Abstract
The black yeast Exophiala (Wangiella) dermatitidis is an increasingly recognized pathogen and a leading cause of severe pheohyphomycosis. Melanin is thought to contribute to the virulence of E. dermatitidis. Whereas the synthesis and the redox properties of melanin have been studied intensively, the influence of melanin and carotenoids on the phagocytosis, the oxidative burst, and the killing of E. dermatitidis by human neutrophils has not been studied. To study their effects on these phenomena, we applied a combination of flow cytometry and a colony-count-dependent method. Using E. dermatitidis wild-type strain 8565 and several melanin-deficient mutants that have been described previously, we demonstrate that melanin prevents this pathogen from being killed in the phagolysosome of the neutrophils. Melanin did not influence the phagocytosis or the oxidative burst of the neutrophils involved. The carotenoids torulene and torularhodine were not found to contribute to the prevention of killing. The ability of E. dermatitidis to block the effects of the neutrophil oxidative burst may critically impair the potential of the host to sufficiently eliminate this fungal pathogen and thus may play an important role in the pathogenesis of phaeohyphomycosis.
Exophiala (Wangiella) dermatitidis is a black yeast frequently isolated from patients suffering from phaeohyphomycosis (38). Due to the distinct neurotropism of E. dermatitidis (1, 26, 36, 37), pheohyphomycosis due to this fungus can be life-threatening. Severe courses are observed mainly in immunocompetent patients in Southeast Asia, whereas disease outside this region occurs mainly in patients with underlying malignancies or in patients with otherwise impaired immunity (37). Beside rare traumatic mycoses due to inoculation of contaminated material from the environment, this zoopathogenic fungus also leads to long-term, mostly subclinical colonization of the lungs of patients with cystic fibrosis (2, 4, 23, 24, 32).
The taxonomy of E. dermatitidis and allied black yeast species is still undefined (21, 37). However, Emmons strain 8656 (8) and its mutants used throughout this study have been genetically analyzed and are phylogenetically highly related to the type strain of E. dermatitidis CBS 207.35, as been shown by analysis of nuclear 18SrRNA genes (25). It is referred to in this report as E. dermatitidis.
Pigments such as melanin and carotenoids are deposited in the cell wall of demataceous hyphomycetes and play an important role in the pathogenicity of these fungi in plants and animals (15, 43, 44, 56). E. dermatitidis and the majority of zoopathogenic dematicious fungi with an ascomycetal affiliation produce melanin endogenously from acetate via the pentaketide pathway with polymerization of endogenous 1,8-dihydroxynaphthalene (DHN) as the last step of synthesis (51). This melanin is different in its synthesis and structure from that of Cryptococcus neoformans, which is synthesized from 3,4-dihydroxyphenylalanine via tyrosinase, with polymerization of dopachrome as the last step of synthesis (42, 56). The redox function of these melanins has been shown to be identical (29, 30). Since melanin-deficient strains of C. neoformans and E. dermatitidis exhibit a decreased virulence, melanin is believed to play a key role in the pathogenicity of these fungi (14, 28, 30, 33, 54, 55). However, the mechanism by which melanin contributes to the virulence of these fungi is poorly understood. In C. neoformans, phagocytosis of a melanized strain by a mouse macrophage-like cell line (J774.16) was less than that of the nonmelanized strain or a melanin-deficient mutant (54). Jacobson et al. demonstrated that the amount of melanin produced by C. neoformans is sufficient to neutralize a large proportion of the cellular oxidant produced by stimulated human macrophages (17, 30). These findings were paralleled for DHN-melanin in E. dermatitidis and Alternaria alternata, which protected these fungi from oxidants produced by permanganate and hypochlorite (29). These data and other studies (19, 28, 55) suggest that the effect of melanin on the survival and the virulence of E. dermatitidis is mainly due to its protective role against oxidants produced by host effector cells or environmental stress. Besides melanin, E. dermatitidis synthesizes the carotenoids 3,4-didehydro-γ-carotene (torulene) and 3,4-didehydro-γ-caroten-16-oic acid (torularhodin) (18). These carotenoids significantly increase post-UV irradiation survival rates of the melanin-deficient E. dermatitidis strain Mel−4 (18), and these authors suggested that the mechanism of carotenoid action is more likely to consist in shielding sensitive molecules or organelles rather than in neutralization of harmful oxidants. However, the influence of these carotenoids on the virulence of E. dermatitidis has not been studied. Dixon et al. (11, 13, 14) observed a reduced virulence in mice when a mutant of E. dermatitidis 8656 (Mel−3), which lacks both melanin and carotenoids, was used, suggesting that the reduced virulence of this mutant is due to a lack of melanin and/or the carotenoids.
Neither the phagocytosis, the oxidative burst, and the killing of black yeasts by human neutrophils nor the effect of melanin or carotenoids on these processes has been studied. The aim of this study was (i) to establish a method for the determination of these phagocyte functions and (ii) to investigate the effect of melanin and carotenoids of E. dermatitidis on these processes. Clarification of these questions should provide a basis on which to investigate the mechanisms leading to the two extremes of E. dermatitidis human infection outcome: long-term colonization of cystic fibrosis (CF) patients with little influence on health and fatal infections in otherwise healthy individuals by the same species, a phenomenon which could not be attributed to differences in strains (52).
We describe here a flow-cytometric method in combination with a colony-count-dependent method to study phagocytosis, oxidative burst, and killing of E. dermatitidis by human neutrophils. Using several melanin- and/or carotenoid-deficient mutants of E. dermatitidis wild-type strain 8656 (ATCC 34100), which has been previously described (18, 19), we studied the effect of melanin and the carotenoids on these processes. The wild-type strain and its melanin and/or carotenoid-defective mutant strains were phagocytized by and evoked an oxidative burst in the human granulocytes to an almost identical degree. However, there was an increased killing of the melanin-deficient strains in comparison to the wild-type strain, an effect which could be reversed by crossfeeding this strain with a precursor substance, thus leading to melanization. In contrast, we observed no influence of the carotenoids on the killing of the respective strains. These results strongly suggest that it is melanin, and not the carotenoid pigments, that plays a role in protecting E. dermatitidis against antifungal activity of neutrophils during infection.
MATERIALS AND METHODS
Fungal strains.
We used the black wild-type strain Emmons 8565 (ATCC 34100) of E. dermatitidis (8), which has been extensively described (20, 27, 45–47, 50). The mutant Mel−3 (ATCC 44504) was isolated as a spontaneous albino mutant of strain 8656 (19). The mutants Mel−1 (ATCC 44502), Mel−2 (ATCC 44503), and Mel−4 (ATCC 58058) had been produced previously by using N-methyl-N-nitroN-nitrosoguanidine mutagenesis (9, 18, 19). The color of colonies and defects in melanin and/or carotenoid synthesis of the respective strains are summarized in Table 1. Strain 8656 produces both melanin and carotenoids and appears as dark brown colonies; colonies of Mel−2 are brown to dark brown due to the oxidative polymerization of DHN in aqueous solution. Colonies of the Mel−1, Mel−3, and Mel−4 mutants are reddish brown, white to opaque, and red-orange, respectively. These appearances are due to scytalone and/or carotenoid synthesis (Mel−1) and not to melanin or to carotenoid synthesis (Mel−3), or are due to carotenoid synthesis only (Mel−4).
TABLE 1.
Melanin and carotenoid production of E. dermatitidis 8656 and its mutants
| Strain | ATCC no. | Colony color | Defect in pigment synthesis | Pigments produced |
|---|---|---|---|---|
| 8656 | 34100 | Black | No defect | Melanin and carotenoids |
| Mel−1 | 44502 | Reddish brown | Production of THN from scytalone (19) | Scytalone and carotenoids |
| Mel−2 | 44503 | Dark brown | Production of melanin from DHN (19) | DHN (unstable), carotenoids |
| Mel−3 | 44504 | Hyaline to white | Production of 1,3,6,8-tetrahydroxynaphthalene from acetate, carotenoid synthesis (9, 19) | None |
| Mel−4 | 58058 | Orange to red | Melanin synthesis (no details known) (18) | Carotenoids, but no melanin precursors |
Culture conditions.
For long-term storage, all strains studied were stored at −70°C by using the MICROBANK system (PRO-LAB Inc., Richmond Hill, Ontario, Canada). Strains were plated on Sabouraud agar (Oxoid, Wesel, Germany) containing 2% glucose and incubated at 37°C for 4 days. One colony of such grown yeast cells was suspended in 30 ml of Sabouraud broth (Oxoid) and incubated at 37°C for 7 days in a tissue culture flask while being rotated and illuminated with a grow light bulb (Philips, Aachen, Germany) to ensure melanin production and the presence of thick-walled yeast cells, which are known to harbor sufficient amounts of melanin (31).
Melanization of Mel−3 mutant was achieved by cross-feeding (19). Yeast cells were cultivated in Sabouraud broth supplemented with 20% (vol/vol) sterile filtrated supernatant of a 7-day-old liquid culture of the Mel−1 mutant. Mel−1 yeast cells accumulate high amounts of scytalone that is secreted into the culture medium and can be utilized by the Mel−3 mutant to synthesize melanin (9). As a control the same procedure was performed with the Mel−2 mutant instead of Mel−3.
Albinization of the wild-type strain 8656 was achieved by growing yeast cells in an acid ascorbate broth (6, 39).
After incubation, the yeast cells were sedimented by centrifugation (3,500 × g, 4°C, 10 min), washed twice with 1 volume of phosphate-buffered saline (PBS; pH = 7.4), and resuspended in 5 ml of PBS. Yeast cells in PBS were kept on ice until further processing.
Phagocytosis assay by using flow cytometry.
To assess the phagocytosis of yeast cells by human neutrophils, sedimented yeast cells from 1 ml of the PBS suspension were incubated with bis-carobxyethyl-carboxyfluorescein-pentaacetoxy-methylester (BCECF/AM; final concentration, 1 μmol/liter) (Boehringer, Mannheim, Germany) for 30 min at 37°C in 1 ml of PBS as previously described for Candida albicans (34). Nonfluorescent BCECF/AM diffuses into the yeast cells and is cleaved by cytoplasmatic esterases to yield the fluorescent, membrane-impermeable product bis-carobyxethyl-carboxyfluorescein (BCECF) that remains trapped in viable yeast cells (3, 5, 34, 35). Then, 5 × 106 of such labeled cells were incubated at 37°C for a maximum of 120 min in 1 ml of heparinized (10 IE = ca. 5USP/ml) whole blood from healthy blood donors (aged 20 to 40 years) in a thermomixer (Eppendorf, Hamburg, Germany) at 1,000 rpm. At 0, 15, 30, 60, and 120 min, 100 μl was removed and immediately mixed with 2 ml of ice-cold lysis buffer (Becton Dickinson, Heidelberg, Germany) to lyse the erythrocytes. These samples were kept on ice for a maximum of 2 h until the isolation of leukocytes and yeast cells by centrifugation (10 min, 4°C, 1,300 rpm; Beckman GS-6R centrifuge) and then washed twice in ice-cold PBS. Leukocytes and yeast cells were resuspended in 500 μl of PBS and analyzed by flow cytometry.
To assess the oxidative burst during the phagocytosis process, unlabeled yeast cells were incubated in heparinized whole blood under identical conditions to those described above. Dihydrorhodamine 123 (DHR) (Molecule Probes, Eugene, Oreg.) was added to a final concentration of 10 mg/liter of blood. DHR is freely permeable, localizes in the mitochondria of neutrophils and, after oxidation by H2O2 and O2− to rhodamine 123 during the respiratory burst, emits a bright green fluorescent signal upon excitation by blue light (488 nm) (47, 48). Flow-cytometric analysis was performed with a FACScan flow cytometer by using Cellquest software (all by Becton Dickinson) (41, 49). The instrument settings were as follows: the forward scatter (FSC) threshold was set at 52; the detectors were set at E00, 338, 528, and 560 for FSC, sideward scatter (SSC), fluorescence 1 (FL1; green fluorescence), and fluorescence 2 (FL2; red fluorescence), respectively. Linear parameters were used for FSC and SSC, whereas logarithmic parameters were used for FL1 and FL2. Neutrophils were selectively analyzed by gating them according to their relative size (FSC) and granularity (SSC), and E. dermatitidis cells according to their relative granularity (SSC) and their green fluorescence (FL1). Both the association of neutrophils with the BCECF/AM-labeled yeast cells and the oxidative burst of the neutrophils induced by unlabeled yeast cells in the presence of DHR are expressed as an increase of the green fluorescence of the neutrophils. In the experiments with labeled E. dermatitidis cells, the concomitant decrease of these free yeast cells was also analyzed.
Killing assay.
To quantify the killing of the E. dermatitidis strains tested by the human neutrophils, strains were cultured as described above. The harvested yeast cells were diluted in PBS to 103 to 104 CFU per ml. Next, 100 μl of diluted cultures and 900 μl of fresh heparinized human blood were mixed and rotated at 37°C for 4 h. Initial viable counts and counts after 10 min, 2 h, and 4 h of rotation were determined by plating 20 μl of the samples and 20 μl of the same samples diluted with 180 μl of PBS prior to plating onto Sabouraud agar.
Microscopy.
To ensure the intracellular location of the yeast cells associated with the neutrophils, representative samples used for determination in flow cytometry were examined by epifluorescence interference contrast microscopy (Leitz DM RB; Leica, Wetzlar, Germany) as previously described (41, 49). From three independent assays of each strain studied, we examined 300 yeast cells with respect to their association with the neutrophils at the beginning, after 30 min, and after 60 min of incubation in heparizined blood. Budding yeast cells were counted as one cell as long as the length of the daughter cell did not exceed half the length of the mother cell.
Statistical methods.
Differences in the phagocytosis, the oxidative burst, and the killing of E. dermatitidis 8656 and its mutants were analyzed by the nonparametric Mann-Whitney U test. Probability values of <0.05 were considered to be significant.
RESULTS
Fluorescence staining of E. dermatitidis.
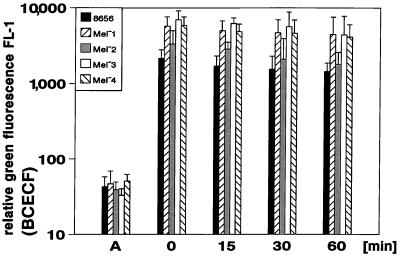
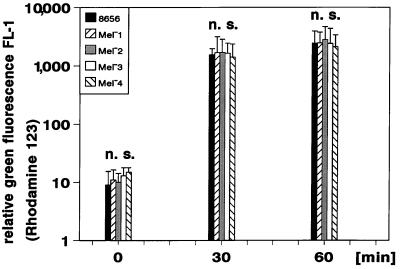
E. dermatitidis strains were stained by incubation for 30 min in PBS containing 1 μmol of BCECF/AM per liter. This procedure led to a stable green fluorescence of the parent strain and all its mutants when excited by the light of the argon laser of the flow cytometer (488 nm). Figure 1 shows the resulting relative green fluorescence of all strains studied from six independent assays (mean values ± standard deviation [SD]). Dark pigmented wild-type strain and the Mel−2 mutant exhibited a lower green fluorescence compared with the Mel−1, Mel−3, and Mel−4 mutants; however, the resulting green fluorescence even of the dark pigmented cells was strong enough to be sufficiently determined in flow cytometry. Fluorescence decreased slightly over the time without affecting the detection either of labeled yeast cells or of the neutrophils after phagocytosis of such cells (Fig. 1). The BCECF-labeled yeast cells were not affected in their viability and showed no difference in their killing kinetics compared with unlabeled cells (data not shown).
FIG. 1.
Staining of E. dermatitidis with BCECF/AM as determined by flow cytometry. Relative green fluorescence results of six independent assays are shown prior to the staining procedure (A), immediately after the staining (0 min), and at 15, 30, and 60 min after the end of coincubation with the dye (mean values ± SD are shown).
Phagocytosis of E. dermatitidis.
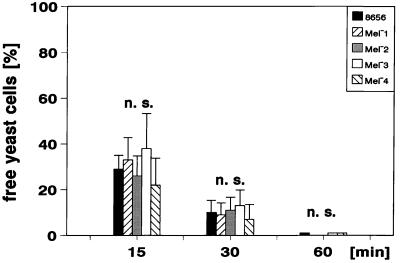
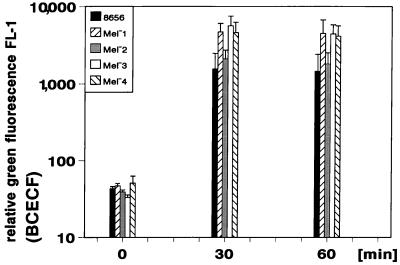
To exclude an effect of activation of neutrophils due to isolation procedures, we used heparinized whole blood to measure the phagocytosis of the different yeast cells. Strain 8656 and all mutants were phagocytozed by the human neutrophils. Figure 2 shows the decrease in free E. dermatitidis cells soon after their incubation in the blood (mean values ± SD; n = 6). By 15 min after the incubation of the fluorescent yeast cells, about 10 to 55% of the initially added 106 yeast cells per ml of blood were not bound by the neutrophils. After 30 min this portion was reduced to about 3 to 25%, and after 60 min all of the yeast cells were bound to or ingested by the neutrophils. The differences in binding and phagocytosis by neutrophils for the different strains tested were not significant. Binding of the yeast cells by the neutrophils resulted in a concomitant increase in green fluorescing neutrophils. Figure 3 shows the results of six independent assays (mean values ± SD). As the dark-pigmented strains were found to be stained to a lower degree (Fig. 1) and the extent of relative green fluorescence of the phagocytyzing neutrophils depended on the fluorescence intensity of the phagocytized yeast cells, the fluorescence of neutrophils was lower after phagocytosis of strains 8656 and Mel−2 compared with the other strains studied (Fig. 3). At a yeast/neutrophil ratio of 0.8 to 1.2, the clearance of free yeast cells is achieved by 40 to 70% of the neutrophils. There were no significant differences regarding either the speed of the clearance of free yeast cells (Fig. 2) or the portion of neutrophils being involved (data not shown) when the wild-type strain and its mutants were compared.
FIG. 2.
Decrease of free E. dermatitidis cells during incubation in heparinized blood as determined by flow cytometry. The portion of free yeast cells compared with the inoculum added to the heparinized blood is shown at the beginning (0 min) and after 15, 30, and 60 min of the incubation of the yeast cells in heparinized blood (mean values ± SD are shown: n = 6; n.s., difference not significant).
FIG. 3.
Kinetics of phagocytosis of E. dermatitidis as determined by flow cytometry. The subsequent increase of relative green fluorescence of neutrophils after association with the respective BCECF-labeled E. dermatitidis strains is shown. The mean values ± SD of six independent assays are displayed.
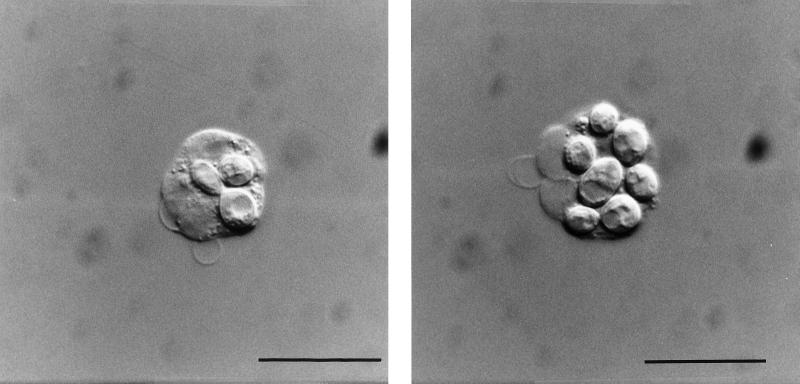
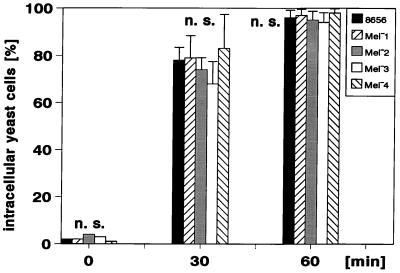
The results presented thus far do not unequivocally prove the intracellular location of the yeast cells after incubation in the heparinized blood. Therefore, we applied a combination of epifluorescence microscopy and simultaneous interference contrast microscopy to representative experimental samples (Fig. 4). After 30 min, between 60 and 98% of the yeast cells were located inside the neutrophils, and after 60 min of incubation nearly all of the yeast cells were located intracellularly within the neutrophils. In all of the strains studied, there were no significant differences concerning the portion of budding yeast cells in relation to their extra- or intracellular location. Figure 5 shows the mean values ± the SD of three independent experiments. Again, there were no significant differences when strain 8656 was compared to its melanin-deficient mutants. Furthermore, epifluorescence interference contrast microscopy and the flow cytometric assay showed an excellent correspondence in the phagocytosis of yeast cells by the neutrophils.
FIG. 4.
Intracellular location of the BCECF/AM-stained E. dermatitidis 8656 as determined by interference contrast microscopy. After 60 min of incubation in heparinized blood, the yeast cells are located within the neutrophils (Bar = 10 μm). (Left panel) Neutrophil with two yeast cells ingested, one of them budding. (Right panel) Neutrophil with maximum number of yeast cells ingested. These microorganisms could be also identified as yeast cells due to their green fluorescence in simultaneously performed epifluorescence microscopy studies.
FIG. 5.
Kinetics of the ingestion of E. dermatitidis as determined by epifluorescence interference contrast microscopy. The portion of intracellularly located yeast cells of 300 yeast cells judged for their association with the neutrophils is shown. The data represent mean values ± SD of three independent counts (n.s., difference not significant).
Oxidative burst evoked by E. dermatitidis.
To determine the production of reactive oxygen intermediates such as O2− or OH− and H2O2 during the respiratory burst, unlabeled yeast cells were incubated in heparinized blood in the presence of DHR (48, 53). Phagocytosis of the E. dermatitidis wild-type strain or its melanin- and/or carotenoid-defective mutants was paralleled by an oxidative burst of the phagocytozing neutrophils, leading to a green fluorescence of the neutrophils involved (Fig. 6). The degree in the oxidative burst was found to be not significantly different when the parent strain 8656 was compared with its melanin-deficient mutants.
FIG. 6.
Kinetics of oxidative burst evoked by phagocytized E. dermatitidis as determined by flow cytometry. The results of studies of relative green fluorescence of neutrophils exhibiting an oxidative burst after the phagocytosis of nonlabeled yeast cells in the presence of DHR (n = 6; mean values ± SD; n.s., difference not significant) are shown.
The wild-type strain is killed to a lesser extent than the Mel− mutants.
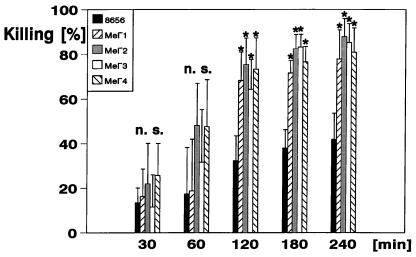
Whereas the melanin-deficient strains showed no significant differences in phagocytosis by the neutrophils or the oxidative burst evoked in the neutrophils compared with the wild-type strain, the mutants showed a striking difference concerning the outcome of the strains in the killing assay. Beginning with ca. 40 CFU per 100 μl of heparinized whole blood, after 4 h the number of CFU decreased to a median of 24.8 CFU (range, 17 to 35 CFU) in the case of the wild-type strain 8656, whereas the median numbers of remaining CFU were 8.83 (range, 3 to 13), 5.17 (range, 3 to 10), 6.00 (range, 2 to 9), and 8.0 (range, 3 to 14) in the cases of the Mel−1, Mel−2, Mel−3, and Mel−4 mutants, respectively. Figure 7 shows the decrease in CFU numbers compared with the inoculum (percent killing, mean values ± SD; n = 6). Whereas the differences between strain 8656 and its mutants were highly significant (P < 0.005), there were no significant differences between all of the melanin-deficient mutants (Fig. 7).
FIG. 7.
Percent killing of E. dermatitidis as determined by colony counts prior to and after incubation in heparinized blood. The killing is displayed as a percentage of the decrease in the number of CFU during the incubation times indicated (n = 6; mean values ± SD) ∗, Significantly different (P < 0.005); n.s., not significantly different from strain 8656 as determined by the Mann-Whitney U test.
Melanin decreases killing of E. dermatitidis.
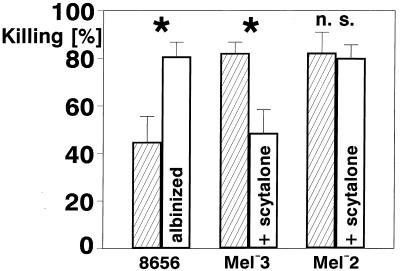
To show unequivocally that strain 8656 is protected by its melanin from being killed by the neutrophils, we albinized this strain by growing it in a low-pH medium (6, 9). The resulting yeast cells appeared as white as the Mel−3 mutant. In addition, we melanized the Mel−3 mutant by using a cross-feeding procedure. After being cultured in the presence of scytalone-containing supernatant of a liquid Mel−1 culture, the yeast cells of Mel−3 were nearly as pigmented as the cells of the wild-type strain as judged by ocular inspection. Albinized yeast cells of strain 8656 and melanized Mel−3 yeast cells were tested in the killing assay as described above, and the percent killing after 4 h was compared with strain 8656 and nonpigmented Mel−3 (Fig. 8, mean values ± SD). After 4 h of incubation in heparinized blood, the reduction in the number of CFU of strain 8656 was a median of 43.5% (range, 31 to 60%) compared with 81% (range, 71 to 89%) after albinization (P ≤ 0.005; n = 6). The respective median values for Mel−3 were 82.5% (range, 74 to 88%) compared with 48.5% (range, 34 to 61%) after melanization (P ≤ 0.005; n = 6). When strain 8656 was compared with the melanized Mel−3 strain and the albinized strain 8656 was compared with Mel−3 there were no statistical differences in the killing rates.
FIG. 8.
Effect of melanin on the killing of strains 8656, Mel−3, and Mel−2. Strain 8656 is compared with its albinized cells, and Mel−3 is compared with its melanized cells after scytalone cross-feeding. Mel−2 cells that are not able to produce melanin from scytalone are also shown as controls. The mean values ± SD are shown; n = 6, ∗, Significantly different according to the Mann-Whitney U test (P < 0.005); n.s., differences not significant.
To exclude an effect of further substances present in the culture supernatant of Mel−1 on the killing, we compared the percent killing of native Mel−2 with that of cross-fed Mel−2 yeast cells. As expected, there was no statistical difference in the percent killing according to both culture conditions.
DISCUSSION
Melanin is thought to play a major role in the pathogenesis of infections by C. neoformans and demataceous hyphomycetes such as E. dermatitidis (12, 15, 28, 30, 33, 43, 44, 54–56). Despite several investigations of the biochemical properties of the melanin of these and other melanin-harboring species, no studies have yet addressed the phagocytosis, oxidative burst, or killing of E. dermatitidis by human neutrophils. We report here for the first time on the application of flow cytometry to study the interactions of E. dermatitidis and neutrophils, and the effect of melanin and/or carotenoid production on these phenomena.
To ensure a high melanin concentration in the cell wall of the E. dermatitidis strains, we cultured the respective fungi in Sabouraud broth for 7 days under constant illumination with a grow light bulb. When illuminated, E. dermatitidis cells are known to develop from sparely melanized thin-walled yeast cells into heavily melanized, thick-walled yeast cells within a few days (31).
Fluorescence-activated cell analysis with flow cytometry has opened up new frontiers in the analysis of phagocyte-microorganism interactions (5, 10, 34, 35, 41, 48, 49, 53). Among the large number of publications that have appeared on its application to the study of phagocytosis, the use of BCECF/AM to stain the respective microorganisms meets various requirements addressed in our study. First, this dye resulted in a prominent and stable green fluorescence of all E. dermatitidis strains studied (Fig. 1). Only black wild-type strain 8656 and the Mel−2 mutant showed a weakening green fluorescence after BCECF/AM staining. This phenomenon is most probably due to a shielding effect of DHN or the melanin localized in the fungal cell wall, which may hamper the BCECF excitation by the argon laser light, as well as the emission of the fluorescence light. Additionally, a direct influence of melanin or DHN on the fluorescence of BCECF cannot be excluded. Nevertheless, even in these highly pigmented yeast cells, green fluorescence upon BCECF/AM staining was well suited for studying the association of such labeled yeast cells with the neutrophils. Second, BCECF/AM is an intracellular stain (3, 5, 10, 34, 35, 41, 49). Such staining should not alter the surface of the respective stained cells and therefore does not influence the interactions between neutrophils and yeast cells; it does not influence viability, growth rate, or colony morphology either in C. albicans (34) or in E. dermatitidis (data not shown).
Martin and Bhakdi reported on the suitability of flow cytometry by using BCECF-stained C. albicans to determine phagocytosis, oxidative burst, and killing of these yeast cells by human neutrophils (34). In contrast to our design, they used isolated leukocytes, and killing was estimated by the lysis of leukocytes with deoxycholate and a subsequent analysis of BCECF-mediated fluorescence in liberated yeast cells (34). We recently found that especially dextran sedimentation for isolation of leukocytes leads to an activation of neutrophils and thereby influences the neutrophil-microorganism interactions (41, 49). To avoid activation of the neutrophils, we used heparinized whole blood instead of isolated neutrophils and determined the killing of the yeast cells by counting the number of CFU prior to and after incubation of the yeast cells in heparinized blood. Once the method described here was applied, lysis of the blood cells by DOC prior to culturing was found not to influence the CFU counts (data not shown). In addition, an inhibiting effect on the phagocytosis of microorganisms by the neutrophils due to the heparin added (10 IE/ml) was previously excluded (41, 49).
BCECF leads to an intracellular stain of the yeast cells (3, 5, 10). Therefore, binding of such labeled cells cannot be excluded by the quenching methods as described by Drevet and Campbell (16). To ensure the intracellular location of the yeast cells associated with the neutrophils, we performed epifluorescence microscopy in combination with interference contrast microscopy of representative experimental samples. These examinations revealed a strong correlation of the reduction of free yeast cells and the appearance of green fluorescence in the neutrophil population after incubation with BCECF-labeled yeast cells (Fig. 2 and 3) compared with the amount of ingested cells (Fig. 5). Therefore, it could be clearly shown that yeast cells bound to the neutrophils are immediately phagocytozed.
We found no differences in the phagocytosis of the wild-type strain and all of its melanin and/or carotenoid-deficient mutants. Wang et al. (54) reported an increased phagocytosis of nonmelanized C. neoformans strains when the mouse macrophage-like cell line J774.16 was used. However, C. neoformans melanin-deficient and wild-type strains were phagocytozed only in the presence of the capsule binding monoclonal antibody 2H1. Since we used only blood of healthy individuals who had previously been shown not to produce detectable concentrations of E. dermatitidis-specific antibodies (22), the observed phagocytosis of E. dermatitidis by neutrophils does not require the presence of specific antibodies.
Oxidative burst of neutrophils was estimated by using DHR, as DHR was found to be superior to other dyes used for this purpose (48, 53). The induction of an oxidative burst in the neutrophils after incubation with the yeast cells further indicates that the yeast cells are not only bound to but are also phagocytozed by the neutrophils. All granulocytes involved in the phagocytosis of unstained E. dermatitidis exhibited a bright green fluorescence in the presence of DHR. The presence of melanin might be expected to lead to reduced oxidation of DHR to the fluorescent dye rhodamin 123 due to competition by the redox function of the melanin. However, DHR is oxidized by H2O2 and negatively charged oxidants (48, 53), whereas melanin reacts mainly with negatively charged oxidants and, except under alkaline conditions (58), not with H2O2 (29, 30). Therefore, the superoxide (O2−), which is formed in the respiratory burst and spontaneously reacts with water to dismutate into hydrogen peroxide (H2O2) and O2 or is converted to other reactive oxygen intermediates such as OH−, will oxidize DHR to rhodamine 123 via H2O2, even if all the reactive oxygen intermediates are neutralized by the melanin. The carotenoids, which are known to be extremely effective in protecting chlorophyll against singlet oxygen by the quenching of chlorophyll triplets (40), are also able to scavenge singlet oxygen without being associated with chlorophyll, but with an inherently lower efficiency (40). In E. dermatitidis, the role of the carotenoids is therefore assumed to be more likely associated with a “shielding” of sensitive molecules or organelles against UV irradiation rather than with the neutralization of harmful oxidants (18). This minor role of the carotenoids concerning protection of phagolysosomal killing is reflected in the lack of protection against the killing of the carotenoid-positive, melanin-deficient strain Mel−4 (Fig. 7).
All of the melanin-deficient E. dermatitidis mutants were killed to a higher degree than the parent strain 8656 (Fig. 7). This increased killing can be unequivocally attributed to the absence of melanin, as albinization of strain 8656 led to a killing comparable to that of the albino mutant Mel−3 (Fig. 8). To further investigate the role of melanin, we performed cross-feeding experiments with supernatants of Mel−1 as a scytalone donor. The mutant Mel−1 has a defect in the production of 1,3,8-trihydroxynaphthalene (THN) from scytalone (Table 1) that accumulates in the Mel−1 cells and is secreted into the medium (9). Scytalone from supernatants of Mel−1 cultures can be utilized by the Mel−3 cells to produce melanin (9, 19), as the mutations affect melanin production in Mel−3 at a step prior to the conversion of scytalone to THN (Table 1) (9, 19). The melanin produced by the Mel−3 cells after cross-feeding has been shown to localize in the outer cell wall, a finding comparable to the localization in the wild-type strain reported in electron microscopic studies (56). Such cross-fed Mel−3 cells were brown to black and were difficult to distinguish from wild-type cells when observed under the light microscope or in pelleted form. These melanized cells showed a killing comparable to that of the wild-type strain, i.e., significantly lower than that of the nonmelanized Mel−3 cells (Fig. 8). To exclude side effects in the cross-feeding assay due to components of the supertants other than scytalone, we performed the same assay with Mel−2 cells as scytalone recipients. The mutation that affects melanin production in this mutant keeps the cells from polymerizing DHN to melanin (19) and thus is located downstream from the production of THN from scytalone. As a consequence, Mel−2 cells are not able to synthesize melanin when exogenously supplemented with scytalone. As expected, the Mel−2 cells showed no significant alteration in their killing rate when supplemented with supernatant of the Mel−1 cells (Fig. 8).
The protection of E. dermatitidis against the reactive oxygen intermediates requires the presence of the complete melanin molecule. The melanin precursor scytalone was found to have no protective function regarding the killing of the yeast cells (Mel−1 in Fig. 7). The protective function of DHN is hard to estimate since DHN is unstable and oxidatively polymerized in aqueous solution to dark, crystalline-type materials (57). These crystalline-type materials led to the dark pigmentation of the Mel−2 strain and were shown to have no protective effect for this mutant regarding the killing by the neutrophils. Even the complete melanin molecule did not protect the E. dermatitidis wild-type strain completely from being killed by the neutrophils. Since killing of the melanized yeast cells was very significantly lower than the nonmelanized mutant strains (Fig. 7; P < 0.005), we did not test muriform yeast cells of E. dermatitidis, i.e., thick-walled, heavily melanized yeast cells, in the present study. In chromoblastomycoses, an infection caused by phylogenetically closely related fungi (e.g., Cladophialophora carrionii, Fonsecaea pedrosoi, Phialophora verrucosa, and Rhinocladiella aquaspersa), sclerotic bodies can be typically seen in the infected tissue (33). They consist of thick-walled, heavily melanized yeast cells. It could be speculated that resistance to killing by the neutrophils of such sclerotic bodies is further enhanced compared with normal yeast cells, thus facilitating this type of chronic fungal infection.
Our findings that the killing of E. dermatitidis strains reaches a maximum of 75.4% after 2 h and 85.2% after 4 h (strain Mel−2) is in excellent agreement with the data presented by Martin and Bhakdi for C. albicans (maximum killing after 2 h, 75%) (34). One possible explanation for this incomplete killing of fungi based on neutrophil phagolysosomal vacuole heterogeneity has been suggested by Cech and Lehrer (7). Dixon et al. reported a striking reduction in mortality in mice compared with the parent strain 8656 after intravenous infection with the Mel−3 mutant (11, 13, 14). However, in chronic infections induced with very high doses of the Mel−3 mutant, mice developed lesions which were indistinguishable from those produced by low concentrations of the wild-type strain (11, 14). Neutrophil phagolysosomal vacuole heterogeneity might account for the incomplete killing of melanin-deficient E. dermatitidis mutant Mel−3 in mice, too, especially after a high-dose intravenous injection. In CF patients with long-term subclinical colonization of the lung, such mechanisms might contribute to the reduced elimination of the fungus.
These results show for the first time that melanin prevents E. dermatitidis from being killed by the phagolysosomal oxidative burst of human neutrophils without affecting either the phagocytosis or the induction of the oxidative burst. Furthermore, the method described here is well suited for investigating the interactions of phagocytes and demataceous hyphomycetes that are pathogenic for humans.
ACKNOWLEDGMENT
We thank Regina Holland for her excellent technical assistance.
REFERENCES
- 1.Ajanee N, Alam M, Holmberg K, Kahn J. Brain abscess caused by Wangiella dermatitidis: case report. Clin Infect Dis. 1996;23:197. doi: 10.1093/clinids/23.1.197. [DOI] [PubMed] [Google Scholar]
- 2.Benett I, Knouse C, Johnson J, Meyer R L, Millard M W. Wangiella dermatitidis from a cystic fibrosis patient. Clin Microbiol Newsl. 1997;19:164–166. [Google Scholar]
- 3.Bhakdi S, Greulich S, Muhly M, Eberspächer B, Becker H, Tiehle A, Hugo F. Potent leukocidal action of Escherichia coli hemolysin mediated by permeabilization of target cell membranes. J Exp Med. 1989;169:838–854. doi: 10.1084/jem.169.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaschke-Hellmessen, R., I. Lauterbach, K. D. Paul, K. Tintelnot, and G. Weissbach. 1994. Detection of Exophiala dermatitidis (Kano) De Hoog 1977 in septicemia of a child with acute lymphatic leukemia and in patients with cystic fibrosis. Mycoses 37(Suppl. 1):89–96. [PubMed]
- 5.Bruning J W, Kardol M J, Arentzen R. Carboxyfluorescein fluorochromasia assays. I. Non-radioactively labeled cell mediated lympholysis. J Immunol Methods. 1980;33:33–44. doi: 10.1016/0022-1759(80)90080-0. [DOI] [PubMed] [Google Scholar]
- 6.Butler M J, Lachance M A. Inhibition of melanin synthesis in the black yeast Phaeococcomyces sp. by growth on low pH ascorbate medium: production of spheroblasts from “albinized” cells. Can J Microbiol. 1987;33:184–187. [Google Scholar]
- 7.Cech P, Lehrer R I. Heterogeneity of human neutrophil phagolysosomes: functional consequences for candidacidal activity. Blood. 1984;64:147–151. [PubMed] [Google Scholar]
- 8.Cooper C R J. Induced parasexual genetic analysis of the imperfect, pathogenic fungus Wangiella dermatitidis: identification of selected morphogenic and biosynthetic complementation groups. Ph.D. thesis. Austin, Tex: University of Texas; 1989. [Google Scholar]
- 9.Cooper C R, Jr, Szaniszlo P J. Melanin as a virulence factor in dematious pathogenic fungi. In: Vanden Bossche H, Stevens D A, Odds F C, editors. Proceedings of the 5th Symposium on Topics in Mycology: Host-Fungus Interplay. Bethesda, Md: National Foundation for Infectious Diseases; 1997. pp. 81–93. [Google Scholar]
- 10.Davey H M, Kell D B. Flow cytometry and cell sorting of heterogeneous microbial populations: the importance of single-cell analyses. Microbiol Rev. 1996;60:641–695. doi: 10.1128/mr.60.4.641-696.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dixon D M, Migliozzi J, Cooper C R, Jr, Solis O, Breslin B, Szaniszlo P J. Melanized and non-melanized multicellular form mutants of Wangiella dermatitidis in mice: mortality and histopathological studies. Mycoses. 1992;35:17–21. doi: 10.1111/j.1439-0507.1992.tb00814.x. [DOI] [PubMed] [Google Scholar]
- 12.Dixon D M, Polak A. In vitro and in vivo drug studies with three agents of central nervous system phaeohyphomycosis. Chemotherapy. 1987;33:129–140. doi: 10.1159/000238485. [DOI] [PubMed] [Google Scholar]
- 13.Dixon D M, Polak A, Conner G W. Mel− mutants of Wangiella dermatitidis in mice: evaluation of multiple mouse and fungal strains. J Med Vet Mycol. 1989;27:335–341. [PubMed] [Google Scholar]
- 14.Dixon D M, Polak A, Szaniszlo P J. Pathogenicity and virulence of wild-type and melanin-deficient Wangiella dermatitidis. J Med Vet Mycol. 1987;25:97–106. doi: 10.1080/02681218780000141. [DOI] [PubMed] [Google Scholar]
- 15.Dixon D M, Polak-Wyss A. The medically important dematiaceous fungi and their identification. Mycoses. 1991;34:1–18. doi: 10.1111/j.1439-0507.1991.tb00613.x. [DOI] [PubMed] [Google Scholar]
- 16.Drevet D A, Campbell P A. Macrophage phagocytosis: use of fluorescence microscopy to distinguish between extracellular and intracellular bacteria. J Immunol Methods. 1991;142:31–38. doi: 10.1016/0022-1759(91)90289-r. [DOI] [PubMed] [Google Scholar]
- 17.Fels A O S, Nathan C F, Cohn Z A. Hydrogen peroxide release by alveolar macrophages from sarcoid patients and by alveolar macrophages from normals after exposure to recombinant interferons alpha A, beta, and gamma and 1,25-dihydroxyvitamin D3. J Clin Investig. 1987;80:381–386. doi: 10.1172/JCI113083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geis P A, Szaniszlo P. Carotenoid pigments of the dematiaceous fungus Wangiella dermatitidis. Mycologia. 1984;76:268–273. doi: 10.1007/BF00410729. [DOI] [PubMed] [Google Scholar]
- 19.Geis P A, Wheeler M H, Szaniszlo P J. Pentaketide metabolites of melanin synthesis in the dematiaceous fungus Wangiella dermatitidis. Arch Microbiol. 1984;137:324–328. doi: 10.1007/BF00410729. [DOI] [PubMed] [Google Scholar]
- 20.Grove S N, Oujezdsky K B, Szaniszlo P J. Budding in the dimorphic fungus Phialophora dermatitidis. J Bacteriol. 1973;115:323–329. doi: 10.1128/jb.115.1.323-329.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haase G. Analysis of genes coding for small-subunit rRNA sequences in studying phylogenetics of dematiaceous fungal pathogens. J Clin Microbiol. 1996;34:2049–2040. doi: 10.1128/jcm.34.8.2049-2050.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haase G, Conrad C, Kentrup H, Schnitzler N. Abstracts of the 49th Annual Meeting of the German Society for Hygiene and Microbiology 1997. Reinbek, Germany: Einhorn Presse Verlag; 1997. Establishment of an assay for detection of Exophiala dermatitidis antibodies using the ABICAP TOOL kit, abstr. P397; p. 103. [Google Scholar]
- 23.Haase G, Skopnik H, Groten T, Kusenbach G, Posselt H G. Long-term fungal cultures from sputum of patients with cystic fibrosis. Mycoses. 1991;34:373–376. doi: 10.1111/j.1439-0507.1991.tb00797.x. [DOI] [PubMed] [Google Scholar]
- 24.Haase G, Skopnik H, Kusenbach G. Exophiala dermatitidis infection in cystic fibrosis. Lancet. 1990;336:188–189. doi: 10.1016/0140-6736(90)91721-l. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 25.Haase G, Sonntag L, van de Peer Y, Uijthof J M, Podbielski A, Melzer-Krick B. Phylogenetic analysis of ten black yeast species using nuclear small subunit rRNA gene sequences. Antonie Leeuwenhoek. 1995;68:19–33. doi: 10.1007/BF00873289. [DOI] [PubMed] [Google Scholar]
- 26.Hiruma M, Kawada A, Ohata H, Ohnishi Y, Takahashi H, Yamazaki M, Ishibashi A, Hatsuse K, Kakihara M, Yoshida M. Systemic phaeohyphomycosis caused by Exophiala dermatitidis. Mycoses. 1993;36:1–7. doi: 10.1111/j.1439-0507.1993.tb00679.x. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs C W, Szaniszlo P J. Microtubule function and its relation to cellular development and the yeast cell cycle in Wangiella dermatitidis. Arch Microbiol. 1982;133:155–161. doi: 10.1007/BF00413531. [DOI] [PubMed] [Google Scholar]
- 28.Jacobson E S, Emery H S. Catecholamine uptake, melanization, and oxygen toxicity in Cryptococcus neoformans. J Bacteriol. 1991;173:401–403. doi: 10.1128/jb.173.1.401-403.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobson E S, Hove E, Emery H S. Antioxidant function of melanin in black fungi. Infect Immun. 1995;63:4944–4945. doi: 10.1128/iai.63.12.4944-4945.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobson E S, Tinell S B. Antioxidant function of fungal melanin. J Bacteriol. 1993;175:7102–7104. doi: 10.1128/jb.175.21.7102-7104.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kester S A, Garrett D C. Morphometry and sterology of the conversion of thin-walled yeasts to phase I yeast cells of Wangiella dermatitidis. Mycologia. 1995;87:153–160. [Google Scholar]
- 32.Kusenbach G, Skopnik H, Haase G, Friedrichs F, Dohmen H. Exophiala dermatitidis pneumonia in cystic fibrosis. Eur J Pediatr. 1992;151:344–346. doi: 10.1007/BF02113255. [DOI] [PubMed] [Google Scholar]
- 33.Kwon-Chung K J, Bennett J E. Medical mycology. Philadelphia, Pa: Lea & Febiger; 1992. [Google Scholar]
- 34.Martin E, Bhakdi S. Quantitative analysis of opsonophagocytosis and of killing of Candida albicans by human peripheral blood leukocytes by using flow cytometry. J Clin Microbiol. 1991;29:2013–2023. doi: 10.1128/jcm.29.9.2013-2023.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin E, Bhakdi S. Flow cytometric assay for quantifying opsonophagocytosis and killing of Staphylococcus aureus by peripheral blood leukocytes. J Clin Microbiol. 1992;30:2246–2255. doi: 10.1128/jcm.30.9.2246-2255.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsumoto M, Padhye A A, Ajello L, Standard P G. Critical review of human isolates of Wangiella dermatitidis. Mycologia. 1984;76:232–249. [Google Scholar]
- 37.Matsumoto T, Matsuda T, McGinnis M R, Ajello L. Clinical and mycological spectra of Wangiella dermatitidis infections. Mycoses. 1993;36:145–55. doi: 10.1111/j.1439-0507.1993.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto T, Padhye A A, Ajello L. Medical significance of the so-called black yeasts. Eur J Epidemiol. 1987;3:87–95. doi: 10.1007/BF00239744. [DOI] [PubMed] [Google Scholar]
- 39.Montijin R C, Van Wolven P, de Hoog G S, Klis F M. β-Glucosylated proteins in the cell wall of the black yeast Exophiala (Wangiella) dermatitidis. Microbiology. 1997;143:1673–1680. doi: 10.1099/00221287-143-5-1673. [DOI] [PubMed] [Google Scholar]
- 40.Moore A L, Joy A, Tom R, Gust G, Moore T A. Photoprotection by carotenoids during photosynthesis: motional dependence of intramolecular energy transfer. Science. 1982;216:982–984. doi: 10.1126/science.216.4549.982. [DOI] [PubMed] [Google Scholar]
- 41.Podbielski A, Schnitzler N, Beyhs P, Boyle M D P. M-related protein (Mrp) contributes to group A streptococcal resistance to phagocytosis by human granulocytes. Mol Microbiol. 1996;19:429–441. doi: 10.1046/j.1365-2958.1996.377910.x. [DOI] [PubMed] [Google Scholar]
- 42.Polachek I, Kwon-Chung K J. Melanogenesis in Cryptococcus neoformans. J Gen Microbiol. 1988;134:1037–1041. doi: 10.1099/00221287-134-4-1037. [DOI] [PubMed] [Google Scholar]
- 43.Polak A. Melanin as a virulence factor in pathogenic fungi. Mycoses. 1990;33:215–224. doi: 10.1111/myc.1990.33.5.215. [DOI] [PubMed] [Google Scholar]
- 44.Polak A, Dixon D M. Pathogenicity of Wangiella dermatitidis. In: Vanden Bossche H, Odds F C, Kerridge D, editors. Dimorphic fungi in biology and medicine. New York, N.Y: Plenum Press; 1993. pp. 307–312. [Google Scholar]
- 45.Roberts R L, Lo R J, Szaniszlo P J. Induction of synchronous growth in the yeast phase of Wangiella dermatitidis. J Bacteriol. 1980;141:981–984. doi: 10.1128/jb.141.2.981-984.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roberts R L, Szaniszlo P J. Temperature-sensitive multicellular mutants of Wangiella dermatitidis. J Bacteriol. 1978;135:622–632. doi: 10.1128/jb.135.2.622-632.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts R L, Szaniszlo P J. Yeast-phase cell cycle of the polymorphic fungus Wangiella dermatitidis. J Bacteriol. 1980;144:721–731. doi: 10.1128/jb.144.2.721-731.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rothe G, Emmendörfer A, Oser A, Roesler J, Valet G. Flow cytometric measurement of the respiratory burst of phagocytes using dihydrorhodamine 123. J Immunol Methods. 1991;138:133–135. doi: 10.1016/0022-1759(91)90074-p. [DOI] [PubMed] [Google Scholar]
- 49.Schnitzler N, Haase G, Büssing A, Kaufhold A, Beyhs P, Podbielski A. Measuring resistance to phagocytosis of group A and G streptococci: comparison of direct bactericidal assay and flow cytometry. Med Microbiol Immunol. 1995;184:17–22. doi: 10.1007/BF00216785. [DOI] [PubMed] [Google Scholar]
- 50.Szaniszlo P J, Hsieh P H, Marlowe J D. Induction and ultrastructure of the multicellular (sclerotic) morphology in Phialophora dermatitidis. Mycologia. 1976;68:117–130. [PubMed] [Google Scholar]
- 51.Thaylor B E, Wheeler M H, Szaniszlo P J. Evidence for pentaketide melanin biosynthesis in dematiaceous human pathogenic fungi. Mycologia. 1987;76:268–273. [Google Scholar]
- 52.Uijthof J M J, de Hoog G S, de Cock A W, Takeo K, Nishimura K. Pathogenicity of strains of the black yeast Exophiala (Wangialla) dermatitidis: an evaluation based on polymerase chain reaction. Mycoses. 1994;37:235–242. doi: 10.1111/j.1439-0507.1994.tb00419.x. [DOI] [PubMed] [Google Scholar]
- 53.Vowells S J, Sekhsaria S, Malech H L, Shalit M, Fleisher T A. Flow cytometric analysis of the granulocyte respiratory burst: a comparison study of fluorescent probes. J Immunol Methods. 1995;178:89–97. doi: 10.1016/0022-1759(94)00247-t. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y, Aisen P, Casadevall A. Crytococcus neoformans melanin and virulence: mechanism of action. Infect Immun. 1995;63:3131–3136. doi: 10.1128/iai.63.8.3131-3136.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, Casadevall A. Susceptibility of melanized and nonmelanized Cryptococcus neoformans to nitrogen- and oxygen-derived oxidants. Infect Immun. 1994;62:3004–3007. doi: 10.1128/iai.62.7.3004-3007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wheeler M H, Bell A A. Melanins and their importance in pathogenic fungi. Curr Top Med Mycol. 1988;2:338–387. doi: 10.1007/978-1-4612-3730-3_10. [DOI] [PubMed] [Google Scholar]
- 57.Wheeler M H, Tolmsoff W J, Bell A A, Mollenhauer H H. Ultrastructure and chemical distinction of melanins formed by Verticillium dahliae from (+)-scytalone, 1,8-dihydroxynaphthalene, catechol, and l-3,4-dihydroxyphenylalanine. Can J Microbiol. 1978;24:289–297. doi: 10.1139/m78-049. [DOI] [PubMed] [Google Scholar]
- 58.Wolfram L J. The mechanisms of hair bleaching. J Soc Cosmet Chem. 1970;21:875–900. [Google Scholar]