Abstract
Tumour hypoxia is a known and extensively researched phenomenon that occurs in both solid and haematological malignancies. As cancer cells proliferate, demand for oxygen can outstrip supply reducing tumour oxygenation. In solid tumours this is contributed to by disorganized blood vessel development. Tumour hypoxia is associated with resistance to treatment, more aggressive disease behaviour and an increased likelihood of metastatic progression. It can be measured using both invasive and non-invasive methods to varying degrees of accuracy. The presence of hypoxia stimulates a complex cellular network of downstream factors including Hypoxia Inducible Factor 1 (HIF1), C-X-C motif chemokine 4 (CXCR4) and Hypoxia‐inducible glycolytic enzyme hexokinase‐2 (HK2) amongst many others. They work by affecting different mechanisms including influencing angiogenesis, treatment resistance, immune surveillance and the ability to metastasize all of which contribute to a more aggressive disease pattern. Tumour hypoxia has been correlated with poorer outcomes and worse prognosis in patients. The correlation between hypoxic microenvironments and poor prognosis has led to an interest in trying to therapeutically target this phenomenon. Various methods have been used to target hypoxic microenvironments. Hypoxia-activated prodrugs (HAPs) are drugs that are only activated within hypoxic environments and these agents have been subject to investigation in several clinical trials. Drugs that target downstream factors of hypoxic environments including HIF inhibitors, mammalian target of rapamycin (mTOR) inhibitors and vascular endothelial growth factor (anti-VEGF) therapies are also in development and being used in combination in clinical trials. Despite promising pre-clinical data, clinical trials of hypoxia targeting strategies have proven challenging. Further understanding of the effect of hypoxia and related molecular mechanisms in human rather than animal models is required to guide novel therapeutic strategies and future trial design. This review will discuss the currently available methods of hypoxia targeting and assessments that may be considered in planning future clinical trials. It will also outline key trials to date in both the solid and haemato-oncology treatment spheres and discuss the limitations that may have impacted on clinical success to date.
Keywords: Hypoxia, Cancer, Haematological, Solid tumours
Background
Hypoxia is a long since recognised and widely agreed upon challenge in cancer medicine. Hypoxia in solid tumours is known to be associated with resistance to chemotherapy and radiotherapy and to promote a more aggressive tumour phenotype contributing to poor patient outcomes. The importance of hypoxia in haematological malignancy is much less studied than in the solid tumour setting, however evidence for the potential importance of bone marrow hypoxia is emerging. Interest in targeting tumour hypoxia to decrease hypoxia-associated treatment resistant mechanisms has existed for many years but has proven challenging. Several different strategies for the targeting of hypoxia have been investigated, including hypoxia activated prodrugs (HAPs) and molecular targeting of hypoxia induced resistance mechanisms. However, uncertainty remains as to the optimal methods to assess tumour hypoxia in human subjects, which contributes to a lack of understanding around which predictive and validated biomarkers of response to hypoxia targeting strategies should be used in the trial setting. Biomarkers assessing hypoxia are not routinely included in these clinical trials investigating hypoxia targeting strategies.
Hypoxia-inducible transcription factors (HIFs)
In response to low oxygen tension, tumour cells activate gene expression programs involved in glucose uptake, metabolism, angiogenesis, proliferation, differentiation and apoptosis. The master regulators to this adaptive response are the hypoxia-inducible transcription factors (HIFs) [1]. Three isoforms of HIFα exist (HIF1α, HIF2α and HIF3α) which differ in structure and function. HIF1α is ubiquitously expressed in cells throughout the body, whereas HIF2α is expressed more abundantly during embryonic development and within vascular endothelium, lung and heart tissue. HIF3α is a repressor of HIF signalling by inhibiting the activity of HIF1/2α. For simplicity henceforth only HIF1/2α are discussed given their role in promoting tumourigenic activity and will be referred to collectively as HIFα. HIFα levels are regulated by the prolyl hydroxylase domains enzymes (PHD 1–3) which, under physiological oxygen tension, hydroxylate HIFα to allow binding of Von Hippel-Lindau (VHL; a tumour suppressor gene), ubiquitination of HIFα and subsequent proteasomal degradation. In lower oxygen tension PHD enzymes are less able to hydroxylate HIFα leading to nuclear translocation and heterodimerisation with HIFβ, and expression of hypoxia response genes via binding to hypoxia-responsive elements (HREs) in their promotor regions. It is the switching on of such gene signatures that improves survival and facilitates proliferation of tumour cells in hypoxic conditions, as well as contributing to angiogenesis, epithelial-to-mesenchymal transition (EMT), avoidance of the immune system and metastatic spread [2]. In addition, increased expression of HIFα causes up regulation of genes involved in glucose metabolism, pH regulation, cellular proliferation and apoptosis, angiogenesis and erythropoiesis [3].
Hypoxia definitions
In its simplest terms, biochemists define hypoxia as a state is which electron transport-mediated cellular metabolism is limited by insufficient oxygen. Tissue hypoxia (be that normal or neoplastic tissue) is perhaps a better description for the phenomenon encountered in 50–60% of all solid tumours and associated with worse patient outcomes, resistance to chemotherapy and radiotherapy, and positively correlates with the extent of metastasis [4–6]. The term hypoxia is often interchangeably used with hypoxaemia which itself defines a blood oxygen partial pressure of less than 80 mmHg (10.6 kPa). Attempts to precisely define tissue hypoxia are hampered by multiple variables including metabolic demand and blood flow rate of the target tissue, arterial oxygen partial pressure, and haemoglobin concentration. It is generally accepted that the critical oxygen partial pressure, at which oxygen consumption is sufficiently reduced to alter intracellular signalling pathways, is 8—10 mmHg [7, 8]. Unregulated tumour growth, rapid cell turnover and invasion through different tissue types drive such hypoxia due to an increased oxygen demand that cannot be compensated by existing vascular access, nor oncogenic angiogenesis. Under normal circumstances hypoxia signalling pathways are activated to allow homeostasis to be achieved, often transiently, under fluctuating metabolic conditions. These signalling’survival’ pathways are hijacked during malignant transformation, the importance of which is underlined by the inclusion of metabolic reprogramming as a fundamental hallmark of cancer [9].
Hypoxia in normal bone marrow
Unlike other normal organs that might provide the site for solid tumour development, bone marrow is considered to be physiologically hypoxic. Direct in vivo measurements of local oxygen tension (pO2) in the bone marrow of live mice have found intravascular pO2 in the range of 15–30 mmHg (mean ~ 23 mmHg, about 3% O2) and extravascular pO2 in the range of 10–25 mmHg (mean ~ 17 mmHg, about 2% O2) despite very high vascular density [10, 11]. Within the bone marrow architecture there are significant variations in the level of hypoxia characterised by two different niches. The endosteal niche is an area closer to the bone. The second area is a more central vascular niche which sits closer to the blood vessels. The endosteal niche is thought to be the most hypoxic area of the bone marrow and contains a higher level of HIF-1α positive cells [12, 13]. Haematopoetic stem cells (HSCs) are mostly found within the endosteal niche suggesting that there may be a role for hypoxia in the stabilisation of HSCs although this view has been challenged [10, 11].
Hypoxia in the pathophysiology of haematological malignancies
The importance of the local hypoxic tumour microenvironment has been studied extensively in solid malignancies and there is emerging evidence to show similar detrimental hypoxia related factors are present within the bone marrow of patients with haematological malignancies.
Animal models have suggested hypoxic bone marrow in mice with multiple myeloma (MM). Comparison of the marrow of control and 5T33MM diseased mice found increased expression HIF1α suggestive of increased hypoxia in the diseased mice [14–16]. This has also been reflected in studies on human bone marrow biopsy specimens where an increased expression of HIF-1α has been demonstrated in the marrow of patients with MM [17–19]. In human subjects, circulating myeloma cells display similar characteristics with higher expression of HIF-1 found when compared to other circulating cells [20].
In acute leukaemia there are studies suggesting that the bone marrow shows a higher degree of hypoxia than in bone marrow without any malignant infiltration and that hypoxia correlates with the degree of infiltration in human and animal samples [21, 22]. Increased levels of the surrogate hypoxia markers HIF1a and Higher Vascular Endothelial Growth Factor A (VEGF-A) have been found when compared to normal bone marrow in samples from patients with Acute Myeloid Leukaemia (AML) [23]. However, HIF expression has also been demonstrated under normal oxygen tension in myeloma, leukaemia and lymphoma cells suggesting that HIF activation may act independently of hypoxia in the setting of haematological malignancy [24]. HIF has been demonstrated to play a role in the survival of cancer stem cells within the bone marrow in both leukaemia and lymphoma [24, 25].
Hypoxia in the pathophysiology of solid tumours
Metastatic spread from tumours represents a major clinical challenge given that it is seen in more than 90% of all cancer-related deaths. Various mechanisms have been characterised by which HIF signalling drives metastatic progression in solid neoplasms. A key early aspect of metastasis is the navigation from the tissue of origin and invasion towards, usually, either the vascular or lymphatic circulation. This is associated with EMT, allowing loss of cell–cell and cell-basement membrane interactions, liberating tumour cells to invade through local structures [26]. Both induction of hypoxia and overexpression of HIF signalling in normoxia can induce EMT and promote local invasion [27, 28]. Capturing information on EMT in the clinical setting has proven challenging given the transient nature of this process and the heterogeneity within tumours, presenting both temporal and spatial obstacles to informative biopsy. There has thus been a focus on liquid biopsies, particularly involving circulating tumour cells (CTCs) to capture EMT gene signatures, although consideration must be given to changes which may occur prior to tumour cells entering the circulation. Cancer therapy affects EMT phenotype of CTCs within breast cancer patients, with those who respond to treatment having more epithelial-like CTCs compared to those with refractory disease who have more mesenchymal-like CTCs [29]. This is in keeping with pre-clinical models of breast and pancreatic cancer highlighting an important role for EMT in chemoresistance [30, 31]. This EMT transition process must be reversible (e.g. cells can transition back to epithelial phenotypes), to also allow cells to extravasate and form metastases. Whether these differences are a cause or consequence of treatment efficacy remains to be delineated.
Escaping detection and targeting by the immune system is key to survival as tumours extend and invade from their tissue of origin. Immunotherapy in the form of checkpoint inhibitors has been an important development in solid oncology in the last decade and remains a key focus for drug development. HIF signalling impacts directly on several key immune cell types, all of which act to promote an immunosuppressive microenvironment [32–35]. T cell receptor (TCR) signal transduction is negatively regulated by HIF1α inhibiting effector T cell-mediated tumour cell targeting [36]. Experiments in both prostate and breast cancer cell lines revealed increased Programmed death-ligand 1 (PD-L1) expression via increased HIF1α expression and subsequent binding at HREs in the PD-L1 promoter in hypoxic (0.5% O2) versus normoxic (20% O2) conditions. This conferred significantly reduced cytotoxic T cell lysis in both a PD-L1 and HIF1α-dependent manner, likely due to interaction with Programmed cell death protein 1 (PD-1) on effector T cells to escape immune detection [37, 38].
In hypoxic conditions, mismatch repair capacity is reduced and leads to a greater level of microsatellite instability. This is in part controlled by HIF signalling at both the transcriptional and translational level, likely as a conserved physiological adaptation to diminished metabolic resource [39, 40]. Inhibition of complimentary repair mechanisms such as with protein poly(ADP-ribose) polymerase-1 (PARP-1) has been hypothesised to generate a synthetic lethal interaction in hypoxic tumour cells [41]. Two phase I clinical trials have investigated the combination of PARP inhibition together with angiogenesis inhibition. The first combined the PARP inhibitor Olaparib with the VEGF inhibitor Bevacizumab in twelve heavily pre-treated patients with advanced refractory solid tumours. Unfortunately, nine of the twelve discontinued due to either disease progression or toxicity to treatment [42]. The second study combined Olaparib with a novel VEGF inhibitor cediranib in 28 patients with either recurrent epithelial ovarian or triple-negative breast cancer. Some putative evidence of efficacy was seen in ovarian cancer patients but 75% of patients discontinued the study due to ≥ grade 3 toxicities [43]. Neither study progressed to later phases of development.
Angiogenesis and hypoxia
Angiogenesis has long been noted to play a role in the pathogenesis and progression of various different types of cancer, with HIF signalling implicated in regulating the process directly for almost as long. The presence of HRE within the VEGF promotor region confirms a direct link but, further, HIF signalling directly or indirectly regulates more than 2% of all genes associated with neovascularisation [44]. Several clinical studies have looked to address this causal relationship via combination therapy in anticancer therapy. Bevacizumab is an established VEGF inhibitor whilst Temsirolimus inhibits mTOR, an upstream regulator of HIF signalling through the PI3K/AKT/mTOR pathway. A phase I clinical trial of these agents in combination with liposomal doxorubicin revealed a 19% objective response rate in 74 breast and gynaecological cancer patients [45]. A larger study using Bevacizumab, Temsirolimus and Sorafenib, an inhibitor of multiple kinases involved in cell proliferation and angiogenesis, found no progression-free survival benefit compared with Bevacizumab monotherapy in 331 advanced renal cell carcinoma patients [46]. A further phase I study combined Bevacizumab with Bortezomib, a proteosome inhibitor which indirectly inhibits HIF signalling through Phosphoinositide 3-kinase (PI3K)/Akt/mTOR deactivation, in 91 patients with advanced refractory solid malignancies. Disappointingly, only 12% of these patients had either an objective response or stable disease at six months [47] and this regimen has not progressed to later phase trials. Importantly, these studies did not include the prospective use of any validated hypoxia or VEGF pathway assays or biomarkers so mechanisms of resistance remain unknown.
Within non-Hodgkin’s lymphoma (NHL), a recent study has compared malignant lymph node biopsies from diagnosis and at recurrence of disease. Reactive lymphadenopathy archival biopsies were analysed as a negative control. The lymphoma cells within lymph node biopsies reviewed at the point of NHL recurrence showed a significantly increased vascular network and higher level of HIF-1a expression suggesting a correlation between angiogenesis, hypoxia and disease progression [48].
Similarly, it has been shown in patients with multiple myeloma that HIF1a and HIF2a were strongly expressed within the myeloma cells alongside an up-regulation of VEGFR. This up-regulation was linked to increased angiogenesis. This was linked to a worse prognosis in MM cases that showed a high vascular density [18]. It is speculated that many of the traditional cytotoxic therapies used to treat patients with multiple myeloma may exert some of their effects through reducing expression of HIF1 (and in turn VEGF) thereby suppressing neo-angiogenesis [49, 50].
Treatment resistance
Evidence exists that hypoxic tumour microenvironments can interfere with the efficacy of traditional chemotherapy agents on tumour activity in both solid and haematological malignancies. It has been shown that hypoxia of bone marrow can lead to arrest of the cell cycle of AML blasts in the G0/G1 phase therefore not reaching the S phase. Cytarabine, a conventional chemotherapy which is the mainstay of much AML treatment, is an S phase dependent drug. When Cytarabine was applied to AML blasts exposed to hypoxic conditions it was shown to have a significantly decreased affect [51]. Hypoxia associated treatment resistance has also been demonstrated in Acute Lymphoblastic Leukaemia (ALL), a study found that blocking HIF1a expression resulted in increased sensitivity to cytotoxic therapy [52].
Several studies within a multiple myeloma population have shown that inhibition of downstream enzymes in the hypoxia pathway can increase susceptibility to cytotoxic therapy. Ikeda et al. demonstrated that exposure to an antibody against hypoxia‐inducible glycolytic enzyme hexokinase‐2 (HK2) increased apoptosis [53]. HK2 has also been found to contribute to an anti-apoptotic effect in myeloma cells whilst in vivo studies have found increased efficacy of the chemotherapy agent melphalan in the presence of an inhibitor of HIF1α [54, 55]. The mechanism by which HIF signalling inhibition sensitises to melphalan therapy remains to be elucidated.
Increased HIF1α expression significantly and inversely correlated with response to Epirubicin therapy [56] and was also shown to be an independent risk factor for resistance to aromatase inhibitor therapy [57] in 187 and 114 oestrogen receptor (ER) positive breast cancer patients, respectively. Histone deacetylation has been shown to stabilise HIF1α as acetylation leads to polyubiquitination and targeting toward proteosomal degradation [58]. Interestingly, histone deacetylase inhibition (HDACi) reduces HIF1α expression through a VHL-independent mechanism [59]. Preclinical work has revealed HDACi can reverse treatment resistance in combination with the VEGF inhibitor pazopanib in sarcoma cell lines [60]. In a phase I trial the HDAC inhibitor Abexinostat was used in combination with Pazopanib in 51 patients with advanced renal cell carcinoma. Tumour regression was seen in seven of 10 patients with previously pazopanib-refractory disease indicating a potential role for HIF signalling in VEGF treatment resistance clinically [61].
HIFα plays a role in chemotherapy resistance through the activation of the multidrug resistance 1 (MDR1) gene in hypoxic conditions. A seven-fold increase in MDR1 was seen via quantitative microarray in epithelial cells exposed to hypoxia [62]. In human lung adenocarcinoma cells under hypoxic stress HIFα and multidrug resistance levels were increased, as was resistance to Adriamycin [63]. Clinically MDR1 is expressed more highly in triple negative breast cancer (TNBC) compared to other breast cancer subtypes which correlates with greater chemoresistance and poorer prognoses [64, 65]. Despite the mounting evidence for targeting multidrug resistance in cancer clinically there has been limited success in either solid or haematological cancers to date [66, 67]. Most of the assessments investigating resistance have been performed retrospectively and examined at the end of trials rather than incorporating prospective biomarkers to understand mechanisms at the outset.
Clinical assessment of hypoxia
There are multiple different methods used for the assessment of hypoxia, each has its advantages and disadvantages, and these are summarised in Table 1. In broad terms these can be broken down into direct methods, tissue-based methods and imaging techniques. The majority of these assessment methods have been investigated in solid malignancies and little evidence is currently available for these methods in haematological malignancies.
Table 1.
Advantages and disadvantages of the methods of assessing hypoxia
| Method | Advantages | Disadvantages |
|---|---|---|
| Oxygen Electrodes |
Around 100 measurements taken- good overview of area No major adverse effects |
Surface lesions only Invasive No repeat measurements Cannot account for necrotic areas- will give discordant results Artifacts-excessive oxygen consumption Technically skilled user and inter-operator variability |
| Phosphorescence quenching |
Real time oxygenation information Readings are independent of tracer concentrations |
Invasive technique Technically skilled user Early in development- limited availability |
| Electron Paramagnetic Resonance oximetry |
Implantable technology- repeated results. Can monitor effects of treatment Absolute pO2 readings |
Invasive- needs a direct probe in situ Early in development- limited availability |
| Endogenous markers |
Not affected by the sampling time or microenvironment It can be correlated within the same sample against other markers of tumour hypoxia |
Cell line specific Can be affected by metabolic factors that vary between cells |
| Dynamic contrast-enhanced magnetic resonance imaging |
Non-invasive Widely available Radiology departments familiar with method and equipped to perform and report imaging Can be repeated to monitor effects of treatment with relative accuracy |
When administered IV mostly absorbed in liver/spleen. Amount in tumours often insufficient to get an accurate reading Cleared within days- limited time period for collecting data When administered into tumour can only read oxygen tension within that area of the tumour Readings significantly affected by temperature |
| Blood-oxygen level dependent magnetic resonance imaging |
Non-invasive Can detect changes in tumour hypoxia over time |
Small movements can lead to poor images and artefact Not a direct measure of oxygenation and therefore independent variables can interfere with measurements |
| Positron emission tomography imaging |
Non-invasive Widely available Familiar method- clinicians and radiology departments used to dealing with images and results Repeated measurements possible Enables the visualization of the hypoxic status of the entire tumour in 3D image |
Varying tracers used result in varying uptake levels and result in some discrimination between hypoxic levels Relatively short half life of tracer means it must be manufactured and imaged within several hours |
| Pimonidazole |
Non-invasive Good prognostic correlation |
Limited availability Requires tumour biopsy after administration of Pimonidazole- tumour needs to be accessible Invasive |
Direct methods
Long considered the gold standard method by many researchers in the field, oxygen electrodes are one of the oldest and most studied methods of direct measurement of hypoxia. The electrodes are polarographic needles inserted directly into a tumour or metastatic lymph node with the purpose of measuring oxygen partial pressure (pO2). They rely on the interaction of oxygen with a sensor on the probe and the method is based on the electro-reduction of oxygen molecules. The sensors measure oxygen at various points along their length and therefore can provide a good overall view of the oxygen levels of the tumour. However, some concern exists as to whether the oxygen electrodes could contribute to seeding of the tumour [68]. There are barriers to the utilisation of oxygen electrodes within the context of a clinical trial, including lack of availability of probes, skilled probe operators, patient acceptability and a reliance on an assessable tumour location. Oxygen electrodes are also particularly ill-suited as a method of hypoxia assessment in haematological malignancies where tumour cells may be predominantly confined within the bone marrow.
Tissue based methods
Tissue based methods of hypoxia assessment all require the removal of a sample of tumour tissue. These are necessarily invasive and reliant on the accessibility of the tumour. However, these methods do allow for the centralisation of hypoxia assessment as part of a clinical trial. Pimonidazole is a 2-nitroimidazole compound which undergoes a nitro reductase catalysed single-electron reduction in the presence of hypoxia. Pimonidazole then binds covalently to cellular compartments in hypoxic cells [69] and can then be detected in poorly oxygenated regions in histological sections from tumours [70]. Pimonidazole has been used to detect hypoxic areas within solid tumours [71–73] and the marrow of AML patients’ populations [74]. Pimonidazole can be safely administered to patients in oral and intravenous forms and after removal of a tumour sample, binding of pimonidazole can be assessed histologically in tumour sections or by flow cytometry using anti-pimonidazole antibodies. As a method of assessing tumour hypoxia, pimonidazole can be considered to give an average of hypoxia during the period of pimonidazole metabolism.
Endogenous markers
There are several endogenous markers that have a role in the assessment of hypoxia within both solid and haematological malignancies. Histological assessment of the levels of these surrogate hypoxia markers is possible using primary antibodies targeted against these proteins. Carbonic anhydrase (CA) is an enzyme that catalyses the reversible hydration of carbon dioxide to carbonic acid. Carbonic anhydrase 9 (CAIX) is strongly induced by hypoxia and has been implicated in hydrogen ion efflux and prevention of cell death in hypoxia [75]. Whilst CAIX has shown some promise of correlation with prognosis in several solid tumours [76, 77], it did not correlate well with other measurements of hypoxia (pimonidazole staining and direct p02 measurements) [78, 79].
Glucose transporter 1 (GLUT-1) is a membrane protein involved in transporting glucose across cell membranes. During hypoxic conditions there is an increased rate of glycolysis and therefore this transporter is up regulated in order to facilitate the increased glucose requirements.
Osteopontin (OPN) is a tumour associated phosphorylated glycoprotein. It is found is a variety of different cells and plays a role in modulating cell adhesion and in angiogenesis. It is known to be upregulated in hypoxic environments [80]. There are several studies which show that osteopontin may act as a surrogate hypoxia marker and therefore as a marker of prognosis in various cancer patient populations [81, 82].
Imaging techniques
Overhauser-enhanced magnetic resonance imaging (OMRI) is essentially a combination of MRI and electron paramagnetic resonance (EPR) methods of assessing for hypoxia which allows for anatomical tissue imaging alongside physiological parameter measurements. Essentially novel contrast medium based on single electron substance allows single enhancement which is influenced by oxygen concentration via low-field MRI scanning. To date this has only been explored in the preclinical setting but offers promise for an accurate measure of tissue hypoxia in cancer patients [83].
Dynamic contrast enhanced MRI can be used to look at perfusion data which in turn can estimate tissue oxygen tension. In both preclinical and initial clinical studies this method has shown a great deal of promise in being able to identify poorly perfused and hypoxic areas of tumour [84, 85]. This is yet to be used prospectively in combination with hypoxia targeting agents.
Blood-oxygen level dependent MRI (BOLD MRI) is a technique used within functional MRI studies which works by relying on the differences in blood flow to determine regional oxygen levels and identify hypoxia. It has been demonstrated to have a high sensitivity to hypoxic regions in the tumours of patients with prostate cancer when compared with both Pimonidazole staining and oxygen electrode readings [71, 86]. Additionally, BOLD-MRI has been shown to reliably yield hypoxic information in patients with breast and cervical cancer [87–89].
Positron emission tomography (PET) imaging is a non-invasive technique which uses radioisotopes to determine the presence of tumour hypoxia. The tracers are given intravenously and the uptake into tissues is caught by using a PET camera. In hypoxic conditions, the tracer is chemically reduced and bonds with thiol-rich proteins and this compound accumulates intracellularly. It has been shown to produce reliable results within cervical cancer and head and neck cancers [90, 91].
Hypoxia targeting strategies
Given that tumour tissues are differentially more hypoxic than their wild type counterparts and hypoxia is associated with increased chemoradiotherapy resistance, hypoxia targeting strategies have been extensively researched in both the pre-clinical and clinical settings. The strategies can be broadly categorised into hypoxia activated prodrugs (HAPs) and drugs that act either up- or down-stream of the HIFα signalling pathway.
Hypoxia-activated Prodrugs (HAPs)
Also known as bio reductive agents, these compounds are selectively reduced under hypoxic conditions to produce activated cytotoxic drugs so have relatively little toxicity to normoxic tissue. The HAPS most extensively investigated in both the preclinical and clinical setting include Tirapazamine (SR-4233), Apaziquone (EO9), PR-104, Banoxantrone (AQ4N) and Evofosfamide (TH-302). They largely exert their cytotoxic effect by interfering with normal DNA replication and, further, tumour cell division and proliferation [92–95]. Evofosfamide is a second-generation HAP and has been of particular interest in recent years. It consists of a dual moiety of bromo-iso-phosphoramide (Br-IPM), a DNA cross-linking mustard prodrug, and 2-nitroimidazole, a bioreductive phosporamide prodrug. Both undergo reduction reactions to activate the prodrugs in hypoxic conditions [96].
HIF Pathway Inhibitors
Pharmacological targeting of the HIF signalling pathway is complicated by its interconnected interactions and redundancy with other signalling pathways. In the last two decades many and varied targeting strategies have been developed including inhibitors of HIF1α transcription, translation, protein stabilisation and heterodimerisation with HIFβ. HIF signalling may be targeted indirectly either through the upstream PI3K/Akt/mTOR pathway (such as the mTOR inhibitors temsirolimus, evorolimus and sirolimus), or downstream, through anti-VEGF therapy (such as Bevacizumab or multiple kinase inhibitors like Lenvatinib and Sorafenib which inhibit VEGFR 1/2/3 alongside fibroblast growth factor receptors (FGFR) 1/2/3/4, Platelet-derived growth factor receptor (PDGFR), c-KIT and the RET oncogene). A detailed review of these different targeting strategies is beyond the scope of this article and has been covered elsewhere [97].
Of interest, given that hydroxylation via PHD proteins plays such a pivotal role in reducing HIFα levels via von Hippel-Lindau (VHL) mediated proteosomal degradation, benzopyranyl 1,2,3-triazole has recently been identified as a novel anticancer agent. This compound increases HIFα hydroxylation and thus subsequent targeting for proteosomal degradation, reduces VEGF expression and angiogenesis in both in vitro and in vivo cancer models as well as showing combination efficacy with the epidermal growth factor receptor (EGFR) receptor gefitinib [98].
Another potentially druggable HIF-related target is Heat shock protein 90 (HSP90) which binds to and stabilises HIFα to increase its activity by; (i) blocking VHL-dependent proteosomal degradation, and (ii) improving HIF heterodimer recruitment of further transcriptional machinery at HREs [99]. Multiple HSP90 inhibitors including Geldanamycin semi-synthetic derivatives such as tanespimycin and farnesyltransferase derivatives have been shown to reduce HIFα levels and downregulate HRE-containing downstream genes in human cancer settings [100, 101]. In a phase I trial, tanespimycin was used in combination with bortezomib for 17 patients with advanced refractory solid tumours but unfortunately no objective responses were seen [102].
Camptothecins (CPTs), including Topotecan, which was originally discovered as part of a HIF-targeted transcriptional activity assay [103]. They are traditional chemotherapeutic agents which act as topoisomerase I inhibitors but also prevent HIF1α accumulation and have been shown to reduce hypoxia-mediated VEGF mRNA expression in human glioma cell lines under hypoxic conditions [104]. Recently CRLX-101 was developed as a first-in-class nano pharmaceutical agent which conjugates a CPT moiety to a polyethene glycol (PEG) co-polymer [105] and has shown higher efficacy and improved tolerability compared with synthetic analogues Topotecan and Irinotecan [106]. It has shown anticancer efficacy in combination with Bevacizumab in triple negative breast cancer mouse models [107] as well as monotherapy for locally advanced rectal cancer [108]. Two phase II clinical trials have explored CRLX-101 in combination with Bevacizumab to treat advanced renal cell carcinoma although sadly neither displayed any improved anticancer efficacy compared with approved treatment agents [109, 110]. Most trials discussed above have not investigated mechanisms of resistance or reasons behind the disappointing efficacy results. There was also limited use of prospective pharmacodynamic biomarkers assessing baseline hypoxia, or changes in hypoxia levels in patients on the trials.
Clinical trials of hypoxia targeting strategies
The most significant advance in HIF pathway targeting strategies came in recent months with The United States Food and Drug Administration (FDA) approval of the HIF2α inhibitor Belzutifan for the treatment of von Hippel-Lindau associated tumours including renal cell carcinomas, central nervous system haemangiomas and pancreatic neuroendocrine tumours. This follows the publication of phase II clinical trial findings by Jonasch et al. [111]. This study recruited patients with renal cell carcinoma secondary to von Hippel-Lindau syndrome and used objective response (including complete and partial responses) as the primary endpoint. Objective response was seen in 49% of patients with renal cell carcinoma, in 77% of co-existing pancreatic neuroendocrine tumours and in 30% of co-existing central nervous system haemangiomas. 100% of co-existing retinal haemangiomas (16 eyes across 12 patients) were graded as showing some improvement following Belzutifan administration. This work follows on from several phase I trials which screened novel HIF2α inhibitors in von Hippel-Lindau associated tumours [112, 113]. A summary of these studies along with recent clinical trials utilising hypoxia-targeting strategies is summarised in Table 2 [102, 109–134].
Table 2.
Summary of published clinical trials using hypoxia targeting strategies
| Target | IMP | Treatment | Trial Phase | Patients Treated | Disease type | Findings | Reference |
|---|---|---|---|---|---|---|---|
| Hypoxia-activated Prodrugs | Evofosfamide (TH-302) | Pazopanib + Evofosfamide | I | 30 | All solid tumours | Partial response in 10%, stable disease in 57%, progressive disease in 23% of patients | (Riedel et al., 2017) [114] |
| Evofosfamide monotherapy in relapsed/refractory leukaemia | I | 49 | Acute myeloid/lymphoid leukaemia | Reduced HIF1a/CAIX but only 6% overall response rate | (Badar et al., 2016) [115] | ||
| Gemcitabine Vs Gemcitabine + Evofosfamide | II | 214 | Pancreatic | Extended progression-free survival (5.6 vs 3.6 months; p = 0.005), greater reduction in tumour burden (p = 0.04) and CA19.9 levels (p = 0.008) with addition of Evofosfamide. No significant difference in overall survival | (Borad et al., 2015) [116] | ||
| Evofosfamide + Dexamethasone ± Bortezomib | I-II | 59 | Multiple myeloma | Stable disease (38/59) or better in 80% patients across all cohorts | (Laubach et al., 2019) | ||
| Doxorubicin Vs Doxorubicin + Evofosfamide | III | 640 | Soft-tissue sarcoma | No survival benefit (18.4 months combination therapy Vs 19.0 months Doxorubicin monotherapy median overall survival) | (Tap et al., 2017) [117] | ||
| Gemcitabine Vs Gemcitabine + Evofosfamide | III | 693 | Pancreatic | Overall survival endpoint not quite met (8.7 months combination therapy Vs 7.6 months Gemcitabine monotherapy; p = 0.059). Median progression-free survival 5.5 months combination therapy V 3.7 months Gemcitabine monotherapy (P = 0.004) | (Van Cutsem et al., 2016) [119] | ||
| Tirapazamine (SR-4233) | Tirapazamine (TPZ) + Carboplatin + Paclitaxel | I | 42 | All solid tumours | 8% complete response, 5% partial response, 60% stable disease, 26% progression of disease | (Lara et al., 2003) [120] | |
| Cisplatin + radiotherapy + Tirapazamine | I | 16 | Oesophageal adenocarcinoma | Three year overall survival 88%, but omission of Tirapazamine needed in latter cycles to avoid dose-limiting toxicity of neutropenia | (Rischin et al., 2001) [121] | ||
| Arterial Embolisation + Tirapazamine | I | 27 | Hepatocellular carcinoma | 60% complete response, 84% objective response | (Abi-Jaoudeh et al., 2021) [122] | ||
| Cisplatin + Etoposide + radiotherapy + Tirapazamine | II | 69 | Limited-stage small cell lung cancer | Median progression-free survival 11 months, median overall survival 21 months | (Le et al., 2009) [123] | ||
| Paclitaxel + Carboplatin ± Tirapazamine | III | 367 | Non-small cell lung cancer | Overall survival end-points not reached, significantly more adverse events leading to treatment cessation when Tirapazamine added to combination therapy (p < 0.05), mostly due to myelosuppression | (Williamson et al., 2005) [124] | ||
| PR-104 | PR-104 + Docetaxel or Gemcitabine | I | 42 | All solid tumours | 9.5% partial response overall, significant myelosuppression prevented further analysis of combo + Gemcitabine | (McKeage et al., 2012) [125] | |
| PR-104 | I | 27 | All solid tumours | No objective responses were observed | (Jameson et al., 2010) [126] | ||
| PR-104 | I-II | 50 | Acute myeloid/lymphoid leukaemia | Objective response in 32% AML and 20% ALL patients | (Konopleva et al., 2015) [127] | ||
| HIF Signalling | Belzutifan | Belzutifan | I | 98 | Renal cell carcinoma | Objective response in 25%, median progression-free survival was 14.5 months | (Choueiri et al., 2021) [112] |
| Belzutifan | II | VHL-associated tumours | Objective response in 49% renal cell carcinomas, 77% pancreatic lesions, 30% CNS haemangioblastomas, 100% retinal haemangioblastomas | (Jonasch et al., 2021) [111] | |||
| PT2385 | PT2385 | I | 51 | Renal cell carcinoma | 2% complete response, 12% partial response, 52% stable disease | (Courtney et al., 2018) [113] | |
| CRLX101 | CRLX101 + Bevacizumab | I-II | 22 | Renal cell carcinoma | 23% partial response, 55% achieving progression-free survival of more than four months | (Keefe et al., 2016) [109] | |
| CRLX101 + Bevacizumab Vs standard of care (SOC) therapy | II | 111 | Renal cell carcinoma | No improvement in progression-free survival (3.7 months CRLX101 + Bevacizumab Vs 3.9 months SOC therapy; p = 0.831) or objective response (5% CRLX101 + Bevacizumab Vs 14% SOC therapy; p = 0.836) | (Voss et al., 2017) [110] | ||
| PX-12 | PX-12 (thioredoxin-1 inhibitor) | I | 38 | All solid tumours | 18% stable disease, as best response observed | (Ramanathan et al., 2007) [128] | |
| PX-12 | I | 14 | All solid tumours | 7% stable disease, as best response observed | (Ramanathan et al., 2012) [129] | ||
| Tanespimycin | Tanespimycin + Bortezomib | I | 17 | All solid tumours | 6% stable disease, as best response observed | (Schenk et al., 2013) [102] | |
| CXCR4 (haematological malignancies) | BL-8040 | BL-8040 + Ara-C | II | 42 | Acute myeloid leukaemia | 29% complete remission ± incomplete haematological recovery. Median overall survival 8.4 months | (Borthakur et al., 2021) [130] |
| Plerixafor | Plerixafor + high-dose cytarabine + etoposide | I | 19 | Acute myeloid/lymphoid leukaemia, myelodysplastic syndrome | 16% objective response, exclusively in acute myeloid leukaemia | (Cooper et al., 2017) [131] | |
| Plerixafor + Decitabine | I | 69 | Acute myeloid/lymphoid leukaemia, myelodysplastic syndrome | 43% objective response | (Roboz et al., 2018) [132] | ||
| Plerixafor + FLAG-IDA | I-II | 41 | Acute myeloid leukaemia | Complete remission ± incomplete haematological recovery in 50% and 47% of primary refractory and early relapse groups respectively | (Martínez-Cuadrón et al., 2018) [133] | ||
| Ulocuplumab | Ulocuplumab + MEC (mitoxantrone + etoposide + cytarabine) | I | 73 | Acute myeloid leukaemia | Complete remission ± incomplete haematological recovery in 51% combination therapy compared with 24–28% in those receiving MEC alone | (Becker et al., 2014) [134] |
Evofosfamide is a second-generation hypoxia-activated prodrug (HAP) consisting of a dual moiety in which bromo-iso-phosphoramide (Br-IPM) is attached to the enzyme responsible for its reduction-dependent activation, 2-nitroimidazole. Tirapazamine generates an oxidative radical following reduction in hypoxic conditions. This occurs preferentially in the nucleus leading to DNA double-strand breaks, chromosomal degradation and ultimately to apoptosis. PR-104 contains a nitrogen mustard moiety which, when activated by reduction in hypoxia, is able to cross-link DNA to prevent further replication. Belzutifan is a small molecule selective HIF2α inhibitor. PT2385 similarly acts as an antagonist of HIF2α. CRLX-101 is a nanopharmaceutical agent which conjugates a camptothecin moiety to a polyethene glycol co-polymer. PX-12 is a small molecule inhibitor of thioredoxin-1 (Trx-1), a redox protein pivotal for HIF1α and VEGF. Tanespimycin is a Geldanamycin semi-synthetic derivative inhibitor of heat shock protein 90 (HSP90) which binds to and stabilises HIF1α. BL-8040 is a CXCR4 antagonist, a downstream target of HIF1a. Plerixafor is similarly a CXCR4 antagonist whilst Ulocuplumab is a fully human IgG4 monoclonal antibody which prevents the binding of CXCR4 to CXCL12
Published clinical literature exists regarding hypoxia-related biomarker analyses to help identify potential markers with therapeutic prognostic value. MicroRNA-210 (miR-210) is upregulated in tissue hypoxia [135] and has been linked to improved tumour cell survival and impaired DNA repair [136, 137]. Ono and colleagues accessed plasma samples from melanoma patients enrolled on a phase III trial and analysed circulating cell-free miR-210 via quantitative Polymerase chain reaction (PCR). They found miR-210 to be significantly higher in metastatic versus primary disease and a significant positive correlation with poorer prognosis (p < 0.001). Interestingly, when analysing sequential serum samples from individual patients miR-210 levels significantly increased in the three-month period prior to disease recurrence (p = 0.012) [138]. Irlam-Jones and colleagues found that miR-210 level significantly and positively correlated with hypoxia signalling, including HIF1α (p = 0.01) and carbonic anhydrase 9 (CAIX) level (p = 0.0004) as well as a 26-gene hypoxia score (p = 0.07), but concluded this did not improve on these established hypoxia biomarkers [139].
CAIX is downstream of and dependent upon HIF signalling. As a metalloenzyme, CAIX catalyses the production of H+ and HCO3− from H2O and CO2 which helps to buffer pH fluctuations in hypoxic tumour microenvironments [140]. Higher CAIX expression was significantly associated with poorer survival outcomes (p = 0.016) in 45 glioblastoma multiforme and anaplastic astrocytoma patients treated with bevacizumab and irinotecan in a phase II clinical trial [141]. Similarly, higher CAIX expression was negatively correlated with two-year loco-regional control (p = 0.001) in 39 head-and-neck squamous cell carcinoma (HNSCC) patients receiving chemoradiation in a prospective imaging trial [142]. A larger cohort of 203 soft tissue sarcoma patients were analysed via immunohistochemistry retrospectively for the hypoxia markers HIF1α, GLUT1 and CAIX following a phase III radiotherapy trial. Whilst HIF1α and GLUT-1 protein expression were not prognostic, high CAIX expression was significantly associated with worse disease-free survival outcomes (p < 0.001) indicating that this downstream factor in HIF signalling may be a more clinically significant prognostication marker [143]. However it is as yet unclear whether this is due to a functional role of CAIX, i.e. in hydrogen ion efflux to promote cancer cell survival, or a result of differential protein stability or staining techniques utilised in this study.
Conclusion
The role of hypoxia in cancer is not in doubt. Hypoxia has been consistently shown to contribute to more aggressive and treatment resistant disease in both solid and haematological malignancies. Hypoxia modulates the growth and characteristics of cancer via an array of highly complex pathways as summarized above, but the many ways in which hypoxia is important in cancer remains an expanding area of research.
The ability to identify hypoxia, measure it with precision and work out in which patients it is especially important, is essential to further progress with hypoxia targeting strategies in the clinical setting. To date, no large studies identifying hypoxia in specific cancer patient populations have been performed. The failure to accurately identify patients with hypoxic tumours, and the lack of integration of validated hypoxia biomarkers into clinical trials, has contributed to disappointing clinical trial results. Whilst the gold standard for measuring hypoxia is currently considered to be oxygen electrodes, there are obvious benefits to using imaging modalities, being non-invasive and independent of operator differences. The development of hypoxia biomarkers provides future promise for alternative effective tools to identify patients who may benefit from clinical trials of hypoxia targeting strategies. However, data is still limited to a handful of tumour types in solid tumours and none currently exists in the haematological malignancy setting. Perhaps one of the biggest flaws to date in clinical trials targeting hypoxia pathways in cancer has been a failure to first pre-screen patients based on established and validated hypoxia biomarkers, and then only enrol those patients with proven hypoxic tumours onto trials of hypoxia targeted agents. Ultimately a panel of biomarkers will probably be needed as we anticipate different hypoxia markers are likely to prove prognostic in different cancer types. Future clinical trials also need to include pharmacodynamic biomarkers of hypoxia so we can also further understand mechanisms of response and resistance to hypoxia targeting strategies.
Figure 1 is an example of how future clinical trials could be designed to propel forward knowledge and experience in this area of cancer research. Performing initial pre-screening assessments using validated hypoxia biomarkers has potential to identify the population of patients where hypoxia is contributing to disease progression. Once this population of patients has been identified their treatment could be supplemented with hypoxia-modulatory agents and outcomes monitored. The results from these trials would allow us to assess for clinically relevant activity and take forward any of the promising agents to further larger later phase clinical trials.
Fig. 1.
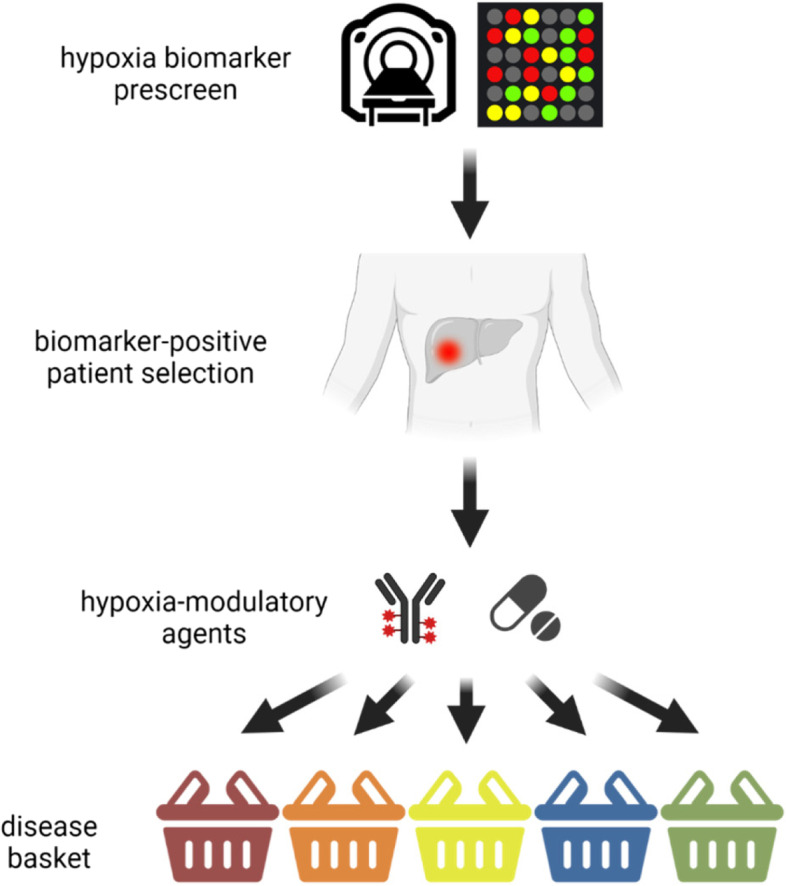
Suggested future clinical trial algorithm for hypoxia biomarker integration. Patients consent for pre-screening to investigate hypoxia biomarker expression in their cancer (detected either via hypoxia based imaging (i.e. pimonidazole) or validated hypoxia gene assays). Only patients that test positive for the hypoxia biomarker can then consent to the main study of a hypoxia modulating agent. Patients are stratified into disease specific cohorts based on the cancer type of interest (e.g. sarcoma, bladder, pancreas) due to the differences in cancer specific outcomes in these cohorts. If any hypoxia-biomarker cohort in this basket design shows sufficient signal of clinically relevant activity (e.g. durable clinical benefit) to warrant further investigation the cohort can then be expanded
Acknowledgements
The figure was created using BioRender.com.
Abbreviations
- ALL
Acute lymphoblastic leukaemia
- AML
Acute myeloid leukaemia
- BOLD MRI
Blood-oxygen level dependent MRI
- Br-IPM
Bromo-iso-phosphoramide
- CA
Carbonic anhydrase
- CAIX
Carbonic anhydrase 9
- CMML
Chronic myelomonocytic leukaemia
- CPT
Camptothecin
- CTC
Circulating tumour cell
- CXCR4
C-X-C motif chemokine 4
- EGFR
Epidermal growth factor receptor
- EMT
Epithelial-to-mesenchymal transition
- EPR
Electron paramagnetic resonance
- ER
Oestrogen receptor
- FDA
The United States Food and Drug Administration
- FGFR
Fibroblast growth factor receptors
- GLUT-1
Glucose transporter 1
- HAPs
Hypoxia-activated prodrugs
- HDAC
Histone deacetylase
- HIF
Hypoxia Inducible Factor
- HK2
Hypoxia‐inducible glycolytic enzyme hexokinase‐2
- HNSCC
Head-and-neck squamous cell carcinoma
- HRE
Hypoxia-responsive elements
- HSC
Haematopoetic stem cells
- HSP90
Heat shock protein 90
- NHL
Non-Hodgkin’s lymphoma
- MDR1
Multidrug resistance 1
- MHC
Major histocompatibility complex
- MM
Multiple myeloma
- mTOR
Mammalian target of rapamycin
- OPN
Osteopontin
- OMRI
Over Hauser-enhanced magnetic resonance imaging
- PARP
Protein poly(ADP-ribose) polymerase
- PCR
Polymerase chain reaction
- PEG
Polyethene glycol
- PDGFR
Platelet-derived growth factor receptor
- PD-L1
Programmed death-ligand 1
- PD-1
Programmed cell death protein 1
- PET
Positron emission tomography
- PHD
Propyl hydroxylase domains enzymes
- PI3K
Phosphoinositide 3-kinase
- TCR
T cell receptor
- TNBC
Triple negative breast cancer
- VEGF
Vascular endothelial growth factor
- VHL
Von Hippel-Lindau
Authors’ contributions
ES and NC conceived the manuscript. SS and BH drafted the manuscript before additions and editing by ES and NC. ES provided overall supervision for the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing financial interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bill Harris and Sana Saleem co-first authors.
References
- 1.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. Available from: https://pubmed.ncbi.nlm.nih.gov/22304911. [DOI] [PMC free article] [PubMed]
- 2.Keith B, Johnson RS, Simon MC. HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2011;12:9–22. Available from: https://pubmed.ncbi.nlm.nih.gov/22169972. [DOI] [PMC free article] [PubMed]
- 3.Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckl). Dove Medical Press; 2015;3:83–92. Available from: https://pubmed.ncbi.nlm.nih.gov/27774485. [DOI] [PMC free article] [PubMed]
- 4.Rankin EB, Giaccia AJ. The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ. 2008;15:678–85. doi: 10.1038/cdd.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–39. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto Y, Ibusuki M, Okumura Y, Kawasoe T, Kai K, Iyama K, et al. Hypoxia-inducible factor 1α is closely linked to an aggressive phenotype in breast cancer. Breast Cancer Res Treat. 2008;110:465–75. doi: 10.1007/s10549-007-9742-1. [DOI] [PubMed] [Google Scholar]
- 7.Krüger W, Mayer W-K, Schaefer C, Stohrer M, Vaupel P. Acute changes of systemic parameters in tumour-bearing rats, and of tumour glucose, lactate, and ATP levels upon local hyperthermia and/or hyperglycaemia. J Cancer Res Clin Oncol. 1991;117:409–15. doi: 10.1007/BF01612759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaupel P, Schaefer C, Okunieff P. Intracellular acidosis in murine fibrosarcomas coincides with ATP depletion, hypoxia, and high levels of lactate and total Pi. NMR Biomed. John Wiley & Sons, Ltd. 1994;7:128–36. 10.1002/nbm.1940070305. [DOI] [PubMed]
- 9.Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell. Elsevier; 2011;144:646–74. 10.1016/j.cell.2011.02.013. [DOI] [PubMed]
- 10.Christodoulou C, Spencer JA, Yeh S-CA, Turcotte R, Kokkaliaris KD, Panero R, et al. Live-animal imaging of native haematopoietic stem and progenitor cells. Nature. 2020;578:278–83. doi: 10.1038/s41586-020-1971-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature [Internet]. 2014;508:269–73. Available from: 10.1038/nature13034. [DOI] [PMC free article] [PubMed]
- 12.Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, et al. Regulation of the HIF-1a Level Is Essential for Hematopoietic Stem Cells. Cell Stem Cell. Elsevier; 2010;7:391–402. 10.1016/j.stem.2010.06.020. [DOI] [PubMed]
- 13.Lee KE, Simon MC. From stem cells to cancer stem cells: HIF takes the stage. Curr Opin Cell Biol. 2012;24:232–5. Available from: https://www.sciencedirect.com/science/article/pii/S0955067412000063. [DOI] [PubMed]
- 14.K Asosingh, H De Raeve, M de Ridder, GA Storme, A Willems, I Van Riet, et al. Role of the hypoxic bone marrow microenvironment in 5T2MM murine myeloma tumor progression. Haematologica. 2005;90:810–7. Available from: https://haematologica.org/article/view/3558. [PubMed]
- 15.Méndez-Ferrer S, Bonnet D, Steensma DP, Hasserjian RP, Ghobrial IM, Gribben JG, et al. Bone marrow niches in haematological malignancies. Nat Rev Cancer. 2020;20:285–98. doi: 10.1038/s41568-020-0245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawano Y, Moschetta M, Manier S, Glavey S, Görgün GT, Roccaro AM, et al. Targeting the bone marrow microenvironment in multiple myeloma. Immunol Rev. John Wiley & Sons, Ltd; 2015;263:160–72. 10.1111/imr.12233. [DOI] [PubMed]
- 17.Sally K. Martin, Peter Diamond, Sharon A. Williams, Luen Bik To, Daniel J. Peet, Nobutaka Fujii, et al. Hypoxia-inducible factor-2 is a novel regulator of aberrant CXCL12 expression in multiple myeloma plasma cells. Haematologica. 2010;95:776–84. Available from: https://haematologica.org/article/view/5590. [DOI] [PMC free article] [PubMed]
- 18.Giatromanolaki A, Bai M, Margaritas D, Bourantas K, Koukourakis M, Sivridis E, et al. Hypoxia and Activated VEGF/Receptor Pathway in Multiple Myeloma. Anticancer Res. 2010;30:2831. Available from: http://ar.iiarjournals.org/content/30/7/2831.abstract. [PubMed]
- 19.Colla S, Storti P, Donofrio G, Todoerti K, Bolzoni M, Lazzaretti M, et al. Low bone marrow oxygen tension and hypoxia-inducible factor-1α overexpression characterize patients with multiple myeloma: role on the transcriptional and proangiogenic profiles of CD138+ cells. Leukemia. 2010;24:1967–70. doi: 10.1038/leu.2010.193. [DOI] [PubMed] [Google Scholar]
- 20.Azab AK, Hu J, Quang P, Azab F, Pitsillides C, Awwad R, et al. Hypoxia promotes dissemination of multiple myeloma through acquisition of epithelial to mesenchymal transition-like features. Blood. 2012;119:5782–94. doi: 10.1182/blood-2011-09-380410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen PØ, Mortensen BT, Hodgkiss RJ, Iversen PO, Christensen IJ, Helledie N, et al. Increased cellular hypoxia and reduced proliferation of both normal and leukaemic cells during progression of acute myeloid leukaemia in rats. Cell Prolif. John Wiley & Sons, Ltd; 2000;33:381–95. 10.1046/j.1365-2184.2000.00183.x. [DOI] [PMC free article] [PubMed]
- 22.Desplat V, Faucher J-L, Mahon FX, dello Sbarba P, Praloran V, Ivanovic Z. Hypoxia Modifies Proliferation and Differentiation of CD34+ CML Cells. Stem Cells. 2002;20:347–54. doi: 10.1634/stemcells.20-4-347. [DOI] [PubMed] [Google Scholar]
- 23.Jabari M, Allahbakhshian Farsani M, Salari S, Hamidpour M, Amiri V, Mohammadi MH. Hypoxia-Inducible Factor1-Α (HIF1α) and Vascular Endothelial Growth Factor-A (VEGF-A) Expression in De Novo AML Patients. Asian Pacific J Cancer Prev. 2019;20:705–10. Available from: http://journal.waocp.org/article_82375.html. [DOI] [PMC free article] [PubMed]
- 24.Wang Y, Liu Y, Malek SN, Zheng P, Liu Y. Targeting HIF1a; Eliminates Cancer Stem Cells in Hematological Malignancies. Cell Stem Cell. Elsevier; 2011;8:399–411. 10.1016/j.stem.2011.02.006. [DOI] [PMC free article] [PubMed]
- 25.Griessinger E, Anjos-Afonso F, Pizzitola I, Rouault-Pierre K, Vargaftig J, Taussig D, et al. A Niche-Like Culture System Allowing the Maintenance of Primary Human Acute Myeloid Leukemia-Initiating Cells: A New Tool to Decipher Their Chemoresistance and Self-Renewal Mechanisms. Stem Cells Transl Med. 2014;3:520–9. doi: 10.5966/sctm.2013-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4:118–32. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- 27.Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. The American Society for Clinical Investigation; 2007;117:3810–20. 10.1172/JCI30487. [DOI] [PMC free article] [PubMed]
- 28.Liu Y, Liu Y, Yan X, Xu Y, Luo F, Ye J, et al. HIFs enhance the migratory and neoplastic capacities of hepatocellular carcinoma cells by promoting EMT. Tumor Biology. 2014;35:8103–14. doi: 10.1007/s13277-014-2056-0. [DOI] [PubMed] [Google Scholar]
- 29.Min Y, Aditya B, Wittner Ben, Stott Shannon, Smas Malgorzata, Ting David, et al. Circulating Breast Tumor Cells Exhibit Dynamic Changes in Epithelial and Mesenchymal Composition. Science (1979). American Association for the Advancement of Science. 2013;339:580–4. 10.1126/science.1228522. [DOI] [PMC free article] [PubMed]
- 30.Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong STC, et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472–6. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525–30. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn J-I, Cheng P, et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. Journal of Experimental Medicine. 2010;207:2439–53. 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed]
- 33.Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, DeNardo DG, et al. Macrophage Expression of Hypoxia-Inducible Factor-1α Suppresses T-Cell Function and Promotes Tumor Progression. Cancer Res. 2010;70:7465. Available from: http://cancerres.aacrjournals.org/content/70/19/7465.abstract. [DOI] [PMC free article] [PubMed]
- 34.Liu G, Bi Y, Shen B, Yang H, Zhang Y, Wang X, et al. SIRT1 Limits the Function and Fate of Myeloid-Derived Suppressor Cells in Tumors by Orchestrating HIF-1alpha–Dependent Glycolysis. Cancer Res. 2014;74:727. Available from: http://cancerres.aacrjournals.org/content/74/3/727.abstract. [DOI] [PubMed]
- 35.White JR, Harris RA, Lee SR, Craigon MH, Binley K, Price T, et al. Genetic amplification of the transcriptional response to hypoxia as a novel means of identifying regulators of angiogenesis. Genomics. 2004;83:1–8. Available from: https://www.sciencedirect.com/science/article/pii/S0888754303002155. [DOI] [PubMed]
- 36.Neumann AK, Yang J, Biju MP, Joseph SK, Johnson RS, Haase VH, et al. Hypoxia inducible factor 1α regulates T cell receptor signal transduction. Proc Natl Acad Sci U S A. 2005;102:17071. Available from: http://www.pnas.org/content/102/47/17071.abstract. [DOI] [PMC free article] [PubMed]
- 37.Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A Mechanism of Hypoxia-Mediated Escape from Adaptive Immunity in Cancer Cells. Cancer Res. 2014;74:665. Available from: http://cancerres.aacrjournals.org/content/74/3/665.abstract. [DOI] [PubMed]
- 38.Shibutani ST, Saitoh T, Nowag H, Münz C, Yoshimori T. Autophagy and autophagy-related proteins in the immune system. Nat Immunol. 2015;16:1014–24. doi: 10.1038/ni.3273. [DOI] [PubMed] [Google Scholar]
- 39.Rodríguez-Jiménez FJ, Moreno-Manzano V, Lucas-Dominguez R, Sánchez-Puelles J-M. Hypoxia Causes Downregulation of Mismatch Repair System and Genomic Instability in Stem Cells. Stem Cells. 2008;26:2052–62. doi: 10.1634/stemcells.2007-1016. [DOI] [PubMed] [Google Scholar]
- 40.Cowman S, Pizer B, Sée V. Downregulation of both mismatch repair and non-homologous end-joining pathways in hypoxic brain tumour cell lines. PeerJ. 2021;9:e11275. Available from: https://peerj.com/articles/11275. [DOI] [PMC free article] [PubMed]
- 41.Scanlon SE, Glazer PM. Multifaceted control of DNA repair pathways by the hypoxic tumor microenvironment. DNA Repair (Amst). 2015;32:180–9. Available from: https://www.sciencedirect.com/science/article/pii/S1568786415001226. [DOI] [PMC free article] [PubMed]
- 42.Dean E, Middleton MR, Pwint T, Swaisland H, Carmichael J, Goodege-Kunwar P, et al. Phase I study to assess the safety and tolerability of olaparib in combination with bevacizumab in patients with advanced solid tumours. Br J Cancer. 2012;106:468–74. doi: 10.1038/bjc.2011.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu JF, Tolaney SM, Birrer M, Fleming GF, Buss MK, Dahlberg SE, et al. A Phase 1 trial of the poly(ADP-ribose) polymerase inhibitor olaparib (AZD2281) in combination with the anti-angiogenic cediranib (AZD2171) in recurrent epithelial ovarian or triple-negative breast cancer. Eur J Cancer. Elsevier; 2013;49:2972–8. 10.1016/j.ejca.2013.05.020. [DOI] [PMC free article] [PubMed]
- 44.Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, et al. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105:659–69. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- 45.Moroney JW, Schlumbrecht MP, Helgason T, Coleman RL, Moulder S, Naing A, et al. A phase I trial of liposomal doxorubicin, bevacizumab, and temsirolimus in patients with advanced gynecologic and breast malignancies. Clin Cancer Res. United States; 2011;17:6840–6. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med8&NEWS=N&AN=21890452. [DOI] [PMC free article] [PubMed]
- 46.Flaherty KT, Manola JB, Pins M, McDermott DF, Atkins MB, Dutcher JJ, et al. BEST: A Randomized Phase II Study of Vascular Endothelial Growth Factor, RAF Kinase, and Mammalian Target of Rapamycin Combination Targeted Therapy With Bevacizumab, Sorafenib, and Temsirolimus in Advanced Renal Cell Carcinoma--A Trial of the ECOG-ACRIN C. J Clin Oncol. United States; 2015;33:2384–91. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med12&NEWS=N&AN=26077237. [DOI] [PMC free article] [PubMed]
- 47.Falchook GS, Wheler JJ, Naing A, Jackson EF, Janku F, Hong D, et al. Targeting hypoxia-inducible factor-1alpha (HIF-1alpha) in combination with antiangiogenic therapy: a phase I trial of bortezomib plus bevacizumab. Oncotarget. United States; 2014;5:10280–92. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med11&NEWS=N&AN=25373733. [DOI] [PMC free article] [PubMed]
- 48.Minoia C, Quero C, Asselti M, Galise I, Marzano AL, Iacobazzi A, et al. Changes in angiogenesis and hypoxia-inducible factor-1α protein expression in relapsed/refractory indolent non-Hodgkin lymphomas. Br J Haematol. John Wiley & Sons, Ltd. 2013;163:640–5. 10.1111/bjh.12560. [DOI] [PubMed]
- 49.Kaluz S, Kaluzová M, Stanbridge E. Proteasomal Inhibition Attenuates Transcriptional Activity of Hypoxia-Inducible Factor 1 (HIF-1) via Specific Effect on the HIF-1α C-Terminal Activation Domain. Mol Cell Biol. American Society for Microbiology. 2006;26:5895–907. 10.1128/MCB.00552-06. [DOI] [PMC free article] [PubMed]
- 50.Shin DH, Chun Y-S, Lee DS, Huang LE, Park J-W. Bortezomib inhibits tumor adaptation to hypoxia by stimulating the FIH-mediated repression of hypoxia-inducible factor-1. Blood. 2008;111:3131–6. doi: 10.1182/blood-2007-11-120576. [DOI] [PubMed] [Google Scholar]
- 51.Drolle H, Wagner M, Vasold J, Kütt A, Deniffel C, Sotlar K, et al. Hypoxia regulates proliferation of acute myeloid leukemia and sensitivity against chemotherapy. Leuk Res. 2015;39:779–85. Available from: https://www.sciencedirect.com/science/article/pii/S0145212615001307. [DOI] [PubMed]
- 52.Frolova O, Samudio I, Benito JM, Jacamo R, Kornblau SM, Markovic A, et al. Regulation of HIF-1α signaling and chemoresistance in acute lymphocytic leukemia under hypoxic conditions of the bone marrow microenvironment. Cancer Biol Ther Taylor Francis. 2012;13:858–70. doi: 10.4161/cbt.20838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ikeda S, Tagawa H. Impact of hypoxia on the pathogenesis and therapy resistance in multiple myeloma. Cancer Sci. John Wiley & Sons, Ltd; 2021;112:3995–4004. 10.1111/cas.15087. [DOI] [PMC free article] [PubMed]
- 54.Hu Y, Kirito K, Yoshida K, Mitsumori T, Nakajima K, Nozaki Y, et al. Inhibition of hypoxia-inducible factor-1 function enhances the sensitivity of multiple myeloma cells to melphalan. Mol Cancer Ther. 2009;8:2329–38. doi: 10.1158/1535-7163.MCT-09-0150. [DOI] [PubMed] [Google Scholar]
- 55.Tsubaki M, Takeda T, Tomonari Y, Koumoto Y, Imano M, Satou T, et al. Overexpression of HIF-1α contributes to melphalan resistance in multiple myeloma cells by activation of ERK1/2, Akt, and NF-κB. Lab Investig. 2019;99:72–84. doi: 10.1038/s41374-018-0114-8. [DOI] [PubMed] [Google Scholar]
- 56.Generali D, Berruti A, Brizzi MP, Campo L, Bonardi S, Wigfield S, et al. Hypoxia-inducible factor-1alpha expression predicts a poor response to primary chemoendocrine therapy and disease-free survival in primary human breast cancer. Clin Cancer Res. United States; 2006;12:4562–8. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med6&NEWS=N&AN=16899602. [DOI] [PubMed]
- 57.Generali D, Buffa FM, Berruti A, Brizzi MP, Campo L, Bonardi S, et al. Phosphorylated ERalpha, HIF-1alpha, and MAPK signaling as predictors of primary endocrine treatment response and resistance in patients with breast cancer. J Clin Oncol. United States; 2009;27:227–34. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med7&NEWS=N&AN=19064988. [DOI] [PubMed]
- 58.Geng H, Harvey CT, Pittsenbarger J, Liu Q, Beer TM, Xue C, et al. HDAC4 Protein Regulates HIF1a; Protein Lysine Acetylation and Cancer Cell Response to Hypoxia. J Biol Chem. Elsevier; 2011;286:38095–102. 10.1074/jbc.M111.257055. [DOI] [PMC free article] [PubMed]
- 59.Qian DZ, Kachhap SK, Collis SJ, Verheul HMW, Carducci MA, Atadja P, et al. Class II Histone Deacetylases Are Associated with VHL-Independent Regulation of Hypoxia-Inducible Factor 1α. Cancer Res. 2006;66:8814–21. doi: 10.1158/0008-5472.CAN-05-4598. [DOI] [PubMed] [Google Scholar]
- 60.Tavallai S, Hamed HA, Grant S, Poklepovic A, Dent P. Pazopanib and HDAC inhibitors interact to kill sarcoma cells. Cancer Biol Ther. Taylor & Francis. 2014;15:578–85. 10.4161/cbt.28163. [DOI] [PMC free article] [PubMed]
- 61.Aggarwal R, Thomas S, Pawlowska N, Bartelink I, Grabowsky J, Jahan T, et al. Inhibiting Histone Deacetylase as a Means to Reverse Resistance to Angiogenesis Inhibitors: Phase I Study of Abexinostat Plus Pazopanib in Advanced Solid Tumor Malignancies. J Clin Oncol. United States; 2017;35:1231–9. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med14&NEWS=N&AN=28221861. [DOI] [PMC free article] [PubMed]
- 62.Jing X, Yang F, Shao C, Wei K, Xie M, Shen H, et al. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol Cancer. 2019;18:157. doi: 10.1186/s12943-019-1089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zunino F, Perego P, Pilotti S, Pratesi G, Supino R, Arcamone F. Role of apoptotic response in cellular resistance to cytotoxic agents. Pharmacology & therapeutics. 1997;76:177–85. Available from: http://europepmc.org/abstract/MED/9535179. [DOI] [PubMed]
- 64.Ma F, Li H, Li Y, Ding X, Wang H, Fan Y, et al. Aldehyde dehydrogenase 1 (ALDH1) expression is an independent prognostic factor in triple negative breast cancer (TNBC). Medicine. 2017;96. Available from: https://journals.lww.com/md-journal/Fulltext/2017/04070/Aldehyde_dehydrogenase_1__ALDH1__expression_is_an.40.aspx. [DOI] [PMC free article] [PubMed]
- 65.Yamada A, Ishikawa T, Ota I, Kimura M, Shimizu D, Tanabe M, et al. High expression of ATP-binding cassette transporter ABCC11 in breast tumors is associated with aggressive subtypes and low disease-free survival. Breast Cancer Res Treat. 2013;137:773–82. doi: 10.1007/s10549-012-2398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mahadevan D, List AF. Targeting the multidrug resistance-1 transporter in AML: molecular regulation and therapeutic strategies. Blood. 2004;104:1940–51. doi: 10.1182/blood-2003-07-2490. [DOI] [PubMed] [Google Scholar]
- 67.Palmeira A, Sousa M, Vasconcelos H, Pinto MM. Three decades of P-gp Inhibitors: Skimming through several Generations and Scaffolds. Curr Med Chem. 2012;19:1946–2025. doi: 10.2174/092986712800167392. [DOI] [PubMed] [Google Scholar]
- 68.Raleigh JA, Dewhirst MW, Thrall DE. Measuring tumor hypoxia. Semin Radiat Oncol. 1996;6:37–45. Available from: https://www.sciencedirect.com/science/article/pii/S1053429696800348. [DOI] [PubMed]
- 69.Lokmic Z, Musyoka J, Hewitson TD, Darby IA. Chapter three - Hypoxia and Hypoxia Signaling in Tissue Repair and Fibrosis. In: Jeon KW, editor. Int Rev Cell Mol Biol. Academic Press; 2012. p. 139–85. Available from: https://www.sciencedirect.com/science/article/pii/B9780123943071000035. [DOI] [PubMed]
- 70.Kizaka-Kondoh S, Konse-Nagasawa H. Significance of nitroimidazole compounds and hypoxia-inducible factor-1 for imaging tumor hypoxia. Cancer Sci. John Wiley & Sons, Ltd; 2009;100:1366–73. 10.1111/j.1349-7006.2009.01195.x. [DOI] [PMC free article] [PubMed]
- 71.Hoskin PJ, Carnell DM, Taylor NJ, Smith RE, Stirling JJ, Daley FM, et al. Hypoxia in Prostate Cancer: Correlation of BOLD-MRI With Pimonidazole Immunohistochemistry—Initial Observations. Int J Radiat Oncol Biol Phys. Elsevier; 2007;68:1065–71. 10.1016/j.ijrobp.2007.01.018. [DOI] [PubMed]
- 72.Hutchison GJ, Valentine HR, Loncaster JA, Davidson SE, Hunter RD, Roberts SA, et al. Hypoxia-Inducible Factor 1α Expression as an Intrinsic Marker of Hypoxia: Correlation with Tumor Oxygen, Pimonidazole Measurements, and Outcome in Locally Advanced Carcinoma of the Cervix. Clin Cancer Res. 2004;10:8405–12. doi: 10.1158/1078-0432.CCR-03-0135. [DOI] [PubMed] [Google Scholar]
- 73.Kaanders JHAM, Wijffels KIEM, Marres HAM, Ljungkvist ASE, Pop LAM, van den Hoogen FJA, et al. Pimonidazole Binding and Tumor Vascularity Predict for Treatment Outcome in Head and Neck Cancer1. Cancer Res. 2002;62:7066–7074. [PubMed] [Google Scholar]
- 74.Portwood S, Lal D, Hsu Y-C, Vargas R, Johnson MK, Wetzler M, et al. Activity of the Hypoxia-Activated Prodrug, TH-302, in Preclinical Human Acute Myeloid Leukemia Models. Clin Cancer Res. 2013;19:6506–19. doi: 10.1158/1078-0432.CCR-13-0674. [DOI] [PubMed] [Google Scholar]
- 75.Potter C, Harris AL. Hypoxia Inducible Carbonic Anhydrase IX, Marker of Tumour: Hypoxia, Survival Pathway and Therapy Target. Cell Cycle. Taylor & Francis; 2004;3:159–62. 10.4161/cc.3.2.618. [PubMed]
- 76.Loncaster JA, Harris AL, Davidson SE, Logue JP, Hunter RD, Wycoff CC, et al. Carbonic Anhydrase (CA IX) Expression, a Potential New Intrinsic Marker of Hypoxia: Correlations with Tumor Oxygen Measurements and Prognosis in Locally Advanced Carcinoma of the Cervix1. Cancer Res. 2001;61:6394–6399. [PubMed] [Google Scholar]
- 77.Chia SK, Wykoff CC, Watson PH, Han C, Leek RD, Pastorek J, et al. Prognostic Significance of a Novel Hypoxia-Regulated Marker, Carbonic Anhydrase IX, in Invasive Breast Carcinoma. J Clin Oncol. Wolters Kluwer; 2001;19:3660–8. 10.1200/JCO.2001.19.16.3660. [DOI] [PubMed]
- 78.Kappler M, Taubert H, Holzhausen H-J, Reddemann R, Rot S, Becker A, et al. Immunohistochemical Detection of HIF-1α and CAIX in Advanced Head-and-Neck Cancer. Strahlentherapie und Onkologie. 2008;184:393–9. doi: 10.1007/s00066-008-1813-7. [DOI] [PubMed] [Google Scholar]
- 79.Le Q-T, Kong C, Lavori PW, O’Byrne K, Erler JT, Huang X, et al. Expression and Prognostic Significance of a Panel of Tissue Hypoxia Markers in Head-and-Neck Squamous Cell Carcinomas. Int J Radiat Oncol Biol Phys. Elsevier. 2007;69:167–75. 10.1016/j.ijrobp.2007.01.071. [DOI] [PubMed]
- 80.Sodhi CP, Phadke SA, Batlle D, Sahai A. Hypoxia Stimulates Osteopontin Expression and Proliferation of Cultured Vascular Smooth Muscle Cells: Potentiation by High Glucose. Diabetes. 2001;50:1482–90. doi: 10.2337/diabetes.50.6.1482. [DOI] [PubMed] [Google Scholar]
- 81.Hui EP, Sung FL, Yu BKH, Wong CSC, Ma BBY, Lin X, et al. Plasma Osteopontin, Hypoxia, and Response to Radiotherapy in Nasopharyngeal Cancer. Clin Cancer Res. 2008;14:7080–7. doi: 10.1158/1078-0432.CCR-08-0364. [DOI] [PubMed] [Google Scholar]
- 82.Raja R, Kale S, Thorat D, Soundararajan G, Lohite K, Mane A, et al. Hypoxia-driven osteopontin contributes to breast tumor growth through modulation of HIF1α-mediated VEGF-dependent angiogenesis. Oncogene. 2014;33:2053–64. doi: 10.1038/onc.2013.171. [DOI] [PubMed] [Google Scholar]
- 83.Golman K, Petersson JS, Ardenkjær-Larsen J-H, Leunbach I, Wistrand L-G, Ehnholm G, et al. Dynamic in vivo oxymetry using overhauser enhanced MR imaging. Journal of Magnetic Resonance Imaging. John Wiley & Sons, Ltd; 2000;12:929–38. 10.1002/1522-2586(200012)12:6%3C929::AID-JMRI17%3E3.0.CO. [DOI] [PubMed]
- 84.Stoyanova R, Huang K, Sandler K, Cho H, Carlin S, Zanzonico PB, et al. Mapping Tumor Hypoxia In Vivo Using Pattern Recognition of Dynamic Contrast-enhanced MRI Data. Transl Oncol. 2012/12/01. Neoplasia Press Inc.; 2012;5:437–47. Available from: https://pubmed.ncbi.nlm.nih.gov/23326621. [DOI] [PMC free article] [PubMed]
- 85.Hillestad T, Hompland T, Fjeldbo CS, Skingen VE, Salberg UB, Aarnes E-K, et al. MRI Distinguishes Tumor Hypoxia Levels of Different Prognostic and Biological Significance in Cervical Cancer. Cancer Res. 2020;80:3993–4003. doi: 10.1158/0008-5472.CAN-20-0950. [DOI] [PubMed] [Google Scholar]
- 86.Chopra S, Foltz WD, Milosevic MF, Toi A, Bristow RG, Ménard C, et al. Comparing oxygen-sensitive MRI (BOLD R2*) with oxygen electrode measurements: A pilot study in men with prostate cancer. Int J Radiat Biol. Taylor & Francis; 2009;85:805–13. 10.1080/09553000903043059. [DOI] [PubMed]
- 87.Liu M, Guo X, Wang S, Jin M, Wang Y, Li J, et al. BOLD-MRI of breast invasive ductal carcinoma: correlation of R2* value and the expression of HIF-1α. Eur Radiol. 2013;23:3221–7. doi: 10.1007/s00330-013-2937-4. [DOI] [PubMed] [Google Scholar]
- 88.Hallac RR, Ding Y, Yuan Q, McColl RW, Lea J, Sims RD, et al. Oxygenation in cervical cancer and normal uterine cervix assessed using blood oxygenation level-dependent (BOLD) MRI at 3T. NMR Biomed. John Wiley & Sons, Ltd; 2012;25:1321–30. 10.1002/nbm.2804. [DOI] [PMC free article] [PubMed]
- 89.Mahajan A, Engineer R, Chopra S, Mahanshetty U, Juvekar SL, Shrivastava SK, et al. Role of 3T multiparametric-MRI with BOLD hypoxia imaging for diagnosis and post therapy response evaluation of postoperative recurrent cervical cancers. Eur J Radiol Open. Elsevier; 2016;3:22–30. 10.1016/j.ejro.2015.11.003. [DOI] [PMC free article] [PubMed]
- 90.Okamoto S, Shiga T, Yasuda K, Ito YM, Magota K, Kasai K, et al. High Reproducibility of Tumor Hypoxia Evaluated by 18F-Fluoromisonidazole PET for Head and Neck Cancer. Journal of Nuclear Medicine. 2013;54:201. Available from: http://jnm.snmjournals.org/content/54/2/201.abstract. [DOI] [PubMed]
- 91.Dehdashti F, Grigsby PW, Lewis JS, Laforest R, Siegel BA, Welch MJ. Assessing Tumor Hypoxia in Cervical Cancer by PET with 60Cu-Labeled Diacetyl-Bis(N4Methylthiosemicarbazone). Journal of Nuclear Medicine. 2008;49:201. Available from: http://jnm.snmjournals.org/content/49/2/201.abstract. [DOI] [PubMed]
- 92.Patterson LH. Rationale for the use of aliphatic N-oxides of cytotoxic anthraquinones as prodrug DNA binding agents: a new class of bioreductive agent. Cancer Metastasis Rev. 1993;12:119–34. doi: 10.1007/BF00689805. [DOI] [PubMed] [Google Scholar]
- 93.Plumb JA, Workman P. Unusually marked hypoxic sensitization to indoloquinone E09 and mitomycin C in a human colon-tumour cell line that lacks DT-diaphorase activity. Int J Cancer. John Wiley & Sons, Ltd; 1994;56:134–9. 10.1002/ijc.2910560124. [DOI] [PubMed]
- 94.Singleton RS, Guise CP, Ferry DM, Pullen SM, Dorie MJ, Brown JM, et al. DNA Cross-Links in Human Tumor Cells Exposed to the Prodrug PR-104A: Relationships to Hypoxia, Bioreductive Metabolism, and Cytotoxicity. Cancer Res. 2009;69:3884. Available from: http://cancerres.aacrjournals.org/content/69/9/3884.abstract. [DOI] [PubMed]
- 95.Wang R, Green DR. Metabolic reprogramming and metabolic dependency in T cells. Immunol Rev. 2012;249:14–26. Available from: https://pubmed.ncbi.nlm.nih.gov/22889212. [DOI] [PMC free article] [PubMed]
- 96.Duan J-X, Jiao H, Kaizerman J, Stanton T, Evans JW, Lan L, et al. Potent and Highly Selective Hypoxia-Activated Achiral Phosphoramidate Mustards as Anticancer Drugs. J Med Chem. American Chemical Society; 2008;51:2412–20. 10.1021/jm701028q. [DOI] [PubMed]
- 97.Masoud GN, Li W. HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5:378–89. Available from: https://www.sciencedirect.com/science/article/pii/S2211383515000817. [DOI] [PMC free article] [PubMed]
- 98.Park K, Lee HE, Lee SH, Lee D, Lee T, Lee YM. Molecular and functional evaluation of a novel HIF inhibitor, benzopyranyl 1,2,3-triazole compound. Oncotarget. Impact Journals LLC; 2017;8:7801–13. Available from: https://pubmed.ncbi.nlm.nih.gov/27999195. [DOI] [PMC free article] [PubMed]
- 99.Hur E, Kim H-H, Choi SM, Kim JH, Yim S, Kwon HJ, et al. Reduction of Hypoxia-Induced Transcription through the Repression of Hypoxia-Inducible Factor-1α/Aryl Hydrocarbon Receptor Nuclear Translocator DNA Binding by the 90-kDa Heat-Shock Protein Inhibitor Radicicol. Mol Pharmacol. 2002;62:975. Available from: http://molpharm.aspetjournals.org/content/62/5/975.abstract. [DOI] [PubMed]
- 100.Han J-Y, Oh SH, Morgillo F, Myers JN, Kim E, Hong WK, et al. Hypoxia-inducible Factor 1α and Antiangiogenic Activity of Farnesyltransferase Inhibitor SCH66336 in Human Aerodigestive Tract Cancer. JNCI: Journal of the National Cancer Institute. 2005;97:1272–86. Available from: 10.1093/jnci/dji251. [DOI] [PubMed]
- 101.Porter JR, Ge J, Lee J, Normant E, West K. Ansamycin inhibitors of Hsp90: nature’s prototype for anti-chaperone therapy. Curr Top Med Chem. 2009;9:1386–1418. doi: 10.2174/156802609789895719. [DOI] [PubMed] [Google Scholar]
- 102.Schenk E, Hendrickson AEW, Northfelt D, Toft DO, Ames MM, Menefee M, et al. Phase I study of tanespimycin in combination with bortezomib in patients with advanced solid malignancies. Invest New Drugs. United States; 2013;31:1251–6. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med10&NEWS=N&AN=23543109. [DOI] [PMC free article] [PubMed]
- 103.Rapisarda A, Uranchimeg B, Scudiero DA, Selby M, Sausville EA, Shoemaker RH, et al. Identification of Small Molecule Inhibitors of Hypoxia-inducible Factor 1 Transcriptional Activation Pathway. Cancer Res. 2002;62:4316. Available from: http://cancerres.aacrjournals.org/content/62/15/4316.abstract. [PubMed]
- 104.Rapisarda A, Uranchimeg B, Sordet O, Pommier Y, Shoemaker RH, Melillo G. Topoisomerase I-Mediated Inhibition of Hypoxia-Inducible Factor 1. Cancer Res. 2004;64:1475. Available from: http://cancerres.aacrjournals.org/content/64/4/1475.abstract. [DOI] [PubMed]
- 105.Cheng J, Khin KT, Jensen GS, Liu A, Davis ME. Synthesis of Linear, β-Cyclodextrin-Based Polymers and Their Camptothecin Conjugates. Bioconjug Chem. American Chemical Society; 2003;14:1007–17. Available from: 10.1021/bc0340924. [DOI] [PubMed]
- 106.Gaur S, Chen L, Yen T, Wang Y, Zhou B, Davis M, et al. Preclinical study of the cyclodextrin-polymer conjugate of camptothecin CRLX101 for the treatment of gastric cancer. Nanomedicine. 2012;8:721–30. Available from: https://www.sciencedirect.com/science/article/pii/S1549963411003649. [DOI] [PubMed]
- 107.Pham E, Yin M, Peters CG, Lee CR, Brown D, Xu P, et al. Preclinical Efficacy of Bevacizumab with CRLX101, an Investigational Nanoparticle–Drug Conjugate, in Treatment of Metastatic Triple-Negative Breast Cancer. Cancer Res. 2016;76:4493. Available from: http://cancerres.aacrjournals.org/content/76/15/4493.abstract. [DOI] [PubMed]
- 108.Clark AJ, Wiley DT, Zuckerman JE, Webster P, Chao J, Lin J, et al. CRLX101 nanoparticles localize in human tumors and not in adjacent, nonneoplastic tissue after intravenous dosing. Proceedings of the National Academy of Sciences. 2016;113:3850. Available from: http://www.pnas.org/content/113/14/3850.abstract. [DOI] [PMC free article] [PubMed]
- 109.Keefe SM, Hoffman-Censits J, Cohen RB, Mamtani R, Heitjan D, Eliasof S, et al. Efficacy of the nanoparticle-drug conjugate CRLX101 in combination with bevacizumab in metastatic renal cell carcinoma: results of an investigator-initiated phase I-IIa clinical trial. Ann Oncol. England; 2016;27:1579–85. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med13&NEWS=N&AN=27457310. [DOI] [PMC free article] [PubMed]
- 110.Voss MH, Hussain A, Vogelzang N, Lee JL, Keam B, Rha SY, et al. A randomized phase II trial of CRLX101 in combination with bevacizumab versus standard of care in patients with advanced renal cell carcinoma. Ann Oncol. England; 2017;28:2754–60. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med14&NEWS=N&AN=28950297. [DOI] [PubMed]
- 111.Jonasch E, Donskov F, Iliopoulos O, Rathmell WK, Narayan VK, Maughan BL, et al. Belzutifan for Renal Cell Carcinoma in von Hippel–Lindau Disease. New England Journal of Medicine. Massachusetts Medical Society; 2021;385:2036–46. 10.1056/NEJMoa2103425. [DOI] [PMC free article] [PubMed]
- 112.Choueiri TK, Bauer TM, Papadopoulos KP, Plimack ER, Merchan JR, McDermott DF, et al. Inhibition of hypoxia-inducible factor-2alpha in renal cell carcinoma with belzutifan: a phase 1 trial and biomarker analysis. Nat Med. United States; 2021;27:802–5. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med18&NEWS=N&AN=33888901. [DOI] [PMC free article] [PubMed]
- 113.Courtney KD, Infante JR, Lam ET, Figlin RA, Rini BI, Brugarolas J, et al. Phase I Dose-Escalation Trial of PT2385, a First-in-Class Hypoxia-Inducible Factor-2alpha Antagonist in Patients With Previously Treated Advanced Clear Cell Renal Cell Carcinoma. J Clin Oncol. United States; 2018;36:867–74. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med15&NEWS=N&AN=29257710. [DOI] [PMC free article] [PubMed]
- 114.Riedel RF, Meadows KL, Lee PH, Morse MA, Uronis HE, Blobe GC, et al. Phase I study of pazopanib plus TH-302 in advanced solid tumors. Cancer Chemother Pharmacol. Germany; 2017;79:611–9. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med14&NEWS=N&AN=28238078. [DOI] [PubMed]
- 115.Badar T, Handisides DR, Benito JM, Richie MA, Borthakur G, Jabbour E, et al. Phase I study of evofosfamide, an investigational hypoxia-activated prodrug, in patients with advanced leukemia. Am J Hematol. 2016/06/25. 2016;91:800–5. Available from: https://pubmed.ncbi.nlm.nih.gov/27169385. [DOI] [PMC free article] [PubMed]
- 116.Borad MJ, Reddy SG, Bahary N, Uronis HE, Sigal D, Cohn AL, et al. Randomized Phase II Trial of Gemcitabine Plus TH-302 Versus Gemcitabine in Patients With Advanced Pancreatic Cancer. J Clin Oncol. United States; 2015;33:1475–81. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med12&NEWS=N&AN=25512461. [DOI] [PMC free article] [PubMed]
- 117.Laubach JP, Liu C-J, Raje NS, Yee AJ, Armand P, Schlossman RL, et al. A Phase I/II Study of Evofosfamide, A Hypoxia-activated Prodrug with or without Bortezomib in Subjects with Relapsed/Refractory Multiple Myeloma. Clinical Cancer Research. 2019;25:478. Available from: http://clincancerres.aacrjournals.org/content/25/2/478.abstract. [DOI] [PMC free article] [PubMed]
- 118.Tap WD, Papai Z, van Tine BA, Attia S, Ganjoo KN, Jones RL, et al. Doxorubicin plus evofosfamide versus doxorubicin alone in locally advanced, unresectable or metastatic soft-tissue sarcoma (TH CR-406/SARC021): an international, multicentre, open-label, randomised phase 3 trial. Lancet Oncol. England; 2017;18:1089–103. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med14&NEWS=N&AN=28651927. [DOI] [PMC free article] [PubMed]
- 119.van Cutsem E, Lenz H-J, Furuse J, Tabernero J, Heinemann V, Ioka T, et al. MAESTRO: A randomized, double-blind phase III study of evofosfamide (Evo) in combination with gemcitabine (Gem) in previously untreated patients (pts) with metastatic or locally advanced unresectable pancreatic ductal adenocarcinoma (PDAC) J Clin Oncol. 2016;34:4007–4007. doi: 10.1200/JCO.2016.34.15_suppl.4007. [DOI] [Google Scholar]
- 120.Lara PNJ, Frankel P, Mack PC, Gumerlock PH, Galvin I, Martel CL, et al. Tirapazamine plus carboplatin and paclitaxel in advanced malignant solid tumors: a california cancer consortium phase I and molecular correlative study. Clin Cancer Res. United States; 2003;9:4356–62. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med5&NEWS=N&AN=14555506. [PubMed]
- 121.Rischin D, Peters L, Hicks R, Hughes P, Fisher R, Hart R, et al. Phase I trial of concurrent tirapazamine, cisplatin, and radiotherapy in patients with advanced head and neck cancer. J Clin Oncol. United States; 2001;19:535–42. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med4&NEWS=N&AN=11208848. [DOI] [PubMed]
- 122.Abi-Jaoudeh N, Dayyani F, Chen PJ, Fernando D, Fidelman N, Javan H, et al. Phase I Trial on Arterial Embolization with Hypoxia Activated Tirapazamine for Unresectable Hepatocellular Carcinoma. J Hepatocell Carcinoma. New Zealand; 2021;8:421–34. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=pmnm&NEWS=N&AN=34041204. [DOI] [PMC free article] [PubMed]
- 123.Le Q-TX, Moon J, Redman M, Williamson SK, Lara PNJ, Goldberg Z, et al. Phase II study of tirapazamine, cisplatin, and etoposide and concurrent thoracic radiotherapy for limited-stage small-cell lung cancer: SWOG 0222. J Clin Oncol. United States; 2009;27:3014–9. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med7&NEWS=N&AN=19364954. [DOI] [PMC free article] [PubMed]
- 124.Williamson SK, Crowley JJ, Lara PNJ, McCoy J, Lau DHM, Tucker RW, et al. Phase III trial of paclitaxel plus carboplatin with or without tirapazamine in advanced non-small-cell lung cancer: Southwest Oncology Group Trial S0003. J Clin Oncol. United States; 2005;23:9097–104. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med6&NEWS=N&AN=16361616. [DOI] [PubMed]
- 125.McKeage MJ, Jameson MB, Ramanathan RK, Rajendran J, Gu Y, Wilson WR, et al. PR-104 a bioreductive pre-prodrug combined with gemcitabine or docetaxel in a phase Ib study of patients with advanced solid tumours. BMC Cancer. England; 2012;12:496. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med9&NEWS=N&AN=23098625. [DOI] [PMC free article] [PubMed]
- 126.Jameson MB, Rischin D, Pegram M, Gutheil J, Patterson A v, Denny WA, et al. A phase I trial of PR-104, a nitrogen mustard prodrug activated by both hypoxia and aldo-keto reductase 1C3, in patients with solid tumors. Cancer Chemother Pharmacol. Germany; 2010;65:791–801. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med8&NEWS=N&AN=20012293. [DOI] [PubMed]
- 127.Marina Konopleva, Peter F. Thall, Cecilia Arana Yi, Gautam Borthakur, Andrew Coveler, Carlos Bueso-Ramos, et al. Phase I/II study of the hypoxia-activated prodrug PR104 in refractory/relapsed acute myeloid leukemia and acute lymphoblastic leukemia. Haematologica. 2015;100:927–34. Available from: https://haematologica.org/article/view/7438. [DOI] [PMC free article] [PubMed]
- 128.Ramanathan RK, Kirkpatrick DL, Belani CP, Friedland D, Green SB, Chow H-HS, et al. A Phase I pharmacokinetic and pharmacodynamic study of PX-12, a novel inhibitor of thioredoxin-1, in patients with advanced solid tumors. Clin Cancer Res. United States; 2007;13:2109–14. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med6&NEWS=N&AN=17404093. [DOI] [PubMed]
- 129.Ramanathan RK, Stephenson JJ, Weiss GJ, Pestano LA, Lowe A, Hiscox A, et al. A phase I trial of PX-12, a small-molecule inhibitor of thioredoxin-1, administered as a 72-hour infusion every 21 days in patients with advanced cancers refractory to standard therapy. Invest New Drugs. United States; 2012;30:1591–6. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med9&NEWS=N&AN=21863237. [DOI] [PubMed]
- 130.Borthakur G, Ofran Y, Tallman MS, Foran J, Uy GL, DiPersio JF, et al. BL-8040 CXCR4 antagonist is safe and demonstrates antileukemic activity in combination with cytarabine for the treatment of relapsed/refractory acute myelogenous leukemia: An open-label safety and efficacy phase 2a study. Cancer. John Wiley & Sons, Ltd; 2021;127:1246–59. 10.1002/cncr.33338. [DOI] [PubMed]
- 131.Cooper TM, Sison EAR, Baker SD, Li L, Ahmed A, Trippett T, et al. A phase 1 study of the CXCR4 antagonist plerixafor in combination with high-dose cytarabine and etoposide in children with relapsed or refractory acute leukemias or myelodysplastic syndrome: A Pediatric Oncology Experimental Therapeutics Investigators’ Co. Pediatr Blood Cancer. John Wiley & Sons, Ltd; 2017;64:e26414. 10.1002/pbc.26414. [DOI] [PMC free article] [PubMed]
- 132.Gail J. Roboz, Ellen K. Ritchie, Yulia Dault, Linda Lam, Danielle C. Marshall, Nicole M. Cruz, et al. Phase I trial of plerixafor combined with decitabine in newly diagnosed older patients with acute myeloid leukemia. Haematologica. 2018;103:1308–16. Available from: https://haematologica.org/article/view/8552. [DOI] [PMC free article] [PubMed]
- 133.Martínez-Cuadrón D, Boluda B, Martínez P, Bergua J, Rodríguez-Veiga R, Esteve J, et al. A phase I-II study of plerixafor in combination with fludarabine, idarubicin, cytarabine, and G-CSF (PLERIFLAG regimen) for the treatment of patients with the first early-relapsed or refractory acute myeloid leukemia. Ann Hematol. 2018;97:763–72. doi: 10.1007/s00277-018-3229-5. [DOI] [PubMed] [Google Scholar]
- 134.Becker PS, Foran JM, Altman JK, Yacoub A, Castro JE, Sabbatini P, et al. Targeting the CXCR4 Pathway: Safety, Tolerability and Clinical Activity of Ulocuplumab (BMS-936564), an Anti-CXCR4 Antibody, in Relapsed/Refractory Acute Myeloid Leukemia. Blood. 2014;124:386. doi: 10.1182/blood.V124.21.386.386. [DOI] [Google Scholar]
- 135.Huang X, Ding L, Bennewith KL, Tong RT, Welford SM, Ang KK, et al. Hypoxia-Inducible mir-210 Regulates Normoxic Gene Expression Involved in Tumor Initiation. Mol Cell. Elsevier; 2009;35:856–67. 10.1016/j.molcel.2009.09.006. [DOI] [PMC free article] [PubMed]
- 136.Crosby ME, Kulshreshtha R, Ivan M, Glazer PM. MicroRNA Regulation of DNA Repair Gene Expression in Hypoxic Stress. Cancer Res. 2009;69:1221. Available from: http://cancerres.aacrjournals.org/content/69/3/1221.abstract. [DOI] [PMC free article] [PubMed]
- 137.Won Kim H, Haider HK, Jiang S, Ashraf M. Ischemic Preconditioning Augments Survival of Stem Cells via miR-210 Expression by Targeting Caspase-8-associated Protein 2 </sup>. Journal of Biological Chemistry. Elsevier; 2009;284:33161–8. 10.1074/jbc.M109.020925. [DOI] [PMC free article] [PubMed]
- 138.Ono S, Oyama T, Lam S, Chong K, Foshag LJ, Hoon DSB. A direct plasma assay of circulating microRNA-210 of hypoxia can identify early systemic metastasis recurrence in melanoma patients. Oncotarget. United States; 2015;6:7053–64. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med12&NEWS=N&AN=25749524. [DOI] [PMC free article] [PubMed]
- 139.Irlam-Jones JJ, Eustace A, Denley H, Choudhury A, Harris AL, Hoskin PJ, et al. Expression of miR-210 in relation to other measures of hypoxia and prediction of benefit from hypoxia modification in patients with bladder cancer. Br J Cancer. England; 2016;115:571–8. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med13&NEWS=N&AN=27441495. [DOI] [PMC free article] [PubMed]
- 140.Swietach P, Hulikova A, Vaughan-Jones RD, Harris AL. New insights into the physiological role of carbonic anhydrase IX in tumour pH regulation. Oncogene. 2010;29:6509–21. doi: 10.1038/onc.2010.455. [DOI] [PubMed] [Google Scholar]
- 141.Sathornsumetee S, Cao Y, Marcello JE, Herndon JE 2nd, McLendon RE, Desjardins A, et al. Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with bevacizumab and irinotecan. J Clin Oncol. United States; 2008;26:271–8. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med7&NEWS=N&AN=18182667. [DOI] [PMC free article] [PubMed]
- 142.Ruhle A, Grosu A-L, Wiedenmann N, Stoian R, Haehl E, Zamboglou C, et al. Immunohistochemistry-based hypoxia-immune prognostic classifier for head-and-neck cancer patients undergoing chemoradiation - Post-hoc analysis from a prospective imaging trial. Radiother Oncol. Ireland; 2021;159:75–81. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med18&NEWS=N&AN=33753155. [DOI] [PubMed]
- 143.Forker L, Gaunt P, Sioletic S, Shenjere P, Potter R, Roberts D, et al. The hypoxia marker CAIX is prognostic in the UK phase III VorteX-Biobank cohort: an important resource for translational research in soft tissue sarcoma. Br J Cancer. England; 2018;118:698–704. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med15&NEWS=N&AN=29235571. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


