Abstract
Background
Seemingly, the Matrix metalloproteinases (MMPs) play a role in the etiopathogenesis of coronavirus disease 2019 (COVID-19). Here in this study, we determined the association of MMP9 rs3918242, MMP3 rs3025058, and MMP2 rs243865 polymorphisms with the risk of COVID-19, especially in those with neurological syndrome (NS).
Methods
We enrolled 500 patients with COVID-19 and 500 healthy individuals. To genotype the target SNPs, the Real-time allelic discrimination technique was used. To determine serum levels of MMPs, Enzyme-linked immunosorbent assay (ELISA) was exerted.
Results
The MMP9 gene rs3918242 and MMP3 gene rs3025058 SNP were significantly associated with increased COVID-19 risk and susceptibility to COVID-19 with NS. The serum level of MMP-9 and MMP-3 was significantly higher in COVID-19 cases compared with the healthy controls. Serum MMP-9 and MMP-3 levels were also higher in COVID-19 subjects with NS in comparison to the healthy controls. The polymorphisms in MMP genes were not associated with serum level of MMPs.
Conclusion
MMP9 and MMP3 gene polymorphisms increases the susceptibility to COVID-19 as well as COVID-19 with neurologic syndrome, but they probably have no role in the regulation of serum MMP-9 and MMP-3 levels.
Keywords: Coronavirus disease 2019, Central nervous system, Matrix metalloproteinases, Genetic polymorphism, Neurological symptoms
Introduction
The recently emerging severe acute respiratory syndrome coronavirus 2 (SARS-CoV‐2) attacks to lungs and several body organs and causes coronavirus disease 2019 (COVID-19). The virus also attacks organs (like heart and kidneys) expressing angiotensin-converting enzyme 2 (ACE2) receptor as the main molecular receptor for S protein of virus [1, 2]. Additionally, neurological manifestations are also reported commonly in patients with COVID-19 [3]. SARS-CoV-2 nucleic acid has been identified by reverse-transcriptase–polymerase chain-reaction (RT-PCR) in the cerebrospinal fluid (CSF) samples of a number of COVID-19 patients [4]. Moreover, virus particles have also been detected in the autopsy samples of brain in a subject [5]. However, it is not clear if the neurological manifestations are due to infection of the Central nervous system (CNS) by SARS-CoV-2 or other possible mechanisms might cause complications related to CNS.
Matrix metalloproteinases (MMPs) are calcium-dependent zinc-containing endopeptidases enzymes that act in the extracellular environment of cells and play a role in degrading extracellular matrix (ECM) and basement membrane by cleaving both matrix and non-matrix proteins. These enzymes are involved in different physiological as well as pathological processes, such as wound healing, morphogenesis, tissue repair and remodeling, inflammation, and angiogenesis [6, 7]. Studies show that MMPs are involved in the facilitation of immune cells infiltration into the CNS through the blood-brain barrier (BBB) [8]. In addition, it was observed that MMP-3 levels are increased in the serum of COVID-19 patients that was correlated with higher levels of inflammatory cytokines [9]. According to a hypothesis, upon entrance of SARS-CoV-2 into human airways, it may pass through the epithelial cells into blood circulation and then infect monocytes. Seemingly, increased permeability of BBB by MMP-9 and enhanced expression of Intercellular adhesion molecule 1 (ICAM-1) on the endothelial cells by Tumor necrosis factor (TNF)-α promotes the migration of infected monocytes to the CNS. Thereupon, monocytes secret inflammatory mediators in the CNS that leads to injury to neurons and oligodendrocytes [10].
Studies show that the genomic sequences of MMP genes are polymorphic that might be involved in the regulation of MMP gene expression [11–14]. Numerous studies have indicated that single nucleotide polymorphisms (SNPs) in the different MMP genes are associated with human diseases [15], especially infectious diseases [16, 17] and neurodegenerative disorders [18, 19]. Taking all, here we intended to disclose the possible association of MMP9 gene rs3918242, MMP3 gene rs3025058, and MMP2 gene rs243865 polymorphisms with the risk of COVID-19 disease. Moreover, the possible involvement of these polymorphisms in the development of COVID-19 associated neurologic symptoms was evaluated.
Study participants and methods
COVID-19 patients and healthy controls
In the current case-control study, 500 subjects with COVID-19 and 500 age and gender matched healthy individuals were recruited (Table 1). COVID-19 patients were diagnosed by Real-time PCR for the infection by SARS-CoV-2 by nasopharyngeal swabs and were selected from those who referred to the intensive care unit (ICU) of Shahid Rajaee hospital of Karaj, Iran. Patients had severe form of the disease and had respiratory failure and decreased oxygen saturation. The neurologic symptoms of the patients were also determined by a neurologist. Individuals in the control group were negative for the SARS-CoV-2 nucleic acid in nasopharyngeal swabs evaluated by Real-time PCR. Before sampling (10 ml of venous blood), all participants signed written informed forms and the local ethical committee of Alborz University of Medical Science approved the protocol of the study (IR.ABZUMS.REC.1399.340).
Table 1.
Baseline characteristics, laboratory findings, and clinical symptoms of the study participants
| Feature | COVID-19 patients (n = 500) |
Healthy controls (n = 500) |
P value |
|---|---|---|---|
| Sex; male/female | 320 (64%)/180 (36%) | 300 (60%)/200 (40%) | > 0.05 |
| Age; year | 56.55 ± 10.31 | 54.35 ± 9.88 | > 0.05 |
| WBC; cells/mm3 | 8521.65 ± 4821.65 | 5278.45 ± 2754.89 | < 0.05 |
| Lymphocyte-total leukocyte ratio | 25.41 ± 15.32 | 31.28 ± 18.54 | < 0.05 |
| Neutrophil-lymphocyte ratio | 8.24 ± 15.48 | 2.89 ± 9.51 | < 0.05 |
| CRP (mg/L) | 4.16 ± 2.52 | 1.12 ± 0.98 | < 0.05 |
| AST (IU/L) | 33.41 ± 9.13 | 25.32 ± 8.58 | < 0.05 |
| ALT (IU/L) | 38.25 ± 8.11 | 26.44 ± 7.86 | < 0.05 |
| LDH (IU/L) | 432.88 ± 99.21 | 316.39 ± 88.54 | < 0.05 |
| Fever; yes/no | 350 (70%)/ 150 (30%) | - | - |
| Cough; yes/no | 190 (38%)/ 310 (62%) | - | - |
| Dyspnea; yes/no | 426 (85.2%)/ 74 (14.8%) | - | - |
| Sputum; yes/no | 140 (28%)/ 360 (72%) | - | - |
| Vomiting/diarrhea; yes/no | 80 (16%)/ 420 (84%) | - | - |
| Neurologic syndrome; yes/no | 72 (14.4%)/ 428 (85.6%) | - | - |
| Delirium* | 42/72 (58.33%) | - | - |
| Encephalitis | 16/72 (22.22%) | - | - |
| Headache | 22/72 (30.55%) | - | - |
COVID-19, Coronavirus disease 2019; WBC, White blood cell; CRP, C-reactive protein; AST, Aspartate aminotransferase; ALT, Alanine aminotransferase; LDH, Lactate dehydrogenase
* Delirium, Encephalitis, and Headache were determined in the 72 cases with Neurologic syndrome
DNA extraction and genotyping of polymorphisms
About 10 ml of perpheral blood was obtained from all case and control subjectes using EDTA containing venipuncture for DNA extraction as well as tubes for serum isolation. The whole blood samples were stored in -20 °C before extracting DNA. The DNA content from whole blood was isolated by exerting the QIAamp DNA Mini Kit (Qiagen, Germany). The quality and quantity of the extracted DNA samples was determined by optical density (OD) at 260/280 nm ratio by a NanoDrop spectrophotometer system (NanoDrop ND-2000 C Spectrophotometer, Thermo Fisher Scientific, USA). Then, MMP9 rs3918242, MMP3 rs3025058, and MMP2 rs243865 polymorphisms were genotyped by Real-time allelic discrimination method using StepOnePlus Real-Time PCR device (Applied Biosystems, Foster City, USA) and TaqMan assays (Applied Biosystems, Foster City, USA). The reaction mixture in each well of 96-microwell plates contained 2 µl DNA (20 ng/µl), 5 µl TaqMan Master Mix (containing Taq DNA polymerase and dNTPs), 0.5 µl TaqMan Genotyping Assay Mix (containing primers and probes; Applied Biosystems, Foster City, USA), and distilled water for reaching a total volume of 15 µl. The thermocycling conditions of the PCR reactions were as follow; initial heating for 60 °C for 30 s followed by 95 °C for 10 min, then 40 cycles of amplification in 95 °C for 15 s and 60 °C for 50 s, and ultimately 60 °C for 45 s.
Serum levels of MMPs
Serum samples were isolated from the venous blood of 80 COVID-19 cases as well as 80 healthy controls to measure the concentration of the MMP-9, MMP-3, and MMP-2 using the enzyme linked immunosorbent assay (ELISA) technique. The OD was determined using a commercial kit (Invitrogen, Thermo Fisher Scientific, San Diego, CA, USA) and an ELISA reader device (Tecan Spectra, Austria).
Statistical analysis
The distribution of the alleles and genotypes was represented as frequency and corresponding percentage. The associations between the different genetic models of polymorphisms and risk of COVID-19 were analyzed by Pearson’s chi square (χ2). To determine the association level, the odds ratios (OR) and corresponding 95% confidence intervals (CI) were calculated.
Genotype distribution of SNPs in the control group was tested to comply with the Hardy–Weinberg Equilibrium (HWE). Exploring for the normality of numeric data distribution was done by the Kolmogorov-Smirnov test. The serum level of MMPs between different groups were compared using the non-parametric Mann-Whitney U-test or the Kruskal-Wallis test. Multivariate logistic regression analysis was conducted to control ORs for confounding factors. Demonstration of numeric data was done by Mean ± standard deviation (SD) and nominal data was presented as numbers and percentage. GraphPad PRISM software v.8.00 (GraphPad Software, Inc., San Diego, CA, USA) was used for data analysis and designing graphs.
Results
Allele and genotype frequencies in COVID-19 patients and healthy controls
Distribution of genotypes for all three SNPs did not deviate from HWE in the control group (Table 2). The genetic comparisons in the MMP9 gene rs3918242 and MMP3 gene rs3025058 SNP was associated with risk of COVID-19, while MMP2 gene rs243865 did not show any significant difference.
Table 2.
Allele and genotype frequencies of MMP9 rs3918242, MMP3 rs3025058, and MMP2 rs243865 polymorphisms in COVID-19 patients and healthy controls and related association analyses
| SNP | Allele /Genotype | COVID-19 (n = 500) N% |
Healthy controls (n = 500) N% |
OR (95% CI) | P |
|---|---|---|---|---|---|
| MMP9 rs3918242 | T vs. C | 192 (19.2) | 134 (13.4) | 1.53 (1.09–2.15) | 0.013 |
| C (Reference) | 808 (80.8) | 866 (86.6) | - | - | |
| TT vs. CC | 30 (6) | 14 (2.8) | 2.40 (0.95–6.05) | 0.061 | |
| CT vs. CC | 132 (26.4) | 106 (21.2) | 1.40 (0.92–2.12) | 0.113 | |
| TT vs. CT + CC | 30 (6) | 14 (2.8) | 2.21 (0.88–5.53) | 0.088 | |
| TT + CT vs. CC | 162 (32.4) | 120 (24) | 1.51 (1.02–2.24) | 0.037 | |
| CC (Reference) | 338 (67.6) | 380 (76) | - | - | |
| HWE | P = 0.17 | ||||
| MMP3 rs3025058 | G vs. C | 238 (23.8) | 168 (16.8) | 1.54 (1.13–2.11) | 0.006 |
| C (Reference) | 762 (76.2) | 832 (83.2) | - | - | |
| GG vs. CC | 42 (8.4) | 6 (1.2) | 7.78 (2.27–26.6) | 0.001 | |
| GC vs. CC | 154 (30.8) | 156 (31.2) | 1.09 (0.74–1.61) | 0.634 | |
| GG vs. GC + CC | 42 (8.4) | 6 (1.2) | 7.55 (2.22–25.6) | 0.0012 | |
| GG + GC vs. CC | 196 (39.2) | 162 (32.47) | 1.34 (0.92–1.94) | 0.113 | |
| CC (Reference) | 304 (60.8) | 338 (67.6) | - | - | |
| HWE | P = 0.06 | ||||
| MMP2 rs243865 | T vs. C | 212 (21.2) | 204 (20.4) | 1.04 (0.77–1.42) | 0.755 |
| C (Reference) | 788 (78.8) | 796 (79.6) | - | - | |
| TT vs. CC | 24 (4.8) | 18 (3.6) | 1.34 (0.54–3.27) | 0.518 | |
| CT vs. CC | 164 (32.8) | 168 (33.6) | 0.98 (0.67–1.43) | 0.926 | |
| TT vs. CT + CC | 24 (4.8) | 8 (3.6) | 1.35 (0.55–3.26) | 0.505 | |
| TT + CT vs. CC | 188 (37.6) | 186 (37.2) | 1.01 (0.70–1.46) | 0.926 | |
| CC (Reference) | 312 (62.4) | 314 (62.8) | - | - | |
| HWE | P = 0.16 |
SNP, Single nucleotide polymorphism; MMP, Matrix metalloproteinase; COVID-19, Coronavirus disease 2019; OR, Odds ratio; 95% CI, 95% Confidence interval; HWE, Hardy-Weinberg equilibrium
It was observed that frequency of the T allele of MMP9 gene rs3918242 was higher in the COVID-19 patients compared to the controls (19.2% vs. 13.4%); hence the T allele was significantly associated with a 1.53-times increased risk of COVID-19 (OR = 1.53, 95%CI = 1.09–2.15; P = 0.013). Additionally, the dominant genetic comparison (TT + CT vs. CC) was significantly associated with a 1.51-times increased risk of COVID-19 (OR = 1.51, 95%CI = 1.02–2.24; P = 0.037). The TT genotype was highly seen in the COVID-19 group and increased COVID-19 risk 2.40 times, even though it was not statistically significant (OR = 2.40, 95%CI = 0.95–6.05; P = 0.061). The recessive TT vs. CT + CC model was observed to be insignificantly associate with a 2.21 times increased COVID-19 risk (OR = 2.21, 95%CI = 0.88–5.53; P = 0.088). The CT genotype did not have statistically significant association with COVID-19 risk, even though it caused a 1.40-times increased COVID-19 risk (OR = 1.40, 95%CI = 0.92–2.12; P = 0.113; Table 2).
The minor G allele of MMP3 gene rs3025058 SNP had a statistically significant and strong association with a 1.54-times increased risk of COVID-19 (OR = 1.54, 95%CI = 1.13–2.11; P = 0.006). Interestingly, the GG genotype was significantly associated with a 7.78-times higher risk of COVID-19 (OR = 7.78, 95%CI = 2.27–26.6; P = 0.001), which was statistically strong association. Moreover, the dominant genetic model (GG vs. GC + CC) had statistically significant association with a 7.55-times increased risk of COVID-19 (OR = 7.55, 95%CI = 2.22–25.6; P = 0.0012). The GC genotype (OR = 1.09, 95%CI = 0.74–1.61; P = 0.634) and dominant GG + GC vs. CC model (OR = 1.34, 95%CI = 0.92–1.94; P = 0.113) had insignificant association with slightly increased COVID-19 risk (Table 2).
For MMP2 gene rs243865, it was detected that the T allele (OR = 1.04), TT genotype (OR = 1.34), dominant TT vs. CT + CC model (OR = 1.35), and recessive TT + CT vs. CC model (OR = 1.01) had statistically insignificant association with a slight increased risk of COVID-19. However, the CT genotype was insignificantly associated with decreased COVID-19 risk (OR = 0.98; Table 2).
Allele and genotype frequencies in COVID-19 patients with neurologic syndrome and healthy controls
Table 3 shows the allele and genotype frequencies of MMP9 gene rs3918242, MMP3 gene rs3025058 SNP, and MMP2 gene rs243865 in COVID-19 patients with neurologic syndrome and healthy controls.
Table 3.
Allele and genotype frequencies of MMP9 rs3918242, MMP3 rs3025058, and MMP2 rs243865 polymorphisms in COVID-19 patients with neurologic syndrome and healthy controls and related association analyses
| SNP | Allele /Genotype | COVID-19 cases with neurologic syndrome (n = 72) N% |
Healthy controls (n = 500) N% |
OR (95% CI) | P |
|---|---|---|---|---|---|
| MMP9 rs3918242 | T vs. C | 32 (22.2) | 134 (13.4) | 1.84 (1.00-3.40) | 0.049 |
| C (Reference) | 112 (77.8) | 866 (86.6) | - | - | |
| TT vs. CC | 6 (8.3) | 14 (2.8) | 3.54 (0.85–14.6) | 0.081 | |
| CT vs. CC | 20 (27.7) | 106 (21.2) | 1.55 (0.69–3.47) | 0.278 | |
| TT vs. CT + CC | 6 (8.3) | 14 (2.8) | 3.15 (0.77–12.8) | 0.107 | |
| TT + CT vs. CC | 26 (36.11) | 120 (24) | 1.78 (0.85–3.74) | 0.122 | |
| CC (Reference) | 46 (63.8) | 380 (76) | - | - | |
| HWE | P = 0.17 | ||||
| MMP3 rs3025058 | G vs. C | 46 (31.9) | 168 (16.8) | 2.23 (1.34–4.02) | 0.002 |
| C (Reference) | 98 (68.1) | 832 (83.2) | - | - | |
| GG vs. CC | 8 (11.1) | 6 (1.2) | 13.25 (2.73–64.2) | 0.001 | |
| GC vs. CC | 30 (41.6) | 156 (31.2) | 1.91 (0.90–4.02) | 0.087 | |
| GG vs. GC + CC | 8 (11.1) | 6 (1.2) | 10.29 (2.20–48.1) | 0.003 | |
| GG + GC vs. CC | 38 (52.7) | 162 (32.47) | 2.33 (1.15–4.72) | 0.018 | |
| CC (Reference) | 34 (47.2) | 338 (67.6) | - | - | |
| HWE | P = 0.06 | ||||
| MMP2 rs243865 | T vs. C | 32 (22.3) | 204 (20.4) | 1.11 (0.61–2.02) | 0.721 |
| C (Reference) | 112 (77.7) | 796 (79.6) | - | - | |
| TT vs. CC | 4 (5.5) | 18 (3.6) | 1.58 (0.32–7.82) | 0.571 | |
| CT vs. CC | 24 (33.3) | 168 (33.6) | 1.01 (0.48–2.16) | 0.959 | |
| TT vs. CT + CC | 4 (5.5) | 8 (3.6) | 1.57 (0.32–7.59) | 0.571 | |
| TT + CT vs. CC | 28 (38.9) | 186 (37.2) | 1.07 (0.52–2.20) | 0.844 | |
| CC (Reference) | 44 (61.2) | 314 (62.8) | - | - | |
| HWE | P = 0.16 |
SNP, Single nucleotide polymorphism; MMP, Matrix metalloproteinase; COVID-19, Coronavirus disease 2019; OR, Odds ratio; 95% CI, 95% Confidence interval; HWE, Hardy-Weinberg equilibrium
The T allele of MMP9 gene rs3918242 was highly represented in COVID-19 patients with neurologic syndrome in comparison to controls (22.2% vs. 13.4%). The analysis indicated that the T allele had statistically significant (but marginal) association with a 1.84-times increased risk of COVID-19 with neurologic syndrome (OR = 1.84, 95%CI = 1.00-3.40; P = 0.049). Even though it was not statistically significant and the CI was wide, the TT genotype was associated with a 3.54 times increased risk of COVID-19 with neurologic syndrome (OR = 3.54, 95%CI = 0.85–14.6; P = 0.081). The CT genotype had also higher expression in the COVID-19 with neurologic syndrome and was insignificantly associated with a 1.55-times increased risk of the COVID-19 with neurologic syndrome (OR = 1.55, 95%CI = 0.69–3.47; P = 0.278). The analysis also revealed that both dominant TT vs. CT + CC (OR = 3.15) and recessive TT + CT vs. CC (OR = 1.78) models had statistically insignificant association with increased risk of COVID-19 with neurologic syndrome.
For MMP3 gene rs3025058, it was seen that the minor G allele was associated with a 2.23-times increased risk of COVID-19 with neurologic syndrome (OR = 2.23, 95%CI = 1.34–4.02; P = 0.002). As well, it was detected that GG genotype had statistically significant association with a strong 13.25-times increased risk of COVID-19 with neurologic syndrome (OR = 13.25, 95%CI = 2.73–64.2; P = 0.001). A statistically significant association was found between the dominant (OR = 2.23, 95%CI = 1.15–4.72; P = 0.018) and the recessive (OR = 10.29, 95%CI = 2.20–48.1; P = 0.003) models and increased (2.23-times and 10.29-times, respectively) risk of COVID-19 with neurologic syndrome (Table 3).
Even though all genetic comparisons for MMP2 gene rs243865 were not statistically significant, they were associated with an increased risk (T allele OR = 1.11, TT genotype OR = 1.58, CT genotype OR = 1.01, dominant model OR = 1.57, recessive model OR = 1.07) of COVID-19 with neurologic syndrome.
Regression analysis
The multivariate logistic regression analysis was performed to adjust ORs of statistically significant comparisons in MMP SNPs for potential confounding factors. It was observed that for the MMP9 rs3918242 SNP in the TT + CT vs. CC model, the ORs were still statistically significant after controlling for the potential confounders, including Age, Sex, Fever, Cough, Dyspnea, Sputum, Vomiting/diarrhea, Delirium, Encephalitis, and Headache. As such, ORs were still statistically significant for MMP3 rs3025058 SNP in both GG vs. CC and GG vs. GC + CC models after controlling for the confounders (Table 4).
Table 4.
Multivariate logistic regression analysis to adjust ORs of genetic models in MMP SNPs for potential confounding factors
| SNP | Genetic model | Confounding factor | OR | 95% CI | P value |
|---|---|---|---|---|---|
| MMP9 rs3918242 | TT + CT vs. CC | Age | 1.48 | (1.11–2.84) | 0.0254 |
| Sex | 1.35 | (1.05–3.04) | 0.0483 | ||
| Fever | 1.59 | (1.12–2.55) | 0.0215 | ||
| Cough | 1.50 | (1.20–2.81) | 0.0134 | ||
| Dyspnea | 1.27 | (1.22–3.10) | 0.0312 | ||
| Sputum | 1.39 | (1.07–2.89) | 0.0380 | ||
| Vomiting/diarrhea | 1.42 | (1.21–2.77) | 0.0244 | ||
| Delirium | 1.55 | (1.30-3.00) | 0.0401 | ||
| Encephalitis | 1.39 | (1.19–3.13) | 0.0219 | ||
| Headache | 1.70 | (1.09–2.17) | 0.0304 | ||
| CC (Reference) | 1.00 | - | - | ||
| MMP3 rs3025058 | GG vs. CC | Age | 6.28 | (1.25–25.88) | 0.0014 |
| Sex | 6.14 | (3.11–20.40) | 0.0054 | ||
| Fever | 5.45 | (2.49–28.02) | 0.0188 | ||
| Cough | 6.11 | (2.87–26.43) | 0.0057 | ||
| Dyspnea | 7.05 | (3.04–29.14) | 0.0084 | ||
| Sputum | 6.28 | (2.65–26.69) | 0.0067 | ||
| Vomiting/diarrhea | 6.70 | (2.99–27.03) | 0.0040 | ||
| Delirium | 7.26 | (2.87–30.14) | 0.0091 | ||
| Encephalitis | 6.81 | (2.14–23.80) | 0.0049 | ||
| Headache | 6.37 | (2.16–26.92) | 0.0304 | ||
| GG vs. GC + CC | Age | 7.14 | (1.88–24.39) | 0.0015 | |
| Sex | 6.36 | (2.16–29.02) | 0.0305 | ||
| Fever | 6.98 | (2.49–24.27) | 0.0058 | ||
| Cough | 6.41 | (2.09–28.65) | 0.0091 | ||
| Dyspnea | 7.25 | (2.14–27.98) | 0.0037 | ||
| Sputum | 7.30 | (2.05–30.07) | 0.0024 | ||
| Vomiting/diarrhea | 7.07 | (2.19–29.60) | 0.0039 | ||
| Delirium | 7.10 | (2.14–28.19) | 0.0109 | ||
| Encephalitis | 6.57 | (2.44–23.01) | 0.0076 | ||
| Headache | 6.51 | (2.10-22.89) | 0.0083 | ||
| CC (Reference) | 1.00 | - | - |
MMP, Matrix metalloproteinase; OR, Odds ratio; CI, Confidence interval
Serum levels of MMPs
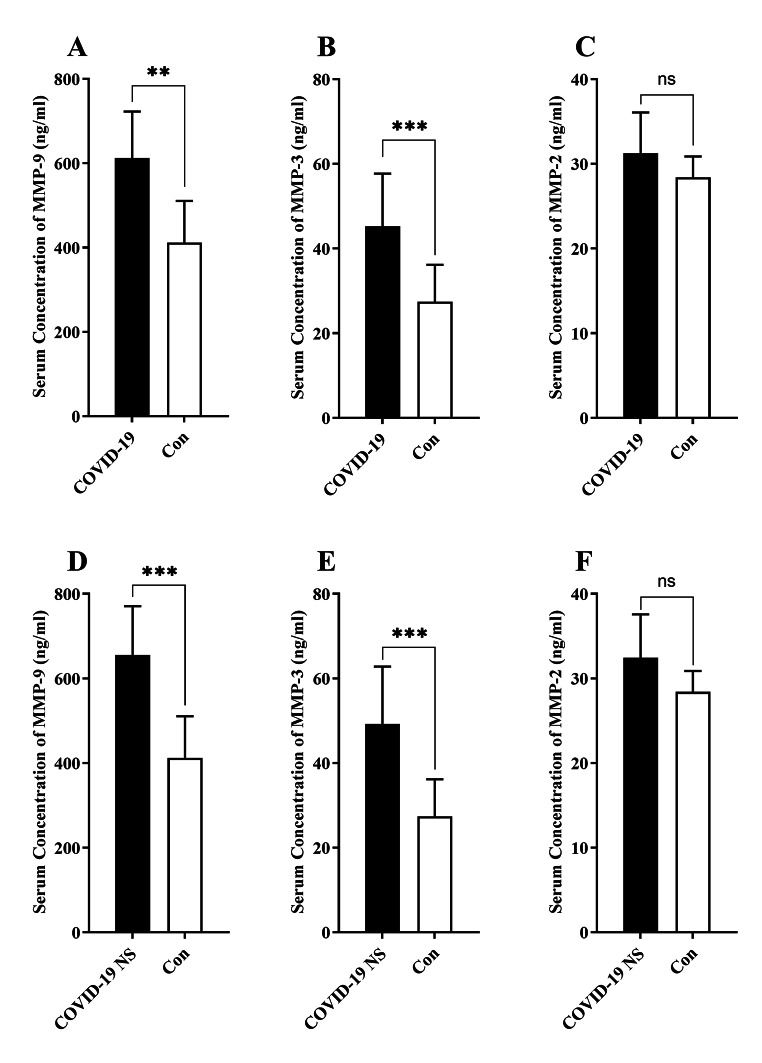
The serum level of MMP-9 was significantly higher in COVID-19 cases (612.32 ± 110.54 ng/ml) compared with the healthy controls (412.25 ± 98.52 ng/ml; P = 0.009; Fig. 1.A). Additionally, serum MMP-3 level was significantly higher in COVID-19 subjects (45.25 ± 12.44 ng/ml) in comparison to the healthy controls (27.44 ± 8.74 ng/ml; P = 0.0005; Fig. 1.B). There was no statistically significant difference in the serum level of MMP-2 between COVID-19 cases and healthy controls (Fig. 1.C).
Fig. 1.
Bar charts demonstrate the serum concentration of MMP-9, MMP-3, and MMP-2 in the COVID-19 subjects and healthy controls (A, B, C). The comparison of the serum levels of MMP-9, MMP-3, and MMP-2 in the COVID-19 patients with neurologic syndrome (NS) compared with healthy controls (D, E, F). The mean comparisons were done by statistical test of Mann-Whitney’s U test (** shows P < 0.01, *** shows P < 0.001; ns, non-significant)
The results indicated significantly higher level of MMP-9 in COVID-19 patients with neurologic syndrome (655.41 ± 115.21 ng/ml) in comparison to healthy controls (412.25 ± 98.52 ng/ml; P = 0.0003; Fig. 1.D). Moreover, there was significantly higher levels of MMP-3 in serum of COVID-19 patients with neurologic syndrome (49.25 ± 13.54 ng/ml) relative to healthy controls (27.44 ± 8.74 ng/ml; P = 0.0007; Fig. 1.E). No significant difference was observed in serum level of MMP-2 between COVID-19 patients with neurologic syndrome and healthy controls (Fig. 1.F).
Association of polymorphisms with serum levels of MMPs
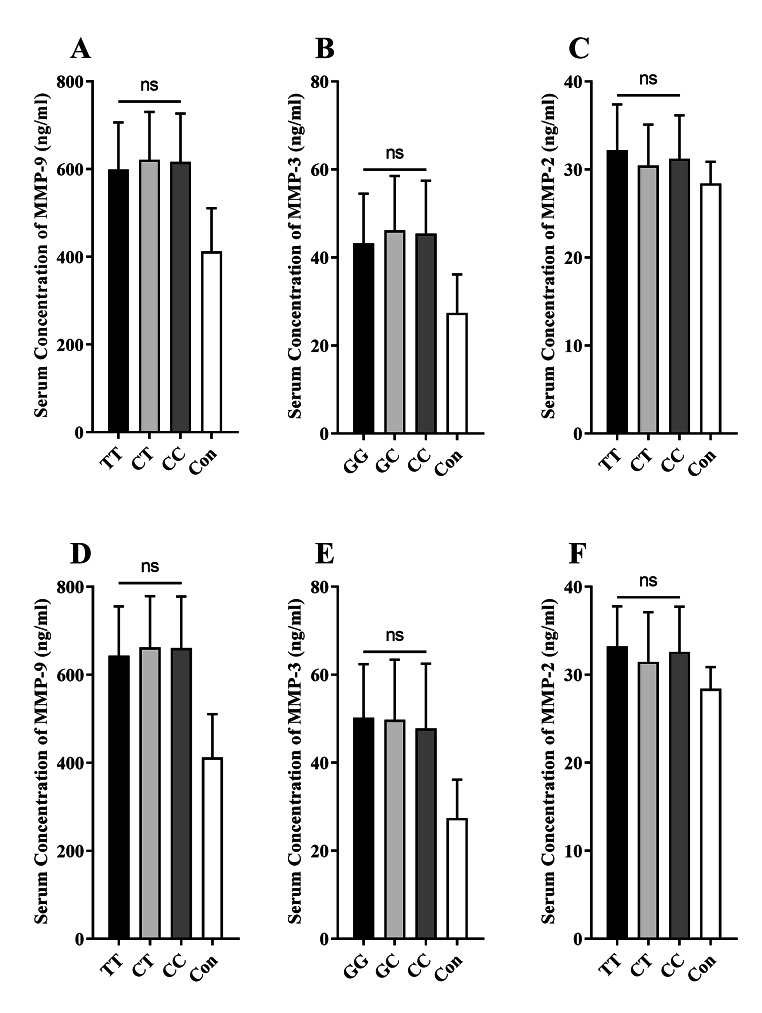
In order to determine if genetic polymorphisms of MMP genes affect the serum level of MMPs, the levels of these enzymes were compared among patients with different genotypes for each SNP. However, it was detected that none of the MMP-9 (Fig. 2.A), MMP-3 (Fig. 2.B), and MMP-2 (Fig. 2.C) had different levels among COVID-19 patients with different three genotypes for MMP9 rs3918242, MMP3 rs3025058, and MMP2 rs243865 polymorphisms, respectively. Additionally, no significant differences were observed in serum levels of MMP-9 (Fig. 2.D), MMP-3 (Fig. 2.E), and MMP-2 (Fig. 2.F) among COVID-19 patients with neurologic syndrome with three different genotypes for MMP9 rs3918242, MMP3 rs3025058, and MMP2 rs243865 polymorphisms, respectively.
Fig. 2.
Bar graphs show the serum concentration of MMP-9, MMP-3, and MMP-2 in the COVID-19 subjects with three different genotypes for MMP9 rs3918242, MMP3 rs3025058, and MMP2 rs243865 polymorphisms, respectively (A, B, C). The comparison of the serum levels of MMP-9, MMP-3, and MMP-2 in the COVID-19 subjects with neurologic syndrome (NS) harboring three different genotypes for MMP9 rs3918242, MMP3 rs3025058, and MMP2 rs243865 polymorphisms, respectively (D, E, F). The mean comparisons were done using the statistical test of Kruskal-Wallis (ns, non-significant)
Discussion
The major target tissue of SARS-CoV-2 is lungs but other tissues like heart and kidney might be involved [1, 2]. Whereases most of the patients with COVID-19 experience a mild form of the disease, the occurrence of acute respiratory distress syndrome (ARDS) is also frequent [20]. In the severe forms of the disease, uncontrolled production of inflammatory cytokines leads to cytokine storm, which in turn promotes intense clinical symptoms and complications [21]. Studies have revealed the critical involvement of MMPs (especially MMP-3) in the pathogenesis of lung-associated disorders, such as pulmonary fibrosis and ARDS. Additionally, MMP-3 deficiency was associated with normal function of lung surfactant, which in turn was related to lung protection during pathological settings [22].
Inflammatory mediators are involved in the stimulation of MMP-3 secretion form endothelial cells and fibroblasts. On the other hand, MMP-3 can also target, and hence regulate, proinflammatory cytokines like interleukin (IL)-1β and TNF‐α [23]. Several reports indicate that inflammation and dysregulated immune responses like cytokine storm and related inflammatory mediators (such as IL‐1β, IL-6 and TNF‐α) contribute to the pathology of COVID-19 [21]. A study by Shi et al. revealed that MMP-3 level was higher in the serum of COVID-19 patients that was correlated with serum levels of IL‐1β and IL‐6 [9]. As a result, MMPs might contribute to the inflammatory state in COVID-19 patients and worsen the clinical presentations of the suffering cases.
It was indicated that SARS-CoV-2 exerts Transmembrane serine protease 2 (TMPRSS2) to prime S protein that facilitates binding to ACE2 and entry to the target cells. Additionally, a TMPRSS2 inhibitor was suggested to block the virus entry and might be used as a therapeutic compound in the COVID-19 patients [24]. Additionally, it was reported that zinc metalloproteases like MMPs might contribute to the cell-cell fusion and entry of coronavirus [25]. As a consequence, MMPs are probably involved in facilitating the entry of virus to host cells. Our experiments also indicated higher serum levels of MMP-9 and MMP-3 in COVID-19 patients. Hence, it is worthy to explore for the potential treatment options in COVID-19 patients through investigating compounds that inhibit the function of MMPs.
Studies have established that rs3918242 as the functional SNP in the promoter region MMP9 gene affect the transcriptional level of this gene [26, 27]. Additionally, in vitro studies revealed that the C–1562 T SNP (rs3918242) is involved in suppressing the binding of nuclear repressor protein to the promoter region in which this SNP is harbored, resulting in promotion of the expression of MMP9 [28]. At the position − 1612/−1617 upstream of the transcription start site of MMP3 gene, insertion of adenosine into the promoter region leads to constitution of a polymonomeric series of six adenosines (which is called allele 6 A), whereas the wild type form occurs with five adenosines (named as allele 5 A). Studies have demonstrated that the presence of the 6 A allele was associated with the downmodulation MMP3 expression [29]. Here we hypothesized that genetic polymorphisms in the MMP genes might alter the protein levels of MMPs and contribute to the development of COVID-19 disease. At first, we detected that the T allele of MMP9 gene rs3918242 SNP (OR = 1.84) as well as the G allele (5 A) of MMP3 gene rs3025058 (OR = 2.3) were associated with increased risk of COVID-19. Moreover, the serum levels of both MMP-9 and MMP-3 were higher in the serum levels of COVID-19 cases. However, it was observed that none of the MMP-9 and MMP-3 had different levels among COVID-19 patients with different three genotypes for MMP9 rs3918242 and MMP3 rs3025058, respectively. As a result, it seems that genetic polymorphisms might not be involved in the regulation of the MMP levels in the COVID-19 patients. It should, however, be noted that there are several genetic polymorphisms in each of these genes that might control the transcription of MMPs that were not evaluated in this study.
It has been reported that MMP-9 play a role in the degradation of the BBB in multiple sclerosis (MS), which is a neurodegenerative disorder [8, 30]. MMP-9 degrades the ECM and myelin basic protein (MBP) in MS patients, resulting in infiltration of the inflammatory immune cells into the CNS [31–34]. It seems that increased permeability of BBB by MMP-9 alongside with promoted expression of ICAM-1 (which mediates the recruitment of immune cells through endothelium) on the endothelial cells by TNF-α facilitates the migration of virus-infected monocytes to the CSF [10]. Additionally, reports have shown the presence of SARS-CoV-2 nucleic acid in the CSF samples of COVID-19 patients [4]. Furthermore, increased number of immune cells in the CSF of COVID-19 cases was reported [35]. Additionally, level of MMP-10 in the spinal fluid was correlated with the level of neurologic dysfunction in COVID-19 cases [36]. Our previous research also revealed that monocytes in the CSF of COVID-19 patients with neurological syndrome secrets high levels of MMP-2, MMP-3, MMP-9, and MMP-12 that might result in disruption of blood-CSF barrier, which in turn might facilitate recruitment of more immunoinflammatory cells into CNS, culminating in presentation of neurological symptoms in the COVID-19 subjects [37].
Here we also observed that the levels of MMP-9 and MMP-3 were higher in the serum samples from COVID-19 cases with neurologic syndrome in comparison to the controls. We also detected significant association of MMP9 gene rs3918242 and MMP3 gene rs3025058 polymorphisms with the risk of COVID-19 with neurologic syndrome. Nonetheless, there were no significant differences in the levels of MMP-9 and MMP-3 in COVID-19 cases with neurologic syndrome harboring three genotypes for MMP9 rs3918242 and MMP3 rs3025058, respectively. Therefore, at least we can prematurely assert that MMP9 rs3918242 and MMP3 rs3025058 might not be involved in regulating the MMP-9 and MMP-3 in COVID-19 cases with neurologic manifestations. Probably other genetic markers in these gens as well as other regulatory mechanisms play a role in the modulation of MMPs in COVID-19 subjects with neurologic symptoms.
Considering all the facts, our attempt to disclose the probable implication of MMPs in risk of COVID-19 revealed that MMP9 gene rs3918242 and MMP3 gene rs3025058 SNP, but not MMP2 gene rs243865, was associated significantly with increased risk of the disease. Additionally, both these SNPs were associated with susceptibility to COVID-19 with neurologic symptoms. Although levels of MMP-9 and MMP-3 was higher in the serum of COVID-19 cases as well as COVID-19 individuals with neurologic syndrome, the related genetic polymorphisms might not be involved in the regulation of corresponding MMPs. Hence, we need to be armed with further investigation to understand the involvement of MMP genetic polymorphisms in raising neurologic complications in the COVID-19 cases.
Acknowledgements
The authors are grateful of the patients for their contribution in this study.
Abbreviations
- MMPs
Matrix metalloproteinases
- COVID-19
Coronavirus disease 2019
- ELISA
Enzyme-linked immunosorbent assay
- SARS-CoV‐2
Severe acute respiratory syndrome coronavirus 2
- ACE2
Angiotensin-converting enzyme 2
- CNS
Central nervous system
- RT-PCR
Reverse-transcriptase–polymerase chain-reaction
- CSF
Cerebrospinal fluid
- ECM
Extracellular matrix
- BBB
Blood-brain barrier
- ICAM-1
Intercellular adhesion molecule 1
- TNF
Tumor necrosis factor
- SNP
Single nucleotide polymorphism
- ICU
Intensive care unit
- OR
Odds ratios
- CI
confidence intervals
- HWE
Hardy–Weinberg Equilibrium
- SD
Standard deviation
- ARDS
Acute respiratory distress syndrome
- IL
Interleukin
- TMPRSS2
Transmembrane serine protease 2
- MS
Multiple sclerosis
- MBP
Myelin basic protein
Author contribution
Samaneh Ramezani; Performed experiments, prepared the draft of the paper, and read the manuscript critically. Fatemeh Ezzatifar; Performed experiments, prepared the draft of the paper, and read the manuscript critically. Tahereh Hojjatipour; Participated in experiments and drafting the paper, and read the manuscript critically. Maryam Hemmatzadeh; Participated in experiments and drafting the paper, and read the manuscript critically. Arezoo Gowhari Shabgah; Participated in experiments and drafting the paper, and read the manuscript critically. Jamshid Gholizadeh Navashenaq; Participated in experiments and drafting the paper, and read the manuscript critically. Saeed Aslani; Participated in experiments and drafting the paper, performed statistical analysis, and read the manuscript critically. Navid Shomali; Participated in experiments and drafting the paper, and read the manuscript critically. Mohsen Arabi; Participated in drafting the paper and read the manuscript critically. Farhad Babaie; Participated in drafting the paper and read the manuscript critically. Farhad Jadidi-Niaragh; Participated in drafting the paper and read the manuscript critically. Ramin Hosseinzadeh; Participated in drafting the paper and read the manuscript critically. Fahimeh Feizisani; Participated in drafting the paper and read the manuscript critically. Sara Khodayar; Participated in drafting the paper and read the manuscript critically. Roghaiyeh Safari; Developed the main idea, designed the work, interpreted the experiments, and read the manuscript critically. Hamed Mohammadi; Developed the main idea, designed the work, interpreted the experiments, and read the manuscript critically.
Funding
This study was financially supported by a grant from the Alborz University of Medical Sciences, Karaj, Iran (Grant No. 99-4209).
Data Availability
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
The study protocol was approved from the local Ethical Review committee located in Alborz University of Medical Sciences (Permission No. IR.ABZUMS.REC.1399.340) and written informed consent form was taken by all subjects.
Research involving human subjects and/or animals
Research carried out here were in compliance with the Helsinki Declaration. The protocol of this study was approved by the Human Research Ethics Committee from the Alborz University of Medical Sciences, Karaj, Iran (Permission No. IR.ABZUMS.REC.1399.340). Written informed consent forms were obtained from patients and healthy controls before blood taking.
Conflict of interest
The authors declare that they have no conflicting interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Roghaiyeh Safari, Email: roghaiyeh.safari@gmail.com.
Hamed Mohammadi, Email: mohamadi.h86@gmail.com, Email: h.mohammadi@abzums.ac.ir.
References
- 1.Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turner AJ, Hiscox JA, Hooper NM. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci. 2004;25(6):291–294. doi: 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoon S-O, Park S-J, Yun C-H, Chung A-S. Roles of matrix metalloproteinases in tumor metastasis and angiogenesis. J Biochem Mol Biol. 2003;36(1):128–137. doi: 10.5483/bmbrep.2003.36.1.128. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro SD. Matrix metalloproteinase degradation of extracellular matrix: biological consequences. Curr Opin Cell Biol. 1998;10(5):602–608. doi: 10.1016/S0955-0674(98)80035-5. [DOI] [PubMed] [Google Scholar]
- 8.Yong VW, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci. 2001;2(7):502. doi: 10.1038/35081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi S, Su M, Shen G, Hu Y, Yi F, Zeng Z, et al. Matrix metalloproteinase 3 as a valuable marker for patients with COVID-19. J Med Virol. 2021;93(1):528–532. doi: 10.1002/jmv.26235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desforges M, Le Coupanec A, Stodola JK, Meessen-Pinard M, Talbot PJ. Human coronaviruses: viral and cellular factors involved in neuroinvasiveness and neuropathogenesis. Virus Res. 2014;194:145–158. doi: 10.1016/j.virusres.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye S. Polymorphism in matrix metalloproteinase gene promoters: implication in regulation of gene expression and susceptibility of various diseases. Matrix Biol. 2000;19(7):623–629. doi: 10.1016/S0945-053X(00)00102-5. [DOI] [PubMed] [Google Scholar]
- 12.Kanamori Y, Matsushima M, Minaguchi T, Kobayashi K, Sagae S, Kudo R, et al. Correlation between expression of the matrix metalloproteinase-1 gene in ovarian cancers and an insertion/deletion polymorphism in its promoter region. Cancer Res. 1999;59(17):4225–4227. [PubMed] [Google Scholar]
- 13.dos Reis ST, Pontes J, Jr, Villanova FE, de Andrade Borra PM, Antunes AA, Dall’oglio MF, et al. Genetic polymorphisms of matrix metalloproteinases: susceptibility and prognostic implications for prostate cancer. J Urol. 2009;181(5):2320–2325. doi: 10.1016/j.juro.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Yong VW, Zabad RK, Agrawal S, DaSilva AG, Metz LM. Elevation of matrix metalloproteinases (MMPs) in multiple sclerosis and impact of immunomodulators. J Neurol Sci. 2007;259(1–2):79–84. doi: 10.1016/j.jns.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Hassanzadeh-Makoui R, Razi B, Aslani S, Imani D, Tabaee SS. The association between Matrix Metallo-proteinases-9 (MMP-9) gene family polymorphisms and risk of Coronary Artery Disease (CAD): a systematic review and meta-analysis. BMC Cardiovasc Disord. 2020;20(1):232. doi: 10.1186/s12872-020-01510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh H, Nain S, Krishnaraj A, Lata S, Dhole TN. Genetic variation of matrix metalloproteinase enzyme in HIV-associated neurocognitive disorder. Gene. 2019;698:41–49. doi: 10.1016/j.gene.2019.02.057. [DOI] [PubMed] [Google Scholar]
- 17.Herbster S, Paladino A, de Freitas S, Boccardo E (2018) Alterations in the expression and activity of extracellular matrix components in HPV-associated infections and diseases. Clinics (Sao Paulo, Brazil) 73 (suppl 1):e551s. 10.6061/clinics/2018/e551s [DOI] [PMC free article] [PubMed]
- 18.Mohammadhosayni M, Khosrojerdi A, Lorian K, Aslani S, Imani D, Razi B, et al. Matrix metalloproteinases (MMPs) family gene polymorphisms and the risk of multiple sclerosis: systematic review and meta-analysis. BMC Neurol. 2020;20(1):218. doi: 10.1186/s12883-020-01804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behl T, Kaur G, Sehgal A, Bhardwaj S, Singh S, Buhas C et al (2021) Multifaceted Role of Matrix Metalloproteinases in Neurodegenerative Diseases: Pathophysiological and Therapeutic Perspectives. Int J Mol Sci 22(3). 10.3390/ijms22031413 [DOI] [PMC free article] [PubMed]
- 20.Han S, Mallampalli RK. The acute respiratory distress syndrome: from mechanism to translation. J Immunol. 2015;194(3):855–860. doi: 10.4049/jimmunol.1402513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ebrahimi N, Aslani S, Babaie F, Hemmatzadeh M, Hosseinzadeh R, Joneidi Z et al (2020) Recent findings on the Coronavirus disease 2019 (COVID-19); immunopathogenesis and immunotherapeutics. International immunopharmacology:107082 [DOI] [PMC free article] [PubMed]
- 22.Yamashita CM, Cybulskie C, Milos S, Zuo YY, McCaig LA, Veldhuizen RA. The effect of matrix metalloproteinase-3 deficiency on pulmonary surfactant in a mouse model of acute lung injury. Can J Physiol Pharmacol. 2016;94(6):682–685. doi: 10.1139/cjpp-2015-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nissinen L, Kähäri V-M (2014) Matrix metalloproteinases in inflammation. Biochimica et Biophysica Acta (BBA)-General Subjects. 1840:2571–25808 [DOI] [PubMed]
- 24.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271–280e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips JM, Gallagher T, Weiss SR (2017) Neurovirulent murine coronavirus JHM. SD uses cellular zinc metalloproteases for virus entry and cell-cell fusion.Journal of virology91 (8) [DOI] [PMC free article] [PubMed]
- 26.Fernandes KS, Brum DG, Sandrim VC, Guerreiro CT, Barreira AA, Tanus-Santos JE. Matrix metalloproteinase-9 genotypes and haplotypes are associated with multiple sclerosis and with the degree of disability of the disease. J Neuroimmunol. 2009;214(1–2):128–131. doi: 10.1016/j.jneuroim.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 27.La Russa A, Cittadella R, De Marco EV, Valentino P, Andreoli V, Trecroci F, et al. Single nucleotide polymorphism in the MMP-9 gene is associated with susceptibility to develop multiple sclerosis in an Italian case-control study. J Neuroimmunol. 2010;225(1–2):175–179. doi: 10.1016/j.jneuroim.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 28.Zhang B, Henney A, Eriksson P, Hamsten A, Watkins H, Ye S. Genetic variation at the matrix metalloproteinase-9 locus on chromosome 20q12. 2–13.1. Hum Genet. 1999;105(5):418–423. doi: 10.1007/s004390051124. [DOI] [PubMed] [Google Scholar]
- 29.Souslova V, Townsend PA, Mann J, van der Loos CM, Motterle A, D’Acquisto F, et al. Allele-specific regulation of matrix metalloproteinase-3 gene by transcription factor NFκB. PLoS ONE. 2010;5(3):e9902. doi: 10.1371/journal.pone.0009902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waubant E. Biomarkers indicative of blood-brain barrier disruption in multiple sclerosis. Dis Markers. 2006;22(4):235–244. doi: 10.1155/2006/709869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woessner JF., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5(8):2145–2154. doi: 10.1096/fasebj.5.8.1850705. [DOI] [PubMed] [Google Scholar]
- 32.Chandler S, Coates R, Gearing A, Lury J, Wells G, Bone E. Matrix metalloproteinases degrade myelin basic protein. Neurosci Lett. 1995;201(3):223–226. doi: 10.1016/0304-3940(95)12173-0. [DOI] [PubMed] [Google Scholar]
- 33.Gijbels K, Proost P, Masure S, Carton H, Billiau A, Opdenakker G. Gelatinase B is present in the cerebrospinal fluid during experimental autoimmune encephalomyelitis and cleaves myelin basic protein. J Neurosci Res. 1993;36(4):432–440. doi: 10.1002/jnr.490360409. [DOI] [PubMed] [Google Scholar]
- 34.Proost P, Vandamme J, Opdenakker G. Leukocyte gelatinase B cleavage releases encephalitogens from human myelin basic protein. Biochem Biophys Res Commun. 1993;192(3):1175–1181. doi: 10.1006/bbrc.1993.1540. [DOI] [PubMed] [Google Scholar]
- 35.Neumann B, Schmidbauer ML, Dimitriadis K, Otto S, Knier B, Niesen W-D et al (2020) Cerebrospinal fluid findings in COVID-19 patients with neurological symptoms. Journal of the neurological sciences 418 [DOI] [PMC free article] [PubMed]
- 36.Remsik J, Wilcox JA, Babady NE, McMillen TA, Vachha BA, Halpern NA, et al. Inflammatory leptomeningeal cytokines mediate COVID-19 neurologic symptoms in cancer patients. Cancer Cell. 2021;39(2):276–283. doi: 10.1016/j.ccell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohammadhosayni M, Mohammadi FS, Ezzatifar F, Gorabi AM, Khosrojerdi A, Aslani S, et al. Matrix metalloproteinases are involved in the development of neurological complications in patients with Coronavirus disease 2019. Int Immunopharmacol. 2021;100:108076. doi: 10.1016/j.intimp.2021.108076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.