drew D, Weiner D, Sarnak M. Low 25-hydroxyvitamin D levels and cognitive impairment in hemodialysis patients. Clin J Am Soc Nephrol. 2013;8(6):979–86. doi: 10.2215/CJN.10651012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buell JS, Scott TM, Dawson-Hughes B, Dallal GE, Rosenberg IH, Folstein MF, Tucker KL. Vitamin D is associated with cognitive function in elders receiving home health services. The journals of gerontology Series A Biological sciences and medical sciences. 2009;64(8):888–95. doi: 10.1093/gerona/glp032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alamdari A, Mozafari R, Tafakhori A, Faghihi-Kashani S, Hafezi-Nejad N, Sheikhbahaei S, Naderi N, Ebadi M, Esteghamati A. An inverse association between serum vitamin D levels with the presence and severity of impaired nerve conduction velocity and large fiber peripheral neuropathy in diabetic subjects. Neurol sciences: official J Italian Neurol Soc Italian Soc Clin Neurophysiol. 2015;36(7):1121–6. doi: 10.1007/s10072-015-2207-0. [DOI] [PubMed] [Google Scholar]
- 41.Hewitt NA, O’Connor AA, O’Shaughnessy DV, Elder GJ. Effects of cholecalciferol on functional, biochemical, vascular, and quality of life outcomes in hemodialysis patients. Clin J Am Soc Nephrol. 2013;8(7):1143–9. doi: 10.2215/CJN.02840312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taskapan H, Baysal O, Karahan D, Durmus B, Altay Z, Ulutas O. Vitamin D and muscle strength, functional ability and balance in peritoneal dialysis patients with vitamin D deficiency. Clin Nephrol. 2011;76(2):110–6. doi: 10.5414/CN107160. [DOI] [PubMed] [Google Scholar]
- 43.Kestenbaum B, de Boer IH. Cholecalciferol in chronic dialysis patients: not strong enough? Clin J Am Soc Nephrol. 2013;8(7):1064–5. doi: 10.2215/CJN.04760513. [DOI] [PubMed] [Google Scholar]
- 44.Fedorov V, Mannino F, Zhang R. Consequences of dichotomization. Pharm Stat. 2009;8(1):50–61. doi: 10.1002/pst.331. [DOI] [PubMed] [Google Scholar]
- 45.Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ. 2006;332(7549):1080. doi: 10.1136/bmj.332.7549.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang F, Befort CA, Wick J, Gajewski BJ. Unifying the analysis of continuous and categorical measures of weight loss and incorporating group effect: a secondary re-analysis of a large cluster randomized clinical trial using Bayesian approach. BMC Med Res Methodol. 2022;22(1):28. doi: 10.1186/s12874-021-01499-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.