Abstract
Introduction
The purpose of this study was to investigate the relation between preoperative cachexia index (CXI) and long‐term outcomes in patients with colorectal liver metastases (CRLM) after hepatic resection.
Method
In all,118 patients who underwent hepatic resection for CRLM were analyzed retrospectively. The relationship between CXI and the long‐term outcomes in patients after hepatic resection was investigated. CXI was calculated based on preoperative skeletal muscle index, serum albumin level, and neutrophil–lymphocyte ratio.
Results
The multivariate analysis showed that extrahepatic lesion (hazard ratio [HR] 2.86, 95% confidence interval [CI] 1.48–5.53, P < .01) and high CXI (HR 0.44, 95% CI 0.20–0.98, P = .04) were independent and significant predictors of disease‐free survival. Moreover, extrahepatic lesion (HR 2.32, 95% CI 1.03–5.22, P = .04), high CXI (HR 0.17, 95% CI 0.05–0.57, P < .01), and curability R 1 or 2 (HR 3.29, 95% CI 1.23–8.78, P = .02) were independent and significant predictors of overall survival.
Conclusion
CXI is a useful prognostic factor for disease‐free survival and overall survival after hepatic resection in CRLM patients.
Keywords: cachexia, colorectal liver metastases, liver resection
We investigate the relationship between preoperative CXI and long‐term outcome after hepatectomy in patients with colorectal liver metastases (CRLM), and this is the first report to show that CXI is a useful prognostic determinant after hepatectomy in patients with CRLM. #10;DFS and OS were predominantly prolonged in the group of patients with high CXI compared to the group of patients with low CXI.

1. INTRODUCTION
Colorectal cancer is the most prevalent malignant tumor in Japan, with an increasing prevalence worldwide. 1 , 2 Overall, 20%–25% of these patients show liver metastases at the time of discovery, and 40%–50% develop colorectal liver metastases (CRLM) after primary lesion resection. 3 Hepatic resection is the only treatment that offers the possibility of prolonging life, with a 5‐y survival rate of 33%–50%. 4 , 5 For improving prognosis, prognostic factors after hepatectomy for CRLM are important. Beppu et al reported prognostic prediction using six preoperative factors: synchronous metastases, primary lymph node positive, tumor number, tumor size, extrahepatic metastatic disease at hepatectomy, and preoperative carbohydrate antigen 19‐9 level. 6 Recent reports suggest that nutritional factors, independent of tumor staging, such as the neutrophil–lymphocyte ratio (NLR), Glasgow Prognostic Score (GPS), prognostic nutrient index (PNI), and platelet–lymphocyte ratio (PLR) predict cancer‐specific survival in various cancers, including CRLM. 7 , 8 In addition, there are also reports on the relationship between sarcopenia and overall survival (OS) and disease‐free survival (DFS) for CRLM. 9 , 10
Cachexia is a complex metabolic disorder characterized by muscle loss with or without loss of fat mass associated with the underlying disorder and associated with more than 50% of cancer deaths and causes death in ~30%. 11 Recently, the “cachexia index (CXI)” has been established, which consists of skeletal muscle index (SMI), serum albumin (Alb) level, and NLR, and may thus comprehensively reflect cachectic status. 12 , 13 , 14 , 15 However, the impact of CXI on the prognosis for digestive cancers has never been reported.
In this study we examined the possibility that CXI could be a better prognostic factor for CRLM, since sarcopenia and NLR have already been reported as prognostic factors for CRLM.
2. METHODS
2.1. Patients
The study period was from May 2007 to October 2017 and included 118 consecutive patients who underwent initial hepatic resection for CRLM at the Department of Surgery, Jikei University Hospital, Tokyo, Japan. Patient data were recorded prospectively and analyzed retrospectively. This study was approved by the Ethics Committee of the Jikei University School of Medicine (27‐177). The requirement for acquisition of informed consent from patients was waived because of the retrospective design of this study and anonymized data.
2.2. Treatment and patient management
All 118 patients without unresectable extrahepatic tumors underwent hepatectomy regardless of the size, number, or location of liver metastases, as long as curative resection left sufficient residual liver. The extent of hepatic resection was determined based on the retention rate of indocyanine green at 15 min (ICGR15). 16 Patients with an estimated residual liver volume of <30% underwent percutaneous transhepatic portal vein embolization.
Contrast‐enhanced computed tomography (CT) and gadoxetic acid‐enhanced magnetic resonance imaging (MRI) were performed for tumor staging according to the criteria of the Japanese Society of Colon Cancer. Liver metastasis was classified as novel H1‐H3 (nH1‐nH3). 17 The novel H classification is as follows: nH1 is isolated lesions of 5 cm or less in size; nH3 is five or more lesions of any size; and nH2 as anything in between nH1 and nH3.
Postoperative complications included surgical site infections and Grade ≥ III complications according to the Clavien–Dindo classification. Surgical site infection was defined as a condition where purulent discharge was observed with or without microbiological evidence in the incision or in an organ or space.
Recurrence of colorectal cancer after hepatic resection for patients with CRLM was defined by newly detected local, hepatic, pulmonary, or extrahepatic tumors by ultrasonography, CT, or MRI with or without an increase in serum carcinoembryonic antigen (CEA) or carbohydrate antigen 19‐9. For recurrent liver or lung metastasis, repeated hepatic or partial lung resection, respectively, or systemic chemotherapy was performed. For local recurrence, tumor resection, radiotherapy, or systemic chemotherapy was performed. For systemic chemotherapy, the patients received infusional 5‐fluorouracil/l‐leucovorin with oxaliplatin (FOLFOX) and/or infusional 5‐fluorouracil/l‐leucovorin with irinotecan (FOLFIRI).
2.3. Cachexia index
A hemogram and chemistry profile were conducted preoperatively. CXI was calculated based on preoperative SMI, Alb, and NLR. The formula for calculating CXI is SMI/(height (m) × height (m)) × Alb/NLR. The SMI was calculated by measuring the major and minor diameters of the iliopsoas muscle at the third lumbar vertebra using preoperative CT images. The SMI was calculated using the formula: iliopsoas major axis (mm) × iliopsoas minor axis (mm) × π/100. The CXI cutoff value was determined by receiver operating characteristic curves 3 yy after surgery for OS. CXI = 22.90 for men and CXI = 16.58 for women were set as cutoff values.
2.4. Analysis of risk factors for recurrence DFS and OS
We investigated the association between clinicopathologic variables and DFS or OS after initial liver resection by univariate and multivariate analyses. The variables include regional lymph node metastases of primary colorectal cancer, novel H classification (nH1‐nH3), tumor size, extrahepatic lesion, resectability (resectable, borderline resectable, or unresectable), neoadjuvant chemotherapy, serum CEA level, CXI, SMI, GPS, 18 serum Alb level, serum C‐reactive protein (CRP) level, PNI, PLR, NLR, intraoperative blood loss, and curability (R0‐R2).
Continuous variables were classified into two groups for the log‐rank test and the Cox proportional hazard regression model based on novel H classification for tumor size, GPS for Alb and CRP, and optimal cutoff values determined by a receiver operating characteristic analysis for CEA, SMI, PNI, PLR, NLR, and intraoperative blood loss.
In addition, we investigated the relation between clinical variables and CXI by univariate analysis. The variables include age, gender, body mass index (BMI), timing of tumor, neoadjuvant chemotherapy, extrahepatic lesion, tumor number, tumor size, serum CEA level, serum Alb level, serum CRP level, GPS, PNI, NLR, PLR, operation time, intraoperative blood loss, postoperative complications, curability, and adjuvant chemotherapy.
2.5. Statistical analysis
The data were expressed as the median (interquartile range). The Mann–Whitney U‐ and Chi‐squared tests were used to compare the continuous and dichotomous variables, respectively. Multivariate analysis of DFS and OS was performed using the Cox proportional regression model with a backward elimination stepwise approach. The survival curve was calculated using the Kaplan–Meier method with the log‐rank test. All P‐values were considered statistically significant if they had an associated probability of <.05.
3. RESULTS
3.1. Patient characteristics
Patients' characteristics are summarized in Table 1. The median age was 66 yy, with a range of 60–75 yy, and 81 (68.6%) study participants were male. The primary tumor site was right‐sided colon in 31 patients (26.3%) and left‐sided colon in 87 patients (73.7%). The resectability at the time of CRLM diagnosis was resectable in 104 patients (88.1%) and borderline or unresectable in 14 patients (11.9%). Neoadjuvant chemotherapy was performed on 41 patients (34.7%). CXI was low in 93 of 118 patients (78.8%). The median follow‐up durations for DFS and OS were 1.01 and 3.03 yy, respectively. The 3‐y DFS and OS rates after hepatic resection for CRLM were 19.5% and 50.8%, respectively.
TABLE 1.
Patients' characteristics
| Variables | Patients (n = 118) |
|---|---|
| Age, yeas | 66 (60–75) |
| Gender | |
| Female | 37 (31.4%) |
| Male | 81 (68.6%) |
| Body mass index, kg/m2 | 22.5 (20.3–24.4) |
| Primary tumor location | |
| Ascending colon | 19 (16.1%) |
| Transverse colon | 12 (10.2%) |
| Descending colon | 10 (8.5%) |
| Sigmoid colon | 36 (30.5%) |
| Rectum | 41 (34.7%) |
| T factor | |
| T1 | 2 (1.7%) |
| T2 | 5 (4.2%) |
| T3 | 72 (61.0%) |
| T4 | 39 (33.1%) |
| Lymph node metastases | |
| Yes | 74 (62.7%) |
| No | 44 (37.3%) |
| Timing of tumor | |
| Synchronous | 74 (62.7%) |
| Metachronous | 44 (37.3%) |
| Novel H classification | |
| nH1 | 44 (37.3%) |
| nH2 | 6 (5.1%) |
| nH3 | 68 (57.6%) |
| Tumor size, mm | 49 (19–45) |
| Resectability | |
| Resectable | 104 (88.1%) |
| Borderline resectable | 12 (10.2%) |
| Unresectable | 2 (1.7%) |
| Neoadjuvant chemotherapy | |
| Yes | 41 (34.7%) |
| No | 77 (65.3%) |
| Serum CEA, ng/mL | 11.1 (4.7–38.7) |
| CXI | |
| High | 25 (21.2%) |
| Low | 93 (78.8%) |
| Serum CEA, ng/mL | 11.1 (4.7–38.7) |
Abbreviations: CEA, carcinoembryonic antigen; CXI, cachexia index.
3.2. Univariate and multivariate analyses of clinicopathological variables in relation to DFS after hepatic resection for CRLM
We investigated the relationship between the clinicopathological variables and DFS after hepatic resection in patients with CRLM (Table 2). Univariate analysis showed a poor DFS in patients with lymph node metastases (P = .03), nH2 or 3 (P = .02), extrahepatic lesion (P < .01), borderline resectable or unresectable (P = .03), and low CXI (P = .03). In multivariate analysis, extrahepatic lesion (HR 2.86, 95% CI 1.48–5.53, P < .01) and high CXI (HR 0.44, 95% CI 0.20–0.98, P = .04) were independent and significant predictors of DFS.
TABLE 2.
Univariate and multivariate analyses of clinicopathological variables in relation to disease‐free survival after hepatic resection for colorectal liver metastases
| Variables | N | DFS univariate analysis | DFS multivariate analysis | ||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P‐value | Hazard ratio (95% CI) | P‐value | ||
| Lymph node metastases | |||||
| Yes | 74 | 1.68 | .03 | NS | |
| No | 44 | (1.04–2.69) | |||
| Novel H classification | |||||
| nH2, nH3 | 74 | 1.73 | .02 | NS | |
| nH1 | 44 | (1.08–2.77) | |||
| Tumor size, mm | |||||
| ≥50 | 25 | 0.93 | .79 | NS | |
| <50 | 93 | (0.54–1.61) | |||
| Extrahepatic lesion | |||||
| Yes | 19 | 2.10 | <.01 | 2.86 | <.01 |
| No | 99 | (1.21–3.64) | (1.48–5.53) | ||
| Resectability | |||||
| Borderline or unresectable | 14 | 1.96 | .03 | NS | |
| Resectable | 104 | (1.05–3.65) | |||
| Neoadjuvant chemotherapy | |||||
| Yes | 41 | 1.37 | .17 | NS | |
| No | 77 | (0.88–2.15) | |||
| Serum CEA, ng/mL | |||||
| ≥10.7 | 60 | 1.20 | .43 | NS | |
| <10.7 | 58 | (0.77–1.86) | |||
| CXI | |||||
| High | 25 | 0.51 | .03 | 0.44 | .04 |
| Low | 93 | (0.28–0.94) | (0.20–0.98) | ||
| SMI | |||||
| High | 87 | 0.7– | .14 | NS | |
| Low | 31 | (0.43–1.13) | |||
| GPS | |||||
| 1 or 2 | 31 | 1.35 | .22 | NS | |
| 0 | 87 | (0.83–2.20) | |||
| Serum Alb | |||||
| ≥3.5 | 97 | 0.92 | .77 | NS | |
| <3.5 | 21 | (0.52–1.63) | |||
| CRP | |||||
| ≥1 | 17 | 1.62 | .10 | NS | |
| <1 | 101 | (0.91–2.88) | |||
| PNI | |||||
| ≥46 | 58 | 0.81 | .34 | NS | |
| <46 | 60 | (0.52–1.25) | |||
| PLR | |||||
| ≥146.6 | 62 | 1.55 | .05 | NS | |
| <146.6 | 56 | (0.99–2.40) | |||
| NLR | |||||
| ≥2.15 | 71 | 1.23 | .37 | NS | |
| <2.15 | 47 | (0.78–1.94) | |||
| Intraoperative blood loss, mL | |||||
| ≥400 | 67 | 1.49 | .08 | NS | |
| <400 | 51 | (0.95–2.35) | |||
| Curability | |||||
| R1 or 2 | 16 | 1.80 | .05 | NS | |
| R0 | 102 | (0.99–3.29) | |||
Abbreviations: Alb, albumin; CEA, carcinoembryonic antigen; CI, confidence interval; CRP, C‐reactive protein; CXI, cachexia index; DFS, disease‐free survival; GPS, Glasgow Prognostic Score; NLR, neutrophil‐lymphocyte ratio; PLR, platelet‐lymphocyte ratio; PNI, prognostic nutrition index; SMI, skeletal muscle mass index.
3.3. Univariate and multivariate analyses of clinicopathological variables in relation to OS after hepatic resection for CRLM
We investigated the association between clinicopathological variables and OS after hepatic resection in patients with CRLM (Table 3). In univariate analysis, OS was poor in patients with extrahepatic lesion (P = .02), low CXI (P < .01), and R1 or 2 (P = .02). In multivariate analysis, extrahepatic lesion (HR 2.32, 95% CI 1.03–5.22, P = .04), high CXI (HR 0.17, 95% CI 0.05–0.57, P < .01), and R1 or 2 (HR 3.29, 95% CI 1.23–8.78, P = .02) were also independent and significant predictors of OS. Kaplan–Meier curve for DFS after hepatic resection for CRLM showed low CXI was significantly associated with worse DFS (P = .03; Figure 1). In addition, low CXI was also significantly associated with worse OS (P < .01; Figure 1).
TABLE 3.
Univariate and multivariate analyses of clinicopathological variables in relation to overall survival after hepatic resection for colorectal liver metastases
| Variables | OS univariate analysis | OS multivariate analysis | |||
|---|---|---|---|---|---|
| N | Hazard ratio (95% CI) | P‐value | Hazard ratio (95% CI) | P‐value | |
| Lymph node metastases | |||||
| Yes | 74 | 1.86 | .06 | NS | |
| No | 44 | (0.98–3.50) | |||
| Novel H classification | |||||
| nH2, nH3 | 74 | 1.46 | .23 | NS | |
| nH1 | 44 | (0.79–2.69) | |||
| Tumor size, mm | |||||
| ≥50 | 25 | 1.25 | .52 | NS | |
| <50 | 93 | (0.64–2.45) | |||
| Extrahepatic lesion | |||||
| Yes | 19 | 2.15 | .02 | 2.32 | .04 |
| No | 99 | (1.12–4.14) | (1.03–5.22) | ||
| Resectability | |||||
| Borderline or unresectable | 14 | 1.76 | .15 | NS | |
| Resectable | 104 | (0.82–3.76) | |||
| Neoadjuvant chemotherapy | |||||
| Yes | 41 | 1.64 | .09 | NS | |
| No | 77 | (0.92–2.92) | |||
| Serum CEA, ng/mL | |||||
| ≥10.7 | 60 | 1.50 | .17 | NS | |
| <10.7 | 58 | (0.84–2.66) | |||
| CXI | |||||
| High | 25 | 0.22 | <.01 | 0.17 | <.01 |
| Low | 93 | (0.08–0.63) | (0.05–0.57) | ||
| SMI | |||||
| High | 87 | 0.66 | .19 | NS | |
| Low | 31 | (0.36–1.22) | |||
| GPS | |||||
| 1 or 2 | 31 | 1.73 | .08 | NS | |
| 0 | 87 | (0.94–3.20) | |||
| Serum Alb | |||||
| ≥3.5 | 97 | 0.75 | .43 | NS | |
| <3.5 | 21 | (0.36–1.54) | |||
| CRP | |||||
| ≥1 | 17 | 1.72 | .13 | NS | |
| <1 | 101 | (0.86–3.46) | |||
| PNI | |||||
| ≥46 | 58 | 0.56 | .05 | NS | |
| <46 | 60 | (0.32–1.01) | |||
| PLR | |||||
| ≥146.6 | 62 | 1.53 | .15 | NS | |
| <146.6 | 56 | (0.86–2.71) | |||
| NLR | |||||
| ≥2.15 | 71 | 1.38 | .31 | NS | |
| <2.15 | 47 | (0.74–2.58) | |||
| Intraoperative blood loss, mL | |||||
| ≥400 | 67 | 1.34 | .33 | NS | |
| <400 | 51 | (0.75–2.43) | |||
| Curability | |||||
| R1 or 2 | 16 | 2.53 | .02 | 3.29 | .02 |
| R0 | 102 | (1.18–5.45) | (1.23–8.78) | ||
Abbreviations: Alb, albumin; CEA, carcinoembryonic antigen; CI, confidence interval; CRP, C‐reactive protein; CXI, cachexia index; DFS, disease‐free survival; GPS, Glasgow Prognostic Score; NLR, neutrophil‐lymphocyte ratio; PLR, platelet‐lymphocyte ratio; PNI, prognostic nutrition index; SMI, skeletal muscle mass index.
FIGURE 1.
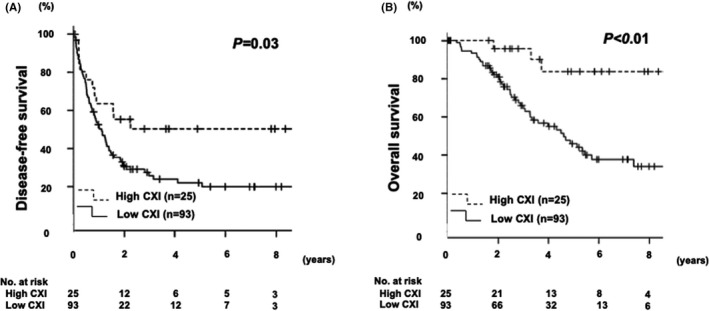
(A) Kaplan–Meier curve for disease‐free survival after hepatic resection for colorectal liver metastasis. (B) Kaplan–Meier curve for overall survival after hepatic resection for colorectal liver metastasis
3.4. Association between clinical variables and CXI
We investigated the association between clinical variables and CXI (Table 4). In univariate analysis, the low CXI group was significantly associated with older age (68 vs. 64 yy, P = .03), lower BMI (22 vs. 23 kg/m2, P = .02), lower Alb (3.8 vs. 4.0 g/dL, P < .01), higher CRP (0.08 vs. 0.21 mg/dL, P < .01), lower PNI (45 vs. 49, P < .01), higher NLR (2.6 vs. 1.4, P < .01), higher PLR (162 vs. 93.5, P < .01), and more postoperative complications (22 vs. 4%, P = .04). The timing of tumor, extrahepatic lesion, the tumor progression including tumor number and tumor size, CEA, and preoperative and postoperative chemotherapy were comparable between the two groups.
TABLE 4.
Univariate analysis of clinical variables in relation to cachexia index
| Variables | Cachexia index | P‐value | |
|---|---|---|---|
| High (n = 25) | Low (n = 93) | ||
| Age, yeas | 64 (56–70) | 68 (61–75) | .03 |
| Gender, female | 7 (28%) | 30 (32%) | .81 |
| Body mass index, kg/m2 | 23 (22–26) | 22 (20–24) | .02 |
| Timing of tumor, synchronous | 12 (48%) | 62 (66.7%) | .11 |
| Neoadjuvant chemotherapy, yes | 10 (40%) | 31 (33.3%) | .64 |
| Extrahepatic lesion, yes | 3 (12%) | 16 (17.2%) | .76 |
| Tumor number | 2 (1–4) | 2 (1–2) | .27 |
| Tumor size, mm | 21 (17–40) | 27 (18–44) | .23 |
| Serum CEA, ng/mL | 9.7 (4.2–28) | 12.4 (4.9–40) | .77 |
| Serum Alb, g/dL | 4.0 (3.8–4.3) | 3.8 (3.5–4.1) | <.01 |
| Serum CRP, mg/dL | 0.08 (0.04–0.15) | 0.21 (0.05–0.44) | <.01 |
| GPS, 1 or 2 | 3 (12%) | 28 (30.1%) | .08 |
| PNI | 49 (47–53) | 45 (41–48) | <.01 |
| NLR | 1.4 (1.1–1.6) | 2.6 (2.0–3.4) | <.01 |
| PLR | 93.5 (69.4–138) | 162 (121–227) | <.01 |
| Operation time, min | 359 (307–436) | 384 (275–496) | .61 |
| Intraoperative blood loss, mL | 490 (120–1050) | 470 (190–1050) | .68 |
| Postoperative complications, yes | 1 (4%) | 20 (22%) | .04 |
| Curability, R1 or 2 | 3 (12%) | 13 (14%) | 1 |
| Adjuvant chemotherapy, yes | 16 (64%) | 57 (61.3%) | 1 |
Abbreviations: Alb, albumin; CEA, carcinoembryonic antigen; CRP, C‐reactive protein; GPS, Glasgow Prognostic Score; NLR, neutrophil‐lymphocyte ratio; PLR, platelet‐lymphocyte ratio; PNI, prognostic nutrition index.
4. DISCUSSION
In recent years, prognostic factors other than cancer staging, such as nutritional predictors and sarcopenia, have been studied. We demonstrated that CXI using NLR and SMI was a prognostic predictor of postoperative outcome in patients who underwent hepatic resection for CRLM. This is the first study to report that a low CXI is closely related to poor clinical outcomes in CRLM patients.
Cancer cachexia is a multifactorial syndrome characterized by a persistent loss of skeletal muscle mass (with or without loss of fat mass) that cannot be completely reversed with conventional nutritional support and leads to progressive functional impairment. In 2011, the European Palliative Care Research Collaborative proposed a new definition and classification of cancer cachexia, which is divided into three levels: pre‐cachexia, cachexia, and refractory cachexia. 19 It has been proposed that various factors and inflammatory cytokines produced by the tumor and host affect different cells of the body, such as muscle cells, hepatocytes, and adipocytes, causing metabolic abnormalities that lead to cachexia. 20 For instance, inflammatory cytokines produced by tumor cells, such as tumor necrosis factor‐α, interleukin (IL)‐6, and IL‐8, are thought to contribute to muscle wasting and atrophy by inducing oxidative stress in skeletal muscles and activating muscle degradation pathways. 21 , 22 Since cachexia has a complex pathophysiology, having a composite index to measure ongoing cachexia is essential rather than using a single index BMI.
The main clinical features of cancer cachexia are: poor nutritional status, systemic inflammation, and loss of muscle mass. Clinical indicators of these features include serum Alb, NLR, and SMI. Each of these has been independently associated with poor prognosis. 23 , 24 , 25 Therefore, we decided that it would be beneficial to investigate whether CXI, along with the known factors, would serve as a better prognostic indicator for CRLM.
In this study the low CXI group had more elderly people with lower BMI, Alb, and PNI, and higher CRP, NLR, and PLR compared to the high CXI group. Hence, we considered that CXI reflects the status of PNI, NLR, and PLR and a comprehensive assessment of nutritional status. On the other hand, no significant difference in the number or size of tumors or the presence or absence of extrahepatic metastases co‐relating to the CXI values was observed. Also, there was no significant difference in surgical techniques such as operative time or intraoperative blood loss. This suggests that CXI reflects musculoskeletal status and nutritional status, not preoperative tumor progression or invasion, and does not contribute to the content of the surgical procedure. Although it has been reported that chemotherapy treatment is discontinued early and inpatient care is very common in the low CXI group due to treatment‐related toxicity, 26 and preoperative chemotherapy induced skeletal muscle loss in patients with breast cancer, 27 our study did not find a difference in the proportion of patients who received neoadjuvant chemotherapy or postoperative adjuvant chemotherapy between the low CXI and high CXI groups. In multivariate analysis, patients with extrahepatic lesions and those with low CXI showed a poor prognosis, in common with both DFS and OS. As a result, low CXI was demonstrated to be an independent and significant risk factor for poor DFS and OS after hepatic resection for CRLM. Moreover, patients with low CXI had more postoperative complications than those with high CXI. These results suggest that prevention or improvement of CXI decline may improve both short‐ and long‐term outcomes.
Several reports proposed that the CXI is a novel index for estimating cachexia that also correlates with prognosis for malignant tumors such as advanced non‐small cell lung cancer (NSCLC), non‐Hodgkin’s lymphoma, and diffuse large B‐cell lymphoma. In diffuse large B‐cell lymphoma, the low CXI group showed a predominant decrease in OS, and in patients with NSCLC and non‐Hodgkin’s lymphoma, the low CXI group showed a predominant decrease in both OS and DFS, which was similar to what was found for patients with CRLM in this study. 12 , 13 , 14 , 15 Therefore, CXI may be associated with treatment outcomes and prognosis in other cancers or diseases, and further research is needed.
Currently, a study is underway in Japan to improve cancer cachexia with the use of anamorelin, a ghrelin receptor agonist, that can be administered orally. 28 In Japan, the use of anamorelin for cancer cachexia was approved on December 11, 2020 for four types of cancers: NSCLC, gastric cancer, pancreatic cancer, and colorectal cancer.. 29 Further clinical studies using anamorelin or novel medications are expected to promote research on improving CXI and the prognosis associated with it as well as to improve research on the clinical outcomes of CRLM.
The limitations of this study include its retrospective nature, the fact that it was conducted at a single university hospital, and the small number of patients. Another limitation is that the follow‐up period was relatively short. Therefore, a large‐scale prospective study is needed to validate the results.
Preoperative CXI is a reliable indicator to assess the DFS and OS of CRLM patients after hepatectomy. The use of this index for clinical decision making will allow a more accurate risk stratification system combined with tumor staging of CRLM. In conclusion, CXI seems to be a useful predictor of poor DFS and OS in patients with CRLM after hepatectomy.
DISCLOSURE
Conflict of interest: The authors have no conflicts of interest or funding to declare.
Ethical approval: This study was approved by the Ethics Committee of the Jikei University School of Medicine (27‐177).
Informed consent: The requirement for acquisition of informed consent from patients was waived because of the retrospective design of this study and anonymized data.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
ACKNOWLEDGMENT
N/A.
Tanji Y, Furukawa K, Haruki K, Taniai T, Onda S, Tsunematsu M, Significant impact of cachexia index on the outcomes after hepatic resection for colorectal liver metastases. Ann Gastroenterol Surg. 2022;6:804–812. doi: 10.1002/ags3.12578
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2. Katanoda K, Kamo K, Saika K, Matsuda T, Shibata A, Matsuda A, et al. Short‐term projection of cancer incidence in Japan using an age‐period interaction model with spline smoothing. Jpn J Clin Oncol. 2014;44(1):36–41. [DOI] [PubMed] [Google Scholar]
- 3. Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244(2):254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer. 2006;94(7):982–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adam R, Kitano Y. Multidisciplinary approach of liver metastases from colorectal cancer. Ann Gastroenterol Surg. 2019;3(1):50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beppu T, Sakamoto Y, Hasegawa K, Honda G, Tanaka K, Kotera Y, et al. A nomogram predicting disease‐free survival in patients with colorectal liver metastases treated with hepatic resection: multicenter data collection as a project study for hepatic surgery of the Japanese society of Hepato‐biliary‐pancreatic surgery. J Hepatobiliary Pancreat Sci. 2012;19(1):72–84. [DOI] [PubMed] [Google Scholar]
- 7. Nakagawa K, Tanaka K, Nojiri K, Kumamoto T, Takeda K, Ueda M, et al. The modified Glasgow prognostic score as a predictor of survival afterhepatectomy for colorectal liver metastases. Ann Surg Oncol. 2014;21(5):1711–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Neal CP, Cairns V, Jones MJ, Masood MM, Nana GR, Mann CD, et al. Prognostic performance of inflammation‐based prognostic indices in patients with resectable colorectal liver metastases. Med Oncol. 2015;32(5):144. [DOI] [PubMed] [Google Scholar]
- 9. Liu YW, Lu CC, Chang CD, Lee KC, Chen HH, Yeh WS, et al. Prognostic value of sarcopenia in patients with colorectal liver metastases undergoing hepatic resection. Sci Rep. 2020;10(1):6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Waalboer RB, Meyer YM, Galjart B, Olthof PB, van Vugt JLA, Grünhagen DJ, et al. Sarcopenia and long‐term survival outcomes after local therapy for colorectal liver metastasis: a meta‐analysis. HPB (Oxford). 2021;24:9–16. [DOI] [PubMed] [Google Scholar]
- 11. Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2(11):862–71. [DOI] [PubMed] [Google Scholar]
- 12. Jafri SH, Previgliano C, Khandelwal K, Shi R. Cachexia index in advanced non‐small‐cell lung cancer patients. Clin Med Insights Oncol. 2015;9:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Go SI, Park MJ, Lee GW. Clinical significance of the cachexia index in patients with small cell lung cancer. BMC Cancer. 2021;21(1):563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karmali R, Alrifai T, Fughhi IAM, Ng R, Chukkapalli V, Shah P, et al. Impact of cachexia on outcomes in aggressive lymphomas. Ann Hematol. 2017;96(6):951–6. [DOI] [PubMed] [Google Scholar]
- 15. Go SI, Park MJ, Park S, Kang MH, Kim HG, Kang JH, et al. Cachexia index as a potential biomarker for cancer cachexia and a prognostic indicator in diffuse large B‐cell lymphoma. J Cachexia Sarcopenia Muscle. 2021;12(6):2211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miyagawa S, Makuuchi M, Kawasaki S, Kakazu T. Criteria for safe hepatic resection. Am J Surg. 1995;169(6):589–94. [DOI] [PubMed] [Google Scholar]
- 17. Beppu T, Imai K, Honda G, Sakamoto K, Kobayashi S, Endo I, et al. Proposal of a novel H category‐based classification of colorectal liver metastases based on a Japanese nationwide survey. J Hepatobiliary Pancreat Sci. 2021;28(4):317–26. [DOI] [PubMed] [Google Scholar]
- 18. Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non‐small‐cell lung cancer. Br J Cancer. 2003;89(6):1028–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–95. [DOI] [PubMed] [Google Scholar]
- 20. Argilés JM, Busquets S, López‐Soriano FJ. Anti‐inflammatory therapies in cancer cachexia. Eur J Pharmacol. 2011;668(Suppl 1):S81–6. [DOI] [PubMed] [Google Scholar]
- 21. Hou YC, Wang CJ, Chao YJ, Chen HY, Wang HC, Tung HL, et al. Elevated serum Interleukin‐8 level correlates with cancer‐related cachexia and sarcopenia: an indicator for pancreatic cancer outcomes. J Clin Med. 2018;7(12):502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Narsale AA, Carson JA. Role of interleukin‐6 in cachexia: therapeutic implications. Curr Opin Support Palliat Care. 2014;8(4):321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin GN, Peng JW, Liu PP, Liu DY, Xiao JJ, Chen XQ. Elevated neutrophil‐to‐lymphocyte ratio predicts poor outcome in patients with advanced non‐small‐cell lung cancer receiving first‐line gefitinib or erlotinib treatment. Asia Pac J Clin Oncol. 2017;13(5):e189–e94. [DOI] [PubMed] [Google Scholar]
- 25. Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31(12):1539–47. [DOI] [PubMed] [Google Scholar]
- 26. Jain R, Handorf E, Khare V, Blau M, Chertock Y, Hall MJ. Impact of baseline nutrition and exercise status on toxicity and outcomes in phase I and II oncology clinical trial participants. Oncologist. 2020;25(2):161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee BM, Cho Y, Kim JW, Ahn SG, Kim JH, Jeung HC, et al. Association between skeletal muscle loss and the response to neoadjuvant chemotherapy for breast cancer. Cancers (Basel). 2021;13(8):1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM, Yan Y, et al. Anamorelin in patients with non‐small‐cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double‐blind, phase 3 trials. Lancet Oncol. 2016;17(4):519–31. [DOI] [PubMed] [Google Scholar]
- 29. Wakabayashi H, Arai H, Inui A. The regulatory approval of anamorelin for treatment of cachexia in patients with non‐small cell lung cancer, gastric cancer, pancreatic cancer, and colorectal cancer in Japan: facts and numbers. J Cachexia Sarcopenia Muscle. 2021;12(1):14–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


