Abstract
Background/Aim: To investigate the association between androgen deprivation therapy (ADT) and changes in the estimated glomerular filtration rate (eGFR) in male Japanese patients with prostate cancer, based on post-hoc analysis of data from a previous prospective study.
Patients and Methods: Among 103 patients with prostate cancer in whom renal function changes were tracked over 5 years, 88 were divided into a group who completed ADT within 3 years (short ADT group; n=47) and a group who continued with ADT for more than 5 years (continuous ADT group; n=41). We compared the groups in terms of the eGFR, calculated based on age and serum creatinine (mg/dl) before ADT initiation and every other year over the next 5 years.
Results: The eGFR decreased by 4.91 and 2.89 ml/min in the short and continuous ADT groups, respectively, over the 5-year period following ADT initiation. The respective decreases in the eGFR were 0.98 and 0.58 ml/min/year. No significant difference in the eGFR was observed between the two groups at any measurement point. Patients treated with ADT showed a decrease in the eGFR of 0.58-0.98 ml/min/year over a 5-year period, which is about twice as high as that of normal Japanese males. No significant difference in the eGFR by ADT duration was observed.
Conclusion: The eGFR decreased by 0.58-0.98 ml/min/year in our ADT patients, which was about twice as high as the rate of decrease in normal Japanese males, and approximately the same as in urine protein-positive male patients. We suggest that a large decrease in the eGFR may not play a role in the development of acute kidney injury. In addition, the duration of ADT does not appear to have a significant effect on the eGFR.
Keywords: Prostate cancer, renal function, androgen deprivation therapy
Prostate cancer has become one of the most prevalent diseases among men in Western countries, and its incidence has also increased in Japan (1). Androgen deprivation therapy (ADT) is the mainstay treatment for localized, locally advanced, and metastatic prostate cancer (2). However, even short-term use of ADT can lead to side-effects, such as osteoporosis, obesity, sarcopenia, changes in lipid levels, and insulin resistance; the risk of diabetes and cardiovascular morbidities is also increased by ADT (3). In addition to these widely recognized side-effects, ADT increases the incidence of acute kidney injury (AKI). Lapi et al. reported that the use of ADT in newly diagnosed patients with non-metastatic prostate cancer was associated with a significantly increased risk of AKI, according to their case–control analysis of medical data extracted from the UK Clinical Practice Research Datalink (linked to the Hospital Episodes Statistics database) (4). They also reported that antiandrogen or estrogen therapy used in combination with ADT significantly increased the incidence of AKI compared to ADT alone. Androgen blockade by gonadotropin-releasing hormone (GnRH) agonists in combination with oral antiandrogens [odds ratio (OR)=4.50], estrogens (OR=4.00), other therapeutics (OR=4.04), and GnRH agonists (OR=1.93) all increased the incidence of AKI. No reports have examined changes in renal function in patients treated by ADT for prostate cancer. Therefore, we conducted a post-hoc study to investigate changes in the renal function of patients who underwent ADT for prostate cancer for over 5 years, using data from a prospective study conducted at our Institute.
Patients and Methods
We had previously conducted a prospective single-arm study of 103 patients with prostate cancer who started ADT between April 2010 and March 2012 at Gunma University Hospital. The primary endpoint was change in bone mineral density. Changes in blood chemistry data (including the serum creatinine level) and side-effects were the secondary endpoints. The present post-hoc study used the data from the prospective study. We enrolled 88 patients in whom changes in renal function from the start of ADT until March 2017 (5 years) were observed. Of the 15 patients in the prospective study excluded from the present investigation, two died from prostate cancer, two died from other cancers, five were transferred, and six were lost to follow-up. We considered the effect of ADT duration on the estimated glomerular filtration rate (eGFR). The 88 patients were divided into a group that completed ADT treatment within 3 years (short ADT group; n=47) and a >5-year ADT treatment group (continuous ADT group). We retrospectively compared the groups in terms of the eGFR, calculated based on age and serum creatinine (mg/dl) before the start of ADT and every other year over the next 5 years (Figure 1). The eGFR was calculated in the prospective study using the Modification of Diet in Renal Disease (MDRD) formula, which has been widely used in other countries (5). In this study, we calculated the eGFR using Matsuo's formula, which is a modified version of the MDRD formula that is more accurate for Japanese populations (6). The formula is as follows:
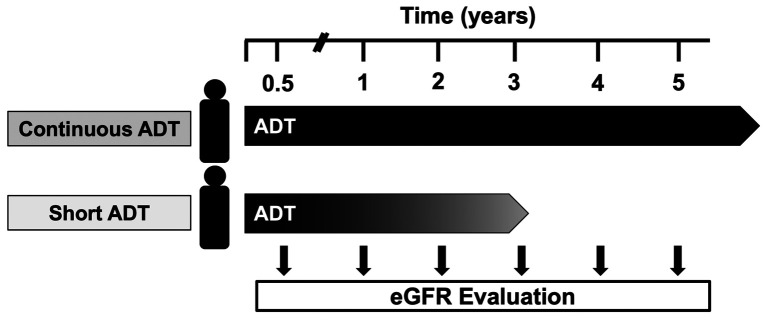
Figure 1. Schematic of the study. The 88 patients enrolled in this study were divided into a short androgen deprivation therapy (ADT) group (<3 years; n=47) and a continuous ADT group (>5 years; n=41). The short ADT group received ADT treatment for an average of 25.4±8.2 months.
eGFR (ml/min/1.73 m2)=194 × serum creatinine (−1.094) × age (−0.287)
SPSS software (ver. 25.0; IBM Corp., Armonk, NY, USA) was used for the statistical analysis. Student's t-test and Wilcoxon rank-sum test were used for statistical analysis in this study, a value of p<0.05 being considered significant. This study was approved by the Ethics Committee for Clinical Studies of Gunma University Hospital (Approval No: 8-5), which also approved the inclusion of patients from other facilities.
Results
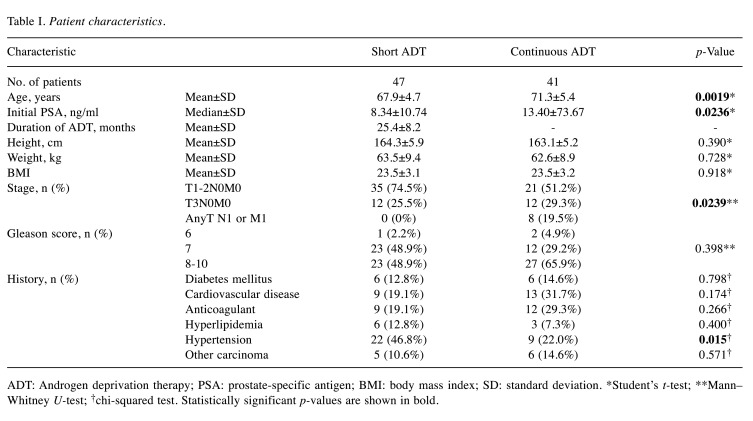
The patient characteristics are shown in Table I. All cases analyzed in this study had an eGFR≥45 ml/min. The mean age of the short ADT group was 67.9±4.7 years and that of the continuous ADT group patients was 71.3±5.4 years. All patients were treated with luteinizing hormone-releasing hormone agonists (leuprorelin or goserelin). The continuous ADT group was significantly older at the start of treatment than the short ADT group (p=0.002, Student’s t-test). All patients in the short ADT group received curative intensity-modulated radiation therapy (75 Gy) and an average of 25.4±8.2 months of ADT treatment. The median prostate-specific antigen (PSA) level in the short ADT group was 8.34±10.7 ng/ml, and that in the continuous ADT group was 13.4±73.67 ng/ml (p=0.024, Student’s t-test). The prostate cancer stage distribution was not significantly different between the short and continuous ADT groups. Significantly more metastatic cases were observed in the continuous ADT group, whereas all cases were of localized cancer in the short ADT group (p=0.024, Mann–Whitney U-test). No significant difference in total Gleason score was observed between the groups (p=0.398, Mann–Whitney U-test). No significant group differences were observed in height, weight, or body mass index. Significantly more patients in the short ADT group had a history of hypertension (p=0.015, chi-squared test). No significant group differences were observed in history of diabetes, heart disease, use of anticoagulants, hyperlipidemia, or the presence of other carcinomas.
Table I. Patient characteristics.
ADT: Androgen deprivation therapy; PSA: prostate-specific antigen; BMI: body mass index; SD: standard deviation. *Student’s t-test; **Mann–Whitney U-test; †chi-squared test. Statistically significant p-values are shown in bold.
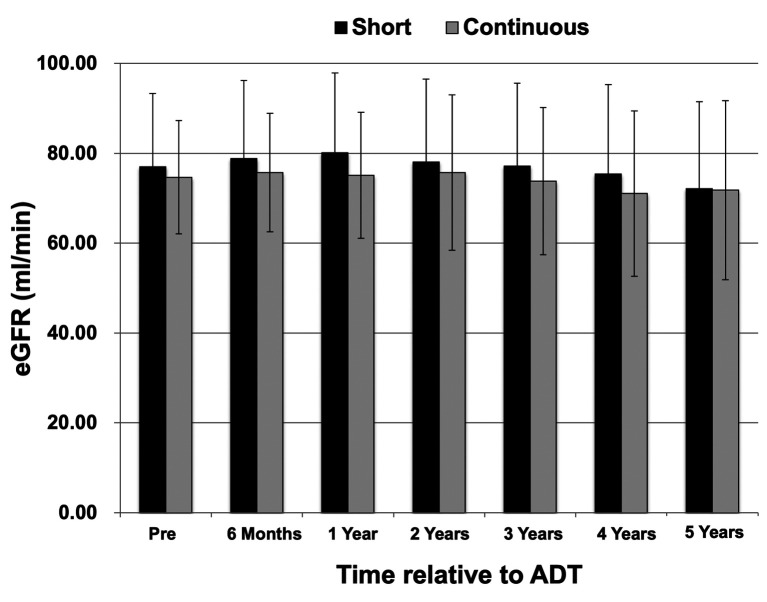
The median eGFR before treatment was 68 ml/min (range=54-75 ml/min) in the short ADT group and 72 ml/min (range=55-81 ml/min) in the continuous ADT group. In both groups, the eGFR at each measurement point before treatment, and at 6 months and 1, 2, 3, 4, and 5 years after treatment are shown in Figure 2. The eGFR decreased by 4.91 ml/min (6.40%) in the short ADT group and by 2.89 ml/min (4.19%) in the continuous ADT group over the 5-year period after the start of treatment. The eGFR decreased by 0.98 ml/min/year in the short ADT group and by 0.58 ml/min/year in the continuous ADT group. No significant group difference in the eGFR was observed at any measurement point. A retrospective check of the medical records revealed no cases in either group of renal dysfunction due to dysuria, such as urinary retention. Moreover, there was no case of AKI in either group.
Figure 2. Estimated glomerular filtration rate (eGFR) over time in the short androgen deprivation therapy (ADT) (<3 years; n=47) and continuous ADT (>5 years; n=41) groups. The eGFR values at each measurement point and before treatment. The eGFR decreased by 4.91 ml/min in the short ADT group and by 2.89 ml/min in the continuous group over the 5-year period following treatment initiation. The eGFR decreased by 0.98 ml/min in the short ADT group and by 0.58 ml/min in the continuous group over the 1-year period following treatment initiation. No significant difference in the eGFR was observed between groups at any measurement point.
Discussion
This is the first study to investigate the association between ADT and changes in eGFR in Japanese patients with prostate cancer. The eGFR did not vary by duration of ADT. ADT has been reported to increase the incidence of cardiovascular disease and metabolic syndrome, which are widely recognized side-effects of this therapy. In a population-based study, Azoulay et al. reported that ADT may increase the risk of stroke/transient ischemic attack (TIA). They reported that current users of GnRH agonists and oral antiandrogens, and patients who underwent bilateral orchiectomy, had a higher risk of stroke/TIA compared to those not undergoing ADT (7). Keating et al. reported that ADT using a GnRH agonist was associated with an increased risk of diabetes and cardiovascular disease. Treatment with a GnRH agonist was associated with a significantly higher risk of incident diabetes [adjusted hazard ratio (aHR)=1.28], incident coronary heart disease (aHR=1.19), myocardial infarction (aHR=1.28), sudden cardiac death (aHR=1.35), and stroke (aHR=1.22) (8). Braga-Basaria et al. suggested that metabolic syndrome is present in more than 50% of patients undergoing ADT for prostate cancer. Additionally, they reported that ADT increased the risk of cardiovascular events, as well as the prevalence of obesity and dyslipidemia (9). Dyslipidemia and hyperglycemia may adversely affect glomerular function by expanding and thickening the interstitial tubular membranes (10). Dyslipidemia is a known risk factor for thrombosis, which increases the risk of AKI in patients with hyperlipidemia undergoing post-cardiac surgery by inducing oxidative stress (10). As mentioned above, many studies have reported on ADT in association with metabolic syndrome and cardiovascular events, but few have determined the relationship between ADT and renal function. In 2013, Lapi et al. conducted the first case–control analysis of ADT based on the medical records of 10,250 patients. They revealed that ADT use was significantly associated with an increased risk of AKI in a cohort of patients with newly diagnosed non-metastatic prostate cancer (4). In 2014, Gandaglia et al. reported that administration of GnRH agonists, but not bilateral orchiectomy, increased the risk of AKI in patients with prostate cancer (11). However, in a letter to the editor, Smith pointed out several problems with the study of Lapi et al. He suggested that AKI is not a known complication of hypogonadism in the general population, and has not been reported as an adverse event in any large randomized study. In addition, he suggested that Lapi et al. did not adequately control for the confounding effects of prostate cancer progression (12). Although Lapi et al. discussed the incidence of AKI (4), no study has reported a change in the eGFR based on serum creatinine in ADT-treated patients with prostate cancer. We agreed with Smith in questioning the development of AKI in patients undergoing ADT, which prompted us to conduct the present study. No significant difference in eGFR was observed between our short (ADT duration <3-year; median, 25.4 months) and continuous ADT groups during the 5-year period following treatment initiation. Regarding the effects of ADT on eGFR, we found that changes in the eGFR were generally reported in elderly men. Imai et al. reported a decline in the eGFR of a general Japanese population in a 10-year follow-up study conducted in 2007. They calculated rates of eGFR decline over 10 years of 0.35, 0.31, 0.37, and 0.42 ml/min/year in those aged 40-49, 50-59, 60-69, and 70-79 years, respectively. In the overall study population, proteinuria occurred in 2.6% of the males. Overall, males with proteinuria had a two-fold higher mean rate of eGFR decline compared to patients without proteinuria (13). The change in eGFR in our ADT-treated patients (0.58-0.98 ml/min/year) was closer to that in patients with proteinuria than in normal men in the report of Imai et al. ADT appears to influence the eGFR to some extent regardless of treatment period. Masuda et al. compared eGFR changes between patients with prostate cancer who underwent 6 months of ADT and those who underwent 12 months of ADT. They reported that both groups had similar reductions in eGFR after the start of ADT, with a trend toward improvement in eGFR at 12 months in the group treated for 6 months, but the reduction remained prolonged in the group treated with ADT for 12 months (14). Although we studied relatively long-term ADT, these reports may be helpful for short-term ADT.
As well as several strengths, our study also had some limitations. Firstly, it was limited by a post-hoc design and small sample size; we were not able to exclude the possibility of selection bias. Furthermore, significant group differences were observed in mean age and PSA levels, and significantly more patients had a history of hypertension in the short ADT group. In addition, the number of patients who showed early progression was significantly higher in the short ADT group. Moreover, all patients in the short ADT group received radiation therapy for curative purposes, and the absence of this therapy in the continuous ADT group may have affected the results. However, no postrenal failure occurred in any case in this study. Secondly, we believe that better comparisons could be made if patients with prostate cancer who did not receive ADT comprised a control group, for example, a group of patients who had undergone radical prostatectomy; this study was unsuitable because it divided the patients into two groups based on the results of the single-arm study. Thirdly, our study was limited by a lack of data on serum testosterone. It is well known that the effects of castration are prolonged after stopping ADT but testosterone recovery in patients in the short ADT group was not assessed. The median duration of ADT treatment in that group was approximately 2 years (median=25.4 months) but no test was performed to determine whether testosterone had recovered after ADT had been discontinued. It was confirmed in all cases that testosterone decreased to castration level 3 months after the start of ADT.
In conclusion, the eGFR decreased by 0.58-0.98 ml/min/y in our ADT patients, which was about twice as high as the rate of decrease in normal Japanese males, and approximately the same as in urine protein-positive male patients. Although the decrease in the eGFR may have been slightly higher than that of normal males, we suggest that a large decrease in the eGFR may not play a role in the development of AKI. In addition, the duration of ADT does not appear to have a significant effect on the eGFR.
Conflicts of Interest
Kazuhiro Suzuki has potential financial conflicts of interest: Consultancies: Takeda Pharmaceutical, Astellas Pharma, Daiichi-Sankyo, AstraZeneca, Sanofi, Janssen, Bayer; Grants received: Takeda Pharmaceutical, Astellas Pharma, Daiichi-Sankyo, Ono Pharmaceutical. All other Authors have declared that no conflicts of interest exist.
Authors’ Contributions
Concept and design: KS, YM; Acquisition of data: YS, SA, YM; Analysis of interpretation of data: YS, SA, YM; Drafting of the manuscripts: YM; Critical revision of the manuscript for important intellectual content: YM; Statistical analysis: YM; Obtaining funding: none; Administrative, technical, or material support: KS; Supervision: KS, YS, SA, MS, HK and HM.
Acknowledgements
The Authors are grateful to Atsuko Ohyama, Hayumi Ohyama and Rie Suzuki (Gunma University Graduate School of Medicine, Department of Urology) for their technical support and collecting samples.
References
- 1.Ito K. Prostate cancer in Asian men. Nat Rev Urol. 2014;11(4):197–212. doi: 10.1038/nrurol.2014.42. [DOI] [PubMed] [Google Scholar]
- 2.Lepor H, Shore ND. LHRH agonists for the treatment of prostate cancer: 2012. Rev Urol. 2012;14(1-2):1–12. [PMC free article] [PubMed] [Google Scholar]
- 3.Isbarn H, Boccon-Gibod L, Carroll PR, Montorsi F, Schulman C, Smith MR, Sternberg CN, Studer UE. Androgen deprivation therapy for the treatment of prostate cancer: consider both benefits and risks. Eur Urol. 2009;55(1):62–75. doi: 10.1016/j.eururo.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lapi F, Azoulay L, Niazi MT, Yin H, Benayoun S, Suissa S. Androgen deprivation therapy and risk of acute kidney injury in patients with prostate cancer. JAMA. 2013;310(3):289–296. doi: 10.1001/jama.2013.8638. [DOI] [PubMed] [Google Scholar]
- 5.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 6.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, Collaborators developing the Japanese equation for estimated GFR Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 7.Azoulay L, Yin H, Benayoun S, Renoux C, Boivin JF, Suissa S. Androgen-deprivation therapy and the risk of stroke in patients with prostate cancer. Eur Urol. 2011;60(6):1244–1250. doi: 10.1016/j.eururo.2011.08.041. [DOI] [PubMed] [Google Scholar]
- 8.Keating NL, O’Malley AJ, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010;102(1):39–46. doi: 10.1093/jnci/djp404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braga-Basaria M, Dobs AS, Muller DC, Carducci MA, John M, Egan J, Basaria S. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol. 2006;24(24):3979–3983. doi: 10.1200/JCO.2006.05.9741. [DOI] [PubMed] [Google Scholar]
- 10.Kambham N, Markowitz GS, Valeri AM, Lin J, D’Agati VD. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59(4):1498–1509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 11.Gandaglia G, Sun M, Hu JC, Novara G, Choueiri TK, Nguyen PL, Schiffmann J, Graefen M, Shariat SF, Abdollah F, Briganti A, Montorsi F, Trinh QD, Karakiewicz PI. Gonadotropin-releasing hormone agonists and acute kidney injury in patients with prostate cancer. Eur Urol. 2014;66(6):1125–1132. doi: 10.1016/j.eururo.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 12.Smith MR. Androgen deprivation therapy and acute kidney injury. JAMA. 2013;310(21):2313. doi: 10.1001/jama.2013.281599. [DOI] [PubMed] [Google Scholar]
- 13.Imai E, Horio M, Yamagata K, Iseki K, Hara S, Ura N, Kiyohara Y, Makino H, Hishida A, Matsuo S. Slower decline of glomerular filtration rate in the Japanese general population: a longitudinal 10-year follow-up study. Hypertens Res. 2008;31(3):433–441. doi: 10.1291/hypres.31.433. [DOI] [PubMed] [Google Scholar]
- 14.Masuda H, Fujimoto A, Kanesaka M, Hou K, Suyama T, Araki K, Kojima S, Naya Y. Renal function improves after the discontinuation of androgen deprivation therapy in japanese patients with prostate cancer. Anticancer Res. 2021;41(9):4443–4446. doi: 10.21873/anticanres.15252. [DOI] [PubMed] [Google Scholar]