Abstract
On December 22, 2021, the United States Food and Drug Administration approved the first main protease inhibitor, i.e., oral antiviral nirmatrelvir (PF-07321332)/ritonavir (Paxlovid), for the treatment of early severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Nirmatrelvir inhibits SARS-CoV-2 infection, but high doses or long-term treatment may cause embryonic developmental toxicity and changes in host gene expression. The chiral structure of nirmatrelvir plays a key role in its antiviral activity. Ritonavir boosts the efficacy of nirmatrelvir by inactivating cytochrome P450 3A4 expression and occupying the plasma protein binding sites. Multidrug resistance protein 1 inhibitors may increase the efficacy of nirmatrelvir. However, Paxlovid has many contraindications. Some patients treated with Paxlovid experience a second round of coronavirus disease 2019 (COVID-19) symptoms soon after recovery. Interestingly, the antiviral activity of nirmatrelvir metabolites, such as compounds 12–18, is similar to or higher than that of nirmatrelvir. Herein, we review the advances and challenges in using nirmatrelvir and its derivatives with the aim of providing knowledge for drug developers and physicians in the fight against COVID-19.
Keywords: Nirmatrelvir, Pharmacology, Pharmacokinetics, Toxicology, Derivatives, COVID-19
Graphical abstract
Highlights
-
•
Nirmatrelvir and its metabolites inhibit SARS-CoV-2 replication by suppressing Mpro.
-
•
Ritonavir increases nirmatrelvir's efficacy by slowing metabolism and occupying PPB sites.
-
•
Nirmatrelvir may cause a second round of COVID-19 symptoms and embryonic developmental toxicity.
-
•
The chiral structure plays a key role in the antiviral activity of nirmatrelvir.
-
•
The antiviral activity of compounds 12–18 is similar to or higher than that of nirmatrelvir.
1. Introduction
Coronavirus disease 2019 (COVID-19) is caused by infection with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and continues to cause deaths and lockdowns as it quickly spreads worldwide [1,2]. Since its first discovery in December 2019, this disease has received considerable attention from researchers around the globe [3,4]. The availability of effective and low-cost oral antiviral drugs for the treatment of early-stage SARS-CoV-2 infection in the community environment is of great importance for disease control and prevention and is therefore a priority for controlling COVID-19, but there remains a crucial need for the development of oral antiviral drugs.
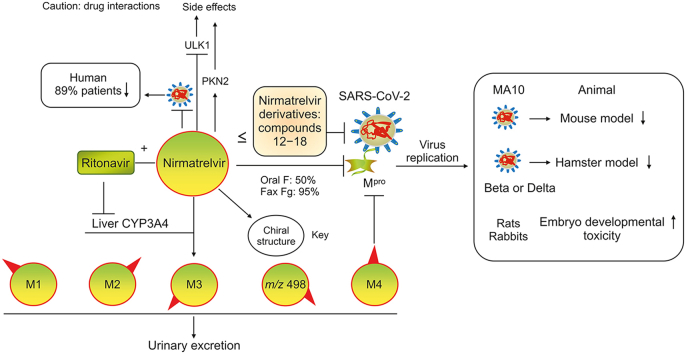
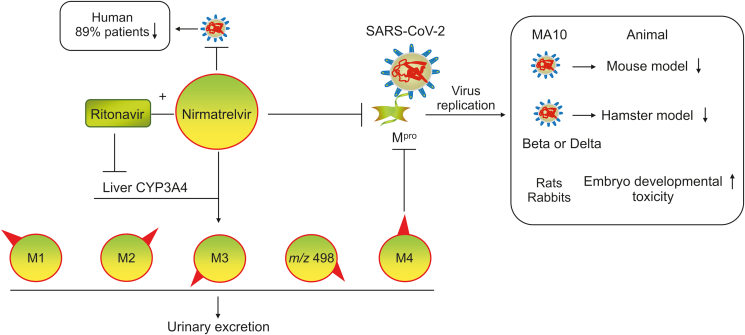
Paxlovid is the first oral antiviral to be approved by the United States Food and Drug Administration (FDA) for the treatment of COVID-19 (approved on December 22, 2021). Paxlovid is a copackaged combination of nirmatrelvir (PF-07321332) and ritonavir tablets, and the recommended dosage is a 2:1 ratio (2 nirmatrelvir tablets and 1 ritonavir tablet). Nirmatrelvir is a reversible covalent inhibitor with a nitrile warhead that targets the main protease (Mpro, also called 3C-like protease) of SARS-CoV-2 [5]. Nirmatrelvir exhibits nanomolar efficacy in suppressing SARS-CoV-2 by binding and suppressing Mpro both in vitro and in vivo and is metabolized mainly by cytochrome P450 3A4 (CYP3A4) [6,7]. Ritonavir boosts the efficacy of nirmatrelvir through the inactivation of CYP3A4 [7,8]. Phase II–III studies have shown that Paxlovid decreases hospital admissions and deaths among patients with severe COVID-19 [9,10], and the cost of Paxlovid is lower than the hospitalization costs estimated by Medicare for patients with COVID-19 [11,12]. In fact, Paxlovid has been approved for use in many countries (such as the United States, China, the United Kingdom, Canada, Australia, the Republic of Korea, Israel, and New Zealand) and European Union member states (such as Germany, France, Italy, the Netherlands, Belgium, and Luxembourg) [13]. Pfizer and the Medicine Patent Pool (MPP) have signed licensing agreements to expand access to Paxlovid to treatment candidates in 95 low- and middle-income countries [14]. In March 2022, 35 generic manufacturers in 13 countries signed agreements with the MPP to produce generic versions of Pfizer's Paxlovid [15]. Paxlovid helps address early disease in the outpatient setting. However, nirmatrelvir may cause embryonic developmental toxicity and changes in host gene expression. Hence, the need for the discovery and development of new antiviral drugs remains urgent. The main goal of this review is to summarize the pharmacology, pharmacokinetics, toxicology, and derivatives of nirmatrelvir, with the aim of providing knowledge for drug developers and physicians that will help them combat the COVID-19 outbreak.
2. Effect of nirmatrelvir on suppressing SARS-CoV-2 infection
2.1. Mechanism through which nirmatrelvir suppresses SARS-CoV-2 infection
2.1.1. Nirmatrelvir suppresses SARS-CoV-2 replication by suppressing its protease Mpro
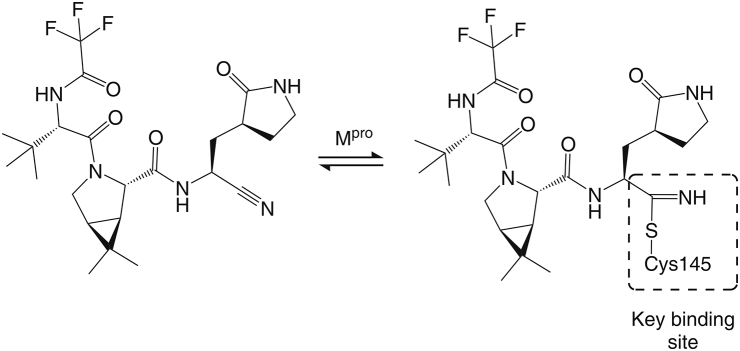
Mpro promotes the replication of Coronaviridae viruses by cleaving the polyprotein when the viral RNA enters the host cells [16,17]. Importantly, SARS-CoV-2 Mpro exhibits >96% sequence identity with that of SARS-CoV; in particular, the residues of the binding pocket of Mpro are highly conserved. Moreover, mammals, including humans, mice, rats, pigs, and monkeys, lack Mpro homologs. Therefore, mammalian proteases do not recognize the Mpro sequence, and an Mpro inhibitor may have fewer side effects than other antiviral therapeutics in mammals [18,19]. Nirmatrelvir forms a reversible thioimidate adduct through the binding of its nitrile carbon to the Mpro Cys145 and then strongly inhibits Mpro activity in coronaviruses (Fig. 1) [20], including SARS-CoV-2, SARS-CoV-1, human coronavirus (HCoV)-HKU1, HCoV-OC43, Middle East respiratory syndrome coronavirus (MERS)-CoV, HCoV-229E, and HCoV-NL63, with half maximal inhibitory concentration (IC50) values ranging from 10 to 100 nM. However, the IC50 of nirmatrelvir against papain-like protease (PLpro) is above 20 μM, which suggests that nirmatrelvir does not suppress PLpro [21]. Therefore, nirmatrelvir has a higher affinity for Mpro than for PLpro.
Fig. 1.
Mechanism and major binding sites of nirmatrelvir for suppressing the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) main protease (Mpro). Nirmatrelvir forms a reversible thioimidate adduct by the binding of its nitrile carbon to the Mpro Cys145 to suppress Mpro. (Reprint from Ref. [20] with permission).
2.1.2. Ritonavir boosts the efficacy of nirmatrelvir by inactivating CYP3A4
Nirmatrelvir is metabolized by CYP3A4, but its rapid metabolism interferes with its efficacy. Interestingly, the CYP3A4 inactivator ritonavir is a pharmacokinetic enhancer of drugs that are used to treat HIV infection, such as darunavir and lopinavir. Ritonavir also slows the metabolism of nirmatrelvir by suppressing CYP3A4 expression to enhance the efficacy of nirmatrelvir [22]. In patients, the metabolic clearance rate of nirmatrelvir (oral, 250 mg) in combination with ritonavir is 3 times slower than that of nirmatrelvir alone (oral, 150 mg). The coadministration of nirmatrelvir and ritonavir results in an approximately 8-fold increase in the plasma nirmatrelvir concentration, which suggests that the plasma concentration of nirmatrelvir is increased by ritonavir [3,23].
2.1.3. Ritonavir may increase the efficacy of nirmatrelvir by occupying the plasma protein binding sites
The total drug concentration in plasma consists of two parts: the free plasma drug concentration and the concentration bound to plasma proteins [24]. Plasma protein binding (PPB) is a dynamic process because drugs can continuously bind to and dissociate from plasma proteins. The free plasma drug concentration maintains drug efficacy [25,26]. Nirmatrelvir exhibits concentration-independent PPB, with PPB values ranging from 37.9% to 74% [3,22,27]. Nirmatrelvir may bind to only plasma proteins. However, the PPB of nirmatrelvir in humans differs among studies (69% or 45.5%). The PPB process is dependent on the concentrations of both drug and plasma proteins, including albumin, α-1-acid glycoprotein, and, to a lesser degree, globulins and lipoproteins [28]. The PPB of nirmatrelvir is independent of the drug concentration, and differences in the plasma protein concentration may be the main cause of the observed differences in PPB, but additional studies are needed.
The PPB of ritonavir is very high (96%–99.5%) in all species tested (including rats, dogs, monkeys, and humans), with values of 99.3%–99.5% in humans, which suggests that the PPB of ritonavir is higher than that of nirmatrelvir [29]. When nirmatrelvir is administered in combination with ritonavir, ritonavir may occupy the PPB sites due to its strong protein binding ability. Nirmatrelvir cannot be fully bound by plasma proteins, leading to increases in its free plasma drug concentration and antiviral efficacy (Fig. S1). Thus, the unbound and bound plasma concentrations of nirmatrelvir/ritonavir should be evaluated to ascertain both their pharmacodynamics and toxicity.
2.1.4. Multidrug resistance protein 1 (MDR1) inhibitors may increase the efficacy of nirmatrelvir
Nirmatrelvir is a substrate of MDR1 (also named P-glycoprotein or adenosine triphosphate-binding cassette subfamily B member 1 (ABCB1)) but not breast cancer resistance protein. The concentration for 50% of the maximal effect (EC50) of nirmatrelvir against SARS-CoV-2 USA-WA1/2020 in infected Vero E6 cells is 74.5 nM in the presence of the MDR1 inhibitor CP-100356, whereas its EC50 increases to 4.48 μM in the absence of CP-100356, which suggests that MDR1 inhibitors increase the antiviral efficacy of nirmatrelvir [3,22,30]. However, the expression of MDR1 is cell-type specific. Only a small fraction of respiratory tract cells, which are infected mainly with SARS-CoV-2, could exhibit detectable MDR1 expression [31]. Further research is needed to determine whether MDR1 inhibitors affect the efficacy of nirmatrelvir.
2.2. Efficacy of nirmatrelvir in suppressing SARS-CoV-2 infection
2.2.1. A broad-spectrum antiviral agent
Nirmatrelvir can impede in vitro infection with multiple human coronaviruses, including SARS-CoV-2, SARS-CoV-1, HCoV-HKU1, HCoV-OC43, MERS-CoV, HCoV-229E, and HCoV-NL63. Nirmatrelvir also suppresses SARS-CoV-2 lineages and its mutant variants, including USA_WA1/2020, C.37 Lambda (G15S), B.1.1.318 (T21I), B.1.2 (L89F), B.1.351 Beta (K90R), P.2 Zeta (L205V), BavPat, B.1.1.7 Alpha, B.1.1.28.1, B.1.617.2 Delta, Gamma P.1, and B.1.1.529 Omicron (P132H). The combination of nirmatrelvir and molnupiravir potently suppresses the replication of the Omicron variant [[32], [33], [34], [35]]. Thus, nirmatrelvir may be a broad-spectrum antiviral agent.
Nirmatrelvir suppresses the replication of different SARS-CoV-2 variants, including Omicron, Delta, and B.1.13, with IC50 values ranging from 7.9 to 10.5 nM, and impedes infection with different SARS-CoV-2 strains with EC50 values ranging from 32.6 to 280 nM. The concentration required for 90% inhibition (EC90) can more accurately predict the effective concentration of an antiviral drug in vivo. The EC90 values of nirmatrelvir in A549 and dNHBE cells infected with SARS-CoV-2 range from 56.1 to 215 nM [3,36,37]. The efficacy of nirmatrelvir against these variants is better than those of acriflavine (ACF), remdesivir, AT-527, molnupiravir, β-d-N4-hydroxycytidine, and ACF [38]. Overall, nirmatrelvir is a broad-spectrum antiviral agent.
2.2.2. Mouse model with SARS-CoV-2 mouse-adapted (MA)10 infection
Nirmatrelvir decreases multifocal pulmonary lesions and the SARS-CoV-2 viral load in a dose-dependent manner in a SARS-CoV-2 MA10 mouse model, which is an important tool for evaluating vaccines and drugs for coronavirus infection [39,40]. Specifically, the oral administration of 300 or 1000 mg/kg nirmatrelvir (b.i.d.) protects against weight loss in female BALB/c mice with SARS-CoV-2 MA10 infection. The 50% cell culture infectious dose values of nirmatrelvir at 0, 300, and 1000 mg/kg b.i.d. in infected mice are approximately log10 3.53, log10 3.02, and log10 4.93, respectively, suggesting that nirmatrelvir reduces the lung viral titers in infected mice by 28.40% and 38.74%, respectively. Nirmatrelvir reduces lung viral antigen levels and multifocal pulmonary lesions in infected mice in a dose-dependent manner [3]. Therefore, nirmatrelvir protects against lung tissue damage and virus replication in a dose-dependent manner in the SARS-CoV-2 MA10 model.
2.2.3. Hamster models with SARS-CoV-2 Beta and Delta infections
The development of new drugs usually requires preclinical studies with multiple animal models prior to clinical application. Interestingly, the oral administration of nirmatrelvir to Syrian golden hamsters completely suppresses intranasal infection with SARS-CoV-2 variants, including Beta and Delta [27]. Nirmatrelvir (125 and 250 mg/kg b.i.d.) suppresses the viral RNA copy numbers and virus titers in the lung tissues of female hamsters after intranasal infection with the Beta virus in a dose-dependent manner. Nirmatrelvir also markedly improves virus-induced weight loss and lung pathology. The 50% tissue culture infective dose values of nirmatrelvir at 0, 125, and 250 mg/kg b.i.d. in Beta virus-infected hamsters are approximately 6 × 104, 0.5 × 104, and 0, respectively, suggesting that nirmatrelvir reduces the lung viral titers in infected hamsters by 91.7% and 100%, respectively. The virus titer was also not detectable in Delta virus-infected hamsters after high-dose treatment, which suggests that 250 mg/kg nirmatrelvir completely inhibits viral replication. In addition, nirmatrelvir (250 mg/kg b.i.d.) completely suppresses Delta virus transmission from infected hamsters to naive hamsters, which suggests that the treatment of infected animals with nirmatrelvir significantly reduces transmission to contacts. However, nirmatrelvir (Paxlovid) does not control virus spread in humans [27,32,41].
2.2.4. Humans (Paxlovid)
As noted above, Paxlovid is a compound preparation of nirmatrelvir and ritonavir. Many clinical trials of Paxlovid, including NCT04962022, NCT04962230, NCT04756531, NCT04960202, NCT05064800, NCT05005312, NCT05032950, and NCT05047601, have been completed [23,32]. In NCT04756531, Paxlovid is characterized as safe and well tolerated. When administered within 3 days of COVID-19 symptom onset, Paxlovid has an efficacy of up to 89% [12,42,43]. Paxlovid is strongly recommended in the WHO COVID-19 treatment guidelines for the treatment of patients with mild and toxic COVID-19 [44]. However, some patients taking Paxlovid experience a second round of COVID-19 symptoms soon after recovery. The FDA is evaluating the rare reports of “viral load rebound” after the completion of Paxlovid treatment [45]. The long-term dosing of symptomatic patients has shown no additional benefit [46], and more studies are needed.
2.3. Pharmacokinetics of nirmatrelvir
2.3.1. Metabolic pathways
Nirmatrelvir is metabolized by CYP3A4 and has four metabolites [3]. Nirmatrelvir exhibits low renal and biliary excretion. The urine biliary excretion rates of nirmatrelvir in rats and monkeys are 17% and 7.0%, respectively. The corresponding biliary excretion index of nirmatrelvir is approximately 43%. However, the kidney is the main organ that eliminates nirmatrelvir in the presence of ritonavir. The exposure, accumulation, urinary recovery, and safety of nirmatrelvir do not substantially differ between Japanese and non-Japanese participants, which suggests that the pharmacokinetics and safety of nirmatrelvir are not affected by ethnicity [22,23,47].
2.3.2. Half-lives (t1/2)
Nirmatrelvir is stable for approximately 6 h at 37 °C in rat, monkey, and human plasma. The required dose of nirmatrelvir is not affected by diet. The t1/2 values in mice, rats, hamsters, monkeys, and humans are 23.5 min, 5.1 h, 26.6 min, 0.8 h, and 59.9 min, respectively, which suggests that the rapid metabolism of nirmatrelvir may require an adjuvant to exhibit increased efficacy [3,27].
2.4. Toxicology of nirmatrelvir
2.4.1. Nirmatrelvir does not show adverse reactions in animals
No adverse reactions have been noted in animal studies. The maximal plasma concentration (cmax) values of nirmatrelvir at the highest dose in rats (1000 mg/kg/day) and monkeys (600 mg/kg/day) were 51.5 μg/mL (corresponding to 103 μM) and 106 μg/mL (corresponding to 212 μM), respectively. No adverse reactions have been detected with the highest dose in rats (1000 mg/kg/day) and monkeys (600 mg/kg/day), which suggests that nirmatrelvir is well tolerated [3].
2.4.2. Reproductive and developmental safety of nirmatrelvir
The administration of nirmatrelvir is associated with reduced fetal weight. However, no other adverse effects have been observed. The oral administration of 1000 mg/kg/day nirmatrelvir for 32 days does not change early embryonic development or fertility in male or female rats. This dose also does not affect fetal body weight or external, visceral, or skeletal morphological development. The period encompassing gestational day (GD) 7 to 19 in rabbits is similar to the first trimester of gestation in humans. Oral dosages of 1000 mg/kg/day nirmatrelvir do not affect early embryonic development in rabbits from GD 7 to 19, which suggests that nirmatrelvir has a favorable reproductive safety profile in humans [30]. A toxicokinetic analysis showed that the effects of exposure to nirmatrelvir are dependent on the dose. Notably, at lower dosages, no fetal malformations have been observed in rats or rabbits. However, developmental toxicity effects, including higher resorption, lower fetal body weight, ossification delays, and increased skeletal variations, have been observed with the highest dosages in rats (75 mg/kg/day) and rabbits (110 mg/kg/day) [30].
Studies have also shown that ritonavir does not affect the development or birth of infants when taken in the first trimester of gestation. Studies of breastfeeding mothers have revealed that ritonavir is excreted into milk and is present at low levels in the blood of nursing infants, but the adverse reactions in breastfed infants are unclear [48]. However, the effects of Paxlovid and nirmatrelvir during breastfeeding have not been investigated in humans [49]. Breastfeeding infants must be carefully monitored if their mothers require treatment with Paxlovid.
2.4.3. Humans (Paxlovid)
Paxlovid is safe in patients who are not at high risk for complications, in children, and in patients with known exposure to COVID-19 (postexposure prophylaxis). Nevertheless, there are certain contraindications, including long-term treatment, hospitalization, and onset of symptoms or signs that began more than 5 days prior. The FDA warns that Paxlovid is not recommended for patients with severely impaired liver or kidney function and acknowledges the following side effects: headache, dysgeusia, myalgia, vomiting, mildly increased blood thyroid-stimulating hormone levels, gastrointestinal (GI) upset, nausea, diarrhea, and elevated blood pressure. The absence of adverse events and liver injury associated with Paxlovid may be due to the relatively short treatment time. The widespread use and abuse of Paxlovid may lead to the development of clinical resistance through an increase in the number of Mpro mutations. Whether long-term Paxlovid treatment has any serious adverse effects remains unclear [32,[49], [50], [51], [52]]. Importantly, the mean cmax of nirmatrelvir in patients treated with an oral dose of nirmatrelvir as a 2250-mg suspension (dosed as three split doses of 750 mg administered at 0, 2, and 4 h) in combination with 100 mg of ritonavir is 32 μM (corresponding to 15.94 μg/mL) [23]. Nirmatrelvir (10 μM) regulates the expression of 278 kinases, among which unc-51-like autophagy-activating kinase 1 (ULK1) (reduced by 36.1%) and protein kinase N2 (PKN2) (enhanced by 26.2%) show the most significant changes [3]. ULK1 plays a key role in autophagy progression. PKN2 is involved in several biological processes, including cytoskeleton organization, cell adhesion, cell cycle, immune response, and glucose metabolism. The side effects of nirmatrelvir may be caused by changes in host gene expression.
Taken together, the data show that nirmatrelvir has good tolerability, pharmacology, pharmacodynamics, pharmacokinetics, and safety (Fig. 2). However, high doses or long-term use may lead to multiple side effects via changes in the expression of host genes. Nirmatrelvir causes embryonic developmental toxicity and a second round of COVID-19 symptoms soon after recovery. Nirmatrelvir (Paxlovid) has many contraindications. The discovery and development of new antiviral drugs remain urgently needed.
Fig. 2.
Pharmacology, pharmacokinetics and toxicology of nirmatrelvir. Nirmatrelvir and its metabolite M4 inhibit severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) replication by suppressing the SARS-CoV-2 main protease (Mpro). Nirmatrelvir is metabolized by liver cytochrome P450 3A4 (CYP3A4) into M1−M4 and m/z 498 and is excreted in the urine. Ritonavir inhibits the metabolism of nirmatrelvir by suppressing CYP3A4 expression. Nirmatrelvir causes fetal developmental toxicity in rats and rabbits.
3. Mechanism and antiviral activity of nirmatrelvir derivatives
The lead structure of nirmatrelvir (compound 1) was patented by Pfizer, and the derivatives and analogs of nirmatrelvir were patented by Pfizer (compounds 2–18), Pardes Biosciences (compounds 19–28) and Enanta Pharmaceuticals (compounds 29–35) (Table S1) [3,22,[53], [54], [55]]. The structures of these compounds are similar to that of nirmatrelvir and form a reversible thioimidate adduct by the binding of nitrile carbon to the Mpro Cys145 to suppress Mpro. Compound 2 has the same structure as nirmatrelvir, with the exception of a different chiral structure. However, the EC50 value of compound 2 (9190 nM) against SARS-CoV-2 in epithelial Vero E6 cells is more than 123-fold higher than that of nirmatrelvir (74.5 nM), which suggests that the chiral structure plays a key role in the antiviral activity of nirmatrelvir [3,22,53]. Compounds 12–14 suppress SARS-CoV-2 infection in epithelial Vero E6 cells, with EC50 values similar to that of nirmatrelvir, whereas compounds 15–18 have EC50 values lower than that of nirmatrelvir, suggesting that the antiviral activity of compounds 12–18 is similar to or higher than that of nirmatrelvir. Notably, compound 18 exhibits the highest potency in suppressing SARS-CoV-2 (7-fold higher than that of nirmatrelvir) [53]. However, the antiviral activity of compounds 12–18 in vivo has not been investigated or reported. New drug development in the future will face the problem of overcoming patent limitations.
Compounds 29–35 suppress SARS-CoV-2 Mpro, with IC50 values lower than 100 nM [55]. However, the antiviral activity of compounds 29–35 in vivo has not been investigated or reported. Interestingly, compound 36 (also known as MPI47) has the same structure as compound 35, with the exception of a different chiral structure. However, the IC50 value of compound 35 (<100 nM) against Mpro in SARS-CoV-2 is more than 7-fold lower than that of compound 36 (720 nM), which suggests that the antiviral activity of compound 35 is higher than that of compound 36. It is worth noting that the EC50 value (>10 μM) of compound 36 against SARS-CoV-2 in 293T cells is higher than that of nirmatrelvir [56], which suggests that compound 36 suppresses SARS-CoV-2 replication with weaker antiviral activity than nirmatrelvir.
The metabolites of nirmatrelvir are mainly oxidative metabolites, including M1 (PF-07329265), M2 (PF-07329266), M3 (PF-07329267), M4 (PF-07329268), and m/z 498. M1–M4, are monohydroxylated metabolites, while m/z 498 is a dehydrogenated metabolite. M3 and M4 suppress Mpro with an inhibition constant (Ki) value (3 nM) similar to that of nirmatrelvir (3.11 nM). M4 is the main metabolite of nirmatrelvir. M4 also suppresses SARS-CoV-2 replication in epithelial Vero E6 cells, but its EC50 value (690 nM) is higher than that of nirmatrelvir (74.5 nM). The additional hydrogen bond donor of nirmatrelvir could reduce its oral absorption and permeability, which suggests that the OH group of M4 is the main cause of the reduced efficacy [[3], [22], [53]]. Many studies are needed to investigate the antiviral activity of M1−M3 and m/z 498 in suppressing SARS-CoV-2. In addition, the antiviral activity of M4 in suppressing SARS-COV-2 in vivo needs to be investigated. The structure of compound 3 is quite similar to that of M4. However, compound 3 has higher potency in suppressing SARS-CoV-2 (2-fold that of M4). It may be possible to develop derivatives (or prodrugs) of nirmatrelvir metabolites as new drugs to fight COVID-19.
4. Several issues of concern
Nirmatrelvir is the active ingredient of Paxlovid, and ritonavir enhances the efficacy of nirmatrelvir by inactivating its metabolic enzyme CYP3A4. However, ritonavir suppresses the expression of CYP3A4 along with MDR1, CYP2D6, CYP2C19, CYP2C8, and CYP2C9 [57]. Thus, several issues regarding Paxlovid treatment must be noted. 1) Certain drugs, such as statins, steroids, azole antifungals, sedative hypnotics, anticoagulants, vitamin K antagonists, oral antithrombotics, antiarrhythmics, opioids, midazolam, benzodiazepines, neuropsychiatric drugs, immunosuppressants, and antiarrhythmic therapies, may lead to life-threatening adverse events [49,52,58,59]. These drugs require empiric adjustment/discontinuation prior to treatment with nirmatrelvir/ritonavir [60,61]. 2) Ritonavir increases tacrolimus exposure by 57-fold and cyclosporine exposure by 5.8-fold. Thus, the doses of calcineurin inhibitors (CNIs) and mammalian target of rapamycin inhibitors (mTORis) for the treatment of solid organ transplant recipients should be reduced when used in combination with nirmatrelvir/ritonavir, and the drug levels should be closely monitored to prevent toxicity due to supratherapeutic CNI and mTORi exposure [59,62]. 3) Paxlovid should not be used with strong CYP3A inducers, such as rifampin, carbamazepine, phenobarbital, phenytoin, and St. John's wort. 4) Melatonin deficiency, which can be induced by advanced age, is a risk factor for COVID-19 severity [63,64]. Drugs such as ritonavir also induce melatonin deficiency by promoting melatonin metabolism [65]. Ritonavir-induced melatonin deficiency should be considered when treating COVID-19 patients with Paxlovid. 5) Providers may overlook the ritonavir component because Paxlovid is marketed under a single name, which increases the risk of toxicity [66]. 6) COVID-19 damages not only the lungs and respiratory system but also other organs, such as the GI tract, liver, pancreas, kidneys, heart, brain, and skin. Notably, blood vessels and endothelial cells (ECs), which are the conduits for virus dissemination to these organs, can be harmed by the virus [67]. However, the biological effects of Paxlovid and nirmatrelvir on blood vessels and ECs have not been investigated. 7) Hypoalbuminemia is an indicator of COVID-19 severity and patient prognosis [68]. The plasma concentrations of unbound and bound nirmatrelvir/ritonavir should therefore be evaluated in these patients. 8) Paxlovid has been approved for the treatment of patients ≥12 years of age who weigh ≥40 kg [69], but the efficacy and safety of Paxlovid in patients aged <12 years or weighing <40 kg are unclear. 9) There may be a need to develop accompanying diagnostic tools.
5. Conclusion
In the present review, we summarize the advances and challenges in using nirmatrelvir and its derivatives from the perspectives of pharmacology, pharmacokinetics, and toxicology. The administration of nirmatrelvir/Paxlovid in animal models (mouse and hamster models) and patients results in a reduction in pulmonary viral titers and tissue pathology. The tolerability, pharmacological, pharmacodynamics, pharmacokinetics, and safety of nirmatrelvir/Paxlovid are good. Thus, we should accelerate their development. However, Paxlovid should be used carefully in clinical practice, and clinicians must personalize treatment and medical orders for individual patients to ensure that the benefits of Paxlovid outweigh the risks. The antiviral activity of some derivatives of nirmatrelvir is higher than that of nirmatrelvir. However, many studies on topics such as the timing of intervention relative to SARS-CoV-2 infection, COVID-19 disease stage, dosage form, solubility, bioavailability, antiviral activity, metabolism and toxicity are needed. Overall, we sincerely hope that many more scientists will focus on nirmatrelvir and its derivatives to develop more new drugs that can be used to achieve victory over the COVID-19 pandemic.
CRediT author statement
Wujun Chen: Writing - Original draft preparation, Supervision, Supervision; Bing Liang: Writing - Original draft preparation, Resources; Xiaolin Wu: Formal analysis, Investigation; Ling Li: Formal analysis, Investigation, Funding acquisition; Chao Wang: Writing - Reviewing and Editing, Project administration; Dongming Xing: Conceptualization, Writing - Reviewing and Editing, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This study was supported by the Qingdao Major Scientific and Technological Project for Distinguished Scholars (Project No.: 20170103), the Laoshan Major Scientific and Technological Project for Distinguished Scholars (Project No.: 20181030), the Natural Science Foundation of Shandong Province (Project No.: ZR2020MH369), the Hospital Pharmacy Research Foundation of Guangdong Province (Project No.: 2022A01), and the Science and Technology Planning Project in Zhuhai (Project No.: ZH2202200090HJL).
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2022.10.005.
Contributor Information
Chao Wang, Email: wangchao20086925@126.com.
Dongming Xing, Email: xdm_tsinghua@163.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Pavan M., Bolcato G., Bassani D., et al. Supervised Molecular Dynamics (SuMD) Insights into the mechanism of action of SARS-CoV-2 main protease inhibitor PF-07321332. J. Enzyme Inhib. Med. Chem. 2021;36:1646–1650. doi: 10.1080/14756366.2021.1954919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korkmaz S., Ateşçelik M., Balcı H.N., et al. Health anxiety, health perception, and Healthy Lifestyle behavior among psychiatric patients during the COVID-19 pandemic. Prim. Care Companion. CNS Disord. 2022;24 doi: 10.4088/PCC.21m03197. [DOI] [PubMed] [Google Scholar]
- 3.Owen D.R., Allerton C.M.N., Anderson A.S., et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;374:1586–1593. doi: 10.1126/science.abl4784. [DOI] [PubMed] [Google Scholar]
- 4.Abu-Freha N., Alsana H., El-Saied S., et al. COVID-19 vaccination among the arab bedouin population: Lessons Learned from a minority population. Int. J. Public Health. 2022;67 doi: 10.3389/ijph.2022.1604133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sendi P., Razonable R.R., Nelson S.B., et al. First-generation oral antivirals against SARS-CoV-2. Clin. Microbiol. Infect. 2022;28:1230–1235. doi: 10.1016/j.cmi.2022.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ullrich S., Sasi V.M., Mahawaththa M.C., et al. Challenges of short substrate analogues as SARS-CoV-2 main protease inhibitors. Bioorg. Med. Chem. Lett. 2021;50 doi: 10.1016/j.bmcl.2021.128333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soriano V., de-Mendoza C., Edagwa B., et al. Oral antivirals for the prevention and treatment of SARS-CoV-2 infection. AIDS Rev. 2022;24:41–49. doi: 10.24875/AIDSRev.22000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.A.K. Singh, A. Singh, R. Singh, et al., An updated practical guideline on use of molnupiravir and comparison with agents having emergency use authorization for treatment of COVID-19, Diabetes Metabol. Syndr. 16 (2022), 102396. [DOI] [PMC free article] [PubMed]
- 9.Vandyck K., Deval J. Considerations for the discovery and development of 3-chymotrypsin-like cysteine protease inhibitors targeting SARS-CoV-2 infection. Curr. Opin. Virol. 2021;49:36–40. doi: 10.1016/j.coviro.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen W., Chen C., Tang J., et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: A meta-analysis. Ann. Med. 2022;54:516–523. doi: 10.1080/07853890.2022.2034936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee T.C., Morris A.M., Grover S.A., et al. Outpatient therapies for COVID-19: How do we choose? Open Forum Infect. Dis. 2022;9 doi: 10.1093/ofid/ofac008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahase E. Covid-19: Pfizer’s Paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ. 2021;375 doi: 10.1136/bmj.n2713. [DOI] [PubMed] [Google Scholar]
- 13.European Medicines Agency COVID-19: EMA recommends conditional marketing authorisation for Paxlovid. https://www.ema.europa.eu/en/news/covid-19-ema-recommends-conditional-marketing-authorisation-paxlovid
- 14.Medicines Patent Pool Pfizer and the Medicines Patent Pool (MPP) sign licensing agreement for COVID-19 oral antiviral treatment candidate to expand access in low- and middle-income countries. https://medicinespatentpool.org/news-publications-post/pfizer-and-the-medicines-patent-pool-mpp-sign-licensing-agreement-for-covid-19-oral-antiviral-treatment-candidate-to-expand-access-in-low-and-middle-income-countries
- 15.Medicines Patent Pool 35 generic manufacturers sign agreements with MPP to produce low-cost, generic versions of Pfizer’s oral COVID-19 treatment nirmatrelvir in combination with ritonavir for supply in 95 low- and middle-income countries. https://medicinespatentpool.org/news-publications-post/35-generic-manufacturers-sign-agreements-with-mpp-to-produce-low-cost-generic-versions-of-pfizers-oral-covid-19-treatment-nirmatrelvir-in-combination-with-ritonavir-for-supply-in-95-low-and
- 16.Zhao Y., Fang C., Zhang Q., et al. Crystal structure of SARS-CoV-2 main protease in complex with protease inhibitor PF-07321332. Protein Cell. 2022;13:689–693. doi: 10.1007/s13238-021-00883-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmad B., Batool M., Ain Q.U., et al. Exploring the binding mechanism of PF-07321332 SARS-CoV-2 protease inhibitor through molecular dynamics and binding free energy simulations. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22179124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Şimşek-Yavuz S., Komsuoğlu Çelikyurt F.I. An update of anti-viral treatment of COVID-19. Turk. J. Med. Sci. 2021;51:3372–3390. doi: 10.3906/sag-2106-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J., Lin C., Zhou X., et al. Structural basis of main proteases of coronavirus bound to drug candidate PF-07321332. J. Virol. 2022;96 doi: 10.1128/jvi.02013-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramos-Guzmán C.A., Ruiz-Pernía J.J., Tuñón I. Computational simulations on the binding and reactivity of a nitrile inhibitor of the SARS-CoV-2 main protease. Chem. Commun. (Camb) 2021;57:9096–9099. doi: 10.1039/d1cc03953a. [DOI] [PubMed] [Google Scholar]
- 21.Brewitz L., Kamps J.J.A.G., Lukacik P., et al. Mass spectrometric assays reveal discrepancies in inhibition profiles for the SARS-CoV-2 papain-like protease. ChemMedChem. 2022;17 doi: 10.1002/cmdc.202200016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eng H., Dantonio A.L., Kadar E.P., et al. Disposition of PF-07321332 (nirmatrelvir), an orally bioavailable inhibitor of SARS-CoV-2 3C-like protease, across animals and humans. Drug Metab. Dispos. 2022;50:576–590. doi: 10.1124/dmd.121.000801. [DOI] [PubMed] [Google Scholar]
- 23.Singh R.S.P., Toussi S.S., Hackman F., et al. Innovative randomized phase 1 study and dosing regimen selection to accelerate and inform pivotal COVID-19 trial of nirmatrelvir. Clin. Pharmacol. Ther. 2022;112:101–111. doi: 10.1002/cpt.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charlier B., Coglianese A., De Rosa F., et al. The effect of plasma protein binding on the therapeutic monitoring of antiseizure medications. Pharmaceutics. 2021;13 doi: 10.3390/pharmaceutics13081208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hochman J., Tang C., Prueksaritanont T. Drug-drug interactions related to altered absorption and plasma protein binding: Theoretical and regulatory considerations, and an industry perspective. J. Pharm. Sci. 2015;104:916–929. doi: 10.1002/jps.24306. [DOI] [PubMed] [Google Scholar]
- 26.Bohnert T., Gan L.-S. Plasma protein binding: From discovery to development. J. Pharm. Sci. 2013;102:2953–2994. doi: 10.1002/jps.23614. [DOI] [PubMed] [Google Scholar]
- 27.Abdelnabi R., Foo C.S., Jochmans D., et al. The oral protease inhibitor (PF-07321332) protects Syrian hamsters against infection with SARS-CoV-2 variants of concern. Nat. Commun. 2022;13 doi: 10.1038/s41467-022-28354-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith D.A., Di L., Kerns E.H. The effect of plasma protein binding on in vivo efficacy: Misconceptions in drug discovery. Nat. Rev. Drug Discov. 2010;9:929–939. doi: 10.1038/nrd3287. [DOI] [PubMed] [Google Scholar]
- 29.Denissen J.F., Grabowski B.A., Johnson M.K., et al. Metabolism and disposition of the HIV-1 protease inhibitor ritonavir (ABT-538) in rats, dogs, and humans. Drug Metab. Dispos. 1997;25:489–501. [PubMed] [Google Scholar]
- 30.Catlin N.R., Bowman C.J., Campion S.N., et al. Reproductive and developmental safety of nirmatrelvir (PF-07321332), an oral SARS-CoV-2 Mpro inhibitor in animal models. Reprod. Toxicol. 2022;108:56–61. doi: 10.1016/j.reprotox.2022.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Vries M., Mohamed A.S., Prescott R.A., et al. A comparative analysis of SARS-CoV-2 antivirals characterizes 3CLpro inhibitor PF-00835231 as a potential new treatment for COVID-19. J. Virol. 2021;95 doi: 10.1128/JVI.01819-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z., Yang L. The age of Omicron variant: Paxlovid raises new hopes of COVID-19 recovery. J. Med. Virol. 2022;94:1766–1767. doi: 10.1002/jmv.27540. [DOI] [PubMed] [Google Scholar]
- 33.Li P., Wang Y., Lavrijsen M., et al. SARS-CoV-2 Omicron variant is highly sensitive to molnupiravir, nirmatrelvir, and the combination. Cell Res. 2022;32:322–324. doi: 10.1038/s41422-022-00618-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.S. Ullrich, K.B. Ekanayake, G. Otting, et al., Main protease mutants of SARS-CoV-2 variants remain susceptible to nirmatrelvir, Bioorg. Med. Chem. Lett. 62 (2022), 128629. [DOI] [PMC free article] [PubMed]
- 35.Drożdżal S., Rosik J., Lechowicz K., et al. An update on drugs with therapeutic potential for SARS-CoV-2 (COVID-19) treatment. Drug Resist. Updates. 2021;59 doi: 10.1016/j.drup.2021.100794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang K.S., Leeuwon S.Z., Xu S., et al. Evolutionary and structural insights about potential SARS-CoV-2 evasion of nirmatrelvir. J. Med. Chem. 2022;65:8686–8698. doi: 10.1021/acs.jmedchem.2c00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vangeel L., Chiu W., De Jonghe S., et al. Remdesivir, molnupiravir and nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern. Antivir. Res. 2022;198 doi: 10.1016/j.antiviral.2022.105252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.A. Dabrowska, A. Szczepanski, P. Botwina, et al., Efficacy of antiviral drugs against the omicron variant of SARS-CoV-2, bioRxiv. 2021. 10.1101/2021.12.21.473268. [DOI]
- 39.Leist S.R., Dinnon K.H., 3rd, Schafer A., et al. A mouse-adapted SARS-CoV-2 induces acute lung injury and mortality in standard laboratory mice. Cell. 2020;183 doi: 10.1016/j.cell.2020.09.050. 1070−1085.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tostanoski L.H., Gralinski L.E., Martinez D.R., et al. Protective efficacy of rhesus adenovirus COVID-19 vaccines against mouse-adapted SARS-CoV-2. J. Virol. 2021;95 doi: 10.1128/JVI.00974-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfizer. Inc., Pfizer shares top-Line results from phase 2/3 EPIC-PEP study of Paxlovid™ for post-exposure prophylactic use. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-shares-top-line-results-phase-23-epic-pep-study. (Accessed 29 April 2022).
- 42.Hammond J., Leister-Tebbe H., Gardner A., et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N. Engl. J. Med. 2022;386:1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.García-Lledó A., Gómez-Pavón J., González Del Castillo J., et al. Pharmacological treatment of COVID-19: An opinion paper. Rev. Esp. Quimioter. 2022;35:115–130. doi: 10.37201/req/158.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Endpoint News Covid-19 roundup: WHO issues ‘strong’ recommendation for Pfizer’s Paxlovid but wants more price transparency. https://endpts.com/covid-19-roundup-who-issues-strong-recommendation-for-pfizers-paxlovid-but-wants-more-price-transparency/
- 45.E.Y. Dai, K.A. Lee, A.B. Nathanson, et al., Viral kinetics of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron infection in mRNAvaccinated individuals treated and not treated with nirmatrelvir-ritonavir, medRxiv. 2022. 10.1101/2022.08.04.22278378. [DOI]
- 46.News U.S. The case for testing Pfizer’s Paxlovid for treating long COVID. https://www.usnews.com/news/top-news/articles/2022-04-18/the-case-for-testing-pfizers-paxlovid-for-treating-long-covid
- 47.Boras B., Jones R.M., Anson B.J., et al. Preclinical characterization of an intravenous coronavirus 3CL protease inhibitor for the potential treatment of COVID-19. Nat. Commun. 2021;12 doi: 10.1038/s41467-021-26239-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lea A.P., Faulds D. Ritonavir. Drugs. 1996;52:541–546. doi: 10.2165/00003495-199652040-00007. [DOI] [PubMed] [Google Scholar]
- 49.Paxlovid for treatment of COVID-19. Med. Lett. Drugs Ther. 2022;64:9–10. [PubMed] [Google Scholar]
- 50.Marzi M., Vakil M.K., Bahmanyar M., et al. Paxlovid: Mechanism of action, synthesis, and in silico study. BioMed Res. Int. 2022;2022 doi: 10.1155/2022/7341493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parums D.V. Editorial: Current status of oral antiviral drug treatments for SARS-CoV-2 infection in Non-Hospitalized patients. Med. Sci. Mon. 2022;28 doi: 10.12659/MSM.935952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vuorio A., Kovanen P.T., Raal F. Cholesterol-lowering drugs for high-risk hypercholesterolemia patients with COVID-19 while on PaxlovidTM therapy. Future Virol. 2022;17:761–765. doi: 10.2217/fvl-2022-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.D.R. Owen, M.Y. Pettersson, M.R. Reese, et al., Inventors; Nitrile-containing antiviral compounds, World Intellectual Property Organization International Bureau Patent WO 2021/250648A1, 16 December 2021.
- 54.L.D. Arnold, A. Jennings, W. Keung, Inventors; Inhibitors of cysteine proteases and methods of use thereof, World Intellectual Property Organization International Bureau Patent WO 2021/252644A1, 16 December 2021.
- 55.Panarese J.D., Davis D., Kenton N.T., et al. 2021. Inventors; Functionalized peptides as antiviral agents, United States patent US 2022/0033383A1, 19 July. [Google Scholar]
- 56.Y.R. Alugubelli, Z.Z. Geng, K.S. Yang, et al., The N-terminal carbamate is key to high cellular and antiviral potency for boceprevir-based SARS-CoV-2 main protease inhibitors, bioRxiv. 2021. https://www.biorxiv.org/content/10.1101/2021.12.18.473330v1.
- 57.Waters L., Marra F., Pozniak A., et al. Ritonavir and COVID-19: Pragmatic guidance is important. Lancet. 2022;399:1464–1465. doi: 10.1016/S0140-6736(22)00280-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heskin J., Pallett S.J.C., Mughal N., et al. Caution required with use of ritonavir-boosted PF-07321332 in COVID-19 management. Lancet. 2022;399:21–22. doi: 10.1016/S0140-6736(21)02657-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lange N.W., Salerno D.M., Jennings D.L., et al. Nirmatrelvir/ritonavir use: Managing clinically significant drug-drug interactions with transplant immunosuppressants. Am. J. Transplant. 2022;22:1925–1926. doi: 10.1111/ajt.16955. [DOI] [PubMed] [Google Scholar]
- 60.McDonald E.G., Lee T.C. Nirmatrelvir-ritonavir for COVID-19. CMAJ. 2022;194 doi: 10.1503/cmaj.220081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.C. Marzolini, D.R. Kuritzkes, F. Marra, et al., Recommendations for the management of drug-drug interactions between the COVID-19 antiviral nirmatrelvir/ritonavir (Paxlovid) and comedications, Clin. Pharmacol. Ther. 2022. 10.1002/cpt.2646. [DOI] [PMC free article] [PubMed]
- 62.Wang A.X., Koff A., Hao D., et al. Effect of nirmatrelvir/ritonavir on calcineurin inhibitor levels: Early experience in four SARS-CoV-2 infected kidney transplant recipients. Am. J. Transplant. 2022;22:2117–2119. doi: 10.1111/ajt.16997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nair A.S. Perioperative melatonin in COVID-19 patients: Benefits beyond sedation and analgesia. Med. Gas Res. 2022;12:41–43. doi: 10.4103/2045-9912.325990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Borges L., Gennari-Felipe M., Dias B.B., et al. Melatonin, zinc, and vitamin C: Potential adjuvant treatment for COVID-19 patients. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.821824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.D.X. Tan, R.J. Reiter, Mechanisms and clinical evidence to support melatonin's use in severe COVID-19 patients to lower mortality, Life Sci. 294 (2022), 120368. [DOI] [PMC free article] [PubMed]
- 66.Fishbane S., Hirsch J.S., Nair V. Special considerations for Paxlovid treatment among transplant recipients with SARS-CoV-2 infection. Am. J. Kidney Dis. 2022;79:480–482. doi: 10.1053/j.ajkd.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tarnawski A.S., Ahluwalia A. Endothelial cells and blood vessels are major targets for COVID-19-induced tissue injury and spreading to various organs. World J. Gastroenterol. 2022;28:275–289. doi: 10.3748/wjg.v28.i3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen C., Zhang Y., Zhao X., et al. Hypoalbuminemia – an indicator of the severity and prognosis of COVID-19 patients: A multicentre retrospective analysis. Infect. Drug Resist. 2021;14:3699–3710. doi: 10.2147/IDR.S327090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.COVID-19 updates: Remdesivir (Veklury) in high-risk outpatients with COVID-19. Med. Lett. Drugs Ther. 2022;64:31–32. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.